Abstract
Background and Aims:
Enhanced recovery after surgery (ERAS) has been applied in various laparoscopic procedures. Intravenous lidocaine (IVL) infusion is used for laparoscopic procedures as a part of ERAS protocols. The study aimed to evaluate the role of IVL infusion in enhanced bowel recovery after laparoscopic renal surgeries.
Material and Methods:
A randomized, double-blind, placebo-control trial was conducted on 80 patients (with American Society of Anesthesiologists physical status I–II) who presented for laparoscopic renal surgeries under general anesthesia. The study period was from Oct 2018 to Sept 2019. By computer-generated codes, patients were randomly divided into two groups: L (lidocaine) and C (control). Group L received an intravenous (IV) bolus (1.5 mg/kg) of 2% lidocaine over 2 min, followed by an IV lidocaine infusion at the rate of 1.5 mg/kg/h until skin closure. Group C received the same volume of bolus followed by normal saline infusion. Patients were monitored for bowel functions, total hospital stay, and total analgesic consumption. Student’s t-test and Chi-square test were used for quantitative data and occurrence of events, respectively. P <0.05 was considered to be statistically significant.
Results:
First bowel sound, flatus, and defecation occurred in 16.4 ± 2.50, 26.7 ± 9.02, and 39.1 ± 6.31 h, respectively, in group L and 18.2 ± 2.90, 32.3 ± 3.11, and 43.3 ± 4.22 h, respectively, in group C (P = 0.006, 0.001, and 0.01, respectively). Total hospital stay was 4.0 ± 0.74 and 5.3±0.0.91 days in groups L and C, respectively (P < 0.001).
Conclusion:
The present study concluded that IVL could enhance the bowel recovery and reduce total hospital stay after laparoscopic renal surgeries.
Keywords: Enhanced recovery, laparoscopic renal surgeries, lidocaine infusion, postoperative pain intensity
Introduction
Modern health care requires reducing complications, shorter recovery times, and improving the prognosis. These can be achieved with the help of minimally invasive laparoscopic surgery and multidisciplinary treatment.[1] Enhanced recovery after surgery (ERAS) protocol has found that intravenous lidocaine (IVL) reduces postoperative opioid consumption.[2] IVL is equally effective as epidural lidocaine for postoperative analgesia, early bowel recovery, and shorter hospital stays.[3] It can play a pivotal role in the ERAS program.[4]
Lidocaine acts by binding with sodium channels. It interacts with anesthetic agents and leads to a synergic effect.[5] It has also shown an anti-inflammatory action and prevents central hyperalgesia.[6,7]
Several human studies evaluated the effects of systemic lidocaine infusion during abdominal surgeries. Its effects on pain relief,[8] cytokine response, bowel function recovery,[9] postoperative nausea and vomiting (PONV), and length of hospital stay[10,11] were studied. IVL infusion has been shown to enhance bowel recovery after surgery. This is thought to be due to reduced postoperative pain,[12] its anti-inflammatory action,[6] and improved gut motility.[13]
Few studies that evaluate the role of perioperative IVL in enhancing recovery after laparoscopic renal surgeries are available.[1,14] Further randomized trials are required to find out the beneficial effects of IVL during laparoscopic renal surgeries like nephrectomy, pyeloplasty, partial nephrectomy, and pyelolithotomy. This randomized, double-blinded, placebo-controlled study was performed at our institute. The primary aim of the study was to evaluate IVL infusion role in enhancing bowel recovery. The secondary objectives were to assess the perioperative hemodynamic stability, the requirement of an inhalational agent, postoperative pain intensity, total analgesic consumption, and total hospital stay.
