Abstract
Many gram-negative bacteria synthesize N-acylhomoserine lactones (AHLs) and then use transcription factors of the LuxR family to sense and respond to AHL accumulation in the environment; this phenomenon is termed quorum sensing. Bacteria produce a variety of AHLs, and numerous bacterial reporter strains, or biosensors, that can detect subsets of these molecules have been constructed. Many of these are based on Escherichia coli because this species does not produce AHLs. However, both Escherichia and Salmonella spp. contain a LuxR homolog named SdiA that can detect exogenous AHL synthesized by other microbial species. In this study we have determined that sdiA of E. coli and Salmonella spp. can activate an RhlR-based biosensor plasmid in response to AHLs other than what the biosensor was designed to detect. SdiA does not activate LuxR-, LasR-, or AhyR-based biosensor plasmids, although the presence of sdiA in E. coli does interfere with the function of the AhyR-based biosensor. Because sdiA interferes with the function of two of the four reporters, we have constructed a set of E. coli biosensor strains that lack sdiA. The set includes control reporters that allow the luxR dependence of responses to be determined.
Bacteria have mechanisms by which they sense their own population density and adjust their behavior accordingly, termed quorum sensing (3). In this way individual bacteria can regulate their activities with others in their population, resulting in coordinated behavior that often resembles that of a multicellular organism. The prototype for gram-negative quorum sensing is the colonization of the light organ of the bobtail squid, Euprymna scolopes, by Vibrio fischeri (5, 6). The bacteria in this relationship use the LuxI enzyme to synthesize primarily N-(3-oxo-hexanoyl)-l-homoserine lactone (oxoC6) (7, 18). This molecule can diffuse freely across bacterial membranes and is bound by the LuxR transcription factor (10). A high population density of V. fischeri in the light organ leads to the accumulation of oxoC6, with the result being light production (22). The LuxR protein activates transcription of the luxICDABEGH operon in response to oxoC6, producing the luciferase enzyme subunits, substrate synthesis enzymes, and more LuxI. Given that light production by an individual bacterium is likely to be ineffective, this presumably prevents individuals from wasting energy until a suitably large population has been assembled. Quorum sensing by the use of LuxR and LuxI homologs has now been documented in numerous gram-negative bacterial species, including many pathogens, which use quorum sensing to regulate genes involved with the colonization of eukaryotic hosts (21).
The LuxR/LuxI homologs from various bacterial species utilize N-acylhomoserine lactones (AHLs) that vary in length, oxidation, and saturation of the acyl chain. For example, Pseudomonas aeruginosa contains two pairs of LuxR/LuxI homologs, neither of which optimally detects oxoC6. LasI synthesizes N-(3-oxo-dodecanoyl)-l-homoserine lactone (oxoC12), which is detected by LasR (15). RhlI synthesizes N-butanoyl-l-homoserine lactone (C4), which is detected by RhlR (16, 17).
Bacterial species of the genera Escherichia, Salmonella, and Klebsiella are unique in that they have a LuxR homolog, SdiA, but they do not contain a LuxI homolog or any other enzyme family that can synthesize AHLs (1). The function of SdiA is most understood in Salmonella enterica serovar Typhimurium. In this organism, SdiA detects AHLs produced by other bacterial genera (14, 19). SdiA then activates two Salmonella-specific loci, the rck (resistance to complement killing) operon, which is carried on the Salmonella virulence plasmid, and srgE (sdiA-regulated gene), which is carried in the chromosome but is of unknown function (2, 19). The rck operon includes six genes, three of unknown function and three that play a role in adhesion to extracellular matrix and/or host cells and resistance to complement killing (1).
