Abstract
The F1Fo-ATP synthase plays an important role in a number of vital cellular processes in plants, animals, and microorganisms. In this study, we constructed a ΔatpD mutant of Mycobacterium smegmatis and demonstrated that atpD encoding the β subunit of the F1Fo-ATP synthase is an essential gene in M. smegmatis during growth on nonfermentable and fermentable carbon sources.
The F1Fo-ATP synthases of bacteria play an important role in a number of vital cellular processes (3, 22). In aerobic bacteria, these enzymes are responsible for ATP generation via oxidative phosphorylation, leading to large amounts of ATP synthesized per substrate oxidized. In anaerobic bacteria, under nonrespiratory growth conditions, these enzymes work primarily as an ATPase, pumping protons to generate a proton motive force, and in some cases, this is coupled to pH homeostasis to prevent intracellular acidification (5, 10).
A recent study reported that the F1Fo-ATP synthase of mycobacteria is the target of the first new antituberculosis drug family (diarylquinolines) to be discovered in 40 years (1). The authors suggest that the drug leads to ATP depletion and an imbalance in pH homeostasis in mycobacterial species, both contributing to decreased ability to survive. Other studies have implicated a potential role for the F1Fo-ATP synthase in the physiology of mycobacteria at acidic pH (8, 17, 18). In this study, we sought to establish a role for this enzyme in the physiology of Mycobacterium smegmatis as a model for understanding its potential role in the biology of other mycobacterial species.
The atpD gene encoding the β subunit of the F1Fo-ATP synthase is an essential gene in M. smegmatis.
The putative atp operon coding for the F1Fo-ATP synthase of M. smegmatis was identified in an unfinished genome database (www.tigr.org). The DNA sequence shows that this operon is similar to those of many bacteria and is colinear to the atp operon of Mycobacterium tuberculosis with the gene order atpBEFHAGDC. In order to disable the F1Fo-ATP synthase of M. smegmatis, we chose to disrupt the atpD gene by allelic exchange mutagenesis by using a strategy adapted from Pelicic et al. (16).
M. smegmatis strain mc2155 (23) and derived mutants (Table 1) were grown with agitation at 37°C in either Luria-Bertani medium supplemented with 0.05% (wt/vol) Tween 80 (Sigma Chemicals) (LBT) or Middlebrook 7H9 broth (Difco Laboratories, Detroit, Mich.) supplemented with sterile Middlebrook ADC enrichment (Becton Dickinson, Cockeysville, Md.). For solid medium, Middlebrook 7H11 was supplemented with ADC and glycerol (0.5% vol/vol) or LBT with 1.5% agar. Unless otherwise stated, M. smegmatis transformants were grown at 28°C for temperature-sensitive vector propagation and 40 to 42°C for allelic exchange mutagenesis. All subcloning steps were performed with Escherichia coli strain DH10B (Table 1) with culturing at 37°C in LB broth or 2× YT broth at an initial pH of 7.0 and LB agar (19). The E. coli plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or informationa | Reference or sourceb |
|---|---|---|
| E. coli DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 endA1 recA1 deoR Δ(ara-leu)7697 araD139 galU galK nupG rspL λ− | 19 |
| M. smegmatis | ||
| mc2155 | Wild-type ept-1 | 23 |
| ST10 | mc2155 carrying pST100 (ΔatpD::aphA-3), Kmr Gmr | This study |
| ST24 | mc2155 ΔatpD1::aphA-3, Kmr | This study |
| ST34 | ST24 harboring pST100, Kmr Gmr | This study |
| ST35 | ST34 with pST100 integrated (ΔatpD2::pST100), Kmr Gmr | This study |
| ST36 | ST35 harboring pST101, Kmr Str/Spr | This study |
| ST40 | mc2155 harboring pST220 (atpB-lacZ), Kmr | This study |
| Plasmids | ||
| pUC18K | Contains aphA-3 cassette, Kmr | 14 |
| LITMUS 28 | E. coli cloning vector, Apr | NEB |
| pPR23 | E. coli-mycobacterial shuttle vector, oriM temps, sacB Gmr | 16 |
| pCG76 | Shuttle vector, temps, Str/Spr | 27 |
| pMV261 | Shuttle vector, BCG Hsp60 promoter, Kmr | 25 |
| pJEM15 | Shuttle vector, Kmr | 26 |
| pLatpD | LITMUS 28 harboring ΔatpD1::aphA-3, Apr | This study |
| pST100 | pPR23 harboring ΔatpD1::aphA-3, Kmr Gmr | This study |
| pST101 | pCG76 harboring PHsp60-atpD+ fusion, Str/Spr, temps | This study |
| pST200 | pMV261 harboring atpD+, Kmr | This study |
| pST220 | pJEM15 harboring atpB-lacZ fusion, Kmr | This study |
Kmr, kanamycin resistance; Gmr, gentamicin resistance; Str, streptomycin resistance; Spr, spectinomycin resistance; Apr, ampicillin resistance; temps, temperature sensitivity.
