Abstract
A citrate synthase (CS) deletion mutant of Agrobacterium tumefaciens C58 is highly attenuated in virulence. The identity of the mutant was initially determined from its amino acid sequence, which is 68% identical to Escherichia coli and 77% identical to Brucella melitensis. The mutant lost all CS enzymatic activity, and a cloned CS gene complemented a CS mutation in Sinorhizobium. The CS mutation resulted in a 10-fold reduction in vir gene expression, which likely accounts for the attenuated virulence. When a plasmid containing a constitutive virG [virG(Con)] locus was introduced into this mutant, the level of vir gene induction was restored to nearly wild-type level. Further, the virG(Con)-complemented CS mutant strain induced tumors that were similar in size and number to those induced by the parental strain. The CS mutation resulted in only a minor reduction in growth rate in a glucose-salts medium. Both the CS mutant and the virG(Con)-complemented CS strain displayed similar growth deficiencies in a glucose-salts medium, indicating that the reduced growth rate of the CS mutant could not be responsible for the attenuated virulence. A search of the genome of A. tumefaciens C58 revealed four proteins, encoded on different replicons, with conserved CS motifs. However, only the locus that when mutated resulted in an attenuated phenotype has CS activity. Mutations in the other three loci did not result in attenuated virulence and any loss of CS activity, and none were able to complement the CS mutation in Sinorhizobium. The function of these loci remains unknown.
Agrobacterium tumefaciens induces the formation of crown gall tumors by transferring a piece of DNA, the T-DNA, from the tumor-inducing (Ti) plasmid into host cells (for reviews, see references 12 and 46). The overproduction of auxin and cytokinin encoded on the T-DNA gives rise to tumors characteristic of the disease. Under laboratory conditions, however, Agrobacterium can transfer DNA to a wide variety of eukaryotic cells, including fungi (5, 34), algae (22), and humans (23). This transfer requires genes outside the T-DNA whose products mediate the interaction of Agrobacterium with its hosts. These genes comprise both the vir regulon on the Ti plasmid and additional genes located elsewhere in the genome. The vir regulon is activated by a two-component system, VirA/G, that responds to two distinct classes of plant signal molecules. Included are phenolic compounds such as acetosyringone (AS), synthesized by wounded plant cells, as well as monosaccharides, such as arabinose, galactose, and glucose, which are components of the plant cell wall (1). Signal molecules only function in an acidic environment, the milieu of the wound site of a plant, and many of the genes required for tumor formation are acid inducible (24). These include the virG locus, which codes for the response regulator that controls expression of the vir regulon.
In addition to the vir genes on the Ti plasmid, numerous other genes, termed chv, located on other replicons, are required for the successful interaction of Agrobacterium with its hosts. As a general rule, it appears that the vir genes are dedicated solely to the interaction of Agrobacterium with its host plants, whereas the chv genes serve dual functions. They operate in the physiology of Agrobacterium growing in the absence of its plant hosts as well as in the interaction of Agrobacterium with its hosts. For example, the gene chvE plays a role in the transport of specific sugars, which the cell can use as a source of carbon, and also plays a role in activating the vir genes (7). Another gene, katA, is concerned with overcoming the plant's host defenses and probably plays a role in the stress response of the cell (45). However, although additional chv genes have been identified and recognized as playing a role in the interaction of Agrobacterium with plants, their precise role in virulence has not been determined (12). A number of chv mutants have been identified that are defective in vir gene induction which are poorly understood (8, 14, 25, 29). Such mutants are avirulent or attenuated in virulence.
One approach to identifying genes important in the interaction of Agrobacterium with its host plants is to randomly mutate the genome by insertion mutagenesis using Tn5 or a derivative such as TnPhoA. This approach continues to reveal new chv genes. For example, this report characterizes one previously unidentified gene important in tumor formation, the gene coding for citrate synthase (CS), the first enzyme of the tricarboxylic acid (TCA) cycle.
Citrate synthase governs the entry of carbon into the TCA cycle (42). This cycle is important in Agrobacterium in that it represents the main pathway for the generation of energy and serves to synthesize precursor metabolites such as α-ketoglutarate, which is converted into glutamate. Agrobacterium also has the genes for the glyoxylate pathway that converts isocitrate to malate (44). This pathway has been shown to be important for pathogenesis of a variety of animal and plant pathogens (28, 41).
Several studies have demonstrated that citrate synthase is important in the interaction of members of the Rhizobiaceae with their hosts. A deletion mutation of CS in Sinorhizobium meliloti resulted in a strain that formed non-nitrogen-fixing nodules on alfalfa (31). This strain could not synthesize fully succinylated succinoglycan, which is required for root hair invasion and nodule development. Therefore, it is not clear if the symbiotic defect resulted from the altered succinoglycan, reduced energy, or limitations in precursor metabolite generation by the TCA cycle. Another member of the Rhizobiaceae, Rhizobium tropici, has two CS genes, one on the chromosome and the other on a plasmid (17, 32). Mutations in either gene did not alter nitrogen fixation, although nodule formation was reduced when the plasmid-borne copy was deleted. When both genes were deleted, however, the cells formed ineffective nodules that were unable to fix nitrogen. Thus, both genes appear to be functional in the nodulation process.
In a continuing program to identify genes of A. tumefaciens involved in its interaction with its plant hosts, we found that a mutant generated by a transposon insertion into a gene of CS resulted in a strain with highly attenuated virulence. This story became more interesting but complicated when the sequence of the genome of A. tumefaciens was analyzed (13, 44), and four genes were identified with characteristic CS domains. Here we identify and characterize the single gene that codes for CS and identify a step in tumor formation at which it acts. We also analyze the sequences and characterize mutations in the other three putative CS genes and conclude that none of these genes have CS activity.
MATERIALS AND METHODS
Strains, plasmids, and media.
