Abstract
The major phenotypes of lon mutations, UV sensitivity and overproduction of capsule, are due to the stabilization of two substrates, SulA and RcsA. Inactivation of transfer mRNA (tmRNA) (encoded by ssrA), coupled with a multicopy kanamycin resistance determinant, suppressed both lon phenotypes and restored the rapid degradation of SulA. This novel protease activity was named Alp but was never identified further. We report here the identification, mapping, and characterization of a chromosomal mutation, faa (for function affecting Alp), that leads to full suppression of a Δlon ssrA::cat host and thus bypasses the requirement for multicopy Kanr; faa and ssrA mutants are additive in their ability to suppress lon mutants. The faa mutation was mapped to the C terminus of dnaJ(G232); dnaJ null mutants have similar effects. The identification of a lon suppressor in dnaJ suggested the possible involvement of heat shock. We find that ssrA mutants alone significantly induce the heat shock response. The suppression of UV sensitivity, both in the original Alp strain and in faa mutants, is reversed by mutations in clpY, encoding a subunit of the heat shock-induced ClpYQ protease that is known to degrade SulA. However, capsule synthesis is not restored by clpY mutants, probably because less RcsA accumulates in the Alp strain and because the RcsA that does accumulate is inactive. Both ssrA effects are partially relieved by ssrA derivatives encoding protease-resistant tags, implicating ribosome stalling as the primary defect. Thus, ssrA and faa each suppress two lon mutant phenotypes but by somewhat different mechanisms, with heat shock induction playing a major role.
Proteases play a pivotal role in metabolic regulation. They degrade many key regulatory proteins as well as abnormal proteins and thereby let cells adjust to varied environmental conditions. In Escherichia coli, five ATP-dependent proteases have been characterized: Lon (or La), ClpAP, ClpXP, ClpYQ (also called HslUV), and FtsH (5, 6). Lon was the first of these proteases to be identified and purified. E. coli mutants defective in the lon protease display a pleiotropic phenotype (7). Two of these phenotypes are sensitivity to DNA-damaging agents and overexpression of capsular polysaccharide. These two phenotypes are due to stabilization of two substrates of the Lon protease, SulA and RcsA, respectively. SulA, an inhibitor of cell division, is induced upon DNA damage (10). In lon+ cells, SulA is degraded very rapidly, with a half-life of less than 2 min; in lon mutant cells, stabilization of SulA results in irreversible filamentation and subsequent cell death (11, 26). RcsA, an unstable positive regulator of genes involved in colanic acid capsular polysaccharide, is degraded by the Lon protease and has a half-life of 5 min in lon+ cells. In lon mutant cells, the accumulation of RcsA (with a half-life of more than 20 min) leads to overexpression of capsular polysaccharide (33, 34). Although lon mutants have profound effects on RcsA and SulA turnover, the residual degradation of SulA (22) and appreciable turnover of RcsA (34) in lon mutant cells led to the prediction that other proteases that can degrade these Lon substrates must exist. The work described here was originally begun to identify this protease(s) and led to the isolation of strains with a phenotype referred to as Alp (alternate Lon protease) (35). Alp strains had the UV resistance and low capsule synthesis of a lon+ strain but were devoid of Lon.
Alp activity was originally detected in a screen of a plasmid genomic library for plasmids that would suppress lon mutant phenotypes. Trempy and Gottesman (35) found that an activity (then designated Alp and now called AlpA), when present in a multicopy plasmid, can suppress both lon mutant phenotypes of sensitivity to DNA-damaging agents and overexpression of capsular polysaccharide. Rapid proteolysis of SulA, as was found in lon+ cells, was found in Δlon cells that carried the AlpA plasmid. This activity, like Lon and Clp, is energy dependent. Further studies revealed that the AlpA open reading frame affected protease activity indirectly, by leading to the inactivation of ssrA (17, 36). Inactivation of ssrA by an insertion mutation mimicked the effect of the Alp plasmid. Thus, the product of the ssrA gene inhibited the expression of the Alp protease activity.
At the time the original Alp work was done, ssrA was known to encode a small stable RNA (10Sa RNA) of unknown function. It has now been shown to encode tmRNA, a molecule with both tRNA-like and mRNA-like properties that acts cotranslationally to add an 11-amino-acid tag to the C termini of proteins whose translation is interrupted or stalled in the absence of a nonsense codon (14). The resulting tagged proteins are then sensitive to degradation by the ClpAP and ClpXP proteases (8). Thus, at first glance, one would expect ssrA mutants, unable to add a degradation signal to polypeptides on stalled ribosomes, to decrease the degradation of certain tagged proteins, contrary to our initial identification of the mutants as promoting a protease activity able to substitute for Lon. Even more puzzling than the role of ssrA was the observation that for full and stable expression of Alp activity, cells also had to harbor a multicopy plasmid carrying a kanamycin resistance element (designated AHA for Alp helper activity) (17).
Since the original publications on the relationship of Alp to ssrA, we and others have shown that ClpYQ, an ATP-dependent protease induced under heat shock conditions, is capable of degrading SulA and RcsA (13, 16, 20, 42). Full suppression of the capsule overproduction or of the UV sensitivity of a lon mutant required overproduction of ClpYQ (42). However, the suppression of capsule synthesis in lon strains, a hallmark of the activity we originally identified genetically and called Alp, was not reversed by mutations in clpY or clpQ (42) and therefore remained unidentified.
The experiments described here were begun with the aim of understanding the basis for the kanamycin resistance gene role and the mechanism of action of Alp. Mutations that bypassed the kanamycin resistance requirement were sought. We report here that a novel allele of dnaJ has the capacity to substitute for AHA and, in fact, can suppress lon phenotypes even in ssrA+ hosts. Studies of this mutant and new information on tmRNA led us to a new understanding of the Alp phenomenon as two separate suppression events, the ClpYQ-dependent degradation of SulA and the inactivation of the capsule synthesis pathway under heat shock stress.
MATERIALS AND METHODS
Bacterial strains, plasmids, and bacteriophages.
All strains used for this study are derivatives of MC4100 (2) and are described in Table 1 or in the text. Derivatives of SG20780 (Δlon cpsB::lacZ) and SG20781 (lon+ cpsB::lacZ) (1) were used in assessing lon phenotypes. Isogenic derivatives of these strains were constructed by P1 transductions (23, 24), as described in Table 1 and the text.
TABLE 1.
