Abstract
Background
Nonadherence/discontinuation of antipsychotic (AP) medications represents an important clinical issue in patients across psychiatric disorders, including schizophrenia spectrum disorders (SSDs). While antipsychotic‐induced weight gain (AIWG) is a reported contributor to nonadherence, a systematic review of the association between AIWG and medication nonadherence/discontinuation has not been explored previously.
Method
A systematic search was conducted in MEDLINE, EMBASE, PsychINFO, CINAHL, and CENTRAL databases, among others, to help identify all studies which explored adherence, study dropouts, AP switching and/or discontinuations attributable to AIWG among individuals with severe mental illness. A meta‐analysis was also completed where applicable.
Results
We identified two categories of studies for the meta‐analysis. Category 1 included three studies, which compared measures of AP adherence or discontinuation across BMI classes/degrees of self‐reported weight gain. When compared to normal weight individuals receiving APs or those who did not report AIWG, individuals who were either overweight or obese or reported weight gain in relation to AP use had an increased odds of AP nonadherence (OR 2.37; 95% CI 1.51–3.73; p = 0.0002). Category 2 had 14 studies which compared measures of discontinuation related to weight gain reported as an adverse effect across different APs. Olanzapine was associated with a 3.32 times (95% CI 2.32–4.74; p < 0.00001) increased likelihood of nonadherence or discontinuation when compared to other APs with lower weight gain liabilities. Similarly, APs with moderate weight gain liability (paliperidone, risperidone, and quetiapine) increased the odds of nonadherence or discontinuation by 2.25 (95% CI 1.31–3.87; p = 0.003) when compared to APs considered to have lower weight gain liability (i.e. haloperidol and aripiprazole). The qualitative summary also confirmed these findings.
Conclusion
This review and meta‐analysis suggests that AIWG influences medication nonadherence/discontinuation, whereby APs with higher weight gain liability are associated with nonadherence/discontinuation. Additional studies are needed to confirm these findings.
Keywords: antipsychotics, discontinuation, nonadherence, schizophrenia, weight gain
Summations
Our findings indicate that antipsychotic‐induced weight gain (AIWG) may be associated with increased nonadherence or discontinuations among individuals with SSDs.
APs with a higher metabolic liability are associated with higher risk of nonadherence or discontinuation attributable to AIWG.
These findings support systematic metabolic monitoring and treatment to help improve physical health and acceptability of treatment with APs.
Limitations
Definitions and measurements of nonadherence are highly heterogenous in the current literature, thereby limiting generalizability of findings.
In many studies, it was unclear whether discontinuations reflected decisions made by the patient versus a healthcare professional.
Given the paucity of studies directly exploring the association between AIWG and nonadherence or discontinuation, we can conclude an association rather than a direct causal relationship between these variables.
1. INTRODUCTION
Antipsychotic (AP) medications are integral to the treatment of psychiatric disorders including schizophrenia spectrum disorders (SSDs) and bipolar disorder. 1 Despite their effectiveness, medication nonadherence and discontinuations remain prevalent. For example, studies in individuals with schizophrenia (SCZ) have reported nonadherence rates between 20% and 89%, with a rate of 49.5% in studies where adherence was measured by a trained professional defined as “taking medications as prescribed at least 75% of the time.” 2 Individuals with bipolar disorder display similar adherence patterns, with approximately 45% of patients reporting to be partially adherent or nonadherent to mood stabilizers and/or AP medications. 3 , 4 AP nonadherence has been shown to increase disease burden through risk of relapse and/or rehospitalization as well as overall mortality. Beyond impairing patients' quality of life, these consequences also translate to substantial increases in healthcare costs. 5
Broadly speaking, medication adherence refers to the degree to which a person complies with the dosing recommendations provided by a healthcare professional. 6 , 7 In practice, nonadherence is complex, reflected by a lack of standardization in assessment tools and adherence thresholds. Patients' own experiences in taking medications can impact adherence and be influenced by a variety of personal and social factors including age, gender, socioeconomic status, level of insight, symptom severity, social support, attitude towards medication, and relationship with treatment providers. Importantly, existing measures of adherence are unable to establish the relative impact of each factor, making it difficult to develop a singular strategy addressing nonadherence. 8
It is perhaps not surprising that side effects associated with medications contribute to non‐adherence. In particular, weight gain is a common and distressing side effect. 9 , 10 , 11 Indeed, AP‐induced weight gain (AIWG) has been ranked as the most concerning side effect among patients calling distress help lines and has been reported to lead to challenges with treatment engagement. 12 , 13 In line with this, clinicians consistently cite weight gain as a major risk factor for non‐adherence to medications across diagnoses. 7 , 14 , 15 Conversely, a small but growing body of evidence suggests a relationship between AP efficacy and AP‐related metabolic effects. In particular, studies indicate that the most efficacious APs also appear to cause the most severe metabolic effects including weight gain. 16 , 17 As a result, the concept of a ‘metabolic threshold’ has been proposed, where clinical improvement is proposed to be at least partially dependent on metabolic adverse effects. 18 , 19 , 20 , 21 This, in turn, is difficult to reconcile with the observation that weight gain and other metabolic adverse effects are distressing and can lead to treatment nonadherence and discontinuation.
The objective of this review was to explore the relationship between AIWG and nonadherence/treatment discontinuation among individuals with severe mental illness receiving APs for an approved indication (i.e., SSDs, bipolar disorder, treatment refractory depression, among others). Since authors generally report on treatment discontinuation more reliably than nonadherence, we chose to examine studies that include at least one of these measures to ensure that our review is as comprehensive as possible. In addition, we included studies examining treatment nonadherence or discontinuation across APs with differential weight gain liabilities to explore whether, despite the proposed concept of a metabolic threshold, metabolic adverse effects remain an issue which may lead individuals to discontinue their AP treatment. Therefore, our specific aims of the study included assessing whether (a) individuals with AIWG are more likely to discontinue or be nonadherent to their AP treatment than those with no AIWG, and (b) individuals taking APs with higher weight gain liability are more likely to be nonadherent/discontinue their AP treatment compared to those on APs with lower weight gain liability.
2. MATERIALS AND METHODS
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. A PROSPERO protocol for this review was registered prior to its commencement and can be found under the following identifier: PROSPERO 2022 CRD42022295769. Our search strategies identified studies which were subsequently grouped into two categories based on any reported measures of nonadherence or discontinuation:
Comparison of body mass index (BMI) categories (as a proxy for AIWG) or degrees of self‐reported weight gain during AP treatment
- Comparison of adherence or discontinuation across APs with differing weight gain liabilities. Studies that reported on discontinuations or nonadherence due to weight gain for either one specific AP or comparator groups which could not be grouped with the other studies in the meta‐analysis were synthesized qualitatively. Based on our search results, we conducted two comparisons for the meta‐analysis:
- Olanzapine (high weight gain liability AP) versus APs with lower propensity for weight gain (this included any other AP besides clozapine) 12
- APs with moderate weight gain liability (i.e., quetiapine, risperidone, and paliperidone, grouped together) versus APs with lower propensities for weight gain (such as aripiprazole, amisulpride, asenapine, haloperidol, ziprasidone, or lurasidone, among others) 12
We also used a similar approach (1 and 2 above) to explore the effect of other metabolic adverse effects (i.e., dysglycemia, dyslipidemia) on treatment nonadherence or discontinuations if sufficient data were reported across the included studies.
2.1. Search strategy
We searched Ovid MEDLINE, Ovid EMBASE, Ovid PsychINFO, Ebsco's CINAHL, Wiley's CENTRAL, Web of Science, and Scopus from inception until July 2023. This was supplemented with searches in ClinicalsTrials.gov and the International Clinical Trials Registry Platform (ICTRP) Search Portal, a manual search of the first 10 pages of Google Scholar, and hand‐searching of reference lists from relevant systematic reviews and meta‐analyses. No restrictions were placed on publication dates. The full search strategy can be found in Table S1.
