Abstract
Although a previous study indicated that the dissimilatory metal-reducing bacterium Shewanella oneidensis MR-1 lacks chemotactic responses to metals that can be used as anaerobic electron acceptors, new results show that this bacterium responds to both Mn(III) and Fe(III). Cells were also shown to respond to another unusual electron acceptor, the humic acid analog anthraquinone-2,6-disulfonate. These results indicate that S. oneidensis is capable of moving towards a number of unusual anaerobic electron acceptors, including some that would normally be insoluble in the environment. Additionally, S. oneidensis was shown to migrate in gradients of several divalent cations under anaerobic conditions. Although responses to the reduced forms of redox-active metals, such as Mn(II) and Fe(II), might indicate that S. oneidensis uses gradients of these metals to locate the insoluble electron acceptors Mn(III/IV) and Fe(III) for dissimilatory purposes, responses to non-redox-active metals, such as Zn(II), suggest that movement towards divalent cations might serve other, potentially assimilatory, purposes.
The γ-proteobacterium Shewanella oneidensis MR-1 (previously known as Shewanella putrefaciens MR-1) (14) is a motile, nonfermenting, facultative anaerobe capable of growth by reduction of many terminal electron acceptors. Anaerobic electron acceptors that can be used by S. oneidensis include fumarate, dimethyl sulfoxide (DMSO), trimethylamine N-oxide (TMAO), the humic acid analog anthraquinone-2,6-disulfonate (AQDS), nitrate (NO3−), nitrite (NO2−), thiosulfate (S2O32−), sulfite (SO32−), elemental sulfur (S0), chromium [Cr(VI)], manganese [Mn(III/IV)], iron [Fe(III)], and uranium [U(VI)] (3, 4, 5, 6, 7, 8, 10). Although many of these electron acceptors are commonly used by facultative or obligate anaerobes, the ability to reduce metals and humic acids is less common. However, both processes are environmentally relevant because of the potential abundance of these electron acceptors in anoxic environments.
The dissimilatory reduction of metals such as iron and manganese plays an important role in their biogeochemical cycling. However, the inherent insolubility of most iron and manganese minerals suggests that colonization of fresh mineral surfaces might occur only after random collision events bring suitable microbes into close proximity with the minerals. Reduction of the mineral surface could then proceed, resulting in the creation of gradients of soluble Fe(II) or Mn(II) in the local environment. Gradients of these reduced metals would also be produced during the abiotic reduction of mineral surfaces.
The possibility that dissimilatory metal-reducing bacteria might use gradients of reduced metals to locate mineral surfaces was first proposed by Childers and coworkers after they observed that Geobacter metallireducens moves up gradients of Fe(II) or Mn(II) (1). However, a previous study showed that Bdellovibrio bacteriovorus, a bacterium that does not use metals for dissimilatory purposes, also responds to Mn(II) as a chemoattractant (11). This study implies that there may be a selective advantage for bacteria to move towards reduced manganese even when it is not associated with anaerobic respiration.
Relatively few studies have examined chemotaxis to anaerobic electron acceptors, and even fewer have looked for responses to metals that can be used as electron acceptors. However, Nealson and coworkers analyzed responses of S. oneidensis MR-1 to several electron acceptors, including Fe(III) and Mn(IV) (9). These authors reported that S. oneidensis MR-1 responds to nitrate, nitrite, fumarate, DMSO, TMAO, and thiosulfate, but not to Fe(III) citrate or colloidal Mn(IV) oxides. We have repeated these studies using two different assay formats and have shown that, under specific conditions, S. oneidensis responds to both Fe(III) and Mn(III). In addition, we have shown that S. oneidensis responds to gradients of the humic acid analog AQDS. Our results also show that, like G. metallireducens, S. oneidensis responds to several divalent cations, including Fe(II), Mn(II), Zn(II), and Ni(II).
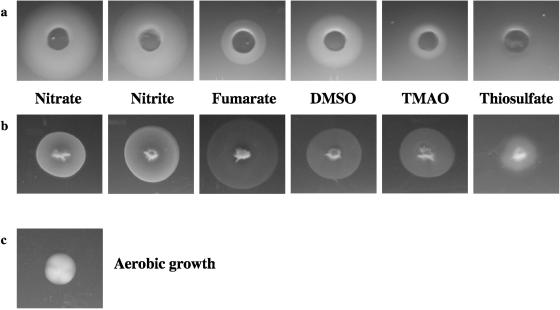
Identification of chemotactic responses to anaerobic electron acceptors by plug-in-pond assays.