Material and Methods
A randomized, double-blind, placebo-control trial was conducted after obtaining institutional ethical committee approval (ref. no. EC/531/2018, dated August 16, 2018). The study was registered under the clinical trial registry of India (CTRI/2018/10/015912). Ninety-eight patients were evaluated for eligibility, and 80 were enrolled for the study. All patients were evaluated and investigated preoperatively as per standard protocol. Written and informed consent was taken from patients who were posted for transperitoneal laparoscopic renal surgery. The study period was from Oct 2018 to Sept 2019 [CONSORT flow diagram shown in Figure 1]. The inclusion criteria were patients of age between 18 and 65 years and having American Society of Anesthesiologists (ASA) physical status I–II. The exclusion criteria were patients having allergy to local anesthetics, severe underlying cardiovascular disease, impaired kidney or liver function, history of daily intake of analgesics, a psychiatric disorder, arrhythmia or seizures, and pretransplant nephrectomy and surgery duration of more than 5 h.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram
The anesthesia technician opened the sealed envelopes having computer-generated randomized codes and prepared the syringe with the study drug or placebo. The syringe was labelled with the patient’s registration number by anaesthesia technician. Patients, doctors (treating surgeons and anesthesiologists), and the nursing staff involved in data collection were blinded to study allocation. We included urological transperitoneal laparoscopic renal procedures like simple nephrectomy, radical nephrectomy, pyeloplasty, partial nephrectomy, and pyelolithotomy, and these patients were taken as the first case in the morning hours.
All patients received standard general anaesthesia (GA) as per the hospital protocol. A large-bore intravenous (IV) cannulation was done on the hand or forearm in the ward. The crystalloid fluid was started intravenously in preoperative ward. Glycopyrrolate (0.004 mg/kg) and metoclopramide (0.15 mg/kg) were given. In the operation theater, standard monitors were used, including electrocardiogram (ECG), capnography (EtCO2), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse oximetry (SpO2) and anesthesia gas monitor (AGM). Patients were randomly divided into two equal groups: L (lidocaine) and C (control). Two minutes before tracheal intubation, patients in group L (n = 40) received IVL bolus (1.5 mg/kg, 2% lidocaine) over 2 min, followed by IVL infusion at the rate of 1.5 mg/kg/h until skin closure. The control group (n = 40) received the same volume of bolus followed by infusion of normal saline. Lidocaine, which is cheaper and easily available, was used as the study drug for group L. Normal saline was used for group C to blind the study drug, as both are clear liquids. All patients received 0.03 mg/kg midazolam and 1 μg/kg fentanyl. After preoxygenation, anaesthesia induction was done with 1 mg/kg propofol and intubation was facilitated with 1 mg/kg succinylcholine or 0.1 mg/kg vecuronium IV and kept on volume control mechanical ventilation. Anesthesia was maintained with oxygen (0.5 l/min), air (0.5 l/min), sevoflurane, and vecuronium with volume control mode of ventilation. The ventilator parameters were kept in such a way to keep the end-tidal CO2 at 35–45 mmHg. Patients were placed in lateral decubitus position depending upon the side of surgery. Endotracheal tube position was checked for bilateral air entry during change of patient position and pneumoperitoneum. All the necessary precautions were taken to avoid injury to the patient during positioning, and pressure points were checked and secured. Bair Hugger patient warming unit was used to prevent hypothermia. Ten milliliters of bupivacaine 0.25% was used for local infiltration before the port placement as a part of the routine protocol in both groups for analgesia during the perioperative period. Intraoperatively, sevoflurane and fentanyl were used in titration to maintain the hemodynamic parameters within ± 20% of the patient’s baseline values. IV paracetamol 1 g was given during the time of skin closure. The study drug infusion was stopped at the end of skin closure. Patients were reversed and extubated on the table within 30 min. Patients were transferred to the post-anesthesia recovery room. They were considered to be dropped if there was conversion from laparoscopic to open surgery, they were lost to follow-up, or surgery duration lasted more than 5 h.
Heart rate (HR), SBP, DBP, MAP, SpO2, and EtCO2 were recorded intraoperatively. End-tidal sevoflurane (Et-Sevo) concentration was recorded every 15 min until the end of surgery. Pain intensity was measured using a visual analog scale (VAS) at 0, 2, 6, 12, and 24 h after surgery. IV paracetamol 1 mg was given every 8 h postoperatively. IV nalbuphine infusion was started at the rate of 20 μg/kg/h for postoperative analgesia for 24 h (nalbuphine infusion preparation: 50 mg in 20 ml sterile water; 2.5 mg/ml). If VAS was more than 3, the postoperative staff were advised to give 2 ml bolus. The instruction was that the bolus dose should not be repeated more than two times in 1 h. Also, 24-h postsurgical consumption of analgesia was recorded. The return of gastrointestinal motility was assessed by auscultation of bowel sound six hourly. The time of first flatus and time of first defecation were recorded by asking twice daily. Nausea, vomiting, ileus, and lidocaine-related complications were documented. The total duration of hospital stay was recorded.