E. coli and Klebsiella spp. also carry sdiA, but the function of sdiA in these organisms is unclear (1). No experiments have been performed on the sdiA genes of Klebsiella spp., and the results from experiments performed with E. coli are difficult to interpret. To date, all information on sdiA in E. coli has been obtained using sdiA overexpression. SdiA overexpression in E. coli results in a large pleiotropic response that is probably not an accurate indication of the natural function (the pleiotropic effect of sdiA overexpression is not observed with Salmonella). The genes affected by sdiA overexpression in E. coli have never been demonstrated to respond significantly to sdiA expressed from its natural position in the chromosome. However, a plasmid-encoded transcriptional fusion to the Salmonella gene srgE is responsive to sdiA and AHL in E. coli. Therefore, although the target genes are not known, sdiA is functional in E. coli (1).
Despite the presence of sdiA in E. coli, this organism has been used as the host organism for several AHL biosensor strains. This is for good reason as E. coli has the advantages of not producing AHLs, being safe, and being easy to manipulate. E. coli carrying a plasmid that encodes both a LuxR homolog and a transcriptional fusion regulated by that LuxR homolog can be used as a biosensor that responds to those AHLs that are specifically bound by that particular LuxR homolog. A description of a set of these biosensor strains was published previously (23, 24). The set consists of a plasmid carrying luxR and a luxR-regulated fusion that optimally detects oxoC6 (pSB401), a plasmid carrying lasR and a lasR-regulated fusion that optimally detects oxoC12 (pSB1075), and a third plasmid carrying rhlR and an rhlR-regulated fusion that optimally detects C4 (pSB406). A description of another plasmid that carries the Aeromonas hydrophila gene ahyR and an ahyR-regulated fusion (pSB536) was published separately (20). Like RhlR of P. aeruginosa, AhyR optimally detects C4. In each case, the fusion is to the luxI homolog fortuitously located adjacent to the luxR homolog (Fig. 1).
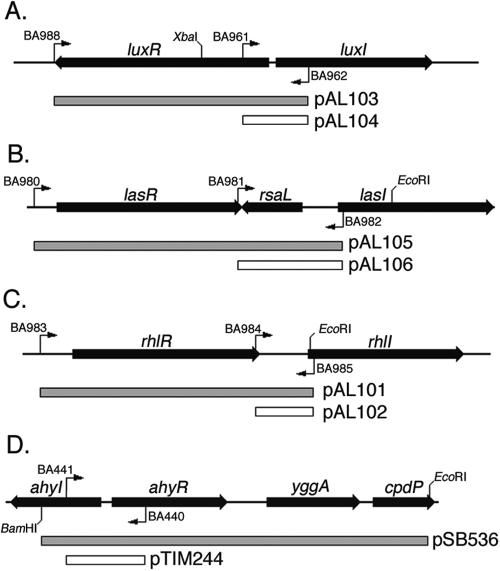
FIG. 1.
Map of the DNA fragments used to make the biosensor plasmids. The PCR primers used and important restriction sites are shown. The DNA sequence of each primer is listed in Table 1. Templates: the luxRI region of V. fischeri (pSB401) (A), the lasRI region of P. aeruginosa (PA01) (B), the rhlRI region of P. aeruginosa (PA01) (C), and the ahyRI region of A. hydrophila (pSB536) (D).
A limitation of these biosensor plasmids is that an increase in light production does not necessarily indicate the presence of AHLs. This is because the luciferase enzyme and the enzymes that produce the substrate for the luciferase reaction require energy, oxygen, and reducing equivalents (8). Therefore, light production can increase and decrease independently of the luxR homolog and AHL. In this study, we have constructed a set of control plasmids that lack the luxR homolog of interest. This control plasmid set allows the luxR dependence of light production to be determined. Additionally, we have found that sdiA of E. coli interferes with two of the biosensors. A set of strains lacking sdiA and carrying the various biosensor plasmids or control plasmids has been assembled for general use.
Construction of AHL biosensor and control plasmids.
