Abstract
Background
A network meta-analysis was performed to evaluate the risk of congenital malformations and other prenatal outcomes in fetuses after exposure to antipsychotic medications and mood stabilizers during pregnancy.
Methods
We searched the PubMed, EMBASE, and Cochrane CENTRAL databases up to 15 December 2023, to identify experimental and observational studies comparing antipsychotic and mood stabilizer treatments with control treatments (no exposure). The primary outcome of the study was the incidence of congenital malformations and the secondary outcomes were preterm birth and spontaneous abortion. Additionally, two authors independently assessed the risk of bias in each domain of the included studies using the ROBINS-I tool and evaluated the quality of evidence using the CINeMA rating tool.
Results
The literature search identified 18,334 potential records, and 22 studies involving 3,042,997 pregnant women were ultimately included. Compared with the unexposed group, quetiapine [odds ratio (OR), 1.19; 95% credible interval (CrI), 1.01–1.39], aripiprazole (OR, 1.30; 95% CrI 1.10–1.65), olanzapine (OR, 1.33; 95% CrI 1.11–1.64), risperidone (OR, 1.43; 95% CrI 1.18–1.77), and lithium (OR, 1.61; 95% CrI 1.07–2.30) were associated with a slightly increased risk of congenital malformations. In contrast, lamotrigine (OR, 1.21; 95% CrI 0.86–1.64), ziprasidone (OR, 1.14; 95% CrI 0.73–1.72), and haloperidol (OR, 1.26; 95% CrI 0.90–1.75) did not show significant differences compared with the unexposed group, with narrower credible intervals.
Conclusions
The evidence from this analysis suggests that, overall, quetiapine has the lowest teratogenic risk when used during pregnancy, making it the safer option for pregnant women. Lamotrigine and haloperidol follow closely behind. At the same time, the use of lurasidone and ziprasidone should be approached with caution, and further clinical studies are necessary to better assess their safety.
Systematic Review Registration
PROSPERO CRD4201811373.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-024-01131-x.
Key Points
| Challenges in treatment choices: The use of antipsychotic medications and mood stabilizers during pregnancy presents a complex decision-making challenge for both doctors and patients, mainly due to the variable and recurrent nature of symptoms associated with schizophrenia and bipolar disorder. |
| Lack of human research: There is a significant deficiency in human research concerning antipsychotic medications and mood stabilizers, which complicates the ability to determine their safety during pregnancy accurately. |
| Findings from network meta-analysis: Our network meta-analysis, which includes eight antipsychotic drugs and four mood stabilizers, indicates that quetiapine may be the safest option with the lowest teratogenic risk. Following quetiapine, lamotrigine and the first-generation antipsychotic Penfluridol/haloperidol also appear safer but still require cautious use in clinical practice. Decisions should be based on an individualized assessment of risks and benefits. |
Introduction
Antipsychotic medications and mood stabilizers, especially atypical antipsychotics, are commonly employed as first-line treatments for psychiatric disorders such as schizophrenia and bipolar affective disorder [1, 2]. The risk of mental illness can increase in response to psychological, hormonal, and lifestyle changes during pregnancy [3]. Mental illness during pregnancy can have adverse effects on women and their children, including poor compliance with medical care and poor nutritional status (too little or too much weight gained during pregnancy) [4, 5]. One study showed a significant increase in both readmission and relapse rates in women with schizophrenia and bipolar disorder after stopping antipsychotic medication [3]. Therefore, for women with stable severe mental illness, medication discontinuation should be avoided unless absolutely necessary. However, the use of these drugs can lead to adverse obstetric outcomes, including birth defects (malformations), miscarriages, and stillbirths. Congenital malformations are usually the primary adverse outcome of using antipsychotics and mood stabilizers during pregnancy [6]. Congenital malformations include defects in one or more identifiable organs or other body parts present at birth or in utero. Malformations can lead to long-term disability, illness, and death [7]. The safety of antipsychotics and mood stabilizers in pregnant women has been assessed in numerous studies, including observational studies and systematic reviews [8–12] and meta-analyses [13–25]. However, the results were not homogeneous, as these studies differed considerably in terms of methodology, specific drugs, and sample size. Ennis and Damkier [19]concluded that exposure to olanzapine in early pregnancy was not associated with an increased risk of congenital malformations, that quetiapine and risperidone increased the risk of congenital malformations, and that the risk assessment for aripiprazole was tentatively unreliable due to the small amount of data available. However, the studies by Ellfolk et al. [26] and Huybrechts et al. [11]revealed that olanzapine and risperidone are associated with an increased risk, and the conclusion regarding the safety of aripiprazole in the study by Freeman et al. [27]was also limited by the small sample size. In contrast, the present meta-analysis yielded information only about the association between antipsychotic use and the risk of congenital malformations, without focusing on specific drugs [21].
Determining the best pharmacological interventions is challenging when relying solely on individual studies or paired analyses. Network meta-analysis (NMA), also known as mixed treatment comparison, goes beyond traditional comparisons: it considers both direct and indirect evidence from various studies simultaneously. NMA helps estimate how well different interventions work in comparison and ranks them, even when direct comparisons between two interventions are missing [28]. Therefore, we conducted an NMA to search all the published literature in several core databases to provide a more precise risk estimation of the association between the use of antipsychotic medications during pregnancy and congenital malformations in children.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. The review protocol was registered (registration number: CRD4201811373) with the International Prospective Register of Systematic Reviews (PROSPERO) (PRISMA checklist is reported in Supplementary materials 2).
Search Strategy and Selection Criteria
We systematically searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to 13 December 2023. We used the following search terms to filter documents: antipsychotic drugs, quetiapine, aripiprazole, clozapine, olanzapine, risperidone, ziprasidone, lurasidone, lamotrigine, lithium, carbamazepine, valproate, pregnancy, newborn, and fetus. To ensure the inclusion of all potentially eligible studies, we also performed manual searches of the reference lists of all relevant meta-analyses and systematic reviews. The detailed search strategy is presented in Supplementary Table 1.
The inclusion criteria were as follows: (1) randomized controlled trials, observational studies (cohort or case‒control studies), and registered studies that reported the association between the use of antipsychotics and/or mood stabilizers during pregnancy and adverse outcomes. These studies included the interventions under investigation, the medical history before and during pregnancy, and the outcomes of interest. (2) The interventions covered by the studies included both monotherapy and polytherapy with antipsychotics or mood stabilizers, first-generation medications (e.g., Penfluridol/haloperidol), second-generation medications (e.g., quetiapine, aripiprazole, olanzapine, risperidone, clozapine, ziprasidone, and lurasidone), and mood stabilizers (e.g., lamotrigine, lithium, valproate, and carbamazepine), with no restrictions on the dose of these medications. Placebo, no exposure, or other single or combined use of antipsychotics and mood stabilizers were considered comparator interventions. (3) The trials included adverse pregnancy outcomes.
Studies were excluded if they met the following conditions: (1) included pregnant women who were not taking the studied medication for mental illness, (2) had incomplete outcome data that precluded analysis, (3) were animal experiments or (4) were articles without available full texts.
Outcome Measures
Primary Outcomes
Major congenital malformations: The proportion of children who presented with any type of major congenital malformation (as defined by the original study authors) was determined. Major congenital malformations included structural abnormalities of the body or organs that were present at birth, impaired viability, and required significant intervention [30].
Secondary Outcomes
1. Preterm birth: Preterm birth was defined as the birth of a live infant before completing 37 weeks of gestation [31].
2. Spontaneous abortion: Spontaneous abortion referred to the spontaneous loss of a pregnancy before 12 weeks (early miscarriage) or from 12 to 24 weeks (late miscarriage) of gestation [32].