Strains used in this study
| Strain | Relevant genotype | Construction, reference, or source |
|---|---|---|
| JK6261 | Δlon cpsB10-lac ssrA::cat | SG20780 + P1 (ssrA::cat); 17 |
| MS101 | Δlon cpsB10-lac ssrA::cat faa (dnaJG232D) | JK6261 + NTG (Lac− MMSr) |
| MS102 | Δlon cpsB10-lac ssrA::cat thr::Tn10 faa | MS101 + P1 (thr::Tn10) |
| MS102 recA | Δlon cpsB10-lac ssrA::cat thr::Tn10 faa recA::kan | MS102 + P1 (recA::kan) |
| MS104 | F′104/MS102 recA | |
| MSG101 | Δlon cpsB10-lac ssrA::cat zaa-101::Tn10 faa+ | MS101 + P1 (Tn10 pool) |
| MSG102 | Δlon cpsB10-lac ssrA::cat zaa-101::Tn10 faa | MS101 + P1 (MSG101) |
| MSG103 | Δlon cpsB10-lac ssrA::cat zaa-101::Tn10 faa | MS101 + P1 (MSG101) |
| NK6024 | pheA::tet ssrA+ | E. coli Genetic Stock Center |
| SG12065 | C600 clpY::cat | Lab strain collection |
| SG12084 | C600 ssrA::cat | Lab strain collection |
| SG20781 | lon+cpsB10::lac | 1 |
| SG20780 | Δlon cpsB10::lac | 1 |
| SG22120 | MC4100 dnaJ::kan | 12 |
| SG22753 | Δlon cpsB10::lac thr::tet faa+ | SG20780 + P1 (MS104) |
| SG22754 | Δlon cpsB10::lac thr::tet faa | SG20780 + P1 (MS104) |
| SG22755 | Δlon cpsB10::lac ssrA::cat thr::tet faa+ | JK6261 + P1 (MS104) |
| SG22756 | Δlon cpsB10::lac ssrA::cat thr::tet faa | JK6261 + P1 (MS104) |
| SG22759 | Δlon cpsB10::lac thr+faa | SG22754 + P1 (SG22120) |
| SG22760 | Δlon cpsB10::lac thr+dnaJ::kan | SG22754 + P1 (SG22120) |
| SG22767 | Δlon cpsB10::lac thr+dnaJ+ | SG22753 + P1 (SG22120) |
| SG22771 | lon+cpsB10::lac thr+dnaJ+ | SG20781 derivative |
| SG22774 | lon+cpsB10::lac thr+dnaJ+ssrA::cat | SG22771 + P1 (SG12084) |
| SG22780 | Δlon cpsB10::lac clpY::cat pheA::tet | SG22783 + P1 (SG12065) |
| SG22781 | Δlon cpsB10::lac clpY::cat pheA::tet faa | SG22784 + P1 (SG12065) |
| SG22782 | Δlon cpsB10::lac pheA::tet clpY::cat dnaJ::kan | SG22785 + P1 (SG12065) |
| SG22783 | Δlon cpsB10::lac pheA::tet | SG22767 + P1 (NK6024) |
| SG22784 | Δlon cpsB10::lac pheA::tet faa | SG22759 + P1 (NK6024) |
| SG22785 | Δlon cpsB10::lac pheA::tet dnaJ::kan | SG22760 + P1 (NK6024) |
| SG22786 | Δlon cpsB10::lac ssrA::cat | SG22783 + P1 (SG12084) |
| SG22787 | Δlon cpsB10::lac ssrA+ | SG22783 + P1 (SG12084) |
| SG22788 | Δlon cpsB10::lac clpY::cat ssrA::cat | SG22780 + P1 (SG12084) |
| SG22789 | Δlon cpsB10::lac clpY::cat ssrA+ | SG22780 + P1 (SG12084) |
| SG22804 | Δlon cpsB10::lac clpY::cat ssrA::cat faa | SG22781 + P1 (SG12084) |
| SG22805 | Δlon cpsB10::lac clpY::cat ssrA+faa | SG22781 + P1 (SG12084) |
| SG22806 | Δlon cpsB10::lac clpY::cat ssrA::cat dnaJ::kan | SG22782 + P1 (SG12084) |
| SG22807 | Δlon cpsB10::lac clpY::cat ssrA+dnaJ::kan | SG22782 + P1 (SG12084) |
| SG22808 | Δlon cpsB10::lac faa ssrA+ | SG22784 + P1 (SG12084) |
| SG22809 | Δlon cpsB10::lac dnaJ::kan ssrA+ | SG22785 + P1 (SG12084) |
| SG22810 | Δlon cpsB10::lac faa ssrA::cat | SG22784 + P1 (SG12084) |
| SG22811 | Δlon cpsB10::lac dnaJ::kan ssrA::cat | SG22785 + P1 (SG12084) |
Mutant isolation and mapping.
The strain JK6261 (17) was mutagenized with nitrosoguanidine (NTG). Cells were grown in LB broth to mid-logarithmic stage; NTG was added at a 1/40 dilution from a stock of 2.5 mg/ml in 95% ethyl alcohol, for a final concentration of 62.5 μg/ml, and incubated at 30°C for 10 min. Cells were centrifuged, washed three times in fresh LB broth, and inoculated into LB broth to grow overnight at 30°C. Dilutions of the overnight culture were plated on MacConkey lactose agar plates and incubated at 30°C. White (Lac−) colonies were purified and checked for their ability to grow on methyl methanesulfonate (MMS) plates (LB agar with 0.025% MMS). About 1 of 100 colonies was Lac−. Isolates that were both Lac− and relatively MMSr (8/80 Lac− colonies tested) were selected for further investigation. The mutants were numbered 1 to 8. The mutant saved for the most extensive further analysis was named MS101 (Table 1).
To characterize and map the mutations, they were first linked to mini-Tn10 insertions. A pool of Tn10 insertions in MC4100, created by infecting MC4100 with λNK561 and pooling the resulting tetracycline-resistant colonies (18, 38), was kindly provided by N. Trun. P1 was grown on the pooled cells and used to transduce the mutant cells, selecting for tetracycline resistance and screening for Lac+ MMSs colonies (wild-type phenotype of JK6261). Candidate Lac+ Tetr derivatives of MS101 were used as donors to grow P1 and to repeat the transduction into MS101; from this process, we identified a Tetr insertion that showed 40% linkage to the mutation. The original Lac+ Tetr insertion was saved as MSG101. The Lac phenotype and the MMS phenotype cosegregated, suggesting that they were due to a single mutation. A Lac− (faa mutant) Tetr and a Lac+ (faa+) Tetr derivative were saved as MSG102 and MSG103, respectively.
Mapping by Hfr and Pl.
MSG102 (faa Tetr) was used as a recipient in Hfr matings with a set of streptomycin-sensitive Hfr donors, each carrying kanamycin-resistant insertions near the origin of transfer (32). Recombinants resistant to both kanamycin and streptomycin were selected for after mating with each Hfr donor, and the recombinants were screened for Tetr. Only in crosses with the donors CAG12204 (KL227; origin of transfer, 6 min, counterclockwise transfer) and CAG12206 (Hfr Hayes; origin of transfer, 96 min, clockwise transfer) were most of the recombinants Tets; this suggested that the tet insertion and the linked faa mutation lay between approximately 95 min and 5 min on the E. coli genome. The tet insertion was further mapped by Pl transduction from MSG103 (Tetr faa+) into a set of recipients carrying kanamycin-resistant transposons at different positions within this region (32). The highest linkage between Tetr and Kanr was found for K80 (zjj-3188, at 99.5 min) and K1 (thr-309, at 0 min). Based on this, a further transduction was carried out with thr::Tn10, which was found to be 50% linked to the faa mutation, suggesting that the faa mutation was close to thr and near the dnaKJ region. A thr::Tn10 faa derivative was saved as MS102.
For further fine-structure mapping of the faa mutation, transducing Kohara phages containing E. coli fragments from the region of the mutation were used (19). Lysates of the phages (Kohara phages 101 to 106 and 673 to 676) were prepared on LE392 (31). We had observed that strains carrying the faa mutant, but not isogenic faa+ derivatives, were temperature sensitive for growth. Note that these strains also carry λ cI+ immunity, associated with the cpsB10::lac insertion (37). Therefore, infection of the Kohara phages was not expected to result in lytic growth but in lysogeny and/or recombination. Cultures of MS101 or MSG102 (both faa mutant strains) were grown in LB broth with maltose and MgSO4, and aliquots were infected with an equal volume of each Kohara phage. After adsorption, 50-μl spots were spotted on LB broth plates and incubated at 42°C overnight.
In the process of mapping the faa mutation by P1 transduction, we found that we were unable to transduce the faa mutation and, in fact, were unable to transduce any markers from the faa mutant host. P1 could lysogenize the strain, but no lysis was observed in spots or after induction. Other phages, including T4 and lambda, were also unable to grow, although they could grow normally on the faa+ transductants, suggesting that these phenotypes were cosegregating with the faa mutation. Therefore, a second copy of the gene was introduced on an F′ plasmid to create a faa+/faa mutant merodiploid strain in the hope that, under these conditions, the strain might become permissive for P1 growth. F′104 carries thr+, known to be tightly linked to the faa region. The F′104 donor CGSC4251 (KL723; his arg) was obtained from the E. coli Genetic Stock Center. The strain was used as a donor in matings with faa mutants carrying either thr::Tn10 or both thr::Tn10 and recA::kan. The donor and recipient strains were grown to mid-logarithmic phase in LB broth and mixed in a 1:1 ratio for 1 h at 32°C. Dilutions from the mating were plated on minimal glucose agar, and Thr+ recombinants were selected. The recA::kan merodiploid did regain its sensitivity to P1 infection and therefore was used as the donor in P1 transductions to introduce the faa mutation into other strains, selecting for Tetr (thr::Tn10) and screening for the faa phenotypes.
Isogenic sets of strains carrying the faa mutation, faa+, or dnaJ::kan were constructed in the following manner. Parental strains were transduced with Pl grown on a merodiploid strain carrying thr::Tn10 and the faa mutant allele [dnaJ(G232D)], selecting for tetracycline resistance and screening for phage resistance and/or temperature sensitivity. Both a temperature-resistant (faa+) and a temperature-sensitive (faa mutant) derivative were saved. Using these as recipients, a subsequent transduction was done from SG22120 (dnaJ::kan thr+), selecting for Thr+ transductants and screening for either kanamycin resistance or phage sensitivity. Transductants that became dnaJ+ were fully phage sensitive; those that carried dnaJ::kan were resistant to lambda derivatives but sensitive to an imm21 dnaJ+-transducing phage. Those that retained the faa mutation [dnaJ(G232D); see below] were resistant to even the imm21 dnaJ+ phage except at very high phage concentrations.