To be as inclusive as possible, any studies with references made to AP “discontinuation,” “nonadherence,” “noncompliance,” “withdrawal,” “dropouts,” or “switch” were captured.
2.2. Study eligibility
Studies were included if they had the following characteristics: (1) patients 16 years of age or older who were taking any AP(s) for an approved (i.e., on‐label) diagnosis classified by International Classification of Diseases (ICD), Diagnostic Statistical Manual (DSM), and/or the World Health Organization (WHO) and using the indication guide provided by Christian et al., 22 (2) reference to a measure of AP nonadherence and/or discontinuation, including decision to switch AP medications (reported as a dichotomous or continuous variable) in relation to weight gain, its proxy (i.e., across weight/BMI classes), or self‐reported degree of weight gain, (3) written in English.
Additionally, for studies to be included in the meta‐analysis, they had to have extractable data presented in one of the following ways: (1) BMI groups or degrees of self‐reported weight gain, (2) at least two AP groups that had nonadherence or discontinuation due to weight gain reported. If weight gain was grouped together with other metabolic effects, the studies were instead included in the qualitative synthesis.
Exclusion criteria included: (1) conference abstracts, commentaries, or letters; (2) lifestyle or pharmacological intervention studies aimed at reducing weight, which were excluded due to potential confounds that could affect our study objectives; (3) for aim 2, AP studies involving comparison with a placebo group as these would not address our research objective; (4) adherence or discontinuation measures not reported as an outcome (e.g., if they are explored as an independent variable instead). There were no other limitations on the type of studies included.
2.3. Data collection
All articles identified in the search were screened at the title/abstract level by six authors (RD, ECCS, EA, KM, MP, and RS) and at the full‐text level by nine authors (RD, ES, JN, EA, KM, MP, RS, KJP, and BH) utilizing the Covidence software. Any conflicts were resolved through discussion.
Data extraction was conducted independently by three authors (RD, ES, JN) using a standardized template consisting of the following headings: (1) country of study, (2) study setting, (3) sample size, (4) diagnosis, (5) percentage of males in the population, (6) mean age, (7) AP use timeframe, (8) type of study (randomized control trial or RCT, cross sectional, or cohort), (9) the definition of how nonadherence or discontinuation was explored in respective studies, (10) AP nonadherence or discontinuation/switch numbers across groups for the meta‐analysis and any AP related data for the qualitative synthesis, (11) other metabolic variables considered in relation to treatment discontinuation (e.g., dyslipidemia, glucose, and HbA1c levels). Study authors were contacted if any further information was needed.
2.4. Data synthesis
Studies were synthesized both qualitatively and quantitatively. All studies included in the meta‐analysis were analyzed using Review Manager (RevMan) 5.4. Data were included in the meta‐analysis if there were reported dichotomous outcomes, such as the odds ratio (OR) itself or the number of outcomes/events provided by AP groupings (therefore allowing RevMan to calculate the respective ORs). To determine the total number of events for each of the two groups used in comparisons for the meta‐analysis, the sample sizes reported in the respective studies were used for each grouping. In the comparator groups, all relevant APs with lower weight gain propensities were grouped together to report these results according to the hierarchy proposed by Dayabandara et al. 12
A random‐effects model was used to calculate the overall effect size of the difference between comparator groups. Study heterogeneity was assessed using the I 2 statistic, with an I 2 ≥ 50% indicating considerable heterogeneity. ORs with 95% confidence intervals were reported for the outcome measures in the meta‐analysis.
2.5. Critical appraisal
The quality of all included studies in the meta‐analysis was assessed independently by three authors (RD, ES, JN). The relevant JBI tools (formerly known as the Joanna Briggs Institute tools) were utilized for cohort and cross‐sectional studies and the Cochrane Risk of Bias (RoB) tool was utilized for RCTs. The list of questions used to conduct the risk of bias assessments can be found in Table S2.
3. RESULTS
3.1. Study selection
A PRISMA diagram shown in Figure 1 illustrates study selection. A total of 33,809 articles were identified from the search, out of which 14,600 duplicates were identified and removed. After title and abstract screening, a total of 1,531 articles were included in the full text review stage, following which 39 articles were deemed eligible for inclusion in this review (17 in the meta‐analysis and 39 in the qualitative portion of the systematic review).
FIGURE 1.
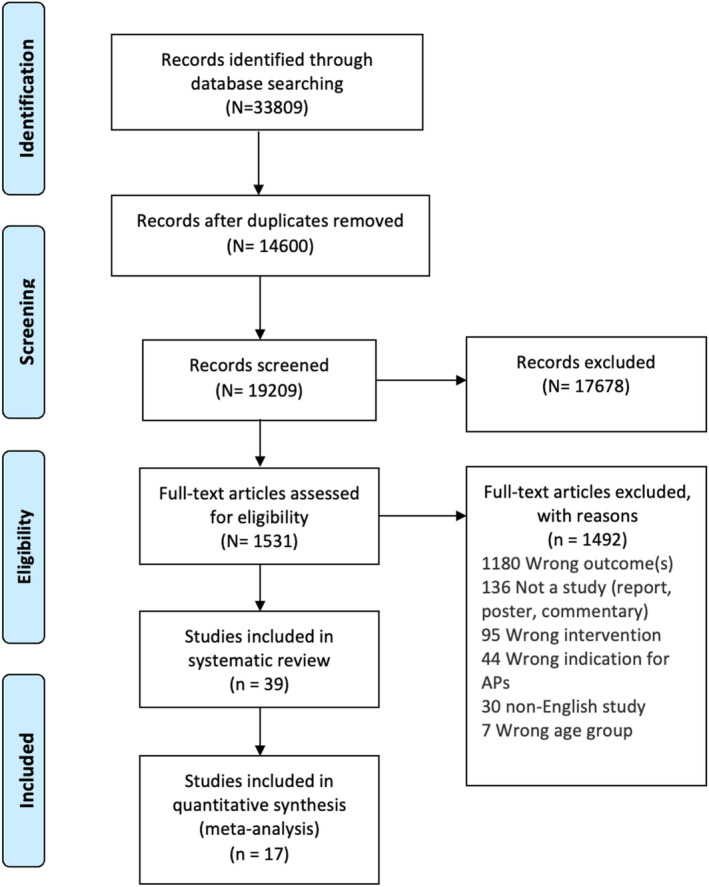
PRISMA diagram.
3.2. Systematic review
3.2.1. Overview of qualitative synthesis
Thirty‐nine studies were included in the qualitative synthesis, inclusive of all studies in the meta‐analysis. These studies were published between 1989 and 2023, with the represented diagnoses being SSDs (e.g., SCZ, schizoaffective disorder, schizophreniform disorder), first episode psychosis, or bipolar disorder.
Studies were conducted in the USA (N = 12), UK (N = 2), Spain (N = 2), Japan (N = 2), China (N = 2), India (N = 2), Germany (N = 2), multiple countries (N = 8) and one each from Austria, Korea, Italy, Australia, Canada, Turkey, and Uganda. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 The mean age ranged from 21.54 to 48.5 years and the percentage of males ranged from 36.1 to 82%. The types of studies included RCT, observational, and cross‐sectional studies. The AP use duration captured ranged from 4 weeks to 9 years, with included studies consisting of a mix of inpatients and outpatients. Out of these 39 studies, 17 studies were included in the meta‐analysis and are discussed in more detail under Section 3.3. Eight studies which couldn't be analyzed in the meta‐analysis had a clear comparator group and are discussed in Subsection Subgroup comparison with comparator groups. Fourteen studies without an active comparator reported on the percentage of patients who discontinued due to weight gain on specific AP agents. Among these studies, four did not report any discontinuations due to AIWG. 25 , 27 , 31 , 33 Eight other studies reported on discontinuation due to AIWG that fell within the range of 0.01%–10%, including risperidone (1.1% and 3.1%), lurasidone (8.2%), paliperidone palmitate (5.1%), olanzapine (2.4% and 5.8%), and clozapine (0.40% and 1.65%). 30 , 39 , 40 , 44 , 45 , 50 , 55 , 58 One study reported a rate of weight gain‐related discontinuation of 22.2% among olanzapine‐treated patients, while another study reported that 15.6% of patients switched APs due to weight gain. 28 , 35 Table S3 highlights discontinuation rates due to weight gain alongside any reported all‐cause discontinuations in the respective studies. All studies were judged to be at low to moderate risk of bias. A detailed study summary table can be found in Tables 1 and 2 (meta‐analysis) and Table S1 (qualitative studies), and the risk of bias assessments can be found in Table S2.