Study Selection and Data Collection
Two reviewers independently screened the titles, abstracts, and full texts of the identified references. Disagreements were resolved by consultation and discussion with a third reviewer. The same process was used to extract the data and assess the methodological quality.
Appraisal of Methodological Quality
As we did not find any randomized controlled studies that met our inclusion and exclusion criteria during the screening process, we included and analyzed only cohort studies in our research. We assessed their methodological quality using the ROBINS-I tool (see Supplementary materials 3) [33, 34]. The ROBINS-I tool is primarily used to assess the risk of bias in the following seven domains for nonrandomized studies: bias due to confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. The categories for bias risk assessment are “low risk,” “moderate risk,” “serious risk,” and “critical risk.” Disagreements were resolved by discussion, and if needed, a third reviewer was consulted to reach a resolution.
Statistical Analysis
We performed an NMA using a random-effects model within a Bayesian framework. The treatments were ranked according to the surface under the cumulative ranking curve (SUCRA), with SUCRA values ranging from 0 to 1; the closer the SUCRA value is to 1, the greater the risk of adverse reactions for that treatment. Odds ratios (ORs) and 95% credible intervals (95% CrIs) were calculated to estimate the effects of different interventions on adverse outcomes. It is important to note that a credibility interval in Bayesian statistics shows the range where an unobserved parameter likely falls with a specific probability. This is similar to the 95% confidence interval used in frequentist statistics but differs in some ways [35]. We also performed a node-splitting analysis to assess local inconsistencies in the network. This analysis identifies possible inconsistencies by separating direct and indirect evidence. Interstudy heterogeneity was assessed using the I2 index, and the degree of heterogeneity was characterized as low, medium, or high based on the first and third quartiles of its empirical distribution. Transitivity assumption was evaluated by comparing the distribution of key study characteristics across studies grouped by comparison (average age, treatment period). To determine the comparative effectiveness of the different interventions, we performed a Bayesian network meta-analysis using a Markov chain Monte Carlo algorithm (i.e., Gibbs sampling). We discarded the first 20,000 iterations (burn-out period) and used the next 50,000 iterations for estimation. Publication bias was detected by comparing adjusted funnel plots. Our analysis was performed by the gemtc and netmeta packages for R 4.0.5 [36–38]and the network package for Stata 14.0 [39, 40].
Certainty Assessment
According to the GRADE guidelines, when studies involve multiple interventions, Salanti and colleagues recommend a more appropriate methodology, which can be implemented through the online tool CINeMA [41, 42]. Therefore, we used CINeMA, an improved online application based on GRADE principles, to assess the level of evidence of the 22 nonrandomized studies included. This method takes into account six factors that may lead to a lower rating: within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency [43]. Although the included studies were all nonrandomized, we used the new Risk of Intervention Bias Assessment Tool for Non-Randomized Studies (ROBINS-I), and in the initial assessment all studies were considered to be high-certainty evidence and downgraded on a case-by-case basis [44]. CINeMA’s evidence ratings are conducted independently by two researchers and if there is a discrepancy between their assessments a third researcher will join the discussion to reach consensus. In addition, CINeMA uses the netmeta R package for network meta-analysis [38].
Results
Literature Search
A total of 18,334 potentially eligible records were identified in the search, and 16,872 records were excluded after title and abstract screening (Fig. 1). In total, the full texts of 354 studies were examined, and 332 studies were excluded. Twenty-two studies provided data on at least one outcome and were included in the quantitative synthesis. For the included studies, the risk of bias assessment and references are shown in the Supplementary Information (see Supplementary materials 3)
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram. The above figure outlines the flow of studies reviewed during the PRISMA-guided systematic review process
Study and Patient Characteristics
We included 22 observational studies (3,042,997 patients) published from 1983 to 2023. The number of patients included in each study ranged from 58 to 1,341,544. All the studies included pregnant women who had taken antipsychotics and/or mood stabilizers for mental illness (including conditions such as schizophrenia, bipolar disorder, and anxiety) during pregnancy. Pregnant women with epilepsy were excluded. A total of 68.1% of the studies examined women in early pregnancy, while 27.2% of the studies examined women throughout pregnancy. A total of 12 intervention measures were included. Fifty percent of the studies included in the Institute included controls who had experienced psychiatric disorders during pregnancy but had not taken antipsychotic medication. Sixteen studies (3,040,761 participants) reported data for the primary outcome (congenital malformations). Seven studies (n = 2381 participants) reported data on preterm birth and six (n = 2553 participants) reported data on spontaneous abortion. The characteristics of included studies are presented in Table 1.
Table 1.
Description of characteristics of included studies
| Author, Year | Design | Indication | Nonexposed group | Interventions | Sample Source |
Age, years (mean ± SD/range) | Time of exposure | Duration of follow-up | Outcomes | Study conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Huybrechts KF (2023) [46] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders |
Quetiapine, aripiprazole, olanzapine, risperidone, haloperidol/ penfluridol |
341,363 |
FGAs1: 28.54 SGAs2: 29.54 NEG: 27.54 |
First trimester | NR | Major congenital malformations. |
(1) Overall, in utero exposure to antipsychotics was not significantly associated with an increased risk of malformations. (2) Olanzapine may be associated with an increased risk of oral clefts, atypical antipsychotics with an increased risk of gastroschisis and brain anomalies, and chlorprothixene with an increased risk of cardiac malformations, but further research is needed. |
| Freeman MP (2021) [27] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders | Aripiprazole | 867 |
EG (ari): 32.4 ± 5.49 NEG: 32.7 ± 4.19 |
First trimester | The follow-up was conducted at 7 months of pregnancy and 12 weeks postpartum. | Major congenital malformations. | The risk of major malformations following early pregnancy exposure to aripiprazole was not significantly increased, but the findings are limited by the relatively small sample size. Larger studies are needed in the future to further validate these results. |
| Ellfolk M, 2021) [26] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders |
Quetiapine, aripiprazole, olanzapine, risperidone, clozapine |
26,139 | NR | First trimester | NR | Major congenital malformations. | Olanzapine use was associated with an increased risk of major congenital malformations and specifically, musculoskeletal malformations. |
| Cohen LS (2018) [47] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders | Quetiapine | 365 |
EG (que): 32.3 ± 4.89 NEG: 33.3 ± 4.07 |
First trimester | The follow-up was conducted at 7 months of pregnancy and 12 weeks postpartum. | Major congenital malformations. | Quetiapine was not a major teratogen. However, considering the uncertainty in risk estimation due to sample size limitations, the study results could only rule out approximately a fivefold increase in the risk of major malformations. |
| Bellet F (2015) [8] | Cohort study | Psychiatric disorders |
Unexposed but unknown disease status |
Aripiprazole | 232 |
EG (ari): 31.8 ± 5.8 NEG: 31.4 ± 5.4 |
4–10 gestational weeks | The follow-up was conducted within 2 months after the expected delivery date. |
(1) All congenital malformations (both major and minor). (2) Preterm birth. (3) Miscarriage. |