The strains and plasmids used in this study are listed in Table 1. A. tumefaciens strains were grown in MG/L or AB minimal medium at 28°C with shaking (6). E. coli strains were grown in Luria-Bertani (LB) medium (35) at 37° with shaking. S. meliloti strains were grown in MG/L or in minimal mannitol (MM NH4) medium (31) at 28°C. Antibiotics were used at the indicated concentrations: for A. tumefaciens, 100, 100, 50, and 5 μg/liter carbenicillin, kanamycin, gentamicin, and tetracycline, respectively; for E. coli, 100, 50, 5, and 10 μg/liter carbenicillin, kanamycin, gentamicin, and tetracycline, respectively; and for S. meliloti, 100 μg/liter kanamycin. Induction of vir gene expression was measured according to published procedures (33) in cells grown in induction broth with arabinose substituting for glucose (6) supplemented with 200 μM AS.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| A. tumefaciens | ||
| C58 | Wild type | 15 |
| A6080 | TnPhoA insertion in C58 | 7 |
| A136 | C58; Ti plasmid cured | 36 |
| Δ1392 | Deletion of CS in C58 | This study |
| Δ4851 | Deletion of CS in C58 | This study |
| Δ5306 | Deletion of CS in C58 | This study |
| Δ5307 | Deletion of CS in C58 | This study |
| S. meliloti | ||
| 1021 | Wild type | 31 |
| Δ1A | CS deletion mutant of S. meliloti 1021 | Unpublished, from Michael Kahn |
| E. coli DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(argF-lacZYA)U169 φ80d lacZ ΔM15 | Invitrogen |
| Plasmids | ||
| pEX18Gm | Gmr, oriT+sacB+, gene replacement vector with MCS from pUC18 | 18 |
| pEX1 | Construct for deleting 1392 | This study |
| pEX2 | Construct for deleting 4851 | This study |
| pEX3 | Construct for deleting 5306 | This study |
| pEX4 | Construct for deleting 5307 | This study |
| pPR1068 | pMAL-c2 derivative, NdeI at the start of MalE | New England Biolabs |
| pBBR1MCS-2 | Kanr, broad-host-range cloning vector | 26 |
| pSM243cd | virB::lacZ fusion | 39 |
| pSM358cd | virE::lacZ fusion | 39 |
| pSY204 | Constitutive virG | 20 |
| pLP111 | tac-1392 in pPR1068 | This study |
| pLP112 | tac-4851 in pPR1068 | This study |
| pLP113 | tac-5306 in pPR1068 | This study |
| pLP114 | tac-5307 in pPR1068 | This study |
| pLP115 | tac-1392 in pBBR1MCS-2 | This study |
| pLP116 | tac-4851 in pBBR1MCS-2 | This study |
| pLP117 | tac-5306 in pBBR1MCS-2 | This study |
| pLP118 | tac-5307 in pBBR1MCS-2 | This study |
Identification of a TnPhoA insertion within Atu1392.
A TnPhoA library in A. tumefaciens C58 generated by Cangelosi et al. (7) was screened for the presence of mutants that were unable to induce tumors on Kalanchoë. Avirulent isolates were further characterized using inverse PCR in order to identify sequences flanking the transposon. One of these strains, designated A6080, contained a transposon insertion in open reading frame (ORF) Atu1392 that was predicted to encode CS based on its nucleotide similarity to the CS of E. coli (http://www.agrobacterium.org/).
Construction of unmarked deletion mutants.
Unmarked deletion mutants in A. tumefaciens were generated as described previously (18). In brief, 1.5-kb regions were amplified from the upstream and downstream regions flanking the region targeted for replacement, using primers that included specific restriction enzyme sites (Table 2). After restriction enzyme digestion, the upstream and downstream fragments were ligated into the vector pEX18Gm using a directional three-way ligation. These plasmids were introduced into strain C58 by electroporation. After incubation for 3 h to allow for homologous recombination, the cells were plated on LB agar with 5% sucrose for the first selection; the colonies which grew on the media containing 5% sucrose were streaked onto Mg/L and Mg/L agar plates with 25 μg/ml gentamicin for the second selection. Deletion mutants cannot grow on Mg/L agar containing 25 μg/ml gentamicin. Mutations were verified by sequencing the junction fragment generated using PCR that spans the open reading frame selected for deletion.
TABLE 2.
Primers used in this study
| Gene | Primer (5′ to 3′) |
|---|---|
| For making deletion mutants | |
| 1392 | |
| Upstream | |
| Sense | CGGGATCCATGACCAGAGCGGACAATGCAACATTC |
| Antisense | TCTAGAACTGATCCTCCGAGAACATGCGGGTG |
| Downstream | |
| Sense | GCTCTAGAGCCACGAAACCCTGTAAGATCAAAG |
| Antisense | AAAAAAAAGCTTTTGCTGGTCATGGGCGGCAACCTC |
| 4851 | |
| Upstream | |
| Sense | CTCTAGACGATAGCACGGGTGTGAGCGAAAAGAAGG |
| Antisense | AACTGCAGTAGCGGTGACTGCGGGCCGAACGACCCCG |
| Downstream | |
| Sense | AACTGCAGCCCACGATCTTGGGCAGAGCTAGGAAAAATC |
| Antisense | AAAAAAAAGCTTATGATATCAGGTCATCGCCAGCCACAG |
| 5306 | |
| Upstream | |
| Sense | CGGAATTCTGGCGATTTCGGTCATGCGTCTTGC |
| Antisense | GCTCTAGAGATTTCGCGCAGCAGTGGAGAACAG |
| Downstream | |
| Sense | GCTCTAGAAGTTTCGGCGGCAACGACATCATCAAG |
| Antisense | AAAAAAAAGCTTATTAGGTTGGCGCGGCTCGCCAAACCG |
| 5307 | |
| Upstream | |
| Sense | CGGGATCCAGGGGCTAAGGCTGGAATTTCTACTG |
| Antisense | GCTCTAGAGAACCCGGCTCCCGGCCTTGATGATG |
| Downstream | |
| Sense | GCTCTAGAGCGCTCACTACAAAAACGCTGGAGC |
| Antisense | AACTGCAGGTTCGTTACAGGATGCTGCCTATAC |
| For functional complementation | |
| 1392 | |
| Sense | GGGGGGGCATATGACGGAAAAAAGCGCTACAGTG |
| Antisense | AAAAAGCTTTTAACGCTTGGAAACCGGAACG |
| 4851 | |
| Sense | GGGGGGGCATATGCAGAAGGTGAGGGGAGAGACAG |
| Antisense | AAAAAGCTTCTATTGCCCCATATAACGGGC |
| 5306 | |
| Sense | GGGGGGGCATATGCTCAGTCATGTAGACGGCCAG |
| Antisense | AAAAAGCTTTCACCTTGGATGTGGACCGATATAG |
| 5307 | |
| Sense | GGGGGGGCATATGCCGACAAGAGCGGGGGTGGCAG |
| Antisense | AAAAAGCTTCTATCGCCCGATGTAACGGGC |
Virulence assays.