TABLE 1.
Summary and characteristics of included studies exploring discontinuation or nonadherence across BMI categories or degree of self‐reported AIWG.
| Study | Dx | Country of study | Objective of study | % Male | Mean age ± SD | N | Study type | Antipsychotic use timeframe | Definition of nonadherence/discontinuation in study | Nonadherence/discontinuation based on or proxy for AIWG used in meta‐analysis | Other metabolic variables considered in relation to treatment discontinuations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Doane 2020 | SCZ | US | Online survey exploring attitudes towards AP treatment, among individuals who have been using APs for at least a 12‐month period at the time of survey | 50 | 41.9 ± 11.0 | 200 | Cross sectional (survey data) | NR – but SCZ dx for at least 1 year | Discontinuation of AP medication across degree of self‐reported weight gain: no weight gain or non‐bothersome weight gain, versus somewhat bothersome or very/extremely bothersome weight gain |
Discontinuation in those reporting bothersome weight gain: 102/124 Discontinuation in those without weight gain or with non‐bothersome weight gain: 48/76 |
N |
| Sliwa 2014 | SCZ | Multiple countries (Costa, Rica, Mexico, Romania, Russia, Ukraine, South Africa, Korea, Taiwan, US) | Post hoc analysis examining metabolic treatment‐related adverse events across BMI categories | 59 | 37.3 ± 10.58 | 644 (29 of these individuals are in underweight category and are not relevant to this study) | Multicentre open‐label transition and extension data from RCT examining treatment with paliperidone palmitate (PP); only patients consistently treated with PP across all study phases were included in the analysis | Median duration of exposure to PP: 204 days (6 to 1009 days) | Discontinuation by patient choice across BMI categories |
Overweight: 1/232 Obese: 0/154 Normal weight: 0/229 Overweight and obese combined: 1/386 |
N |
| Weiden 2004 | SCZ | US | National survey examining self‐report of AP non‐compliance across BMI categories, and in relation to self‐report of distress around weight gain | NR directly (included in the logistic regression model) | NR directly (included in the logistic regression model) | 239 | Cross sectional (survey data) | NR directly (logistic regression model) |
AP non‐compliance reported across BMI categories and according to subjective distress over “weight gain.” Non‐compliance was defined as “self‐report of missing any AP medication in the previous month” |
Overweight: 34/88 Obese: 41/89 Normal weight: 16/62 Overweight and obese combined: 75/177 The total number of events in each group are approximations so that the reported OR could be reflected in the meta‐analysis. |
N |
Abbreviations: AP, antipsychotic; BMI, body mass index; NR, not reported; PP, paliperidone palmitate; SCZ, schizophrenia.
TABLE 2.
Summary and characteristics of included studies with AP comparisons.
| Study | Dx | Country of study | Setting | % Male | Mean age ± SD | N | Study type | Antipsychotic use timeframe | Definition of nonadherence/discontinuation in study | Weight gain‐related nonadherence/discontinuation used in meta‐analysis | Other metabolic variables considered in relation to treatment discontinuations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ader 2008 | SCZ or schizoaffective or schizophreniform disorder | US | All patients |
OLZ: 58.1 RIS: 7 8.6 |
OLZ: 39.6 ± 8.3 RIS: 39.8 ± 7.6 |
59 | Double‐blind RCT | 24‐week treatment on RIS or OLZ | Treatment discontinuation due to weight gain. |
OLZ: 1/31 RIS: 0/28 |
N |
| Bobes 2003 | SCZ | Spain | Outpatients |
OLZ: 61.4 HAL: 60.5 RIS: 64.2 QUE:51.2 |
HAL: 41.2 ± 12.3 OLZ: 35.7 ± 12.8 RIS: 36.1 ± 11.5 QUE: 32.2 ± 10.5 |
636 evaluated | Cross‐sectional, retrospective, naturalistic | At least 4 weeks | Treatment discontinuation due to weight gain. |
OLZ: 8/133 HAL: 0/39 RIS: 1/96 QUE: 0/4 For OLZ versus other APs comparison: HAL, RIS, and QUE combined: 1/139 For RIS, QUE and PP versus other APs comparison: RIS and QUE combined: 1/100 and compared with HAL Reported n here = number of patients with weight gain data recorded in the UKU with information about requirement of any related action |
N |
| Citrome 2015 | SCZ | US | Multicentre (outpatients) | Total: 67 | 43.3 ± 11.0 | 500 | Open label randomized switch study | Previous AP use timeframe not reported. However, there were 12 weeks of iloperidone monotherapy | Discontinuation of APs due to weight gain who were then switched to iloperidone to combat weight gain (i.e., switch study). |
OLZ: 39/155 ARI: 16/170 RIS: 22/175 ARI and RIS combined: 38/345 |
N |
| Hofer 2017 | SCZ spectrum disorders | Austria | Inpatients and outpatients | Total: 59.3 | 35.5 ± 9.2 | 194 | Post‐hoc analysis of a naturalistic study | Follow up was for a maximum of 12 months | Treatment discontinuation due to weight gain. |
OLZ: 1/49 RIS: 2/33 AMI: 0/39 ARI: 0/13 QUE: 0/22 SER: 0/8 ZIP: 0/24 ZOT: 0/6 All APs other than OLZ combined for comparison = 2/145 |
Y |
| Huang 2018 | First episode, drug‐naive SCZ patients | China | Inpatients and outpatients |
PP: 60.7 OLZ: 70 |
PP: 21.54 ± 5.60 OLZ: 23.79 ± 5.89 |
57 | RCT | 13‐week study assessing efficacy of PP and OLZ in patients | Discontinuation |
OLZ: 0/29 PP: 1/28 |
N |
| Kinon 2006 | SCZ, schizophreniform disorder, or schizoaffective disorder | US | Inpatients and outpatients | Total: 64.4 | 39.53 ± 10.85 | Total: 1627 | Post‐hoc pooled analysis of four double‐blind RCTs | 24–28‐week exposure to OLZ versus other APs | Discontinuation |
OLZ: 3/822 RIS: 0/167 QUE: 0/175 ZIP: 0/463 All APs other than OLZ combined for comparison: 0/805 |
N |
| Kishi 2021 | SCZ and related psychotic disorders | Japan | Inpatients | NR | NR | 157 | Retrospective chart review | Ranged from 2 to 48 months | Treatment discontinuation due to weight gain. |
PP: 2/41 RIS LAI: 0/58 ARI: 0/58 PP and RIS combined: 2/99 |
N |
| Matsuzaki 2021 | SCZ or schizoaffective disorder | Japan | Hospital |
ASE: 39.1 OLZ: 36.7 |
ASE: 42.0 ± 15.3 OLZ: 43.8 ± 15.6 |
95 | Retrospective chart review | Initiated on either ASE or OLZ, with discontinuation assessed at 1 and 6 months | Treatment discontinuation due to weight gain. |
OLZ: 7/49 ASE: 1/46 |
N |
| McEvoy 2014 | SCZ, schizoaffective disorder | US | Multicentre (inpatients and Outpatients) |
PP: 73.1 HAL: 75.9 |
PP: 43 ± 12.6 HAL: 45 ± 12.3 |
311 randomized, 290 included in primary analysis | Double‐blind RCT | Up to 24 months | Treatment discontinuation due to weight gain. |
PP: 7/145 HAL: 2/145 |
N |
| Montes 2007 | SCZ or schizoaffective disorder | Spain | Multicentre (outpatients) | Total: 50.7 | 35.0 ± 11.7 | 67 | Open‐label, flexible‐dose, prospective | Previous AP use timeframe: 31.4 months. ZIP treatment was reported to be for 6 months. | Switching due to weight gain |