S. oneidensis strain MR-1 was obtained from the American Type Culture Collection (ATCC 700550). Cells for use in chemotaxis assays were grown aerobically in Luria-Bertani (LB) medium at 30°C on a rotary shaker at 240 rpm. Mn(III) pyrophosphate and Fe(III) citrate were prepared as described previously (2). Stock solutions of NaNO3, NaNO2, fumaric acid, DMSO, TMAO, Na2S2O3, and AQDS were prepared from commercially available laboratory-grade chemicals. Hard agar (1.5% [wt/vol]) plugs (∼1.0-cm diameter) containing 20 mM of each electron acceptor to be tested were allowed to equilibrate overnight at room temperature in a Coy anaerobic chamber (5% H2, 95% N2). Soft agar (0.5% [wt/vol]) was prepared using equal volumes of overnight-grown culture (∼1010 cells/ml) and molten (∼45°C) LM basal medium (0.2 g yeast extract/liter, 0.1 g peptone/liter, 10 mM HEPES [pH 7.4], 10 mM NaHCO3). Because Mn(III) can be reduced abiotically by HEPES, assays analyzing responses to Mn(III) were performed in the absence of this buffer. Chloramphenicol (3 μg/ml) was added to the soft agar prior to pouring to prevent growth that might be confused with chemotactic responses. Duplicate sets of plates were prepared in which the redox indicator dye resazurin was incorporated into the soft agar to confirm that no oxygen was present in the assays. Plates were checked for responses periodically for 24 h. Plates were photographed immediately after their removal from the anaerobic chamber.
As shown by Nealson and coworkers, S. oneidensis responded to nitrate, nitrite, fumarate, DMSO, TMAO, and thiosulfate as attractants (Fig. 1a), although the response to thiosulfate was weak. No response was seen to Fe(III) citrate. Mn(III) pyrophosphate was used to study responses to oxidized manganese, and a strong response was observed to plugs containing soluble Mn(III). However, the cells formed several bands of different intensities at different distances from the plug (not shown). Because the multiple bands might have been caused by a combination of an attractant response to Mn(III) and a repellent response to pyrophosphate (or vice versa), responses to pyrophosphate were analyzed in the absence of Mn(III). Cells moved away from the Na pyrophosphate-containing plugs, indicating that Na pyrophosphate is a repellent for S. oneidensis. Thus, some cells in the Mn(III) pyrophosphate assay may have been moving towards Mn(III), whereas others may have been moving away from pyrophosphate. The lack of other chelating agents that solubilize Mn(III) make it impossible to test this hypothesis.
FIG. 1.
Chemotaxis assays showing responses to a range of anaerobic electron acceptors in either a plug-in-pond assay format (a) where cells accumulate around an agar plug containing the chemoattractant or a swarm plate assay format (b) where cells stabbed into soft agar swarm away from the initial stab as the attractant is metabolized. Weak responses to thiosulfate may have been caused by the disproportional reduction of thiosulfate which leads to an accumulation of hydrogen sulfide. The growth of cells on soft agar under aerobic conditions (c) is shown to contrast growth with swarming.
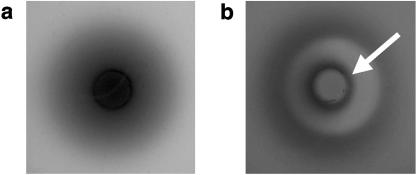
S. oneidensis was also shown to accumulate around plugs containing the humic acid analog AQDS. Under anoxic conditions, this response was obscured because the cells secrete a diffusible redox-active molecule that reduces the colorless AQDS to orange anthrahydraquinone-2,6-disulfonate (AHDS) (10). Responses to AQDS became obvious only after the plates were exposed to an aerobic environment. Figure 2a shows the location of AHDS both in and around an agar plug after 24 h of incubation. Figure 2b shows the location of AHDS in the same assay after a brief exposure to an aerobic environment. Under these conditions, AHDS was rapidly reoxidized to AQDS in areas of the plate where no cells were present (for example, in the agar plug itself). Thus, a band of cells that had moved towards AQDS could be identified surrounding the plug.
FIG. 2.
