Abstract
Objective
Debate exists regarding the true pathogenicity of cerebral infundibula (CI). Pre-aneurysmal lesions and benign anatomical variants have both been proposed. In this study, we present the largest single cohort series on the natural history of CI.
Methods
Retrospective review of prospective surveillance of 420 CI was undertaken in a single tertiary cerebrovascular centre. All CI diagnosed by a neuroradiologist, diagnosed on either a Magnetic resonance angiography (MRA), Computed tomography angiography (CTA) or Digital subtraction angiography (DSA) were eligible for inclusion. Imaging and demographic characteristics were recorded at baseline. CI growth and aneurysm transformation were the outcomes of interest. Groupwise comparison was conducted via Fischer exact testing. Kaplan Meir curves and Cox proportional hazard ratios were used to assess variables of interest with respect to time on surveillance.
Results
402 patients with 420 CI were surveyed over 2418 infundibula-years. Eleven CI (2.62%) grew on surveillance, and three (0.7%) transformed into aneurysms. Median time to growth was 85 months (36-263) and median time to aneurysm transformation was 112 months (96-142). Of the CI that grew, male sex and CI >2 mm at diagnosis were significant predictors of growth (all p<0.05). Of the CI that grew in surveillance, 2/11 (18.2%) transformed into aneurysms (p=0.001). Aneurysm transformation occurred at a rate of 1.27 per 1000 infundibula years. CI growth on surveillance (p=0.00016) and size at diagnosis (p=0.038) remained significant predictors of aneurysm transformation on Kaplan Meir curves.
Conclusions
The transformation of a CI to an aneurysm occurs at a low rate. A history of growth on surveillance imaging represents significant risk for aneurysm transformation.
Keywords: Neurosurgery, Intracranial aneurysm, Infundibulum, Disease progression
INTRODUCTION
A cerebral infundibulum (CI) is a dilatation of a cerebral vessel, described as being funnel shaped and arising at the origin of a branching vessel. Histological evaluation of CI suggests they are distinct from aneurysms [15]. Definition of CI vary; however, they often include size limitations and preclusions to where the continuing vessel may arise from them [4]. Previous literature describes a prevalence of 7-25% [8]. Historically CI have been regarded as anatomical variants with minimal clinical significance. However, case reports of infundibular growth and rupture resulting in subarachnoid hemorrhage exist [1,3,5,6,9,12,14,17]. This has led to some arguing that CI represent pre-aneurysmal lesions [1,4,5,6,8,12,15]. An abundance of literature and guidelines are present with respect to the treatment and surveillance of patients with unruptured intracranial aneurysms [2,10,11,18].
There are no large or adequately powered cohort studies which examine the risk of infundibula growth or aneurysm formation. Subsequently, there is little literature to inform surveillance or treatment guidelines for CI. In this study, we aim to define the natural history of CI to determine whether they are a benign entity or whether surveillance is required to assess for growth or aneurysm transformation. Further, we aim to examine risk factors for growth and aneurysm transformation to inform surveillance paradigms. To our knowledge, this represents the largest observational study in literature regarding the natural history of infundibula.
MATERIALS AND METHODS
Database formulation and demographics
The patient population for this study was derived from an electronic database consisting of sequential presentations to a tertiary neurosurgical centre between 2000 and 2021. Cerebral infundibula in this database were either under prospective surveillance due to the patient having other cerebral aneurysm warranting surveillance, or they were patients with isolated cerebral infundibula which were under surveillance. Prospective surveillance of isolated infundibula was departmental policy during the study period due to perceived risk of aneurysm transformation. Surveillance consisted of yearly computed tomography angiogram (CTA) or magnetic resonance angiogram (MRA) for at least the first 5 years; thereafter, follow-up intervals varied (median follow-up 60 months, range 14 to 263 months). Demographic and clinical variables, including age, sex and medical co-morbidities were entered into the database at baseline. In total, we retrieved 402 patients with 420 cerebral infundibula. This study was approved by the local ethics committee.
