Abstract
Maternal immune activation (MIA), a maternal stressor, increases risk for neuropsychiatric diseases, such as Major Depressive Disorder in offspring. MIA of toll-like receptor 7 (TLR7) initiates an immune response in mother and fetuses in a sex-selective manner. The paraventricular nucleus of the hypothalamus (PVN), a brain region that is sexually dimorphic and regulates hypothalamic-pituitary-adrenal (HPA) stress responses, have been tied to stress-related behaviors (i.e., depression, anxiety, social impairments). The current study characterized the sex-selective impact of mid-gestational TLR7 activation on PVN vasculature of adult offspring based on a prior study of excess prenatal glucocorticoid stress. The PVN of offspring were evaluated to determine if fetal MIA impacted vascular leakage in the brains of adult mice with or without restraint stress. Timed-pregnant female mice were administered the TLR7 agonist Resiquimod (RQ) or saline vehicle on embryonic day (E) 12.5. Basal and restraint stress-induced corticosterone was measured to examine changes in stress response. Mice were perfused transcardially with fluorescein isothiocyanate (FITC) to assess blood vessel integrity. Sections with FITC-labeled blood vessels through the PVN of offspring were immunolabeled for Glial Fibrillary Acidic Protein (GFAP; astrocytic end feet) and IBA-1 (microglia). MIA with RQ led to elevated levels of plasma corticosterone 60-minutes after restraint in offspring, suggesting prenatal RQ impairs glucocorticoid negative feedback. Blood-brain barrier integrity was assessed. Adult offspring of RQ injected dams showed greater leakage in the PVN (greater in males than females). GFAP+ colocalization with FITC-labeled vessels was lower in the PVN of offspring from RQ treated dams, potentially contributing to the observed increased FITC leakage. Microglia were examined in relation to the vasculature as an indicator of a neuroimmune response. Data show IBA-1+ cells greater in size and number in the PVN with closer proximity to blood vessels after maternal injection of RQ in a male-selective manner. Microglia were unchanged in females from RQ-treated dams but were smaller in size after restraint. This study provides support for sex-selective influences of fetal immune antecedents for altered brain vascular and blood brain barrier development and adult neuroendocrine function that could indicate a PVN locus for increased susceptibility for adult disorders.
Keywords: Maternal immune activation, neurovascular, blood-brain barrier, astrocytes, microglia, depression
1. Introduction
Fetal brain programming is heavily influenced by the physiological environment of the mother(Sheng et al., 2023). Stressors ranging from immune insults to psychological stressors lead to elevated maternal glucocorticoids (GCs) and inflammatory cytokines(Schepanski et al., 2018; Sheng et al., 2020). Stimulation or inhibition of the maternal immune system during middle to late gestation can lead to dysregulation of fetal brain circuitry, behavior, and cerebral vasculature(Frahm and Tobet, 2015; Gilman et al., 2016; Goldstein et al., 2021; Goldstein et al., 2014). Such phenotypes increase risk for associated mood and autonomic disorders, including Major depressive disorder (MDD) and cardiometabolic disease(Bale et al., 2010; Dearing et al., 2022; Goldstein et al., 2014; Madhavpeddi et al., 2022). MDD is sex-selective, with a 2x higher incidence in women(Handa et al., 2022; Sheng et al., 2021), while heart disease shows twice the incidence in men than women(Bots et al., 2017).
The paraventricular nucleus (PVN) is a sexually dimorphic nucleus in the brain and the central regulator of the HPA axis (Borrow et al., 2019; Heck et al., 2020). The PVN is a nexus for the integration of inputs from other brain regions(Cottrell and Seckl, 2009), and plays a key role in the central response to environmental stressors. Dysregulation of PVN development or circuitry negatively alters the stress response and increases susceptibility for stress-related disorders later in life in rodent (Brunton, 2013; Grundwald and Brunton, 2015) and human studies (Bale, 2011; Lautarescu et al., 2020). The current study examined neuroendocrine stress outputs in adults, indicative of potential HPA stress axis changes that were programmed in utero when mice were exposed to maternal immune activation (MIA). In previous experiments, a toll-like receptor (TLR) 7 agonist Resiquimod (RQ) was injected during mid-gestation leading to an increase in peripheral immune responses in mother and fetus(Sheng and Tobet, 2024). Offspring of RQ-injected mothers exhibited developmental delay and stress-related behavioral symptoms, including social impairments, and anxiety- and anhedonia-like behaviors, in a sex-dependent manner. As these behavioral phenotypes can be regulated by PVN neurons, the current study focused on investigating cellular changes, such as vasculature impairment, in this region(Frahm et al., 2018; Frahm and Tobet, 2015).
The PVN is 2–3 times more densely vascularized than surrounding regions in the brain(Frahm et al., 2012; Palkovits et al., 1984). The blood-brain barrier (BBB) offers protection from potentially harmful peripheral compounds and is comprised of endothelial cells connected by tight junctions, astrocytic end-feet, and pericytes. Overexposure to glucocorticoids (GC) during fetal development lowered blood vessel density in the PVN of juvenile mice(Frahm and Tobet, 2015) and revealed a potential relationship between BBB integrity in the PVN and depression-like behavior in adulthood (Frahm, 2018). Studies in humans have associated BBB disruption with MDD and other neurological diseases(Labonte et al., 2017; Menard et al., 2017), driven by reduced tight junctions between endothelial cells(Dion-Albert et al., 2022b; Greene et al., 2020) and alterations in astrocytes surrounding capillary endothelia(Rajkowska and Miguel-Hidalgo, 2019; Rajkowska and Stockmeier, 2013). Microglia are important regulators of central immune function and may indicate locations of impaired BBB function and elevated vascular leakage in disease(Ronaldson and Davis, 2020; Sequeira and Bolton, 2023). Several studies with rodent offspring of mothers exposed to TLR 3 activation by poly I:C reported changes in microglial morphology and secreted chemokines and cytokines in areas of tissue damage or infiltration of harmful substances (Block et al., 2022; Guma et al., 2021; Loayza et al., 2023; Loewen et al., 2023; Zhao et al., 2022). As a follow up to a previous report on offspring behavioral changes (Sheng and Tobet, 2024), the current study tests the hypothesis that exposure to fetal immune stress predisposed the offspring to be more sensitive to a second stressor in adulthood with an altered acute stress response and impaired BBB integrity in the adult PVN.
2. Methods
2.1. Mice
Adult female C57BL/6 N female mice (sexually naïve, 6–8 weeks old) were monitored daily by vaginal lavage for 1 week to identify estrous cyclicity. Females on day of proestrus were time-mated with a C57BL/6 N adult stud male (8 weeks old) and removed the following day to their own cage. This day was noted as embryonic day (E) 0. Upon successful pregnancy, females were injected with RQ (HY-13740, MedChemExpress; s.c. 2 mg/kg body weight) dissolved in phosphate buffered saline (0.05 M PBS) or vehicle (VEH; PBS) on E12.5. Pregnant females were allowed to parturition and noted as postnatal day (P) 0 (Fig. 1A). Sex of neonates was determined by anogenital separation on P0 and all litters were culled to 6 pups (3 male, 3 female; randomized selection of pups for each sex) to avoid litter sex bias(Agnish and Keller, 1997). Litters were culled to equal numbers of males and females to reduce variability of maternal behavior toward one sex (usually males) and litter-size to optimize pup growth and development. One pup of each sex from each litter was used in all studies to avoid a litter effect. Mice were housed with ad libitum access to food and water and on a 12:12 light: dark cycle (lights on at 06:00 and off at 18:00). All mice were euthanized by isoflurane delivered in a sealed chamber at a fill rate of 30 – 70 % chamber volume per minute until breathing ceased, consistent with Colorado State University’s Institutional Animal Care and Use Committee. This was followed by exsanguination by intracardial perfusion at 4 mL/min with fluorescein isothiocyanate (FITC; 1 μg/mL) phosphate buffered saline and 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) to fix tissues according to American Veterinary Medical Association approved methods. FITC is a small, 496 kDa molecule, which can bind to proteins in extracellular matrix and cell surfaces in and outside of blood vessels allows visualization of blood vessels and surrounding tissues when leakage occurs (Frahm 2014, modified from (Miyata and Morita, 2011)). All procedures were approved by Colorado University Lab Animal Resources and Institutional Animal Care and Use Committee Guidelines under protocol #1567.
Fig. 1. Experimental timeline and plasma corticosterone (CORT) measurements indicative of impaired HPA axis stress reactivity in adult offspring.