Chemotaxis to AQDS in a plug plate assay. Darker areas represent the location of the reduced form of AQDS, AHDS, which is orange in color. (a) Location of AHDS both in and around the plug under anaerobic conditions. (b) Location of AHDS after briefly exposing the plate to aerobic conditions. A brief exposure to aerobic conditions causes reoxidation of the AHDS. This reoxidation occurs faster where no cells are present. The arrow denotes the location of the cells that have accumulated around the agar plug.
Identification of chemotactic responses to anaerobic electron acceptors by swarm plate assays.
Swarm plate assays differ from plug-in-pond assays in that the bacteria are stabbed into soft agar and generate a gradient of the chemoattractant by metabolic activity (e.g., by reduction of electron acceptors during anaerobic respiration). The cells then move up the self-generated gradients. Thus, swarm plate assays do not necessarily measure chemotaxis but instead may measure the ability of cells to detect energetically favorable environments using a process called “energy taxis” (12). Although chemotaxis and “energy taxis” are mechanistically distinct, both responses allow cells to move in favorable directions and require a chemotaxis signal transduction system.
Swarm plates were prepared using the same set of electron acceptors that were tested previously in the plug-in-pond assays. Electron acceptors were added to the soft agar (0.4% LM agar) to a final concentration of 2 mM, and excess (20 mM) sodium lactate was added as the electron donor. Fe(III) citrate was added to swarm plates at higher concentrations (10 mM) to facilitate observation of the oxidized and reduced iron by color changes (oxidized iron is orange, and reduced iron is colorless). Plates were also prepared with no anaerobic electron acceptor. These plates were incubated either anaerobically or aerobically. All the plates were allowed to equilibrate in the anaerobic chamber for ∼2 days prior to inoculation. Resazurin was added to duplicate sets of plates to show that the cells were not moving in oxygen gradients. Cells for inoculation of the swarm plates were grown aerobically at 30°C on LB agar, scraped off, and stabbed into swarm agar with sterile pipette tips. Plates were examined periodically over the course of 48 h (or longer) and then either photographed in the anaerobic chamber or removed from the chamber for photographic documentation.
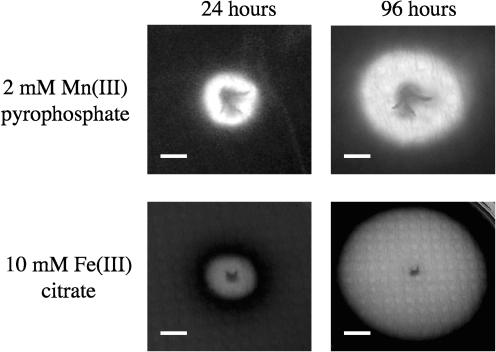
Results of swarm plate assays with the electron acceptors nitrate, nitrite, fumarate, DMSO, TMAO, and thiosulfate are shown in Fig. 1b. In each case, the cells formed a swarm with a sharply defined edge, although the response to thiosulfate was again weak, and the swarm was more diffuse. Plates that contained no anaerobic electron acceptor and that were incubated under anaerobic conditions showed no swarms. Plates that contained no anaerobic electron acceptor and that were incubated under aerobic conditions showed growth rather than swarming (Fig. 1c). Responses to Fe(III) citrate and Mn(III) pyrophosphate are shown in Fig. 3. In these assays swarms of cells are not seen. Instead, an expanding zone of reduced metal is observed around the initial stab. The location of a moving band of cells at the interface between the oxidized and reduced metals was determined microscopically after sections of agar were sliced from the swarm plates. Responses to AQDS were again difficult to interpret because a large diffuse area of AHDS was observed surrounding the initial stab (again due to the production of the diffusible redox-active molecule that reduces AQDS). However, as previously, reoxidation of the AHDS in these plates by exposure to an aerobic environment showed that a swarm of cells was present in the soft agar. Thus, the swarm plate assays indicate that S. oneidensis moves towards a wide range of anaerobic electron acceptors, including Fe(III), Mn(III), and AQDS.
FIG. 3.
Swarm plate assays showing responses to 2 mM Mn(III) pyrophosphate (top) and Fe(III) citrate (bottom) after 24 and 96 h. The respiring cells localize at the interface between the oxidized and reduced forms of the metals. Bars, 1 cm.