No significant association was found between embryonic exposure to aripiprazole and major malformations. However, larger prospective studies are needed to further elucidate the reproductive safety of aripiprazole. |
| Huybrechts KF (2016) [11] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders |
Quetiapine, aripiprazole olanzapine, risperidone, ziprasidone |
1,341,544 |
SGAs2: 25.39 ± 6.42 NEG: 24.01 ± 5.77 |
First trimester | NR | All congenital malformations. | Early pregnancy use of antipsychotics (APs) generally did not significantly increase the risk of congenital malformations, particularly cardiac malformations. However, the slight increase in malformation risk observed with risperidone warranted further investigation. |
| McKenna K (2005) [69] | Cohort study | Psychiatric disorders | Not exposed to teratogenic drugs, no mental illness or use of psychotropic drugs | Olanzapine | 185 | NR | First trimester | The follow-up was conducted 3 to 4 months after the expected delivery date. | Major congenital malformations. | Atypical antipsychotics did not appear to be associated with an increased risk of major malformations. |
| Diav-Citrin O (2005) [61] | Cohort study | Psychiatric disorders | Not exposed to teratogenic drugs | Haloperidol/penfluridol | 709 |
EG (hal/pen): 32 (28–36) NEG: 30 (27–33) |
First trimester | The follow-up could extend up to 6 years, but most follow-ups were completed within the first 2 years after the infant's birth. |
(1) Major congenital malformations. (2) Miscarriage. (3) Preterm birth. |
Pregnant women using haloperidol or penfluridol had a higher risk of preterm birth compared to the control group, but there were no significant differences in the risks of congenital malformations and miscarriage. |
| Patorno E (2017) [70] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders |
Lamotrigine, lithium |
1,325,563 |
EG (lit)3: 25.6 ± 6.1 NEG: 24.0 ± 5.8 |
First trimester | NR | All congenital malformations. | Lithium use by mothers in the first trimester of pregnancy was associated with an increased risk of cardiac malformations. |
| Cohen LS (2023) [55] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders |
Quetiapine, lurasidone, aripiprazole |
1477 |
EG (lur): 33.2 ± 4.92 EG (que): 32.0 ± 4.80 NEG: 32.6 ± 4.18 |
First trimester | The follow-up was conducted at 7 months of pregnancy and 12 weeks postpartum. | Major congenital malformations. | Lurasidone and quetiapine did not appear to be major teratogens, but additional information is needed to refine the risk estimates. |
| Habermann (2013) [71] | Cohort study | Psychiatric disorders | NR |
Aripiprazole, quetiapine, olanzapine, risperidone, clozapine, ziprasidone |
447 |
SGAs2: 32 (27–36) |
First trimester | The follow-up was conducted 8 weeks after the delivery date. | Major congenital malformations. | The study did not find that second-generation antipsychotics (SGAs) posed a significant teratogenic risk. |
| Kulkarni J (2014) [72] | Cohort study | Psychiatric disorders | NR |
Clozapine, olanzapine, quetiapine, risperidone, |
124 |
SGAs2: 32.67 ± 4.7 |
First trimester | The follow-up was conducted every 6–8 weeks during pregnancy. | Congenital malformations. | The rate of congenital anomalies in Australia was higher than the expected rate, particularly for congenital heart defects. |
| Viguera, A.C (2023) [73] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders | Olanzapine | 1207 |
EG (ola): 33.6 ± 4.56 NEG: 32.5 ± 4.08 |
First trimester | The follow-up was conducted at 7 months of pregnancy and 12 weeks postpartum. | Major congenital malformations. | Exposure to olanzapine in early pregnancy did not significantly increase the risk of major malformations, the study found. |
| Bodén R (2012) [74] | Cohort study | Bipolar disorder | NR |
Lamotrigine, lithium, valproate, carbamazepine |
266 | NR | During pregnancy | NR | Major congenital malformations. | Infants born to women with bipolar disorder had an increased risk of preterm birth, regardless of whether the mother received mood stabilizer treatment. Infants of untreated women also faced a higher risk of microcephaly and neonatal hypoglycemia. |
| Diav-Citrin O (2014) [75] | Cohort study | Psychiatric disorders | Unexposed with psychiatric disorders | Lithium | 184 |
EG (lit): 32 ± 6 NEG: 31 ± 6 |
During pregnancy | The follow-up was conducted within the first 2 years after birth in most cases. |
(1) Major congenital malformations (2) Miscarriage. (3) Preterm delivery. |
Lithium treatment during pregnancy was associated with a higher incidence of fetal cardiovascular abnormalities. |
| Källén B (1983) [76] | Cohort study | Psychiatric disorders | Exposed to psychotropic drug but unexposed to lithium with psychiatric disorders | Lithium | 79 | NR | First trimester | NR | Malformations. | The use of lithium in early pregnancy was found to possibly increase the risk of perinatal mortality and malformations in infants; therefore, it is recommended to avoid lithium during early pregnancy. |
| Vigod SN (2015) [9] | Cohort study | Psychiatric disorders | NR |
Quetiapine, olanzapine, risperidone |
834 |
SGAs2: 28.8 ± 6.1 NEG: 26.7 ± 6.3 |
During pregnancy | NR | Preterm birth. | Antipsychotic drug use in pregnancy had a minimal evident impact on important maternal medical and short-term perinatal outcomes. However, the rate of adverse outcomes is high enough to warrant a careful assessment of maternal and fetal well-being among women prescribed an antipsychotic drug in pregnancy. |
| Sørensen MJ (2015) [77] | Cohort study | Psychiatric disorders | NR | Quetiapine, olanzapine, lithium, risperidone, aripiprazole, ziprasidone, haloperidol | 743 | NR | During pregnancy | NR | Spontaneous abortion. | The increased risk of spontaneous miscarriage observed among women who were treated with antipsychotic medications during pregnancy was likely due to confounding factors. |
| Sakai T (2017) [78] | Cohort study | Psychiatric disorders | NR |
Aripiprazole, olanzapine, quetiapine |
165 | NR | NR | NR | Miscarriage. | Aripiprazole may be associated with miscarriage. However, safety information regarding the use of aripiprazole during pregnancy is very limited. Further research is recommended. |
| Jacobson SJ (1992) [79] | Cohort study | Psychiatric disorders | Not exposed to teratogenic drugs | Lithium | 286 |
EG (lit): 30 ± 5.3 NEG: 29.8 ± 5.3 |
First trimester | The postpartum follow-up was conducted when the average age was 61 weeks. |
(1) Spontaneous abortion. (2) Premature birth. |
Lithium was not considered a significant human teratogen. Women with major affective disorders who wished to have children could continue lithium therapy, provided that adequate screening tests, including level II ultrasound and fetal echocardiography, were performed. |
| Newport DJ (2007) [6] | Cohort study | Psychiatric disorders | NR |
Haloperidol, olanzapine, quetiapine, risperidone |
58 |
EG (hal): 30.5 ± 5.1 EG (ola): 30.8 ± 5.3 EG (que): 31.7 ± 6.8 EG (ris): 27.7 ± 4.4 |
During pregnancy proximate | Follow-up was conducted monthly for participants during pregnancy. | Preterm delivery. | All four antipsychotics (haloperidol, olanzapine, quetiapine, and risperidone) exhibited incomplete placental passage, with quetiapine showing the lowest placental passage among the medications studied. |
| Poels EMP (2022) [80] | Cohort study | Bipolar spectrum disorder | Unexposed with psychiatric disorders. | Lithium | 99 | NR | During pregnancy | NR | Premature birth. | There was no evidence of significant changes in neuropsychological function in children exposed to lithium in utero. |
Ari Aripiprazole, EG Exposure group, Hal Haloperidol, Hal/Pen Haloperidol/Penfluridol, Lit Lithium, Lur Lurasidone, NEG Nonexposed group, NR Not reported, Ola Olanzapine, Que Quetiapine, Ris Risperidone
1FGAs refer to all first-generation antipsychotics in this study
2SGAs refer to all second-generation antipsychotics in this study
3Age was not reported for lamotrigine
4Only the mean value was presented
Results of the Network Meta-analysis
Overall Congenital Malformations
The NMA of overall congenital malformations included 16 cohort studies in which 12 interventions were assessed and 3,040,761 pregnant women were included. The network plot is shown in Fig. 2. Quetiapine, aripiprazole, and olanzapine were included in the largest number of comparison studies, followed by risperidone. However, lurasidone, valproate, and carbamazepine were mentioned in only a small number of studies.