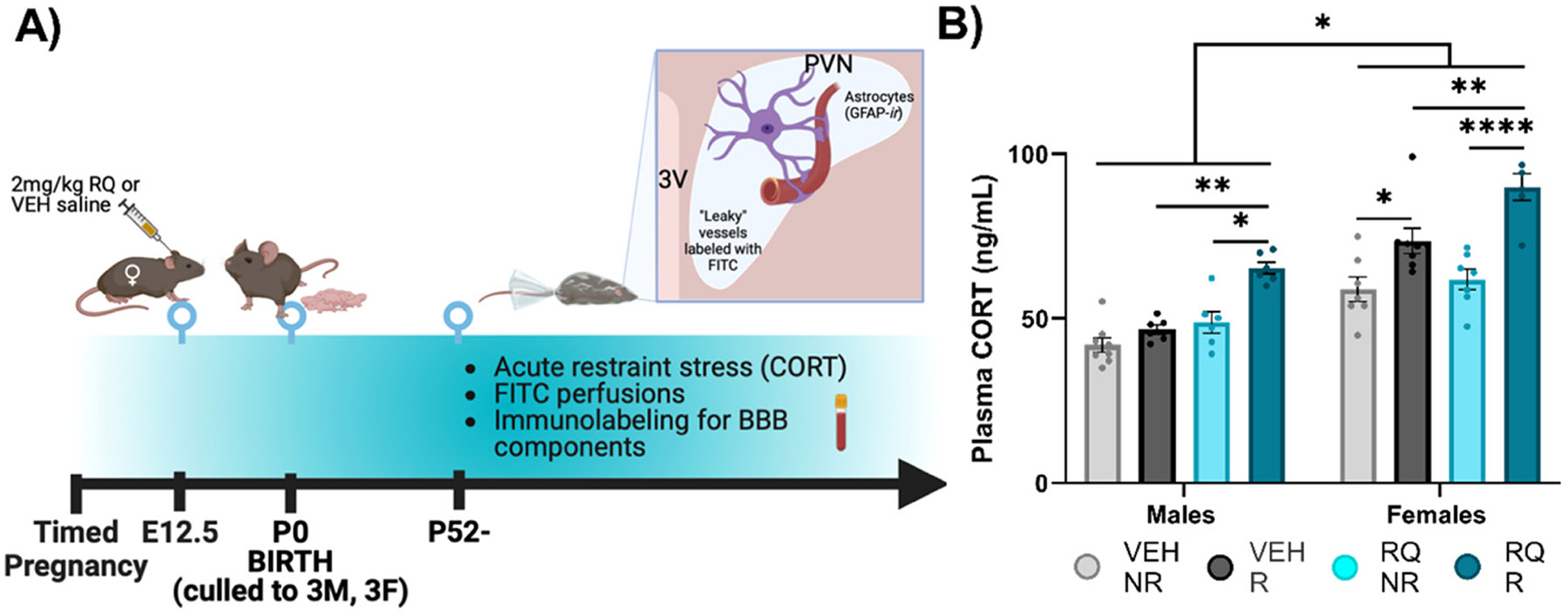
(A) Experimental timeline. (B) In males, plasma CORT was elevated in RQ-R adult offspring compared to the other groups. Plasma CORT was greater in restraint/recovery groups in adult female offspring (VEH-R/RQ-R vs. NR) and overall greater with prenatal RQ treatment (RQ-NR/RQ-R vs. VEH). E = embryonic day, P = postnatal day, HPA = Hypothalamic-Pituitary-Adrenal, VEH = vehicle, RQ = Resiquimod, NR = no restraint, R = restraint H-recovery, CORT = corticosterone, PVN = paraventricular nucleus of the hypothalamus, 3 V = 3rd ventricle, FITC = fluorescein isothiocyanate, IBA-1 = ionizing binding calcium adaptor molecule-1. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Error bars represented as +/− SEM. n = 6–9 mice / group.
2.2. Acute restraint and plasma corticosterone assays
Adult (P52) C57 male and female offspring were restrained inside a plastic 50 mL conical for 20-minutes with restricted movement followed by 60-minutes of recovery. The 50 mL conical was a spatially constricted tube with a breathing hole on one end and holes along the lateral sides for increased ventilation. After the 60-minutes of recovery in the home cage, the animal was euthanized by inhalation of isoflurane in a sealed chamber until breathing was ceased. Animal was euthanized by intracardial perfusion as previously described above(Frahm and Tobet, 2015). Restraint stress was performed between 09:00 and 14:00 to avoid diurnal elevations in corticosterone. Cardiac blood was collected and placed into chilled 0.5 M EDTA/aprotinin tubes 2μg/mL; Sigma-Aldrich, St. Louis, MO). Blood was centrifuged in a Beckman J6 centrifuge at 2000 rpm at 4 °C for 10 minutes. Separated plasma was stored at −20° C until assayed. Plasma corticosterone levels were measured by Enzyme-Linked ImmunoSorbent Assay (ELISA) per manufacturer’s guidelines (Arbor Assays, Ann Arbor, MI; cat no. K014-H1; Limit of detection 7.7 μg/mL mean intra-assay CV = 8.5 %). 5uL of plasma samples (run in duplicates) were combined with dissociation reagent provided with the ELISA kit (Catalog # X058. This allowed dissociation of the corticosterone from corticosteroid binding globulin. The optical density of each sample was determined at a wavelength of 450 nm in Azure biosystems AO microplate reader (Azure Biosystems, Inc, Dublin, CA). The optical density readings for the standards and samples were used to calculate the concentration of corticosterone. A standard curve was generated using the online tool from “MyAssays” through Arbor Assays. The sample concentrations were calculated from the %B/B0 curve and multiplied by the dilution factor to obtain the neat sample values. Values were analyzed with GraphPad Prism (v10, La Jolla, CA) by 3-Way ANOVA to examine the effect of prenatal RQ treatment X sex X restraint. Šídák’s multiple comparisons post-hoc analysis was performed, where appropriate.
2.3. Immunolabeling for blood vessels (FITC), astrocytes (GFAP), and microglia (IBA-1)
Mice were perfused intracardially with FITC; (1 μg/mL) in PBS (pH 7.4.), followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4.) (as described above). Brains were dissected and post-fixed in 4 % paraformaldehyde overnight followed by immersion in 0.05 M PBS until processing. Immunohistochemistry methods were followed as previously described(Frahm et al., 2018; Frahm and Tobet, 2015). Briefly, brains from male and female mice were sectioned coronally at 50 μm with a vibrating microtome (Leica VT1000S). Free-floating sections were collected in 0.05 M PBS, followed by treatment with 0.1 M glycine to neutralize unreacted aldehydes. Sections were then incubated in 0.5 % sodium borohydride prior to being placed in blocking serum [0.5 % Triton X-100 (Tx), 1 % hydrogen peroxide, 5 % normal goat serum (NGS)]. Sections were then incubated in primary antisera for 48 h at 4°C against glial fibrillary acidic protein (GFAP; 1:250, AB_10013382; Z0334, Dako) for astrocytes and ionized calcium binding adaptor molecule 1 (IBA-1; 1:1000, 0.1 mg/mL, Cat# 019–19741, Wako) for microglia. After 48 hours, all sections were washed at room temperature in 0.05 M PBS with 1 % NGS and 0.02 % Tx. The tissue was incubated in secondary antiserum containing Cy3 conjugated anti-rabbit (1:500), 1 % NGS, and 0.32 % Tx for 2 hours and washed in 0.05 M PBS. Sections were mounted onto SuperFrost slides and cover-slipped with Aqua-Poly/Mount prior to imaging.
2.4. Imaging and analysis
FITC was detected using a 505/530 nm emission filter on a confocal microscope (Carl Zeiss LSM880) with an Axiocam 503 monochromatic camera. GFAP and IBA-1 immunoreactivity (-ir) were imaged with Cy3, detected with a 585/615 emission filter. Images were acquired for the PVN and two control regions [cerebral cortex (CX) and lateral hypothalamus (LH)]. All images were taken in 10 μm z-stacks with two optical sections for every 1 μm using a 40x/0.95 Corr M27 (Plan-Apochromat) oil immersion objective. Images were analyzed by an investigator blinded to treatment groups and sex. For FITC extravascular leakage, z-stack images were compiled in FIJI (ImageJ, v1.54 f) using a maximum intensity z-projection. The mean gray area of a 2.97 ×2.97 μm selection (area = 8.793μm2) inside (intravascular FITC) and outside (extravascular FITC), directly adjacent to the selection on the inside of the blood vessel, was measured. The ratio of extravascular FITC to intravascular FITC was then determined by dividing the mean gray area of the outside selection by that of the inside adjacent selection to account for differences in quality of the perfusion. This ratio was taken 12 times for each image and averaged for the single output value per image. For GFAP and IBA-1 in proximity to FITC (blood vessels), images of FITC-labeled blood vessels and GFAP-ir or IBA-1-ir were independently z-projected to max intensity and thresholded in FIJI (GFAP threshold to 20 % intensity, IBA-1 threshold to 10 % intensity). The colocalization plug-in was used to determine the percent of colocalization of GFAP or IBA-1-ir with proximity (within 2 pixels = 1.18 μm) of FITC-labeled blood vessels. The number, area size, and total percent immunoreactivity of IBA-1 positive cells was also measured using Analyze Particles on thresholded images in ImageJ. Total percent area immunoreactivity was also measured for GFAP-labeled cells. FITC leakage analysis and immunolabeled protein values were statistically analyzed with GraphPad Prism (v10, La Jolla, CA) by 3-Way ANOVA to examine the effect of prenatal sex x RQ treatment X restraint. Šídák correction factor was used for multiple comparisons post-hoc analysis.