Isogenic sets of plasmids that differ only in the presence or absence of the luxR homolog of interest were constructed. The plasmids carrying the luxR homolog contain fragments essentially identical to those used in the biosensor plasmids described previously (23, 24), whereas the control reporters carry an abbreviated, but otherwise identical, version of the full fragments, so that the luxR homolog is absent and only the luxI promoter fusion remains. Unlike the previous reporters, these sensors are all in the same plasmid backbone. The exception is the ahyR biosensor, for which we used the original reporter plasmid pSB536 and constructed only a control plasmid lacking the ahyR gene (20). All plasmids were constructed by using PCR to amplify the appropriate luxR homolog (lasR, luxR, or rhlR) along with the luxR-regulated promoter located adjacent to each homolog (the lasI, luxI, or rhlI promoter). Primers used are shown in Fig. 1, and the DNA sequence is listed in Table 1. The control plasmids were made by amplifying only the promoter region without the luxR homolog. The resulting fragments are shown in Fig. 1. These were cloned into the pCR 2.1-TOPO vector (Invitrogen) and then removed as EcoRI fragments. The EcoRI fragments were then cloned into pSB401 that had been digested with EcoRI, replacing the luxRI′ fragment of pSB401. This places each fragment upstream of the promoterless Photorhabdus luminescens luxCD ABE operon in pSB401. The ahyI promoter fragment was cloned into the pSB377 EcoRI site to match the origin of replication and antibiotic resistance marker of the ahyR-containing plasmid pSB536 (20, 23, 24) (Fig. 1D). Each construct was confirmed using PCR screening, restriction digestion, and DNA sequencing. The plasmids were then transformed into wild-type and sdiA mutant strains of E. coli and S. enterica (WM54, JLD271, 14028, and BA612) (Table 2). Used in conjunction with the plasmids containing the functional luxR homolog (referred to as luxR+, lasR+, rhlR+, or ahyR+), the control plasmids of each set (lacking luxR, lasR, rhlR, or ahyR) allow the luxR dependence of any response to be determined. Comparison of the reporters in the presence and absence of sdiA allows the influence of sdiA to be determined.
TABLE 1.
Oligonucleotides used
| Name | DNA sequence |
|---|---|
| BA440 | GGGATCACAGGCCAGCATATGGTTG |
| BA441 | GAAGATGCTGGGCAGCATGTAATCC |
| BA961 | GCTCTAGACAATAGGAACTTCCCTATGTGTCGTCGGCATTTATGT |
| BA962 | GAGAGATCTCATTATAGTCATACCAACCTCCCTT |
| BA980 | GCGTCTAGAGAATAGGAACTTCCCTCTCGGACTGCCGTACAACGT |
| BA981 | GCTCTAGAGAATAGGAACTTCCCATTTGGGTCTTATTACTCTCTG |
| BA982 | GAGAGATCTGCGCCGACCAATTTGTACGATCAT |
| BA983 | GCTCTAGAGAATAGGAACTTCCCAACGGTGCTGGCATAACAGATA |
| BA984 | GCTCTAGAGAATAGGAACTTCCCCGCGGCGCTGGGTCTCATCTGA |
| BA985 | GAGAGATCTCAGCGATTCAGAGAGGAATTCGATC |
| BA988 | GCTCTAGAGAATAGGAACTTCCCTTAATTTTTAAAGTATGGGCAA |
TABLE 2.
