Abstract
In Pseudomonas aeruginosa, N-acylhomoserine lactone signals regulate the expression of several hundreds of genes, via the transcriptional regulator LasR and, in part, also via the subordinate regulator RhlR. This regulatory network termed quorum sensing contributes to the virulence of P. aeruginosa as a pathogen. The fact that two supposed PAO1 wild-type strains from strain collections were found to be defective for LasR function because of independent point mutations in the lasR gene led to the hypothesis that loss of quorum sensing might confer a selective advantage on P. aeruginosa under certain environmental conditions. A convenient plate assay for LasR function was devised, based on the observation that lasR mutants did not grow on adenosine as the sole carbon source because a key degradative enzyme, nucleoside hydrolase (Nuh), is positively controlled by LasR. The wild-type PAO1 and lasR mutants showed similar growth rates when incubated in nutrient yeast broth at pH 6.8 and 37°C with good aeration. However, after termination of growth during 30 to 54 h of incubation, when the pH rose to ≥ 9, the lasR mutants were significantly more resistant to cell lysis and death than was the wild type. As a consequence, the lasR mutant-to-wild-type ratio increased about 10-fold in mixed cultures incubated for 54 h. In a PAO1 culture, five consecutive cycles of 48 h of incubation sufficed to enrich for about 10% of spontaneous mutants with a Nuh− phenotype, and five of these mutants, which were functionally complemented by lasR+, had mutations in lasR. The observation that, in buffered nutrient yeast broth, the wild type and lasR mutants exhibited similar low tendencies to undergo cell lysis and death suggests that alkaline stress may be a critical factor providing a selective survival advantage to lasR mutants.
Cell-cell communication is important for gene expression in the ubiquitous bacterium Pseudomonas aeruginosa. Two signal molecules which are involved in this communication, i.e., N-(3-oxododecanoyl)-homoserine lactone (OdDHL) and N-butyryl-homoserine lactone (BHL), are produced by the LasI and RhlI enzymes, respectively. These signal molecules are released into the medium, where they accumulate as cells grow to high densities and reach a “quorum.” The signals can diffuse into the cells and, above certain threshold levels, activate their cognate transcription factors, LasR and RhlR, respectively, resulting in induction or repression of target genes (11, 53, 67). The hierarchically superior LasRI system regulates the expression of the RhlRI system (40, 65). Microarray data suggest that the LasRI and RhlRI systems together control the expression of 6 to 12% of all chromosomal genes in P. aeruginosa, depending on the experimental conditions (17, 49, 63). Because of the hierarchical organization of quorum-sensing control, some target genes are regulated specifically by LasR with OdDHL, whereas many other target genes require both LasR with OdDHL and RhlR with BHL for optimal expression (17, 49, 63).
Numerous virulence factors of the opportunistic pathogen P. aeruginosa are positively controlled by quorum sensing, e.g., elastase, exotoxin A, staphylolytic enzyme, phospholipase C, alkaline protease, lipase, galactophilic lectin, pyocyanin, hydrogen cyanide (HCN), and rhamnolipids (12, 59, 65, 67). In several animal models, quorum-sensing-negative mutants of P. aeruginosa display reduced virulence, by comparison with the quorum-sensing-proficient parental strains (39, 47), suggesting that quorum sensing may give the bacterium a competitive advantage in pathogenic interactions with the host. Yet, a naturally occurring lasR mutant of P. aeruginosa has been isolated from a wound (14) and an rhlR mutant from a urinary tract infection (54). Defects in the lasR and rhlR genes have also been observed repeatedly in clinical P. aeruginosa isolates obtained from mechanically ventilated patients (7). Furthermore, in another study (4), 12 out of 66 environmental and clinical P. aeruginosa isolates had insertion, missense, or nonsense mutations in lasR. P. aeruginosa PA103, a clinical isolate with low exoprotease activity, can be complemented for protease expression by the cloned lasR+ gene of strain PAO (13). Thus, the question arises whether under some environmental conditions loss of LasR control might give P. aeruginosa a selective advantage.
For comparative purposes, we have used P. aeruginosa PAO1 (presumed wild-type) strains from different laboratory strain collections. Two of these strains attracted our interest because of their unusually strong pigmentation on nutrient agar plates. Both turned out to bear point mutations in the lasR gene, resulting in loss of quorum-sensing function. We then found experimental conditions under which the lasR-negative PAO1 sublines outcompete a lasR+ PAO1 wild type.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli and P. aeruginosa strains were routinely grown in nutrient yeast broth (NYB; 25 g Oxoid nutrient broth, 5 g Oxoid yeast extract per liter) or on nutrient agar plates at 37°C (55). When required, antibiotics were added to the media at the following concentrations: tetracycline (Tc), 25 μg ml−1 (E. coli) or 125 μg ml−1 (P. aeruginosa); ampicillin (Ap), 100 μg ml−1 (E. coli); kanamycin (Km), 50 μg ml−1 (E. coli); gentamicin (Gm), 10 μg ml−1; spectinomycin (Sp), 25 μg ml−1 (E. coli) or 1,000 μg ml−1 (P. aeruginosa); carbenicillin (Cb), 250 μg ml−1. To counterselect E. coli S17-1 donor cells in matings with P. aeruginosa for gene replacement, chloramphenicol (Cm) was used at a concentration of 10 μg ml−1; enrichment for Tc-sensitive cells was performed with Cb at 2,000 μg ml−1 and Tc at 20 μg ml−1 (43, 46). Biolog GN microplates for gram-negative strain identification were used as recommended by the manufacturer. Growth on different carbon sources was tested on OS minimal medium (34) supplemented with 0.1% (wt/vol) adenosine, inosine, or hypoxanthine; 0.1% (wt/vol) (NH4)2SO4 was used as a nitrogen source. Growth was scored after 2 to 3 days. For nucleoside hydrolase assays, the strains were grown in 100 ml of a medium containing 