3. Results
3.1. Plasma corticosterone indicative of impaired HPA axis stress reactivity in adult offspring
Plasma CORT levels were assessed following a 60 min recovery period after a 20 min restraint stress (Fig. 1). ANOVA (3-way; Sex x RQ treatment x Restraint) revealed main effects of prenatal RQ treatment [F (1, 46) = 25.16, P = 0.0001], restraint [F (1, 46) = 51.17, P = 0.0001] and sex [F (1, 46) = 83.05, P = 0.0193] (Fig. 1B). An interaction of sex x restraint [F (1, 46) = 5.884, P = 0.0193] was evident in the graph (Fig. 1B) as females had reliably greater CORT responses than males after restraint. An interaction of prenatal RQ treatment x restraint [F (1, 46) = 7.903, P = 0.0072] (Fig. 1B) was evident in the more reliably greater CORT levels in prenatal RQ-offspring after restraint. The 3-way interaction was not statistically significant (P > 0.10). Detailed post hoc tests showed that VEH-treated female offspring exhibited 20 % higher levels of CORT by restraint (VEH-NR vs. VEH-R p = 0.0131) and a 27.1 % increase in CORT in RQ-treated animals with restraint (RQ-NR vs. RQ-R p = 0.0001). RQ-R females additionally showed 11.7 % higher CORT than VEH-R females (p = 0.0064). In males, post hoc Šídák multiple comparisons tests showed a significant difference in levels of plasma CORT in RQ males that underwent restraint compared to VEH males (28.5 % higher in RQ-R vs. VEH-R p = 0.0032). RQ-R males showed 26.5 % higher levels of plasma CORT after restraint compared to RQ-NR (p = 0.0129). In contrast, CORT levels in VEH-R males were not different from levels seen in VEH non-restrained controls within an hour of the restraint.
3.2. BBB permeability in the PVN was impaired in adult offspring of RQ injected mothers
Total vascular area in the PVN as indicated by the area of the fluorescent blood vessels in the region of the mid-PVN was similar across all offspring. The permeability of the BBB in the PVN was greater in adult offspring exposed to MIA (Fig. 2). Vascular leakage was determined by the ratio of extravascular to intravascular FITC (mean gray area intensity). ANOVA (3-way; Sex x RQ treatment x Restraint) showed significantly greater leakage from FITC-labeled blood vessels in the PVN of males and females with main effects of prenatal RQ treatment (Fig. 2A; [F (1, 65) = 144.8, P = 0.0001]) and restraint [F (1, 65) = 16.21, P = 0.0002]. Interestingly, the 3-way ANOVA showed a significant interaction of sex x restraint [F (1, 65) = 16.21, P = 0.0027] was driven by evidence that restraint appeared to selectively drive additional leakage in male offspring independent of prenatal exposure. In males the comparisons between VEH-NR and VEH-R (p = 0.0045) and between RQ-NR and RQ-R (p = 0.0190) were both significant while neither were noticeably different in females. A significant interaction of sex x RQ treatment [F (1, 65) = 4.940, P = 0.027] was driven by evidence that RQ-offspring regardless of restraint stress showed more leakage in RQ-males than RQ-females. RQ-Males had 33.3 % higher FITC leakage regardless of restraint (VEH-NR vs. RQ-NR p = 0.0001, VEH-R vs. RQ-R p = 0.0001, Šídák’s post-hoc test). RQ females also displayed 25.6 % greater FITC leakage in the PVN of VEH-NR vs. RQ-NR (p = 0.00011) and VEH-R and RQ-R (p = 0.0001) groups. Leakage from FITC-labeled blood vessels was selective to the PVN. As in a prior study (Frahm et al., 2018) (Frahm) two control regions that were in the same sections (LH, CX) showed little evidence of leakage between groups. In the LH, there were main effects of prenatal RQ treatment [F (1, 55) = 6.551, P = 0.0133] and restraint [F (1, 55) = 12.78, P = 0.0007]. The main effects were tempered by an interaction of prenatal RQ treatment x restraint [F (1, 55) = 12.04, P = 0.0010] due to greater leakage in Veh-offspring exposed to restraint stress. This can be traced by Šídák’s post-hoc analysis primarily to a 15 % higher FITC leakage in the LH of females by restraint (VEH-NR vs VEH-R, p = 0.0164) (Fig. 2B). It was similar in males, but not statistically significant. In the CX, 3-Way ANOVA did not reveal a main or interaction effect.
Fig. 2. BBB permeability in the PVN is impaired in adult offspring of RQ injected mothers.
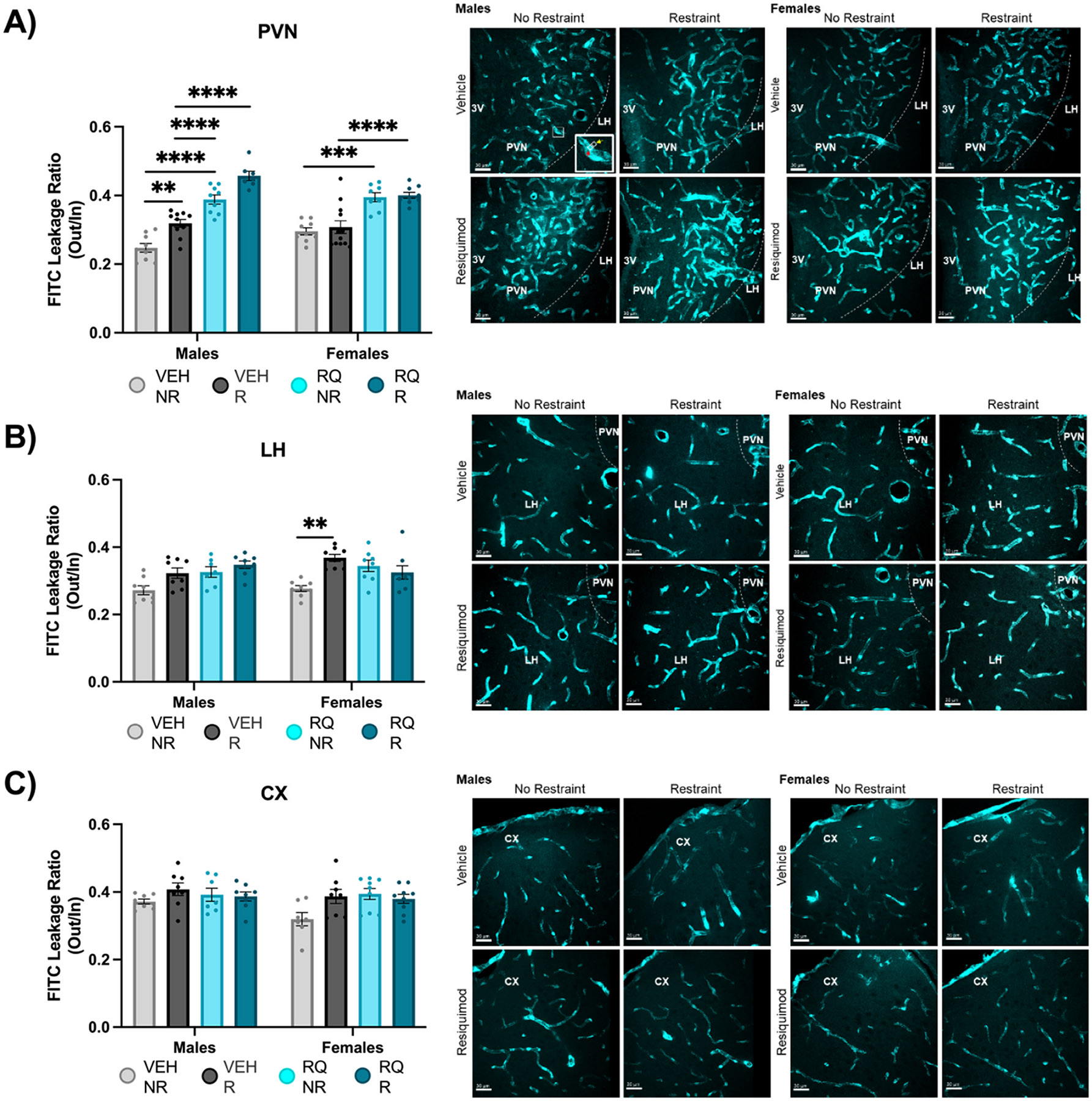
Permeability was determined by FITC leakage in the (A) PVN, (B) LH, and (C) CX. Representative images to the right of each graph. The enlarged image in the bottom right corner of Male VEH-NR in (A) shows how the FITC ratio out (yellow arrow): in (red arrow) was measured. Mean gray area of the extravascular FITC (8.793μm2 selection) was divided by the mean gray area of the intravascular FITC (8.793μm2 selection) to obtain FITC leakage ratio. VEH = vehicle, RQ = Resiquimod, NR = no restraint, R = restraint, PVN = Paraventricular Nucleus of the Hypothalamus, LH = Lateral Hypothalamus, CX = Cortex. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Error bars represented as +/− SEM. n = 8–10 animals / group.