Virulence was assayed in two ways. The first involved inoculating A. tumefaciens cells on Kalanchöe leaves. Cells were grown in MG/L medium overnight at 28°C, collected by centrifugation, resuspended in sterile water at a final concentration of 2.0 at an optical density at 600 nm (OD600), and 1 μl was inoculated onto wounded Kalanchöe diagremontiana leaves. Plants were scored for tumors after 4 weeks of incubation at 25°C.
For quantitative analysis of virulence, the assay described by Banta et al. (2) was used. Briefly, overnight cultures of A. tumefaciens were adjusted to an OD600 of ∼0.5 in 50 ml MG/L, and 20 ml was cocultivated with ∼0.6-cm round leaf explants of Nicotiana tobacum on hormone-free MS medium supplemented with 300 μM AS. After 2 days, the leaf pieces were transferred to hormone-free MS medium containing vancomycin (200 μg/ml) and timentin (200 μg/ml) and cultured at 25°C in the dark. After 10 to 12 days, the numbers of tumors on each leaf piece were scored from 0 to 5 by comparison to a standard, with 0 representing tumors induced by A136 and 5 representing the tumors induced by C58, the parental wild-type strain.
Quantitation of vir gene expression.
The reporter plasmids pSM243cd and pSM358cd, containing a virB::lacZ and a virE::lacZ fusion, respectively, were introduced into C58 and the citrate synthase mutant by electroporation. Cells were grown in induction broth for 24 h, and β-galactosidase levels were measured as described previously (30). Plasmid pSY204 (20) containing the constitutively active virG locus (N54D) was introduced into Agrobacterium strains containing the reporter plasmids by electroporation to determine its effect on vir gene induction assayed by β-galactosidase production of the reporter genes.
Citrate synthase assay.
Forty-milliliter cultures were grown overnight (18 h) at 28°C in AB medium, and bacteria were harvested by centrifugation (6,000 × g, 10 min) at 4°C. The cell pellets were washed twice in 40 ml lysis buffer (20 mM Tris-HCl, 1 mM EDTA, 10 mM MgCl2, adjusted to pH 8.0) and resuspended in 2 ml of lysis buffer, supplemented with 2 mg of lysozyme and 20 μl Focus-Protease Arrest (CALBIOCHEM). After incubation for 30 min at room temperature, the cells were sonicated on ice six times for 15 s each. Following centrifugation (15 min, 12,000 × g, 4°C), the supernatant was analyzed. The total protein concentration was measured using the Bio-Rad protein assay kit using bovine serum albumin as a standard.
Citrate synthase was assayed spectrophotometrically at 412 nm as described by Sere (38). The standard reaction mixture of 1.0 ml contained 0.3 mM acetyl coenzyme A (acetyl-CoA), 0.5 mM oxaloacetate (OAA), 0.1 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and 60 μg of protein extract. Fifty microliters of OAA was added to start the reaction, and measurements were taken every minute for 3 min at OD412 using Hitachi spectrophotometer U-2000. The synthesis of the product, CoA, was linear during the time of the assay. The reaction mixture without either the crude extract or OAA served as controls, and neither showed any change in OD during the time of the assay. Values were calculated as nmol of CoA produced/min/mg protein.
Functional complementation of citrate synthase in Sinorhizobium.
To determine which of the putative A. tumefaciens citrate synthase genes could complement a CS mutation in S. meliloti, we placed each of the four putative CS genes under the control of the tac promoter. Primer pairs containing unique NdeI and HindIII restriction sites were used to amplify the CS coding regions from A. tumefaciens genomic DNA using Ex-Taq (Takara Bio) (Table 2). Each of the A. tumefaciens CS PCR products was digested with NdeI and HindIII and then ligated into pPR1068, creating pLP111, pLP112, pLP113, and pLP114. The EcoRV-HindIII fragments of pLP111, pLP112, pLP113, and pLP114 containing tac-CS were cloned into SmaI- and HindIII-digested pBBR1MCS-2, generating pLP115, pLP116, pLP117, and pLP118. These plasmids were verified by sequencing. Each of these plasmids, with a different putative CS gene (see Table 1), was introduced into the S. meliloti wild-type strain and the S. meliloti CS mutant Δ1A by electroporation. The Kanr colonies were grown in MG/L medium and streaked onto plates containing MM NH4 plates ± arabinose. Since the CS mutant of S. meliloti can only grow on MM NH4 medium supplemented with arabinose (10, 31), the gene capable of complementing the CS mutation could be readily identified.
RESULTS
TnPhoA mutant.
In a search for mutations in genes which are important in the virulence of A. tumefaciens, we screened a previously constructed transposon library of C58 which had been constructed using the gene fusion transposon TnPhoA (7). Mutants were scored for virulence on Kalanchöe leaves. One avirulent mutant, A6080, had a TnPhoA insertion within the coding region of Atu1392, whose predicted product, based on sequence comparison with the E. coli genome, was citrate synthase.
Role of Atu1392 in virulence.
Once it was determined that an insertion in the Atu1392 gene led to a highly attenuated phenotype, an unmarked in-frame deletion mutation was constructed to ensure that the insertion in CS was indeed the cause of the attenuated phenotype. After the mutation was verified by sequencing across the lesion junctions (data not shown), the mutant was inoculated onto Kalanchöe leaves to assay its tumor-forming ability. The Δ1392 strain was highly attenuated (Fig. 1), confirming our original findings in the TnPhoA insertion mutant A6080.