Fig. 2.
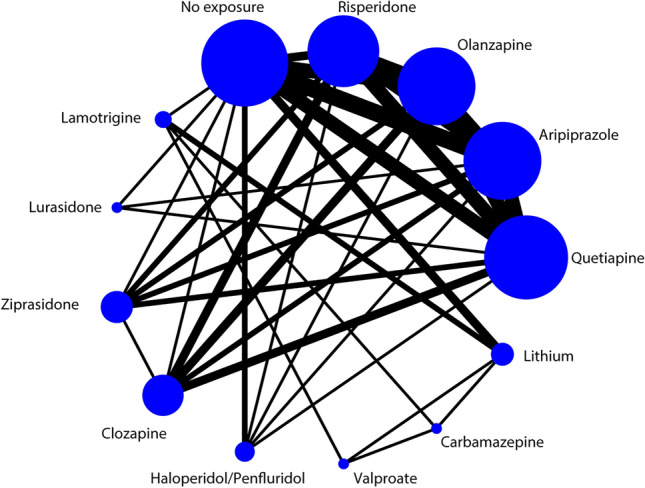
Network plot for congenital malformations. Footnotes: The size of the circle represents the number of participants. The width of the line represents the number of studies
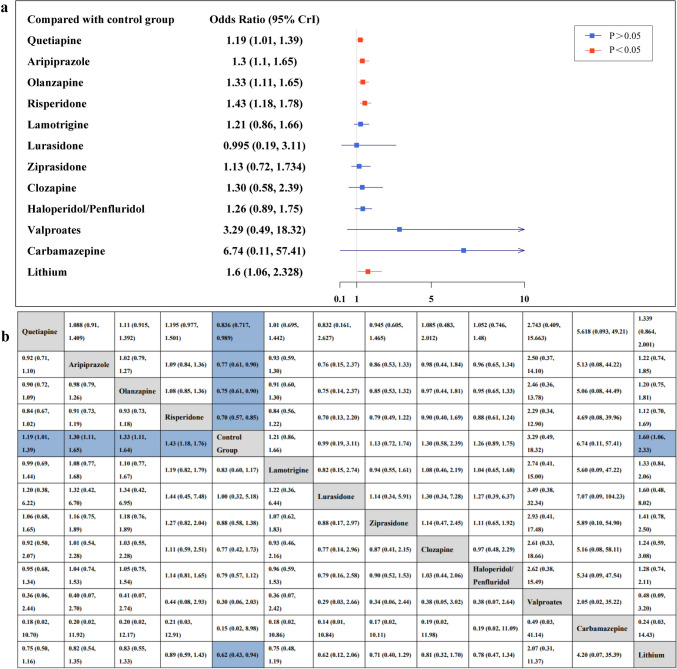
Figure 3 shows the forest plot (a) comparing each intervention with the control group for congenital malformations, and the league table (b) for pairwise comparisons. Compared with the unexposed group, the quetiapine (OR, 1.19; 95% CrI 1.01–1.39), aripiprazole (OR, 1.3; 95% CrI 1.1–1.65), olanzapine (OR, 1.33; 95% CrI 1.11–1.64), risperidone (OR, 1.43; 95% CrI 1.18–1.77), and lithium (OR, 1.61; 95% CrI 1.07–2.3) groups all had a significantly increased risk of congenital malformations. In contrast, there was no difference between the other seven medication groups and the control group. Among these drugs, the drugs with narrower CrI were lamotrigine (OR, 1.21; 95% CrI 0.86–1.64), ziprasidone (OR, 1.14; 95% CrI 0.73–1.72), and haloperidol/penfluridol (OR, 1.26; 95% CrI 0.9–1.75). These treatments were at least as effective as those in the unexposed group. In the comparisons between the two drug treatments, we did not find statistical evidence suggesting any other differences between antipsychotic drugs.
Fig. 3.
The risk of congenital malformations for the group exposed to antipsychotic medications and mood stabilizers compared with the control group. Extreme values that exceed the x axis are indicated with arrows. Red lines represent p-values less than 0.05, indicating statistical significance, while blue lines represent p-values greater than 0.05, indicating no statistical significance. B The risk ratios (95% credible intervals) between treatment groups in the network meta-analysis. Blue squares indicate statistically significant results. The results in the lower-left triangle should be read from left to right; for example, the risk ratio for congenital malformations for the aripiprazole group compared with the unexposed group is 1.3 (95% credible interval, 1.1–1.65). Results in the upper-right triangle should be read from right to left; for example, the risk ratio for congenital malformations for the unexposed group compared with the olanzapine group is 0.75 (95% credible interval, 0.61–0.9).
Tables 10 and 14 in Supplementary file 1 provide a two-by-two comparison of outcomes for spontaneous abortion and preterm birth.
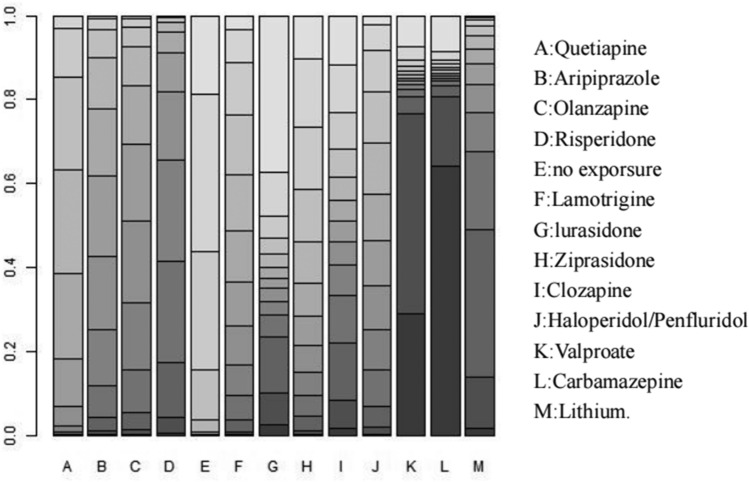
Ranking probabilities
All interventions were ranked using the SUCRA method, which is a summary statistic of cumulative rankings and ranking probabilities. Figure 4 shows the cumulative probability curves for the risk of congenital malformations associated with each drug in the network. In terms of congenital anomaly outcomes, the safest treatment, except for no exposure (SUCRA = 0.11), was lurasidone (SUCRA = 0.32), followed by ziprasidone (SUCRA = 0.33), quetiapine (SUCRA = 0.34), lamotrigine (SUCRA = 0.39), and haloperidol/penfluridol (SUCRA = 0.45). Carbamazepine had the highest risk (SUCRA = 0.87).
Fig. 4.
Rank probabilities of associations between the use of different types of antipsychotic medications and mood stabilizers with the risk of congenital malformations. The intensity of the color distinguishes the rank probabilities of the effect of each medicine on the risk of congenital malformations from the first to the eighth ranking
The SUCRA values for the secondary endpoints are shown in the Supplementary materials 1.
Secondary Outcomes
For preterm birth, we included seven cohort studies (2381 pregnant women) that evaluated six intervention measures and an control group. For natural abortion, we included six cohort studies (2553 pregnant women) that evaluated seven intervention measures and an control group. Due to the limited sample size included for the secondary outcomes, the results are not sufficiently precise and there were no statistically significant findings. Therefore, forest plots for preterm birth and spontaneous abortion, along with pairwise comparison results and SUCRA values, are presented only in the Supplementary material 1.