Definition of an infundibular dilatation
The diagnosis of cerebral infundibula was made using either digital subtraction angiography (DSA), MRA or CTA in all cases. Diagnosis was made in all cases by an expert neuroradiologist. All infundibula diagnosis were retrospectively verified prior to inclusion in this study.
The radiographic inclusion criteria for the diagnosis of CI in this study were as follows:
1. There were no restrictions on arterial location.
2. The lesion had to be funnel shaped, with a base larger than its apex.
3. The cerebral infundibula had to be located at the origin of the branching vessel.
4. The branching vessel had to continue from the apex of the cerebral infundibula.
5. No irregularities to the lesion
Some authors define these lesions as having an ostium less than 3 mm in diameter, we included lesions with an ostium wider than 3 mm in this study [4].
Thirteen patients (n=13) that were initially entered into the database and were subsequently excluded based on retrospective review radiographic criteria. These lesions had the following exclusion criteria.
1. More than one branching vessel from the apex.
2. The branching vessel arose from the wall lesion before the apex.
3. Irregularities in shape or were not funnel shaped.
Radiographic variables and outcomes
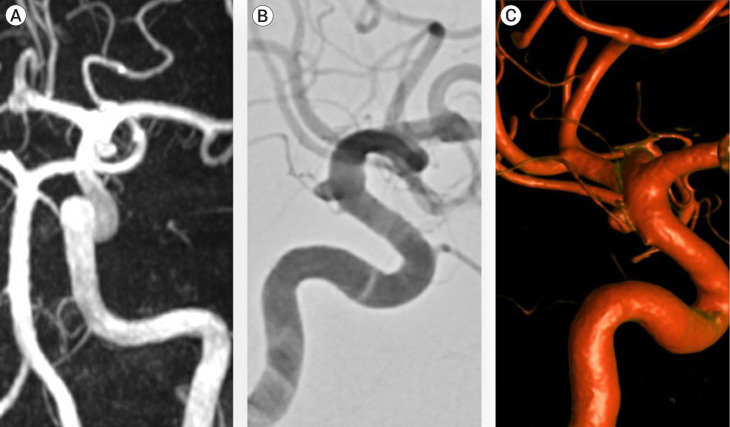
All patients had imaging available electronically and measurements were made using the hospital digital imaging system. The maximum size, height, and neck width were measured on a 0.1 mm scale in line with what has been reported elsewhere [4]. Radiographic outcomes of interest were infundibula growth and aneurysm transformation. Growth was defined as an increase in size of 1 mm compared to the index scan. Denovo aneurysm formation from an infundibulum was defined as radiographic features more in keeping with an aneurysm. In all cases, this was loss of its funnel shape. (Fig. 1)
Fig. 1.
(A–C) Sequential angiographic images of an internal carotid artery infundibulum which demonstrated growth and aneurysm transformation over a 5-year observation period
Statistical analysis
All statistical analysis was performed in R [13]. Categorical data was expressed as frequency and percentages. Continuous data was examined for normality, via Shapiro–Wilk normality test [16]. Data was subsequently expressed as median and range for nonparametric data and mean and standard deviation for parametric data. Non-parametric testing was by means of Mann-Whitney U test, whilst parametric analysis was via t-tests. Categorical data was compared via Fischer exact testing. For all data analysis a significance value of 0.05 was set.
To build regression models that predicted growth and aneurysm formation, all demographic, clinical and radiographic variables were tested via univariate Cox proportional hazards regression to assess relationships to both outcomes of interest [7]. Variables with a p value of <0.1 were considered for multivariate regression. Variable independence was verified using Spearman’s correlation co-efficient prior to multivariate regression modelling. A forward stepwise multivariate Cox proportional hazard regression model was then performed in order of the magnitude beta coefficients. Variables were added until all significant variables were used, or the model became non-significantly different in prediction when the additional variable was added. Subsequent hazard ratios were derived from regression modelling.
