Abstract
Glyphosate is a widely used active ingredient in agricultural herbicides, inhibiting the biosynthesis of aromatic amino acids in plants by targeting their shikimate pathway. Our gut microbiota also facilitates the shikimate pathway, making it a vulnerable target when encountering glyphosate. Dysbiosis in the gut microbiota may impair the gut-brain axis, bringing neurological outcomes. To evaluate the neurotoxicity and biochemical changes attributed to glyphosate, we exposed mice with the reference dose (RfD) set by the U.S. EPA (1.75 mg/Kg-BW/day) and its hundred-time-equivalence (175 mg/Kg-BW/day) chronically via drinking water, then compared a series of neurobehaviors and their fecal/serum metabolomic profile against the non-exposed vehicles (n = 10/dosing group). There was little alteration in the neurobehavior, including motor activities, social approach, and conditioned fear, under glyphosate exposure. Metabolomic differences attributed to glyphosate were observed in the feces, corresponding to 68 and 29 identified metabolites with dysregulation in the higher and lower dose groups respectively compared to the vehicle-control. There were less alterations observed in the serum metabolome. Under 175 mg/Kg-BW/day of glyphosate exposure, the aromatic amino acids (phenylalanine, tryptophan, and tyrosine) were reduced in the feces but not in the serum of mice. We further focused on how tryptophan metabolism was dysregulated based on the pathway analysis, and identified the indole-derivatives were more altered compared to the serotonin and kynurenine derivatives. Together, we obtained a three-dimensional dataset that records neurobehavioral, fecal metabolic, and serum biomolecular dynamics caused by glyphosate exposure at two different doses. Our data showed that even under the high dose of glyphosate irrelevant to human exposure, there were little evidence that supported the impairment of the gut-brain axis.
Keywords: Glyphosate, Roundup, Neurotoxicity, Metabolomics, LC-MS, Herbicide
Introduction
Glyphosate-based herbicides (GBHs), such as Roundup, have been the primary herbicides used globally for inhibiting weed in genetically engineered agriculture. The coformulants of GBHs are complex and proprietary, but glyphosate [N-(phosphonomethyl)glycine] is the major active component of GBHs. Glyphosate critically undermines the shikimate pathway in plants by inhibiting the 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase, which causes failures to their biosynthesis of aromatic amino acids and other secondary metabolites (Boocock and Coggins 1983; Zabalza et al. 2017) (Figure 1A). The shikimate pathway exists in plants and microorganisms but not mammals, which is a major reason that glyphosate is considered as a safer herbicide. Together with other conventional toxicological assessments, the United States Environmental Protection Agency (EPA) has classified glyphosate in the least toxic category (category IV, practically non-toxic and non-irritating) (Williams et al. 2000). However, the human body harbors massive microorganisms in the gastrointestinal tract, namely the gut microbiota, which encompasses kaleidoscopic functions that help maintain physiological homeostasis to their host. By computational modelling, it is believed that most of the bacteria inhabiting in the human gut encompass EPSP synthase, which would render them sensitive to the inhibition by glyphosate (Mesnage and Antoniou 2020). Thus, it is reasonable to suspect that gut dysbiosis attributed by glyphosate can happen, leading to the disruption of host homeostasis.
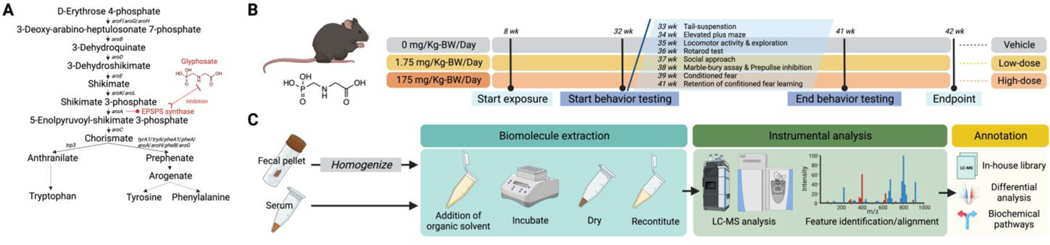
Figure 1.
Schematic study design of the neurobehavior and biochemical evaluation of mice chronically exposed to glyphosate via drinking water. (A) Shikimate pathway that can be facilitated by species of the gut microbiota in mammals. The mode of action for glyphosate in perturbing the gut microbiota is the inhibition of 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase thus abrogating the shikimate pathway. (B) The mice were randomized by body weight and assigned to the vehicle control (0 mg/Kg-BW/day), low-dose (1.75 mg/Kg-BW/day), or high-dose group (175 mg/Kg-BW/day), which the exposure to glyphosate by drinking water started at the age of 8 weeks and continued until the endpoint of study (42nd week) (C) Experimental workflow of the nontargeted metabolomic analysis on feces and serums of mice. Serum and homogenized feces were extracted for metabolites and concentrated to maximize the detection by LC-MS. Signals recorded by the LC-MS analysis were deconvoluted, filtered to avoid backgrounds, identified for molecular features, and aligned across samples. The molecular features were then examined for difference among the groups, matched with our LC-MS in-house library, and analyzed for biochemical pathways.
The impact of glyphosate on the gut microbiota was initially brought to concern in agricultural poultry, with in vitro evidence showing a reduction in growth of beneficial bacteria (e.g., Enterococcus faecalis) isolated from chicken feces (Shehata et al. 2012). Soon, the interaction between glyphosate and gut microorganisms was evaluated in vivo in animals including honey bees (Motta et al. 2018) and rodents (Lozano et al. 2018; Mao et al. 2018). The studies that applied rodent models as a surrogate to the toxicodynamics of glyphosate in humans has yet met consensus, while some studies showed significant perturbation (Aitbali et al. 2018; Lozano et al. 2018), and other concluded safety (i.e., absence of dysbiosis) under environmental exposure levels (Mao et al. 2018; Nielsen et al. 2018). Those studies that observed significant dysbiosis of the gut bacteria further argued that the inhibition of the shikimate pathway practiced by the gut microbiome may attenuate the level of aromatic amino acids in the gastrointestinal tract, which subsequently reduces the absorbed amount of the host. Also, the dysbiosis resulting from glyphosate can influence the dynamics of bacterial-produced secondary metabolites that have essential roles in the host physiology. Both potential mechanisms raised concerns on the more systemic toxicity of glyphosate (Aitbali et al. 2018; Samsel and Seneff 2013).
Gut microbiota-derived metabolites contain multiple neurotransmitters that are central regulators of the gut-brain axis, and can modulate neurodevelopment, neurobehavior, and other neurological events (Saad et al. 2022; Strandwitz 2018). Whether glyphosate demonstrates neurotoxicity by disrupting the gut microbiota remains debatable, with some studies observing significant decrease of neurotransmitters (e.g., serotonin, dopamine) in the brain after glyphosate exposure (Ait Bali et al. 2017; Martínez et al. 2018), and others concluding little alteration (Hernández-Plata et al. 2015). Also, the conclusion on the neurobehavioral effects of glyphosate has yet met consistency as well, which may be contributed by factors including dosing scenarios, experimental designs, test species, and commercial products (Moser et al. 2022).