3.3. Astrocytes less localized with PVN vessels of offspring from RQ-injected mothers
Astrocyte localization measured by GFAP-ir relative to blood vessels was altered in offspring of RQ-injected mothers. The percent of FITC-labeled blood vessel coverage by GFAP-ir was measured in the PVN (Fig. 3A). ANOVA (3-way - Sex x RQ treatment x Restraint) revealed a main effect of prenatal RQ treatment in the PVN [F (1, 50) = 46.96, P = 0.0001] indicating that RQ-offspring had reliably less blood vessel coverage than VEH-offspring. The main effect of sex was not significant, however, the significant interaction of sex by prenatal treatment indicated that there was a sex difference among RQ-offspring [F (1, 50) = 5.109, P = 0.0282]. In females, the percent of FITC-labeled blood vessels by GFAP was 30 % less in RQ no restraint and restraint groups compared to VEH groups in the PVN (RQ-NR vs. VEH-NR p = 0.0002, RQ-R vs. VEH-R p = 0.0006). Males showed 20 % less blood vessel coverage by GFAP in offspring of RQ-injected mothers without restraint with Šídák’s post-hoc analysis test (RQ-NR vs. VEH-NR p = 0.0451). There was no apparent influence of adult restraint for either main effect of interaction. Overall, there was no significant effect of total GFAP-ir in the PVN (Fig. 3B). Despite notable differences in the relationship of GFAP immunoreactive astrocytes to blood vessels among the groups, there were no significant effects of sex or treatment on the total area of GFAP-ir area (Fig. 3B). Therefore, astrocyte differences were driven by their distribution rather than their size or number.
Fig. 3. Astrocytes were less co-localized with PVN vessels of offspring from RQ-injected mothers.
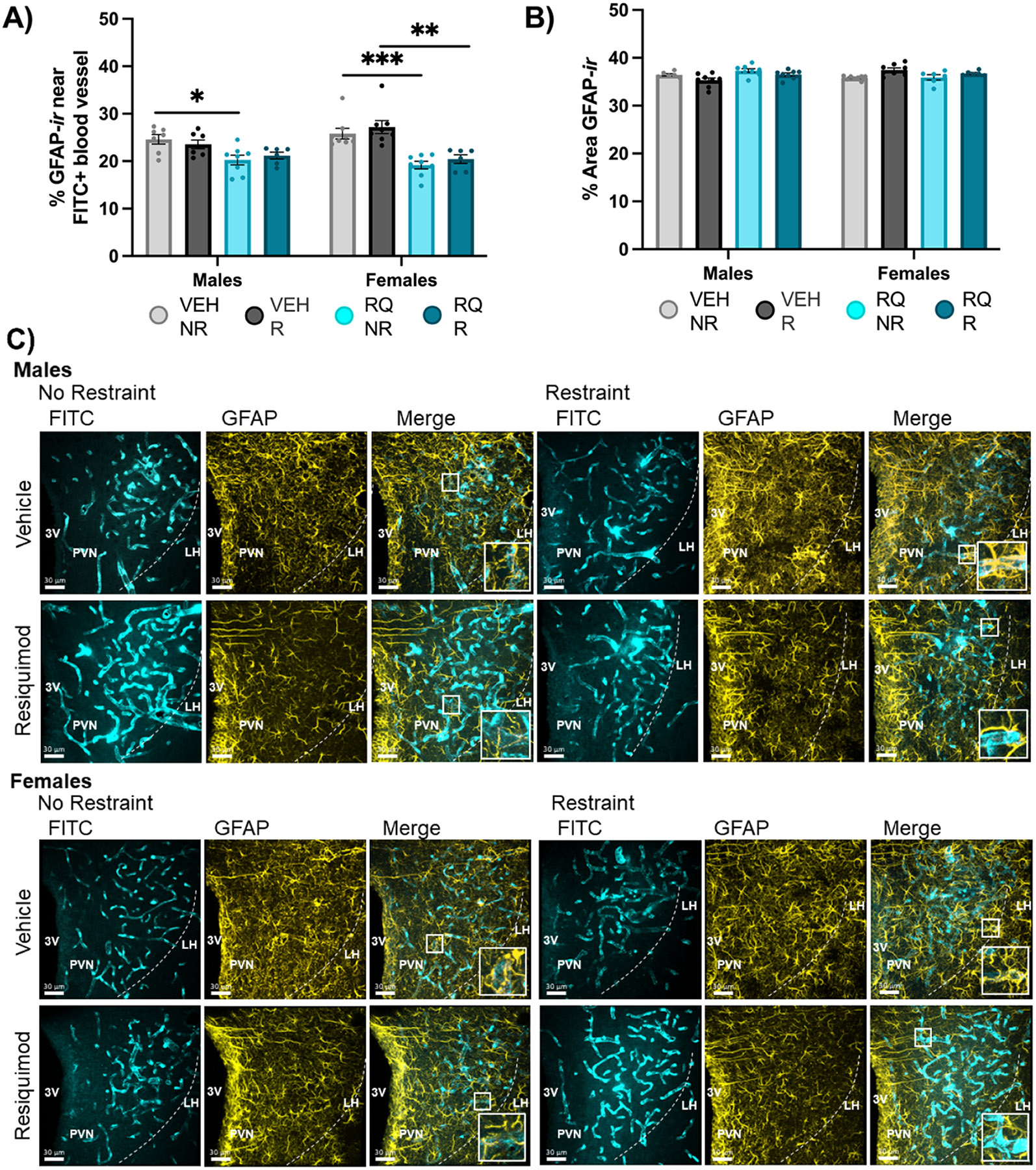
(A) GFAP-labeled astrocyte coverage of PVN blood vessels was lower in male and female adult mice exposed to prenatal RQ. (B) The total immunoreactivity denoted by ”% Area GFAP-ir” was higher in VEH-R females compared to no restraint counterparts. (C) Representative images. VEH = vehicle, RQ = Resiquimod, NR = no restraint, R = restraint, PVN = Paraventricular Nucleus of the Hypothalamus, 3 V = 3rd ventricle, FITC = fluorescein isothiocyanate, GFAP = glial fibrillary acidic protein, F = Female, M = Male. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Error bars represented as +/− SEM. n = 6–8 animals / group.
3.4. Microglial localization and morphology altered in offspring of RQ-injected mothers
Microglia are not a component of the BBB but may serve as indicators of BBB permeability if they respond to leaked molecular signals. Offspring of maternal RQ-injection displayed different microglia localization relative to blood vessels in the PVN, along with alterations in size and number. The percent of IBA-1-positive microglia within 2 pixels (1.18 μm) proximity to FITC-labeled blood vessels were evaluated in the PVN (Fig. 4A). 3-way ANOVA showed main effects of sex [F (1, 53) = 4.141, P = 0.0469], prenatal RQ [F (1, 53) = 27.14, P = 0.0001], and adult restraint [F (1, 53) = 27.14, P = 0.0076]. However, an examination of the significant interaction of sex x prenatal RQ treatment in indicates that the prenatal RQ treatment was selectively impactful in males [F (1, 53) = 6.091, P = 0.0169]. The significant interaction of and sex x restraint [F (1, 53) = 6.129, P = 0.0165] indicates that the acute restraint stress was selectively impactful in females. In RQ-males, microglia were in closer proximity to blood vessels than VEH-males. In females, regardless of RQ fetal exposure, only restraint stress in adults led to microglia to be further from blood vessels. There was 33.3 % more IBA-1 immunoreactive cells in proximity to blood vessels in RQ compared to VEH males (Šídák’s post-hoc test, RQ-NR vs. VEH-NR p = 0.0254, RQ-R vs. VEH-R p = 0.0008). In females there was 25.8 % less immunoreactive IBA-1 in proximity to blood vessels in VEH-treated offspring (VEH-R vs. VEH-NR, p = 0.0146). There was no effect of RQ in microglia proximity to FITC-labeled blood vessels in females. There was, however, 20 % more immunoreactive IBA-1 in proximity to FITC-labeled vessels in RQ-R males than RQ-R females (p = 0.0117).