RESULTS
Patient population
In total 402 individual patients, incorporating 420 CI were analysed. Sixteen patients had two CI, and a single patient had three (Table 1). 35.95% (n=124) were diagnosed on DSA, 34.29% (n=150) were diagnosed on computed tomography angiography (CTA) and 29.74% (n=143) were diagnosed on magnetic resonance angiography (MRA). Posterior communicating artery (PCOM) represented the most common arterial location of the CI, accounting for 49.05% (n=206). Other cerebral aneurysms were present in 58.5% (n=235) of patients. In total, 11 CI (2.64%) grew during follow-up. This gave a rate of growth of 4.66 per 1,000 infundibula-years. Three patients (0.71%) developed aneurysms from infundibula on prospective surveillance. This gave a rate of aneurysm development of 1.27 per 1,000 infundibula years.
Table 1.
Patient demographics
| Patient characteristics | N= 402 | ||
| Demographics | Count | % | |
| Sex | |||
| Male | 285 | 70.9% | |
| Female | 113 | 28.1% | |
| Unknown | 3 | 1% | |
| Age at diagnosis | 55.85 ± 12.54 | ||
| Duration of follow-up (months) | 69.27 ± 38.81 | ||
| Number of infundibula | 420 | ||
| Infundibulum per patient | Count | % | |
| Singular | 385 | 95.7% | |
| Two | 16 | 4.0% | |
| Three | 1 | 0.2% | |
| Diagnosis means | Count | % | |
| DSA | 124 | 36.0% | |
| MRA | 143 | 29.7% | |
| CTA | 150 | 34.3% | |
| ARTERY TERRITORY | Count | % | |
| ACA | 10 | 2.4% | |
| ACOM | 10 | 2.4% | |
| AICA | 2 | 0.5% | |
| ANTERIOR CHOROIDAL | 30 | 7.1% | |
| BASILAR | 7 | 1.7% | |
| ICA TERMINUS | 96 | 22.9% | |
| MCA | 25 | 6.0% | |
| OPHTHALMIC | 10 | 2.4% | |
| ORBITOFRONTAL | 1 | 0.2% | |
| PCA | 12 | 2.9% | |
| PCOM | 206 | 49.1% | |
| PICA | 2 | 0.5% | |
| SCA | 7 | 1.7% | |
| VERTEBRAL | 2 | 0.5% | |
| Growth on surveillance | 11 | 2.64% | |
| Size of infundibulum | |||
| >3 mm | 50 | 11.9% | |
| <3 mm | 352 | 88.1% | |
| Vascular history | |||
| Previous history of subarachnoid haemorrhage | |||
| Yes | 263 | 65.4% | |
| No | 145 | 36.1% | |
| Uncertain | 4 | 1.0% | |
| Hypertension history | |||
| Yes | 215 | 53.5% | |
| No | 147 | 36.6% | |
| Uncertain | 40 | 9.9% | |
| Smoking status | |||
| Ex-Smoker | 7 | 1.7% | |
| Current | 211 | 52.5% | |
| Nonsmoker | 131 | 32.6% | |
| Uncertain | 53 | 13.2% | |
| Family history of subarachnoid haemorrhage | |||
| Prior family history | 20 | 5.0% | |
| No family history | 202 | 50.3% | |
| Uncertain | 180 | 44.8% | |
| Other cerebral aneurysm present | |||
| Yes | 235 | 58.5% | |
| No | 167 | 41.5% | |
DSA, digital subtraction angiography; MRA, magnetic resonance angiography; CTA, computed tomography angiography
The posterior communicating artery represented the most common location of CI, accounting for 49% (n=206/420) of total CI in the study. The distribution of CI with respect to artery location was statistically significantly different than would be expected by chance alone (p<0.001). ICA terminus was included as a location when it was unclear the exact named branching vessel and thus location may be understated (Table 2).
Table 2.
