Abstract
The kdpFABC operon of Escherichia coli, coding for the high-affinity K+ transport system KdpFABC, is transcriptionally regulated by the products of the adjacently located kdpDE genes. The KdpD protein is a membrane-bound sensor kinase consisting of a large N-terminal domain and a C-terminal transmitter domain interconnected by four transmembrane segments (the transmembrane segments together with the C-terminal transmitter domain of KdpD are referred to as CTD), while KdpE is a cytosolic response regulator. We have cloned and sequenced the kdp operon from a nitrogen-fixing, filamentous cyanobacterium, Anabaena sp. strain L-31 (GenBank accession. number AF213466). The kdpABC genes are similar in size to those of E. coli, but the kdpD gene is short (coding only for 365 amino acids), showing homology only to the N-terminal domain of E. coli KdpD. A kdpE-like gene is absent in the vicinity of this operon. Anabaena KdpD with six C-terminal histidines was overproduced in E. coli and purified by Ni2+-nitrilotriacetic acid affinity chromatography. With antisera raised against the purified Anabaena KdpD, the protein was detected in Anabaena sp. strain L-31 membranes. The membrane-associated or soluble form of the Anabaena KdpD(6His) could be photoaffinity labeled with the ATP analog 8-azido-ATP, indicating the presence of an ATP binding site. The coproduction of Anabaena KdpD with E. coli KdpD-CTD decreased E. coli kdpFABC expression in response to K+ limitation in vivo relative to the wild-type KdpD-CTD protein. In vitro experiments revealed that the kinase activity of the E. coli KdpD-CTD was unaffected, but its phosphatase activity increased in the presence of Anabaena KdpD(6His). To our knowledge this is the first report where a heterologous N-terminal domain (Anabaena KdpD) is shown to affect in trans KdpD-CTD (E. coli) activity, which is just opposite to that observed for the KdpD-N-terminal domain of E. coli.
In bacteria, K+ plays an important role in the maintenance of intracellular pH, cell turgor, and control of cellular enzyme activities (10) and is also known to positively regulate the expression of certain osmoresponsive genes (26). In bacteria the intracellular K+ levels are maintained by a variety of K+ uptake and efflux systems. The KdpFABC complex (K+- dependent ATPase), encoded by the kdpFABC operon (2), is an inducible, high-affinity K+ transporter synthesized by Escherichia coli during potassium limitation (11). The expression of the kdpFABC operon is regulated by the products of the adjacent kdpDE genes (27). The membrane-bound sensor kinase KdpD and the cytosolic response regulator KdpE constitute a typical prokaryotic two-component (sensor kinase/response regulator) signal transduction system (37).
The E. coli KdpD (894 amino acids, 99 kDa) consists of a cytosolic N-terminal domain (NTD) and a cytosolic C-terminal domain interconnected by four transmembrane segments (the transmembrane segments together with the C-terminal transmitter domain of KdpD are referred to as CTD) (40). In response to appropriate stimuli, KdpD becomes autophosphorylated. The phosphoryl group is transferred to KdpE, and the phosphorylated KdpE (KdpE∼P) activates transcription of the kdpFABC operon (24). KdpD is a bifunctional enzyme, i.e., it phosphorylates KdpE and is also responsible for dephosphorylation of KdpE∼P. This phosphatase activity is stimulated by ATP or its nonhydrolyzable analogs (17). The C-terminal domain resembles the transmitter domain of other kinases and carries the conserved His673, critical for phosphotransfer reactions (35) and all the other essential features of sensor histidine kinases. The NTD has been shown to be essential for maximal kdpFABC expression in E. coli (28). KdpD lacking the NTD responds poorly to loss of turgor or K+ limitation in vivo and shows deregulated (ATP independent) phosphatase activity in vitro (17). However, the exact stimulus that is sensed by KdpD is not clear (for more details, see references 5, 16, 23, and 32).
kdp homologues were initially shown to be present among gram-negative bacteria (36). Genome sequence analyses have now revealed that gram-positive bacteria and Archaea also possess kdp homologues. Apte and Alahari (3) have earlier demonstrated the presence of the KdpB polypeptide in three strains of Anabaena. Anabaena KdpB protein has been shown to be present in the cytoplasmic membrane under K+-limiting conditions (1).
To understand the organization and regulation of expression of the kdp genes in Anabaena spp., we cloned and sequenced the kdp operon (GenBank accession number AF213466) from a heterocystous, filamentous cyanobacterium, Anabaena sp. strain L-31. In comparison to the E. coli kdpFABC operon, kdpF is missing, but a new gene, kdpG, coding for a small hydrophobic protein was found between kdpB and kdpC. Interestingly, only a short kdpD gene (coding for 365 amino acids) was observed, while a kdpE-like gene was absent in the vicinity of the Anabaena sp. strain L-31 kdp operon. Anabaena KdpD showed homology to the NTD of E. coli KdpD, while the transmembrane segments and the C-terminal domain (which exhibits the histidine kinase activity) were missing. Genome sequencing has revealed the presence of similar short versions of kdpD in other bacteria, such as Synechocystis sp. strain PCC 6803 (20), Anabaena sp. strain PCC 7120 (21), and Deinococcus radiodurans R1 (39), but they have not been characterized from any of them. We report here on the cloning and the in vivo expression of a naturally short KdpD homolog from Anabaena sp. strain L-31, and show that it affects, in trans, the activity of the membrane-bound E. coli KdpD-CTD.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The filamentous, heterocystous, nitrogen-fixing freshwater cyanobacterium Anabaena sp. strain L-31 (33) was grown under axenic conditions. Stock cultures were maintained in standard BG-11 medium (8) without combined nitrogen. E. coli DH5α [F−φ80dlacZΔM15 Δ(lac-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96relA1] was employed as the carrier for the plasmids. E. coli BL21(DE3)pLysS [F− ompT hsdSB(rB − mB−) gal dcm (DE3) pLysS(Cmr)] (Novagen) was used to overproduce Anabaena KdpD with a six-histidine tag (6His). E. coli TKW22692 (kdpD6-92 trkA405 trkD1) was used for in vivo studies of KdpD/Δ12-395 (CTD) with Anabaena KdpD(6His). E. coli TKR2000 (Δ kdpFABCDE trkA405 trkD1 atp706) was used for coexpression of E. coli KdpD-CTD and Anabaena KdpD(6His). pBD6-92 (17) was digested with XmaI and HindIII and the 1.2-kb DNA fragment was transferred to similarly digested pBAD33 (13) vector to obtain pBD3/Δ12-395.