0.15% (wt/vol) K2HPO4, 0.02% (wt/vol) MgSO4 · 7 H2O, 0.2% (wt/vol) NH4Cl, 0.4% (wt/vol) peptone, and 0.02% (wt/vol) yeast extract (58). In survival tests, P. aeruginosa cultures were grown in triplicate in 20 ml of NYB supplemented with 0.05% (vol/vol) Triton X-100 (in 50-ml Erlenmeyer flasks). In some cases, 100 mM K phosphate buffer, pH 7.0, was incorporated into the medium prior to inoculation or 60 μM 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS; chemically synthesized according to reference 41 and provided by P. Williams) was added after 24 h of growth. Cultures were inoculated at an initial optical density at 600 nm (OD600) of 0.02, and incubated at 37°C with shaking. Growth and cell lysis were followed by determining OD600 values and viable counts (CFU) were obtained by plating suitable dilutions made in saline (0.9% NaCl) on nutrient agar. Mixed cultures grown in the same conditions as described above were coinoculated with 50% of PAO1 and 50% of PAO6395; the concentrations of the inocula required were estimated from OD600 measurements. To discriminate between the wild type and a lasR mutant, 100 colonies isolated at each time point from three independent cultures were replicated from nutrient agar onto OS-adenosine medium. After 48 h of incubation, the lasR mutants did not grow on this medium whereas the wild type did.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or oligonucleotide | Description or sequence (5′-3′) | Reference or origin |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAO1 | chl-2,a otherwise presumed wild type | 22 |
| PAO1-J | PAO1 lasR subline | National Food Research Institute, Tsukuba, Japan, via Y. Itoh |
| PAO1-D | PAO1 lasR subline | DSM, Braunschweig, Germany, via K.-E. Jaeger |
| PAO2324 | met-9020 catA1 nar-9011 tyu-9009 puuD6 hcn | 30 |
| PAO6330 | ΔlasR1 derivative of PAO1 | 42 |
| PAO6379 | PAO1, nuh::Ω-Sp/Sm; Spr Smr | This study |
| PAO6395 | ΔlasR derivative of PAO1 | This study |
| PDO7 | recA::Ω-Hg | 23 |
| E. coli | ||
| DH5α | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 deoR λ(φ80dlacZΔM15) | 48 |
| SM10/λpir | thi-1 thr-1 leuB26 tonA21 lacYI supE44 recA chromosome::RP4-2 Tcr::Mu Kmr/λpir | 31 |
| S17-1 | pro thi hsdR recA Tpr Smr; chromosome::RP4-2 Tc::Mu, Km::Tn7 | 52 |
| Plasmids | ||
| pBLS-II SK | pBluescript cloning vector; ColE1 replicon; Apr | Stratagene |
| pHP45Ω | ColE1 replicon carrying a Ω-Sp/Sm cassette; Spr Smr Apr | 45 |
| pME3087 | Suicide vector, ColE1-replicon; Tcr | 62 |
| pME3280a | Mini-Tn7 gene delivery vector; Gmr Apr | 69 |
| pME3827 | pME6001 carrying lasR on a 1.1-kb PvuI-KpnI fragment; Gmr | 42 |
| pME3848 | pME3087 carrying the lasR gene with an internal 0.36-kb | This study |
| deletion on a BamHI-EcoRI fragment; Tcr | ||
| pME3872 | pME3280a carrying lasR+ on a 1.1-kb [PvuI]-KpnI fragment; Apr Gmr | 7 |
| pME3873 | pUK21 containing nuh on a 1.5-kb insert; Kmr | This study |
| pME3877 | pME6014 carrying nuh′-′lacZ translational fusion; Tcr | This study |
| pME3882 | pME6031 with nuh′-′lacZ on a 3.3-kb BamHI-XhoI fragment from pME3877; Tcr | This study |
| pME3883 | pME6010 carrying nuh; Tcr | This study |
| pME3890 | pME3087 with nuh::Ω-Sp/Sm on a 3.5-kb insert; Tcr Spr Smr | This study |
| pME6010 | pACYC177-pVS1 shuttle vector; Tcr | 15 |
| pME6014 | Cloning vector derived from pME6010 for translational ′lacZ fusions; Tcr | 16 |
| pME6031 | pACYC177-pVS1 shuttle vector; Tcr | 15 |
| pUK21 | Cloning vector, ColE1 replicon; Kmr | 61 |
| pUX-BF13 | Helper plasmid containing Tn7 transposition functions; R6K replicon; Apr | 20 |
| Primers | ||
| las1 | CGCCGAACTGGAAAAGTGGC, upstream of lasR (Fig. 1) | |
| las2 | TGAGAGGCAAGATCAGAGAG, downstream of lasR (Fig. 1) | |
| LG1 | AGTGAACCCGGGGACCAGGTGTG, with an underlined XmaI restriction site, upstream of lasR (Fig. 1) | |
| LG2 | ATCGAGAATTCGCCAGCAACCG, with an underlined EcoRI restriction site, in lasI (Fig. 1) | |
| LG3 | TTTAGATCTCGTGCTGCTTTCGCGTC, with an underlined BglII restriction site, in lasR (Fig. 1) | |
| LG4 | AAAAGATCTCCCGCCGCGTAGCGGC, with an underlined BglII restriction site, near 3′ end of lasR (Fig. 1) | |
| P1-KpnI | AAAAGGTACCTCGACCTGGCCGCCCTGATCG, with an underlined KpnI restriction site, upstream of nuh | |
| P2-HindIII | AAAAAAGCTTCGCGGTGCTGGCCAACCTGA, with an underlined HindIII restriction site, downstream of nuh | |
| P3-BamHI | AAAAGGATCCTCGACCTGGCCGCCCTGATCG, with an underlined BamHI restriction site, upstream of nuh | |
| P4-PstI | GTTTCTGCAGCGGACAGAGGAAGGCT, with an underlined PstI restriction site, annealing to the first 8 codons of nuh | |
| Tn7-1 | AACATGGCCAAGTCGGTCACC-3, in glmS downstream of the Tn7 attachment site | |
| Tn7-3 | CCAGCCCGGTCGTAATGCTC, in lasR |
chl-2, spontaneous chloramphenicol resistance.
DNA manipulation and cloning procedures.
Small-scale preparations of plasmid DNA were carried out by the cetyltrimethylammonium bromide method (6), and large-scale preparations were performed using Nucleobond AX100 columns (Macherey Nagel). Chromosomal DNA was extracted from P. aeruginosa and purified as described elsewhere (46). Restriction enzyme digestions, ligations, and agarose gel electrophoresis were performed using standard methods (48). Restriction fragments were purified from agarose gels using the Gene Clean II kit (Bio 101). Transformation of E. coli and P. aeruginosa strains was carried out by electroporation (9). Oligonucleotide primers used are listed in Table 1. PCR products were sequenced on both strands with the Big Dye Terminator cycle sequencing kit and an ABI-PRISM 373 automatic sequencer according to the manufacturer's recommendations (Applied Biosystems). Alignment of nucleotide and deduced amino acid sequences was performed using the Genetics Computer Group program GAP or ClustalW (http://www.ebi.ac.uk/clustalw/#).
Characterization of strains PAO1-D and PAO1-J and spontaneous Nuh-negative mutants.
Chromosomal DNA from PAO1 (control), PAO1-D, PAO1-J, and spontaneous Nuh− mutants as templates and primers las1 and las2 (7) (Table 1) were used for PCR amplification of the 1.24-kb lasR region. Three independently obtained PCR products from each strain were sequenced entirely.