Glyphosate, as a post-emergent and widely applied herbicide has raised public health concerns in its possible effects on inducing gut dysbiosis and neurological disorder. Yet, there remains a scarcity of toxicological data in supporting the health risk assessment and regulatory decision-making, especially with multiple dimensions (e.g., conjugations of neurobehavior and biochemical analyses), next-generation methods, and a more chronic dosing scheme. We address this gap by designing a standardized battery of neurobehavior phenotyping to comprehensively evaluate the neurotoxicity of glyphosate in conjugation to profiling the biochemical landscape with next-generation mass spectrometry metabolomics (Figure 1B, 1C). The chronic reference dose (RfD) for glyphosate (1.75 mg/Kg-BW/day) set by the U.S. EPA and a hundred-time-equivalence (175 mg/KG-BW/day) were administered to 8-week-old mice via drinking water for over 6 months. We phenotyped for a series of their neurobehaviors during the exposure, and profiled the fecal and serum biochemicals at the study endpoint to investigate the dynamics of chemical landscape under the exposure. Our data showed little difference in the neurological behaviors contributed by glyphosate. There were significant alterations of the aromatic amino acids in the feces but not in the serum under glyphosate exposure. Together, our comprehensive evaluation on neurobehavioral effects and high-resolution metabolomics provided new data for the assessment of safety in glyphosate use and exposure.
Methods
Chemicals and reagents
All reagents and chemicals, unless otherwise specified, were obtained from Thermo Fisher Scientific (Rockford, IL), including the glyphosate (P/N AC466180050) used for the animal exposure. All stable-isotope labeled standards were provided by CDN Isotopes (Pointe-Claire, Quebec, Canada).
Animal Exposure
SPF grade C57BL/6J mice (~7 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and provided a standard pelleted rodent diet and water ad libitum under the following environmental conditions: 22°C, 40–70% humidity, and a 12:12 hour light:dark cycle in the static microisolator cages with Bed-O-Cob combination. All 30 mice were housed in the University of North Carolina at Chapel Hill animal facility for 1 week before the experiments. Then, the mice were randomly assigned by their body weight to the vehicle control, low-dose, and high-dose groups at the 8th week of the mice’s age. The drinking water provided to the low- and high-dose groups contained 1.75 and 175 mg/Kg-BW/day glyphosate, while the vehicle control group were provided with drinking water without glyphosate. The drinking water for each group was renewed weekly, and the concentration of glyphosate was adjusted to reach the concentration that provides the mean exposure of 1.75 or 175 mg/Kg-BW/day for the low- and high-dose groups based on the weekly measured body weight and water consumption data. After 24 weeks (6 months, at 32nd week-age of mice) of exposure, the mice were evaluated by a battery of behavioral tests (Figure 1B) that lasted for 9 weeks. The mice were collected for fecal pellets, then euthanized with carbon dioxide and necropsied to collect their tissues and serum at their 42nd week-age. The exposure lasted until the euthanasian. The animal use in this study was approved by The University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Mice behavioral tests
At the 24-week-exposure time point, the mice were comprehensively phenotyped for their behaviors. Tests were carried out by an experimenter blinded to exposure group. The mice were evaluated in a standardized battery of assays, which included the tail-suspension test for paw clasping, elevated plus maze test for anxiety-like behavior, open field test for locomotor activity and exploration, rotarod test for motor coordination, three-chamber choice test for social approach, marble-bury assay for exploratory digging, acoustic startle responses for prepulse inhibition, and the conditioned fear learning test. The regimen of behavior phenotyping is listed in Table S1 and Figure 1B. More stressful procedures, such as the conditioned fear learning, were carried out near the end of the regimen to limit carry-over effects between tests. The detailed description of the method for each behavioral test is provided in the Supporting Information.
Biospecimen sample preparation
The fecal pellets of mice were collected after the behavioral evaluation was finished, then the mice were euthanized by carbon dioxide and immediately collected for serum. Prior to extracting the biomolecules, 30 mg of the fecal pellet from each mouse was added with a weight-adjusted volume (100 μL per 30 mg) of water, and mechanically homogenized by glass-bead-beating. The extraction of biomolecules was facilitated by adding methanol that contains stable isotope-labeled ([D5]-glutamine, [D2]-𝛾-aminobutyric acid, [D3]-tryptophan, [D2]-indole-3-propionic acid, [D2]-indole-3-acetic acid, [D13]-acetylcholine in 500 nM; [D4]-serotonin in 250 nM; and [D4]-kynurenic acid in 50 nM). Weight- or volume-adjusted internal-standard-contained methanol was added to each sample (400 μL per 30 mg fecal pellet, or 180 μL per 20 μL serum) and incubated under 4 °C for 1 hour. The supernatant of each sample was collected by centrifuging under 15,000 ×g for 10 minutes, dried with SpeedVac®, and reconstituted in 2% acetonitrile in water (150 μL and 100 μL for fecal and serum samples, respectively).
LC-ESI-MS analysis for fecal and serum metabolomic
The LC-ESI-MS analysis for the processed biospecimens was facilitated by a Thermo Fisher Scientific Vanquish UHPLC coupled to a Q Exactive mass spectrometer connected by a heated electrospray ionization (HESI) source, using parameters and data processing protocols documented in detail in previous articles (Guo et al. 2020; Hsiao et al. 2022; Lai et al. 2021) and in the Supporting Information. Briefly, the biomolecules were separated by reverse-phase liquid chromatography, recording the retention time (RT), and the m/z in high-resolution by the mass analyzer. The molecular signatures (i.e., RT-m/z combinations) were deconvoluted, aligned across samples, and matched against an LC-MS database we built in-house to annotate the biochemicals detected.
Data interpretation of the metabolomic data
The matched biomolecules were further matched with multiple identifiers (PubChem, HMDB, KEGG, and ChEBI) to validate, cross-reference, and maximize capability to reveal relationships among these molecules. The over-representation enrichment analysis was done by the MetaboAnalystR (ver 3.0) package in R, using all matched biomolecules as the baseline reference. An enriched pathway was defined with the following criteria: (1) an enrichment ratio, which is defined as the ratio of observed and expected number of metabolites, based on the number of metabolites in each pathway and the total detected metabolites, larger than 1; (2) If a pathway involves more than 5 biochemicals, the number of observed, enriched compounds is larger than 3; if a pathway involves less than 5 biochemicals, the number of observed, enriched compounds is larger than 2.