Convergence, Heterogeneity, and Consistency in Models
All models have successfully converged. The potential scale reduction factor (PSRF) value for the model of congenital malformation outcomes is 1.02, while the PSRF values for the models of preterm birth and miscarriage outcomes are both 1. Inconsistency tests revealed that the results of the direct, indirect, and network analyses of congenital anomalies, preterm birth, and spontaneous abortion outcomes were consistent with good agreement. There was very low heterogeneity in the primary outcome among the studies. For the secondary outcomes, heterogeneity was low in most trials, with moderate heterogeneity in a small number of studies (see Supplementary materials 1).
Risk of Bias Across Studies
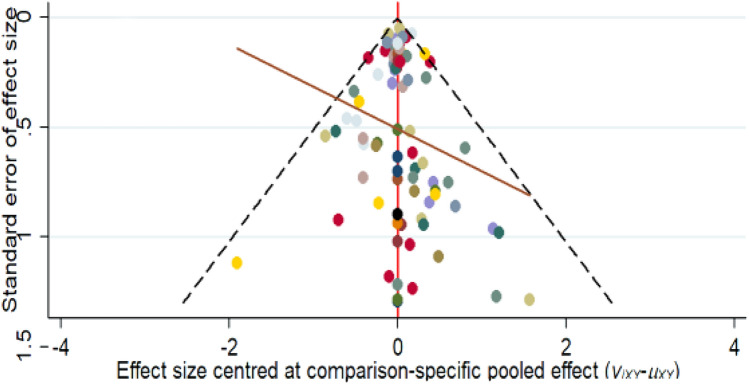
The adjustment of the comparison funnel plot aimed to account for potential biases and asymmetries in the distribution of the study results (Fig. 5). This plot illustrates the effect of study size on effect estimates. Interpretation of adjusted funnel plots requires careful consideration of funnel plot symmetry; if the distribution of the funnel plots is approximately symmetric, publication bias or a low probability of a small study size effect is suggested. In contrast, the points of our funnel plot are evenly distributed and largely symmetrical on both sides of the centerline, with no additional points outside the boundary. This symmetry suggests that the observed treatment effect is unlikely to be influenced by publication bias or small study effects.
Fig. 5.
Comparison of adjusted funnel plots for the outcomes. No funnel plot is presented for spontaneous abortion (k = 6) or preterm birth (k = 7) outcomes due to the small number of studies (k = 9) included in the network meta-analysis
Methodological Quality Results and Certainty of Evidence
We assessed 22 cohort studies using the ROBINS-I risk of bias assessment tool (see Tables 1 and 2 in Supplement 3). Overall, these studies exhibited a moderate to high risk of bias (specifically, 9 studies were classified as moderate risk and 13 studies as serious risk). The included studies primarily demonstrated high risk of bias in areas related to participant selection, deviations from intended interventions, and missing data.
The quality of evidence for the primary outcomes, assessed using the CINeMA tool, ranged from moderate to very low (specifically, 6 comparisons were rated as moderate, 16 as low, and 58 as very low). The downgrading was primarily due to high risk of bias within studies and imprecision. The comparisons classified as moderate risk involved control groups compared with quetiapine, aripiprazole, olanzapine, lithium, risperidone, and the comparison between aripiprazole and quetiapine. For complete information regarding CINeMA, see Supplementary materials 4.
Discussion
Globally, substance use during pregnancy remains a significant public health concern, as it can lead to a range of adverse neonatal outcomes. In our network meta-analysis, we examined the associations between the use of antipsychotic drugs and mood stabilizers during pregnancy and the risks of congenital malformations, preterm birth, and spontaneous abortion. We included a total of 22 studies covering 12 commonly used antipsychotics and mood stabilizers. The results indicated that for the primary outcome—the risk of congenital malformations—the treatment groups for quetiapine, risperidone, aripiprazole, olanzapine, and lithium had a higher risk of fetal congenital malformations compared with the unexposed group. In contrast, the following drugs did not show a statistically significant risk to fetal development compared to the control group: lurasidone (SUCRA = 0.32), ziprasidone (SUCRA = 0.33), lamotrigine (SUCRA = 0.39), and haloperidol (SUCRA = 0.45), and these drugs ranked relatively high in safety.
Second-Generation Antipsychotics
Our network meta-analysis (NMA) showed that most second-generation antipsychotics, such as quetiapine, aripiprazole, olanzapine, and risperidone, slightly increased the risk of congenital malformations compared with the control group. The increase in risk was small for all drugs; even for risperidone, which had the highest risk, the odds ratio (OR) was less than 1.5 times higher than the control group. This is below the commonly cited threshold of a twofold increase in risk for clinical significance in this field [45]. Based on SUCRA values, quetiapine (SUCRA = 0.34) was significantly less risky than the other three drugs (with SUCRA values of 0.50, 0.54, and 0.65, respectively). Consistent with our findings, a 2023 cohort study that included 21,751 participants using second-generation antipsychotics found that among the four drugs studied, quetiapine (absolute risk: 4.24%) and aripiprazole (absolute risk: 4.16%) had lower risks [46]. However, because quetiapine had a narrower confidence interval, its risk estimate may be more precise, which could also be attributed to the larger sample size for quetiapine (n = 11,065) compared with aripiprazole (n = 4523).
Another cohort study (n = 365) indicated that quetiapine does not significantly increase the risk of congenital malformations [47]. Although this finding is not consistent with our results, the study also noted that due to the limited sample size, it could only rule out more than a fivefold increase in risk, similar to the known teratogenic risk of valproate. Therefore, a slight increase in risk may have gone undetected [47]. In our study, the total sample size for quetiapine was 18,545 individuals. The results showed that, compared with the control group, quetiapine had an odds ratio (OR) of 1.19 with a 95% credible interval (CrI) of 1.01–1.38, suggesting a slight increase in the risk of congenital malformations. However, since the OR for quetiapine is only slightly above 1 and the lower limit of the credible interval is close to 1 (1.01), this slight increase in risk may not be sufficient to change clinical practice, especially when the potential therapeutic benefits of quetiapine outweigh the risks. The Canadian Network for Mood and Anxiety Treatments (CANMAT) and the International Society for Bipolar Disorders (ISBD) guidelines, published in 2018, recommend quetiapine as a first-line treatment for acute mania in bipolar disorder and also suggest its use for treating bipolar depression [48]. Given the complexity of bipolar disorder, patients may switch between different disease states (depression, mania, mixed episodes) or different risk phases (risk of mania/mixed episodes or depression) [49]. Lithium and quetiapine have been proven effective in all these scenarios, making them the preferred medications for maintenance therapy [50]. To further investigate the reproductive safety of quetiapine, we reviewed studies related to its bioavailability during pregnancy. A prospective observational study reported that the placental penetration rate of quetiapine (mean = 23.8%) was significantly lower compared with haloperidol (mean = 65.5%), risperidone (mean = 49.2%), and olanzapine (mean = 72.1%) [51]. The placental passage ratio determines the extent of fetal exposure to the drug, which could be one of the reasons why quetiapine may have a lower teratogenic risk compared with other medications. In our study of second-generation antipsychotics, lurasidone and ziprasidone are classified as new atypical antipsychotics in the treatment of schizophrenia and depression. Due to their relatively short time in clinical use, data on these drugs are limited, with 137 and 729 infants exposed, respectively. Although these two drugs rank first and second in SUCRA rankings, this does not indicate that they are safe in terms of teratogenic risk [52]. In fact, a simulation study has shown that treatments with fewer studies often rank higher in network meta-analyses (NMA) [53]. Additionally, we assessed the quality of evidence using the CINeMA method, and the findings indicated that the evidence comparing lurasidone and ziprasidone to control groups was of very low quality. Although research on these newer antipsychotics remains relatively limited, existing studies have suggested that ziprasidone does not significantly increase the risk of congenital malformations [11]. In a study involving 697 fetuses, 26 cases of congenital malformations were reported. After adjusting for potential confounding factors, the relative risk for ziprasidone was 0.88, with a 95% confidence interval of 0.60–1.28, indicating no significant association with the risk of congenital malformations [11]. Lurasidone, alongside clozapine, is the only antipsychotic drug to receive a Category B rating in the US pregnancy safety classification. Animal studies have not shown teratogenic effects for lurasidone, but relevant human data remain limited [54]. The most recent study, conducted by Cohen LS et al. in 2023, involved 137 pregnant women treated with lurasidone. The results suggest that lurasidone does not appear to cause major teratogenic effects, but due to the small sample size, more data are needed to accurately assess the associated risks [55].