The ability of cells to localize at the interface of oxidized and reduced terminal electron acceptors in swarm plate assays could indicate that the cells swim up gradients of the oxidized form of the electron acceptor, a response that presumably requires “energy taxis.” Alternatively, the cells might swim away from the reduced form of the electron acceptor, a response that would require chemotaxis. For example, Fe(III) might be an attractant, or Fe(II) might be a repellent. To resolve this question, plug-in-pond assays testing responses to reduced metals were performed to determine whether Fe(II) and Mn(II) are repellents for S. oneidensis.
Behavioral responses of S. oneidensis to Mn(II) and Fe(II).
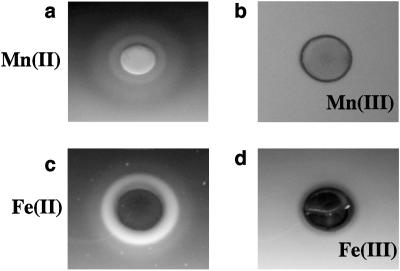
Stock solutions of MnCl2 and FeCl2 were prepared and added to hard agar at 20 mM. Mn(II) and Fe(II) elicited clear attractant responses (Fig. 4a and c). The difference from the expected result (that these reduced metals would act as repellents for S. oneidensis) and the actual result (that these reduced metals are attractants) might have been caused by differences in the swarm and plug-in-pond assay formats, particularly because of the absence of an electron acceptor in the plug-in-pond assays. Therefore, additional assays were performed in which electron acceptors were incorporated into the soft agar surrounding the plugs during the assay. In these assays, 20 mM Fe(II) or Mn(II) was added to the agar plugs, and 2 mM Fe(III) or Mn(III) was added to the soft agar. In the presence of these electron acceptors, no response to the reduced metals was observed (Fig. 4b and d), indicating that S. oneidensis responds to reduced metals only in the absence of electron acceptors.
FIG. 4.
Plug plate assays showing responses to Mn(II) (upper row) and Fe(II) (lower row). Responses to Mn(II) were screened for in the absence (a) or presence (b) of Mn(III) pyrophosphate. Responses to Fe(II) were screened for in the absence (c) or presence (d) of Fe(III) citrate.
Behavioral responses to non-redox-active divalent cations.
The ability of S. oneidensis to respond behaviorally to Mn(II) and Fe(II) might suggest that, in the absence of electron acceptors, this microbe moves up gradients of these reduced metals to locate mineral surfaces. However, alternative explanations are also possible. One is that S. oneidensis might be attracted to Fe(II) because it can be used in assimilatory pathways (for incorporation into cytochromes and iron-sulfur proteins). Assimilation of Mn(II) would be harder to rationalize as a selective advantage, although S. oneidensis does appear to encode a manganese-dependent inorganic pyrophosphatase. To test this possibility, the ability of S. oneidensis to respond to other divalent cations that are not redox active but that are involved in assimilatory pathways was examined. NiCl2 and ZnCl2 were incorporated in agar plugs at 20 mM, and plug-in-pond assays were performed in the absence of electron acceptors. Plugs containing Zn(II) elicited a clear attractant response (not shown). The attractant response to Ni(II) was less obvious, although the cells did appear to move towards nickel transiently, after ∼8 h of incubation.
Responses to Zn(II) and Ni(II) were also tested under aerobic conditions (since neither metal is redox active). In these assays, Zn(II) again elicited a clear attractant response, but Ni(II) elicited a repellent response. Repellent responses to toxic metals, such as nickel, have been reported previously under aerobic conditions (13). Because zinc is a required trace element under both aerobic and anaerobic conditions, whereas nickel is a required trace element for some bacteria only under anaerobic conditions, the observed responses to these metals can be understood as behavioral adaptations to assimilatory requirements.
Behavioral responses to metals and anaerobic electron acceptors.