NEB, New England Biolabs.
To construct a vector to delete the atpD gene (i.e., pST100 ΔatpD::aphA-3 [Fig. 1A]), primers were designed to amplify (Expand high-fidelity PCR system) approximately 1,000 bp flanking the putative atpD gene. The primer pair AtpD4 (5′-AAATTTGAATTCCAAGAAGGCCTAGGTAACAG-3′) and AtpD3 (5′-AAATTTGGTACCTACGCGACCCGCGGTCTTTT-3′) and the primer pair AtpD2 (5′-AAATTTTCTAGACTGGACGACCTGGCGAAGAA-3′) and AtpD1 (5′-AAATTTAAGCTTGTTGCCTGCGAAGTACCTCA-3′) were used to amplify the 5′ and 3′ flanks, respectively. The resulting amplicons were sequenced to confirm fidelity and were then ligated simultaneously with a kanamycin resistance cassette (KpnI-XbaI fragment containing aphA-3 from pUC18K) (14) into EcoRI-HindIII-digested LITMUS 28 (New England Biolabs). The assembled atpD knockout construct (ΔatpD::aphA-3 with 1,000 bp of homologous flanks) was then subcloned as a 2.8-kb AvrII fragment into the XbaI site of the delivery vector pPR23 (thus creating pST100) (Fig. 1A). The expected double crossover would result in a nonpolar deletion-insertion at the atpD locus, eliminating 94% of the atpD coding sequence in exchange for the kanamycin resistance marker. pST100 was electroporated into M. smegmatis mc2155 (0.2-cm-gap cuvette at 2.5 kV, 1,000 Ω, and 25 μF) and transformants screened for gentamicin and kanamycin resistance and confirmed by electroduction (2).
FIG. 1.
(A) Key plasmids used in the generation of ST36 (ΔatpD::aphA-3). Allelic exchange delivery vector pST100 carries DNA amplified from the atp operon of M. smegmatis mc2155. The in trans ΔatpD complementing vector pST101 carries atpD under the constitutive control of the Mycobacterium bovis BCG Hsp60 promoter (Phsp60). (B) The genetic consequences of homologous recombination with ΔatpD::aphA-3 (see the text for detailed explanation).
Recombinants were selected by plating cells from a log-phase culture (optical density at 600 nm, 0.7 to 1.0) of a pST100 transformant (strain ST10) onto LBT agar supplemented with sucrose and kanamycin and incubated at 42°C. pST100 carries a temperature-sensitive mycobacterial origin of replication and sacB counterselection to facilitate screening for a double crossover (16). For mc2155 derivatives, kanamycin, gentamicin, streptomycin, and spectinomycin were used at final concentrations of 20, 5, 20, and 20 μg/ml, respectively. Sucrose was used at a final concentration of 10% (wt/vol). Colonies that appeared were screened for gentamicin sensitivity (loss of vector) and kanamycin resistance (allelic exchange of atpD for ΔatpD::aphA-3). Colonies displaying the desired phenotype (kanamycin resistance, gentamicin resistance, and sucrose resistance) were analyzed by colony blot hybridization to confirm the absence of the delivery vector (pPR23), indicating that the putative mutants had likely undergone double crossover. Genomic DNA from putative ΔatpD mutants displaying the desired phenotype (kanamycin resistance, gentamicin resistance, sucrose resistance, and growth at 42°C) was digested with SmaI, and the fragments were separated by agarose gel electrophoresis, blotted onto nylon membrane and then probed with the 3′ flanking fragment, designated RF (see Fig. 1B). To further confirm the loss of the wild-type atpD allele, primer pair AtpD4 and AtpD1 or AtpDcompF and AtpDcompR was used to amplify the region containing the atpD loci, with the product size reflecting the presence of wild-type atpD or the deletion of atpD.