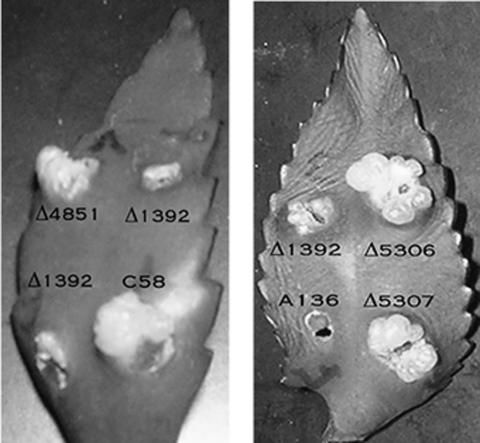
FIG. 1.
Virulence assay on Kalanchöe leaves. Wild-type (C58), Δ1392, Δ4851, Δ5306, Δ5307, and A136 (a negative control) strains were grown in MG/L media as described in Materials and Methods, and 1 μl of each culture was used to inoculate Kalanchöe leaves. Photos were taken 30 days after inoculation.
To gain a more quantitative measure of the tumor-inducing ability of the mutant, we assayed its ability to form tumors on tobacco (Nicotiana tabacum) leaf disks. For each assay, we examined 40 leaf disks and scored the number and severity (from 0 to 5) of tumors arising on each disk. The data in Table 3 confirm that Δ1392 is highly attenuated, yielding about half the number of tumors as the wild-type strain. The tumors that did form were generally smaller than those formed by C58.
TABLE 3.
Virulence assay of putative CS mutants on tobacco leaf explants
| Strain | Mean no. of tumors/explanta |
|---|---|
| Δ1392 | 2.0 ± 0.3 |
| Δ4851 | 4.4 ± 0.2 |
| Δ5306 | 4.3 ± 0.2 |
| Δ5307 | 3.8 ± 0.2 |
| Wild type (C58) | 4.5 ± 0.2 |
| Δ1392 with virG(Con) | 4.0 ± 0.2 |
| A136 | 0 ± 0 |
This assay was carried out as described in Materials and Methods using 40 tobacco leaf explants. Δ4851, Δ 5306, and Δ5307 have deletions in genes that were also annotated as CS. The scale of 0 to 5 takes into account both the numbers of tumors and their sizes.
vir gene expression.
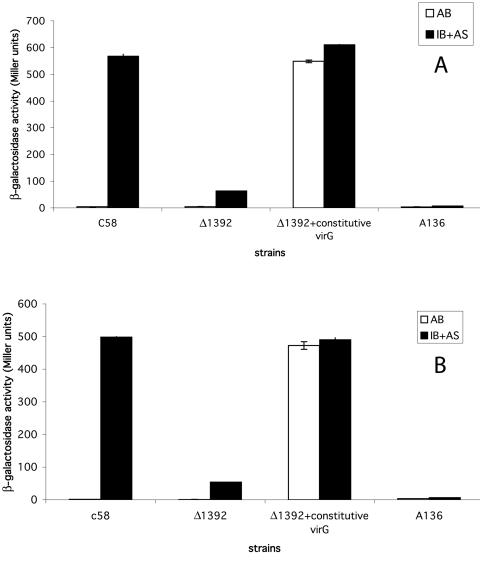
Since CS is a key enzyme in carbohydrate metabolism as well as amino acid synthesis, the possibility existed that the reduction in virulence might be related to vir gene induction, which requires certain sugars for maximum induction. To determine if vir gene induction was affected, reporter plasmids containing a virE2::lacZ fusion (pSM358cd) and a virB1::lacZ fusion (pSM243cd) were introduced by electroporation into the mutant, a strain lacking the Ti plasmid (A136), and the parental strain, C58. The resultant strains were assayed for β-galactosidase activity after growing for 24 h in either AB minimal medium or induction broth containing arabinose ± AS (Fig. 2). As expected, in AB minimal medium (pH 7.0) without AS, no induction of either the virB or virE genes was detected in any of the strains. In the presence of AS in induction broth, however, the level of induction in Δ1392 was reduced about 10-fold in both reporters as compared to the parental strain. Supplementation of the induction medium with the TCA intermediates citrate, succinate, fumarate, and malate, as well as glutamate, didn't alter vir gene expression (data not shown). We speculate that this reduced level of vir gene induction may be sufficient to account for the attenuated virulence of the Δ1392 mutant.
FIG. 2.
Expression of virB and virE in C58, Δ1392, and Δ1392 with a virG(Con) locus (N54D). A. tumefaciens strains containing a virB::lacZ fusion or virE::lacZ fusion were incubated in induction broth with arabinose substituting for glucose for 24 h ± acetosyringone, and β-galactosidase activities were measured as described in Materials and Methods. (A) virB expression; (B) virE expression.
Effect of a constitutive virG on vir gene expression and virulence of Δ1392.
The fact that the induction of both the virB and virE operons was reduced to the same degree suggests that the defect might be in an early stage in the activation process prior to the binding of activated VirG to the vir boxes of the individual operons. If so, it should be possible to restore vir gene induction with a constitutive virG mutation [virG(Con); N54D] which does not require plant signal molecules and acidic conditions in order to activate the vir regulon (20). Accordingly, we introduced virG(Con) (N54D) into Δ1392 and measured β-galactosidase activity as before (Fig. 2). The presence of virG(Con) (N54D) in Δ1392 increased vir gene induction of both reporters to levels observed in wild-type cells. These data suggest that the defect in vir gene induction involves signal recognition or transduction prior to the action of the response regulator VirG.
If the reduction in vir gene expression in Δ1392 plays a role in the attenuated phenotype, then the addition of virG(Con) (N54D) should restore virulence. To test this possibility, we compared the virulence of Δ1392 with Δ1392 containing virG(Con) (N54D) using the semiquantitative tobacco disk assay. The data in Table 3 demonstrate that the constitutive virG gene does in fact increase the virulence of Δ1392 significantly. From these data, we conclude that the reduction in vir gene induction, which results from the CS mutation, is the most important reason for the attenuated phenotype of the CS mutant.