Results
Study design and quality assessment
The mice were assigned to the vehicle control, low-dose (1.75 mg-glyphosate/L in water) and high-dose (175 mg-glyphosate/L in water) groups for chronic exposure to glyphosate via drinking water. The body weight of the mice remained similar among groups until a month after the exposure, and this difference in weight continued throughout the study (Figure S1A). For instance, before conducting the behavior phenotyping (32nd week age), the mice in the low-dose group had the highest weight of 31.35 ± 0.42 mg, followed by the vehicle-control and the high-dose group, which the body weights were respectively 30.52 ± 0.90 and 28.79 ± 0.57 mg. The weekly water consumption had milder changes compared to the body weight (Figure S1B). The exposure remained 1.75 and 175 mg-glyphosate/Kg-BW/day for the low- and high-dose groups as glyphosate-contained drinking water for these two groups were adjusted weekly to the corresponding concentration of glyphosate calculated from the measurements in body weight and water consumption.
Behavioral changes under chronic exposure to glyphosate
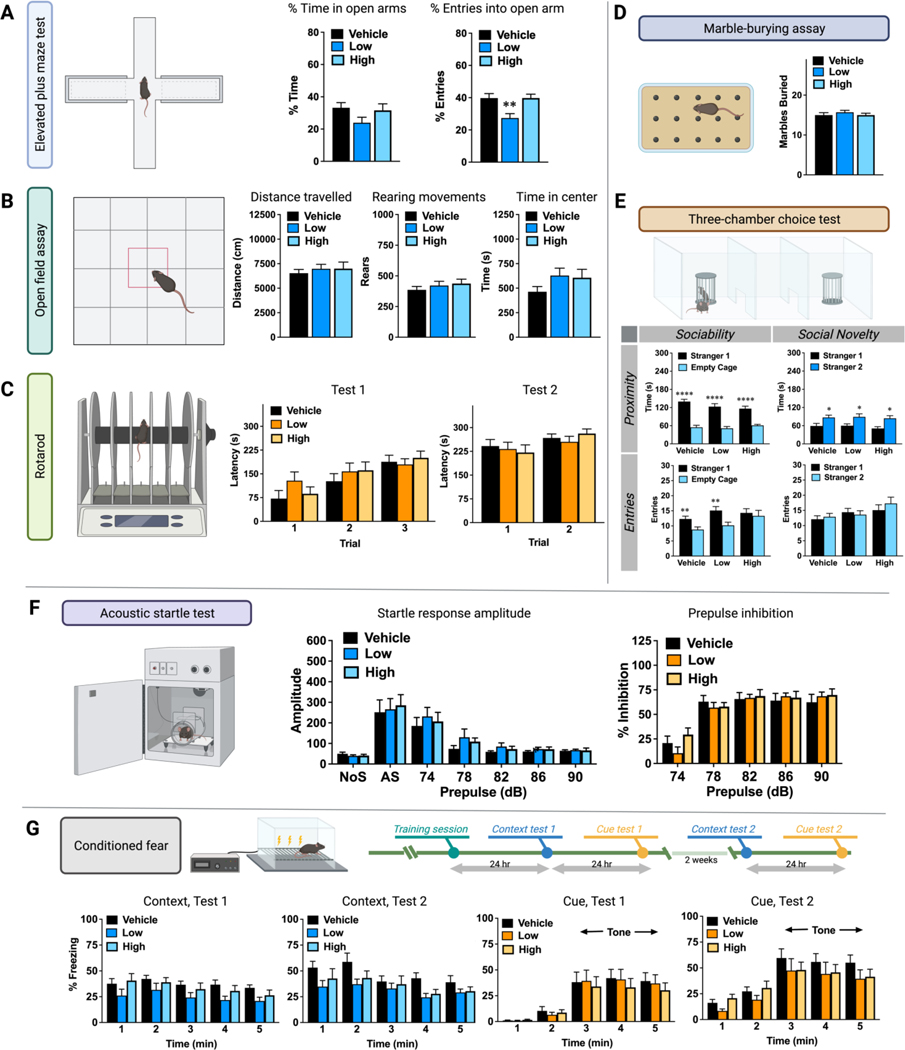
A standardized battery of neurobehavioral tests was examined in the vehicle-control, low-dose, and high-dose mice (Figure 1B). No effects of exposure were found in the tail suspension test. In each group, the mean scores from the test were very close to zero, indicating a lack of paw-clasping or other aberrant phenotypes (data not shown). In the elevated plus maze test for anxiety-like behavior, the groups had similar percent time spent on the open arms, versus the closed arms; however, the low-dose exposure group had significantly decreased percent entries into the open arms, in comparison to both the vehicle and high-dose groups (p = 0.0027) (Figure 2A), suggesting mice given treatment with low-dose glyphosate had increased anxiety in the plus maze, albeit only by one measure. The open field assay is another index of anxiety-like behavior. As shown in Figure 2B, there were no effects of the herbicide exposure on any of the measures taken during the 1-hour-test. Similarly, the three groups had comparable motor coordination in a rotarod assay (Figure 2C), and in the marble-bury test for exploratory digging (Figure 2D).
Figure 2.
Evaluation of behavior of mice exposed to glyphosate at 0 (vehicle), 1.75 (low), and 175 (high) mg/Kg-BW/Day via drinking water for at least 32 weeks until each of the specific behavior test were conducted. (A) Percentage of time and entries in the open arms in the elevated plus maze test. (B) Activity and exploration, which included traveled distance, rearing movements, and time in center in the open field assay (1 hour in a novel open field). (C) Motor coordination of two tests on an accelerating rotarod. (D) Number of buried marbles for exploratory digging via marble-burying assay. (E) Sociability and social novelty preference during a 3-chamber choice test. The upper panel shows the time spent within 5 cm proximity of each cage for a 10-min-test. The lower panel shows the number of entries into each side. (F) Results of the acoustic startle test, which included the amplitude of startle responses and prepulse inhibition. (G) Levels of freezing during conditioned fear tests. The first context test was conducted 24 hr after the training session, and the second test was followed two weeks after. The test for cue learning was conducted 24 hr after each context test. Bar plots are shown as mean ± standard error of mean (SEM). N = 10 for each group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In addition, no significant effects of exposure were found for performance in the 3-chamber test. Mice were first presented with a choice between spending time in proximity to an unfamiliar mouse (stranger 1), versus an empty cage, as an index of sociability. All three groups had robust preference for investigating the stranger mouse, versus the empty cage (within-treatment comparisons following repeated measures ANOVA, significant effect of side, p < 0.0001) (Figure 2E, Sociability × Proximity panel). In the test for social novelty, all three groups demonstrated the typical switch in preference toward the newly-introduced mouse (stranger 2) mouse, versus stranger 1 (within-treatment comparisons following repeated measures ANOVA, significant effect of side, p < 0.05) (Figure 2E, Social Novelty × Proximity panel). Transitions during the 3-chamber test can serve as an index of general exploration and activity, as well as social preference. During the sociability test, the vehicle and low-dose groups made significantly more entries into the side containing the stranger mouse, versus the empty cage (within-treatment comparison following repeated measures ANOVA, significant effect of side, p < 0.01). In contrast, the high-dose exposure group failed to show significant side preference (Figure 2E, Sociability × Entries panel). No differences in side entries were observed during the test for social novelty (Figure 2E, Social Novelty × Entries panel).