OLZ: 37/40 RIS: 17/20 HAL: 0/3 FLZ: 0/1 QUE: 0/1 AMI: 0/1 QUE + RIS: 0/1 All APs other than OLZ combined for comparison = 17/27 |
Y |
| Piparva 2011 | Psychotic disorder | India | Outpatients | Total: 70.27 | Unknown (any age included) | 84 | Observational, prospective | Up to 18 months of follow up exploring adverse drug reactions on various atypical APs | Treatment discontinuation due to weight gain. |
OLZ: 4/34 CLZ: 0/6 RIS: 0/38 QUE: 0/5 ARI: 0/1 All APs other than OLZ and CLZ combined for comparison: 0/44 |
N |
| Shajahan 2009 | SCZ, persistent delusional disorders, schizoaffective disorder and depressive disorder with psychotic symptoms | UK | Outpatients |
ARI: 58 QUE: 52 |
ARI: 39.6 (37.3 to 41.9) QUE: 36.7 (34.1 to 39.3) |
221 | Retrospective chart review (electronic) |
ARI: 488 days (403 to 572) QUE: 450 days (361 to 540) |
Treatment discontinuation due to weight gain. |
ARI: 0/89 QUE: 3/132 |
N |
| Wang 2022 | SCZ | Multicentre | Inpatients and outpatients | Total: 77.9 |
PP: 33.42 ± 11.77 OLZ: 33.10 ± 8.48 |
PP: 45 OLZ: 41 |
Double‐blind RCT | 12 weeks on PP extended release or OLZ | Treatment discontinuation due to weight gain |
OLZ: 1/41 PP: 0/45 |
N |
| Zhou 2023 | SCZ | China | Inpatients and outpatients | Total: 50.69 | 25.13 ± 7.10 | 493 | Open‐label RCT | Assigned to a different AP after <4 weeks of continuous treatment with the previous AP (cumulative lifetime AP exposure <12 weeks) | Switched APs due to weight gain within the first 4 weeks after randomization. |
OLZ: 16/163 ARI: 1/161 RIS: 11/169 All APs other than OLZ combined for comparison: 12/330 |
N |
Abbreviations: AMI, amisulpride; ARI, aripiprazole; ASE, asenapine; CLZ, clozapine; Dx, diagnosis; FLZ, fluphenazine; HAL, haloperidol; NR, not reported; OLZ, olanzapine; PP, paliperidone palmitate; QUE, quetiapine; RIS, risperidone; SCZ, schizophrenia; SER, sertindole; ZIP, ziprasidone; ZOT, zotepine.
Subgroup comparison with comparator groups
A total of eight studies included in this review reported on nonadherence or discontinuation related to weight gain across comparator groups but did not have any extractable data. 24 , 34 , 36 , 40 , 43 , 46 , 52 , 53 The studies were published between 2005 and 2023, and predominantly included individuals with SSDs (Table S3).
Four of the eight studies reported data that could be used to calculate percentage of weight gain related discontinuations between comparator groups. Akkaya et al. 24 reported that 1.33% of individuals in the “atypical” AP group discontinued versus none in the “typical” AP group. Essock et al. 34 reported that 3.2% of individuals in the polypharmacy group had discontinuations due to weight gain, while the monotherapy group had no discontinuations due to weight gain. Lieberman et al. 46 reported on discontinuation due to weight gain and/or metabolic side effects from the CATIE study. This study was a double‐blind RCT where patients were randomly assigned to olanzapine, quetiapine, perphenazine, risperidone, or ziprasidone for up to 18 months. The study found that 9% of individuals on olanzapine discontinued due to weight gain/metabolic effects compared to 1%–4% in the other AP groups. 46 Kim et al. 40 conducted a switch study that reported on weight gain in patients that switched to paliperidone from risperidone vs. from other APs (non‐risperidone group). They found that 1.1% switched from risperidone due to weight gain versus 2.2% who switched APs due to weight gain in the non‐risperidone group. 40
Among the remaining four studies reporting on discontinuation or nonadherence due to AIWG across agents, Gray et al. 36 found that weight gain was positively and significantly correlated with adherence (Pearson's correlation coefficient = 0.49, p < 0.001) in prisoners using APs (N = 44). Additionally, Mustafa et al. 52 reported higher weight gain in first episode psychosis patients that discontinued their AP medications compared to those who did not (t(245) = 4.19, p < 0.001). Perkins et al. 53 used health records to explore the association between weight gain and nonadherence in individuals with SCZ or bipolar disorder compared to psychiatric controls. They reported that weight gain of 7% or greater increased the odds of a medication switch in the first 180 days (OR = 1.60, 95% CI: 1.17, 2.18). 53 Finally, a study by Kule et al. 43 looked at adherence to typical APs among individuals with SCZ from Uganda. They found that, although not significant, increased weight was linked to an OR of 6.66 (95% CI: 0.73–60.51, p = 0.092) for self‐reported nonadherence. 43
3.3. Meta‐analysis
3.3.1. First category of studies—measures of AP nonadherence/discontinuation across BMI proxy categories
Three studies (n = 1054) were found to address this question. 32 , 57 , 60 Two studies compared AP nonadherence between overweight/obese and normal weight individuals with the weight groups acting as a proxy for AIWG. One study reported nonadherence defined as patient self‐reports of not taking their AP medications, as directed across subjective categories of either no AIWG or bothersome AIWG. In the study by Doane et al., authors reported on the number of patients that stopped taking their APs with or without the agreement of their healthcare team (although the authors defined the latter group as being nonadherent, data from both groups are included in our review as they still represent discontinuation). 32 Similarly, Sliwa et al. 57 reported on reasons for AP discontinuation whereas Weiden et al. 60 defined nonadherence as a patient missing medication at least some of the time (as indicated by a score of at least 1 on a 5‐point scale ranging from 0 being “never” to 4 being “almost always”). These studies were combined for the meta‐analysis. A summary of these studies can be found in Table 1. Overweight or obese individuals were 2.37 times more likely to be classified as nonadherent in relation to AP use when compared to the normal weight group (OR = 2.37; 95% CI: 1.51, 3.73; p = 0.0002) (Figure 2).
FIGURE 2.
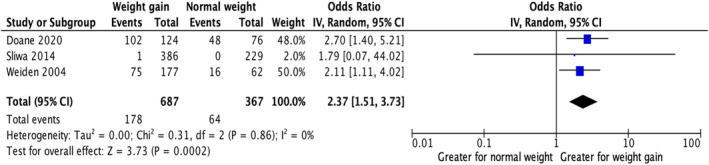
Nonadherence/discontinuation in individuals who are overweight or obese compared to those who are normal weight.
3.3.2. Second category of studies—APs with greater weight gain liability versus APs with lower weight gain liability
Olanzapine comparison
Eleven studies (n = 3528) compared nonadherence or discontinuation due to weight gain between individuals treated with olanzapine, a high weight gain liability AP, versus other APs that have lower weight gain liabilities (i.e., aripiprazole, risperidone, haloperidol, and asenapine, among others). 23 , 26 , 29 , 37 , 38 , 41 , 48 , 51 , 54 , 59 , 61 Individuals on olanzapine were 3.32 times more likely to be nonadherent/discontinue their AP medications when compared to individuals on APs with lower propensities for weight gain (OR = 3.32; 95% CI: 2.32, 4.74; p < 0.00001; Figure 3).
FIGURE 3.