Isogenic derivatives were eventually constructed carrying, in addition to the faa and dnaJ mutations, ssrA::cat insertions and, in some cases, clpY::cat insertions (Table 1). To allow chloramphenicol-resistant markers in both ssrA and clpY to be introduced, pheA::tet was introduced by P1 transduction into the thr+ dnaJ+, dnaJ::kan, or faa derivative. pheA is closely linked to ssrA. clpY::cat was introduced into these strains as needed, selecting for chloramphenicol resistance, and then ssrA::cat was introduced by selecting for Phe+ on glucose minimal plates. Transductants were screened for growth properties (primarily temperature sensitivity) that were characteristic of the ssrA mutant strains, and both ssrA+ and ssrA mutant transductants were saved for further work.
PCR and sequence analysis of the faa region.
In order to identify the precise mutation leading to the faa phenotypes, the faa region was amplified by PCR and sequenced. Three different overlapping fragments were made and sequenced. Primers used for PCR and sequencing extended from Faa1, starting at the 3′ end of talB (at approximately nucleotide 9140) and extending to Faa6 at the 5′ end of nhaA, for a region of 8,300 nucleotides. Sequencing was carried out in the NCI DNA core facility.
Measurement of temperature sensitivity, MMS, and UV sensitivity.
Overnight cultures of the relevant strains grown in LB broth at 30°C were diluted into LB medium and grown to an optical density at 600 nm (OD600) of 0.45. Serial dilutions were made in LB broth, spotted on LB agar plates (with antibiotics where necessary), and incubated at various temperatures overnight. Efficiency of plating was calculated by comparing the titers of colonies at a given temperature to those at 30°C.
In parallel, serial dilutions were also spotted onto LB agar-MMS plates (0.01 to 0.02% MMS in different trials) or onto LB agar plates that were exposed to UV light for various times from 1 to 10 s. Efficiency of plating was estimated from colony formation overnight at 30°C. In experiments with cells carrying pUC18 or pUC4K, or those carrying the ssrA plasmids, cells were grown in the presence of ampicillin and spotted on plates carrying ampicillin. In experiments with cells carrying pJK2-2 (pACYC184 Kanr) (17) as well as the ssrA plasmids, growth and plating were done in the presence of kanamycin and ampicillin.
SulA turnover.
Strains were grown in LB medium at 32°C to an OD600 of 0.3, and MMS was added to a final concentraion of 0.05% (0.1% in some trials) to induce the SOS response and SulA synthesis. Growth was continued for 30 min in MMS. Spectinomycin was added to a final concentration of 100 μg/ml to stop further protein synthesis. At various times after the addition of spectinomycin, samples were taken and precipitated with 5% ice-cold tricarboxylic acid (TCA). Precipitated pellets were treated as previously described (42, 44). Resuspended samples normalized to the OD at time zero were subjected to 12% sodium dodecyl sulfate (SDS)-NuPAGE gels (Invitrogen), transferred, and probed with anti-SulA antibody. To estimate the amounts of SulA and other proteins in the cells, serial dilutions were used for gel electrophoresis and analyzed by Western blotting with appropriate antibodies. The blots were developed with the ECL system (Amersham) and quantified by scanning with an Eagle Eye II scanner (Stratagene, La Jolla, CA).
Heat shock induction measurement.
Overnight cultures were diluted and grown in LB broth at 30°C to an OD of 0.3. Samples were taken and precipitated with 5% TCA under cold conditions. For a heat shock control, cells were shifted to 42°C for 10 min and samples were taken. Samples were used for electrophoresis on 12% SDS-NuPAGE (Invitrogen). The protein gels were stained with a Colloidal Blue kit (Invitrogen). To estimate the amount of the protein, the gels were dried and scanned with an Eagle Eye II scanner. In addition, gels were transferred and used for Western blotting for ClpQ and RcsA. The antibodies for these experiments have been described previously (12, 42).
Measurement of cps-lac expression and RcsA.
Cultures of strains were grown in M63 Casamino Acids (CAA) glucose B1 minimal medium with appropriate antibiotics at 30°C or 32°C, and samples were removed at an OD600 of 0.5 for an assay of β-galactosidase activity. Units are machine units, which are about 25 times lower than Miller units. Samples were also removed for Western blot analysis, as described above, using anti-RcsA antibody (12).
RESULTS
Search for chromosomal mutations affecting Alp activity: isolation of a mutant with an alp Up phenotype.
Alp suppression of lon mutants can be detected in Δlon strains carrying a cpsB::lac fusion. This fusion is expressed at low levels in a lon+ strain (Lac−) but at high levels when RcsA is stabilized in the lon mutant strain (Lac+); the lon mutant strain is also sensitive to SOS induction (sensitive to UV light) relative to the lon+ strain (Fig. 1). When the full Alp phenotype is expressed, the strain becomes Lac− and resistant to UV or SOS-inducing treatments such as MMS (17). However, the full phenotype requires both a mutation in ssrA (ssrA::cat) and a plasmid encoding kanamycin resistance (Fig. 1). Without the plasmid, a strain carrying the Δlon-510 mutation, a cpsB10::lac fusion, and a mutation in ssrA (ssrA::cat) (JK6261) is lac+ and only weakly MMSr (17) (see below).
FIG. 1.
Isolation of faa mutants. The capsule synthesis and UV sensitivity of lon mutants were used to first isolate Alp strains (17, 35) and, in this paper, faa mutants that proved to mimic the Alp phenotype. The status of capsule synthesis was determined from expression of a cps-lac fusion on MacConkey plates; UV sensitivity was determined as described in Materials and Methods. pUC4K is a plasmid derivative of pUC18 carrying kan.
JK6261, without the Kanr plasmid, was mutagenized with NTG, and eight strong Lac− MMSr mutants were isolated (Up phenotype). The ssrA+ allele was reintroduced by P1 transduction with a linked pheA::Tn10 marker, screening for loss of chloramphenicol resistance. All eight mutants also rendered the strain temperature sensitive for growth. Seven of the eight mutants retained the Lac− MMSr phenotype when ssrA+ was introduced. In the eighth mutant, ssrA+ derivatives were partially Lac+ and only moderately resistant to MMS. We selected this mutation to study further, since we were interested in understanding the basis of the ssrA::cat role in suppressing the lon mutation. We designate the new mutation faa (for function affecting alp) and the strain carrying it MS101 (Fig. 1). In retrospect, we suspect that at least some, if not all, of the seven other mutations (not saved) may be alleles of the same gene, since all were temperature sensitive, a phenotype of the faa mutation (see below).
Genetic analysis of the faa mutation.
To map the faa mutation, P1 phage propagated on a pool of Tn10 insertions in MC4100 (18) was transduced into MS101, selecting for Tetr and screening for Lac+ (loss of the faa mutant phenotype). Of four such Lac+ colonies obtained, two became sensitive to chloramphenicol (the resistance marker inserted in ssrA) and were only partially Lac+, as was found previously upon the introduction of ssrA+. Therefore, these transductants contain Tn10 insertions linked to the ssrA+ gene and replaced ssrA::cat with ssrA+. However, the colonies were still temperature sensitive, suggesting that the faa mutation alone confers temperature sensitivity.
Two tetracycline-resistant MMS-sensitive transductants that retained the ssrA::cat marker were identified, consistent with the introduction of faa+ linked to Tetr. Subsequently, the Tn10 insertions were linked to faa mapped by using Hfr conjugation and Pl transduction (see Materials and Methods). Both were close to thr and very close to the dnaKJ operon at 0.2 min on the chromosome.
We attempted to move the faa mutation to a nonmutagenized background by P1 transduction from a Tetr faa mutant recombinant from the transduction described above. However, we found that strains carrying the faa mutation were resistant to phage P1 and the T4 derivative used for generalized transduction; they were also resistant to lambda infection. Therefore, transductions to move the faa mutation required the construction of merodiploids for the faa region.
We did a complementation analysis of faa strains by introducing F′104, a plasmid expected to harbor the faa region, to test whether the faa mutation was dominant or recessive. F′104 was introduced into MS102, a thr::Tn10 faa-1 ssrA::cat cpsB::lac Δlon strain, and its recA::kan derivative by mating, and thr+ exconjugates were checked for the faa mutant phenotype (Lac−). In the rec+ host, most of the exconjugates become Tets and fully Lac+. The loss of tetracycline resistance suggests that there is a strong selection for recombination in this region, leading to marker rescue for both thr (Tets) and faa (Lac+). In a recA::kan derivative, Thr+ transconjugants were moderately Lac+ but not as Lac+ as the faa+ isogenic strain, suggesting that faa is incompletely recessive to the wild-type allele on the F′ plasmid. We were also able to grow P1 on the merodiploid and use it to transduce the faa allele to a new host. Thus, F′104 was able to partially complement all of the defects due to the faa mutation in the merodiploid strains.