Startle magnitude reflects responsivity to stimuli in the environment. As shown in the middle panel of Figure 2F, the three treatment groups had similar startle amplitudes at every decibel level. The three groups also had comparable prepulse inhibition of startle responses (Figure 2F, right panel). Figure 2G depicted the results from the conditioned fear tests. In the first test for contextual learning, the low-dose group had generally reduced levels of freezing, in comparison to the vehicle-control group (repeated measures ANOVA, main effect of treatment, p = 0.0479). Post-hoc analyses for each minute of the test did not reveal any further significant differences. No effects of exposure were evident in the second test, conducted 2 weeks later. All three groups had comparable levels of freezing in the tests for cue-dependent learning, conducted in a modified test chamber. Notably, there were no effects of glyphosate exposure on the typical increase in freezing observed at minute 3, with the onset of the tone.
Exploratory and differential analysis on the biomolecular landscape in feces and serum
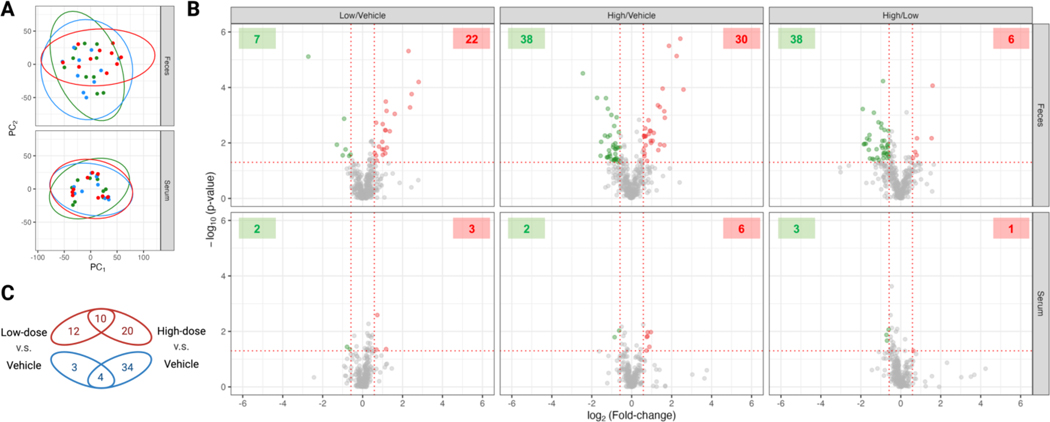
After evaluating the effects in neurological behaviors, we sought to investigate the modulation of the chemical landscapes in the gut flora and host exposed to glyphosate. Facilitated by the high-resolution LC-MS analysis, we aligned 46,113 molecular features (i.e., signatures of RT-m/z combination) in the fecal samples, and 370 metabolites from our in-house database can be matched. For the serum samples, 28,031 features were detected, and 285 metabolites can be assigned. There was little statistical clustering that corresponded to the exposure of glyphosate for both feces and serum samples (Figure 3A). In the differential analysis of the 370 metabolites annotated in the fecal samples, 29 (22 up- and 7 down-regulated), 68 (30 up- and 38 down-regulated), and 43 (6 up- and 38 down-regulated) compounds were significantly different when comparing the low-dose group against the vehicle-control group, the high-dose group against the vehicle-control group, and the high-dose group against the low-dose group, respectively (Figure 3B). Compared to the absence of exposure to glyphosate, low-dose and high-dose exposures respectively accounted for alterations in 7.8% (29/370) and 18.3% (68/370) of metabolites detected in our analysis. The modulation on fecal biomolecules is dose-dependent, as 20 and 12 metabolites showed difference only in the high-dose and low-dose group, with 10 metabolites having intersected up-regulations under the two exposure levels (Figure 3C). Interestingly, the high-dose-exposure to glyphosate resulted in a significant increase in the down-regulated metabolites, which is not observed under the low-dose-exposure. In contrast to the more apparent dysregulation glyphosate resulted in feces, the metabolites in serum remained mostly unchanged under glyphosate exposure, which high- and low-dose-exposure only resulted in 8 and 5 altered metabolites.
Figure 3.
Exploratory and differential analysis on the untargeted LC-MS analysis on the fecal and serum samples of mice exposed to glyphosate. (A) Clustering result from principal component analysis in the fecal (upper panel) and serum (lower panel) samples of mice exposed to 0 (vehicle, green dots), 1.75 (low-dose, blue dots), and 175 (high-dose, red dots) mg/Kg-BW/day in drinking water. (B) Volcano plots of the matched compounds in the fecal (upper panel) and serum (lower panel) samples. The x-axis coordinates the binary logarithm of fold changes and the y-axis is the negative common logarithm of p-value from pairwise Student t-test of low-dose against vehicle groups (left panels), high-dose against vehicle groups (middle panels), and high-dose against low-dose groups (right panels) in the fecal and serum samples. The fecal and serum samples were respectively detected and annotated to 370 and 285 compounds. The significantly altered compounds were colored in green (down-regulated) and red (up-regulated) dots, and the number of down- and up-regulated metabolites were respectively annotated in the green and red boxes in the upper corners of each facet. Significance was defined as the fold-change larger than 1.5 in both directions and p-value under 0.05 in any pair of comparison. (C) Venn diagram summary of significantly altered compounds in the low- and high-dose groups respectively against the vehicle control group. The number of the up-regulated compounds in the low-dose group (left) and in the high-dose group (right) are annotated in red ellipses, and the number of down-regulated compounds in the low-dose group and in the high-dose group are annotated in green ellipses.