The results of this study indicate that the respiratory flexibility displayed by S. oneidensis is mirrored by an equivalent flexibility in behavioral responses to electron acceptors. Our data suggest that S. oneidensis can respond to the electron acceptors nitrate, nitrite, fumarate, DMSO, TMAO, thiosulfate, Fe(III), Mn(III), and AQDS. Most of these data agree with the previous study by Nealson and coworkers (9). Neither study observed responses to Fe(III) citrate in plug-in-pond assays. However, our results suggest that S. oneidensis can respond to Fe(III) in swarm plate assays. This result suggests that S. oneidensis does not use chemotaxis to locate soluble Fe(III) but may use “energy taxis” to move towards this energetically favorable electron acceptor. Our data also suggest that S. oneidensis can move towards soluble Mn(III). Although responses to Mn(III) pyrophosphate were difficult to interpret in the plug-in-pond assay, clear responses were observed in the swarm plate assay. Thus, S. oneidensis may respond to Mn(III) using the same mechanism used for responding to Fe(III), i.e., “energy taxis.” However, the possibility remains that S. oneidensis may respond to soluble Mn(III) using true chemotaxis. In either case, as far as we are aware, this report documents for the first time behavioral responses by bacteria to oxidized metals. Lastly, responses to an unusual electron acceptor, AQDS, were also observed during this study, although these responses were obscured under anoxic conditions, because S. oneidensis produces a redox-active molecule capable of AQDS reduction.
The ability of S. oneidensis to move towards anaerobic electron acceptors, including metals, using either chemotaxis or “energy taxis,” suggests that bacteria with extensive respiratory versatility may use a variety of mechanisms to locate environments favorable for growth. The ability of S. oneidensis to respond to Mn(II) and Fe(II) might be an additional example of this versatility. Observation of this same phenomenon in G. metallireducens and S. oneidensis suggests that several dissimilatory metal-reducing bacteria may respond to the reduced forms of certain metals in order to locate mineral surfaces containing oxidized metals. The possibility that S. oneidensis moves towards Fe(II) and Mn(II) for assimilatory purposes seems unlikely because the responses were not observed when the cells were actively respiring. In contrast, responses to zinc were observed under both anaerobic conditions (where no electron acceptor was available) and aerobic conditions, suggesting that these responses might be associated with assimilatory metabolism.
In conclusion, we propose that S. oneidensis has two distinct mechanisms for locating Fe(III) or Mn(III) mineral surfaces. Firstly, it can respond to soluble forms of the oxidized metals, using “energy taxis.” Secondly, it can respond chemotactically to reduced forms of these metals in the absence of electron acceptors. Thus, S. oneidensis may gain a competitive advantage over other dissimilatory metal-reducing bacteria in the colonization of mineral surfaces in anoxic environments.
Acknowledgments
This work was supported by the start-up funding package provided to M.J.W. by the Whiting School of Engineering, Johns Hopkins University.
REFERENCES
- 1.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 2.Kostka, J., and K. H. Nealson. 1998. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria, p. 58-78. In R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (ed.), Techniques in microbial ecology. Oxford University Press, Inc., Oxford, England.
- 3.Krause, B., and K. H. Nealson. 1997. Physiology and enzymology involved in denitrification by Shewanella putrefaciens. Appl. Environ. Microbiol. 63:2613-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovley, D. R., E. J. P. Phillips, and D. J. Lonergan. 1989. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl. Environ. Microbiol. 55:700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovley, D. R., E. J. P. Phillips, Y. A. Gorby, and E. R. Landa. 1991. Microbial reduction of uranium. Nature 350:413-416. [Google Scholar]
- 6.Moser, D. P., and K. H. Nealson. 1996. Growth of the facultative anaerobe Shewanella putrefaciens by elemental sulfur reduction. Appl. Environ. Microbiol. 62:2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 8.Myers, C. R., B. P. Carstens, W. E. Antholine, and J. M. Myers. 2000. Chromium(VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Appl. Microbiol. 88:98-106. [DOI] [PubMed] [Google Scholar]
- 9.Nealson, K. H., D. P. Moser, and D. A. Saffarini. 1995. Anaerobic electron acceptor chemotaxis in Shewanella putrefaciens. Appl. Environ. Microbiol. 61:1551-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 11.Straley, S. C., A. G. LaMarre, L. J. Lawrence, and S. F. Conti. 1979. Chemotaxis of Bdellovibrio bacteriovorus toward pure compounds. J. Bacteriol. 140:634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor, B. L., I. B. Zhulin, and M. S. Johnson. 1999. Aerotaxis and other energy-sensing behavior in bacteria. Annu. Rev. Microbiol. 53:103-128. [DOI] [PubMed] [Google Scholar]
- 13.Tso, W., and J. Adler. 1974. Negative chemotaxis in Escherichia coli. J. Bacteriol. 118:560-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]