Strains and plasmids used
| Strain or plasmid | Genotype or description | Source or referencea |
|---|---|---|
| Strains | ||
| WM54 | E. coli K-12 ΔlacX74 | Bill Metcalf |
| JLD271 | WM54 sdiA271::Cam | Wanner mutagenesis, deleting nt 8137 to 8856 of accession no. AE000284 and replacing with Camr of pKD3 (4) |
| PAO1 | Wild-type P. aeruginosa | 9 |
| 14028 | Wild-type S. enterica serovar Typhimurium | American Type Culture Collection |
| BA612 | 14028 sdiA1::mTn3 | 2 |
| Plasmids | ||
| pSB377 | luxCDABE transcriptional fusion vector, Ampr ColE1 origin | 24 |
| pSB401 | luxR+luxI::luxCDABE; Tetr p15A origin | 23 |
| pSB1075 | lasR+lasI::luxCDABE; Ampr ColE1 origin | 23 |
| pSB536 | ahyR+ahyI::luxCDABE; Ampr ColE1 origin | 20 |
| pTIM244 | ahyI::luxCDABE; Ampr ColE1 origin | PCR with pSB536 template and oligos BA440 and BA441; cloned into pSB377/EcoRI |
| pAL101 | rhlR+rhlI::luxCDABE; Tetr p15A origin | PCR with PA01 template and oligos BA983 and BA985; cloned into pSB401/EcoRI |
| pAL102 | rhlI::luxCDABE; Tetr p15A origin | PCR with PA01 template and oligos BA984 and BA985; cloned into pSB401/EcoRI |
| pAL103 | luxR+luxI::luxCDABE; Tetr p15A origin | PCR with pSB401 template and oligos BA988 and BA962; cloned into pSB401/EcoRI |
| pAL104 | luxI::luxCDABE; Tetr p15A origin | PCR with pSB401 template and oligos BA961 and BA962; cloned into pSB401/EcoRI |
| pAL105 | lasR+lasI::luxCDABE; Tetr p15A origin | PCR with PA01 template and oligos BA980 and BA982; cloned into pSB401/EcoRI |
| pAL106 | lasI::luxCDABE; Tetr p15A origin | PCR with PA01 template and oligos BA981 and BA982; cloned into pSB401/EcoRI |
Abbreviations: nt, nucleotides; oligos, oligonucleotides.
LuxR and LasR.
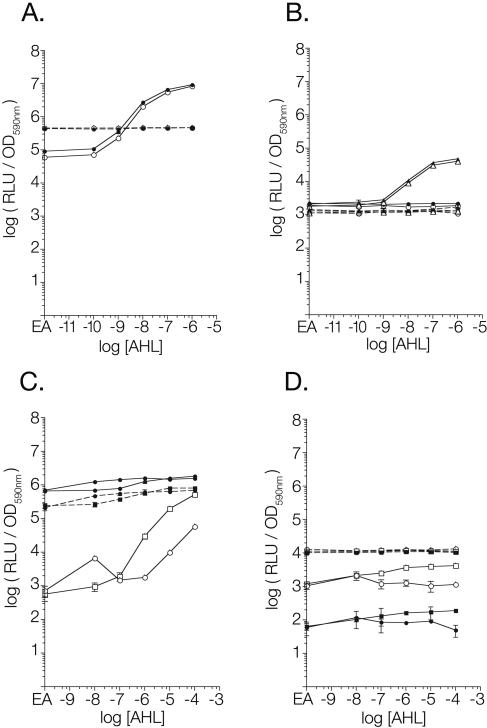
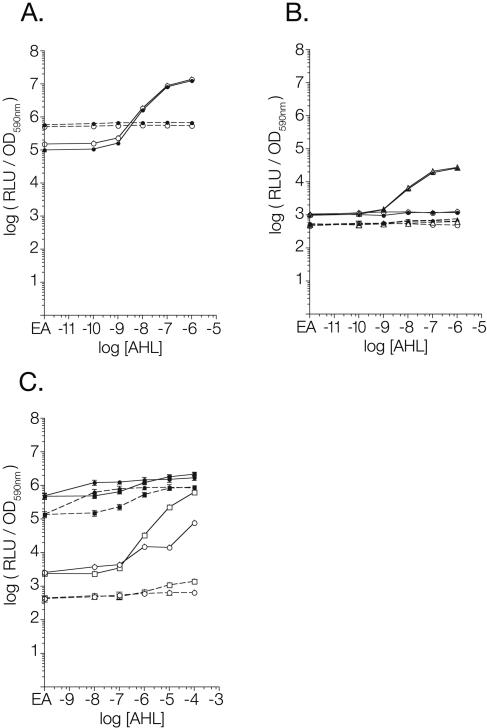
The luxR+ and lasR+ biosensors respond to 10 nM oxoC6 and 10 nM oxoC12 in E. coli, respectively, while the isogenic luxR- and lasR-negative plasmids do not detect AHL (Fig. 2A and 2B). Under the conditions tested, the presence or absence of sdiA did not have an effect on the response to AHL by either the biosensor or the control plasmids. Similar results were observed using Salmonella as the host strain (the wild-type strain 14028 versus the sdiA mutant strain BA612) (Fig. 3A and 3B). For unknown reasons, the basal level of luminescence of the luxR-negative control plasmid is higher than that of the luxR+ plasmid. However, this does not affect the utility of the control plasmid in determining the luxR dependence of reporter activity. Therefore, the luxR and lasR biosensors are able to respond appropriately to the presence and absence of exogenous AHLs, and sdiA does not contribute to, or interfere with, AHL detection.