Alteration of shikimate-pathway-related aromatic amino acids under glyphosate exposure
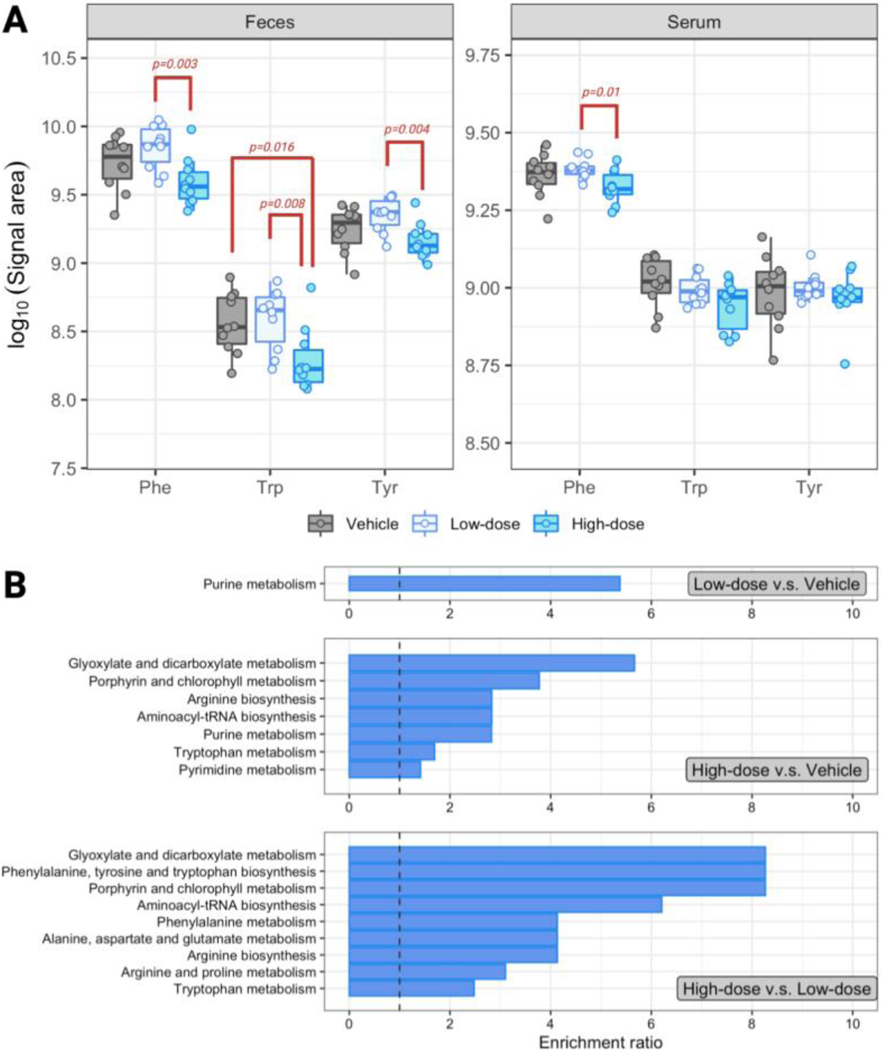
Among the metabolites we analyzed in the fecal and serum samples of mice exposed to glyphosate, we were most interested in the aromatic amino acids that may be vulnerably perturbed by glyphosate. Compared to the absence of exposure to glyphosate in drinking water, the high-dose group had lower levels of the three aromatic amino acids in feces, but only the levels of tryptophan were significantly down-regulated (p = 0.016) (Figure 4A). Although without statistical significance, the levels of aromatic amino acids increased slightly under the low-dose-exposure. Due to the nonlinearity in dose-response, the high-dose group had significantly decreased aromatic amino acids compared to the low-dose group, which the fold-changes for phenylalanine, tryptophan, and tyrosine were respectively 0.56 (p = 0.003), 0.52 (p = 0.008), and 0.65 (p = 0.004) (Figure 4A). The perturbation of glyphosate on the levels of fecal aromatic amino acids was not pervaded through circulation, as little difference was observed in serum between the high- and low-dose groups against the vehicle-control. To be specific, tryptophan and tyrosine in serum showed similar levels among the three groups. Serum phenylalanine was significantly lower in the high-dose than in the low-dose group, which the fold-change was 0.88 (p = 0.01).
Figure 4.
Alteration of key metabolites of mice exposed to glyphosate. (A) Common logarithm of the signal area of phenylalanine (Phe), tryptophan (Trp), and tyrosine (Tyr) in the feces (left panel) and serum (right panel) of mice exposed to 0 (grey), 1.75 (light blue), and 175 (sky blue) mg/Kg-BW/day glyphosate in drinking water. (B) Biochemical pathways significantly altered in the comparison of the low-dose group against the vehicle group (upper panel), high-dose group against the vehicle group (middle panel), and the high-dose group against the low-dose group (lower panel). The enrichment ratio is calculated by MetaboAnalyst by dividing the observed number to the expected number of altered metabolites.
Modulation of biochemical pathways under glyphosate exposure
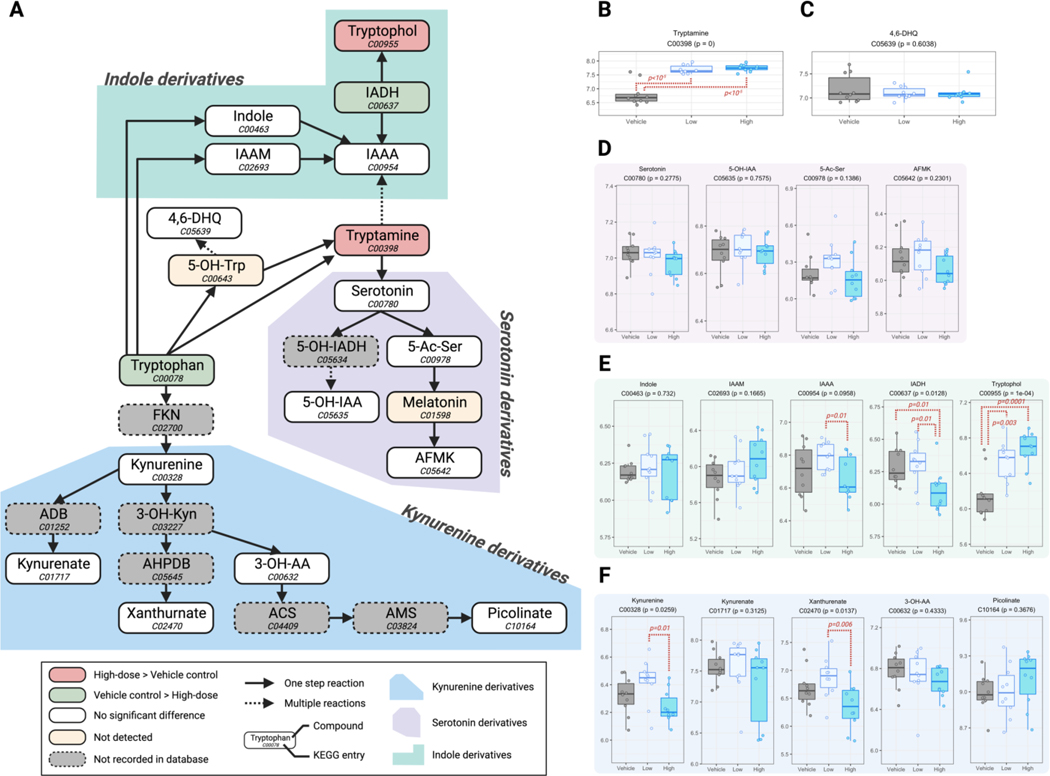
From the nontargeted metabolomic analysis, we observed a larger overall biomolecular modulation in the feces, but not in the serum, under glyphosate exposure. To identify the specific biochemical pathways perturbed by the glyphosate, we further performed over-representation enrichment analysis on the 29, 68, and 43 dysregulated metabolites retrieved from the differential analysis of the low-dose versus vehicle, high-dose versus vehicle, and high-dose versus low-dose groups, using the total detected 370 fecal metabolites as the baseline reference. The 29 dysregulated compounds in the low-dose group (compared to the vehicle control) only showed enrichment in the purine metabolism pathway (Figure 4B). The 68 altered compounds in the high-dose group were enriched in 7 biochemical pathways, which includes tryptophan metabolism. In addition, there were 9 enriched pathways from the 43 metabolites that presented different levels between the high- and low-dose groups. Chemicals involved in the tryptophan metabolism showed enrichment in the dysregulation caused by higher exposure of glyphosate (i.e., when comparing the high-dose against the vehicle-control group, and the high-dose against the low-dose group), and tryptophan was attenuated in the high-dose group (Figure 4A). Together, we are interested if the perturbed tryptophan level can also affect downstream biomolecules of the tryptophan metabolism. Among the biochemicals involved in the biotransformation of tryptophan mapped by KEGG, we were able to detect 17 metabolites in the feces of our subjects. Some of the species can be categorized into three of the main sub-pathways of tryptophan metabolism, which 4, 5, and 5 of them respectively belong to the serotonin, indole, and kynurenine derivatives. The simplified map of the sophisticated tryptophan metabolism that focused on the 17 metabolites we detected is presented in Figure 5A, with the levels of each detected metabolite illustrated in Figure 5B–F. The kynurenine derivatives remained in similar levels compared to the vehicle-control under low- or high-dose exposure of glyphosate, but kynurenine and xanthurenate showed decrease in the high-dose group compared to the low-dose group (p = 0.01 and 0.006, respectively). Tryptamine, the product of metabolizing tryptophan by bacterial tryptophan decarboxylase, was significantly up-regulated under the exposure of low- and high-dose glyphosate, which the levels were 4.9 (p = 4.8×10−6) and 5.4 (p = 1.7 ×10−6) times higher than in the vehicle-control group, respectively (Figure 5B). However, subsequent products of tryptamine, serotonin and its downstream metabolites were not influenced by glyphosate (Figure 5D). Tryptophan is the substrate of the gut microbiome to formulate a variety of indole derivatives. We found the direction of regulation inconsistent in the indole derivatives in the feces under glyphosate exposure. For example, the high-dose group had significantly higher level of tryptophol (fold-change 2.92, p = 1.1×10−4), but lower level of indole-3-acetaldehyde (IAAA) (fold-change 0.66, p = 0.014) compared to the unexposed group.