Plasmid and mutant constructions.
An in-frame deletion removing codons 101 to 222 was generated in the lasR gene of strain PAO1. This deletion includes the helix-turn-helix motif and most of the putative OdDHL binding domain (Fig. 1) and was obtained as follows. A 620-bp XmaI-BglII fragment including the first 100 codons of lasR was PCR amplified using primers LG1 and LG3 (Table 1) and linked to a 630-bp BglII-EcoRI fragment containing the last 18 codons of lasR, which had been PCR amplified using primers LG4 and LG2. The resulting 1.25-kb fragment was cloned, via an XmaI-BamHI linker from pBluescript-II, into the suicide plasmid pME3087 digested with BamHI and EcoRI, resulting in plasmid pME3848. After conjugation with PAO1 as the recipient and E. coli S17-1/pME3848 as the donor, Tc-resistant transconjugants having a chromosomally integrated pME3848 were selected. Cb enrichment provided Tc-sensitive colonies which were unable to use adenosine as the only carbon source; one isolate (PAO6395) was verified by PCR for the presence of the 0.36-kb deletion in lasR using primers LG1 and LG2 (Fig. 1); the LasR-negative phenotype was checked by assaying for OdDHL, BHL, elastase, rhamnolipids, HCN, and pyocyanin.
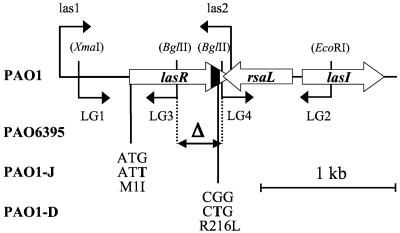
FIG. 1.
The 2.2-kb region of P. aeruginosa PAO1 with lasR, rsaL, and lasI (56). Artificial restriction sites on primers used for cloning and constructions are shown in parentheses; LG3 and LG4 border the deletion from codons 101 to 222 (inclusive) in the lasR gene of PAO6395. Mutations in codon 1 of PAO1-J and in codon 216 of PAO1-D are indicated. The predicted helix-turn-helix motif of the LasR protein is located in the C-terminal region (60); codons 190 to 220, corresponding to this motif, are indicated in black in the lasR gene.
Plasmid pME3873, carrying the PAO1 nuh gene, was obtained as follows. A 1.5-kb fragment, amplified by PCR using PAO1 chromosomal DNA as a template and primers P1-KpnI and P2-HindIII, was digested with KpnI and HindIII, and cloned into pUK21. A nuh′-′lacZ translational fusion was constructed by PCR using pME3873 as template and primers P3-BamHI and P4-PstI. The resulting 270-bp PCR product containing the nuh promoter region and the 5′ end of nuh was digested with BamHI and PstI and joined to the ′lacZ reporter gene in the vector pME6014, such that the ninth codon of nuh was fused in-frame to the 8th codon of lacZ in the resulting construct pME3877. The nuh′-′lacZ fusion was subcloned on a 3.3-kb BamHI-XhoI fragment into pME6031, resulting in plasmid pME3882. In PAO6379, the nuh gene was interrupted by the insertion of a spectinomycin-streptomycin resistance cassette (Ω-Sp/Sm). The 2-kb cassette was excised from pHP45Ω and inserted into the EcoRI site of pME3873. A 3.5-kb KpnI-XbaI fragment, carrying the interrupted gene nuh:: Ω-Sp/Sm, was subcloned into the suicide plasmid pME3087, giving pME3890. This plasmid was mobilized into PAO1 by the donor cell E. coli S17-1, and gene replacement was carried out as above. Tc-sensitive, Sp-resistant clones were checked by Southern blotting (data not shown).
Complementation of lasR mutants.
Complementation with the lasR+ gene was carried out using either the multicopy plasmid pME3827 (Table 1) or a mini-Tn7-based system for single-copy insertion into chromosomes of gram-negative bacteria (20, 69). In the latter procedure, P. aeruginosa lasR recipient strains, grown overnight at 43°C, were mixed with the helper strain E. coli SM10λpir/pUX-BF13 and the donor E. coli S17-1/pME3872, carrying the mini-Tn7-lasR construct (Table 1). Site-specific chromosomal insertion of mini-Tn7-lasR+ was revealed by PCR using primers Tn7-1 and Tn7-3 (Table 1), with the formation of a typical 2.0-kb band. Complementation by the lasR+ allele was confirmed by growth on OS-adenosine medium and by assaying for OdDHL, BHL, and elastase.
Enzyme assays.
For nucleoside hydrolase determination, P. aeruginosa cells grown overnight in 100 ml of the medium of Terada et al. (58) were harvested at 4°C by centrifugation, washed, and broken by sonication as a 20% (wet weight per volume) suspension in 50 mM Tris-HCl, pH 7.3. After centrifugation at 10,000 rpm for 30 min, the supernatant was dialyzed overnight at 4°C (Spectra/Por Float-A-Lyzer membrane, cutoff 5,000 Da; Spectrumlabs), providing a crude extract. Nucleoside hydrolase activity was determined at 37°C by measuring the formation of ribose from 5 mM inosine or adenosine in 50 mM HEPES, pH 7.3; the assay volume was 800 μl including 50 μl or 100 μl of extract. The reaction was terminated after 5 or 10 min by the addition of 100 μl of 1.0 M HCl. Reducing sugar was measured by the addition of 100 μl of 1.05 M NaOH, 0.3 ml of 0.12% (wt/vol) 1,9-dimethyl-1,10-phenanthroline, and 0.3 ml of a reagent containing 4% (wt/vol) Na2CO3, 1.6% (wt/vol) glycine, and 0.045% CuSO4 · 5H2O. Following color development at 95°C for 8 min, the absorbance at 450 nm was measured, in parallel with a standard curve prepared with ribose (35). Protein concentrations were determined by the Bradford method with bovine serum albumin as a standard. Specific activity is expressed as nmol of ribose formed per min and mg of protein.
For β-galactosidase assays, P. aeruginosa strains were cultivated with shaking in 20 ml NYB supplemented with 0.05% (vol/vol) Triton X-100 in 50-ml Erlenmeyer flasks at 37°C. β-Galactosidase-specific activities were determined by the Miller method (48).
Elastase production was estimated by measuring elastolytic activities of cells grown in 20 ml NYB (in 50-ml Erlenmeyer flasks) for 16 h against elastin Congo red (Sigma), as previously described (33).
Assays for OdDHL, BHL, rhamnolipids, HCN, and pyocyanin.