FIG. 2.
Response of biosensors to AHL and sdiA in E. coli. Overnight cultures of each strain were subcultured 1:100 into 200 μl of LB broth containing AHL or the solvent control (0.1% ethyl acetate) in a 96-well plate with black side walls. The plate was incubated at 37°C for 9 hours before being placed into a Wallac Victor2 multimode plate reader for recording of the culture optical density at 590 nm (OD590nm) and the luminescence of the culture in relative light units (RLU). Data were analyzed using Prism 3.0 graphing software. (A) LuxR biosensor set pAL103 (luxR+) and pAL104 (luxR negative). (B) LasR biosensor set pAL105 (lasR+) and pAL106 (lasR negative). (C) RhlR biosensor set pAL101 (rhlR+) and pAL102 (rhlR negative). (D) AhyR biosensor set pSB536 (ahyR+) and pTIM244 (ahyR negative). Closed symbols, sdiA+; open symbols, sdiA negative; solid lines, luxR homolog present; dashed lines, luxR homolog absent; circles, oxoC6; triangles, oxoC12; squares, C4. EA on the x axis represents an AHL concentration of zero in which there is only the solvent ethyl acetate. Each point represents the mean of results from triplicate cultures, with error bars representing standard deviations.
FIG. 3.
Response of biosensor plasmids to AHL and sdiA in Salmonella. Data were obtained and analyzed as described for E. coli in the legend to Fig. 2. (A) LuxR biosensor set pAL103 (luxR+) and pAL104 (luxR negative). (B) LasR biosensor set pAL105 (lasR+) and pAL106 (lasR negative). (C) RhlR biosensor set pAL101 (rhlR+) and pAL102 (rhlR negative). Closed symbols, sdiA+; open symbols, sdiA negative; solid lines, luxR homolog present; dashed lines, luxR homolog absent; circles, oxoC6; triangles, oxoC12; squares, C4. EA on the x axis represents an AHL concentration of zero in which there is only the solvent ethyl acetate. Each point represents the mean of results from triplicate cultures, with error bars representing standard deviations. OD590nm, optical density at 590 nm.
AhyR.
In the absence of sdiA, the ahyR reporter system works as expected in E. coli. During growth in the presence of C4 at 100 nM or higher concentrations, the ahyR+ plasmid produces more luminescence than when it is grown in the presence of the solvent control or oxoC6 (Fig. 2D). However, the maximal difference achieved did not exceed fourfold at 100 μM C4. This attribute of the system does not make it particularly useful as a biosensor, and we have found that the rhlR system is much better (see below). Therefore, this reporter was not tested further in Salmonella. For unknown reasons, the basal level of luminescence from the ahyR-negative plasmid is more than an order of magnitude higher than the ahyR+ plasmid. This is similar to what was observed with the luxR reporter set. As with the luxR set, the control plasmid shows no AHL-dependent activation of the ahyI promoter in the absence of ahyR.