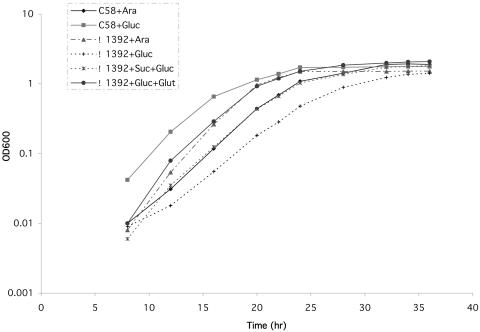
A second possible explanation for the attenuated virulence and reduced vir gene expression of the CS mutant is the fact that the mutant grows more slowly than the parental strain in a glucose-salts (AB) medium (Fig. 3). If this is the case, then the Δ1392 strain carrying the virG(Con) locus (N54D), which results in increased virulence, should have its growth rate restored to the parent C58 strain. However, the addition of the virG(Con) (N54D) to either C58 or Δ1392 did not alter their growth rates (data not shown). Thus, we conclude that the reduced growth rate of Δ1392 in minimal medium cannot account for its attenuated virulence.
FIG. 3.
Growth curve of Δ1392 and wild-type (C58) in AB minimal medium with various supplements. Log-phase cultures of C58 and Δ1392 were inoculated into AB minimal medium with the various supplements indicated with a starting OD600 of 0.001. Cells were grown at 28°C. Optical density at 600 nm was measured at 2-h intervals over a 36-h period. C58+Ara, C58 was grown in AB minimal medium with 0.4% arabinose as the sole carbon source; C58+Gluc, C58 was grown in AB minimal medium with 0.4% glucose as the sole carbon source; Δ1392+Suc+Gluc, Δ1392 was grown in AB minimal medium with 0.4% succinate and 0.4% glucose as carbon sources; Δ1392+Gluc+Glut, Δ1392 was grown in AB minimal medium with 0.4% glucose and 0.4% glutamate as carbon sources.
Growth properties of the CS mutant.
Our initial experiments to characterize the physiology of Δ1392 looked at its growth in AB glucose-salts medium with various supplements (Fig. 3). Since a CS mutant of S. meliloti is a glutamate auxotroph, we grew the wild-type C58 strain and the Δ1392 mutant in AB minimal medium, with and without glutamate. Without glutamate, the mutant grew more slowly and growth leveled off before that of the parent strain. Addition of 0.4% sodium glutamate (final concentration), however, stimulated growth and restored its generation time in log phase to approximately the same as that of the parental strain. Although the CS mutant does have a significant requirement for this amino acid, we conclude that, unlike in S. meliloti, a CS mutation does not lead to glutamate auxotrophy. Apparently the cells are capable of synthesizing glutamate through a pathway that does not involve the conversion of citrate to α-ketoglutarate by CS. The pathway leading to glutamate is unclear. The fact that succinate stimulated the growth of Δ1392 (Fig. 3) implies that succinate may be a precursor. The growth curves also suggest that, like S. meliloti (10), Agrobacterium is able to convert arabinose to glutamate since growth of the Δ1392 mutant on AB medium supplemented with arabinose is virtually identical to the growth observed in AB medium supplemented with glutamate.
Enzymatic assay for citrate synthase activity.
To confirm the conclusion that Atu1392 codes for CS based on a bioinformatics analysis, we assayed crude extracts from Δ1392 and the parent C58 strain for CS activity. The Δ1392 mutant showed no demonstrable CS activity (2 ± 1 nmol/min/mg of protein), whereas the C58 strain clearly did (192 ± 18 nmol/min/mg of protein).
Genetic complementation of CS-negative mutant.
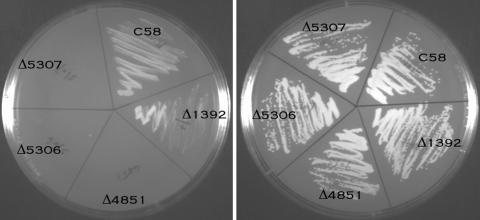
To confirm the biochemical data, we next demonstrated that a cloned Atu1392 gene could complement a CS mutation of S. meliloti by the following procedure (31). Due to the defect in CS, the S. meliloti CS mutant is a glutamate auxotroph. Arabinose, which is readily converted to α-ketoglutarate by S. meliloti (10), serves as a good source of glutamate and was used to supplement MM NH4 medium (31, 37). Although the wild-type strain of S. meliloti grows on MM NH4 medium, the CS-negative mutant only grew if the this medium was supplemented with a source of glutamate such as arabinose. Following electroporation of the plasmid which overexpresses the CS gene, the cells of S. meliloti were plated on MM NH4 medium containing arabinose and kanamycin to select for cells that contained the electroporated plasmid. Colonies were streaked onto MM NH4 plates with and without arabinose. Colonies containing the Atu1392 locus grew on both media (Fig. 4).
FIG. 4.
Functional complementation of A. tumefaciens CS in Sinorhizobium. The vectors, each containing one of the cloned putative CS genes, were introduced by electroporation into S. meliloti Δ1A, a mutant with a mutation in CS. The cells were plated on MM NH4 medium with arabinose and kanamycin. Colonies were then streaked out onto MM NH4± arabinose. Growth in the absence of arabinose indicates that the cell has a functional CS.
Phylogenetic analyses of Atu4581, Atu5306, and Atu5307.