Distribution on infundibula
| CI growth status | Count | Percent |
|---|---|---|
| Increased | 11 | 2.62% |
| ACOM | 1 | 9.09% |
| BASILAR | 1 | 9.09% |
| ICA TERMINUS | 5 | 45.45% |
| MCA | 1 | 9.09% |
| PCOM | 3 | 27.27% |
| Stable | 406 | 96.67% |
| ACA | 10 | 2.46% |
| ACOM | 9 | 2.22% |
| AICA | 2 | 0.49% |
| ANTERIOR CHOROIDAL | 30 | 7.39% |
| BASILAR | 6 | 1.48% |
| ICA TERMINUS | 91 | 22.41% |
| MCA | 24 | 5.91% |
| OPTHALMIC | 10 | 2.46% |
| ORBITOFRONTAL | 1 | 0.25% |
| PCA | 12 | 2.96% |
| PCOM | 200 | 49.26% |
| PICA | 2 | 0.49% |
| SCA | 7 | 1.72% |
| VERTEBRAL | 2 | 0.49% |
| Uncertain | 3 | 0.71% |
| PCOM | 3 | 100.00% |
CI, cerebral infundibula; ACOM, anterior cerebral artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCOM, posterior communicating artery; ACA, anterior cerebral artery; AICA, anterior inferior cerebellar artery; PCA, posterior cerebral artery; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery
Out of the 11 CI that grew the most common location was the terminus of the Internal carotid artery (n=5/11; 45.5%). Although, amongst the CI that grew, no one artery was overrepresented than would be expected by chance alone (p=0.21). Arterial location groupings with respect to traditional aneurysm risk stratification tools, including PHASES 2 and ISUIA 10 provided some predictive value on the univariate Cox hazard regression modelling. Specifically, ‘Posterior circulation’ of ISUIA (HR: 0.22, 95% CI 0.06, 0.86; p=0.029) and ‘other’ grouping on PHASES (HR: 0.22, 0.06, 0.87; p=0.032) demonstrated protective effects on CI growth (Table 3).
Table 3.
Infundibula diagnosis modality by change in size
| Row labels | Increased | Stable | Grand total |
|---|---|---|---|
| CTA | 6 | 141 | 147 |
| DSA | 1 | 123 | 124 |
| MRA | 4 | 139 | 143 |
| Grand total | 11 | 403 | 414 |
CTA, computed tomography angiography; DSA, digital subtraction angiography; MRA, magnetic resonance angiography
There was a preponderance for growing infundibula to be diagnosed on imaging modalities other than DSA (10/11 vs 1/11; p=0.006). For these lesions, DSA was often used to confirm diagnosis. Though, the means of imaging modality did not directly predict growth (Table 4).
Table 4.
Univariate Cox hazard regression analysis for infundibula growth
| Characteristic | N | HR | 95% CI | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis | 407 | 1.02 | 0.96, 1.07 | 0.6 |
| Sex | 418 | |||
| Female | — | — | ||
| Male | 4.84 | 1.36, 17.3 | 0.015 | |
| Imaging modality | 420 | |||
| CTA | — | — | ||
| MRA | 0.45 | 0.11, 1.82 | 0.3 | |
| DSA | 0.13 | 0.02, 1.12 | 0.064 | |
| Artery characteristics | ||||
| Size of infundibula | 420 | |||
| <2 mm | — | — | ||
| 2-3 mm | 13.4 | 1.64, 109 | 0.015 | |
| >3 mm | 19.7 | 1.78, 218 | 0.015 | |
| IUSIA grouping | 420 | |||
| Anterior circulation | ||||
| Cavernous | NC | >0.9 | ||
| Posterior circulation | 0.22 | 0.06, 0.86 | 0.029 | |
| PHASES grouping | 420 | |||
| ICA | ||||
| MCA | 0.67 | 0.07, 6.06 | 0.7 | |
| Other | 0.22 | 0.06, 0.87 | 0.032 | |
| Multiple infundibula present | 420 | 1.03 | 0.13, 8.36 | >0.9 |
| Vascular risk factors | ||||
| Smoker | 364 | |||
| No | — | — | ||
| Yes | NC | >0.9 | ||
| Family history of subarachnoid Haemorrhage | 419 | |||
| No | — | — | ||
| Yes | NC | >0.9 | ||