Unlike with luxR or lasR, the presence of sdiA interferes with the function of the ahyR+ biosensor, pSB536. Reporter activity of the ahyR+ plasmid is reduced between 10- and 100-fold by the presence of sdiA (Fig. 2D). Reporter activity of the control plasmid is similar in the presence and absence of sdiA, indicating that SdiA does not directly activate or repress the ahyI promoter itself. Because interference by sdiA requires the presence of ahyR, this suggests that SdiA and AhyR may oligomerize in the cell, forming inactive complexes. Alternatively, SdiA may compete with AhyR for binding to the ahyI promoter but fail to activate transcription. Regardless of the mechanism, these results show that sdiA of E. coli interferes with the ahyR-dependent activation of the ahyI promoter. Therefore, if used, the ahyR reporter system should be used only in an sdiA mutant background, though the rhlR reporter system is preferable.
RhlR.
In an E. coli sdiA mutant background, the rhlR+ biosensor detects 1 μM C4 or 10 μM oxoC6, while the rhlR-negative plasmid does not detect AHLs (Fig. 2C). The values for the rhlR-negative controls in the sdiA mutant background are not visible in Fig. 2C because the values at every point were slightly above or below zero. This indicates that, as with the luxR and ahyR plasmid pairs, the rhlR+ and rhlR-negative plasmids have different basal levels of luminescence. In this case that of the rhlR+ plasmid is higher. However, the fold induction of the rhlR+ nears three orders of magnitude (913-fold) in response to 100 μM C4, making the rhlR biosensor much more useful than the ahyR biosensor described above. Similar results with the rhlR reporters were seen by using Salmonella host strains (Fig. 3C).
The most striking result is that the presence of sdiA in the host strain strongly activates the rhlI promoter, with the addition of AHL increasing expression only a further two- to threefold. Essentially, sdiA is constitutively activating the rhlI promoter to a level that is achieved by rhlR only in the presence of 100 μM C4 (Fig. 2C). Interestingly, Winson et al. used sdiA+ E. coli host strains when describing the rhlR biosensor system, and they too observed only a two- to threefold induction in response to AHL (23). Based on our results, we believe that they were observing almost full induction of the reporter, even in the absence of AHL, due to the presence of sdiA in the host strain. With the removal of sdiA, the rhlR reporter system becomes much more useful, with a 53-fold response to 1 μM C4 and a 913-fold response to 100 μM C4.
Of the LuxR homologs discussed here, RhlR is the most closely related to SdiA (12). Given that sdiA is activating the rhlI promoter, it appears that RhlR and SdiA may have an overlapping DNA binding specificity, although they have diverged in the AHLs detected. While RhlR optimally detects C4, SdiA optimally detects oxoC6 and oxoC8, the same AHLs detected by LuxR and TraR. However, SdiA binds a fairly wide range of AHLs at what are likely to be physiologically relevant concentrations, possibly because SdiA has no corresponding AHL synthase gene and instead is used to detect AHLs produced by other species (1, 14, 19). Most intriguing is that sdiA is activating the rhlI promoter in the absence of AHL. One possibility is that SdiA binds DNA regardless of AHL. Upon binding AHL, a conformational change that allows activation of transcription may take place. Since the rhlI promoter is not a native promoter for SdiA, for unknown reasons binding of SdiA alone may be sufficient for activation. This would be consistent with recent findings in which AhyR and RhlR were found to bind DNA regardless of the presence of AHL (11, 13). Additionally, an in vivo DNA methylation protection assay suggested that RhlR binds its target DNA sequence in a different conformation when AHL is present. The AHL-bound conformation was competent for transcription activation, while the other conformation was not (13). Future biochemical studies of SdiA and RhlR will be required to determine the mechanism by which sdiA causes activation of the rhlI promoter. Whatever the mechanism, sdiA should not be present in rhlR biosensor strains.