P1 grown on two MS102 recA::kan/F′104 isolates (MS104 and MS105) was used to transduce thr::Tn10 into JK6261 (Δlon ssrA::cat cpsB::lac). One out of three tetracycline-resistant transductants obtained became Lac− MMSr(Ts), as expected if the faa mutation was linked to thr::Tn10 and faa was responsible for the full set of phenotypes. The Lac− MMSr(Ts) transductant of JK6261 (named SG22756) and the isogenic Lac+ MMSs(Tr) (named SG22755) strains, as well as equivalent strains made as described in Materials and Methods, were used in the studies described below.
We were able to use the temperature sensitivity of the faa mutation for fine-structure mapping by using phages from the Kohara collection (19). Using Kohara phages carrying DNA in the faa region, we found that Kohara phages 101 and 102 could rescue the temperature sensitivity of the faa mutants, while neighboring phage 103 did not. All temperature-resistant colonies obtained had reverted all the other phenotypes. The approximately 7-kb overlapping region carried by Kohara phages 101 and 102 includes dnaK and dnaJ, the htpY heat shock gene (25), and several unidentified open reading frames.
The 8.3-kb region containing these genes, between talB and nhaA, was sequenced from the isogenic faa+ and faa mutant transductants SG22755 and SG22756. Three mutations were found in the faa mutant strain compared to the wild type. Two mutations were silent changes in yaaI, the gene just upstream of dnaK. The third mutation was a G-to-A change in codon 232 of dnaJ, changing GGT to GAT. This changes a highly conserved glycine to aspartate; we believe this change is responsible for the set of phenotypes, in part because a known dnaJ insertion mutant has similar phenotypes (see below).
Properties of the faa mutation in the presence and absence of ssrA.
The dnaJ(G232D) mutation will be referred to as faa hereafter. The faa mutation was introduced by P1 transduction into a set of strains with and without the ssrA::cat and lon alleles (Materials and Methods; Table 1) and tested for temperature sensitivity, capsule expression, and UV sensitivity (Table 2). In addition, isogenic strains that carried a dnaJ::kan null allele in place of the faa allele were constructed, and the properties of strains carrying this mutation were compared to those of the faa mutant (Table 2). Both dnaJ alleles {dnaJ::kan and faa [dnaJ(G232D)]} rendered cells temperature sensitive and unable to grow bacteriophage lambda, consistent with previous work on dnaJ. More unexpectedly, phage P1 also grew poorly in these strains, although it grew more poorly in the faa mutant (data not shown). Although DnaJ and DnaK were known to play an essential role in the replication of the lysogenic form of bacteriophage P1 (39), we are not aware of other reports of a role for these chaperones in the lytic growth of phage P1. The target for DnaJ/DnaK function in lytic growth has not been identified.
TABLE 2.
Suppression of lon phenotypes in ssrA and faa strains
| Strain | Relevant genotype | Lac phenotypea | UV resistanceb | EOPc
|
||
|---|---|---|---|---|---|---|
| 37°C | 39°C | 42°C | ||||
| SG222771 | lon+ssrA+dnaJ+ | − | 0.2 | 1 | 1 | 1 |
| SG22787 | Δlon | ++ | 0.001 | 1 | 1 | 1 |
| SG22786 | Δlon ssrA::cat | + | 0.007 | 1 | 1 | 1 |
| SG22786/pUC18 | Δlon ssrA::cat/pUC18 | + | 0.006 | .1 | 1 | 1 |
| SG22786/pUC4K | Δlon ssrA::cat/pUC4K | − | 0.06 | 1 | 1 | 0.001 |
| SG22810 | Δlon ssrA::cat faa | − | 0.2 | 0.001 | 0.001 | 0.0001 |
| SG22811 | Δlon ssrA::cat dnaJ::kan | − | 0.03 | 0.001 | 0.001 | 0.0001 |
| SG22808 | Δlon faa | − | 0.3 | 1 | 0.01 | 0.001 |
| SG22809 | Δlon dnaJ::kan | − | 0.03 | 0.05 | 0.001 | 0.0001 |
Phenotypes were determined from the appearance of colonies on MacConkey lactose plates after overnight incubation at 30°C. −, Lac− strains; +, Lac+ strains; ++, strongly Lac+ strains.
EOP values were obtained from 3-s doses. Cultures grown at 30°C were spotted on LB broth (or LB ampicillin plates where appropriate) and exposed to UV light (dose, 0.2 J/m2/s).
Cultures grown at 32°C were diluted and spotted on LB broth (or LB ampicillin plates where appropriate) and then grown at 37, 39, or 42°C, as indicated.
Combining either faa or dnaJ::kan with a mutation in ssrA caused more severe temperature sensitivity, suggesting that these dnaJ alleles and the ssrA mutation might affect the same pathway (Table 2). We note that, in previous work, we found that the alp strain (Δlon ssrA::cat/Kanr) was also somewhat temperature sensitive, which we confirm here. While the Alp strain (SG22786/pUC4K) had an efficiency of plating (EOP) of 1 at 39°C, it had an EOP of 0.001 at 42°C (Table 2, line 5). The parental strain, or the strain with a vector not carrying the kanamycin resistance gene (pUC18), had an EOP of 1 at 42°C (Table 2, line 4).
Heat shock induction and the involvement of ClpYQ.
dnaJ mutations express heat shock proteins at an elevated level (43). The identification of the faa mutation as an allele of dnaJ suggested that induction of the heat shock response, and therefore participation of other heat shock-induced proteases, such as ClpYQ, might be involved in suppression of lon mutants in the Alp strains. Because ClpYQ overproduction is known to suppress lon mutants (16, 42), we had considered this possibility earlier. However, when mutant clpY or clpQ was introduced into the Alp+ strain, the capsule phenotype (low capsule expression) was not suppressed (42).
We reconsidered the interpretation of those experiments, given the finding of an Alp-like phenotype for the faa mutation and based on the observations we had made in other work on the effect of a dnaJ::kan mutation on capsule expression (discussed below). First, we asked if ClpYQ played a role in the suppression of the UV resistance (rather than capsule synthesis) in Alp and lon faa strains. Figure 2 shows that, in fact, ClpYQ is essential for UV resistance in these mutants. lon dnaJ::kan, lon faa, and lon ssrA/pUC4K strains were all more UV resistant than the lon mutant parent strain (Fig. 2; Table 2), and all clpY mutant derivatives were 100 to 1,000 times more UV sensitive than their clpY+ parents (Fig. 2). This is consistent with a major role for ClpYQ in the degradation of SulA and therefore in the UV resistance of these strains.
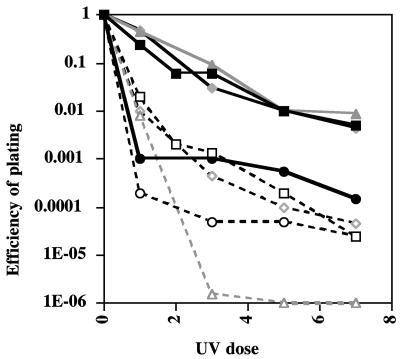
FIG. 2.
Reversal of UV resistance phenotype in clpY mutants. Cells were grown and exposed to UV light as described in Materials and Methods. The UV dose is expressed in seconds. EOP is shown for four pairs of clpY+ (filled symbols) and clpY::cat (open symbols) strains. Circles, SG22787 (Δlon-510 clpY+) and SG22789 (Δlon-510 clpY::cat); squares, SG22786/pUC4K (Δlon-510 ssrA::cat clpY+/pUC4K, original Alp strain) and SG22788/pUC4K (Δlon-510 ssrA::cat clpY::cat/pUC4K); triangles, SG22808 (Δlon-510 faa clpY+) and SG22805 (Δlon-510 faa clpY::cat); and diamonds, SG22809 (Δlon-510 dnaJ::kan clpY+) and SG22807 (Δlon-510 dnaJ::kan clpY::cat). Results are representative of three separate trials on these same strains.
Mutating clpY also increased the UV sensitivity of the lon mutant strain (Fig. 2). This extends our previous observations of a role for ClpYQ in SulA degradation at higher temperature (42) and confirms a role for ClpYQ in SulA degradation under normal growth conditions (13, 30).