| Other aneurysm present on imaging | 420 | |||
| No | — | — | ||
| Yes | 0.71 | 0.20, 2.50 | 0.6 | |
| Hypertension | 378 | |||
| No | — | — | ||
| Yes | 0.55 | 0.14, 2.22 | 0.4 | |
| Antiplatelets | 372 | |||
| No | — | — | ||
| Yes | 0.47 | 0.06, 3.79 | 0.5 | |
| Previous subarachnoid haemorrhage History | 416 | |||
| No | — | — | ||
| Yes | 1.84 | 0.53, 6.38 | 0.3 |
HR, hazard ratio; N, number; 95% CI, 95% confidence interval; CTA, computed tomography angiogram; MRA, magnetic resonance angiogram; DSA: digital subtraction angiography
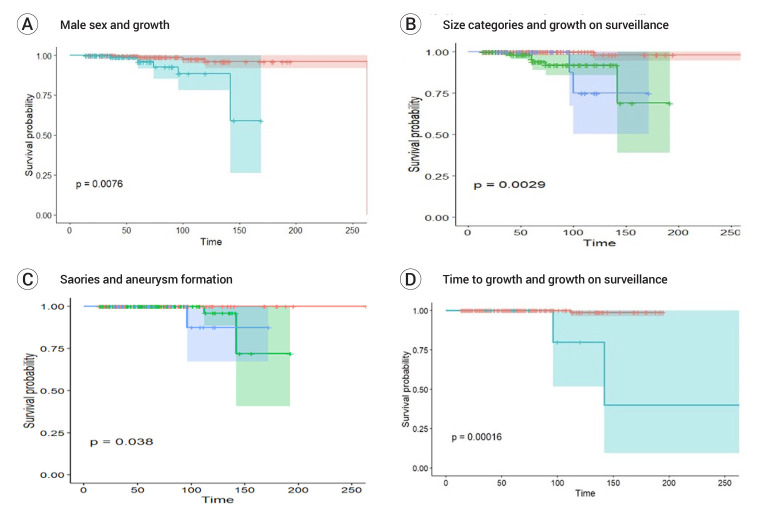
On univariate regression analysis size grouping (<2 mm vs 2-3 mm vs >3 mm), male sex and posterior circulation per ISUIA and ‘other’ per PHASES, yielded statistically significant predictors of CI growth. Initial diagnosis on DSA yielded near significant prediction (p=0.064). In multivariate modelling, size grouping and male sex remained statistically significant predictors. Kaplan Meir graphs are presented in Fig. 2.
Fig. 2.
Kaplan Meier curves for outcomes of interest (A) Kaplan Meier Curve for time to growth for male sex as the variable (Blue: male sex, Red: female sex). (B) Kaplan Meier curve for time to growth for size groupings as the variable (Red: <2 mm in size); Blue: 2-3 mm in size, Green >3 mm in size. (C) Kaplan Meier Curve for time to aneurysm transformation for size groupings as the variable (Red <2 mm in size; Blue: 2-3 mm in size, Green >3 mm in size). (D) Kaplan Meier Curve for time to aneurysm transformation for growth on surveillance as the variable (Red: no growth; Blue: growth on surveillance)
Time to growth and size at diagnosis were independent of each other (r=0.04; p >0.05). The risk factors for CI growth combined are displayed in the below table. In the presence of lowest combination risk factors (ie female sex and <2 mm), CI followed for 10 years or more, grew at a rate of 1 in 33. At 10 years of follow-up, this gave a sensitivity of 88.9% (95% CI 51.75% to 99.72%) and specificity of 4.17% (0.11% to 21.12%) in predicting CI growth. Notably, 7 of the 11 CI grew after 5 years follow-up. Risk of CI growth was equal over the entire study duration, except in CI surveyed for 138 months or more. The odds of growth in those surveyed for 138 months or more was 5.51 (95% CI: 1.094-27.759; p=0.019), compared to those surveyed less (Table 5).
Table 5.
Multivariate Cox hazard regression model in predicting infundibula growth
| Characteristic | HR | 95% CI | p-value |
|---|---|---|---|
| Male sex | 4.35 | 1.18, 16.0 | 0.027 |
| Size grouping | |||
| <2 mm | — | — | |
| 2-3 mm | 11.2 | 1.37, 92.0 | 0.024 |
| >3 mm | 22.0 | 1.97, 246 | 0.012 |
HR, hazard ratio; 95% CI, 95% confidence interval
Three CI transformed into aneurysms on surveillance. Aneurysm transformation occurred at a median follow-up of 112 Months (range: 96-142). All aneurysms showed a history of growth on surveillance, and all were >2 mm at diagnosis. In univariate Kaplan Meir analysis, both growth on surveillance and size grouping predicted aneurysm formation predicted aneurysm formation. The hazard ratios for growth on surveillance was 87.8 (95% CI 7.75, 1,995; p<0.001). Given no aneurysms formed in the reference size group (<2 mm), infinite estimates prevented calculation of hazard ratio with respect to size grouping.