The sample size was calculated based on the time of the first flatus passed, the time of the first defecation, and total hospital stay as variables. Based on previous studies, Song et al.[15] observed that the time of first flatus passage and defecation after laparoscopic cholecystectomy was 20 ± 11 and 41 ± 16 h, respectively, in the lidocaine group and 29 ± 10 and 57 ± 14 h, respectively, in the placebo group. Total hospital stays after laparoscopic colorectal surgeries in the lidocaine and placebo groups were 4.70 ± 1.29 and 5.90 ± 1.97 days, respectively.[16] The OpenEpi software module was used for sample size calculation by keeping a 95% confidence interval, and the power of the test was 80%. The sample size was found to be 22, 14, and 31 in each group for the time of first flatus passed, first defecation, and total hospital stay, respectively. The total sample size was kept at 80 patients to accommodate all variables, protocol violations, and loss of follow-up. The statistical analysis was done using Statistical Package for Social Science (SPSS) Version 15.0. Normally distributed continuous variables were compared with the Student’s t-test. Testing the significance of the occurrence of events was done by Chi-square test. All values were considered statistically significant if the P value was less than 0.05.
Results
In the present study, 18 patients were excluded from 98 enrolled patients [Figure 1]. Five patients (three in group L and two in group C) dropped out due to conversion to open surgery in four and lost follow-up in one [Figure 1]. Data obtained from 75 patients were analyzed. The distributions of patients were comparable in both groups concerning demographic data, physical status, duration of anesthesia, and types of surgery [Table 1].
Table 1.
Demographic data
| Group C (n=38) | Group L (n=37) | P | |
|---|---|---|---|
| AGE (years) | 44.57±10.29 | 43.21±12.9 | 0.615 |
| SEX (M: F) | 25:13 | 21:11 | 0.988 |
| HT (cm) | 167.26±5.24 | 166.37±7.21 | 0.544 |
| WT (kg) | 65.58±11.12 | 67.31±16.07 | 0.60 |
| ASA (I/II/III) (n) | 17/14/5 | 17/16/4 | 0.43 |
| BMI | 23.24±3.69 | 24.85±4.95 | 0.116 |
| Duration of surgery (min) | 174.73±16.8 | 179.86±19.23 | 0.223 |
| Duration of anesthesia (min) | 180.34±14.7 | 184.26±14.21 | 0.244 |
| Types of Renal surgery (n) | |||
| Lap Nephrectomy (Simple/radical/donor) | 25 (10/12/3) | 24 (8/12/4) | 0.734 |
| Lap Pyeoloplasty | 8 | 6 | |
| Lap Paritial Nephrectomy | 5 | 7 |
The values of AGE, HT (height), WT (weight), Duration of surgery and anesthesia shown as Mean±SD, P value is calculated by using student t- test. For Gender, ASA (American Society of Anaesthesiology physical status) and Types of surgery P value was calculated using 2×2 Chi-square test
Time for first bowel sound, flatus, and defecation was 16.4 ± 2.50, 26.7 ± 9.02, and 39.1 ± 6.31 h, respectively, in group L and 18.2 ± 2.90, 32.3 ± 3.11, and 43.3 ± 4.22 h, respectively, in group C (P = 0.006, 0.001, and 0.01, respectively) [Table 2a]. Total hospital stay was 4.0 ± 0.74 and 5.3±0.0.91 days in group L and group C, respectively, which was found to be significantly lower in group L compared to group C (P < 0.001) on using the Student’s t-test [Table 2a].
Table 2a.