For extraction of N-acyl-homoserine lactones, P. aeruginosa strains were grown in 200 ml NYB supplemented with 0.05% (vol/vol) Triton X-100 (in 500 ml-Erlenmeyer flasks) at 37°C to an OD600 of 2.0 to 2.5. Cells were removed by centrifugation; supernatants were adjusted to pH 5, filtered (pore size, 0.45 μm), and extracted three times with 80 ml of dichloromethane in a separating funnel. The extract was processed and analyzed for OdDHL and BHL by reverse-phase thin-layer chromatography as described previously (18). Nuh− mutants obtained in the enrichment experiment were tested qualitatively for OdDHL and BHL production by cross-streaking against the indicator strains previously described (18). Rhamnolipids, HCN, and pyocyanin were quantified in culture supernatants as described before (19, 46).
RESULTS
Discovery of lasR mutations in P. aeruginosa PAO1 sublines.
A PAO1 subline from a German strain collection (PAO1-D) and another, independent PAO1 subline from a Japanese strain collection (PAO1-J) showed a darker bluish pigmentation on nutrient agar and on Pseudomonas isolation agar (Difco) than did our PAO1 laboratory strain, which had been maintained at −80°C since 1970. These differences in pigmentation were due to various amounts of pyocyanin produced (Table 2), hinting at a potential dysfunction of the quorum-sensing machinery in strains PAO1-D and PAO1-J. According to a previous report (8), a lasR mutation causes pyocyanin overproduction in stationary phase. Both strains were found to be defective for OdDHL production and had reduced BHL levels (in strain PAO1-J) or did not produce detectable amounts of BHL (in strain PAO1-D) (Table 2). Strain PAO1-D appeared to be defective for twitching motility, probably as a consequence of a secondary mutation as previously observed (2), whereas PAO1-J had twitching motility. Three LasRI/RhlRI-regulated extracellular products, elastase, rhamnolipids, and HCN (32, 38, 42, 65), were produced in strongly reduced amounts by both PAO1-D and PAO1-J, compared with PAO1 (Table 2). To verify that a lasR defect could cause the quorum-sensing phenotypes observed, we constructed an in-frame lasR deletion mutant, PAO6395, in a PAO1 background. This strain and PAO1-D had the same quorum-sensing-negative phenotypes (Table 2). When the lasR+ allele from strain PAO1 was introduced, as a single copy on a mini-Tn7 vector, into the chromosomes of strains PAO1-J, PAO1-D, and PAO6395, the production of OdDHL, BHL, elastase, rhamnolipids, and HCN was restored to wild-type levels (data not shown). These data indicate that the three mutant strains owed their phenotypes to defective LasR function.
TABLE 2.
Phenotypic characterization of strain PAO1 and derivatives
| Strain (genotype) | Concn of:
|
Elastase activityc (A495) | Amt of rhamnolipidsd (μg/109 bacteria) | HCN concne (nmol/109 bacteria) | Growth on adenosinef | ||
|---|---|---|---|---|---|---|---|
| Pyocyanina (μg/ml) | OdDHLb (μM) | BHLb (μM) | |||||
| PAO1 | 4.7 ± 0.6 | 0.22 ± 0.06 | 3.9 ± 0.2 | 0.9 ± 0.2 | 7.5 ± 2.2 | 2.90 ± 0.30 | +++ |
| PAO1-J (lasR) | 20.7 ± 1.1 | <0.05 | 0.2 ± 0.1 | 0.1 ± 0.1 | <1.0 | 0.63 ± 0.15 | + |
| PAO1-D (lasR) | 20.6 ± 2.8 | <0.05 | <0.1 | 0.1 ± 0.0 | <1.0 | 0.43 ± 0.06 | + |
| PAO6395 (ΔlasR) | 15.9 ± 3.4 | <0.05 | 0.1 ± 0.1 | 0.1 ± 0.0 | <1.0 | 0.40 ± 0.00 | + |
Pyocyanin was quantified in triplicate cultures grown for 48 h as previously described (46).
OdDHL and BHL concentrations were quantified by thin-layer chromatography, using Agrobacterium tumefaciens NTL4/pZLR4 and Chromobacterium violaceum CV026 as indicators, as previously described (18). Assays were done three times.
Elastase activity was estimated for P. aeruginosa strains grown in triplicate NYB cultures (7, 33). Arbitrary A495 units are used.
Rhamnolipids were quantified (19) for P. aeruginosa strains grown in triplicate NYB cultures.
Concentrations of HCN were determined in triplicate for strains grown under semianaerobic conditions (19).
Growth on OS medium with adenosine as the only C source was scored after 2 days. +++, good growth of single colonies; +, faint growth in primary streak.
The lasR alleles of strains PAO1-J and PAO1-D were isolated by PCR amplification and sequenced. In PAO1-J, the original ATG initiation codon of the lasR gene was mutated to ATT (Fig. 1). This rare initiation codon reduces translational efficiency to about 2% in E. coli (57) and is expected to have a similar consequence in P. aeruginosa, given the similar translation initiation mechanisms in both organisms (27). In PAO1-D, the lasR gene contained a point mutation in codon 216 (CGG→CTG), resulting in an arginine→leucine change (Fig. 1). Arg-216 lies in α-helix 9, which is the DNA recognition sequence in the helix-turn-helix motif of LuxR-type transcription factors including LasR (60). Therefore, Arg-216 appears to have an essential function in target DNA recognition by the LasR protein.
Development of a plate assay for LasR function.