DISCUSSION
Multiple guidelines and decision making tools exist for the assessment and management of intracranial aneurysms, however, none exist for CI. We analysed 420 CI for radiological growth, rupture and aneurysm transformation for over one decade. This is the largest natural history study of CI. We derived several important conclusions regarding the natural history of infundibula from this study. (Box 1)
• CI represent a benign entity in most, progression to aneurysm occurs at 1.27 per 1,000 infundibula years.
• Growth and aneurysm transformation occur after prolonged periods of time (>5 years).
• CI size predicts growth and aneurysm formation, but no absolute size cutoff exists with
Box 1: Seminal study findings
Overall, the absolute risk of CI growth on surveillance in our population was 2.6%. CI growth occurred at a rate 4.66 per 1,000 infundibula years. However, CI growth was difficult to predict. Growth is probably a random event, however, it has been believed that hemodynamic factor plays a major role contributing to the progression of an infundibulum to true aneurysm such as a well-developed posterior communicating artery (fetal type P-com).4 In this study, of the 206, PCOM infundibula, 111 had a fetal type PCOM and of the 11 CIs which grew during follow-up, only 3 P-coms (3/11, 27.27%) were included, however, there was no fetal type P-com. This was asserted by the lack of relationship between CI size and time to growth (r=0.04; p>0.05). CI which was >2 mm at diagnosis was more likely to grow (HR: 11.2; 95% CI: 1.37-92.0; p=0.024). Similarly, male patients were also more likely to have CI that grew (HR=4.35; 95% CI: 1.18-16.0; p=0.027). No other features were associated with growth. Despite being able to elicit the risk factors for growth of CI on surveillance, when combined, these risk factors yielded a predictive sensitivity which was suboptimal for clinical use (Sensitivity=88.9%; 95% CI 51.75% to 99.72%) (Table 6). The longest period of time to growth occurred at 263 months. Many of the CI showed growth late during their follow-up (7/11, after 5 years). This would suggest that growth occurs after a prolonged period on surveillance (Table 7).
Table 6.
Growth of CI at follow-up periods and risk factors
| Total |
3 years |
5 years |
10 years |
|||||
|---|---|---|---|---|---|---|---|---|
| Stable | Growth | Stable | Growth | Stable | Growth | Stable | Growth | |
| Female | 279 | 5 | 219 | 0 | 139 | 1 | 21 | 4 |
| <2 mm | 182 | 2 | 140 | 0 | 86 | 0 | 1 | 1 |
| 2-3 mm | 84 | 2 | 68 | 0 | 44 | 1 | 8 | 2 |
| >3 mm | 13 | 1 | 11 | 0 | 9 | 0 | 2 | 1 |
| Male | 109 | 6 | 85 | 0 | 41 | 3 | 3 | 5 |
| <2 mm | 70 | 0 | 47 | 1 | 19 | 0 | 1 | 0 |
| 2-3 mm | 34 | 5 | 33 | 0 | 18 | 3 | 2 | 4 |
| >3 mm | 5 | 1 | 3 | 1 | 4 | 0 | 0 | 1 |
| Total | 388 | 11 | 303 | 1 | 180 | 4 | 24 | 9 |
Table 7.