Fig. 4. Microglia localization, size, and number differed among offspring of prenatally injected mothers.
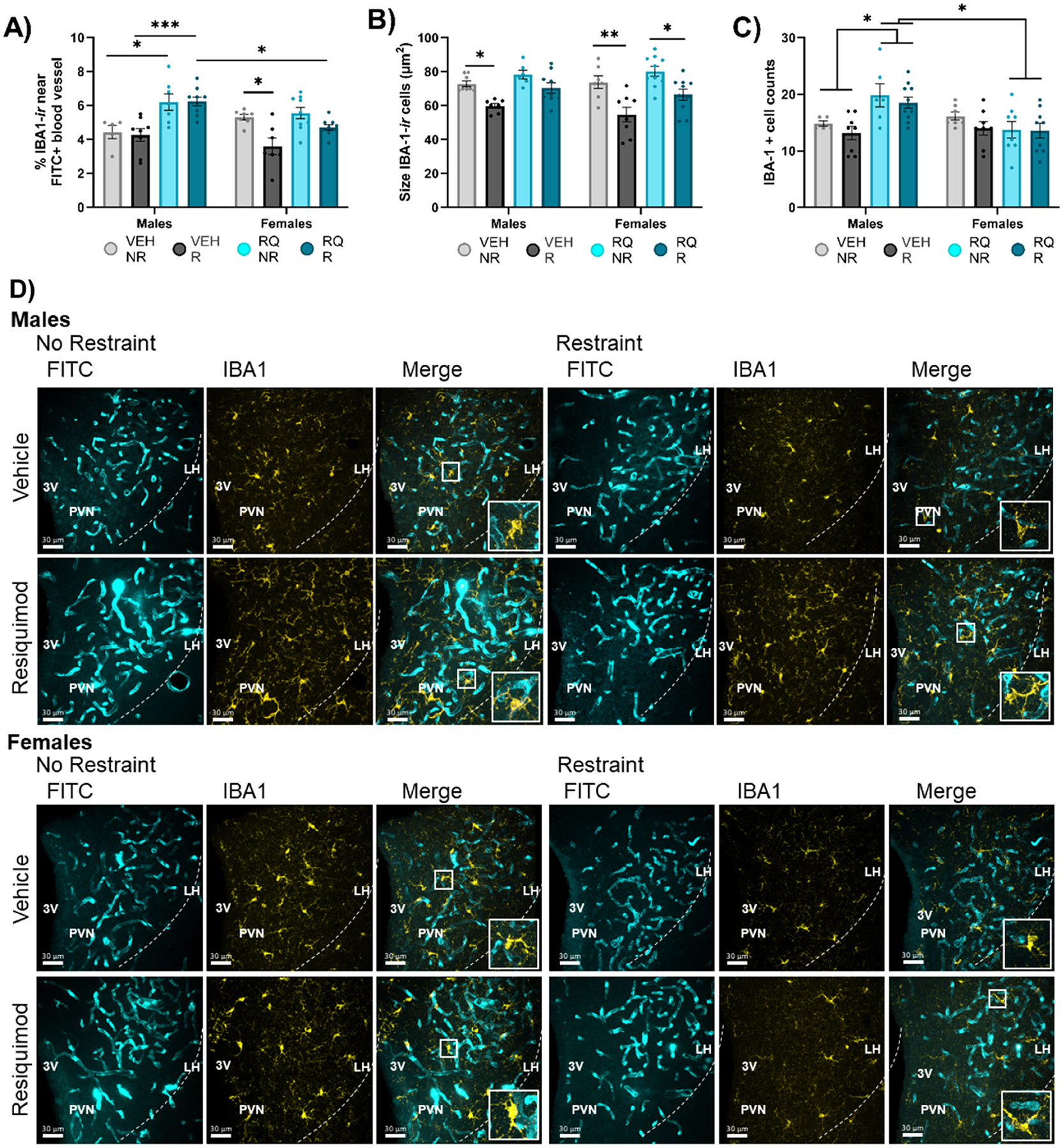
(A) Proximity of microglia to blood vessels (within 1.18 μm), (B) average size of IBA-1-labeled microglia cells, and (C) the number of IBA-1-labeled cells in the PVN. (D) Representative images. VEH = vehicle, RQ = Resiquimod, NR = no restraint, R = restraint, PVN = Paraventricular Nucleus of the Hypothalamus, 3 V = 3rd ventricle, FITC = fluorescein isothiocyanate, IBA1 = ionizing binding calcium adapter molecule-1. *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. Error bars represented as +/− SEM. n = 8–10 animals / group.
The average size of IBA-1 immunoreactive cells in the PVN (Fig. 4B) differed by fetal RQ treatment. ANOVA indicated a main effect of Restraint [F (1, 57) = 36.44, P = 0.0001] but not sex or prenatal treatment. Adult restraint stress led to smaller IBA1 immunoreactive microglia. In males, IBA-1 positive cells in the PVN were 15.6 % smaller in VEH-treated animals with restraint (VEH-R vs. VEH-NR, p = 0.0483) with Šídák’s post-hoc analysis test. In females, IBA-1 positive cells in the PVN were 22.1 % smaller VEH-treated offspring with restraint (VEH-R vs. VEH-NR p – 0.0014) and 12.5 % smaller in RQ-treated offspring with restraint (RQ-R vs. RQ-NR p = 0.0114) according to Šídák’s post-hoc analysis test.
The number of IBA-1 immunoreactive cells in mid-PVN sections differed as a function of fetal exposure. There was statistically significant main effect of prenatal exposure [F (1, 54) = 4.423, P = 0.0401]. However, an examination of the significant interaction of sex by prenatal exposure indicates that the prenatal RQ treatment was selectively impactful in males [F (1, 54) = 13.47, P = 0.0006]. The data show that maternal RQ led to greater microglial numbers in RQ-male versus VEH-male, and more IBA-1 labeled cells in the RQ-treated males than RQ-treated females (male RQ-R vs. female RQ-R p = 0.0272, male RQ-NR vs. female RQ-NR p = 0.0276 by Šídák’s) (Fig. 4D) regardless of adult restraint. In males, Šídák’s post-hoc analysis test showed 25 % more IBA-1 immunoreactive cells in the PVN in RQ-treated compared to VEH-treated offspring (p = 0.0172).
4. Discussion
The current study examined the neuroendocrine stress response and BBB integrity in the PVN of adult offspring from mothers injected with the TLR7/8 agonist RQ. Additionally, an adult stress was tested to evaluate whether exposure to fetal immune stress predisposed offspring to be more sensitive to a second stressor in adulthood. The results suggest HPA axis activity was impaired in offspring of RQ injected mothers. BBB integrity in the PVN was compromised with greater leakage and changes in astrocytes and microglia morphology and vascular proximity in offspring of RQ injected mothers. There was little to no change in BBB integrity in control regions (motor CX or LH) indicating this effect was at least partially PVN-selective. Given that the PVN is more densely vascularized than surrounding regions and plays a critical role regulating homeostasis and stress responses in clinical studies, dysregulation of its BBB could alter neuronal signaling and act as a potential mechanism for increased risk of adult neuropsychiatric disorder.