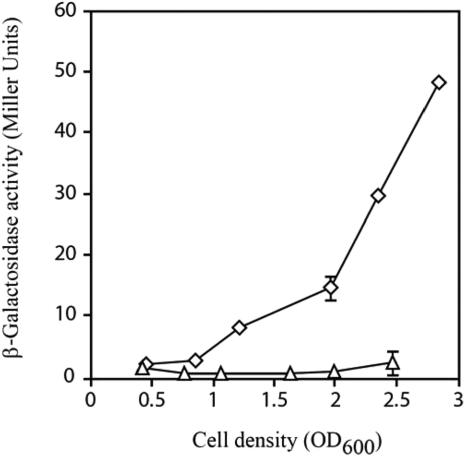
In Biolog GN microplates, which contain 95 different carbon and/or nitrogen sources, strain PAO1 utilized inosine, whereas the lasR mutants were handicapped on this substrate (data not shown). In P. aeruginosa, inosine is formed by deamination from adenosine and degraded by a nucleoside hydrolase to hypoxanthine plus ribose; subsequent degradation of hypoxanthine to glyoxylate plus urea occurs in several steps (30). The nuh (PA0143) gene, whose deduced protein product has 40% amino acid sequence identity with the RihC nucleoside hydrolase of E. coli (44), is inducible about fivefold by the LasRI system, as evidenced by microarrays and proteomics (1, 17, 49, 50, 63). We cloned the nuh gene of strain PAO1 (see Materials and Methods). A nuh′-′lacZ translational fusion carried by pME3877 was expressed in a cell density-dependent manner in the wild-type PAO1 but showed low expression in the lasRI mutant PAO6330 (Fig. 2), confirming the transcriptomic and proteomic data. To verify the nucleoside hydrolase function of the nuh gene, we measured this activity in cell extracts with adenosine or inosine as the substrate. Whereas the wild-type PAO1 manifested nucleoside hydrolase activities of 70 to 80 U per mg of protein with either substrate, the nuh mutant PAO6379 showed a strongly reduced activity (Table 3), thus confirming the predicted function of the gene, and the lasRI mutant PAO6330 exhibited a fourfold decrease of activity, compared to the wild type (Table 3). The nuh mutant PAO6379 as well as the quorum-sensing mutants PAO6330 (lasRI) and PAO6395 (lasR) were impaired in the utilization of adenosine and inosine as the sole carbon source, whereas strain PAO1 grew well on these substrates (Table 3). The complemented nuh mutant PAO6379, carrying pME3883 (Table 1), grew like the wild type. Furthermore, three clinical P. aeruginosa isolates which carry mutations in lasR (7) did not grow on adenosine or inosine (data not shown). Strain PAO2324 (puuD), which is blocked in hypoxanthine degradation (30), was used as a control; this strain was impaired in the utilization of adenosine, inosine, and hypoxanthine. Mutations in the gacA or vfr genes, which are required for optimal lasR expression (11, 46), or in the rhlR gene did not cause a Nuh-negative phenotype, i.e., these mutants grew on adenosine. These results are consistent with the existence of an adenosine catabolic pathway in which the LasR-controlled nuh enzyme hydrolyzes inosine. Thus, growth on adenosine provides a simple and reliable method to distinguish between the wild type and lasR-negative strains, and we used this routinely to monitor the viability of these strains in the mixed culture and enrichment experiments described below.
FIG. 2.
Influence of a lasRI deletion on nuh expression. β-Galactosidase expression from a translational nuh′-′lacZ fusion on pME3882 was determined in PAO1 (⋄) and PAO6330 (lasRI) (▵). Each result is the mean ± standard deviation from three measurements. Bacterial growth in NYB medium reached a plateau at about an OD600 of 3.
TABLE 3.
Nucleoside hydrolase activity of P. aeruginosa strains and their ability to grow on adenosine and its metabolic products
| Strain and genotype | Nucleoside hydrolase sp act (nmol min−1 mg protein−1)
|
Growth onb:
|
|||
|---|---|---|---|---|---|
| Adenosinea | Inosinea | Adenosine | Inosine | Hypoxanthine | |
| PAO1 wild type | 80.2 ± 20.4 | 68.8 ± 17.5 | +++ | +++ | +++ |
| PAO6379 nuh | 1.7 ± 0.5 | 1.0 ± 0.2 | + | + | +++ |
| PAO6330 lasRI | 17.9 ± 5.8 | 18.2 ± 6.4 | + | + | +++ |
| PAO6395 lasR | NDc | ND | + | ++ | +++ |
| PAO2324 puuD | ND | ND | − | + | − |
Used as the substrate.
Growth on OS minimal medium containing adenosine, inosine, or hypoxanthine as the only C source was scored as follows: +++, good growth of single colonies; ++, growth in primary and secondary streaks; +, faint growth in primary streak; −, absence of growth. For PAO2324 testing, 1 mM methionine was included.
ND, not determined.
Selective advantage of lasR mutants during the death phase.
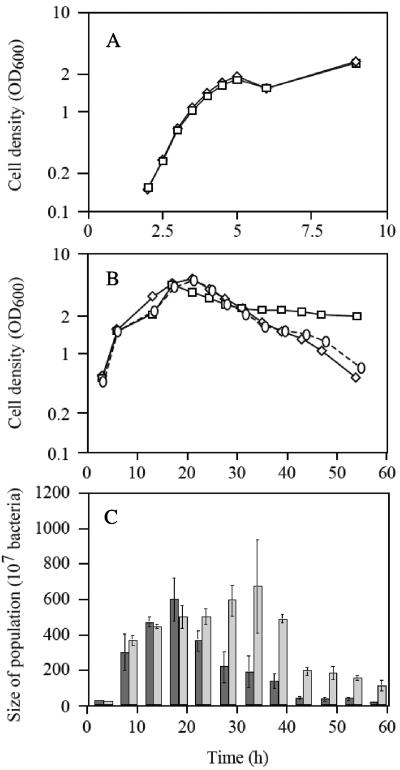
To determine the possible causes for the emergence of lasR-negative P. aeruginosa strains, we grew the wild type PAO1 and the engineered lasR mutant PAO6395 separately in NYB with good aeration. Both strains had the same doubling time of 29 ± 1 min and reached stationary phase after 20 to 24 h, whereby the lasR mutant reached a slightly lower maximal cell density, according to OD600 measurements (Fig. 3A and B). Marked differences were observed after 36 to 54 h of incubation: the wild-type PAO1 showed a stronger decline than did the lasR mutant PAO6395 (Fig. 3B). The PAO6395/PAO1 ratio calculated from cell densities was nearly constant for the initial 30 h of incubation but subsequently went up to 80% after 54 h (Fig. 3B). This differential behavior was confirmed by viable counts (see below; Table 4). In a separate experiment, strain PAO1-J showed the same reduced death rate as did PAO6395 (data not shown). In a mixed culture of strains PAO1 and PAO6395, the initial 50:50 ratio (based on CFU values) did not change significantly for ∼20 h, but thereafter increased to >90% after 54 h (Fig. 3C), whereas the cell densities (OD600 values) of the mixture were roughly intermediate between those of the pure cultures (Fig. 3A and B). This experiment, when repeated with a PAO1-PAO1-J mixture, gave the same selective advantage for the lasR mutant after 48 h (data not shown). We conclude that a functional lasR gene is important for cell lysis and death under the conditions used.
FIG. 3.
Growth and lysis of PAO1 and PAO6395. The strains were grown separately (continuous line) or in mixed culture (dashed line [0]) in 20 ml NYB at 37°C with shaking. Cell densities were estimated by OD600 measurements for PAO1 (wild type; ⋄) and PAO6395 (lasR; □) during exponential phase for 10 h (A) and during the death phase occurring between 20 and 54 h (B). Each point represents the mean of three measurements; standard deviations are too small to be seen. (C) Total viable cells (CFU/ml) in three parallel mixed cultures were estimated by plating two suitable dilutions on nutrient agar, and PAO6395 was distinguished from PAO1 by spotting 100 colonies from each culture onto OS-adenosine medium. Dark grey bars, PAO1; light grey bars, PAO6395.
TABLE 4.