Cloning of the Anabaena kdpD gene.
Anabaena sp. strain L-31 chromosomal DNA was prepared as described previously (4). Degenerate primers D2 (GGN GTN GGN AAA ACC TAN CGN ATG TT) and D3 (CGY AAN GCN AGY TCY CTS AGN GC) were used to amplify the Anabaena sp. strain L-31 chromosomal DNA with 2.5 U Red Taq (Sigma). The 0.56-kb PCR product was cloned into pUC18 to yield vector pABD500 using the Sure Clone kit (Pharmacia) and sequenced. Anabaena sp. strain L-31 chromosomal DNA was digested with different restriction endonucleases, resolved on 0.8% agarose gel, and transferred to a nylon membrane (0.45 μm, Schleicher and Schuell). The 0.56-kb pABD500 insert was labeled with digoxigenin by the random priming method and hybridized to the restriction-digested Anabaena sp. strain L-31 DNA. The hybridization and posthybridization washes and chemiluminescent detection were carried out according to the manufacturer's instructions (Roche Biochemicals).
Construction of Anabaena KdpD(6His).
Anabaena sp. strain L-31 chromosomal DNA was amplified with primers KdpDoefwd 5′ GCG CCC ATG GCT AAT TAC CAA TTA AAA 3′, KdpDoerev 5′ GCG GAT CCC TAT TAG TGA TGG TGA TGG TGA TGG CGT TTG GAT GAG ACT TTT TCG CTG GC 3′. The forward primer contained a site for the restriction enzyme NcoI (underlined), while the reverse primer contained six His codons followed by an in-frame stop codon with the last sense codon of the Anabaena sp. strain L-31 kdpD (the Anabaena sp. strain L-31 sequence is underlined). The 1.1-kb PCR product obtained was cloned into pBluescript SK− vector (pBScD1) and sequenced to confirm the identity of the Anabaena kdpD(6His). The Anabaena kdpD(6His) fragment was transferred as an NcoI-BamHI or NcoI-KpnI fragment to the pET16(b) vector (pETD1) or pBAD24 vector (pBADD1). The plasmid DNA was sequenced on both strands by the dideoxy chain termination reaction (SEQLAB, Goettingen, Germany).
Overproduction, detection, and purification of Anabaena KdpD(6His).
E. coli BL21(DE3)/pLysS/pETDI was grown in complex KML medium (1% tryptone, 0.5% yeast extract, 1% KCl) with appropriate antibiotics to an optical density at 600 nm (OD600) of ∼1.0 and induced with 1 mM isopropylthiogalactopyranoside (IPTG) for 3 h. The cells were shock frozen in liquid nitrogen, thawed on ice, and resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, pH 8.0) and sonicated (10-sec bursts at 200 to 300 W with a 10-sec cooling period between each burst). The cytosolic and membrane fractions were prepared as described before (19) and aliquots of each were electrophoretically resolved on 11% sodium dodecyl sulfate (SDS)-polyacrylamide gels. Anabaena KdpD(6His) was detected by staining with Coomassie brilliant blue or on Western blots using the monoclonal penta-His antibody (QIAGEN). For purification purposes, the E. coli BL21(DE3)/pLysS/pETD1 membrane containing Anabaena KdpD(6His) was solubilized with the detergent Aminoxid WS 35 (1% final concentration) for 30 min at 4°C with gentle stirring. The solubilized mixture was centrifuged at 240,000 × g for 45 min. The membrane-solubilized or cytosolic Anabaena KdpD(6His) was allowed to bind to Ni2+-nitrilotriacetic acid (NTA)-agarose with gentle shaking at 4°C for 1 h. Binding to the Ni2+-NTA resin slurry, washing, and subsequent elution steps were performed as described (QIAexpressionist). To raise antisera against Anabaena KdpD, Anabaena KdpD(6His) purified from E. coli membranes was employed for immunization. The primary and booster immunizations (subcutaneous injections in the back of a rabbit) and collection of the antisera were performed as described (29).
Photoaffinity labeling of Anabaena KdpD(6His) with 8-azido-[α-32P]ATP.
Everted vesicles from E. coli BL21(DE3)/pLysS containing Anabaena KdpD(6 His) were washed once with buffer of low ionic strength (1 mM Tris, pH 7.5, 3 mM EDTA). Vesicles with or without the His-tagged Anabaena KdpD or the purified Anabaena KdpD(6His) were incubated with 5 μCi of 8-azido-[α-32P]ATP (20 Ci/mmol) in lysis buffer (total reaction volume, 25 μl) containing 10 mM MgCl2, in the presence or absence of 2 mM ATP, for 5 min at room temperature. Cross-linking was achieved by irradiating with UV light at 302 nm for 0.5 min. All samples were immediately subjected to electrophoresis on 11% SDS-polyacrylamide gels. The gels were dried and the radiolabeled proteins detected by a PhosphorImager (Molecular Dynamics). Gels were also stained with Coomassie blue to confirm equal protein loading.
Coexpression, coelution, and phosphorylation of Anabena KdpD(6His) and E. coli KdpD-CTD.
For in vivo coexpression experiments E. coli TKW22692 transformed with pBluescript or pBScD1 was grown in K115 medium (12) to an OD600 of 1.0. Cells were collected by centrifugation and, after washing, immediately resuspended in the same volume of medium containing 0.1 mM K+. After 30 min the cells were centrifuged, lysed in SDS sample buffer, and semi-dry blotted onto a nitrocellulose membrane. The expression of the kdpFABC operon was monitored using antisera raised against purified E. coli KdpC (30).