When the A. tumefaciens C58 genome was scanned for genes encoding CS, it was observed that four genes had been annotated as putatively encoding this enzyme (Table 4). In addition to their overall amino acid similarity, all four contain a histidine residue within a consensus signature sequence of 13 amino acids, which plays a critical role in the citrate synthase catalytic activity (21). Interestingly, E. coli, S. meliloti, and Brucella melitensis (the latter two of which are closely related to A. tumefaciens) have only a single copy of the CS gene (3, 9, 31). The size of the four putative CS proteins in Agrobacterium varies widely, as does the identity they share with the CS proteins of the other bacteria (Table 4). The A. tumefaciens gene that resembles most closely the CS gene of the other three genera is Atu1392 (Table 4). Not only is it most similar in overall sequence and size to the other three CS proteins, but the signature sequence of 13 amino acids is identical in all four genera (21). The other three putative CS proteins of Agrobacterium show three to six amino acid variations in the signature sequence (Table 4). Interestingly, the amino acids that vary are in the same position in all three proteins.
TABLE 4.
Comparision of putative CS sequences of A. tumefaciens C58 and other organisms
| Organism | Length of protein (amino acids) | % Amino acid identity to:
|
Signature sequencea | |||
|---|---|---|---|---|---|---|
| S. meliloti | E. coli K-12 | B. melitensis | Atu1392 | |||
| E. coli K-12 | 427 | 68 | 100 | 63 | 68 | GFGHRVYKNYDPR |
| S. meliloti | 429 | 100 | 68 | 80 | 88 | GFGHRVYKNYDPR |
| B. melitensis | 430 | 80 | 63 | 100 | 77 | GFGHRVYKNYDPR |
| A. tumefaciens | ||||||
| Atu1392 | 430 | 88 | 68 | 77 | 100 | GFGHRVYKNYDPR |
| Atu4851b | 336 | 30 | 31 | 16 | 15 | GFRHPLYPDGDPR |
| Atu5306 | 367 | 26 | 25 | 26 | 24 | GFGHRIYRVRDPR |
| Atu5307 | 407 | 17 | 17 | 17 | 17 | GFRHPLYPDGDPR |
The underlined amino acids point out differences from Atu1392.
Atu4851 shares 99% amino acid identity with Atu5307 in the C-terminal region.
Virulence assay of other three putative CS genes.
To determine whether these three putative genes play a role in tumor formation, deletion mutants were constructed in each of the three strains and inoculated onto Kalanchöe leaves and tobacco leaf disks. The Δ4851, Δ5306, and Δ5307 mutants showed no significant decrease in the size and number of tumors (Fig. 1; Table 3).
Biochemical and genetic analysis of Atu4851, Atu5306, and Atu5307.
To determine whether Atu4851, Atu5306, and Atu5307 have CS activity, we assayed crude extracts from each of the deletion mutants, as well as the parent strain, C58. All three deletion mutants had the same CS activity as the C58 strain (data not shown).
To confirm the biochemical data, we determined whether any of the three loci could complement the CS mutation in S. meliloti that the Atu1392 locus complemented. We carried out the same procedure as for the Atu1392 locus. As shown in Fig. 4, none of the loci complemented the mutation. All of the biochemical and genetic data are consistent and indicate that the only locus that encodes CS is Atu1392.
DISCUSSION
The data in this paper add another chromosomal gene to the list of genes that are required for full virulence of Agrobacterium. Many of the previously isolated attenuated mutants have defects in vir gene induction (8, 14, 27, 29), and this study adds another example. However, in only one case, that of chvE (7), is it clear that the mutation plays a direct role in determining the level of vir gene induction. For maximum vir gene induction, ChvE with its bound sugar must interact with the periplasmic domain of the sensor protein, VirA. In the case of other mutants displaying reduced vir gene induction, the effect of the mutation appears to be indirect. This is apparently true in the present case. Since the mutation in the present study involves an enzyme of carbohydrate metabolism, it may not be surprising that the defect involves the VirA/G regulatory system, which involves a monosaccharide signal. Since a virG(Con) (N54D) locus in the cell almost completely overcomes both the reduced vir gene induction, as well as the attenuated virulence resulting from the mutation, this in fact seems to be the case. However, with our present knowledge of how AS and sugars interact with VirA and how VirG activates the vir gene regulon, it is difficult to understand how or why a mutation in CS reduces vir gene expression at an early stage. The addition of intermediates of the TCA cycle and glutamate did not elevate the level of vir gene induction. Conceivably, an accumulation of intermediates such as acetate, pyruvate, or oxaloacetate, which would be expected to result from the CS mutation, might in some way interfere with uptake or function of inducing sugars or AS.
Another possible explanation for the reduced level of vir gene expression is that the CS mutation reduces the level of the sugar binding protein, ChvE. Since ChvE is involved in the transport of sugars into the cell, it would not be surprising if certain components of sugar metabolism were involved in the regulation of chvE at the transcriptional or posttranscriptional level. Further, this protein is far more critical for the expression of vir genes in strain C58, the strain studied in this paper, than in the more commonly studied strain, A348. The latter strain combines the chromosomally encoded ChvE protein of C58 with the VirA/G Ti plasmid-encoded regulon from strain A6. In strain C58, the ChvE protein is absolutely essential for vir gene expression. A mutation in chvE eliminates vir gene induction even in the presence of high levels of AS (11). Thus, if the CS mutation reduced the level of chvE significantly, it is conceivable that vir gene expression would also be reduced significantly. Another explanation involves possible changes in the level of VirA and/or VirG. Since it has been shown recently that elevated levels of VirA can inhibit the functioning of VirG (4, 43), alterations in the level of VirA might reduce vir gene expression. These possibilities are being explored.
Another intriguing observation relates to the nutritional requirements of both Δ1392 and its parent, C58. Surprisingly, the deletion mutant which lacks measurable in vitro CS activity as assayed in crude extracts has only a partial requirement for glutamate (Fig. 3), whereas a deletion of the CS gene in E. coli and S. meliloti confers an absolute requirement for glutamate on the mutants (16, 31). This suggests that, in E. coli and S. meliloti, the only path to glutamate is through citrate and that citrate is only synthesized by the first step of the TCA cycle. It appears that Agrobacterium has another pathway for generating glutamate that is not present in the other two bacteria. Since succinate stimulates the growth of Δ1392 cells (Fig. 3), succinate may be a precursor of glutamate. However, there are other explanations. Through a bioinformatics analysis of its genome, Agrobacterium appears to code for the enzyme citrate lyase, Atu2788, an alternative enzyme to CS, which can also condense oxaloacetate and acetate to form citrate. A paralog of citrate lyase was not annotated in the S. meliloti genome but was found in the E. coli genome.