Phosphate-buffered NYB largely prevents cell death of P. aeruginosa strains growing individually
| Strain and genotype | Growth mediuma | Incubation time (h) | Cell density (OD600) | Lysis (%) | Viable countb (107 CFU/ml) | Death (%) |
|---|---|---|---|---|---|---|
| PAO1 wild type | NYB | 24 | 5.38 ± 0.29 | (0)c | 970 ± 156 | (0) |
| 48 | 1.35 ± 0.11 | 75 | 160 ± 21 | 84 | ||
| PAO6395 ΔlasR | NYB | 24 | 3.93 ± 0.15 | (0) | 753 ± 65 | (0) |
| 48 | 2.90 ± 0.15 | 26 | 288 ± 30 | 62 | ||
| PAO1 wild type | NYB-Pi | 24 | 4.78 ± 0.21 | (0) | 987 ± 123 | (0) |
| 48 | 3.29 ± 0.29 | 31 | 737 ± 71 | 25 | ||
| PAO6395 ΔlasR | NYB-Pi | 24 | 5.10 ± 0.22 | (0) | 1,443 ± 132 | (0) |
| 48 | 3.49 ± 0.13 | 26 | 1,103 ± 59 | 24 |
NYB-Pi is NYB buffered with 100 mM K phosphate, pH 7.0.
Viable counts were determined by plating on nutrient agar.
(0), 1% lysis evident (baseline).
Enrichment for spontaneous quorum-sensing (lasR) mutants during serial transfer of strain PAO1 in aerated cultures.
In a reconstruction experiment done in triplicate, strain PAO1 was incubated aerobically in NYB (20 ml) at 37°C for 48 h, diluted 1:200 into fresh NYB, and incubated again for 48 h. This cycle was repeated several times. After each cycle, an appropriately diluted sample was plated on nutrient agar and 100 colonies were scored for growth on adenosine. After 1, 3, 5, and 7 cycles, the proportions of Nuh− colonies were 1, 4, 11, and 27%, respectively. Small colony variants appeared after cycles 4 to 6, and translucent morphotypes were observed after cycles 6 to 8. Among 80 Nuh− colonies obtained from cycles 1 to 6, none was small and 50 could be functionally complemented for growth on adenosine by the multicopy plasmid pME3827 carrying lasR+. Ten clones from cycles 3 to 5, whose growth on adenosine was restored by pME3827, were chosen at random and analyzed further; 5 showed low or undetectable N-acyl-homoserine lactone and elastase levels, and all were mutated in lasR. One isolate had a point mutation in codon 117 (CCG→CTG) causing a proline→leucine change in the autoinducer-binding domain of LasR. One isolate had a −1 frameshift mutation (loss of G at position 447), and three isolates had a major deletion or rearrangement in lasR, as evidenced by the absence of a typical 1.24-kb lasR fragment in PCR with primers las1 and las2 (Table 1). The remaining five Nuh− clones tested were elastase negative, but N-acyl-homoserine lactone positive, and none was mutated in lasR. These clones were not analyzed further. From this experiment, we conclude that serial transfer in aerated NYB can indeed select for lasR mutants, but also for a variety of other mutants, some of which have a Nuh− phenotype.
Potential causes of cell lysis and death.
Aerobic incubation conditions were found to be important for cell lysis and death: in oxygen-limited cultures (obtained in tightly closed 125-ml bottles containing 60 ml of NYB), PAO1 and a lasR mutant had similar survival abilities during 54 h of incubation (data not shown). As the Pseudomonas quinolone signal (PQS) can trigger autolysis in P. aeruginosa colonies grown on rich, solid media (5), we tested whether the addition of 60 μM PQS to strain PAO6395 (ΔlasR), which had been cultivated in NYB with aeration for 24 h, would restore wild-type lysis over the following 28 h of incubation. However, no such effect was observed. In a control, added PQS did not enhance lysis of strain PAO1 either (data not shown). Thus, it is likely that PQS signaling is not involved in the lysis phenomenon that we observe in PAO1 grown in aerated NYB, in contrast to the established role of PQS in the autoplaquing phenomenon (5).
In E. coli, autolytic cell death can be induced by DNA damage, via activation of the RecA protein (26). A recA mutant derived from PAO1, PDO7, was compared with PAO1; both strains showed similar levels of lysis when grown in NYB (data not shown), suggesting that a major role of RecA in cell death of PAO1 is unlikely under the conditions used. In E. coli, mutation in the stationary-phase sigma factor RpoS can confer a growth advantage under conditions of alkaline pH (10). We measured the pH in cultures of PAO1 and PAO6395 incubated in standard NYB medium: the pH values rose from 6.8 initially to ≥9 at the end of incubation for both strains, and the beginning of lysis was correlated with pH values ≥8.5. When the two strains were grown in NYB buffered with 100 mM K phosphate, pH 7.0, the extent of both lysis and death was reduced to low levels which were similar for the wild type and the lasR mutant (Table 4). In particular, the two strains had comparable cell population densities after 48 h (Table 4). We therefore suggest that an alkaline, low-osmotic-strength environment favors cell lysis and death more strongly in the wild type than in lasR-negative mutants.
DISCUSSION
In the light of recent genomic data (1, 17, 49, 50, 63), it appears that the network of LasR/RhlR-regulated genes in P. aeruginosa is much vaster than assumed earlier when quorum sensing was essentially perceived as a mechanism to control virulence (39, 47, 67). In fact, N-acyl-homoserine lactone-mediated quorum sensing modulates a number of central metabolic functions such as glucose catabolism and denitrification (49, 63). As pointed out by Manefield and Turner (28), little is known about the ecological roles of quorum sensing. In this study, we have shown that the LasRI system is important for catabolism of adenosine and inosine and that LasR is involved in the control of cell lysis and death when the organism is incubated in a rich liquid medium with good aeration.
It has long been known that various P. aeruginosa strains, when cultivated on rich solid media, can form zones of lysis (“autoplaques”) within the bacterial lawn after 1 to 2 days of incubation (3). Although common and widespread in P. aeruginosa, autoplaque formation is a highly variable phenomenon (3). Induction of pyocins or prophages has been considered as a possible cause of autoplaque formation (3). P. aeruginosa PAO produces three pyocins (R2, F2, and S2), and two more are predicted from genome analysis (36). Pyocins are inducible by DNA-damaging agents, and it has been proposed that such induction could be mediated by activated RecA protein (29). As the recA mutant PDO7 was not significantly different from the wild-type PAO1 in terms of lytic death under our experimental conditions, an involvement of pyocin production remains uncertain. Moreover, transcriptome analysis of early-stationary-phase P. aeruginosa cells (49, 50, 63) fails to reveal an effect of quorum sensing on the expression of pyocin genes or on other genes that might be obvious candidates for initiating cell lysis, e.g., the genes for a filamentous phage present in the PAO chromosome (66) or for peptidoglycan hydrolases (autolysins) (24). In the same vein, prophage Pf1-mediated cell death in biofilms does not depend on quorum sensing (64). Autoplaque formation is enhanced in P. aeruginosa pqsL mutants, which overproduce PQS, and abolished by secondary mutations blocking PQS biosynthesis, suggesting that PQS may have a function in triggering autoplaque formation (5). LasR function is required for optimal transcriptional expression of the pqs biosynthetic gene cluster (12, 41, 49). However, our observation that addition of PQS to a lasR mutant in stationary phase did not restore lysis to the wild-type level argues against an involvement of PQS under our experimental conditions. For all these reasons, we suggest that autoplaque formation and cell lysis after growth in NYB are unrelated phenomena.