4.1. Offspring of RQ injected mothers displayed delays in acute-stress induced HPA axis negative feedback in adulthood
The PVN of the hypothalamus acts as the central regulator of the HPA axis stress response. PVN receives signals in relation to stressful stimuli from the environment and subsequently signals to the periphery to stimulate the release of GCs. The data in the current study show females were more sensitive to adult stressors measured by CORT secretion, in agreement with previous studies (Handa et al., 1994, 1985; Simerly et al., 1985). Data further indicate that in adult offspring of RQ-injected mothers, there was hyperactive HPA axis function with impaired negative feedback following acute stress. A dysregulation of the neuroendocrine stress response in adulthood may lead to abnormal levels of stress hormones and associated pathologies. These findings align with previous studies that have shown fetal overexposure to exogenous GCs (e.g., Dexamethasone)(Barbazanges et al., 1996; Frahm et al., 2018; Hiroi et al., 2016; O’Regan et al., 2004; Sheng et al., 2023), maternal high fat diet(Niu et al., 2019; Sasaki et al., 2014; Sheng et al., 2023; Sullivan et al., 2014; Sullivan et al., 2012; Sullivan et al., 2011) and maternal caloric restriction(Akitake et al., 2015; Levay et al., 2008; Sheng et al., 2023) led to impaired HPA axis function and stress-related behavioral phenotypes (Sheng and Tobet, in press). When offspring were examined in adulthood after excess fetal GC treatment, the area of GFAP immunoreactive astrocytes was decreased in females compared to controls, the area of desmin immunoreactive pericytes was greater in males, and elevated depression-like behavior as indicated by a tail-suspension test in males and females(Frahm et al., 2018). The developmental origins of health and disease hypothesis posits perturbations in the maternal environment influence brain development. These fetal derived factors can drive adult risk for neuropsychiatric disorders, including MDD and cardiometabolic disease(Gilman et al., 2016; Goldstein et al., 2011; Grundwald and Brunton, 2015; Harris and Seckl, 2011; Kestering-Ferreira et al., 2021; Lin et al., 2023; Niu et al., 2019; Seckl and Holmes, 2007). Many reports suggest disruption in the PVN, the central regulator of the HPA axis, as a common pathway associated with these disorders(Goldstein et al., 2019; Herman et al., 2012; Herman and Tasker, 2016; Myers et al., 2012). In the current study, fetal exposure to maternal RQ injection led to elevated levels of CORT 60-minutes after restraint, indicating improper negative feedback to the HPA stress axis. Dysregulation of the HPA axis could further be linked to impaired BBB integrity and alter downstream neuroendocrine function and stress-related behaviors(Sheng and Tobet, 2024).
The PVN is an important anatomical region of stress regulation, however, other areas are involved in the HPA axis regulation of negative feedback, including the anterior pituitary and various extrahypothalamic regions (e.g., HIPP). PVN neurons may not be the only contributors to an altered stress response and other pathways to glucocorticoid release may involve more complicated circuits that may or may not include PVN neurons. Such pathways may include activation of the sympathetic nervous system where catecholamines are released by the adrenal medulla and stimulate the adrenal cortex to produce cortisol (Schaeuble and Myers, 2022), circadian rhythms regulated by the SCN (Miller et al., 2022), and low blood glucose levels that trigger the adrenal glands to release glucocorticoids(Dearing et al., 2022). Therefore, plasma levels of corticosterone alone do not provide direct evidence that the PVN is involved in the changes in stress response. Future investigations might benefit from measurements of CRH and/or ACTH to elucidate a more complete picture of the role of maternal RQ injection on HPA axis acute stress circuitry in offspring that differs by sex, in particular given that females in the current study showed higher levels of CORT response to RQ and restraint than males.
4.2. Offspring of RQ injected mothers displayed greater leakage from capillaries in the PVN
The BBB protects the brain by preventing harmful compounds from infiltrating into the CNS(Dion-Albert et al., 2022a). In mice, the BBB begins developing embryonically at ~;E11.5 and continues to mature through postnatal life (Frahm and Tobet, 2015; Haddad-Tovolli et al., 2017; Saili et al., 2017). Maternal stress can perturb BBB formation and lead to downstream influx of toxins and peripheral immune cellular and secretory components into the brain (e.g., inflammatory T cells) (Dudvarski Stankovic et al., 2016; Zhao et al., 2022). Reduced BBB integrity driven by maternal immune stress (i.e., inflammatory response during pregnancy with elevated cytokines, viral or bacterial infection during pregnancy) has been linked with increased susceptibility to neuropsychiatric pathologies, including MDD and autism spectrum disorders, among others(Arnone, 2022; Kealy et al., 2020; Ronaldson and Davis, 2020; Wu et al., 2022). While many maternal immune studies on BBB permeability have focused in cortical, hippocampal, or cerebellar regions and their downstream influence over adult neuropsychiatric disorders, the unusually dense vasculature of the PVN led to the hypothesis that the BBB in the PVN of the hypothalamus might be a site of anatomic and functional importance in the etiology of these disorders (Goldstein et al., 2014). In fact, in previous work by our collaborators in a clinical imaging study of maternal prenatal immune dysregulation on stress response circuitry abnormalities in adult offspring, the anterior hypothalamus (in which PVN is located) and connectivity with the hippocampus, were significantly impacted and by sex, effects that were retained into adulthood(Goldstein et al., 2021). In the present study, BBB permeability as assessed by FITC leakage was higher in the PVN of adult male and female offspring from RQ injected mothers. FITC leakage was selective to the PVN, with little change in leakage in control regions taken from the same sections. This effect was exacerbated with acute restraint stress in males. Such results demonstrate a level of sex-selectivity for susceptibility to BBB leakage in the PVN following a stressor in adulthood if previously exposed to excess maternal immune stimulation during prenatal development.
Astrocytes are an important component of a functional BBB. In the current study, adult offspring of RQ injected mothers showed lower GFAP-ir and coverage of GFAP-labeled astrocyte end feet near blood vessels in the PVN. Reduced coverage of astrocytes surrounding blood vessels of the PVN correlates with increased permeability, as suggested by higher leakiness found of FITC in the adjacent extravascular areas (Supplementary Figure 1). Astrocytic endfeet are shown to mediate water flow through a bi-directional regulatory channel, aquaporin 4 (Rajkowska and Miguel-Hidalgo, 2019; Wang et al., 2018). The current data show that paracellular FITC leaks into extravascular space in the PVN in adult offspring of RQ injected mothers. This effect could be a consequence of reduced GFAP-labeled astrocyte endfeet near blood vessels in the adult offspring.
Mechanisms that influence astrocyte coverage of the blood vessels could be programmed during early development, including genetic mutations or epigenetic modifications involved in end-feet function. Overall, these findings are consistent with a hypothesis that changes in astrocytes might provide a mechanism that leads to a leaky BBB in PVN capillaries in adulthood, a potential causal pathway for understanding associations with neuropsychiatric disorders. BBB components beyond astrocytes have been examined within the context of prenatal GC exposure (Frahm et al., 2018), and MIA studies have shown disruption in endothelial tight junction proteins and mRNA expression (Claudin-5, Claudin-3, occludin, etc.) (Collignon et al., 2024; Dion-Albert et al., 2022b; Menard et al., 2017). Future studies are needed to determine if maternal injection of RQ has a similar effect on BBB pericytes, endothelia, and tight junction proteins in the PVN of maternally stressed offspring.
4.3. Prenatal RQ treatment led to sex-selective changes in microglia in the PVN
Microglia are resident immune cells in the CNS that drive inflammatory effects after MIA from fetal life to adulthood (Bilbo et al., 2018; Block et al., 2022; Loayza et al., 2023; Ozaki et al., 2020; Prins et al., 2018; Schaafsma et al., 2017). In the present study changes in anatomic localization and morphology of PVN microglia were examined in adult offspring of RQ injected mothers. Microglia were closer to blood vessels in the PVN of RQ-males than VEH males. Females did not show an effect of maternal RQ-injection on microglial coverage of PVN blood vessels but did have lower microglial coverage on PVN blood vessels following restraint stress. These data suggest sex-selective responses of microglial located in the PVN. Other groups have shown that pregnant mice treated with the TLR7 agonist, imiquimod, resulted in greater microglial gene expression, including chemokines (Ccl2, Ccl6, Cxcl10) and pro-inflammatory cytokines (Tnf-α, IL-6)(Missig et al., 2020). Unfortunately, this study did not assess sex as a variable, a crucial factor when investigating microglia in the context of sex-selective neuropsychiatric disorders(Lenz and McCarthy, 2015; Lenz et al., 2013; McCarthy et al., 2017).
Maternal immune stress leads to morphological changes in adult microglia. The decrease in size induced by restraint in VEH males and all females in the current study could suggest the PVN microglia are reacting to stress, potentially indicating disrupted BBB function or becoming protective to some degree. Additional studies are needed to fully evaluate the role of these microglia. Microglial size did not change with restraint in RQ-males, indicating less functional microglia caused by exposure to maternal injection of RQ. Activated microglia often migrate to “injured” sites, phagocytose harmful compounds or cells in a region, and secrete inflammatory cytokines to recruit additional innate immune cells to help repair damaged tissue and neurons(Sequeira and Bolton, 2023). Data in the current study demonstrate impaired integrity of the BBB within the bounds of the PVN, suggesting a role for microglia to help “repair” leaky vascular tissue by releasing vascular growth factors and phagocytose harmful compounds from the periphery. An examination of microglial secretory products in the current RQ-paradigm might better illuminate the functional role of microglia in BBB integrity following maternal stress.