Total analgesic consumption and bowel function
| Group C (n=38) | Group L (n=37) | P | |
|---|---|---|---|
| Intraoperative Fentanyl consumption (µg) | 137±26 | 93±16 | <0.001* |
| Postoperative : Total Nalbuphine Consumption (mg) | 55.7±5.67 | 52.3±8.23 | 0.042* |
| First Bowel sound (Hours) | 18.2±2.90 | 16.4±2.50 | 0.006* |
| First flatus (Hours) | 32.3±3.11 | 26.7±9.02 | 0.001** |
| First Defecation (Hours) | 43.3±4.22 | 39.1±6.31 | 0.01* |
| Total Hospital Stay (Day) | 5.3±0.0.91 | 4.0±0.74 | <0.001** |
Data are presented as mean±SD. *P<0.05, **P<0.005
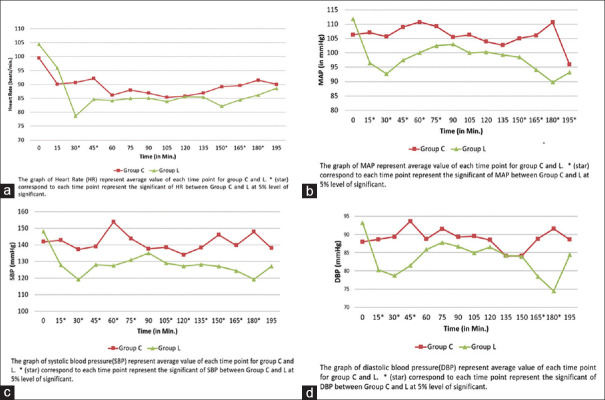
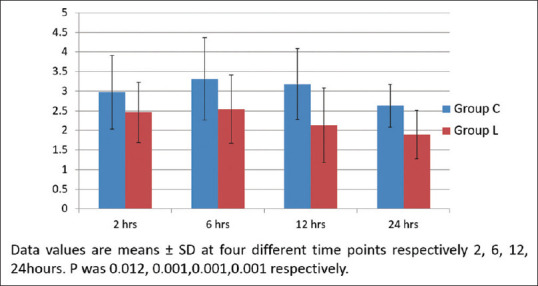
Intraoperative HR, SBP, DBP, and MAP remained significantly lower in group L compared to group C [Figure 2]. The need for Et-Sevo concentration was significantly lower in group L compared to group C [Figure 3]. VAS score was significantly lower in group L compared to group C at four different time points: second hour (2.45 ± 0.76 (L) vs. 2.9 ± 0.94 (C), P = 0.012), sixth hour (2.54 ± 0.86 (L) vs. 3.31 ± 1.04 (C), P = 0.001), 12th hour (2.13 ± 0.94 (L) vs. 3.18 ± 0.89 (C), P < 0.001), and 24th hour (1.89 ± 0.61 (L) vs. 2.63 ± 0.54(C), P = 0.001) [Figure 4]. Intraoperative fentanyl consumption was 93 ± 16 and 137 ± 26 μg in group L and group C, respectively, which was statistically significant (P < 0.01) [Table 2a]. Postoperatively, total nalbuphine consumption was 52.3 ± 8.23 and 55.7 ± 5.67 mg in groups L and C, respectively (P = 0.042) [Table 2a]. It suggested that opioid analgesic consumption was significantly lower in group L compared to group C during the perioperative period.
Figure 2.
Intraoperative hemodynamic stability: (a) intraoperative heart rate, (b) intraoperative mean arterial blood pressure, (c) intraoperative systolic blood pressure, (d) intraoperative diastolic blood pressure
Figure 3.
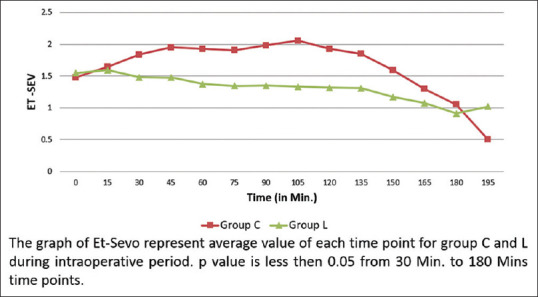
End-tidal sevoflurane (Et-Sevo) concentration
Figure 4.
Visual analog score (0–10)
PONV was significantly lower in group L compared to group C (P < 0.01) on using the Chi-square test [Table 2b].
Table 2b.
Postoperative -Adverse events
| Adverse events | Group C (%) (n=38) | Group L (%) (n=37) | P |
|---|---|---|---|
| Nausea | 6 (15.8) | 2 (5.4) | 0.137 |
| Vomiting | 7 (18.4) | 1 (2.7) | 0.021* |
Data are presented as percentage. Chi-square test was applied *P<0.05
Discussion
The present study demonstrated that perioperative use of IVL augmented bowel recovery and total hospital stay. The study observed that it improved intraoperative hemodynamic stability, reduced the requirement of Et-Sevo concentration, and reduced opioid consumption, postoperative pain intensity, and PONV in group L compared to group C.