The fact that lasR-negative mutants of strain PAO1 evolved in two different strain collections and that this occurred by two different point mutations strongly suggests that loss of LasR function can confer a selective advantage on P. aeruginosa. As we have shown here, this selective advantage (i) does not operate during growth but rather during death, (ii) is evident under alkaline environmental conditions, and (iii) is strong enough to enrich for lasR mutants in a PAO1 wild-type culture after five incubation cycles of 48 h each. As a single 48-h cycle enriches ≥10-fold for a lasR mutant over the wild type (Fig. 3C), five cycles can be expected to reveal spontaneous lasR-negative mutants if these arise with a frequency of about 10−7 in a population, which is a realistic assumption for an average bacterial gene. When P. aeruginosa grows in nutrient-rich media, it usually produces an excess of ammonia, resulting in an alkaline environment. At pH 8.6, the lactone ring of BHL is hydrolyzed entirely and that of OdDHL partially within 6 h (68). Thus, under alkaline conditions, N-acyl-homoserine lactones lose biological activity and mutants that do not produce and respond to these molecules, e.g., lasR mutants, could have a selective advantage. Interestingly, lasR-negative P. aeruginosa strains have been isolated repeatedly from hospitalized patients and natural environments (4, 7, 13, 14, 54), raising the possibility that such strains might have been selected in environments of high pH, created by the bacteria themselves or perhaps imposed by hospital hygiene. However, in the cycling experiment, other mutants were also selected, with unidentified genetic defects outside lasR. At least some of these mutants produced N-acyl-homoserine lactones but appeared to be unable to respond to OdDHL (our unpublished observations). Other mutants retained a functional quorum-sensing machinery and manifested altered colony morphology.
What is the significance of adenosine utilization in the quorum-sensing regulon? When N-acyl-homoserine lactones are synthesized from S-adenosylmethionine and an acyl donor, methylthioadenosine (MTA) is a by-product (37). MTA can be recycled to give methionine. In P. aeruginosa, the first step of this recycling pathway is catalyzed by MTA phosphorylase (mtnP), which produces methylthioribose-1-phosphate and adenine (51). Adenine is a good nitrogen source, but a poor carbon source, for strain PAO1 (data not shown), suggesting that direct degradation of adenine may not be a major pathway. Instead, adenine may be converted to adenosine, which is effectively catabolized to inosine, hypoxanthine, xanthine, urate, and finally glyoxylate (30). It is therefore conceivable that the nucleoside hydrolase encoded by the nuh gene participates in the recycling of the adenosine moiety of MTA. Thus, high levels of N-acyl-homoserine lactones would favor MTA recycling by inducing nuh. From a practical point of view, the adenosine utilization-negative phenotype of lasR mutants provides a useful and simple tool to distinguish them from the wild type.
Repeated transfer of P. aeruginosa PAO1 from one shake culture to another could well be the major cause for the emergence of lasR-negative laboratory strains. The fact that these strains overproduce pyocyanin in late exponential to stationary phase (8) and therefore turn dark blue on plates might have given the intuitive impression of a strongly “wild” phenotype. Unfortunately, the original strain 1 (21) was lost and later replaced by a chloramphenicol-resistant subline called PAO1, in which the chloramphenicol marker appears to be unstable (22, 25), making it difficult to reconstruct the precise genealogy of PAO1 strains stocked in various laboratories. At any rate, when P. aeruginosa strains are newly isolated from an environment, they should be stocked immediately from fresh overnight cultures, by conservation at −80°C or lyophilization.
Acknowledgments
We thank Cornelia Reimmann for performing the pyocyanin assay; Yoshifumi Itoh and Karl-Erich Jaeger for providing strains PAO1-J and PAO1-D, respectively; Paul Williams for a gift of PQS; Dennis Ohman for providing strain PDO7; Paul Rainey, Ted Farmer, and Cornelia Reimmann for discussion; and Nazife Beqa for excellent technical help.
This study was supported by the Swiss National Foundation for Scientific Research (31-56608.99) and the program “Génie Biomédical.”