In this report we have provided to the scientific community a description of an isogenic set of biosensor strains that detect a range of AHLs. This set utilizes the transcription factors LasR, LuxR, and RhlR and was based on the original set published by Winson et al. (23, 24). We have added a control strain for each transcription factor that lacks the luxR homolog, thus allowing a definitive determination of luxR dependence for the observed responses. Additionally, we have determined that sdiA in the E. coli host strain is a serious impediment to the use of the RhlR biosensor. To avoid any potential complications arising from sdiA, the entire reporter set is available in an sdiA mutant background.
Acknowledgments
We are grateful to Ross Larue for amplifying and cloning the inserts used to construct the biosensor plasmids, to Jessica Dyszel for construction of JLD271, and to Max Teplitski for pTIM244. We are indebted to Dietz Bauer for the use of his Victor2 plate reader.
This publication was supported by Public Health Service grant 5 RO1 AI050002-04 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ahmer, B. M. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 52:933-945. [DOI] [PubMed] [Google Scholar]
- 2.Ahmer, B. M. M., J. V. Reeuwijk, C. D. Timmers, P. J. Valentine, and F. Heffron. 1998. Salmonella typhimurium encodes an SdiA homolog, a putative quorum sensor of the LuxR family, that regulates genes on the virulence plasmid. J. Bacteriol. 180:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109:421-424. [DOI] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlap, P. V. 1999. Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 1:5-12. [PubMed] [Google Scholar]
- 6.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 7.Hanzelka, B. L., and E. P. Greenberg. 1996. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J. Bacteriol. 178:5291-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastings, J. W. 1996. Chemistries and colors of bioluminescent reactions: a review. Gene 173:5-11. [DOI] [PubMed] [Google Scholar]
- 9.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirke, D. F., S. Swift, M. J. Lynch, and P. Williams. 2004. The Aeromonas hydrophila LuxR homologue AhyR regulates the N-acyl homoserine lactone synthase, AhyI positively and negatively in a growth phase-dependent manner. FEMS Microbiol. Lett. 241:109-117. [DOI] [PubMed] [Google Scholar]
- 12.Lerat, E., and N. A. Moran. 2004. The evolutionary history of quorum-sensing systems in bacteria. Mol. Biol. Evol. 21:903-913. [DOI] [PubMed] [Google Scholar]
- 13.Medina, G., K. Juarez, B. Valderrama, and G. Soberon-Chavez. 2003. Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J. Bacteriol. 185:5976-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael, B., J. N. Smith, S. Swift, F. Heffron, and B. M. Ahmer. 2001. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J. Bacteriol. 183:5733-5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaefer, A. L., D. L. Val, B. L. Hanzelka, J. E. Cronan, and E. P. Greenberg. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA 93:9505-9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, J. N., and B. M. Ahmer. 2003. Detection of other microbial species by Salmonella: expression of the SdiA regulon. J. Bacteriol. 185:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swift, S., A. V. Karlyshev, L. Fish, E. L. Durant, M. K. Winson, S. R. Chhabra, P. Williams, S. Macintyre, and G. S. Stewart. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 179:5271-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swift, S., P. Williams, and G. S. A. B. Stewart. 1999. N-Acylhomoserine lactones and quorum sensing in proteobacteria, p. 291-313. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 22.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winson, M. K., S. Swift, L. Fish, J. P. Throup, F. Jorgensen, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. Stewart. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 163:185-192. [DOI] [PubMed] [Google Scholar]
- 24.Winson, M. K., S. Swift, P. J. Hill, C. M. Sims, G. Griesmayr, B. W. Bycroft, P. Williams, and G. S. Stewart. 1998. Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol. Lett. 163:193-202. [DOI] [PubMed] [Google Scholar]