The in vitro studies were performed with E. coli TKR2000 cells cotransformed with pBADDI and pBD3/Δ12-395. The cells were grown in KML medium containing 0.2% arabinose to an OD600 of ∼1.0 and harvested by centrifugation, and everted membranes were prepared as described before (19). The kinase assay was performed as described previously (17). The dephosphorylation of KdpE∼32P by E. coli KdpD-CTD in the presence or absence of Anabaena KdpD(6His) with ATP-γ-S was performed as described before (15). For coelution experiments E. coli TKR2000/pBADD1/pBD3/Δ12-395 cells coexpressing Anabaena KdpD(6His) and E. coli KdpD-CTD were lysed by sonication, and the lysate was solubilized with 1% Aminoxid. The solubilized proteins (5 mg/ml total protein) were allowed to bind to Ni2+-NTA resin at 4°C for 1 h. The Ni2+-NTA resin was thoroughly washed with buffer B [50 mM Tris/HCl, pH 7.5, 10% (vol/vol) glycerol, 0.3 M NaCl, 0.04% Aminoxid, 10 mM imidazole], and the bound proteins were eluted by increasing the imidazole concentration to 200 mM.
Analytical procedures.
Protein concentrations were determined by a modified Lowry method (25) with bovine serum albumin as the standard. Proteins were separated on SDS-polyacrylamide gel electrophoresis (PAGE) using 11% or 12.5% polyacrylamide gels (22). For microsequencing the Coomassie-stained protein bands were cut from polyacrylamide gels, transferred to a polyvinylidene difluoride membrane (Millipore) by electroblotting, and sequenced on an Applied Biosystems A473a protein sequencer. A dendrogram depicting evolutionary relationships between 20 different KdpD protein sequences was constructed with the ClustalW software program, available at www.clustalw.genome.ad.jp.
Nucleotide sequence accession number.
The nucleotide sequence of Anabaena kdpD has been submitted to GenBank (accession number AF213466).
RESULTS
Cloning and sequence analysis of kdpD from Anabaena sp strain L-31.
Two degenerate PCR primers used to amplify the Anabaena sp. strain L-31 kdpD from chromosomal DNA were based on the conserved amino acid sequences GVGKT(W/Y)(A/R)ML and ALR(E/K)LAL present in KdpD-NTD. The deduced amino acid sequence of the 0.56-kb product showed high similarity to the KdpD-NTD of different bacteria (e.g., 68% with Synechocystis sp. strain 6803, 37% with E. coli). This Anabaena kdpD gene fragment, when used as a probe on Southern blots with restriction-digested Anabaena sp. strain L-31 DNA, showed single bands at 3.1 kb (HindIII), 5.5 kb (EcoRV), and 2.8 kb (EcoRV and ClaI) (data not shown). The 3.1-kb region of the HindIII or the 2.8-kb region of the EcoRV and ClaI restriction-digested Anabaena sp. strain L-31 chromosomal DNA was eluted from the gel and ligated to the HindIII-digested pUC19 or EcoRV- and ClaI-digested pBluescript SK−, respectively.
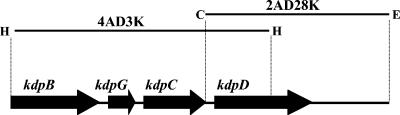
The colonies obtained upon transformation were hybridized to the digoxigenin-labeled pABD500 insert to identify the larger genomic Anabaena kdpD fragment. Two clones, 4AD3K (3.1-kb HindIII fragment in pUC19) and 2AD28K (2.8-kb ClaI-EcoRV fragment in pBluescript SK−) were identified by colony hybridizations with the Anabaena sp. strain L-31 kdpD probe and sequenced. The two plasmids showed an overlap of 974 bp, with p2AD28K containing the entire kdpD gene (Fig. 1).
FIG. 1.
Location of Anabaena sp. strain L-31 kdp genes in p4AD3K and p2AD28K. The kdpB, kdpG, kdpC, and kdpD ORFs are shown by thick arrows. The p4AD3K insert contains the 3′ end of Anabaena kdpB, complete kdpG, kdpC, and 840 bp of the Anabaena kdpD gene. The p2AD28K insert shows a 974-bp overlap with the p4AD3K insert and contains the entire kdpD gene. Restriction sites: H, HindIII; C, ClaI; and E, EcoRV.
The Anabaena sp. strain L-31 kdpD gene was located immediately downstream of kdpC in the kdp operon, while no “kdpE-like” gene was found downstream of kdpD (Fig. 1). The Anabaena sp. strain L-31 kdpD open reading frame (ORF) was found to be 1,098 bp in length and encoded a KdpD protein of only 365 amino acids, compared to 894 amino acids in E. coli KdpD. The Anabaena KdpD protein corresponded only to the NTD of E. coli KdpD, while the four transmembrane segments and the C-terminal transmitter domain found in other full-length KdpDs were missing. All the conserved amino acids present in KdpD-NTD, especially the slightly modified Walker A and Walker B motifs (14, 38), were also present in Anabaena KdpD.
Overproduction and purification of Anabaena KdpD(6His) from E. coli.
To facilitate its biochemical characterization and purification, Anabaena KdpD with six additional histidine codons in-frame was cloned into the pET16b vector (pETD1) and transformed into E. coli BL21(DE3)/pLysS. Upon induction with IPTG, the E. coli BL21(DE3)/pLysS/pETD1 cells synthesized a 42-kDa protein that could clearly be seen on SDS gels. The identity of the protein was confirmed by Western blot analysis using monoclonal penta-His antibodies (data not shown).