Therefore, considering SUCRA values, statistical significance, and clinical relevance, we conclude that quetiapine may be the most reasonable choice among second-generation antipsychotics when treating psychiatric disorders such as bipolar disorder and schizophrenia in pregnant women, from both a teratogenic risk and clinical practice perspective.
First-Generation Antipsychotics
Haloperidol, a butyrophenone derivative, belongs to the first-generation antipsychotics. Although the use of second-generation antipsychotics among pregnant women has increased over the past decade [56], the use of first-generation antipsychotics, particularly haloperidol, remains relatively high [57]. This may be because haloperidol is generally less expensive and remains one of the commonly used medications for managing severe agitation crises, even during pregnancy [58, 59]. However, studies on the reproductive safety of haloperidol are relatively limited, and we currently have to rely on a small body of research data for analysis. Haloperidol is classified as Category C in the US Food and Drug Administration (FDA) pregnancy categories, indicating that animal studies have shown potential adverse effects on the fetus, but human studies are insufficient. In animal studies, haloperidol caused congenital malformations, including cleft palate and minor malformations, in rats when used at doses higher than those recommended for humans [60]. A multicenter prospective study in 2005 involved 188 pregnant women using haloperidol and 27 using Penfluridol [61]. The results showed no significant difference in the rate of congenital abnormalities between children exposed to these drugs and those who were not. These results are consistent with our study, which shows that the odds ratio for congenital malformations associated with haloperidol use is 1.259 (95% CrI 0.894–1.746). This suggests that haloperidol may be associated with a slightly increased risk of malformations, though it is not statistically significant (as the credible interval includes 1). Nevertheless, considering that the study was conducted in 2005, its findings may not fully reflect current clinical practice. Based on the SUCRA evaluation, the safety of haloperidol is considered moderate. Therefore, its use during pregnancy should be approached with caution and reserved for cases where there is a clear clinical need and no suitable alternatives are available, with close monitoring of fetal development. Additionally, more data and further research are necessary to clarify its potential teratogenic risks.
Mood Stabilizers
Mood stabilizers play a crucial role in all phases of bipolar disorder, including the acute and maintenance phases [48]. In our study, the drugs analyzed include lamotrigine, lithium, carbamazepine, and valproate. The outcomes for the lamotrigine group were similar to those of the unexposed group, while lithium significantly increased the risk of congenital malformations. Due to insufficient sample sizes and low evidence quality, this study could not reliably determine the risks associated with carbamazepine and valproate. However, other studies [62,63]have shown that valproate is more teratogenic than carbamazepine and lamotrigine. Carbamazepine has also been associated with an increased risk of various congenital malformations, such as microcephaly, craniofacial defects, growth retardation, and cardiac abnormalities [63, 64]. Lamotrigine has a better safety profile compared with other mood stabilizers, with a SUCRA value second only to quetiapine. Numerous cohort studies have analyzed the relationship between the use of mood stabilizers during pregnancy and the incidence of congenital malformations, showing that lamotrigine does not increase the incidence of congenital malformations, with an overall risk ranging from 2% to 4.6% [62, 65–68]. Therefore, from the perspective of congenital malformations, lamotrigine is a relatively safe mood stabilizer for use during pregnancy, though its application should still be carefully evaluated based on the specific clinical situation.
Strengths of this Study
Our study has several strengths. First, to our knowledge, this is the first study to compare and rank the safety of antipsychotics and mood stabilizers during pregnancy in terms of adverse obstetric outcomes. Second, we used the ROBINS-I tool, recommended by the Cochrane Scientific Committee, to assess the risk of bias in nonrandomized intervention studies. This tool is particularly suited for cohort studies, as it reframes certainty assessments and focuses on key factors addressed by randomization, such as confounding and selection bias, making the evaluation results more reliable and easier to interpret. Lastly, compared with previous studies, our analysis is based on a larger sample size, enabling us to detect subtle risks that might have been missed in smaller studies.
Limitations of this Study
The limitations of this study are as follows. First, we did not assess differences in medication dosages, as although dose-response relationships have been observed, this information was rarely reported in the included studies. Second, there was significant variability in the amount of data available for each intervention, which is another limiting factor; for example, data on second-generation antipsychotics were generally more abundant than for other types of medications. Third, although randomized controlled trials (RCTs) can provide the most reliable evidence regarding the effects of antipsychotics and mood stabilizers during pregnancy, the ethical and practical challenges associated with conducting such trials make them difficult to implement. As a result, our study included only cohort studies, which may introduce greater bias and result in lower overall evidence quality.
Lastly, our conclusions are somewhat limited, as our study focused on the relationship between the use of antipsychotics and mood stabilizers and the risk of congenital malformations, while we did not obtain reliable results regarding preterm birth and spontaneous miscarriage.
Conclusion
The current network meta-analysis (NMA) provides the latest insights into the relationship between the use of antipsychotics and mood stabilizers during pregnancy and the risks of congenital malformations, preterm birth, and miscarriage. The results indicate that among second-generation antipsychotics, quetiapine is currently the most reasonable choice in terms of teratogenic risk and clinical application. Additionally, the use of lurasidone and ziprasidone should be approached with caution, and further clinical research is necessary to assess their safety better. The use of the first-generation antipsychotic haloperidol remains a viable option, but fetal development should be closely monitored. Among mood stabilizers, lamotrigine appears to be relatively safer. Although the teratogenic risk associated with drug exposure is a significant consideration in pregnancy outcomes for women with psychiatric disorders, the use of the lowest effective dose of a single medication during pregnancy remains the ideal approach. These decisions should be based on an individualized risk-benefit assessment considering the patient’s course and severity of illness, and should be made with careful consideration and full patient awareness. We hope this study contributes to the existing evidence and provides further guidance and reference for clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank Professor Tang Yanqing and Dr. Wang Yucheng for their thoughtful guidance and valuable suggestions throughout the writing of this paper.
Declarations
Funding
No research funding was received for this work.
Conflicts of Interest/Competing Interests
The authors declare no potential conflicts of interest.
Availability of Data and Material
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
The code from the current study is available upon reasonable request.
Author Contributions
Enhui Wang: Conceptualization, methodology, investigation, data curation, software, formal analysis, writing—original draft, visualization. Yilin Liu: Investigation, data curation, writing—original draft, visualization. Yucheng Wang: Writing—review and editing, validation. Yifang Zhou: Supervision, writing—review and editing. Xinyu Han: Investigation, data curation. Lingli Zhang: Methodology. Yanqing Tang: Writing—review and editing, supervision, project administration.