Univariate Cox hazard regression analysis for aneurysm formation
| Characteristic | N | HR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at diagnosis | 407 | 1.02 | 0.92, 1.13 | 0.7 | |
| Sex | 418 | ||||
| Female | — | — | |||
| Male | 7.11 | 0.64, 78.7 | 0.11 | ||
| Imaging modality | 420 | ||||
| CTA | — | — | |||
| MRA | 1.58 | 0.14, 17.4 | 0.7 | ||
| DSA | NC | ||||
| Artery characteristics | 420 | ||||
| Size >3 mm at diagnosis | 420 | ||||
| Multiple infundibula present | 420 | NC | |||
| Growth on surveillance | 420 | 31.8 | 2.57, 394 | <0.001 | |
| Size of infundibula | 420 | ||||
| <2 mm | — | — | |||
| 2-3 mm | NC | 0.99 | |||
| >3 mm | NC | 0.99 | |||
| IUSIA grouping | 420 | ||||
| Anterior circulation | — | — | |||
| Cavernous | 1.17 | 0.11-25.2 | 0.99 | ||
| Posterior circulation | NC | 0.99 | |||
| PHASES grouping | 420 | ||||
| ICA | — | — | |||
| MCA | NC | 0.99 | |||
| Other | 0.65 | 0.06-14.0 | 0.7 | ||
| Vascular risk factors | |||||
| Smoker | 364 | ||||
| No | — | — | |||
| Yes | 3.92 | NC | >0.9 | ||
| Family history of Subarachnoid haemorrhage | 419 | ||||
| No | — | — | |||
| Yes | NC | >0.9 | |||
| Other intracranial aneurysm on imaging | 420 | ||||
| No | — | — | |||
| Yes | 1.10 | 0.09, 13.3 | >0.9 | ||
| Hypertension | 378 | ||||
| No | — | — | |||
| Yes | NC | ||||
| Antiplatelets | 372 | ||||
| No | — | — | |||
| Yes | 2.12 | 0.19, 23.3 | 0.5 | ||
| Previous history of Subarachnoid haemorrhage | 416 | ||||
| No | — | — | |||
| Yes | NC | >0.9 |
95% CI, 95% confidence interval; CTA, computed tomography angiogram; MRA, magnetic resonance angiogram; DSA, digital subtraction angiography
Three (0.7%) of the four hundred and twenty infundibula transformed into aneurysms during their surveillance. Transformation to an aneurysm occurred at a rate of 1.27 per 1,000 infundibula years. Overall, this represents a rare occurrence. In all cases, aneurysm diagnosis was made after 5 years of radiological surveillance. Risk factors for this were growth on surveillance imaging (HR: 87.8; 95% CI 7.75-1,995; p<0.001) and larger size at diagnosis (p=0.038). In the absence of both risk factors, no CI progressed to aneurysm formation. Therefore, growth of a CI on sequential imaging probably represents a lesion that is “pre-aneurysmal” as opposed to a CI.
There are several limitations to this study. Firstly, this study was a single centre cohort which may limit the generalisability of these findings. A multicentre study is ultimately required to validate the findings of this study. There is also a probable selection bias inherent in the setting of this study. There may have been incidental CI not referred to our centre during the study period. Risk of growth and aneurysm transformation may therefore be overstated in this study as no CI came to attention due to rupture in this study. Naturally, intra and inter-observer bias is present when reporting small changes within lesions or even when defining a vascular dilatation as an infundibulum or an aneurysm. Another limitation of the paper is the use of CTA and MRA for surveillance of infundibula, as many clinicians believe that DSA is necessary, however in our study, DSA was used to confirm diagnosis and given the additional invasive risks of cerebral angiography, not routinely used to monitor seemingly low risk lesions. Overall, further prospective multicentre studies with strict radiographic CI diagnosis criteria are required to validate these findings reported in this study.
CONCLUSIONS
In conclusion, CI grow and transform into aneurysms; although, this is a rare event. Size of the CI and male sex predict CI growth, though do so imperfectly. If growth occurs, it often occurs after prolonged periods of follow-up. CI that show growth on interval imaging likely represent a “pre-aneurysmal” lesion and not a CI. Future multicentre large prospective cohorts are required to confirm the findings of this study.