Another explanation to account for the nutritional data is that one or more of the other three loci annotated as CS coding genes code for proteins which have some CS activity which could supply the glutamate necessary to achieve the growth observed. If this is the explanation, the activity must be low enough that it is not measurable in crude extracts or that the genes were not expressed under the culture conditions we employed. Further, none of the three loci complemented a CS mutation as measured by growth of CS-negative cells of S. meliloti in the absence of glutamate (arabinose). Isolating a triple mutant lacking these three other loci should help provide insight into this possibility.
Although it is clear from biochemical and genetic data that the Atu1392 gene codes for citrate synthase, it is not at all clear for what activity or activities the other three genes code, if indeed they code for functional proteins. The same biochemical and genetic data that strongly suggest that Atu1392 codes for citrate synthase strongly indicate that the three genes code for one or more other activities. The fact that they have related signature sequences with an invariant histidine suggests that their enzymatic activity or activities may be related to CS, but their substrates are unknown. One possibility is that one or more of the proteins catalyze the condensation of oxaloacetate and propionate to form methyl isocitrate. Such an activity was demonstrated in E. coli and shown to be the previously identified CSII (40). Salmonella enterica serovar Typhimurium LT2 has also been shown to have this activity (19). Like E. coli, Agrobacterium can grow on propionate as a sole source of carbon and energy after 4 to 5 days of incubation. However, demonstrating such activity in crude extracts of Agrobacterium has not proved fruitful thus far. There are other possible substrates. However, these possibilities are limited by the fact that mutations in the individual genes did not result in any growth requirement and therefore cannot be involved in essential biosynthetic reactions.
Acknowledgments
We thank Melanie Sarreal, Liz Jorgenson, and Grace Bae for excellent technical assistance. Marion Brodhagen, Nic Pinel, and Ram Samudrala provided valuable insights and helpful discussions. Many thanks to Michael Kahn for providing the S. meliloti CS deletion mutant.
This work was supported by NIH grant GM 32618 to E.W.N. M.S. was supported by a scholarship under the Commission on Higher Education Staff Development Project, Ministry of Education, Thailand.
REFERENCES
- 1.Ankenbauer, R. G., and E. W. Nester. 1993. The Agrobacterium Ti plasmid and crown gall tumorigenesis: a model for signal transduction in host-pathogen interactions, p. 67-104. In J. Kurjan and B. L. Taylor (ed.), Signal transduction. Academic Press Inc., San Diego, Calif..
- 2.Banta, L. M., R. D. Joerger, V. R. Howitz, A. M. Campbell, and A. N. Binns. 1994. Glu-255 outside the predicted ChvE binding site in VirA is crucial for sugar enhancement of acetosyringone perception by Agrobacterium tumefaciens. J. Bacteriol. 176:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 4.Brencic, A., Q. Xia, and S. C. Winans. 2004. VirA of Agrobacterium tumefaciens is an intradimer transphosphorylase and can actively block vir gene expression in the absence of phenolic signals. Mol. Microbiol. 52:1349-1362. [DOI] [PubMed] [Google Scholar]
- 5.Bundock, P., A. den Dulk-Ras, A. Beijersbergen, and P. J. Hooykaas. 1995. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J. 14:3206-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cangelosi, G. A., E. A. Best, G. Martinetti, and E. W. Nester. 1991. Genetic analysis of Agrobacterium. Methods Enzymol. 204:384-397. [DOI] [PubMed] [Google Scholar]
- 7.Cangelosi, G. A., R. G. Ankenbauer, and E. W. Nester. 1990. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc. Natl. Acad. Sci. USA 87:6708-6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles, T. C., and E. W. Nester. 1993. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 175:6614-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dilworth, M. J., R. Arwas, I. A. McKay, S. Saroso, and A. R. Glenn. 1986. Pentose metabolism in Rhizobium leguminosarum MNF300 and in Cowpea Rhizobium NGR234. J. Gen. Microbiol. 132:2733-2742. [Google Scholar]
- 11.Doty, S. L., M. C. Yu, J. I. Lundin, J. D. Heath, and E. W. Nester. 1996. Mutational analysis of the input domain of the VirA protein of Agrobacterium tumefaciens. J. Bacteriol. 178:961-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelvin, S. B. 2003. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67:16-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodner, B., G. Hinkle, S. Gattung, N. Miller, M. Blanchard, B. Qurollo, B. S. Goldman, Y. Cao, M. Askenazi, C. Halling, L. Mullin, K. Houmiel, J. Gordon, M. Vaudin, O. Iartchouk, A. Epp, F. Liu, C. Wollam, M. Allinger, D. Doughty, C. Scott, C. Lappas, B. Markelz, C. Flanagan, C. Crowell, J. Gurson, C. Lomo, C. Sear, G. Strub, C. Cielo, and S. Slater. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323-2328. [DOI] [PubMed] [Google Scholar]
- 14.Gray, J., J. Wang, and S. B. Gelvin. 1992. Mutation of the miaA gene of Agrobacterium tumefaciens results in reduced vir gene expression J. Bacteriol. 174:1086-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton, R. H., and M. Z. Fall. 1971. The loss of tumor-initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia 27:229-230. [DOI] [PubMed] [Google Scholar]