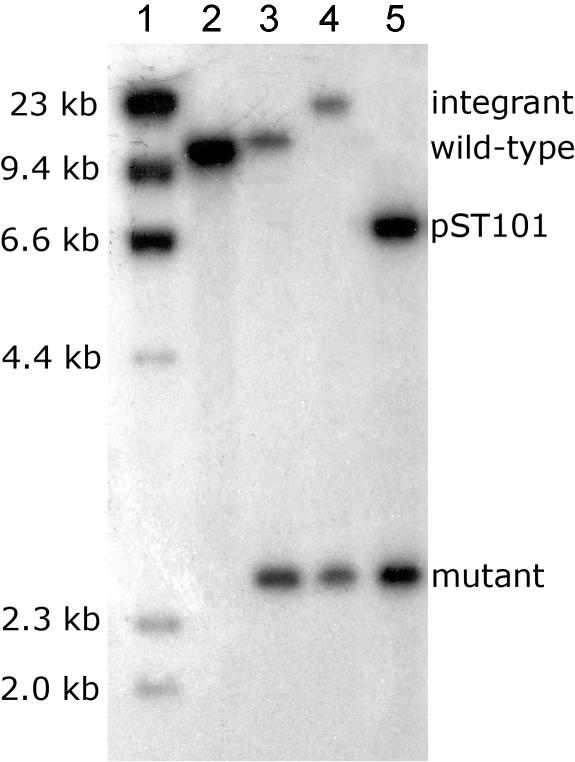
Southern hybridization analysis and PCR revealed that the putative mutants carried a second atpD locus (designated atpD2). SmaI-digested chromosomal DNA, probed with RF (Fig. 1B), hybridized to a single SmaI fragment of approximately 10.7 kb in the parental strain (Fig. 2, lane 2). In these mutants, we expected a double crossover with pST100 to generate a single 2.7-kb hybridizing band. Unexpectedly, two hybridizing bands were found in all mutants screened: the expected 2.7-kb band and an additional 10.7-kb band (Fig. 2, lane 3). These results suggested that atpD and the surrounding region have been duplicated in M. smegmatis, and only one copy of atpD had been successfully deleted from this mutant (designated ST24 [ΔatpD1::aphA-3]). It has been reported that M. smegmatis contains a large duplication (approximately 250 kb or greater) in its genome (7), and this may explain the two copies of atpD observed here. The growth rate and cell yield of ST24 were identical to those of strain mc2155 in M63 minimal medium containing either succinate or glucose, suggesting that a second copy of atpD (designated atpD2) was indeed present (data not shown).
FIG. 2.
Southern hybridization of SmaI-digested genomic DNA was used to confirm double crossover events, which generate a 2.7-kb fragment instead of the wild-type 10.7-kb fragment. In ST35 (ΔatpD1; atpD2::pST100), a 16-kb band is generated by the integration of pST100 in place of the wild-type fragment. The double knockout mutant ST36 (ΔatpD1 ΔatpD2 pST101) carries atpD in trans, thus shows the 2.7-kb double crossover band (a doublet) and a 7.0-kb band derived from pST101. Lane 1, λ DNA (HindIII digest); lane 2, mc2155 (wild type); lane 3, ST24; lane 4, ST35; lane 5, ST36.