If ClpYQ causes UV resistance by the degradation of SulA in the Alp strains but not in lon mutant strains that are wild type for ssrA and dnaJ, it suggests that ClpYQ is induced in the Alp strains. ClpYQ is known to be heat shock regulated (3), and dnaJ mutants are known to perturb heat shock regulation (43). However, heat shock induction has not previously been described for ssrA mutants. We examined heat shock induction in the faa, dnaJ::kan, and Alp strains in a number of ways. The quantitation of ClpQ levels, one measure of heat shock induction, is shown in Fig. 3. A 10-min heat shock increased the level of ClpQ about 2.5 times. Under low-temperature conditions (30°C), all of the strains that showed ClpYQ-dependent UV resistance (the lon, dnaJ, and ssrA/pUC4K strains [Table 2; Fig. 2] and the faa ssrA strain [Table 2]) had ClpQ levels significantly higher than those achieved after heat shock. Even the strain with ssrA alone or with a vector control had significant induction of heat shock proteins (Fig. 3, last column). In a repeat of this experiment comparing the lon ssrA/pUC18 and lon ssrA/pUC4K strains, the pUC4K strain reproducibly had 1.7- to 1.8-times-higher ClpQ levels than those of the pUC18 derivatives (data not shown).
FIG. 3.
ClpQ levels in UV-resistant strains. Strains were grown in LB broth at 30°C to an OD600 of 0.5, and samples were taken for SDS gel electrophoresis and Western blotting, as described in Materials and Methods. Levels in the gels were quantitated as described in Materials and Methods, and the results were normalized to the level of ClpQ in the lon mutant grown at 30°C, set equal to 1 (column 1). lon, heat; results after 10 min of heat treatment. Results are the averages of at least two determinations. The following strains were used: SG22787 (Δlon-510), SG22808 (Δlon-510 faa), SG22809 (Δlon-510 dnaJ::kan), SG22786 (Δlon-510 ssrA::cat), SG22786/pUC18, SG22786/pUC4K, SG22810 (Δlon-510 faa ssrA::cat), and SG22774 (lon+ ssrA::cat).
We confirmed that this induction was not specific to ClpQ but was also true for other heat shock proteins, first by examining the levels of the major heat shock proteins, DnaK and GroEL, by Coomassie staining; these levels were also elevated in the ssrA mutants (data not shown). In addition, we used reverse transcription-PCR to measure the levels of the message of the small heat shock proteins IbpA and IbpB. Again, the levels of both messages were significantly induced in a lon ssrA mutant although not as much as those in the lon faa mutant (data not shown). Thus, the induction of heat shock in the ssrA mutant is general and not confined to ClpYQ.
Fate of SulA in faa and ALP strains.
The requirement for ClpYQ for UV resistance of Alp and faa strains strongly suggests that degradation of SulA by ClpYQ leads to UV resistance. We had previously demonstrated that SulA is degraded more rapidly in Alp strains (35). We repeated that measurement and also examined SulA turnover in the faa mutant and in clpY mutant derivatives of these strains. The expression of sulA was induced at early log phase by the addition of 0.05% MMS. After 30 min of induction, further protein synthesis was stopped by the addition of spectinomycin (100 μg/ml). TCA-precipitated samples taken at various time points after the addition of spectinomycin were analyzed by Western blotting with anti-SulA antibody.
The results generally agreed with the results of the UV sensitivity measurements. In a lon strain, SulA was relatively stable (half-life of 20 to 25 min) (Fig. 4A and B), consistent with the UV sensitivity of that strain (Table 2; Fig. 2). In this host, mutation of clpY further stabilized SulA (half-life of >50 min) (Fig. 4); the clpY strain was also more UV sensitive (Fig. 2). In the lon ssrA/pUC4K strain, SulA could barely be detected, consistent with rapid degradation (as previously observed) (Fig. 4B, bottom panel); the UV resistance of this strain can be explained by the low levels of SulA (Fig. 2; Table 2). As expected if rapid SulA degradation is due to ClpYQ, SulA was present and stable in the clpY derivative of the lon ssrA/pUC4K strain (Fig. 4B). Consistent with this result, the clpY derivative was more UV sensitive than the clpY+ parent (Fig. 2).
FIG. 4.
SulA turnover. Cells were grown, induced, and treated as described in Materials and Methods. (A) SulA turnover, normalized to that at 15 min after spectinomycin addition, from the data in Fig. 5B. Filled squares, SG22787 (Δlon-510 clpY+); open squares, SG22789 (Δlon-510 clpY::cat); filled triangles, SG22808 (Δlon-510 faa clpY+); and open triangles, SG22805 (Δlon-510 faa clpY::cat). (B) Western blots of protein turnover after addition of spectinomycin. Because the antibiotic takes a few minutes to work, the first point shown is 15 min after spectinomycin addition. Bottom left, SG22786/pUC4K (Δlon-510 ssrA::cat+/pUC4K) (original Alp strain); bottom right, SG22788/pUC4K (Δlon-510 ssrA::cat clpY::cat/pUC4K). Other strains were as defined for panel A.
The lon faa strain is somewhat more complicated to understand. The lon faa strain is significantly more UV resistant than a lon mutant (Table 2; Fig. 2). However, in this lon faa strain, SulA was degraded at a rate comparable to that in the lon strain (20-min half-life) (Fig. 4A and B). In another turnover experiment (data not shown), SulA was degraded slightly more rapidly in the lon faa strain. Why is the lon faa strain more UV resistant? The accumulation of SulA at time zero was reduced about 2.5-fold (Fig. 4B), for reasons that are not clear. Even when SulA turnover is further blocked, as in the lon faa clpY strain (Fig. 4), the level of accumulation was still lower than in the lon or lon clpY strain, suggesting that the lower level of accumulation may reflect less synthesis. However, the lon faa clpY strain was very UV sensitive (Fig. 2). Therefore, stabilizing the somewhat reduced level of SulA is sufficient to restore UV sensitivity.
Thus, the clpY mutant, which restores UV sensitivity to the lon faa and lon ssrA/pUC4K strains, also stabilizes SulA. These results are consistent with the levels of UV resistance of the Alp and lon faa strains resulting at least in part from the ClpYQ-dependent degradation of SulA. In addition, it is possible that some SulA is not functional (and therefore does not contribute to UV sensitivity) in the faa mutant.
Fate of RcsA.
RcsA, a positive regulator of the cps genes, is a substrate of Lon. The level of cps gene expression is high in lon mutants because RcsA is stable (and therefore accumulates) and low in lon+ strains because RcsA is rapidly degraded (34). We had observed that strains carrying a dnaJ::kan mutation did not express the cps operon, even in a lon mutant (reference 12; Y. Jubete, M. Maurizi, and S. Gottesman, unpublished data). Studies of this phenomenon suggest that RcsA was present but inactive, leading to the conclusion that RcsA requires DnaJ/DnaK for activity. With the identification of the faa mutation as an allele of dnaJ, we considered the possibility that the suppression of cps synthesis by the faa mutation is due to inactive RcsA and that a similar explanation might be relevant to the original Alp strains. If that is the case, clpY mutations would not be expected to relieve the problem, since RcsA would still be present but inactive. If RcsA is aggregated, it might not be accessible to degradation by ClpYQ in any case.
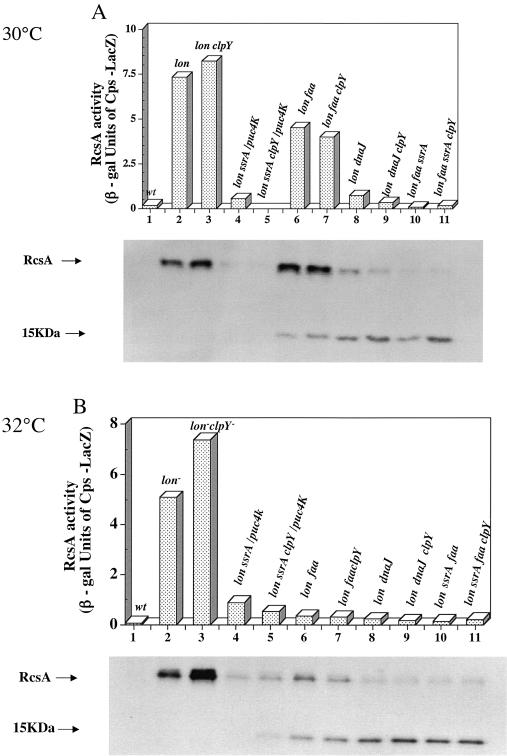
This model predicts that RcsA would be present but inactive in the Alp and faa strains, with or without ClpY. We measured cps-lac expression and RcsA accumulation in these strains at 30°C and 32°C (Fig. 5). In the control lon mutant and lon clpY mutant strains, cps-lac expression levels were high at both temperatures, as were RcsA levels, as expected (Fig. 5, lanes 2 and 3). Both cps-lac expression and RcsA levels were slightly higher in the clpY mutant, consistent with some degradation of RcsA by ClpYQ under these conditions. In the Alp strains (lon ssrA/pUC4K), with or without clpY, the level of cps-lac expression was low as was the level of accumulation of RcsA (28% of the lon mutant level in one trial and below the level of detection in another) (Fig. 5, lanes 4 and 5). Thus, there is decreased accumulation of RcsA, rather than evidence of accumulation of inactive RcsA, in these strains. This result could be explained either by rapid degradation by something other than Lon and ClpYQ or by decreased synthesis (or both). At 32°C, the levels of RcsA were somewhat higher, and some of the cleaved form characteristic of inactive RcsA (see below) was found, suggesting that at this temperature, ClpYQ may contribute to RcsA turnover, but the stable RcsA accumulating in the clpY mutant remains inactive. Either low levels of RcsA or inactive RcsA would explain the failure to see suppression of the lon ssrA/pUC4K strain by a clpY mutation on lactose MacConkey plates, usually tested at 32°C (42).