Figure 5.
Impact of glyphosate exposure to tryptophan metabolism in the fecal metabolome of mice. (A) Partial pathway map of the mammalian tryptophan metabolism and how it can be perturbed by the exposure of glyphosate. Compounds and their KEGG entries are presented in nodes. The colors of nodes indicate the following information: red, significant up-regulation in the high-dose group compared to the vehicle control; green, significant down-regulation in the high-dose group compared to the vehicle control; white, detected without significant difference between the high-dose group and the vehicle control (but may have difference between the high-dose and low-dose groups); yellow: not detected in the samples though the compound exist in our LC-MS in-house library; grey, not recorded in our LC-MS in-house library. Arrows that connect the nodes are solid and dashed to indicate one-step reactions and multiple reactions. The annotating backgrounds in blue, purple, and green indicate the kynurenine, serotonin, and indole sub-pathways under the sophisticated tryptophan metabolism. (B-F) Boxplots of the common logarithm in signal areas of tryptamine (B), 4,6-DHQ (C), serotonin derivatives (D), indole derivatives (E), and kynurenine derivatives (F) in the fecal samples of mice in the vehicle control (grey), low-dose (light blue), or high-dose (sky blue) groups. The subtitle of the boxplot annotated the KEGG entry of the compound and the p-value of the group-wise F-test. Significant differences in any two groups were annotated in red dashed brackets with their corresponding p-values in the Student t test. 4,6-DHQ: 4,6-Dihydroxyquinoline; 5-OH-IAA: 5-Hydroxyindoleacetate; 5-Ac-Ser: N-Acetylserotonin; AFMK: Formyl-N-acetyl-5-methoxykynurenamine; IAAM: Indole-3-acetamide; IAAA: Indole-3-acetate; IADH: Indole-3-acetaldehyde; 3-OH-AA: 3-Hydroxyanthranilate.
Discussion
The increased application of GBHs in agriculture was based on the belief that their long-term adverse health effect under chronic exposure is minimal, due to rationales including the absence of target (i.e., shikimate pathway) in human. However, members of the gut microbiota may utilize the shikimate pathway for synthesizing or cross-feeding nutrients, and the exposure to glyphosate may perturb and cause local or systemic adverse effects to the host. Since the shikimate pathway contributes to precursors of neurotransmitters such as the aromatic amino acids, and previous literature show concerns of glyphosate in neurotoxicity (Costas-Ferreira et al. 2022; Moser et al. 2022), we aimed to analyze the neurobehavioral and biochemical influence of chronic exposure to glyphosate comprehensively.
Under a chronic exposure to glyphosate at the RfD (1.75 mg/Kg-BW/day) and its hundred-time-equivalence (175 mg/Kg-BW/day) for over 6 months, the neurobehavioral phenotypes showed little difference compared to the vehicle-control group. Neurobehavioral normalities were inspected from basic properties of the central nervous system (e.g., fear, classical conditioning) to anxiety, exploratory desires, motor behaviors, and social motivations. There were other studies that evaluated behavioral changes in rodents under glyphosate exposure, with assays similar to our phenotyping regimen, but most of the studies exposed the rodents to doses higher than the human relevance. For example, Brammer exposed Wister rats to diets containing 0, 2,000, 6,000, or 20,000 mg/Kg glyphosate for 52 weeks (n = 11–12/sex/dose), and observed female rats given 5,000 and 20,000 ppm dietary glyphosate had lower motor activities, but not in other sex-dose combinations (Brammer 2001). Nufarm laboratories, an agricultural chemical company, also exposed Wistar rats to diets containing 0, 1,500, 5,000, or a higher dose (15,000 for the first 48 weeks and 24,000 for 4 weeks after) mg/Kg glyphosate, and observed difference neither in motor activity nor functional observational battery (Nufarm 2009). There were also studies that applied subacute, repeated administrations of glyphosate. Rats were exposed to 0, 50, 100, or 150 mg/Kg-BW/day of glyphosate via intraperitoneal injections, three times a week, and for two weeks (n = 16–17/dose/timepoint) (Hernández-Plata et al. 2015). The study observed lower motor activities 3 hours after injections in all exposed groups. This lower effect only remained after 2 days in the 100 and 150 mg/Kg-BW/day groups, but not in the lower 50 mg/Kg-BW/day group. The dosing scenarios and study design vary significantly in the limited toxicological assessments conducted on glyphosate, and this has been one of the major issues in harmonizing and concluding the neurotoxicity of glyphosate, as stated in a recent systemic review on this topic by Moser et al. (Moser et al. 2022). With few assessments made on chronic glyphosate exposure, we choose to focus on the dosages around the RfD (practicing 1× and 100×RfD) to scrutinize the neurobehavioral effects. The calculation of daily exposure dosage using the RfD (1.75 mg/Kg-BW/day) and the 100×RfD on a 80 kg human results in 140 mg and 14 g for glyphosate, which were both significantly higher than environmental and even occupational settings of glyphosate exposure (Add literature). However, our data showed that even under this irrelevantly high dose did not affect the neurobehaviors of mice. Several reasons may contribute to the absence of change in neurobehaviors under glyphosate exposure in our study, including that (1) we applied lower dosage of glyphosate compared to previous studies; (2) we exposed mice to glyphosate by drinking water but not diets or oral gavage, which are more common ways for previous assessments (Moser et al. 2022; Portier 2020); (3) most of the previous studies that exposed glyphosate to rodents via diet or drinking water used an universal concentration in food or water throughout the exposure scheme, not accounting for changes in consumptions of food and water (due to reasons such as gained body weight); and (4) mice may be less sensitive in neurobehavioral change by glyphosate, as past alterations were majorly observed in rats. It is also noted that some previous evaluations on glyphosate administrated the GBH product that contained unidentified coformulants, and resulted inconsistently among studies. Coformulants in herbicides/fungicides/pesticides have been evidenced to cause toxicity in cases such as Amistar (Straw and Brown 2021) and maybe in GBHs such as Roundup (Defarge et al. 2016; Mesnage et al. 2019). Extensive systemic reviews have been made on summarizing past studies on neurotoxicity attributed to glyphosate and GBHs, but they reached conclusion that more quality data are needed, especially with chronic exposure at human-relevant concentrations to evaluate the long-term effects (Moser et al. 2022).