5. Conclusions
In summary, the current study demonstrated maternal injection with RQ led to neuroendocrine dysfunction that was selective for PVN. Improper PVN function was associated with sex selective differences in vasculature integrity, and alterations in astrocyte and microglial cell interactions with PVN capillaries. Interestingly, FITC leak was exacerbated in males overall compared to females, but these data were not fully paralleled by other cellular changes (astrocytes, microglia) in the BBB. Future studies may help tease out interactions between these and other cell types in the BBB (e.g., tight junctions, claudins, matrix metalloproteinases) to better understand sex differences in BBB integrity and function. Given the 3-fold greater vascularization of the PVN relative to most other brain regions, this location of injury may be particularly critical. We previously showed that offspring of mothers injected with RQ demonstrated more stress-related behavioral phenotypes linked to HPA axis dysregulation. The data, taken as a whole, are consistent with the hypothesis that maternal immune stress leads to changes in BBB integrity in the PVN that influence neuroendocrine signaling, and potentially influencing susceptibility to neuropsychiatric pathologies.
Supplementary Material
Acknowledgements
We thank Connie King for expert technical assistance. We thank Derek B. Schaeuble, Ph.D., Jill M. Goldstein, Ph.D., and Taben Hale, Ph.D. for critical comments on the manuscript.
Funding
ORWH-SCORE NIMH U54-MH118919 (Goldstein, Tobet, mPIs)
Abbreviations:
- ACTH
Adrenocorticotropic hormone
- BBB
Blood-brain barrier
- CRH
Corticotropin releasing hormone
- CORT
Corticosterone
- CX
Cerebral cortex
- E
Embryonic day
- FITC
Fluorescein isothiocyanate
- GC
Glucocorticoid
- GFAP
Glial fibrillary acidic protein
- HIPP
Hippocampus
- HPA
Hypothalamic-pituitary-adrenal
- IBA-1
Ionized binding calcium adaptor molecule –1
- IL
Interleukin
- -ir
Immunoreactivity
- MDD
Major depressive disorder
- MIA
Maternal immune activation
- NR
No restraint
- P
Postnatal day
- PVN
Paraventricular nucleus
- R
Restraint
- RQ
Resiquimod
- TLR
Toll-like receptor
- VEH
Vehicle
Footnotes
CRediT authorship contribution statement
Stuart A. Tobet: Writing – review & editing, Supervision, Software, Resources, Investigation, Funding acquisition, Conceptualization. Luke A. Schwerdtfeger: Writing – review & editing, Investigation, Conceptualization. Jonathan R. Christenson: Writing – review & editing, Investigation, Data curation. Julietta A Sheng: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbii.2024.100081.
References
- Agnish ND, Keller KA, 1997. The rationale for culling of rodent litters. Fundam. Appl. Toxicol 38, 2–6. [DOI] [PubMed] [Google Scholar]
- Akitake Y, Katsuragi S, Hosokawa M, Mishima K, Ikeda T, Miyazato M, Hosoda H, 2015. Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutr. Res 35, 76–87. [DOI] [PubMed] [Google Scholar]
- Arnone D., 2022. Blood-brain barrier perturbations, psychiatric disorders, and new opportunities for refining disease models. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 957–959. [DOI] [PubMed] [Google Scholar]
- Bale TL, 2011. Sex differences in prenatal epigenetic programming of stress pathways. Stress 14, 348–356. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ, 2010. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S, 1996. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J. Neurosci 16, 3943–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK, 2018. Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol 299, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block CL, Eroglu O, Mague SD, Smith CJ, Ceasrine AM, Sriworarat C, Blount C, Beben KA, Malacon KE, Ndubuizu N, Talbot A, Gallagher NM, Chan Jo Y, Nyangacha T, Carlson DE, Dzirasa K, Eroglu C, Bilbo SD, 2022. Prenatal environmental stressors impair postnatal microglia function and adult behavior in males. Cell Rep. 40, 111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow AP, Heck AL, Miller AM, Sheng JA, Stover SA, Daniels RM, Bales NJ, Fleury TK, Handa RJ, 2019. Chronic variable stress alters hypothalamic-pituitary-adrenal axis function in the female mouse. Physiol. Behav 209, 112613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots SH, Peters SAE, Woodward M, 2017. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2, e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, 2013. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction 146, R175–R189. [DOI] [PubMed] [Google Scholar]
- Collignon A, Dion-Albert L, Menard C, Coelho-Santos V, 2024. Sex, hormones and cerebrovascular function: from development to disorder. Fluids Barriers CNS 21, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR, 2009. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav. Neurosci 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing C, Handa RJ, Myers B, 2022. Sex differences in autonomic responses to stress: implications for cardiometabolic physiology. Am. J. Physiol. Endocrinol. Metab 323, E281–E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion-Albert L, Cadoret A, Doney E, Kaufmann FN, Dudek KA, Daigle B, Parise LF, Cathomas F, Samba N, Hudson N, Lebel M, Signature C, Campbell M, Turecki G, Mechawar N, Menard C, 2022b. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun 13, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion-Albert L, Bandeira Binder L, Daigle B, Hong-Minh A, Lebel M, Menard C, 2022a. Sex differences in the blood-brain barrier: Implications for mental health. Front Neuroendocr. 65, 100989. [DOI] [PubMed] [Google Scholar]
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MHH, 2016. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 131, 347–363. [DOI] [PubMed] [Google Scholar]
- Frahm KA, Tobet SA, 2015. Development of the blood-brain barrier within the paraventricular nucleus of the hypothalamus: influence of fetal glucocorticoid excess. Brain Struct. Funct 220, 2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm KA, Schow MJ, Tobet SA, 2012. The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling. Horm. Metab. Res 44, 619–624. [DOI] [PubMed] [Google Scholar]
- Frahm KA, Handa RJ, Tobet SA, 2018. Embryonic Exposure to Dexamethasone Affects Nonneuronal Cells in the Adult Paraventricular Nucleus of the Hypothalamus. J. Endocr. Soc 2, 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Cherkerzian S, Buka SL, Hahn J, Hornig M, Goldstein JM, 2016. Prenatal immune programming of the sex-dependent risk for major depression. Transl. Psychiatry 6, e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Buka SL, Fitzmaurice G, Hornig M, Gillman M, O’Toole S, Sloan RP, 2011. Sex-specific impact of maternal-fetal risk factors on depression and cardiovascular risk 40 years later. J. Dev. Orig. Health Dis 2, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Handa RJ, Tobet SA, 2014. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front Neuroendocr. 35, 140–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Hale T, Foster SL, Tobet SA, Handa RJ, 2019. Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology 44, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Cohen JE, Mareckova K, Holsen L, Whitfield-Gabrieli S, Gilman SE, Buka SL, Hornig M, 2021. Impact of prenatal maternal cytokine exposure on sex differences in brain circuitry regulating stress in offspring 45 years later. Proc. Natl. Acad. Sci. USA 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C, Hanley N, Campbell M, 2020. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry 10, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundwald NJ, Brunton PJ, 2015. Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner. Psychoneuroendocrinology 62, 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma E, Snook E, Spring S, Lerch JP, Nieman BJ, Devenyi GA, Chakravarty MM, 2021. Subtle alterations in neonatal neurodevelopment following early or late exposure to prenatal maternal immune activation in mice. Neuroimage-Clin. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad-Tovolli R, Dragano NRV, Ramalho AFS, Velloso LA, 2017. Development and Function of the Blood-Brain Barrier in the Context of Metabolic Control. Front Neurosci. 11, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA, 1985. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol. Reprod 32, 855–864. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA, 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav 28, 464–476. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Sheng JA, Castellanos EA, Templeton HN, McGivern RF, 2022. Sex Differences in Acute Neuroendocrine Responses to Stressors in Rodents and Humans. Cold Spring Harb. Perspect. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Seckl J, 2011. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav 59, 279–289. [DOI] [PubMed] [Google Scholar]