References
- 1.Barbui C, Conti V, Purgato M, Cipriani A, Fortino I, Rivolta AL, et al. Use of antipsychotic drugs and mood stabilizers in women of childbearing age with schizophrenia and bipolar disorder: Epidemiological survey. Epidemiol Psychiatric Sci. 2013;22:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet (London, England). 2014;384:1789–99. [DOI] [PubMed] [Google Scholar]
- 4.The American college of obstetricians and gynecologists committee opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268–71. [DOI] [PubMed]
- 5.Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, US Preventive Services Task Force (USPSTF), et al. Screening for depression in adults: US preventive services task force recommendation statement. JAMA. 2016;315:380–7.26813211 [Google Scholar]
- 6.Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, et al. Atypical antipsychotic administration during late pregnancy: Placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164:1214–20. [DOI] [PubMed] [Google Scholar]
- 7.Corsello G, Giuffrè M. Congenital malformations. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):25–9. [DOI] [PubMed] [Google Scholar]
- 8.Bellet F, Beyens M-N, Bernard N, Beghin D, Elefant E, Vial T. Exposure to aripiprazole during embryogenesis: a prospective multicenter cohort study. Pharmacoepidem Dr S. 2015;24:368–80. [DOI] [PubMed] [Google Scholar]
- 9.Vigod SN, Gomes T, Wilton AS, Taylor VH, Ray JG. Antipsychotic drug use in pregnancy: High dimensional, propensity matched, population based cohort study. BMJ (Clinical Research Ed). 2015;350: h2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LS, Viguera AC, McInerney KA, Freeman MP, Sosinsky AZ, Moustafa D, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts general hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry. 2016;173:263–70. [DOI] [PubMed] [Google Scholar]
- 11.Huybrechts KF, Hernández-Díaz S, Patorno E, Desai RJ, Mogun H, Dejene SZ, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiat. 2016;73:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen I, McCrea RL, Sammon CJ, Osborn DPJ, Evans SJ, Cowen PJ, et al. Risks and benefits of psychotropic medication in pregnancy: Cohort studies based on UK electronic primary care health records. Health Technol Assess (Winchester, England). 2016;20:1–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile S, Fusco ML. Schizophrenia and motherhood. Psychiatry Clin Neurosci. 2019;73:376–85. [DOI] [PubMed] [Google Scholar]
- 14.Damkier P, Videbech P. The safety of second-generation antipsychotics during pregnancy: a clinically focused review. CNS Drugs. 2018;32:351–66. [DOI] [PubMed] [Google Scholar]
- 15.Thomson M, Sharma V. Weighing the risks: the management of bipolar disorder during pregnancy. Curr Psychiatry Rep. 2018;20:20. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother. 2015;16:1335–45. [DOI] [PubMed] [Google Scholar]
- 17.Cuomo A, Goracci A, Fagiolini A. Aripiprazole use during pregnancy, peripartum and lactation. A systematic literature search and review to inform clinical practice. J Affect Disord. 2018;228:229–37. [DOI] [PubMed] [Google Scholar]
- 18.Mehta TM, Van Lieshout RJ. A review of the safety of clozapine during pregnancy and lactation. Arch Women’s Mental Health. 2017;20:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116:315–20. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin CG, Blackwell KA, Bartley C, Hay M, Yonkers KA, Bloch MH. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol. 2015;125:1224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Brauer R, Man KKC, Alfageh B, Mongkhon P, Wong ICK. Prenatal exposure to antipsychotic agents and the risk of congenital malformations in children: a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87:4101–23. [DOI] [PubMed] [Google Scholar]
- 22.Andrade C. Major congenital malformations associated with exposure to second-generation antipsychotic drugs during pregnancy. J Clin Psychiatry. 2021;82:21f14252. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan DL, Byatt N, Dossett EC. Long-acting injectable antipsychotic medications in pregnancy: a review. J Acad Consultation-Liaison Psychiatry. 2022;63:53–60. [DOI] [PubMed] [Google Scholar]
- 24.Betcher HK, Montiel C, Clark CT. Use of antipsychotic drugs during pregnancy. Curr Treat Options Psychiatry. 2019;6:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppola D, Russo LJ, Kwarta RF, Varughese R, Schmider J. Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf. 2007;30:247–64. [DOI] [PubMed] [Google Scholar]
- 26.Ellfolk M, Leinonen MK, Gissler M, Kiuru-Kuhlefelt S, Saastamoinen L, Malm H. Second-generation antipsychotic use during pregnancy and risk of congenital malformations. Eur J Clin Pharmacol. 2021;77:1737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman MP, Viguera AC, Góez-Mogollón L, Young AV, Caplin PS, McElheny SA, et al. Reproductive safety of aripiprazole: Data from the massachusetts general hospital national pregnancy registry for atypical antipsychotics. Arch Women’s Mental Health. 2021;24:659–67. [DOI] [PubMed] [Google Scholar]
- 28.Bafeta A, Trinquart L, Seror R, Ravaud P. Reporting of results from network meta-analyses: methodological systematic review. BMJ (Clinical Research Ed). 2014;348: g1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations - PubMed [Internet]. [cited 2024 Aug 14]. https://pubmed.ncbi.nlm.nih.gov/26030634/. Accesssed 14 Aug 2024. [DOI] [PubMed]
- 30.Anomalies ES of C. EUROCAT guide 1.3 and reference documents: Instructions for the registration and surveillance of congenital anomalies. 2005;
- 31.Wang R, Shi Q, Jia B, Zhang W, Zhang H, Shan Y, et al. Association of preterm singleton birth with fertility treatment in the US. JAMA Netw Open. 2022;5: e2147782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SEM, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22:116–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin Res ed). 2016;355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JAC, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Chapter 25: Assessing risk of bias in a non-randomized study [last updated October 2019]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 65 [Internet]. Version 6.5. Cochrane; 2024. www.training.cochrane.org/handbook.
- 35.Hespanhol L, Vallio CS, Costa LM, Saragiotto BT. Understanding and interpreting confidence and credible intervals around effect estimates. Braz J Phys Ther. 2019;23:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valkenhoef G van. gemtc: network meta-analysis using Bayesian methods. [Internet]. 2024 [cited 2024 Aug 14]. https://github.com/gertvv/gemtc.
- 37.Shim SR, Kim S-J, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41: e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balduzzi S, Rücker G, Nikolakopoulou A, Papakonstantinou T, Salanti G, Efthimiou O, et al. netmeta: an R package for network meta-analysis using frequentist methods. J Stat Softw. 2023;106:1–40.37138589 [Google Scholar]
- 39.StataCorp L. Stata statistical software (version release 14). College Station, TX: Author. 2015;464:14
- 40.White IR. Network meta-analysis. [cited 2024 Aug 14]; Available from: 10.1177/1536867X1501500403
- 41.Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Undertaking network meta-analyses. Cochrane Handbook Syst Rev Interv [Internet]. 2019;11:285–320. 10.1002/9781119536604.ch11. [Google Scholar]
- 42.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17: e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16: e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019;111:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grigoriadis S, VonderPorten EH, Mamisashvili L, Roerecke M, Rehm J, Dennis C-L, et al. Antidepressant exposure during pregnancy and congenital malformations: Is there an association? A systematic review and meta-analysis of the best evidence. J Clin Psychiatry. 2013;74:e293-308. [DOI] [PubMed] [Google Scholar]
- 46.Huybrechts KF, Straub L, Karlsson P, Pazzagli L, Furu K, Gissler M, et al. Association of in utero antipsychotic medication exposure with risk of congenital malformations in nordic countries and the US. JAMA Psychiat. 2023;80:156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen LS, Góez-Mogollón L, Sosinsky AZ, Savella GM, Viguera AC, Chitayat D, et al. Risk of major malformations in infants following first-trimester exposure to quetiapine. Am J Psychiatry. 2018;175:1225–31. [DOI] [PubMed] [Google Scholar]
- 48.Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.E V, M B, Tg S, Af C, T S, Jr C, et al. Bipolar disorders. Nat rev, Dis primers [Internet]. 2018 [cited 2024 Oct 5];4. Available from: https://pubmed.ncbi.nlm.nih.gov/29516993/. Accessed 5 Oct 2024.