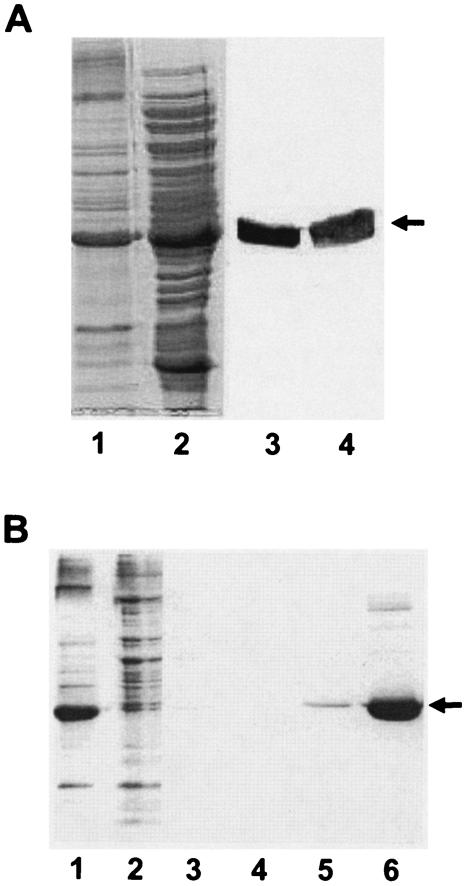
Membrane and cytosolic fractions were prepared from E. coli BL21(DE3)/pLysS/pETD1 cells containing the overproduced Anabaena KdpD(6His). Electrophoretic resolution of these fractions followed by Western blotting and immunodetection (with anti-penta-His) revealed that the Anabaena KdpD(6His) protein was present in both the cytosolic and membrane fractions (Fig. 2A). Anabaena KdpD(6His) was purified to near homogeneity from E. coli cytosolic and solubilized membrane fractions by affinity chromatography using Ni2+-NTA-agarose. In Fig. 2B the purification steps are only shown for the membrane fraction. The identity of Anabaena KdpD(6His) was confirmed by immunoblotting using the monoclonal penta-His antibodies and by N-terminal amino acid microsequencing (data not shown).
FIG. 2.
(A) Localization of Anabaena KdpD(6His) in E. coli cell fractions. Membranes and cytosolic fractions of BL21(DE3)/pLysS/pETDI were prepared as described in Materials and Methods. For comparison, the membrane pellet obtained after the ultracentrifugation step was resuspended (in lysis buffer) in a volume equal to that of the supernatant (cytosol). Lane 1, membrane vesicles (20 μl); lane 2, cytosol (20 μl). The samples were also electroblotted onto a nitrocellulose membrane and probed with the monoclonal anti-His antibody. Detection was carried out by a secondary anti-mouse immunoglobulin G coupled to alkaline phosphatase (lanes 3 and 4). Anabaena KdpD(His6) is indicated by an arrow. (B) Purification of Anabaena KdpD(6His) from E. coli membranes. Anabaena KdpD(6His) was solubilized with 1% Aminoxid WS35 from E. coli BL21(DE3)/pLysS/pETDI membranes and purified by affinity chromatography on an Ni-NTA matrix. The samples were resolved on an SDS-polyacrylamide (11%) gel and stained with Coomassie brilliant blue. Lane 1, solubilized membranes (10 μg); lane 2, unbound fraction (10 μg); lane 3, 10 mM imidazole wash; lane 4, 20 mM imidazole wash; lane 5, 40 mM imidazole wash; lane 6, 200 mM imidazole elution (5 μg). The position of Anabaena KdpD(His6) is indicated by an arrow.
In vivo synthesis of the KdpD protein in Anabaena sp. strain L-31.
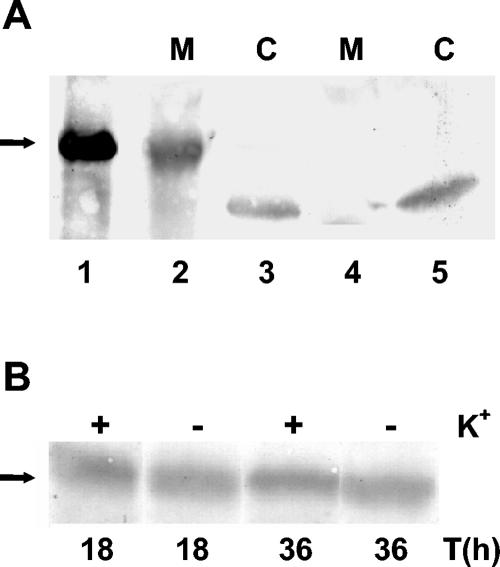
Polyclonal antisera were raised against Anabaena KdpD(6His) (purified from E. coli membranes) and used to detect the KdpD protein in Anabaena sp. strain L-31. The 42-kDa Anabaena KdpD protein could be clearly detected in the Anabaena sp. strain L-31 membrane fraction, while no protein was observed in the cytosol (Fig. 3A). The 39-kDa band seen in the cytosol appears to be a nonspecific signal, as it was also observed with the preimmune serum (control).
FIG. 3.
(A) Localization of KdpD in Anabaena sp. strain L-31. The antiserum raised against purified KdpD(6His) was used to detect the KdpD protein from Anabaena sp. strain L-31. Anabaena sp. strain L-31 membrane (M) and cytosolic (C) fractions from cells grown in BG11K5 medium were prepared as described (1). About 200 μg of protein was loaded in lanes 2 to 5, while 150 ng of purified Anabaena KdpD(6His) was loaded in lane 1. Lanes 1 to 3 were probed with the Anabaena KdpD(6His) antiserum, while lanes 4 and 5 were probed with the control preimmune serum. (B) Effect of K+ limitation on kdpD expression in Anabaena sp. strain L-31. Anabaena sp. strain L-31 cells were grown in BG11K5 medium and then transferred to BG11 medium containing 0 mM or 5 mM K+. At the times indicated, the cells were harvested, membranes were isolated, 200 μg total membrane protein was electrophoresed, and Anabaena KdpD (shown by the arrow) was detected as before.