Upon discovery of atpD2, pST100 was reintroduced into ST24 (creating ST34) and double crossover at the atpD2 locus was executed through a two-step approach (Fig. 1B): (i) pST100 was forced to integrate into atpD2 by selection for gentamicin resistance at 42°C (generating strain ST35), and (ii) atpD was then supplied in trans on the ΔatpD complementing vector pST101, allowing the resolution of the integrated pST100 backbone and simultaneous allelic exchange at atpD2 (generating strain ST36).
Putative atpD double knockouts were screened for kanamycin and sucrose resistance and then confirmed by Southern blot analysis and PCR. Integration of pST100 at atpD2 resulted in a shift from a 10.7-kb hybridizing band to a 16-kb hybridizing band (Fig. 2, lane 4). The hybridization profile also confirmed that only two copies, rather than multiple copies, of atpD are present in the genome of M. smegmatis. Integrants exhibited slow growth rates and poor cell yields, implying that the integration was polar. In support of this, Southern analysis showed that all legitimate integrants had the same genotype, indicating a bias toward the continued expression of atpD from an intact atp operon and promoter (data not shown). To select for a second crossover (and resolution of the intervening sequence), ST35 was grown on kanamycin and sucrose at 42°C. This consistently resulted in colonies with gentamicin resistance (336/336 tested), indicating that the screen was ineffective (likely due to sacB mutation) or that atpD was essential for growth of M. smegmatis. To address the latter hypothesis, an atpD complementing vector (temperature-sensitive origin of replication), pCG76 harboring atpD+ (pST101), was constructed to increase the probability of a double crossover at atpD2. The ΔatpD complementing plasmid, pST101 (Fig. 1A), was constructed by amplifying atpD with primers AtpDcompF (5′-AAATTTAGATCTGCAAGCCCCTTTCAGGAAGC-3′) and AtpDcompR (5′-AAATTTAAGCTTGACATGGCAATCACAGCTTG-3′) and cloning the resulting product as a BglII-HindIII fragment immediately downstream of the BCG Hsp60 promoter (PHsp60) carried by pMV261 (25), thus allowing the constitutive expression of atpD. The PHsp60-atpD fusion (PHsp60-atpD) was then ligated (as a XbaI-NheI fragment) into the XbaI site of pCG76.
Introduction of pST101 (atpD in trans) into ST35 resulted in the spontaneous excision of the integrated pST100 plasmid backbone, the hybridization profiles indicating that pST101 transformants had undergone a double crossover at atpD2 (Fig. 2, lane 5; see Fig. 1B for schematic representation). This is consistent with the incompatibility of pAL5000-derived vectors described by Stolt and Stoker (24). A double crossover mutant was selected and designated ST36 (Fig. 2, lane 5).
Characterization of the ΔatpD::aphA-3 mutant.
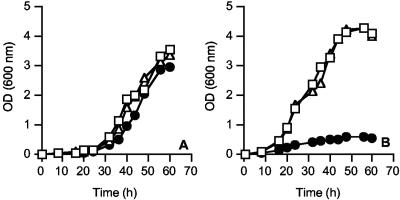
To determine if atpD was essential in M. smegmatis, ST36 cultures were grown at 28°C and 42°C in LBT supplemented with 20 mM glucose (fermentable carbon source) and compared to the single-ΔatpD1::kan mutant ST24 and the wild-type strain mc2155 (Fig. 3A and B). A starter culture of ST36 was grown to mid-log phase (optical density at 600 nm, <1.0) at 28°C in LBT and then was used to inoculate 5-ml LBT broths (supplemented with 20 mM glucose) prewarmed to either 28°C or 42°C. Growth was followed by measuring the optical density at 600 nm. The ST24 ΔatpD1 mutant and wild-type strain mc2155 were used as controls in all experiments.
FIG. 3.
The effect of conditional (temperature-sensitive) atpD expression on the growth of ST36. The growth of ST36 (•) at 28°C (A) and 42°C (B). Wild-type mc2155 (□) and ST24 (Δ) were used as control organisms. All cultures were grown in LBT containing 20 mM glucose. The growth curves shown are representative of three individual experiments in which the data did not differ by more than 10%. OD, optical density.