FIG. 5.
RcsA accumulation and activity. Activity of cps-lac fusion after growth at 30°C (A) and at 32°C (B) in M63 glucose CAA and Western blot of RcsA accumulation. Cells were grown to an OD600 of 0.5, and samples were taken for Western blotting and for an assay of the cps-lac fusion. Lanes 1, SG22771 (lon+); lanes 2, SG22787 (lon mutant); lanes 3, SG22789 (lon clpY); lanes 4, SG22786/pUC4K (lon ssrA/pUC4K); lanes 5, SG22788/pUC4K (lon ssrA clpY/pUC4K); lanes 6, SG22808 (lon faa); lanes 7, SG22805 (lon faa clpY); lanes 8, SG22809 (lon dnaJ::kan); lanes 9, SG22807 (lon dnaJ::kan clpY); lanes 10, SG22810 (lon faa ssrA); and lanes 11, SG22804 (lon faa ssrA clpY). β gal, β-galactosidase.
There was a difference between lon faa and lon dnaJ strains as a function of temperature. For the lon dnaJ strain, the level of cps-lac expression was low at both temperatures (Fig. 5, lanes 8 and 9). RcsA levels in the dnaJ::kan strains were moderate (lower than in the lon mutant but higher than in the lon ssrA/pUC4K derivatives), but most of the protein was present as a truncated band. clpY mutants had relatively little effect on RcsA levels. Therefore, consistent with previous observations, dnaJ::kan leads to accumulation of inactive RcsA; the protein may be resistant to degradation by ClpYQ because it is aggregated (12). We do not know what cleaves RcsA or whether the cleavage is a consequence of aggregation or is the primary cause of inactivation of RcsA. The lon faa strain appears to act more like the lon mutant at 30°C and like the lon dnaJ::kan strain at 32°C, suggesting that this point mutant is somewhat temperature sensitive for DnaJ activity. At 30°C, the level of cps-lac expression was high (62% of that of the wild type); however, it was low at 32°C (6% of that of the wild type) (Fig. 5, lanes 6 and 7). RcsA levels in the lon faa strains were comparable to those in the lon dnaJ::kan mutant strains, but the proportion of RcsA present in the truncated form was higher at 32°C (inactive protein) than at 30°C (active protein). Overall, these results demonstrate that, unlike SulA, the suppression of cps::lac expression in dnaJ or faa mutants is due to inactive RcsA rather than ClpYQ-dependent degradation.
In other experiments (Fig. 5, lanes 10 and 11), we found that introducing the ssrA mutation into the lon faa strain led to low activity even at 30°C; consistent with the results with the faa strain, RcsA was primarily cleaved at both 30 and 32°C. This was not affected significantly by a clpY mutation. The lon faa ssrA combination also produced the highest level of heat shock induction (Fig. 3). We believe that, in these strains, the ssrA mutant stimulation of heat shock induction exacerbates the defect in the faa mutant strain, as if the temperature had been raised.
Basis for ssrA induction of heat shock proteins.
The basis for induction of the heat shock response in dnaJ mutants is believed to be the loss of the rapid degradation of sigma 32 (43). DnaJ/DnaK/GrpE are needed for proper regulation of the heat shock response, probably because they help remove sigma 32 from the core RNA polymerase, making it accessible to the FtsH protease. How then do ssrA mutants cause heat shock induction?
One model would suggest that abnormal protein accumulation is the problem. In ssrA mutants, proteins with incomplete C termini that would otherwise be tagged by tmRNA and degraded by the Clp proteases may accumulate in the cell and become targets for both chaperones and other proteases. The accumulation of unfolded or misfolded proteins is known to induce the heat shock response (28). Possibly the absence of Lon protease, known to degrade many misfolded proteins, also contributes to the accumulation of these proteins. However, Western blots of lon+ and lon+ ssrA strains showed a 2.9-fold increase in levels of ClpYQ, only slightly less than that seen in the lon mutant strain (Fig. 3).
Another prediction of this model is that the ssrA mutant should not be complemented by ssrA derivatives that append nondegradable tags to proteins. This was tested with a set of four plasmids, one carrying the wild-type ssrA gene and two carrying ssrA derivatives, ssrA(DD) and ssrA0, capable of cotranslational tagging but resulting in a released protein that is not recognized by ClpXP or ClpAP (40). The final ssrA derivative, ssrA(UG), is nonchargeable and should act like a null allele (40). These plasmids were introduced into a lon ssrA strain carrying a compatible Kanr plasmid, pJK2-2, that provides the Alp helper activity (17). UV sensitivity, cps-lac expression, and RcsA and ClpQ accumulation were determined for these strains (Fig. 6; data not shown). The results were similar for both UV sensitivity and cps-lac expression. In both cases, the plasmids bearing stable tags, ssrA0 and ssrA(DD), complemented to similar extents as the ssrA+ parent, while the strain with the null allele ssrA(UG) retained the properties of the ssrA mutant parent. The results for the ssrA(UG) strain were indistinguishable from those for the strain without an ssrA plasmid (not shown) and were very similar to those previously seen for a lon ssrA strain carrying the pUC4K plasmid (Fig. 2).
FIG. 6.
Complementation of ssrA::cat/pUC4K. A set of plasmids carrying ssrA derivatives was introduced into SG22786/pJK2-2 (Δlon-510 ssrA::cat/pACYC184-Kanr) and assayed for cps-lac expression, RcsA levels, and UV sensitivity, as described in Materials and Methods. Top left panel, cps-lac activity. Lane 1, ssrA+ plasmid; lane 2, ssrA(UG) plasmid; lane 3, ssrA(DD) plasmid; and lane 4, ssrA0 plasmid. The ssrA(UG) allele cannot be charged and acts as a negative control that does not interact with stalled ribosomes at all (40). The ssrA(DD) and ssrA0 derivatives add nondegradable tags to proteins but should release stalled ribosomes (40). Bottom left panel, RcsA levels (determined as described in Materials and Methods). Right panel, UV sensitivity. Cells were grown and exposed to UV light as described in Materials and Methods. Open squares, ssrA+ plasmid; filled diamonds, ssrA(UG) plasmid; open circles, ssrA(DD) plasmid; and filled circles, ssrA0 plasmid. β gal, β-galactosidase.
Accumulation of RcsA paralleled cps-lac expression, confirming the reduced RcsA levels in an ssrA host carrying a Kanr plasmid, even with a different plasmid than in Fig. 5, and demonstrating that suppression of the defect in cps-lac expression is accompanied by higher levels of RcsA. These results suggest, somewhat surprisingly, that it is a direct or indirect consequence of ribosome jamming, rather than the accumulation of released but nondegraded proteins, that leads to the suppression of lon phenotypes via heat shock induction. ClpQ levels for these four strains generally followed the expected pattern; they were almost twofold higher in the ssrA(UG) strain than in the ssrA+ strain and were intermediate in the two other strains (data not shown).
DISCUSSION
The work described here demonstrates a new pathway for induction of the heat shock response and the complex effects of chaperones and proteases on specific protein substrates. The results are summarized in Table 3; each section of the table demonstrates a somewhat different outcome of heat shock induction. Both the Alp strain and the faa mutant cause heat shock induction but by different mechanisms. ClpYQ is known to be induced as part of the heat shock response and has previously been shown to be capable of degrading both SulA and RcsA (13, 16, 42). Therefore, heat shock induction causes higher levels of ClpQ (Fig. 3) and, presumably, ClpY. In the Alp strain, this results in UV resistance due to increased ClpYQ-dependent degradation of SulA. In the lon faa strain, we observed modestly decreased synthesis along with ClpYQ-dependent degradation, again resulting in UV resistance. Suppression of capsule synthesis in these two strains reflects very different, and not entirely understood, mechanisms. In the Alp strain, RcsA is rapidly degraded or synthesis is significantly decreased in a manner that is mostly ClpYQ independent. In the lon faa strain, RcsA accumulates but is inactive.
TABLE 3.