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Archer CR, Silbert S. Infundibula may be clinically significant. Neuroradiology. 1978 Oct;15(5):247–51. doi: 10.1007/BF00342917. [DOI] [PubMed] [Google Scholar]
- 2.Backes D, Vergouwen MDI, Tiel Groenestege AT, Bor ASE, Velthuis BK, Greving JP, et al. PHASES Score for prediction of intracranial aneurysm growth. Stroke. 2015 May;46(5):1221–6. doi: 10.1161/STROKEAHA.114.008198. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Connolly S, Feldmann E, Hanley DF, Kase CS, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007 Oct;116(16):e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Moosa S, Ding D, Raper DMS, Burke RM, Lee CC, et al. Infundibular dilations of the posterior communicating arteries: pathogenesis, anatomical variants, aneurysm formation, and subarachnoid hemorrhage. J Neurointerv Surg. 2016 Aug;8(8):791–5. doi: 10.1136/neurintsurg-2015-011827. [DOI] [PubMed] [Google Scholar]
- 5.Coupe NJ, Athwal RK, Marshman LAG, Brydon HL. Subarachnoid hemorrhage emanating from a ruptured infundibulum: case report and literature review. Surg Neurol. 2007 Feb;67(2):204–6. doi: 10.1016/j.surneu.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 6.Cowan JA, Barkhoudarian G, Yang LJS, Thompson BG. Progression of a posterior communicating artery infundibulum into an aneurysm in a patient with Alagille syndrome. Case report. J Neurosurg. 2004 Oct;101(4):694–6. doi: 10.3171/jns.2004.101.4.0694. [DOI] [PubMed] [Google Scholar]
- 7.Cox DR. Regression models and life‐tables. Journal of the Royal Statistical Society: Series B (Methodological) 1972 Jan;34(2):187–202. [Google Scholar]
- 8.Ebina K, Ohkuma H, Iwabuchi T. An angiographic study of incidence and morphology of infundibular dilatation of the posterior communicating artery. Neuroradiology. 1986 Dec;28(1):23–9. doi: 10.1007/BF00341761. [DOI] [PubMed] [Google Scholar]
- 9.Endo S, Furuichi S, Takaba M, Hirashima Y, Nishijima M, Takaku A. Clinical study of enlarged infundibular dilation of the origin of the posterior communicating artery. Journal of Neurosurgery. 1995 Sep;83(3):421–5. doi: 10.3171/jns.1995.83.3.0421. [DOI] [PubMed] [Google Scholar]
- 10.Harbaugh RE. The international study on unruptured intracranial aneurysms (ISUIA): New prospective data. Neurosurgery. 2003 Oct;53(4):1. [Google Scholar]
- 11.Mocco JD, Brown RD, Torner JC, Capuano AW, Fargen KM, Raghavan ML, et al. Aneurysm morphology and prediction of rupture: An international study of unruptured intracranial aneurysms analysis. Neurosurgery. 2018 Apr;82(4):491–6. doi: 10.1093/neuros/nyx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohyama T, Ohara S, Momma F. Fatal subarachnoid hemorrhage due to ruptured infundibular widening of the posterior communicating artery -case report. Neurol Med Chir (Tokyo) 1994 Mar;34(3):172–5. doi: 10.2176/nmc.34.172. [DOI] [PubMed] [Google Scholar]
- 13.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: R: A language and environment for statistical computing. Available online at https://www.R-project.org/ [Google Scholar]
- 14.Radulovic D, Nestorovic B, Rakic M, Janosevic V. Enlargement to a saccular aneurysm and subsequent rupture of infundibular widening of posterior communicating artery. Neurochirurgie. 2006 Dec;52(6):525–8. doi: 10.1016/s0028-3770(06)71360-1. [DOI] [PubMed] [Google Scholar]
- 15.Saltzman GF. Infundibular widening of the posterior communicating artery studied by carotid angiography. Acta radiol. 1959 Jun;51(6):415–21. doi: 10.3109/00016925909171114. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965 Dec;52(3/4):591–611. [Google Scholar]
- 17.Takahashi C, Fukuda O, Hori E, Kameda H, Endo S. A case of infundibular dilatation developed into an aneurysm and rupturing after the rupture of an aneurysm 10 years ago. No Shinkei Geka. 2006 Jun;34(6):613–7. [PubMed] [Google Scholar]
- 18.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003 Jul;362(9378):103–10. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]