- 50.Kessing LV. Why is lithium [not] the drug of choice for bipolar disorder? a controversy between science and clinical practice. Int J Bipolar Disord. 2024;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daud ANA, Bergman JEH, Oktora MP, Kerstjens-Frederikse WS, Groen H, Bos JH, et al. Maternal use of drug substrates of placental transporters and the effect of transporter-mediated drug interactions on the risk of congenital anomalies. PLoS ONE. 2017;12: e0173530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orzelska-Górka J, Mikulska J, Wiszniewska A, Biała G. New atypical antipsychotics in the treatment of schizophrenia and depression. Int J Mol Sci. 2022;23:10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kibret T, Richer D, Beyene J. Bias in identification of the best treatment in a bayesian network meta-analysis for binary outcome: a simulation study. Clin Epidemiol. 2014;6:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruz MP. Lurasidone HCl (latuda), an oral, once-daily atypical antipsychotic agent for the treatment of patients with schizophrenia. P & T Peer-Rev J Formul Manag. 2011;36:489–92. [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen LS, Church TR, Freeman MP, Gaccione P, Caplin PS, Kobylski LA, et al. Reproductive safety of lurasidone and quetiapine: update from the national pregnancy registry for psychiatric medications. J Women’s Health. 2002;2023(32):452–62. [DOI] [PubMed] [Google Scholar]
- 56.Robiyanto R, Schuiling-Veninga CCM, Bos JHJ, Hak E, van Puijenbroek EP. Exposure to psychotropic drugs before and during pregnancy: What has changed over the last two decades? Arch Women’s Mental Health. 2023;26:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toh S, Li Q, Cheetham TC, Cooper WO, Davis RL, Dublin S, et al. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Arch Women’s Mental Health. 2013;16:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geddes J, Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: Systematic overview and meta-regression analysis. BMJ (Clinical Research Ed). 2000;321:1371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pratt JP, Chandler-Oatts J, Nelstrop L, Branford D, Pereira S, Johnston S. Establishing gold standard approaches to rapid tranquillisation: a review and discussion of the evidence on the safety and efficacy of medications currently used. J Psychiatric Intensive Care. 2008;4:43–57. [Google Scholar]
- 60.Abdel-Hamid HA, Abdel-Rahman MS, Abdel-Rahman SA. Teratogenic effect of diphenylhydantoin and/or Penfluridol in mice. J Appl Toxicol: JAT. 1996;16:221–5. [DOI] [PubMed] [Google Scholar]
- 61.Diav-Citrin O, Shechtman S, Ornoy S, Arnon J, Schaefer C, Garbis H, et al. Safety of haloperidol and penfluridol in pregnancy: A multicenter, prospective, controlled study. J Clin Psychiatry. 2005;66:317–22. [DOI] [PubMed] [Google Scholar]
- 62.Campbell E, Kennedy F, Russell A, Smithson WH, Parsons L, Morrison PJ, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and ireland epilepsy and pregnancy registers. J Neurol Neurosurg Psychiatry. 2014;85:1029–34. [DOI] [PubMed] [Google Scholar]
- 63.Weston J, Bromley R, Jackson CF, Adab N, Clayton-Smith J, Greenhalgh J, et al. Monotherapy treatment of epilepsy in pregnancy: Congenital malformation outcomes in the child. Cochrane Db Syst Rev. 2016;11:CD010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas SV, Ajaykumar B, Sindhu K, Francis E, Namboodiri N, Sivasankaran S, et al. Cardiac malformations are increased in infants of mothers with epilepsy. Pediatr Cardiol. 2008;29:604–8. [DOI] [PubMed] [Google Scholar]
- 65.Cunnington M, Tennis P, International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64:955–60. [DOI] [PubMed] [Google Scholar]
- 66.Hernández-Díaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–9. [DOI] [PubMed] [Google Scholar]
- 67.Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305:1996–2002. [DOI] [PubMed] [Google Scholar]
- 68.Vajda FJE, O’Brien TJ, Lander CM, Graham J, Eadie MJ. The teratogenicity of the newer antiepileptic drugs—an update. Acta Neurol Scand. 2014;130:234–8. [DOI] [PubMed] [Google Scholar]
- 69.McKenna K, Koren G, Tetelbaum M, Wilton L, Shakir S, Diav-Citrin O, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66:444–9 (quiz 546). [DOI] [PubMed] [Google Scholar]
- 70.Patorno E, Huybrechts KF, Hernandez-Diaz S. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;377:893–4. [DOI] [PubMed] [Google Scholar]
- 71.Habermann F, Fritzsche J, Fuhlbrück F, Wacker E, Allignol A, Weber-Schoendorfer C, et al. Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J Clin Psychopharmacol. 2013;33:453–62. [DOI] [PubMed] [Google Scholar]
- 72.Kulkarni J, Worsley R, Gilbert H, Gavrilidis E, Van Rheenen TE, Wang W, et al. A prospective cohort study of antipsychotic medications in pregnancy: The first 147 pregnancies and 100 one year old babies. PLoS ONE. 2014;9: e94788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Viguera AC, Freeman MP, Kobylski LA, Rossa ET, Gaccione P, Chitayat D, et al. Risk of major malformations following first-trimester exposure to olanzapine: preliminary data from the massachusetts general hospital national pregnancy registry for psychiatric medications. J Clin Psychopharmacol. 2023;43:106–12. [DOI] [PubMed] [Google Scholar]
- 74.Bodén R, Lundgren M, Brandt L, Reutfors J, Andersen M, Kieler H. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: Population based cohort study. BMJ (Clinical research ed). 2012;345: e7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diav-Citrin O, Shechtman S, Tahover E, Finkel-Pekarsky V, Arnon J, Kennedy D, et al. Pregnancy outcome following in utero exposure to lithium: a prospective, comparative, observational study. Am J Psychiatry. 2014;171:785–94. [DOI] [PubMed] [Google Scholar]
- 76.Källén B, Tandberg A. Lithium and pregnancy. Acta Psychiatr Scand. 1983;68:134–9. [DOI] [PubMed] [Google Scholar]
- 77.Sørensen MJ, Kjaersgaard MIS, Pedersen HS, Vestergaard M, Christensen J, Olsen J, et al. Risk of fetal death after treatment with antipsychotic medications during pregnancy. PLoS ONE. 2015;10: e0132280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakai T, Ohtsu F, Mori C, Tanabe K, Goto N. Signal of miscarriage with aripiprazole: a disproportionality analysis of the japanese adverse drug event report database. Drug Saf. 2017;40:1141–6. [DOI] [PubMed] [Google Scholar]
- 79.Jacobson SJ, Jones K, Johnson K, Ceolin L, Kaur P, Sahn D, et al. Prospective multicentre study of pregnancy outcome after lithium exposure during first trimester. Lancet (London, England). 1992;339:530–3. [DOI] [PubMed] [Google Scholar]
- 80.Poels EMP, Schrijver L, White TJH, Roza SJ, Zarchev MG, Bijma H, et al. The effect of prenatal lithium exposure on the neuropsychological development of the child. Bipolar Disord. 2022;24:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.