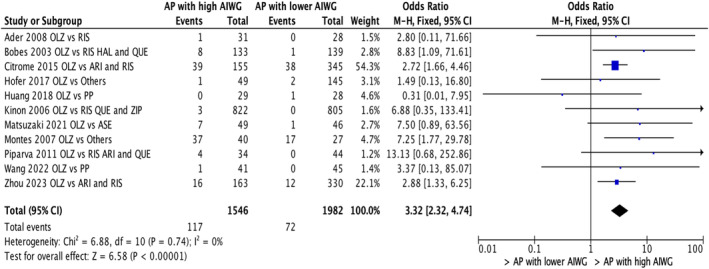
Nonadherence/discontinuation in individuals on APs with high weight gain liability versus APs with lower weight gain liability (Olanzapine grouping vs. others).
Paliperidone, risperidone, and quetiapine comparison
Six studies (n = 1482) compared nonadherence or discontinuation due to weight gain between individuals either on paliperidone, risperidone, or quetiapine with those on APs associated with lower risk of weight gain such as aripiprazole and haloperidol. 26 , 29 , 42 , 49 , 56 , 61 The subgroup analysis revealed that individuals who reported nonadherence or discontinuations due to weight gain were 2.25 times more likely to have been treated with paliperidone, risperidone or quetiapine compared to other APs (OR = 2.25; 95% CI: 1.31, 3.87; p = 0.003; Figure 4).
FIGURE 4.
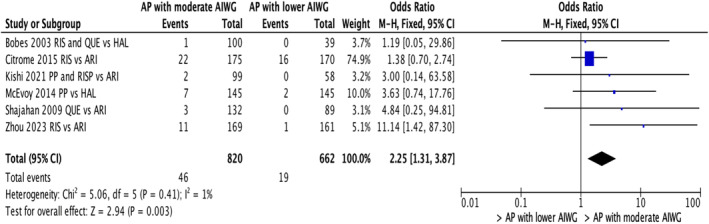
Nonadherence/discontinuation in individuals on APs with moderate weight gain liability versus APs with lower weight gain liability (Paliperidone, risperidone and quetiapine grouping vs. others).
3.3.3. Other metabolic parameters
Among all studies included in the meta‐analysis, only two studies 37 , 51 reported on nonadherence or discontinuation related to metabolic parameters other than weight. These parameters included glucose, including non‐fasting blood glucose and changes in glucose metabolism as reported in the respective studies, total cholesterol, triglycerides, low‐density lipoprotein (LDL), and high‐density lipoprotein (HDL). Montes et al. 51 reported that 13.5% of those who switched to ziprasidone from previous APs did so because of occurrence of dyslipidemia or worsening of prior dyslipidemia, while 9% switched due to glucose intolerance. In the study by Hofer et al., two patients on risperidone dropped out due to alterations in glucose metabolism, although the authors did not specify the nature of these changes. 37
4. DISCUSSION
Nonadherence to AP medications due to weight gain has been a longstanding clinical challenge; however, a comprehensive analysis of the data is lacking. To address this gap in the literature, we conducted a systematic review and meta‐analysis which examined nonadherence or discontinuation of AP treatment in relation to AIWG through two specific research objectives. First, we compared nonadherence or discontinuations across proxies of weight gain measured via BMI categories or degree of self‐reported weight gain. Second, we compared AIWG‐related discontinuation or measures of nonadherence across APs with differing weight gain liabilities.
This systematic review and meta‐analysis supports the position that AIWG is an important factor in AP nonadherence/discontinuation among individuals with severe mental illness, specifically SSDs. Our review demonstrates that individuals receiving APs with higher metabolic liability have an increased risk of discontinuation due to weight gain when compared to APs with lower metabolic liability.
As per our first objective, our findings indicate that individuals with elevated BMI or who self‐reported weight gain and associated distress have greater odds of being nonadherent or discontinuing AP treatment compared to those who are normal weight or those who fail to report bothersome weight gain. Our findings are in keeping with the limited body of literature examining discontinuation due to weight gain related to other psychotropic agents such as lithium in bipolar disorder 62 and antidepressants in major depressive disorder. 63 Together, this evidence supports that weight gain can be a contributing factor in AP nonadherence or discontinuation. This suggests that weight gain is important to monitor clinically as AIWG, or concerns regarding AIWG, can be a deterrent in AP adherence, which in turn can negatively impact clinical outcomes. In line with this, Doane et. al 32 noted that 38% of survey respondents reported that novel APs in the development pipeline should, as a priority, have a lower risk of weight gain. Our qualitative synthesis offers further support regarding this concern, confirming that APs can be a contributor to weight gain resulting in subsequent nonadherence to medications, particularly early‐on in treatment. 35 , 58 It must be emphasized though that the cross‐sectional nature of studies included in this review demonstrate an association rather than a direct causal relationship between AIWG and nonadherence/discontinuation. Future prospective studies directly investigating this association are required, as is research extending findings beyond SSDs to other disorders where APs remain an important component of symptom management.
Our secondary research objective confirmed that individuals prescribed APs with greater weight gain risk are more likely to be nonadherent or discontinue AP treatment versus those taking AP agents associated with lower metabolic liability. Further to this point, individuals treated with olanzapine, paliperidone, risperidone, and quetiapine demonstrated significantly increased odds of stopping their medication due to weight gain when compared to those using APs with lower weight gain propensity. For example, we observed a more than threefold increase in the odds of AIWG‐related discontinuation on olanzapine compared to APs with lower AIWG (Figure 3). Given the finding that olanzapine was associated with a higher risk of weight‐gain related discontinuations, we conducted an exploratory meta‐regression analyzing the effect of olanzapine dose on the discontinuation‐related odds ratios (N = 9). While the results did not reveal a significant association (coefficient = −0.148, p = 0.935 not shown), this finding should be interpreted with caution as a minimum of 10 studies is normally needed for reliability of reported results. Similar to olanzapine, Figure 4 shows that the use of paliperidone, risperidone, and quetiapine is associated with more than a twofold increase in the odds of AIWG‐related discontinuations compared to aripiprazole and haloperidol. These findings are generally supported by our qualitative synthesis in which so‐called “metabolically neutral” APs were associated with less frequent discontinuations due to weight gain compared to APs with higher metabolic liabilities. However, despite a 2–3‐fold increased odds of discontinuation due to AIWG for the higher metabolic liability agents, it is noteworthy that the absolute values of these events were low. 26 , 46 (see Table 2 for details).
Indeed, research shows that clinical efficacy is a major contributor to AP adherence, 7 and the studies included in this review suggest that symptom control may be as important as avoiding weight gain for some patients. For example, two thirds (67%) of patients in the study by Doane et al. noted that they would prefer to be on an AP medication that would provide a small to large improvement in psychiatric symptoms despite a slight worsening in side effects. 32 Similarly, Hofer et al. found that patients with less improvement or worsening of their psychiatric symptoms were more likely to stop taking their medications than those with a good clinical response. 37 This could explain why many patients prefer to stay on high‐efficacy APs like olanzapine despite weight gain. In another study, Ader et al. found that although olanzapine‐treated patients were more likely to discontinue treatment due to weight gain than risperidone‐treated patients, they were also less likely to discontinue due to poor clinical response and/or worsening psychopathology. 23 This finding is consistent with evidence that olanzapine remains one of the most efficacious APs despite being associated with the highest metabolic liability alongside clozapine. 12 Similarly, in the CATIE study, despite higher discontinuation due to weight gain with olanzapine versus other APs, olanzapine, overall had a lower rate of discontinuation. 46
It is also interesting to note that our review included four studies that reported on weight gain‐related discontinuations due to clozapine ranging from none to very few. 24 , 39 , 45 , 54 While the small number of studies prevents us from conducting an accurate assessment of clozapine's effects, the low discontinuation rates observed in these studies are in line with previous literature. Although clozapine remains the AP with most evidence supporting efficacy and effectiveness in treatment‐resistant schizophrenia, clozapine's status as an AP with high metabolic liability cannot be refuted. Thus, this could lend support to the concept of “metabolic threshold” related to weight gain; however, this question will need further exploration in future studies, particularly among individuals treated with clozapine. Taken together, these observations highlight important and complex risk–benefit considerations whereby AP efficacy must be weighed against AP side effects.