To our knowledge, there is little analysis done using next-generation LC-MS technology in the metabolome exposed to glyphosate in mice, but there were some analyses conducted in rats (Hu et al. 2021) and zebrafish (Giommi et al. 2022). Our capture in the fecal and serum metabolome of mice exposed chronically to glyphosate by drinking water, under stringent and rigorous quality control (Figure S2) showed that the perturbation majorly appeared in the feces, where the gut microbiota can directly interact with ingested glyphosate, and cause changes to their ecology, thus leading to differences in secondary metabolites. The serum profile of biochemicals were stable, as little changes were observed. Hu et al. exposed dams and pups of Sprague Dawley rats to 1.75 mg/Kg-BW/day via drinking water for around 50 days, and observed very distinctive statistical clusters in the urinary metabolome profiles for the dams and the pups (Hu et al. 2021). Moreover, there were sex-specific differences observed in the pups (but not in the dams). They also identified alterations of the gut microbiota profile by 16S rRNA sequencing. It was believed that in humans, glyphosate is rapidly but incompletely absorbed after ingestion (around 20% of administered dose), and absorbed glyphosate is mainly eliminated unchanged via feces or after the biotransformation to aminomethylphosphonic acid (AMPA) (Soares et al. 2021). Glyphosate itself is hard to be ionized by electrospray, thus hard to be detected in LC-MS, thus AMPA in urine has been a common surrogate to estimate glyphosate exposure (Zoller et al. 2020). The urine compared to the fecal metabolome may differ more under the existence and effect of AMPA, and rats may have different sensitivity to glyphosate compared to mice, as aforementioned, which may explain the distinctive results in our study.
There were investigations on how glyphosate modulate neurotransmitters in animal models, but different conclusions were drawn. For instance, Martínez et al. oral-gavaged 0, 35, 75, 150, or 800 mg-glyphosate/Kg/day to Wistar male rats for 6 days, and analyzed the levels of dopamine, norepinephrine, serotonin, and their related metabolites in various brain regions (Martínez et al. 2018). They concluded a no-observed-effect level (NOEL) at 35 mg/Kg/day, as they detected general decrease in the neurotransmitter, such as reduced serotonin in the striatum of rats in every group with the exposure above 35 mg-glyphosate/Kg/day, and down-regulated dopamine in the prefrontal cortex of rats exposed to 150 and 800 mg-glyphosate/Kg/day. In contrast, another study that intraperitoneally injected at most 150 mg-glyphosate/Kg/day (3 injections per week for 2 weeks) to rats detected no change in dopamine, serotonin, and other measured neurotransmitter (Hernández-Plata et al. 2015). Their distinctive result may imply the involvement of the gut microbiota, as ingested glyphosate by diet can interact with the dynamics of microbiota-modulated metabolites by altering the microbiota community, but intraperitoneal injection of glyphosate avoided direct encounter. Our data showed decreases of aromatic amino acids in the feces, supporting the hypothesis that glyphosate can disrupt the gut microbiota. However, these aromatic amino acids show less difference in the circulation, and neurotransmitters such as serotonin and dopamine were similar among groups in the feces and serum (Figure 5, Figure S4, and Figure S5). Factors associated with the different results between the study by Martínez et al and ours include (1) our study had a longer exposure duration (Martínez et al. exposed glyphosate by diet for 6 days) which may allow metabolic adaption and compensation; (2) sensitivity difference between mice and rats; (3) bioavailability difference in glyphosate exposed by diet or drinking water (Martínez et al. 2018).
There were limitations on our investigation. Tissues in the brain were not analyzed for the aromatic amino acids and neurotransmitters due to the absence of changes observed in the circulation (i.e., serum). In addition, the bottleneck for next-generation metabolomics and nontargeted MS analysis have been the reliable assignment of chemicals to the detected molecular features (Chaleckis et al. 2019). Although we have diligently constructed a validated metabolic database to allow confident metabolite assignment across a wide range of biochemical pathways, there remain plenty of unknown compounds, including those with significant difference (Figure S3).
The implementation of glyphosate has brought huge economic benefits on human agriculture, yet concerns were raised on its impact in ecology and public health. Previous toxicological assessments on glyphosate focused on acute or subacute adverse effects and carcinogenicity. More recent works have gained insight on how glyphosate may harm human health indirectly by perturbing our gut microbiota. However, previous research often applied doses that are not human-relevant; moreover, impacts under chronic exposure scenarios of glyphosate were under-investigated. To address the lack of data, we chronically authority-suggested RfD of glyphosate to mice and evaluated neurobehaviors and metabolic profiles extensively. The perturbation caused by glyphosate were majorly observed in the feces, which may imply disruption of the gut microbiota. However, there were critically fewer metabolic changes in the serum, which suggested that the glyphosate-disrupted gut microbiota did not significantly impair the host physiological normality. Following this observation, our data show little evidence that glyphosate can impair the gut-brain axis from the metabolomic and neurobehavioral standpoint, even at high doses that are irrelevant to human exposure. We also urge that more toxicological assessment on glyphosate using different exposure scheme and analytical platforms are needed in the future.
Supplementary Material
Funding
The research was supported by the UNC Superfund Research program (P42ES031007), University of North Carolina Center for Environmental Health and Susceptibility grant (P30ES010126), and by the UNC Intellectual and Developmental Disabilities Research Center (NICHD; P50 HD103573; PI: Gabriel Dichter).
Footnotes
Competing Interest
The authors declare they have no actual or potential competing financial interests.
Data Availability
Data are available from the corresponding author upon reasonable request.