REFERENCES
- 1.Arevalo-Ferro, C., M. Hentzer, G. Rell, A. Görg, S. Kjelleberg, M. Givskov, K. Riedel, and L. Eberl. 2003. Identification of quorum-sensing regulated proteins in the opportunistic pathogen Pseudomonas aeruginosa by proteomics. Environ. Microbiol. 5:1350-1369. [DOI] [PubMed] [Google Scholar]
- 2.Beatson, S. A., C. B. Whitchurch, A. B. T. Semmler, and J. S. Mattick. 2002. Quorum sensing is not required for twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 184:3598-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berk, R. S. 1963. Nutritional studies on the “auto-plaque” phenomenon in Pseudomonas aeruginosa. J. Bacteriol. 86:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrol, S., A. Olliver, G. B. Pier, A. Andremont, and R. Ruimy. 2003. Transcription of quorum-sensing system genes in clinical and environmental isolates of Pseudomonas aeruginosa. J. Bacteriol. 185:7222-7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Argenio, D. A., M. W. Calfee, P. B. Rainey, and E. C. Pesci. 2002. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J. Bacteriol. 184:6481-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Sal, G., G. Manfioletti, and C. Schneider. 1988. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 16:9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dénervaud, V., P. TuQuoc, D. Blanc, S. Favre-Bonté, V. Krishnapillai, C. Reimmann, D. Haas, and C. van Delden. 2004. Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J. Clin. Microbiol. 42:554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diggle, S. P., K. Winzer, A. Lazdunski, P. Williams, and M. Cámara. 2002. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 184:2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farinha, M. A., and A. M. Kropinski. 1990. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol. Lett. 58:221-225. [DOI] [PubMed] [Google Scholar]
- 10.Farrell, M. J., and S. E. Finkel. 2003. The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J. Bacteriol. 185:7044-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum-sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher, L. A., S. L. McKnight, M. S. Kuznetsova, E. C. Pesci, and C. Manoil. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 184:6472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamood, A. N., J. Griswold, and J. Colmer. 1996. Characterization of elastase-deficient clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:3154-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heeb, S., Y. Itoh, T. Nishijyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria CHA0. Mol. Plant-Microbe Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- 16.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Høiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heurlier, K., V. Dénervaud, G. Pessi, C. Reimmann, and D. Haas. 2003. Negative control of quorum sensing by RpoN (σ54) in Pseudomonas aeruginosa PAO1. J. Bacteriol. 185:2227-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heurlier, K., F. Williams, S. Heeb, C. Dormond, G. Pessi, D. Singer, M. Cámara, P. Williams, and D. Haas. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol. 186:2036-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Højberg, O., U. Schnider, H. V. Winteler, J. Sørensen, and D. Haas. 1999. Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil. Appl. Environ. Microbiol. 65:4085-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holloway, B. W. 1955. Genetic recombination in Pseudmonas aeruginosa. J. Gen. Microbiol. 13:572-581. [DOI] [PubMed] [Google Scholar]
- 22.Holloway, B. W. 1969. Genetics of Pseudomonas. Bacteriol. Rev. 33:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horn, J. M., and D. E. Ohman. 1988. Transcriptional and translational analyses of recA mutant alleles in Pseudomonas aeruginosa. J. Bacteriol. 170:1637-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadurugamuwa, J. L., and T. J. Beveridge. 1996. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178:2767-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Köhler, T., C. van Delden, L. K. Curty, M. M. Hamzehpour, and J. C. Pechère. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183:5213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma, J., A. Campbell, and S. Karlin. 2002. Correlations between Shine-Dalgarno sequences and gene features such as predicted expression levels and operon structures. J. Bacteriol. 184:5733-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manefield, M., and S. L. Turner. 2002. Quorum sensing in context: out of molecular biology and into microbial ecology. Microbiology 148:3762-3764. [DOI] [PubMed] [Google Scholar]
- 29.Matsui, H., Y. Sano, H. Ishihara, and T. Shinomiya. 1993. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J. Bacteriol. 175:1257-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto, H., S. Ohta, R. Kobayashi, and Y. Terawaki. 1978. Chromosomal location of genes participating in the degradation of purines in Pseudomonas aeruginosa. Mol. Gen. Genet. 167:165-176. [DOI] [PubMed] [Google Scholar]
- 31.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohman, D. E., S. J. Cryz, and B. H. Iglewski. 1980. Isolation and characterizarion of a Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 142:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ornston, L. N., and R. Y. Stanier. 1966. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. J. Biol. Chem. 241:3776-3786. [PubMed] [Google Scholar]
- 35.Parkin, D. W., B. A. Horenstein, D. R. Abdulah, B. Estupinán, and V. L. Schramm. 1991. Nucleoside hydrolase from Crithidia fasciculata. Metabolic role, purification, specificity, and kinetic mechanism. J. Biol. Chem. 266:20658-20665. [PubMed] [Google Scholar]
- 36.Parret, A. H. A., and R. De Mot. 2002. Bacteria killing their own kind: novel bacteriocins of Pseudomonas and other γ-proteobacteria. Trends Microbiol. 10:107-112. [DOI] [PubMed] [Google Scholar]
- 37.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson, J. P., M. Feldman, B. H. Iglewski, and A. Prince. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pesci, E. C., J. B. J. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pessi, G., and D. Haas. 2000. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J. Bacteriol. 182:6940-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. G. Holden, M. Cámara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen, C., and L. B. Møller. 2001. The RihA, RihB, and RhiC ribonucleoside hydrolases of Escherichia coli. J. Biol. Chem. 276:884-894. [DOI] [PubMed] [Google Scholar]
- 45.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 46.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 47.Rumbaugh, K. P., J. A. Griswold, and A. N. Hamood. 2000. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect. 2:1721-1731. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuster, M., A. C. Hawkins, C. S. Harwood, and E. P. Greenberg. 2004. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 51:973-985. [DOI] [PubMed] [Google Scholar]
- 51.Sekowska, A., V. Dénervaud, H. Ashida, K. Michoud, D. Haas, A. Yokota, and A. Danchin. 4 March 2004, posting date. Bacterial variations on the methionine salvage pathway. BMC Microbiology 4:9. [Online.] http://www.biomedcentral.com/1471-2180/4/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-790. [Google Scholar]
- 53.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 54.Sokurenko, E. V., V. Tchesnokova, A. T. Yeung, C. A. Oleykowski, E. Trintchina, K. T. Hughes, R. A. Rashid, J. M. Brint, S. L. Moseley, and S. Lory. 2001. Detection of simple mutations and polymorphisms in large genomic regions. Nucleic Acids Res. 29:e111. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanisich, V. A., and B. W. Holloway. 1972. A mutant sex factor of Pseudomonas aeruginosa. Genet. Res. 19:91-108. [DOI] [PubMed] [Google Scholar]
- 56.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 57.Sussman, J. K., E. L. Simons, and R. W. Simons. 1996. Escherichia coli translation initiation factor 3 discriminates the initiation codon in vivo. Mol. Microbiol. 21:347-360. [DOI] [PubMed] [Google Scholar]
- 58.Terada, M., M. Tatibana, and O. Hayaishi. 1967. Purification and properties of nucleoside hydrolase from Pseudomonas fluorescens. J. Biol. Chem. 242:5578-5585. [PubMed] [Google Scholar]
- 59.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. D. Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieira, J., and J. Messing. 1991. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100:189-194. [DOI] [PubMed] [Google Scholar]
- 62.Voisard, C., C. T. Bull, C. Keel, J. Laville, M. Maurhofer, U. Schnider, G. Défago, and D. Haas. 1994. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches, p. 67-89. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere microorganisms. VCH, Weinheim, Germany.
- 63.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb, J. S., L. S. Thompson, S. James, T. Charlton, T. Tolker-Nielsen, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 66.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 67.Winzer, K., and P. Williams. 2001. Quorum-sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291:131-143. [DOI] [PubMed] [Google Scholar]
- 68.Yates, E. A., B. Philipp, C. Buckley, S. Atkinson, S. R. Chhabra, R. E. Sockett, M. Goldner, Y. Dessaux, M. Cámara, H. Smith, and P. Williams. 2002. N-Acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70:5635-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuber, S., F. Carruthers, C. Keel, A. Mattart, C. Blumer, G. Pessi, C. Gigot-Bonnefoy, U. Schnider-Keel, S. Heeb, C. Reimmann, and D. Haas. 2003. GacS sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol. Plant-Microbe Interact. 16:634-644. [DOI] [PubMed] [Google Scholar]