The ERAS protocol is a combination of multimodal interventions that are applied perioperatively. The key components include preoperative counseling, regional anesthesia, optimal pain control, prevention of PONV, early enteral nutrition, and ambulation.[17] These protocols are expected to reduce surgical stress and complication rates. Over the last decades, laparoscopic surgeries and ERAS protocols have been studied for various kinds of surgery. Still, only a few studies focused on the role of IVL in enhancing recovery after laparoscopic renal surgeries.
Lidocaine is an antiarrhythmic and local anesthetic agent. It also has analgesic, anti-nociceptive, immuno-modulating, anti-inflammatory properties and opioid-sparing effects.[18,19] It acts mainly by blockade of voltage-gated open and inactive sodium channels.[20] The therapeutic plasma concentration is 0.5–5 μg/ml. It shows toxicity at a concentration above 5 μg/ml. IVL should be given to maintain effective therapeutic steady-state concentration with fewer side effects.[21] Therapeutic plasma levels can be achieved faster if a bolus is given before IV infusion. IVL infusion should be based on ideal body weight and should be reduced after 24 h to avoid the toxic level.[22]
Previous laparoscopic studies examined the postoperative outcome, and postoperative bowel recovery was significantly earlier during laparoscopic nephrectomy,[14] cholecystectomy,[15] and colon surgeries.[16] Tikuisis et al.[16] observed significantly early bowel movement in group L (26.97 ± 2.30 h) compared to the placebo group (32.97 ± 2.86 h) during laparoscopic colon surgeries (P < 0.001) Tauzin-Fin et al.[14] found that the time of first flatus passed in lidocaine and placebo groups was 27 ± 7 and 48 ± 15 h, respectively (P < 0.001). Song et al.[15] observed that the time to first flatus passage and defecation in the lidocaine group (20 ± 11 and 41 ± 16 h, respectively) was significantly earlier than in the placebo group (29 ± 10 and 57 ± 14 h, respectively). The current study observed that first flatus and defecation were significantly earlier in L group, that is, 26.7 ± 9.02 h (first flatus) and 39.1 ± 6.31 h (first defecation), compared to C group, that is, 32.3 ± 3.11 h (first flatus) and 43.3 ± 4.22 h (first defecation)]. Tauzin-Fin et al.[14] noted that total hospital stay was reduced with IVL (lidocaine 6.5 ± 1.5 days vs. placebo 7.5 ± 3 days). Patient can go home 1 day earlier. But there was no statistically significant value due to several nonmedical problems.[14] Tikuisis et al.[16] noticed that patients who received lidocaine stayed in hospital 1.2 days lesser than the patients who received placebo (P < 0.01). The current study also observed a similar result. There was a significant reduction in total hospital stay in L group (4.0 ± 0.74 days) compared to C group (5.3 ± 0.91 days).
Abdelazim Abdelhalim Hegazy and Wakel[23] found that IVL infusion attenuated the hemodynamic responses during laparoscopic cholecystectomy. There were significant reductions in HR, SBP, and DBP in the lidocaine group compared to placebo. Wang et al.[24] obtained the same result during laparoscopic gynecologic surgeries. The present study also obtained similar results during laparoscopic renal surgeries. Dennis et al.[25] observed that there was a reduction of 13%–21% in MAC-Minimum Alveolar Concentration of isoflurane requirement to maintain hemodynamic stability during laparoscopic cholecystectomy in obese patients with IVL. Omar and Aboushanab[26] found that Et-Sevo concentration was significantly higher in group C than in group L at all time points during the procedure. Bazin et al.[27] also observed similar results during major abdominal surgery. Equivalent results were found in the current study.
Song et al.[15] found that perioperative fentanyl consumptions in the lidocaine group and control group were 98.27 ± 16.33 and 187.49 ± 19.76 μg, respectively, during laparoscopic cholecystectomy, which is highly significant. The current study showed a comparable result during the intraoperative period. Nalbuphine is a synthetic agonist–antagonist opioid analgesic. Nalbuphine infusion was used because of its lesser adverse effects compared to morphine, like PONV, respiratory depression, addiction, itching, etc.[28] Nalbuphine and morphine were equally effective in patient-controlled IV analgesia after laparoscopic resection of colon cancer.[29] Weibel et al.[2] observed in a review article that IVL has an opioid-sparing effect with a high level of evidence. In the current study, there was a significant reduction of nalbuphine consumption in L group (52.3 ± 8.23 mg) compared to group C (55.7±5.67mg).