The current systematic review, while providing important clinical insights into AIWG in relation to treatment adherence or discontinuation, has limitations. One of the major challenges relates to a lack of a standardized definition for “nonadherence.” Further to this point, a two‐part literature review of over 450 studies identified 10 unique categories of methods to assess medication adherence, each with its own strengths and weaknesses. 8 , 64 For example, subjective or indirect methods such as self‐report or reports from care givers or treatment providers can lead to adherence being overestimated. Objective or direct methods (e.g., blood and urine samples, pill count, re‐fill records, electronic monitoring), while generally considered more accurate, can be affected by patient behavior (e.g., medication stockpiling or disposal). Even the use of a medication event monitoring system (MEMS), which is considered the current “gold standard,” may be affected by erroneous opening of pill containers. 8 , 65 Furthermore, thresholds for determining adherence range widely. 7 These challenges in defining adherence likely contributed to the observed heterogeneity of adherence measures (as seen for objective 1) and/or a failure to report on adherence altogether (as seen for objective 2). The latter largely restricted our review of discontinuation data reported during AP treatment trials, thereby limiting the real‐world generalizability of the findings.
In objective 1, interpreting our findings in a clinical context was limited by the use of proxy measures for weight gain which were assumed to be related to AP use. As such, the reported associations of AIWG with nonadherence may have been influenced by various other factors linked to weight gain and obesity, including illness‐related lifestyle factors (e.g., diet, reduced physical activity), and concomitant medications. Regarding objective 2, a related challenge included the fact that the majority of studies failed to distinguish between discontinuation based on patient decision alone versus discussion and agreement with a healthcare practitioner. Patients may be taken off medications by a healthcare professional for various reasons, whereas patients discontinuing a drug by themselves can pose added challenges and consequences. Thus, the reason behind why patients stop taking their medications is crucial in this line of research and needs to be elucidated in future work.
While our search identified many other studies that included data related to AIWG or nonadherence, the majority of reports failed to examine associations between these two variables, precluding their inclusion in the present review. This subsequently contributed to the paucity of data which could be extracted, particularly pertaining to objective 1. Another challenge limiting data inclusion was reporting of discontinuation data due to metabolic side effects as a general category. These studies were included in the qualitative synthesis but were unable to distinguish the relative importance of weight gain. Furthermore, only two studies included in this review reported the impact of changes in specific metabolic parameters other than weight on treatment discontinuation. As such, no concrete conclusions regarding the impact of metabolic changes other than weight gain on adherence or discontinuation could be made.
To conclude, notwithstanding the limitations noted, the present findings suggest that AIWG is associated with AP nonadherence or treatment discontinuation. While a primary clinical focus may be managing AIWG from a physical health perspective, the potential negative impact in terms of AP medication nonadherence must also be considered. Accordingly, adequately addressing the metabolic side effects is critical on several levels. Notably, we have entered an era where our arsenal of drugs to treat obesity and related metabolic disorders is growing at a rapid pace. 66 , 67 Several existing agents (i.e., metformin) and more novel classes of medications (i.e., glucagon like peptide 1‐receptor agonists, GLP1‐RAs) appear to show a beneficial effect on AIWG alongside a favorable tolerability and safety profile. 68 , 69 Such interventions, alongside reduction of metabolic risk factors in a population vulnerable to cardiovascular disease, may also have meaningful adjunctive benefits in terms of treatment adherence. In keeping with this observation, a recent retrospective chart review demonstrated that co‐commencement of metformin with clozapine is associated with increased treatment continuation at 1 year as well as concomitant beneficial effects on weight. 69 Going forward, it will be both important and interesting to examine long‐term effects of metabolic interventions on AP adherence and patients' attitudes towards their medications.
Moreover, other intrinsic or extrinsic factors which might contribute to medication nonadherence besides weight gain (such as AP efficacy) should also be explored to investigate their cumulative effects on treatment nonadherence or discontinuations. To this last point, while our work can neither refute nor support the concept of a “metabolic threshold,” it is clear that APs such as olanzapine, despite associations with greater effectiveness, are more likely to be associated with treatment discontinuation due to weight gain as compared to lower liability APs. It is therefore imperative that future studies place a greater emphasis on the collection and reporting of metabolic side effects, with special attention paid to weight‐specific outcomes.
AUTHOR CONTRIBUTIONS
RD, GR, SMA, and MKH were involved in the conceptualization of the paper. RD and ECCS conducted the systematic review searches, study screening, data analyses, and manuscript writing. JN, EA, KM, MP, RS, KJP and BH were involved in study screening and data extraction. GHM, MON, and BE were involved in critically reviewing the manuscript. All authors have agreed to the final submitted version.
FUNDING INFORMATION
No specific funding was received for this study.
CONFLICT OF INTEREST STATEMENT
MKH has received consultant and speaker fees from Alkermes, consultant fees from Merck, and holds an investigator‐initiated grant from Merck. GR has received research support from HLS Therapeutics. BE is part of the Advisory Board of Eli Lilly Denmark A/S, Janssen‐Cilag, Lundbeck Pharma A/S, and Takeda Pharmaceutical Company Ltd; and has received lecture fees from Bristol‐Myers Squibb, Boehringer Ingelheim, Otsuka Pharma Scandinavia AB, Eli Lilly Company, and Lundbeck Pharma A/S. SMA has received honoraria from HLS Therapeutics and has served on the advisory board for Boehringer Ingelheim, Canada. All other authors report no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1111/acps.13758.
Supporting information
Data S1: Supporting Information.
De R, Smith ECC, Navagnanavel J, et al. The impact of weight gain on antipsychotic nonadherence or discontinuation: A systematic review and meta‐analysis. Acta Psychiatr Scand. 2025;151(2):109‐126. doi: 10.1111/acps.13758
Riddhita De and Emily C. C. Smith are shared first authorship.