Heat shock responses of SulA and RcsA
| Protein | Effect(s) of heat shock induced by:
|
|
|---|---|---|
| Alp strain (lon ssrA/pUC4K)a | dnaJ strains (lon faa)b | |
| SulA | Increased ClpYQ-dependent degradation | Decreased synthesis and ClpYQ-dependent degradation |
| RcsA | Increased ClpYQ-independent degradation and/or decreased synthesis | Inactive protein (cleaved) |
The Alp strain induces heat shock by ribosome stalling.
The dnaJ mutant strains induce heat shock by sigma 32 stabilization.
Heat shock induction by ssrA mutants.
Induction of heat shock by the faa mutant was not surprising. dnaJ mutants have long been known to perturb heat shock regulation, leading to increased amounts of the heat shock proteins (43). The faa mutant described here is an allele of dnaJ that also induces heat shock (Fig. 3).
We report here that ssrA mutants also induce heat shock. In ssrA mutants, the ClpQ heat shock protein was induced to a higher level than that found after 10 min of heat treatment (Fig. 3). ssrA encodes tmRNA, an important component of quality control of translation. When an mRNA ends without a translation stop codon or translation is paused for one of a number of reasons, tmRNA directs the addition of an 11-amino-acid tag to the C terminus of the translating polypeptide, allowing release from the ribosome and targeting for proteolysis by ClpXP and other proteases (14). Therefore, heat shock induction could reflect ribosome pausing or stalling not relieved by tmRNA and/or accumulation of protein fragments that are not rapidly degraded. These mechanisms can be distinguished by the use of ssrA variants that are able to relieve ribosome stalling but add peptide tags not recognized by the protease (41). Our results (Fig. 6) suggest that induction of heat shock in ssrA mutants is tied to the ribosome pausing that is likely to occur in the absence of tmRNA. Both phenotypes of the ssrA mutant strain are complemented by plasmids encoding ssrA derivatives that release jammed ribosomes but that should still accumulate nondegradable protein fragments.
Two major mechanisms are known to contribute to induction of the heat shock response: regulation of translation and regulation of sigma 32 stability (43). The temperature-sensitive secondary structure of the rpoH mRNA interferes with translation of sigma 32 at low temperatures but not at high temperatures (27). Possibly the ribosomal stalling associated with ssrA mutants disrupts this secondary structure, specifically allowing additional rounds of sigma 32 translation. This model suggests that other mechanisms that might cause ribosome pausing would have similar effects. In a recent study of the global transcriptional effects of antibiotics acting on the translation process, heat shock was found to be induced with a number of antibiotics, particularly with azaleucine, which is believed to mischarge leu-tRNA (29), possibly leading to stalling at leucine codons. We noted a number of such codons in the region of the message known to be involved in translational regulation (27).
The other major regulatory mechanism of sigma 32 accumulation is regulated changes in the stability of the protein, dependent upon FtsH degradation and modulated by DnaJ/DnaK availability. It is this mechanism that is affected by dnaJ mutants (43). Possibly the absence of ssrA causes the accumulation of partially translated proteins, for some of which there may be cotranslational protein folding; in this model, these protein fragments would bind DnaJ and DnaK efficiently while on the ribosome but not if released, possibly because they are exported proteins. The additivity of heat shock induction by an ssrA mutant in combination with a dnaJ mutant (Fig. 3; temperature sensitivity of SG22810 and SG22811 in Table 2) supports a direct effect on sigma 32 translation rather than an effect on DnaJ and DnaK availability, since DnaJ is absent or defective in these strains.
SulA and RcsA are treated differently.
The induction of the heat shock protease ClpYQ is sufficient to explain the suppression of the UV sensitivity of a lon mutant; clpY mutants restore UV sensitivity and increase the half-life of SulA significantly (Table 2; Fig. 2). However, our results suggest that suppression of capsule overproduction is not due to ClpYQ-dependent degradation, although overproduction of ClpYQ from a plasmid can suppress capsule overproduction (42). RcsA, a transcriptional regulator, is the Lon substrate that is responsible for the capsule overproduction phenotype (34). We have previously noted that RcsA is very prone to aggregation (12). In dnaJ mutants (lon dnaJ::kan or lon faa), RcsA accumulates in spite of the induction of ClpYQ (Fig. 5); a clpY mutation does not restore capsule production. We suggest that, in this case, aggregation is more rapid than ClpYQ-dependent degradation. As a result, while RcsA accumulates, it is inactive, so capsule is not made (Fig. 5). In addition, a portion of RcsA is subject to cleavage by an unknown protease to release a 15-kDa product. This cleavage is seen in dnaJ mutants or in wild-type cells incubated briefly at high temperature; it is more pronounced in cells grown in rich medium than in cells grown in minimal medium (data not shown). The inverse correlation of cleavage and activity is most clear by comparing the RcsA activities and amounts in a lon faa strain at 30°C and 32°C (Fig. 5, lanes 6 and 7); RcsA is primarily uncleaved and active at 30°C but is both inactive and mostly cleaved at 32°C. We do not know if these changes in the efficiency of RcsA cleavage reflect differences in the levels of the protease or differences in the state of RcsA. We also do not know if the remaining fragment of RcsA has any biological activity. Certainly capsule synthesis is off in all cells in which we see the cleaved product accumulate, but the cleavage may well be secondary to RcsA inactivation rather than the cause of it. However, it is clear that mutating clpY does not restore capsule production and does not significantly change the amounts or cleavage of RcsA (Fig. 5). In previous experiments in which RcsA was overproduced in a dnaJ mutant, we demonstrated that it became aggregated (12). In those experiments, no truncated band was observed, but the electrophoresis and transfer conditions may have interfered with its detection.
In a recent paper by Kuo and coworkers (20), effects of ClpYQ on capsule synthesis and RcsA accumulation were found, primarily when both ClpY and ClpQ were overproduced. Under those conditions, likely to reflect even higher levels of the ClpYQ protease than under the heat shock conditions studied here, it is certainly possible that RcsA degradation occurs more quickly than aggregation. Since Kuo et al. did not directly measure the turnover of RcsA, we cannot easily compare their results with ours.
We still do not fully understand the reasons for suppression of capsule synthesis in the Alp strain. In Alp strains (lon ssrA/pUC4K), RcsA accumulates to only 28% of the level found in the lon strain, and this accumulation is not significantly increased in a clpY mutant (Fig. 5, lanes 4 and 5). We did not detect significant amounts of cleaved protein, although a bit was detected in the clpY mutant (Fig. 5) (32°C). The same decrease in RcsA accumulation is evident in the experiments for which results are shown in Fig. 6; the RcsA level in the ssrA null strain was >5-fold lower than in the complemented strains. This low accumulation of RcsA is reversed, albeit with inactive protein, if a faa or dnaJ mutation is introduced into the Alp strain (data not shown), suggesting that under heat shock induction conditions, RcsA is degraded by yet other proteases in the absence of Lon and ClpYQ.
The role of chaperones in the presentation of substrates to proteases is complex. In some cases, chaperone mutants lead to slower degradation, while in other cases, the mutants show more rapid degradation (15). The results presented here agree with and extend our previous suggestion that chaperones may only indirectly help protein degradation by keeping protein substrates in a soluble state (9). We predict that, in cases where the dnaJ or dnaK allele speeds degradation, the degradation may well be due to higher levels of the heat shock proteases, such as Lon and ClpYQ. In cases where the alleles slow degradation, substrates may become aggregated and inaccessible to the proteases in spite of the higher protease levels.
We were led to understand that ssrA mutants cause heat shock induction indirectly by the isolation of the faa mutation, an unusual allele of dnaJ. The faa allele has two noteworthy characteristics beyond those found in a dnaJ null mutant. It blocks lytic P1 growth, and it is partially dominant to the wild-type gene. This dominance is sufficient to block the growth of an incoming lambda that carries the wild-type gene. The mutation, G232D, is in C-terminal domain I of DnaJ, based on sequence homology between DnaJ and the yeast homolog Ydj1, for which a structure complexed with a peptide substrate is available (21; S. Wickner, unpublished observations). In contrast to the behavior of strains containing G232D, a deletion in DnaJ extending from amino acids 230 to 238 resulted in only slightly decreased DnaJ activity in vivo and in vitro (4). Pl is known to require the action of DnaJ/DnaK/GrpE for activation of RepA for plasmid growth but had not previously been known to require these chaperones for lytic growth. We do not know which target(s) is responsible for this requirement or the characteristics of this mutation that make it more defective for P1 growth than the null mutation. It is tempting to speculate that this mutation leads to binding and failure to rapidly release some substrates, including a P1 protein that may require chaperone activity for function. This would be consistent with the partial dominance of this mutation as well.