E. coli KdpD has been shown to be located in the cell membrane, and the amount of this protein increases with K+ limitation (35). To test whether this also holds true for Anabaena KdpD, cells were grown in the presence or absence of 5 mM K+ and the membranes were isolated. When probed with Anabaena KdpD antiserum, an almost equal amount of KdpD was seen under all conditions (Fig. 3B). Thus, the Anabaena kdpD gene appeared to be constitutively expressed, and K+ limitation did not enhance its expression level.
Detection of ATP binding to Anabaena KdpD(6His) by photoaffinity labeling with 8-azido-[α-32P]ATP.
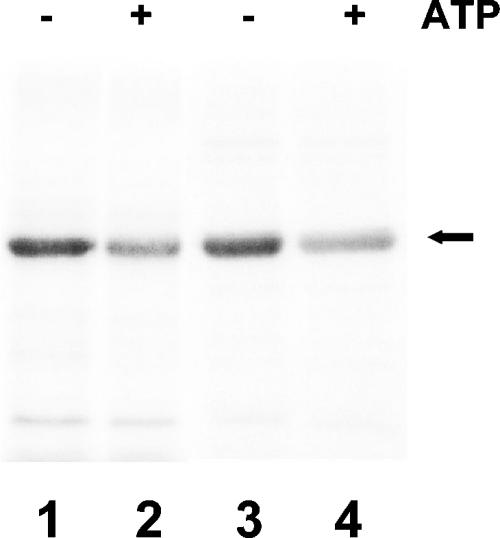
Anabaena KdpD has a region similar to the Walker A (GMAPGVGKT) and Walker B (LVLVD) motifs (38). These sites have been proposed to play an important role in modulating the activity of E. coli KdpD (17). The NTD of E. coli KdpD has been shown to bind 8-azido[α-32P]ATP (14). Everted E. coli BL-21 membrane vesicles or purified Anabaena KdpD(6His) were labeled with 8-azido[α-32P]ATP in the presence or absence of 2 mM unlabeled ATP. Both membrane-bound and purified Anabaena KdpD could be labeled with 8-azido[α-32P]ATP, and binding was significantly reduced in the presence of 2 mM ATP (Fig. 4).
FIG. 4.
Detection of ATP binding by Anabaena KdpD(6His) by photoaffinity labeling with 8-azido-[α-32P]ATP. E. coli BL21(DE3)/pLysS everted membranes containing Anabaena KdpD(6His) (lanes 1 and 2) and purified cytosolic Anabaena KdpD(6His) (lanes 3 and 4) were labeled with 8-azido-[α-32P]ATP in the presence or absence of 2 mM ATP as indicated. After electrophoresis on 11% SDSpolyacrylamide gels, the radiolabeled proteins were detected by a PhosphorImager system (Molecular Dynamics). Anabaena KdpD(6His) is shown by an arrow.
In vivo interaction of Anabaena KdpD with E. coli KdpD-CTD.
E. coli strain TKW22692 carries the kdpABCE genes, but the kdpD gene is truncated (KdpD-NTD deletion; kdpD/Δ12-395). This truncated KdpD does not affect the ability of this strain to grow normally at very low K+ concentrations (0.1 mM), but the expression of the kdpFABC operon is significantly reduced (28). However, when transformed with pBScD1, which constitutively produces the Anabaena KdpD(6His) (data not shown), strain TKW22692 grew poorly at 0.1 mM K+, while at 115 mM K+ the growth of TKW22692/pBScD1 and TKW22692/pBluescript was identical.
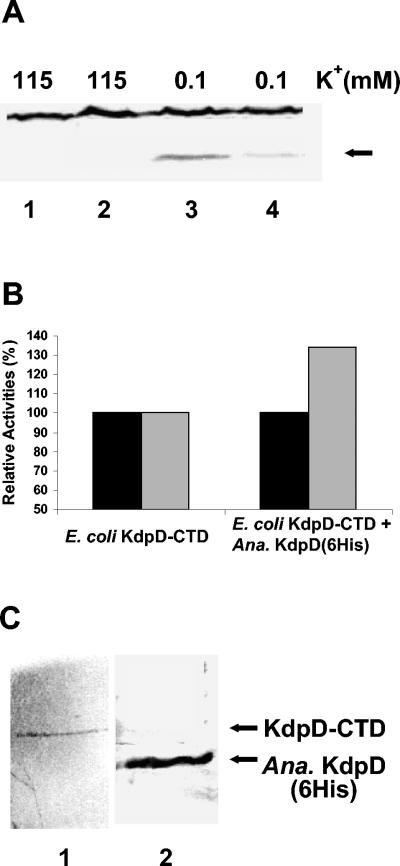
Based on this observation, kdp expression in E. coli TKW22692 with and without Anabaena KdpD(6His) was tested by monitoring the levels of KdpC in response to K+ limitation. A decrease in KdpC production was observed when Anabaena KdpD(6His) was coproduced with E. coli KdpD-CTD (Fig. 5A). This indicated the ability of Anabaena KdpD(6His) to interact with E. coli KdpD-CTD in vivo. In this case the interaction decreased the ability of KdpD-CTD to bring about kdp expression.
FIG. 5.