Gary Remington, Sri Mahavir Agarwal, and Margaret K. Hahn are shared senior authorship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Johnsen E, Kroken RA. Drug treatment developments in schizophrenia and bipolar mania: latest evidence and clinical usefulness. Ther Adv Chronic Dis. 2012;3(6):287‐300. doi: 10.1177/2040622312462275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lacro JP, Dunn LB, Dolder CR, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):15489. [DOI] [PubMed] [Google Scholar]
- 3. Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8(3):232‐241. doi: 10.1111/j.1399-5618.2006.00314.x [DOI] [PubMed] [Google Scholar]
- 4. Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58(6):855‐863. doi: 10.1176/ps.2007.58.6.855 [DOI] [PubMed] [Google Scholar]
- 5. Semahegn A, Torpey K, Manu A, Assefa N, Tesfaye G, Ankomah A. Psychotropic medication non‐adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta‐analysis. Syst Rev. 2020;9(1):17. doi: 10.1186/s13643-020-1274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028‐3035. doi: 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 7. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1‐46. quiz 47–48. [PubMed] [Google Scholar]
- 8. Velligan DI, Lam YWF, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32(4):724‐742. doi: 10.1093/schbul/sbj075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chue P, Cheung R. The impact of weight gain associated with atypical antipsychotic use in schizophrenia. Acta Neuropsychiatr. 2004;16(3):113‐123. doi: 10.1111/j.0924-2708.2004.00067.x [DOI] [PubMed] [Google Scholar]
- 10. Spertus J, Horvitz‐Lennon M, Abing H, Normand SL. Risk of weight gain for specific antipsychotic drugs: a meta‐analysis. NPJ Schizophr. 2018;4(1):12. doi: 10.1038/s41537-018-0053-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perkins DO. Adherence to antipsychotic medications. J Clin Psychiatry. 1999;60(suppl 21):445. [PubMed] [Google Scholar]
- 12. Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva V. Antipsychotic‐associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231‐2241. doi: 10.2147/NDT.S113099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fakhoury WK, Wright D, Wallace M. Prevalence and extent of distress of adverse effects of antipsychotics among callers to a United Kingdom National Mental Health Helpline. Int Clin Psychopharmacol. 2001;16(3):153‐162. doi: 10.1097/00004850-200105000-00004 [DOI] [PubMed] [Google Scholar]
- 14. Johnson FR, Özdemir S, Manjunath R, Hauber AB, Burch SP, Thompson TR. Factors that affect adherence to bipolar disorder treatments: a stated‐preference approach. Med Care. 2007;45(6):545‐552. [DOI] [PubMed] [Google Scholar]
- 15. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002;63(12):15093. [DOI] [PubMed] [Google Scholar]
- 16. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta‐analysis. Lancet Psychiatry. 2020;7(1):64‐77. doi: 10.1016/S2215-0366(19)30416-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma E, Rao NP, Venkatasubramanian G. Association between antipsychotic‐induced metabolic side‐effects and clinical improvement: a review on the Evidence for “metabolic threshold”. Asian J Psychiatry. 2014;8:12‐21. doi: 10.1016/j.ajp.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 18. Kim DD, Barr AM, Fredrikson DH, Honer WG, Procyshyn RM. Association between serum lipids and antipsychotic response in schizophrenia. Curr Neuropharmacol. 2019;17(9):852‐860. doi: 10.2174/1570159X17666190228113348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raben AT, Marshe VS, Chintoh A, Gorbovskaya I, Müller DJ, Hahn MK. The complex relationship between antipsychotic‐induced weight gain and therapeutic benefits: a systematic review and implications for treatment. Front Neurosci. 2018;11:741. Accessed December 30, 2022. doi: 10.3389/fnins.2017.00741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma E, Rao NP, Venkatasubramanian G, et al. Relation between weight gain and clinical improvement: is there a metabolic threshold for second generation antipsychotics? Psychiatry Res. 2011;1(186):155. doi: 10.1016/j.psychres.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 21. Smith ECC, Au E, Pereira S, et al. Clinical improvement in schizophrenia during antipsychotic treatment in relation to changes in glucose parameters: a systematic review. Psychiatry Res. 2023;328:115472. doi: 10.1016/j.psychres.2023.115472 [DOI] [PubMed] [Google Scholar]
- 22. Christian R, Saavedra L, Gaynes BN, et al. Tables of FDA‐approved indications for first‐ and second‐generation antipsychotics. Future Research Needs for First‐ and Second‐Generation Antipsychotics for Children and Young Adults. Agency for Healthcare Research and Quality (US); 2012. Accessed December 9, 2023. https://www.ncbi.nlm.nih.gov/books/NBK84656/ [PubMed] [Google Scholar]
- 23. Ader M, Garvey WT, Phillips LS, et al. Ethnic heterogeneity in glucoregulatory function during treatment with atypical antipsychotics in patients with schizophrenia. J Psychiatr Res. 2008;42(13):1076‐1085. doi: 10.1016/j.jpsychires.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akkaya C, Sarandol A, Cangur S, Kirli S. Retrospective database analysis on the effectiveness of typical and atypical antipsychotic drugs in an outpatient clinic setting. Hum Psychopharmacol Clin Exp. 2007;22(8):515‐528. doi: 10.1002/hup.882 [DOI] [PubMed] [Google Scholar]
- 25. Ascher‐Svanum H, Stensland M, Zhao Z, Kinon BJ. Acute weight gain, gender, and therapeutic response to antipsychotics in the treatment of patients with schizophrenia. BMC Psychiatry. 2005;5:3. doi: 10.1186/1471-244X-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bobes J, Rejas J, Garcia‐Garcia M, et al. Weight gain in patients with schizophrenia treated with risperidone, olanzapine, quetiapine or haloperidol: results of the EIRE study. Schizophr Res. 2003;62(1–2):77‐88. doi: 10.1016/S0920-9964(02)00431-0 [DOI] [PubMed] [Google Scholar]
- 27. Chavda RK, Laxmi L, Nair BS, Gandewar K. Efficacy and tolerability of aripiprazole in patients with schizophrenia & schizoaffective disorders. Indian J Psychiatry. 2004;46(2):150‐155. [PMC free article] [PubMed] [Google Scholar]
- 28. Chue P, Malla A, Bouchard RH, et al. The long‐term clinical benefit and effectiveness of switching to once‐daily quetiapine extended release in patients with schizophrenia. Curr Med Res Opin. 2013;29(3):227‐239. doi: 10.1185/03007995.2012.762903 [DOI] [PubMed] [Google Scholar]
- 29. Citrome L, Weiden PJ, Alva G, et al. Switching to iloperidone: an omnibus of clinically relevant observations from a 12‐week, open‐label, randomized clinical trial in 500 persons with schizophrenia. Clin Schizophr Relat Psychoses. 2015;8(4):183‐195. doi: 10.3371/CSRP.CIWE.103114 [DOI] [PubMed] [Google Scholar]
- 30. Covell NH, McEvoy JP, Schooler NR, et al. Effectiveness of switching from long‐acting injectable fluphenazine or haloperidol Decanoate to long‐acting injectable risperidone microspheres. J Clin Psychiatry. 2012;73(5):669‐675. doi: 10.4088/JCP.11m07074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Hert M, Mittoux A, He Y, Peuskens J. Metabolic parameters in the short‐ and long‐term treatment of schizophrenia with sertindole or risperidone. Eur Arch Psychiatry Clin Neurosci. 2011;261(4):231‐239. doi: 10.1007/s00406-010-0142-x [DOI] [PubMed] [Google Scholar]
- 32. Doane MJ, Sajatovic M, Weiden PJ, et al. Antipsychotic treatment experiences of people with schizophrenia: patient perspectives from an online survey. Patient Prefer Adherence. 2020;14:2043‐2054. doi: 10.2147/PPA.S270020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Durgam S, Greenberg WM, Li D, et al. Safety and tolerability of cariprazine in the long‐term treatment of schizophrenia: results from a 48‐week, single‐arm, open‐label extension study. Psychopharmacology (Berl). 2017;234(2):199‐209. doi: 10.1007/s00213-016-4450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Essock SM, Schooler NR, Stroup TS, et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702‐708. doi: 10.1176/appi.ajp.2011.10060908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graham KA, Perkins DO, Edwards LJ, Barrier RC, Lieberman JA, Harp JB. Effect of olanzapine on body composition and energy expenditure in adults with first‐episode psychosis. Am J Psychiatry. 2005;162(1):118‐123. doi: 10.1176/appi.ajp.162.1.118 [DOI] [PubMed] [Google Scholar]
- 36. Gray R, Bressington D, Lathlean J, Mills A. Relationship between adherence, symptoms, treatment attitudes, satisfaction, and side effects in prisoners taking antipsychotic medication. J Forensic Psychiatry Psychol. 2008;19(3):335‐351. doi: 10.1080/14789940802113493 [DOI] [Google Scholar]