The growth of the single and double atpD knockout mutants at 28°C proceeded at the same rate as that of the wild-type strain mc2155 (Fig. 3A). The growth rates of ST24 and the wild-type mc2155 were also the same at 42°C. ST36 did not show significant growth at 42°C (pST101 supplying atpD in trans is temperature sensitive at 42°C and cannot replicate), thus demonstrating that atpD was indeed essential for growth (Fig. 3B). Equivalent results were obtained with succinate as the sole carbon and energy source (data not shown).
Expression of atpB-lacZ is constitutive and not regulated by growth rate or low pH.
To examine the role of F1Fo-ATPase in the physiology of M. smegmatis, we studied the expression of the atp operon under various growth conditions. To monitor F1Fo-ATPase expression, we constructed an in trans transcriptional atpB-lacZ fusion (strain ST40). To construct an atpB-lacZ fusion, approximately 900 bp upstream of the putative atpB gene was amplified by using the primer pair PatpOF (5′-AATGTCGGATCCGCAGAAAGTCGTCAGGTCAG-3′) and PatpOR (5′-ACGGCAGGATCCAGAATGGTGTCGCCATTGAA-3′). The resulting amplicon was ligated as a BamHI fragment into the promoterless lacZ vector pJEM15 (26) to create an atpB-lacZ transcriptional fusion (plasmid pST220). The construct was electroporated into M. smegmatis mc2155 and putative transformants (designated ST40) confirmed by PCR and DNA sequencing.
The transcriptional activity of atpB-lacZ was then evaluated under various growth regimens. For atpB-lacZ expression studies, M. smegmatis was cultured in M63 minimal medium supplemented with 0.05% (wt/vol) Tween 80 and a 40 mM concentration of glucose, glycerol, succinate, glutamate, or fructose. Cells were harvested and atpB-lacZ expression determined by β-galactosidase assays as previously described (26). Data given in Miller units (MU) are the mean values of three independent experiments with the standard deviations of the means reported. When strain ST40 was grown over the pH range of 5.5 to 8.0, no significant change in atpB-lacZ activity was observed (approximately 250 ± 35 MU). Furthermore, if cells were resuspended at a very acidic pH (pH 3.0 for 2 h), no upregulation of atpB-lacZ expression was observed (data not shown).
To study the effect of the carbon source on atpB-lacZ expression, ST40 was grown in M63 medium with various carbon sources (the specific rates of growth on each carbon source are as follows: succinate, 0.17 h−1; glucose, 0.12 h−1; fructose, 0.21 h−1; glutamate, 0.09 h−1; and glycerol, 0.27 h−1). The level of atpB-lacZ expression on nonfermentable carbon sources (i.e., succinate, 361 ± 58 MU; glutamate, 358 ± 32 MU; and glycerol, 342 ± 49 MU) was high compared to those of the substrates that were not strictly coupled to oxidative phosphorylation (i.e., the levels of expression for glucose and fructose were 274 ± 25 MU and 216 ± 30 MU, respectively). No discernible trend between growth rate and atpB-lacZ expression was observed.
These results demonstrate that atpD and, therefore, the F1Fo-ATP synthase are essential for growth of M. smegmatis on both fermentable and nonfermentable carbon sources. This finding is congruent with those of Sassetti et al. (21), in whose study atpD (and the other genes in the atp operon) were reported to be essential genes by Himar1-based transposon mutagenesis in M. tuberculosis H37Rv (21). The essentiality of the F1Fo-ATP synthase in the growth of species of other bacterial genera (e.g., Helicobacter pylori, Lactococcus lactis, and Listeria monocytogenes) has also been documented (4, 11, 13); however, in none of these studies has the essential requirement been defined experimentally.