- Heck AL, Sheng JA, Miller AM, Stover SA, Bales NJ, Tan SML, Daniels RM, Fleury TK, Handa RJ, 2020. Social isolation alters hypothalamic pituitary adrenal axis activity after chronic variable stress in male C57BL/6 mice. Stress 23, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, 2016. Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation. Front Endocrinol. (Lausanne) 7, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B, 2012. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz. J. Med Biol. Res 45, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, Carbone DL, Zuloaga DG, Bimonte-Nelson HA, Handa RJ, 2016. Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neuroscience 320, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy J, Greene C, Campbell M, 2020. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett 726, 133664. [DOI] [PubMed] [Google Scholar]
- Kestering-Ferreira E, Tractenberg SG, Lumertz FS, Orso R, Creutzberg KC, Wearick-Silva LE, Viola TW, Grassi-Oliveira R, 2021. Long-term Effects of Maternal Separation on Anxiety-Like Behavior and Neuroendocrine Parameters in Adult Balb/c Mice. Chronic Stress (Thousand Oaks) 5, 24705470211067181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, Lorsch ZS, Hamilton PJ, Calipari ES, Hodes GE, Issler O, Kronman H, Pfau M, Obradovic ALJ, Dong Y, Neve RL, Russo S, Kazarskis A, Tamminga C, Mechawar N, Turecki G, Zhang B, Shen L, Nestler EJ, 2017. Sex-specific transcriptional signatures in human depression. Nat. Med 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautarescu A, Craig MC, Glover V, 2020. Prenatal stress: Effects on fetal and child brain development. Int Rev. Neurobiol 150 17–40. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM, 2015. A starring role for microglia in brain sex differences. Neuroscientist 21, 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM, 2013. Microglia are essential to masculinization of brain and behavior. J. Neurosci 33, 2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levay EA, Paolini AG, Govic A, Hazi A, Penman J, Kent S, 2008. Anxiety-like behaviour in adult rats perinatally exposed to maternal calorie restriction. Behav. Brain Res 191, 164–172. [DOI] [PubMed] [Google Scholar]
- Lin C, Lei Q, Yu M, Lin Y, Lu H, Huang Y, Wang H, Xu H, Lin Y, 2023. Maternal High-Fat Diet Multigenerationally Programs HPA Function and Behaviors in Male Rat Offspring. Endocrinology 164. [DOI] [PubMed] [Google Scholar]
- Loayza M, Lin S, Carter K, Ojeda N, Fan LW, Ramarao S, Bhatt A, Pang Y, 2023. Maternal immune activation alters fetal and neonatal microglia phenotype and disrupts neurogenesis in mice. Pedia Res 93, 1216–1225. [DOI] [PubMed] [Google Scholar]
- Loewen SM, Chavesa AM, Murray CJ, Traetta ME, Burns SE, Pekarik KH, Tremblay ME, 2023. The Outcomes of Maternal Immune Activation Induced with the Viral Mimetic Poly I:C on Microglia in Exposed Rodent Offspring. Dev. Neurosci 45, 191–209. [DOI] [PubMed] [Google Scholar]
- Madhavpeddi L, Hammond B, Carbone DL, Kang P, Handa RJ, Hale TM, 2022. Impact of angiotensin II receptor antagonism on the sex-selective dysregulation of cardiovascular function induced by in utero dexamethasone exposure. Am. J. Physiol. Heart Circ. Physiol 322, H597–H606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, Lenz KM, 2017. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat. Rev. Neurosci 18, 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonte B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ, 2017. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci 20, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Bodemeier Loayza Careaga M, Handa RJ, Wu TJ, 2022. The Effects of Chronic Variable Stress and Photoperiod Alteration on the Hypothalamic-Pituitary-Adrenal Axis Response and Behavior of Mice. Neuroscience 496, 105–118. [DOI] [PubMed] [Google Scholar]
- Missig G, Robbins JO, Mokler EL, McCullough KM, Bilbo SD, McDougle CJ, Carlezon WA Jr, 2020. Sex-dependent neurobiologieal features of prenatal immune activation via TLR7. Mol. Psychiatry 25, 2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Morita S, 2011. A new method for visualization of endothelial cells and extravascular leakage in adult mouse brain using fluorescein isothiocyanate. J. Neurosci. Methods 202, 9–16. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP, 2012. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol. Neurobiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Wu X, Ying A, Shao B, Li X, Zhang W, Lin C, Lin Y, 2019. Maternal high fat diet programs hypothalamic-pituitary-adrenal function in adult rat offspring. Psychoneuroendocrinology 102, 128–138. [DOI] [PubMed] [Google Scholar]
- O’Regan D, Kenyon CJ, Seckl JR, Holmes MC, 2004. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am. J. Physiol. Endocrinol. Metab 287, E863–E870. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Kato D, Ikegami A, Hashimoto A, Sugio S, Guo Z, Shibushita M, Tatematsu T, Haruwaka K, Moorhouse AJ, Yamada H, Wake H, 2020. Maternal immune activation induces sustained changes in fetal microglia motility. Sci. Rep 10, 21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Pattou E, Herman JP, Kordon C, 1984. Mapping of LH-RH-containing projections to the mediobasal hypothalamus by differential deafferentation experiments. Brain Res 298, 283–288. [DOI] [PubMed] [Google Scholar]
- Prins JR, Eskandar S, Eggen BJL, Scherjon SA, 2018. Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment? J. Reprod. Immunol 126, 18–22. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, 2019. Glial Pathology in Major Depressive Disorder: An Approach to Investigate the Coverage of Blood Vessels by Astrocyte Endfeet in Human Postmortem Brain. Methods Mol. Biol 1938, 247–254. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA, 2013. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP, 2020. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow. Metab 40, S6–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saili KS, Zurlinden TJ, Schwab AJ, Silvin A, Baker NC, Hunter ES 3rd, Ginhoux F, Knudsen TB, 2017. Blood-brain barrier development: Systems modeling and predictive toxicology. Birth Defects Res 109, 1680–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan PO, 2014. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 272, 92–101. [DOI] [PubMed] [Google Scholar]
- Schaafsma W, Basterra LB, Jacobs S, Brouwer N, Meerlo P, Schaafsma A, Boddeke E, Eggen BJL, 2017. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis 106, 291–300. [DOI] [PubMed] [Google Scholar]
- Schaeuble D, Myers B, 2022. Cortical-Hypothalamic Integration of Autonomic and Endocrine Stress Responses. Front Physiol. 13, 820398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepanski S, Buss C, Hanganu-Opatz IL, Arck PC, 2018. Prenatal Immune and Endocrine Modulators of Offspring’s Brain Development and Cognitive Functions Later in Life. Front Immunol. 9, 2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC, 2007. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat. Clin. Pr. Endocrinol. Metab 3, 479–488. [DOI] [PubMed] [Google Scholar]
- Sequeira MK, Bolton JL, 2023. Stressed Microglia: Neuroendocrine-Neuroimmune Interactions in the Stress Response. Endocrinology 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JA, Tobet SA, 2024. Maternal immune activation with toll-like receptor 7 agonist during mid-gestation alters juvenile and adult developmental milestones and behavior. J. Neuroendocr, e13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, Handa RJ, 2020. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front Behav. Neurosci 14, 601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JA, Tan SML, Hale TM, Handa RJ, 2021. Androgens and Their Role in Regulating Sex Differences in the Hypothalamic/Pituitary/Adrenal Axis Stress Response and Stress-Related Behaviors. Androg. Clin. Res Ther 2, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JA, Handa RJ, Tobet SA, 2023. Evaluating different models of maternal stress on stress-responsive systems in prepubertal mice. Front. Neurosci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA, 1985. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology 40, 501–510. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, Grove KL, 2011. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 93, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Nousen EK, Chamlou KA, Grove KL, 2012. The Impact of Maternal High-Fat Diet Consumption on Neural Development and Behavior of Offspring. Int J. Obes. Suppl 2, S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Nousen EK, Chamlou KA, 2014. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav 123, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu M, Liu C, Han J, Lang W, Gao Y, Lu C, Wang S, Hou S, Zheng N, Wang D, Chen Y, Zhang Y, Zhang HL, Zhu J, 2018. Blood Brain Barrier Permeability Could Be a Biomarker to Predict Severity of Neuromyelitis Optica Spectrum Disorders: A Retrospective Analysis. Front Neurol. 9, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Yin Y, Du L, 2022. Blood-Brain Barrier Dysfunction in the Pathogenesis of Major Depressive Disorder. Cell Mol. Neurobiol 42, 2571–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Dai W, Chen HY, Jacobs RE, Zlokovic BV, Lund BT, Montagne A, Bonnin A, 2022. Prenatal disruption of blood-brain barrier formation via cyclooxygenase activation leads to lifelong brain inflammation. Proc. Natl. Acad. Sci. USA 119, e2113310119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