- 37. Hofer A, Radner V, Edlinger M, Kemmler G, Rettenbacher MA, Fleischhacker WW. Why do indiviuals with schizophrenia drop out of observational clinical trials? Psychiatry Res. 2017;256:1‐5. doi: 10.1016/j.psychres.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 38. Huang M, Yu L, Pan F, et al. A randomized, 13‐week study assessing the efficacy and metabolic effects of paliperidone palmitate injection and olanzapine in first‐episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:122‐130. doi: 10.1016/j.pnpbp.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 39. Khan AA, Ashraf A, Baker D, et al. Clozapine and incidence of myocarditis and sudden death – long term Australian experience. Int J Cardiol. 2017;238:136‐139. doi: 10.1016/j.ijcard.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 40. Kim EY, Chang SM, Shim JC, et al. Long‐term effectiveness of flexibly dosed paliperidone extended‐release: comparison among patients with schizophrenia switching from risperidone and other antipsychotic agents. Curr Med Res Opin. 2013;29(10):1231‐1240. doi: 10.1185/03007995.2013.816277 [DOI] [PubMed] [Google Scholar]
- 41. Kinon BJ, Liu‐Seifert H, Adams DH, Citrome L. Differential rates of treatment discontinuation in clinical trials as a measure of treatment effectiveness for olanzapine and comparator atypical antipsychotics for schizophrenia. J Clin Psychopharmacol. 2006;26(6):632‐637. doi: 10.1097/01.jcp.0000245563.06660.0f [DOI] [PubMed] [Google Scholar]
- 42. Kishi T, Sakuma K, Okuya M, Hatano M, Iwata N. Outcomes of patients with schizophrenia who discontinued long‐acting injectable antipsychotic therapy due to adverse events: a chart review. Neuropsychopharmacol Rep. 2021;41(3):422‐425. doi: 10.1002/npr2.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kule M, Kaggwa MM. Adherence to typical antipsychotics among patients with schizophrenia in Uganda: a cross‐sectional study. Schizophr Res Treat. 2023;2023:e7035893. doi: 10.1155/2023/7035893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lambert M, Holzbach R, Moritz S, Postel N, Krausz M, Naber D. Objective and subjective efficacy as well as tolerability of olanzapine in the acute treatment of 120 patients with schizophrenia spectrum disorders. Int Clin Psychopharmacol. 2003;18(5):251. [DOI] [PubMed] [Google Scholar]
- 45. Leppig M, Bosch B, Naber D, Hippius H. Clozapine in the treatment of 121 out‐patients. Psychopharmacology (Berl). 1989;99(Suppl):S77‐S79. doi: 10.1007/BF00442565 [DOI] [PubMed] [Google Scholar]
- 46. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209‐1223. doi: 10.1056/NEJMoa051688 [DOI] [PubMed] [Google Scholar]
- 47. Liu‐Seifert H, Adams DH, Kinon BJ. Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post‐hoc analysis of four atypical antipsychotic drugs. BMC Med. 2005;3(1):21. doi: 10.1186/1741-7015-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsuzaki H, Hatano M, Iwata M, Yamada S. Treatment continuation of asenapine or olanzapine in Japanese schizophrenia patients: a propensity score matched study. Neuropsychiatr Dis Treat. 2021;17:3655‐3661. doi: 10.2147/NDT.S343840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McEvoy JP, Byerly M, Hamer RM, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA. 2014;311(19):1978‐1987. doi: 10.1001/jama.2014.4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miller S, Do D, Gershon A, et al. Longer‐term effectiveness and tolerability of adjunctive open lurasidone in patients with bipolar disorder. J Clin Psychopharmacol. 2018;38(3):207‐211. doi: 10.1097/JCP.0000000000000867 [DOI] [PubMed] [Google Scholar]
- 51. Montes JM, Rodriguez JL, Balbo E, et al. Improvement in antipsychotic‐related metabolic disturbances in patients with schizophrenia switched to ziprasidone. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):383‐388. doi: 10.1016/j.pnpbp.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 52. Mustafa S, Joober R, Lepage M, Iyer S, Shah J, Malla A. Predictors of ‘all‐cause discontinuation’ of initial oral antipsychotic medication in first episode psychosis. Schizophr Res. 2018;201:287‐293. doi: 10.1016/j.schres.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 53. Perkins AJ, Khandker R, Overley A, et al. Association of antipsychotic‐related weight gain with treatment adherence and switching using electronic medical records data. Prim Care Companion CNS Disord. 2023;25(2):46053. doi: 10.4088/PCC.22m03310 [DOI] [PubMed] [Google Scholar]
- 54. Piparva KG, Buch JG, Chandrani KV. Analysis of adverse drug reactions of atypical antipsychotic drugs in psychiatry OPD. Indian J Psychol Med. 2011;33(2):153‐157. doi: 10.4103/0253-7176.92067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosso G, Pessina E, Martini A, Di Salvo G, Maina G. Paliperidone palmitate and metabolic syndrome in patients with schizophrenia: a 12‐month observational prospective cohort study. J Clin Psychopharmacol. 2016;36(3):206‐212. doi: 10.1097/JCP.0000000000000494 [DOI] [PubMed] [Google Scholar]
- 56. Shajahan P, Keith S, Majjiga C, et al. Comparing the effectiveness of aripiprazole and quetiapine in schizophrenia and related psychoses: a naturalistic, retrospective chart review study. J Clin Psychiatry. Published online. 2009;70:7. [DOI] [PubMed] [Google Scholar]
- 57. Sliwa JK, Fu DJ, Bossie CA, Turkoz I, Alphs L. Body mass index and metabolic parameters in patients with schizophrenia during long‐term treatment with paliperidone palmitate. BMC Psychiatry. 2014;14(1):52. doi: 10.1186/1471-244X-14-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Treuer T, Hoffmann VP, Chen AKP, et al. Factors associated with weight gain during olanzapine treatment in patients with schizophrenia or bipolar disorder: results from a six‐month prospective, multinational, observational study. World J Biol Psychiatry. 2009;10(4–3):729‐740. doi: 10.1080/15622970903079507 [DOI] [PubMed] [Google Scholar]
- 59. Wang D, Wei N, Hu F, et al. Paliperidone extended release versus olanzapine in treatment‐resistant schizophrenia: a randomized, double‐blind, multicenter study. J Clin Psychopharmacol. 2022;42(4):383‐390. doi: 10.1097/JCP.0000000000001573 [DOI] [PubMed] [Google Scholar]
- 60. Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. 2004;66(1):51‐57. doi: 10.1016/S0920-9964(02)00498-X [DOI] [PubMed] [Google Scholar]
- 61. Zhou T, Pu C, Huang Z, et al. Weight changes following treatment with aripiprazole, risperidone and olanzapine: a 12‐month study of first‐episode schizophrenia patients in China. Asian J Psychiatry. 2023;84:103594. doi: 10.1016/j.ajp.2023.103594 [DOI] [PubMed] [Google Scholar]
- 62. Öhlund L, Ott M, Oja S, et al. Reasons for lithium discontinuation in men and women with bipolar disorder: a retrospective cohort study. BMC Psychiatry. 2018;18(1):37. doi: 10.1186/s12888-018-1622-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Balsikci A, Uzun O, Erdem M, Doruk A, Cansever A, Ates MA. Side effects that cause noncompliance to antidepressant medications in the course of outpatient treatment. Klin Psikofarmakol Bül‐Bull Clin Psychopharmacol. 2014;24(1):69‐75. doi: 10.5455/bcp.20120827114140 [DOI] [Google Scholar]
- 64. Velligan DI, Maples NJ, Pokorny JJ, Wright C. Assessment of adherence to oral antipsychotic medications: what has changed over the past decade? Schizophr Res. 2020;215:17‐24. doi: 10.1016/j.schres.2019.11.022 [DOI] [PubMed] [Google Scholar]
- 65. Velligan D, Mintz J, Maples N, et al. A randomized trial comparing in person and electronic interventions for improving adherence to oral medications in schizophrenia. Schizophr Bull. 2013;39(5):999‐1007. doi: 10.1093/schbul/sbs116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. eClinicalMedicine. 2023;58:101882. doi: 10.1016/j.eclinm.2023.101882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De R, Prasad F, Stogios N, et al. Promising translatable pharmacological interventions for body weight management in individuals with severe mental illness – a narrative review. Expert Opin Pharmacother. 2023;24(16):1823‐1832. doi: 10.1080/14656566.2023.2254698 [DOI] [PubMed] [Google Scholar]
- 68. Agarwal SM, Stogios N, Faulkner GEJ, Hahn M. Pharmacological interventions for the prevention of antipsychotic‐induced weight gain in people with schizophrenia: a cochrane systematic review and meta‐analysis. Schizophr Bull. 2023;49:833‐835. doi: 10.1093/schbul/sbad037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stogios N, Maksyutynska K, Navagnanavel J, et al. Metformin for the prevention of clozapine‐induced weight gain: a retrospective naturalistic cohort study. Acta Psychiatr Scand. 2022;146(3):190‐200. doi: 10.1111/acps.13462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