References
- Ait Bali Y, Ba-Mhamed S, Bennis M (2017) Behavioral and Immunohistochemical Study of the Effects of Subchronic and Chronic Exposure to Glyphosate in Mice. Frontiers in Behavioral Neuroscience 11 doi: 10.3389/fnbeh.2017.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitbali Y, Ba-M’hamed S, Elhidar N, Nafis A, Soraa N, Bennis M (2018) Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicology and Teratology 67:44–49 doi: 10.1016/j.ntt.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Boocock MR, Coggins JR (1983) Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett 154(1):127–33 doi: 10.1016/0014-5793(83)80888-6 [DOI] [PubMed] [Google Scholar]
- Brammer A (2001) Glyphosate acid: Two year dietary toxicity and oncogenicity study in rats. Unpublished report No. CTL/PR1111, study No. PR1111.. Zeneca Agrochemicals, Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, England. [Google Scholar]
- Chaleckis R, Meister I, Zhang P, Wheelock CE (2019) Challenges, progress and promises of metabolite annotation for LC-MS-based metabolomics. Curr Opin Biotechnol 55:44–50 doi: 10.1016/j.copbio.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Costas-Ferreira C, Durán R, Faro LRF (2022) Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. International journal of molecular sciences 23(9) doi: 10.3390/ijms23094605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defarge N, Takács E, Lozano VL, et al. (2016) Co-Formulants in Glyphosate-Based Herbicides Disrupt Aromatase Activity in Human Cells below Toxic Levels. Int J Environ Res Public Health 13(3) doi: 10.3390/ijerph13030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giommi C, Ladisa C, Carnevali O, Maradonna F, Habibi HR (2022) Metabolomic and Transcript Analysis Revealed a Sex-Specific Effect of Glyphosate in Zebrafish Liver. International journal of molecular sciences 23(5) doi: 10.3390/ijms23052724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Chou W-C, Lai Y, et al. (2020) Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370(6516):eaay9097 doi: 10.1126/science.aay9097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Plata I, Giordano M, Díaz-Muñoz M, Rodríguez VM (2015) The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male Sprague-Dawley rat. NeuroToxicology 46:79–91 doi: 10.1016/j.neuro.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Hsiao Y-C, Liu C-W, Robinette C, Knight N, Lu K, Rebuli ME (2022) Development of LC-HRMS untargeted analysis methods for nasal epithelial lining fluid exposomics. Journal of Exposure Science & Environmental Epidemiology doi: 10.1038/s41370-022-00448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lesseur C, Miao Y, et al. (2021) Low-dose exposure of glyphosate-based herbicides disrupt the urine metabolome and its interaction with gut microbiota. Scientific Reports 11(1):3265 doi: 10.1038/s41598-021-82552-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Liu C-W, Yang Y, Hsiao Y-C, Ru H, Lu K (2021) High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nature Communications 12(1):6000 doi: 10.1038/s41467-021-26209-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano VL, Defarge N, Rocque L-M, et al. (2018) Sex-dependent impact of Roundup on the rat gut microbiome. Toxicology Reports 5:96–107 doi: 10.1016/j.toxrep.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q, Manservisi F, Panzacchi S, et al. (2018) The Ramazzini Institute 13-week pilot study on glyphosate and Roundup administered at human-equivalent dose to Sprague Dawley rats: effects on the microbiome. Environmental Health 17(1):50 doi: 10.1186/s12940-018-0394-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez MA, Ares I, Rodríguez JL, Martínez M, Martínez-Larrañaga MR, Anadón A (2018) Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ Res 161:212–219 doi: 10.1016/j.envres.2017.10.051 [DOI] [PubMed] [Google Scholar]
- Mesnage R, Antoniou MN (2020) Computational modelling provides insight into the effects of glyphosate on the shikimate pathway in the human gut microbiome. Curr Res Toxicol 1:25–33 doi: 10.1016/j.crtox.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage R, Benbrook C, Antoniou MN (2019) Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem Toxicol 128:137–145 doi: 10.1016/j.fct.2019.03.053 [DOI] [PubMed] [Google Scholar]
- Moser VC, Morris-Schaffer K, Richardson JR, Li AA (2022) Glyphosate and neurological outcomes: A systematic literature review of animal studies. Journal of Toxicology and Environmental Health, Part B 25(4):162–209 doi: 10.1080/10937404.2022.2083739 [DOI] [PubMed] [Google Scholar]
- Motta EVS, Raymann K, Moran NA (2018) Glyphosate perturbs the gut microbiota of honey bees. Proc Natl Acad Sci U S A 115(41):10305–10310 doi: 10.1073/pnas.1803880115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LN, Roager HM, Casas ME, et al. (2018) Glyphosate has limited short-term effects on commensal bacterial community composition in the gut environment due to sufficient aromatic amino acid levels. Environmental Pollution 233:364–376 doi: 10.1016/j.envpol.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Nufarm (2009) Glyphosate technical: dietary com- bined chronic toxicity/carcinogenicity study in the rat. Project Number: 2060–0012. Nufarm Asia Sdn Bhd [Google Scholar]
- Portier CJ (2020) A comprehensive analysis of the animal carcinogenicity data for glyphosate from chronic exposure rodent carcinogenicity studies. Environ Health 19(1):18 doi: 10.1186/s12940-020-00574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AK, Akour A, Mahboob A, AbuRuz S, Sadek B (2022) Role of Brain Modulators in Neurodevelopment: Focus on Autism Spectrum Disorder and Associated Comorbidities. Pharmaceuticals (Basel) 15(5) doi: 10.3390/ph15050612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsel A, Seneff S (2013) Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases. Entropy 15(4) doi: 10.3390/e15041416 [DOI] [Google Scholar]
- Shehata A, Schrödl W, Alnassan A, Hafez H, Krueger M (2012) The Effect of Glyphosate on Potential Pathogens and Beneficial Members of Poultry Microbiota In Vitro. Current microbiology 66 doi: 10.1007/s00284-012-0277-2 [DOI] [PubMed] [Google Scholar]
- Soares D, Silva L, Duarte S, Pena A, Pereira A (2021) Glyphosate Use, Toxicity and Occurrence in Food. Foods 10(11) doi: 10.3390/foods10112785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz P (2018) Neurotransmitter modulation by the gut microbiota. Brain Res 1693(Pt B):128–133 doi: 10.1016/j.brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straw EA, Brown MJF (2021) Co-formulant in a commercial fungicide product causes lethal and sub-lethal effects in bumble bees. Sci Rep 11(1):21653 doi: 10.1038/s41598-021-00919-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31(2 Pt 1):117–65 doi: 10.1006/rtph.1999.1371 [DOI] [PubMed] [Google Scholar]
- Zabalza A, Orcaray L, Fernández-Escalada M, Zulet-González A, Royuela M (2017) The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic Biochem Physiol 141:96–102 doi: 10.1016/j.pestbp.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Zoller O, Rhyn P, Zarn JA, Dudler V (2020) Urine glyphosate level as a quantitative biomarker of oral exposure. International Journal of Hygiene and Environmental Health 228:113526 doi: 10.1016/j.ijheh.2020.113526 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.