In other bacteria, the F1Fo-ATP synthase has been shown to be dispensable for growth on fermentable carbon sources (6, 9, 20, 29), in which increased glycolytic flux can compensate for the loss of oxidative phosphorylation. This strategy does not appear to be exploited by M. smegmatis: the F1Fo-ATP synthase is essential for growth even on fermentable substrates, suggesting that ATP production from substrate level phosphorylation alone, despite increased glycolytic flux, may be insufficient to sustain the growth of these bacteria. This would be reflected by an extraordinarily high value for the amount of ATP required to synthesize a mycobacterial cell, a possibility that requires further investigation. Alternatively, or in conjunction with a high ATP demand for growth, the ATP synthase may be an obligatory requirement for the oxidation of NADH by providing a sink for translocated protons during NADH oxidation coupled with oxygen reduction. Such strict coupling would imply that mycobacteria do not support uncoupled respiration: either they lack a conduit for proton reentry in the absence of the F1Fo-ATP synthase or they are unable to adjust the proton permeability of the cytoplasmic membrane to allow a futile cycle of protons to operate. Recently, we have shown that the cytoplasmic membrane of M. smegmatis is extremely impermeable to protons (27).
The oxidation of NADH by aerobic bacteria is critical for continuous metabolic flux, and in the absence of fermentative metabolism, NADH oxidation will be carried out primarily by NADH dehydrogenases operating in conjunction with a respiratory chain. Weinstein et al. (30) have identified genes for two classes of NADH:menaquinone oxidoreductases in the genome of M. tuberculosis. NDH-1 is a proton-translocating NADH dehydrogenase (encoded by nuoABCDEFGHIJKLMN)and NDH-2 is a non-proton-translocating NADH dehydrogenase present in two copies (ndh and ndhA) (30). Mutagenesis studies have established that both NDH-1 (21) and ndhA (12) are dispensable for growth in vitro, but the lack of a viable strain with a disrupted or deleted ndh gene suggests that this gene is essential for growth. Deleterious point mutations in ndh of M. smegmatis are pleiotropic, conferring temperature-sensitive growth arrest and multiple-amino-acid auxotrophy. Some mutants also have a 25-fold-reduced NADH dehydrogenase activity, implying that NDH-2 is the primary enzyme responsible for NADH oxidation and it is essential for the viability of M. smegmatis (15, 28).
Several new antitubercular drugs have recently been reported (1, 30). It is particularly noteworthy that both classes of drugs target oxidative phosphorylation in mycobacteria. The first class, diarylquinolines (i.e., R207910) target the F1Fo-ATP synthase (1), and the second class, phenothiazine analogs, target the NADH:menaquinone oxidoreductase (30), suggesting that NADH oxidation via aerobic respiration coupled with oxidative phosphorylation is essential for the growth of mycobacteria. Future research will focus on the relationship between these two important metabolic processes in mycobacteria.
Acknowledgments
This work was funded by a New Zealand Lottery Health Grant.
REFERENCES
- 1.Andries, K., P. Verhasselt, J. Guillemont, H. W. Gohlmann, J. M. Neefs, H. Winkler, J. Van Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. de Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis, and V. Jarlier. 2004. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223-227. [DOI] [PubMed] [Google Scholar]
- 2.Baulard, A., C. Jourdan, A. Mercenier, and C. Locht. 1992. Rapid mycobacterial plasmid analysis by electroduction between Mycobacterium spp. and Escherichia coli. Nucleic Acids Res. 20:4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, P. D. 1997. The ATP synthase—a splendid molecular machine. Annu. Rev. Biochem. 66:717-749. [DOI] [PubMed] [Google Scholar]
- 4.Cotter, P. D., C. G. Gahan, and C. Hill. 2000. Analysis of the role of the Listeria monocytogenes F0F1-ATPase operon in the acid tolerance response. Int. J. Food Microbiol. 60:137-146. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedl, P., J. Hoppe, R. P. Gunsalus, O. Michelsen, K. von Meyenburg, and H. U. Schairer. 1983. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 2:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galamba, A., K. Soetaert, X. M. Wang, J. De Bruyn, P. Jacobs, and J. Content. 2001. Disruption of adhC reveals a large duplication in the Mycobacterium smegmatis mc2155 genome. Microbiology 147:3281-3294. [DOI] [PubMed] [Google Scholar]