(A) In vivo interaction of Anabaena KdpD(6His) with E. coli KdpD-CTD. E. coli TKW22692 transformed with pBScD1 or the empty vector was grown in K115 medium and then subjected to K+ limitation as indicated. Thirty minutes after K+ downshock, total protein was extracted from the cells and KdpC (arrow) was detected with the E. coli KdpC antiserum. Lanes 1 and 3, TKW22692/pBluescript; lanes 2 and 4, TKW22692/pBScD1. (B) In vitro interaction of Anabaena KdpD(6His) with E. coli KdpD-CTD. The kinase activity (black bars) and the phosphatase activity (grey bars) of E. coli KdpD-CTD in the presence and in the absence of Anabaena KdpD(6His) were determined. The data are presented as percentages of the initial rates relative to E. coli KdpD-CTD. The kinase reaction was initiated by addition of [γ-32P]ATP to everted membrane vesicles containing E. coli KdpD-CTD with or without Anabaena KdpD(6His). For phosphatase measurements, purified KdpE∼32P was added to the vesicles in the presence of 1 mM ATP-γ-S. For both assays, aliquots were removed at definite time points and added to SDS sample buffer to stop the reaction. The proteins were separated by SDS-PAGE, and the radiolabeled proteins were quantified with a PhosphorImager (Molecular Dynamics). For KdpD-CTD (100% values), the phosphorylation activity was 2.5 pmol KdpD∼P min−1 mg protein−1 and the phosphatase activity was 0.01 pmol of Pi released min−1 mg protein−1. (C) Coelution of Anabaena KdpD (6His) and E. coli KdpD-CTD. The E. coli TKR2000/pBADD1/pBD3/Δ12-395 cell lysate was used for coelution experiments as described in Materials and Methods. The elution fraction was resolved on SDS-PAGE and probed separately with E. coli KdpD (lane 1) and Anabaena KdpD(6His) (lane 2) antisera.
In vitro interaction of Anabaena KdpD(6His) with E. coli KdpD-CTD.
The kinase and phosphatase activities of E. coli KdpD-CTD in the presence and absence of Anabaena KdpD(6His) were tested. Since E. coli kdpD/Δ12-395 (cloned in the pBAD18 vector) was not compatible with pBADD1 (as they have similar origins of replication), E. coli kdpD/Δ12-395 was transferred to pBAD33 (compatible with pBAD24) (13), and the resulting pBD3/Δ12-395 was used for in vitro coexpression. Everted vesicles of strain TKR2000 cotransformed with pBD3/Δ12-395 and pBADD1 were prepared, and the coproduction of Anabaena KdpD(6His) and E. coli KdpD-CTD was checked on Western blots using penta-His and E. coli KdpD antisera, respectively (data not shown).
The kinase activity of KdpD-CTD remained unaltered in the presence of Anabaena KdpD(6His), but the phosphatase activity of KdpD-CTD was about 34% higher in the presence of Anabaena KdpD(6His) (Fig. 5B). In other control experiments E. coli TKR2000/pBADD1 vesicles expressing Anabaena KdpD(6His) could not dephosphorylate KdpE∼P at all (data not shown). These data demonstrated the ability of Anabaena KdpD(6His) to modulate E. coli KdpD-CTD activity and provided indirect evidence for an interaction between the two proteins.
Coelution of E. coli KdpD-CTD with Anabaena KdpD(6His).
To obtain direct evidence for the interaction between the two proteins, coelution experiments with Anabaena KdpD(6His) and E. coli KdpD-CTD were performed. The E. coli TKR2000/pBADD1/pBD3/Δ12-395 cell lysate was solubilized with the detergent Aminoxid, and the solubilized proteins were allowed to bind to the Ni2+-NTA slurry. After removal of unbound proteins, the bound proteins were eluted with 200 mM imidazole. The protein in the eluted fraction were resolved on SDS-PAGE and probed with E. coli KdpD or Anabaena KdpD(6His) antiserum separately. E. coli KdpD-CTD could be clearly detected in the eluted fraction along with Anabaena KdpD(6His) (Fig. 5C). This confirmed the ability of Anabaena KdpD(6His) to interact directly with E. coli KdpD-CTD.
DISCUSSION
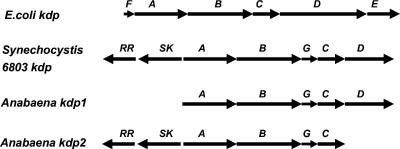
The full-length KdpD protein of E. coli and other bacteria is the only known bacterial sensor kinase with a large cytosolic NTD, comprising almost 400 amino acids (17). The Anabaena sp. strain L-31 kdpD ORF codes for a short KdpD protein of 365 amino acids showing homology only to the KdpD-NTD of E. coli and other bacteria. The kdpDE genes in other bacteria are located either downstream of kdpABC (E. coli and Clostridium acetobutylicum [34, 37]) or upstream of kdpABC (Mycobacterium tuberculosis [9]). Although a short version of kdpD is present downstream of kdpC in Anabaena sp. strain L-31, no kdpE-like gene has been found 1.5 kb downstream or upstream of kdpD or kdpA, respectively. Two other cyanobacteria, Anabaena sp. strain PCC 7120 and Synechocystis sp. strain PCC 6803, also have short kdpD genes at comparable positions in their kdp operons (20, 21; www.kazusa.or.jp) (Fig. 6). The presence of short versions of kdpD is not restricted to cyanobacteria. Other bacteria, such as Deinococcus radiodurans R1, Bacillus anthracis, Leptospirillum ferrooxidans, and Myxococcus xanthus, also display truncated kdpD genes.
FIG. 6.
Organization of the kdp genes in different bacteria. The arrowhead indicates the direction of transcription. SK, sensor kinase; RR, response regulator.
Prior to this study, information was lacking on the regulation of KdpD expression in Anabaena spp. or any other bacteria that encode a short kdpD gene. To specifically address this question, polyclonal antisera were raised against purified Anabaena KdpD(6His). Anabaena KdpD, as detected by the specific antiserum, was only observed in the membrane fraction of Anabaena sp. strain L-31 and not in the cytosol. Similarly, E. coli KdpD-NTD has been shown to be attached to the E. coli cell membrane (14).
In E. coli the amount of KdpD protein synthesized depends on the K+ concentration of the medium. The level of E. coli KdpD in K+-rich medium is very low but increases severalfold under K+ limitation (35) due to the readthrough of the kdpFABC operon into the kdpDE operon during transcription. Anabaena sp. strain L-31 kdpD was observed to be constitutively expressed but, unlike E. coli kdpD, its level did not increase with K+ limitation. This suggests that a readthrough of the kdpABGC operon into kdpD is probably absent in Anabaena sp. strain L-31 or that expression of the operon is not regulated at the transcriptional level.