A remaining mystery, and the one that inspired the experiments described here, is the function of the kanamycin resistance gene on the pUC4K plasmid in promoting SulA and RcsA degradation (17). The phenotype is independent of kanamycin treatment and independent of the plasmid backbone (pBR322 or pACYC184) but requires multiple copies of the plasmid. Therefore, we believe that multiple copies of the Kanr gene, rather than anything else about the plasmid, are necessary. The kanamycin resistance determinant encodes an aminoglycoside phosphotranferase, inactivating kanamycin by phosphorylating it. Since kanamycin itself acts at the ribosome, possibly the resistance enzyme can also recognize (or mimic) some part of the ribosome. We could not detect any induction of heat shock by the pUC4K Kanr plasmid in a Δlon ssrA+ strain, suggesting that it accentuates the ssrA effect rather than doing anything on its own (for instance, encoding a misfolded protein). Consistent with a helping effect with ssrA, there was a more consistent increase in heat shock induction for the pUC4K plasmid than for the pUC18 plasmid in a lon ssrA host. If, as we have suggested here, the ssrA induction of heat shock occurs as a consequence of stalled ribosomes, the Kanr gene product may act at the same step, either causing stalling or somehow blocking alternative mechanisms for releasing stalling. This should be possible to test in the future.
The results reported here provide some explanation for the Alp phenotype and demonstrate that this phenotype is in part due to different behaviors for the two Lon substrates, RcsA and SulA, when heat shock is induced. These results provide a new example of the delicate balance between proper protein folding, aggregation, and degradation, particularly for these naturally unstable Lon substrates, and provide new insight into the physiological importance of tmRNA in relieving stalled ribosomes.
Acknowledgments
We thank Sue Wickner for performing the modeling of the dnaJ(G232D) mutation and for comments on the manuscript and Michael Maurizi, Sue Wickner, and members of their laboratories as well as other members of our laboratories for helpful discussions and advice throughout this work.
Hussain Munavar was supported by the S. N. and S. Pradhan Fellowship for a portion of this work and by the Fogarty International Center for another portion.
REFERENCES
- 1.Brill, J. A., C. Quinlan-Walshe, and S. Gottesman. 1988. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 170:2599-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadaban, M. J., and S. N. Cohen. 1979. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc. Natl. Acad. Sci. USA 76:4530-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang, S.-E., I. G. P. B. Burland, D. L. Daniels, and F. R. Blattner. 1993. Sequence analysis of four new heat-shock genes constituting the hslTS/ibpAB and hslVU operons in Escherichia coli. Gene 134:1-6. [DOI] [PubMed] [Google Scholar]
- 4.Goffin, L., and C. Georgopoulos. 1998. Genetic and biochemical characterization of mutations affecting the carboxy-terminal domain of the Escherichia coli molecular chaperone DnaJ. Mol. Microbiol. 30:329-340. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman, S. 2003. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19:565-587. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman, S. 1987. Regulation by proteolysis, p. 1308-1312. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 8.Gottesman, S., E. Roche, Y.-N. Zhou, and R. T. Sauer. 1998. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12:1338-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman, S., S. Wickner, and M. R. Maurizi. 1997. Protein quality control: triage by chaperones and proteases. Genes Dev. 11:815-823. [DOI] [PubMed] [Google Scholar]
- 10.Huisman, O., and R. D'Ari. 1981. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature 290:797-799. [DOI] [PubMed] [Google Scholar]
- 11.Huisman, O., R. D'Ari, and S. Gottesman. 1984. Cell division control in Escherichia coli: specific induction of the SOS SfiA protein is sufficient to block septation. Proc. Natl. Acad. Sci. USA 81:4490-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jubete, Y., M. R. Maurizi, and S. Gottesman. 1996. Role of the heat shock protein DnaJ in the Lon-dependent degradation of naturally unstable proteins. J. Biol. Chem. 271:30798-30803. [DOI] [PubMed] [Google Scholar]
- 13.Kanemori, M., H. Yanagi, and T. Yura. 1999. The ATP-dependent HslVU/ClpQY protease participates in turnover of cell division inhibitor SulA in Escherichia coli. J. Bacteriol. 181:3674-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keiler, K. C., P. R. H. Waller, and R. T. Sauer. 1996. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science 271:990-993. [DOI] [PubMed] [Google Scholar]
- 15.Keller, J. A., and L. D. Simon. 1988. Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol. Microbiol. 2:31-41. [DOI] [PubMed] [Google Scholar]
- 16.Khattar, M. M. 1997. Overexpression of the hslVU operon suppresses SOS-mediated inhibition of cell division in Escherichia coli. FEBS Lett. 414:402-404. [DOI] [PubMed] [Google Scholar]
- 17.Kirby, J. E., J. E. Trempy, and S. Gottesman. 1994. Excision of a P4-like cryptic prophage leads to Alp protease expression in Escherichia coli. J. Bacteriol. 176:2068-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleckner, N., J. Bender, and S. Gottesman. 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol. 204:139-180. [DOI] [PubMed] [Google Scholar]
- 19.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-508. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, M. S., K. P. Chen, and W. F. Wu. 2004. Regulation of RcsA by the ClpYQ (HslUV) protease in Escherichia coli. Microbiology 150:437-446. [DOI] [PubMed] [Google Scholar]
- 21.Li, J., Z. Qian, and B. Sha. 2003. The crystal structure of the yeast Hsp40 Ydj1 complexed with its peptide substrate. Structure 11:1475-1483. [DOI] [PubMed] [Google Scholar]
- 22.Maurizi, M. R., P. Trisler, and S. Gottesman. 1985. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J. Bacteriol. 164:1124-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1972. Experiments in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Missiakas, D., C. Georgopoulos, and S. Raina. 1993. The Escherichia coli heat shock gene htpY: mutational analysis, cloning, sequencing, and transcriptional regulation. J. Bacteriol. 175:2613-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizusawa, S., and S. Gottesman. 1983. Protein degradation in Escherichia coli: the lon gene controls the stability of the SulA protein. Proc. Natl. Acad. Sci. USA 80:358-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita, M. T., Y. Tanaka, T. S. Kodama, Y. Kyoguoku, H. Yanagi, and T. Yura. 1999. Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev. 13:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsell, D. A., and R. T. Sauer. 1989. Induction of a heat shock-like response by unfolded protein in Escherichia coli: dependence on protein level not protein degradation. Genes Dev. 3:1226-1232. [DOI] [PubMed] [Google Scholar]
- 29.Sabina, J., N. Dover, L. J. Templeton, D. R. Smulski, D. Söll, and R. A. LaRossa. 2003. Interfering with different steps of protein synthesis explored by transcriptional profiling of Escherichia coli K-12. J. Bacteriol. 185:6158-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seong, I. S., J. Y. Oh, S. J. Yoo, J. H. Seol, and C. H. Chung. 1999. ATP-dependent degradation of SulA, a cell division inhibitor, by the HslVU protease in Escherichia coli. FEBS Lett. 456:211-214. [DOI] [PubMed] [Google Scholar]
- 31.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stout, V., A. Torres-Cabassa, M. R. Maurizi, D. Gutnick, and S. Gottesman. 1991. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 173:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres-Cabassa, A. S., and S. Gottesman. 1987. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 169:981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trempy, J. E., and S. Gottesman. 1989. Alp, a suppressor of Lon protease mutants in Escherichia coli. J. Bacteriol. 171:3348-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trempy, J. E., J. E. Kirby, and S. Gottesman. 1994. Alp suppression of Lon: dependence on the slpA gene. J. Bacteriol. 176:2061-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trisler, P., and S. Gottesman. 1984. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J. Bacteriol. 160:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trun, N. J., and S. Gottesman. 1990. On the bacterial cell cycle: Escherichia coli mutants with altered ploidy. Genes Dev. 4:2036-2047. [DOI] [PubMed] [Google Scholar]
- 39.Wickner, S. H. 1990. Three Escherichia coli heat shock proteins are required for P1 plasmid DNA replication: formation of an active complex between E. coli DnaJ protein and the P1 initiator protein. Proc. Natl. Acad. Sci. USA 87:2690-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Withey, J., and D. Friedman. 1999. Analysis of the role of trans-translation in the requirement of tmRNA for λimmP22 growth in Escherichia coli. J. Bacteriol. 181:2148-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Withey, J. H., and D. I. Friedman. 2003. A salvage pathway for protein structures: tmRNA and trans-translation. Annu. Rev. Microbiol. 57:101-123. [DOI] [PubMed] [Google Scholar]
- 42.Wu, W.-F., Y. N. Zhou, and S. Gottesman. 1999. Redundant in vivo proteolytic activities of Escherichia coli Lon and the ClpYQ (HslUV) protease. J. Bacteriol. 181:3681-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yura, T., M. Kanemori, and M. T. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 44.Zhou, Y., and S. Gottesman. 1998. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J. Bacteriol. 180:1154-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]