- 8.Jones, P. C. 2001. Introduction of a carboxyl group in the first transmembrane helix of Escherichia coli F1F0 ATPase subunit c and cytoplasmic pH regulation. J. Bacteriol. 183:1524-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klionsky, D. J., W. S. A. Brusilow, and R. D. Simoni. 1984. In vivo evidence for the role of the ɛ subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J. Bacteriol. 160:1055-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi, H. 1985. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J. Biol. Chem. 260:72-76. [PubMed] [Google Scholar]
- 11.Koebmann, B. J., D. Nilsson, O. P. Kuipers, and P. R. Jensen. 2000. The membrane-bound H+-ATPase complex is essential for growth of Lactococcus lactis. J. Bacteriol. 182:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdam, R. A., S. Quan, D. A. Smith, S. Bardarov, J. C. Betts, F. C. Cook, E. U. Hooker, A. P. Lewis, P. Woollard, M. J. Everett, P. T. Lukey, G. J. Bancroft, W. R. Jacobs, Jr., and K. Duncan. 2002. Characterization of a Mycobacterium tuberculosis H37Rv transposon library reveals insertions in 351 ORFs and mutants with altered virulence. Microbiology 148:2975-2986. [DOI] [PubMed] [Google Scholar]
- 13.McGowan, C. C., T. L. Cover, and M. J. Blaser. 1997. Analysis of F1F0-ATPase from Helicobacter pylori. Infect. Immun. 65:2640-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miesel, L., T. R. Weisbrod, J. A. Marcinkeviciene, R. Bittman, and W. R. Jacobs, Jr. 1998. NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J. Bacteriol. 180:2459-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piddington, D. L., A. Kashkouli, and N. A. Buchmeier. 2000. Growth of Mycobacterium tuberculosis in a defined medium is very restricted by acid pH and Mg2+ levels. Infect. Immun. 68:4518-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao, M., T. L. Streur, F. E. Aldwell, and G. M. Cook. 2001. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology 147:1017-1024. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Santana, M., M. S. Ionescu, A. Vertes, R. Longin, F. Kunst, A. Danchin, and P. Glaser. 1994. Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants. J. Bacteriol. 176:6802-6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 22.Senior, A. E., S. Nadanaciva, and J. Weber. 2002. The molecular mechanism of ATP synthesis by F1F0-ATP synthase. Biochim. Biophys. Acta 1553:188-211. [DOI] [PubMed] [Google Scholar]
- 23.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 24.Stolt, P., and N. G. Stoker. 1996. Functional definition of regions necessary for replication and incompatibility in the Mycobacterium fortuitum plasmid pAL5000. Microbiology 142:2795-2802. [DOI] [PubMed] [Google Scholar]
- 25.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, S. B. Snapper, R. G. Barletta, W. R. Jacobs, and B. B. Bloom. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 26.Timm, J., E. M. Lim, and B. Gicquel. 1994. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J. Bacteriol. 176:6749-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran, S. L., M. Rao, C. Simmers, S. Gebhard, K. Olsson, and G. M. Cook. 2005. Mutants of Mycobacterium smegmatis unable to grow at acidic pH in the presence of the protonophore carbonyl cyanide m-chlorophenylhydrazone. Microbiology 151:665-672. [DOI] [PubMed] [Google Scholar]
- 28.Vilcheze, C., T. R. Weisbrod, B. Chen, L. Kremer, M. H. Hazbon, F. Wang, D. Alland, J. C. Sacchettini, and W. R. Jacobs, Jr. 2005. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob. Agents Chemother. 49:708-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Z., D. B. Hicks, A. A. Guffanti, K. Baldwin, and T. A. Krulwich. 2004. Replacement of amino acid sequence features of a- and c-subunits of ATP synthases of alkaliphilic Bacillus with the Bacillus consensus sequence results in defective oxidative phosphorylation and nonfermentative growth at pH 10.5. J. Biol. Chem. 279:26546-26554. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein, E. A., T. Yano, L. S. Li, D. Avarbock, A. Avarbock, D. Helm, A. A. McColm, K. Duncan, J. T. Lonsdale, and H. Rubin. 2005. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl. Acad. Sci. USA 102:4548-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]