Anabaena KdpD contains a slightly modified Walker A (ATP binding) motif. A similar binding site in E. coli KdpD-NTD has been shown to play a role in regulating the phosphatase activity of the KdpD protein (17). Membrane-bound E. coli KdpD-NTD has been shown to bind 8-azido-ATP. Anabaena KdpD(6His) in its membrane-bound form or in its soluble form also binds 8-azido-ATP, indicating the presence of a functional ATP binding site. We have earlier constructed a chimeric KdpD (AnacoliKdpD) from Anabaena KdpD and E. coli KdpD-CTD and studied some of its properties in vivo and in vitro (6). The hybrid protein, like E. coli KdpD, showed very rapid ATP-dependent phosphatase activity in vitro. Therefore, the ATP binding site in Anabaena KdpD may play a regulatory role in kdpFABC expression mediated by AnacoliKdpD in E. coli.
The NTD of KdpD has been shown to regulate the E. coli KdpD phosphatase activity (17), which in turn has been implicated in the regulation of kdpFABC expression. KdpD mutants that show low or negligible phosphatase activity exhibit constitutive kdpFABC expression (7). Conversely, KdpD mutants that show high phosphatase activity exhibit reduced kdpFABC expression in vivo (18). E. coli KdpD-NTD has been shown to complement in trans the membrane-anchored KdpD-CTD, resulting in restoration of signal transduction in vivo (14). However, our experiments show that coexpression of Anabaena KdpD(6His) with E. coli KdpD-CTD decreased kdpFABC expression in vivo and increased the phosphatase activity of the E. coli KdpD-CTD studied in vitro.
Coelution data shown here clearly indicated the ability of Anabaena KdpD(6His) to interact with E. coli KdpD-CTD. The results show that a heterologous NTD (Anabaena KdpD) can affect the KdpD-CTD (E. coli) activity, however, this modulation is different from that observed with the KdpD-NTD from E. coli. Therefore, this observation lends support to the notion that the NTDs from various organisms might exert different effects on the in vivo and in vitro activities of KdpD-CTD proteins. Furthermore, it is conceivable that, in contrast to the intact KdpD protein, the separation of domains (NTD and CTD) allows more flexibility with respect to interaction not only with CTD domains, but also with additional proteins. Evidence in this direction stems from the recent observation that the KdpD-NTD of Mycobacterium tuberculosis interacts with the lipoproteins LprJ and LprF (31).
How the short KdpD functions in vivo in Anabaena sp. strain L-31 is not clear. The genome of Anabaena sp. strain L-31 (our unpublished results) and that of the cyanobacterium Anabaena sp. strain PCC 7120 (21) show the presence of a second (kdp2) operon encompassing the kdp2ABGC genes but lacking kdpD. Interestingly, in both Anabaena strains, upstream of the kdpA2 gene two ORFs exist, which code for a putative sensor kinase and a response regulator (Fig. 6). The sensor kinase is a membrane-anchored polypeptide consisting of N-terminal transmembrane segments followed by a cytosolic histidine kinase domain (reminiscent of E. coli KdpD-CTD), while the response regulator shows homology to the general class of bacterial response regulators.
Our results indicate that Anabaena KdpD can interact with a membrane-anchored histidine kinase (E. coli KdpD-CTD) and modulate its activity. Therefore, it is conceivable that in Anabaena sp. strain L-31, KdpD may interact in vivo with a membrane-bound histidine kinase and modulate its activity. The search for partners for Anabaena KdpD is currently being pursued in our laboratory.
Acknowledgments
We thank Kirsten Jung and Ralf Heermann for helpful suggestions in the beginning of this work, Roland Schmid for microsequencing, Sudha Premachandran for help with antiserum production, and Anne Steinbrügge for expert technical assistance.
This work was supported by Grants-in-Aid from the Deutsche Forschungsgemeinschaft (to K.A.), from the DLR (to A.B., H.R., and S.K.A.), and from the Fonds der Chemischen Industrie (to K.A.).
REFERENCES
- 1.Alahari, A., A. Ballal, and S. K. Apte. 2001. Regulation of the potassium-dependent Kdp-ATPase expression in the nitrogen-fixing cyanobacterium Anabaena torulosa. J. Bacteriol. 183:5778-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altendorf, K., and W. Epstein. 1996. The Kdp-ATPase of Escherichia coli, p. 403-420. In A. G. Lee (ed.), Biomembranes, vol. 5: ATPases. JAI Press, London, England.