Tauzin-Fin et al.[14] noticed a significant reduction of pain intensity during rest and coughing for 48 h after laparoscopic nephrectomy. Song et al.[15] found that the VAS score was significantly lower in the lidocaine group during second and sixth hours. The present study noticed comparable results at the first, second, 12th, and 24th hours. Wang et al.[24] observed that the incidences of PONV were significantly lower in the lidocaine group compared to the control group after laparoscopic gynecologic surgeries. The current study made a similar observation, and no lidocaine-related adverse effects were found during the study period.
Various mechanisms led to early return of bowel movements in the above studies. IVL reduces postoperative pain, which can help to improve the bowel function. It has anti-inflammatory effects, which can help to reduce inflammation in the gut and promote healing. IVL may modulate intestinal reflexes, enhancing gut motility and promoting faster return to normal bowel function.
There were certain limitations of the present study. There was no bispectral (BIS) monitoring to measure the depth of anesthesia. IVL was used as per the previously studied regimen. Measurement of lidocaine plasma concentration was not done to monitor the therapeutic level. Different types of renal surgeries with various pathologies like infection, malignancy, and anatomical abnormality were included in the present study. These etiological factors may affect the outcome and hospital recovery. The pain score was analyzed with IV paracetamol usage and continuous infusion of nalbuphine in the first 24 h. No records were available on pain scores and analgesic usage after 24 h.
Conclusion
The perioperative use of IVL infusion enhances the recovery in patients undergoing laparoscopic renal surgeries by augmenting the bowel recovery and shortening the total hospital stay. It also has some beneficial effects like reducing the requirement of an inhalational agent, opioid-sparing effect, and lesser incidence of PONV. It can be the key component of ERAS protocols during laparoscopic renal surgeries.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil
References
- 1.Miao C, Yu A, Yuan H, Gu M, Wang Z. Effect of enhanced recovery after surgery on postoperative recovery and quality of life in patients undergoing laparoscopic partial nephrectomy. Front Oncol. 2020;10:513874. doi: 10.3389/fonc.2020.513874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. doi: 10.1002/14651858.CD009642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swenson BR, Gottschalk A, Wells LT, Rowlingson JC, Thompson PW, Barclay M, et al. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: A randomized clinical trial. Reg Anesth Pain Med. 2010;35:370–6. doi: 10.1097/AAP.0b013e3181e8d5da. [DOI] [PubMed] [Google Scholar]
- 4.Helander EM, Webb MP, Bias M, Whang EE, Kaye AD, Urman RD. A Comparison of multimodal analgesic approaches in institutional enhanced recovery after surgery protocols for colorectal surgery: Pharmacological agents. J Laparoendosc Adv Surg Tech A. 2017;27:903–8. doi: 10.1089/lap.2017.0338. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Laster MJ, Eger EI, 2nd, Sharma M, Sonner JM. Lidocaine, MK-801, and MAC. Anesth Analg. 2007;104:1098–102. doi: 10.1213/01.ane.0000260318.60504.a9. [DOI] [PubMed] [Google Scholar]
- 6.Castro I, Carvalho P, Vale N, Monjardino T, Mourao J. Systemic anti-inflammatory effects of intravenous lidocaine in surgical patients: A systematic review and meta-analysis. J Clin Med. 2023;12:3772. doi: 10.3390/jcm12113772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee IW, Schraag S. The use of intravenous lidocaine in perioperative medicine: Anaesthetic, analgesic and immune-modulatory aspects. J Clin Med. 2022;11:3543. doi: 10.3390/jcm11123543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigneault L, Turgeon AF, Cote D, Lauzier F, Zarychanski R, Moore L, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: A meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58:22–37. doi: 10.1007/s12630-010-9407-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–8. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Herroeder S, Pecher S, Schonherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: A double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. 2006;97:640–6. doi: 10.1093/bja/ael217. [DOI] [PubMed] [Google Scholar]