- 16.Heinzen, R. A., and L. P. Mallavia. 1987. Cloning and functional expression of the Coxiella burnetii citrate synthase gene in Escherichia coli. Infect. Immun. 55:848-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Lucas, I., M. A. Pardo, L. Segovia, J. Miranda, and E. Martinez-Romero. 1995. Rhizobium tropici chromosomal citrate synthase gene. Appl. Environ. Microbiol. 61:3992-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 19.Horswill, A. R., and J. C. Escalante-Semerena. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. J. Bacteriol. 181:5615-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, S., Y. Song, S. Q. Pan, and E. W. Nester. 1993. Characterization of a virG mutation that confers constitutive virulence gene expression in Agrobacterium. Mol. Microbiol. 7:555-562. [DOI] [PubMed] [Google Scholar]
- 21.Karpusas, M., B. Branchaud, and S. J. Remington. 1990. Proposed mechanism for the condensation reaction of citrate synthase: 1.9-A structure of the ternary complex with oxaloacetate and carboxymethyl coenzyme A. Biochemistry 29:2213-2219. [PubMed] [Google Scholar]
- 22.Kumar, S. V., R. W. Misquitta, V. S. Reddy, B. J. Rao, and M. V. Rajam. 2004. Genetic transformation of the green alga-Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci. 166:731-738. [Google Scholar]
- 23.Kunik, T., T. Tzfira, Y. Kapulnik, Y. Gafni, C. Dingwall, and V. Citovsky. 2001. Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl. Acad. Sci. USA 98:1871-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, L., Y. Jia, Q. Hou, T. C. Charles, E. W. Nester, and S. Q. Pan. 2002. A global pH sensor: Agrobacterium sensor protein ChvG regulates acid-inducible genes on its two chromosomes and Ti plasmid. Proc. Natl. Acad. Sci. USA 99:12369-12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, Z., M. Jacobs, D. A. Schaff, C. A. McCullen, and A. N. Binns. 2001. ChvD, a chromosomally encoded ATP-binding cassette transporter-homologous protein involved in regulation of virulence gene expression in Agrobacterium tumefaciens. J. Bacteriol. 183:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machael, E. K., H. E. Philip, D. S. Hill, G. T. Robertson, M. A. Farris, R. Martin Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 27.Mantis, N. J., and S. C. Winans. 1993. The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J. Bacteriol. 175:6626-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, and D. G. Russell, Jr. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 29.Metts, J., J. West, S. H. Doares, and A. G. Matthysse. 1991. Characterization of three Agrobacterium tumefaciens avirulent mutants with chromosomal mutations that affect induction of vir genes. J. Bacteriol. 173:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Mortimer, M. W., T. R. McDermott, G. M. York, G. C. Walker, and M. L. Kahn. 1999. Citrate synthase mutants of Sinorhizobium meliloti are ineffective and have altered cell surface polysaccharides. J. Bacteriol. 181:7608-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardo, M. A., J. Lagunez, J. Miranda, and E. Martinez. 1994. Nodulating ability of Rhizobium tropici is conditioned by a plasmid-encoded citrate synthase. Mol. Microbiol. 11:315-321. [DOI] [PubMed] [Google Scholar]
- 33.Peng, W.-T., Y.-W. Lee, and E. W. Nester. 1998. The phenolic recognition profiles of the Agrobacterium tumefaciens VirA protein are broadened by a high level of the sugar binding protein ChvE. J. Bacteriol. 180:5632-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piers, K. L., J. D. Heath, X. Liang, K. M. Stephens, and E. W. Nester. 1996. Agrobacterium tumefaciens-mediated transformation of yeast. Proc. Natl. Acad. Sci. USA 93:1613-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sciaky, D., A. L. Montoya, and M. D. Chilton. 1978. Fingerprints of Agrobacterium Ti plasmids. Plasmid 1:238-253. [DOI] [PubMed] [Google Scholar]
- 37.Somerville, J. E., and M. L. Kahn. 1983. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J. Bacteriol. 156:168-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srere, P. A. 1969. Citrate synthase. Methods Enzymol. 13:3-11. [Google Scholar]
- 39.Stachel, S. E., and P. C. Zambryski. 1986. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell 46:325-333. [DOI] [PubMed] [Google Scholar]
- 40.Textor, S., V. F. Wendisch, A. A. De Graaf, U. Muller, M. I. Linder, D. Linder, and W. Buckel. 1997. Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch. Microbiol. 168:428-436. [DOI] [PubMed] [Google Scholar]
- 41.Vereecke, D., K. Cornelis, W. Temmerman, M. Jaziri, M. Van Montagu, M. Holsters, and K. Goethals. 2002. Chromosomal locus that affects pathogenicity of Rhodococcus fascians. J. Bacteriol. 184:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weitzman, P. D., and M. J. Danson. 1976. Citrate synthase. Curr. Top. Cell Regul. 10:161-204. [DOI] [PubMed] [Google Scholar]
- 43.Wise, A. A., L. Voinov, and A. N. Binns. 2005. Intersubunit complementation of sugar signal transduction in VirA heterodimers and posttranslational regulation of VirA activity in Agrobacterium tumefaciens. J. Bacteriol. 187:213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood, D. W., J. C. Setubal, R. Kaul, D. E. Monks, J. P. Kitajima, V. K. Okura, Y. Zhou, L. Chen, G. E. Wood, N. F. Almeida, L. Woo, Y. Chen, I. T. Paulsen, J. A. Eisen, P. D. Karp, D. Bovee, P. Chapman, J. Clendenning, G. Deatherage, W. Gillet, C. Grant, T. Kutyavin, R. Levy, M.-J. Li, E. McClelland, A. Palmieri, C. Raymond, G. Rouse, C. Saenphimmachak, Z. Wu, P. Romero, D. Gordon, S. Zhang, H. Yoo, Y. Tao, P. Biddle, M. Jung, W. Krespan, M. Perry, B. Gordon-Kamm, L. Liao, S. Kim, C. Hendrick, Z.-Y. Zhao, M. Dolan, F. Chumley, S. V. Tingey, J.-F. Tomb, M. P. Gordon, M. V. Olson, and E. W. Nester. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317-2323. [DOI] [PubMed] [Google Scholar]
- 45.Xu, X.Q., and S. Q. Pan. 2000. An Agrobacterium catalase is a virulence factor involved in tumorigenesis. Mol. Microbiol. 35:407-414. [DOI] [PubMed] [Google Scholar]
- 46.Zupan, J., T. R. Muth, O. Draper, and P. Zambryski. 2000. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 23:11-28. [DOI] [PubMed] [Google Scholar]