- 3.Apte, S. K., and A. Alahari. 1994. Role of alkali cations (K+ and Na+) in cyanobacterial nitrogen fixation and adaptation to salinity and osmotic stress. Indian J. Biochem. Biophys. 31:267-279. [PubMed] [Google Scholar]
- 4.Apte, S. K., and R. Haselkorn. 1990. Cloning of salinity stress-induced genes from salt tolerant nitrogen-fixing cyanobacteruim Anabaena torulosa. Plant Mol. Biol. 15:723-733. [DOI] [PubMed] [Google Scholar]
- 5.Asha, H., and J. Gowrishankar. 1993. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as a signal for transcriptional control. J. Bacteriol. 181:4528-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballal, A., R. Heermann, K. Jung, M. Gassel, S. K. Apte, and K. Altendorf. 2002. A chimeric Anabaena/ Escherichia coli KdpD protein (Anacoli KdpD) functionally interacts with E. coli KdpE and activates kdp expression in E. coli. Arch. Microbiol. 178:141-148. [DOI] [PubMed] [Google Scholar]
- 7.Brandon, L., S. Dorus, W. Epstein, K. Altendorf, and K. Jung. 2000. Modulation of KdpD phosphatase implicated in the physiological expression of the Kdp ATPase of Escherichia coli. Mol. Microbiol. 38:1086-1092. [DOI] [PubMed] [Google Scholar]
- 8.Castenholz, R. W. 1988. Culturing methods for cyanobacteria. Methods Enzymol. 167:68-93. [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Epstein, W. 2003. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75:293-320. [DOI] [PubMed] [Google Scholar]
- 11.Epstein, W. 1992. Kdp, a bacterial P-type ATPase whose expression and activity are regulated by turgor pressure. Acta Physiol. Scand. 146:193-199. [PubMed] [Google Scholar]
- 12.Epstein, W., and M. Davies. 1970. Potassium-dependent mutants of Escherichia coli K-12. J. Bacteriol. 101:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heermann, R., K. Altendorf, and K. Jung. 2000. The hydrophilic N-terminal domain complements the membrane-anchored C-terminal domain of the sensor kinase KdpD of Escherichia coli. J. Biol. Chem. 275:17080-17085. [DOI] [PubMed] [Google Scholar]
- 15.Heermann, R., A. Fohrmann, K. Altendorf, and K. Jung. 2003. The transmembrane domains of the sensor kinase KdpD of Escherichia coli are not essential for sensing K+ limitation. Mol. Microbiol. 47:839-848. [DOI] [PubMed] [Google Scholar]
- 16.Jung, K., and K. Altendorf. 2003. Stimulus perception and signal transduction by the KdpD/KdpE system of Escherichia coli, p. 53-58. In P. Dürre and B. Friedrich (ed.), Regulatory networks in prokaryotes. Horizon Scientific Press, Wymondham, England.
- 17.Jung, K., and K. Altendorf. 1998. Truncation of amino acids 12-228 causes deregulation of the phosphatase activity of the sensor kinase KdpD of Escherichia coli. J. Biol. Chem. 273:17406-17410. [DOI] [PubMed] [Google Scholar]
- 18.Jung, K., and K. Altendorf. 1998. Individual substitutions of clustered arginine residues of the sensor kinase KdpD of Escherichia coli modulate the ratio of kinase to phosphatase activity. J. Biol. Chem. 273:26415-26420. [DOI] [PubMed] [Google Scholar]
- 19.Jung, K., B. Tjaden, and K. Altendorf. 1997. Purification, reconstitution and characterization of KdpD, the turgor sensor of Escherichia coli. J. Biol. Chem. 272:10847-10852. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, et al. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, et al. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205-213; 227-253. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Laimins, L. A., D. B. Rhoads, and W. Epstein. 1981. Osmotic control of kdp operon expression in Escherichia coli. Proc. Natl. Acad. Sci. USA. 78:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima, K., A. Sugiura, H. Momoi, and T. Mizuno. 1992. Phosphotransfer signal transduction between two regulatory factors involved in the osmoregulated kdp operon in Escherichia coli. Mol. Microbiol. 6:1777-1784. [DOI] [PubMed] [Google Scholar]
- 25.Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al., which is more generally applicable. Anal. Biochem. 83:346-356. [DOI] [PubMed] [Google Scholar]
- 26.Polarek, J. W., G. Williams, and W. Epstein. 1992. The products of kdpDE operon are required for expression of the Kdp-ATPase of Escherichia coli. J. Bacteriol. 174:2145-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince, W. S., and M. R. Villarejo. 1990. Osmotic control of proU transcription is mediated through direct action of K+ glutamate on the transcription complex. J. Biol. Chem. 265:17673-17679. [PubMed] [Google Scholar]
- 28.Puppe, W., P. Zimmann, K. Jung, M. Lucassen, and K. Altendorf. 1996. Characterization of truncated forms of the KdpD protein, the sensor kinase of the K+-translocating Kdp system of Escherichia coli. J. Biol. Chem. 271:25027-25034. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning. a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Siebers, A., and K. Altendorf. 1988. The K+-translocating Kdp-ATPase from Escherichia coli. Purification, enzymatic properties and production of complex- and subunit-specific antisera. Eur. J. Biochem. 178:131-140. [DOI] [PubMed] [Google Scholar]
- 31.Steyn, A. J. C., J. Joseph, and B. R. Bloom. 2003. Interaction of the sensor module of Mycobacterium tuberculosis H37Rv KdpD with members of the Lpr family. Mol. Microbiol. 47:1075-1089. [DOI] [PubMed] [Google Scholar]
- 32.Sugiura, A., K. Hirokawa, K. Nakashima, and T. Mizuno. 1994. Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol. Microbiol. 14:929-938. [DOI] [PubMed] [Google Scholar]
- 33.Thomas, J. 1970. Absence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. Nature (London) 228:181-183. [DOI] [PubMed] [Google Scholar]
- 34.Treuner-Lange, A., A. Kuhn, and P. Dürre. 1997. The kdp system of Clostridium acetobutylicum: cloning, sequencing, and transcriptional regulation in response to potassium concentration. J. Bacteriol. 179:4501-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voelkner, P., W. Puppe, and K. Altendorf. 1993. Characterisation of the KdpD protein, the sensor kinase of the K+-translocating system of Escherichia coli. Eur. J. Biochem. 217:1019-1026. [DOI] [PubMed] [Google Scholar]
- 36.Walderhaug, M. O., E. D. Litwack, and W. Epstein. 1989. Wide distribution of homologs of Escherichia coli Kdp K+-ATPase among gram-negative bacteria. J. Bacteriol. 171:1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walderhaug, M. O., J. W. Polarek, P. Voelkner, J. M. Daniel, J. E. Hesse, K. Altendorf, and W. Epstein. 1992. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J. Bacteriol. 174:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker, J. E., M. Saraste, M. Runswick, and N. J. Gay. 1982. Distantly related sequences in α-and β-subunits of ATP synthase, myosin kinases and other ATP requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, et al. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmann, P., W. Puppe, and K. Altendorf. 1995. Membrane topology analysis of the sensor kinase KdpD of Escherichia coli. J. Biol. Chem. 270:28282-28288. [DOI] [PubMed] [Google Scholar]