- 12.Rollins KE, Javanmard-Emamghissi H, Scott MJ, Lobo DN. The impact of peri-operative intravenous lidocaine on postoperative outcome after elective colorectal surgery: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2020;37:659–70. doi: 10.1097/EJA.0000000000001165. [DOI] [PubMed] [Google Scholar]
- 13.Chen PC, Lai CH, Fang CJ, Lai PC, Huang YT. Intravenous infusion of lidocaine for bowel function recovery after major colorectal surgery: A Critical appraisal through updated meta-analysis, trial sequential analysis, certainty of evidence, and meta-regression. Front Med (Lausanne) 2021;8:759215. doi: 10.3389/fmed.2021.759215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauzin-Fin P, Bernard O, Sesay M, Biais M, Richebe P, Quinart A, et al. Benefits of intravenous lidocaine on post-operative pain and acute rehabilitation after laparoscopic nephrectomy. J Anaesthesiol Clin Pharmacol. 2014;30:366–72. doi: 10.4103/0970-9185.137269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song X, Sun Y, Zhang X, Li T, Yang B. Effect of perioperative intravenous lidocaine infusion on postoperative recovery following laparoscopic Cholecystectomy-A randomized controlled trial. Int J Surg. 2017;45:8–13. doi: 10.1016/j.ijsu.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Tikuisis R, Miliauskas P, Samalavicius NE, Zurauskas A, Samalavicius R, Zabulis V. Intravenous lidocaine for post-operative pain relief after hand-assisted laparoscopic colon surgery: A randomized, placebo-controlled clinical trial. Tech Coloproctol. 2014;18:373–80. doi: 10.1007/s10151-013-1065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Zhao Q, Bai B, Ji G, Liu Y. Enhanced recovery after surgery programs for laparoscopic abdominal surgery: A systematic review and meta-analysis. World J Surg. 2018;42:3463–73. doi: 10.1007/s00268-018-4656-0. [DOI] [PubMed] [Google Scholar]
- 18.Gordh T. Lidocaine: The origin of a modern local anesthetic. 1949. Anesthesiology. 2010;113:1433–7. doi: 10.1097/ALN.0b013e3181fcef48. [DOI] [PubMed] [Google Scholar]
- 19.Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78:1229–46. doi: 10.1007/s40265-018-0955-x. [DOI] [PubMed] [Google Scholar]
- 20.Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: A new therapeutic indication? Anesthesiology. 2000;93:858–75. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg L PB, Tan C, Nikfarjam M. Pharmacokinetics and pharmacodynamics of lignocaine: A review. World J Anesthesiol. 2015;4:17–29. [Google Scholar]
- 22.Greenwood E, Nimmo S, Paterson H, Homer N, Foo I. Intravenous lidocaine infusion as a component of multimodal analgesia for colorectal surgery-measurement of plasma levels. Perioper Med (Lond) 2019;8:1. doi: 10.1186/s13741-019-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelazim Abdelhalim Hegazy MMME-S, Wakel AMMA. Attenuation of pneumoperitoneum-induced hypertension by intraoperative lidocaine infusion in laparoscopic cholecystectomy. Egypt J Hosp Med. 2019;76:4436–44. [Google Scholar]
- 24.Wang T, Liu H, Sun JH, Wang L, Zhang JY. Efficacy of intravenous lidocaine in improving post-operative nausea, vomiting and early recovery after laparoscopic gynaecological surgery. Exp Ther Med. 2019;17:4723–9. doi: 10.3892/etm.2019.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis PB, Davis K, Kuppuswamy B, Sahajanandan R. Intraoperative lidocaine infusion reduces analgesic and anesthetic requirements in patients with high body mass index undergoing laparoscopic cholecystectomy. Indian Anaesth Forum. 2020;21:23–32. [Google Scholar]
- 26.Omar AM, Aboushanab OH. Effect of intravenous lidocaine infusion on sevoflurane requirements as monitored by bispectral index: A randomized double-blinded controlled study. Egypt J Anaesth. 2013;29:235–9. [Google Scholar]
- 27.Bazin P, Padley J, Ho M, Stevens J, Ben-Menachem E. The effect of intravenous lidocaine infusion on bispectral index during major abdominal surgery. J Clin Monit Comput. 2018;32:533–9. doi: 10.1007/s10877-017-0035-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen MK, Chau SW, Shen YC, Sun YN, Tseng KY, Long CY, et al. Dose-dependent attenuation of intravenous nalbuphine on epidural morphine-induced pruritus and analgesia after cesarean delivery. Kaohsiung J Med Sci. 2014;30:248–53. doi: 10.1016/j.kjms.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Zhang R, Liu T. Effect of nalbuphine on patient controlled intravenous analgesia after radical resection of colon cancer. Oncol Lett. 2020;19:2533–8. doi: 10.3892/ol.2020.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]