Abstract
Fifteen novel carbazole alkaloids, euchrestifolines A–O (1–15), were obtained from Murraya euchrestifolia. Their structures were elucidated by spectroscopic analysis, Mosher’s ester, calculated ECD, and transition metal complex ECD methods. Notably, euchrestifolines A–C (1–3) are the first naturally occurring pyrrolidone carbazoles to be identified, while euchrestifolines D–F (4–6) represent rare carbazole alkaloids containing a phenylpropanyl moiety; euchrestifoline G (7) features a unique benzopyranocarbazole skeleton. More importantly, these compounds exhibited significant anti-ferroptotic activity, along with inhibitory effects of nitric oxide (NO) production and notable cytotoxicity. This study marks the first disclosure of carbazole's inhibitory effects against ferroptosis, and the EC50 values of some carbazoles ranging from 0.04 to 1 μM, substantially lower than the positive control, ferrostatin-1. In sum, this research not only enhances our understanding of carbazole alkaloids but also opens new avenues for the discovery of ferroptosis-related leading compounds.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13659-024-00483-7.
Keywords: Murraya euchrestifolia, Carbazole, Benzopyranocarbazole, Anti-ferroptosis, NO inhibition, Cytotoxicity
Introduction
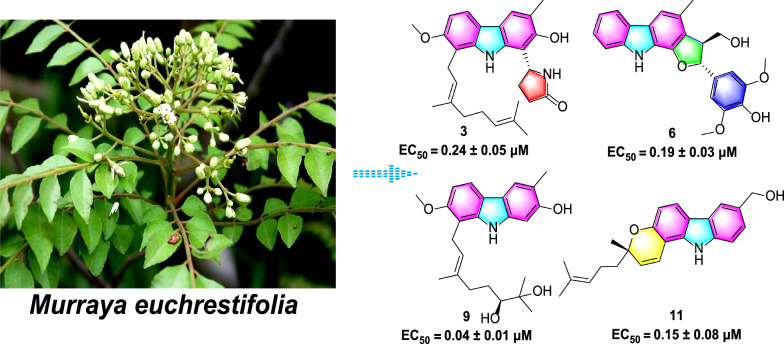
Murraya euchrestifolia Hayata, an evergreen tree, is widely distributed in Chinese Guangdong, Guangxi, and Hainan provinces [1]. Their leaves and twigs have been widely used by local people for the treatment of inflammation and pain [2]. Previous phytochemical investigations indicated that there were abundant carbazole alkaloids [3–6] and essential oil [2] in M. euchrestifolia. It has been reported that the carbazole alkaloids in Murraya plants possess unique structures and demonstrate effective anticancer, anti-diabetic, pain-relieving, anti-inflammatory, and antimicrobial properties [7–10].
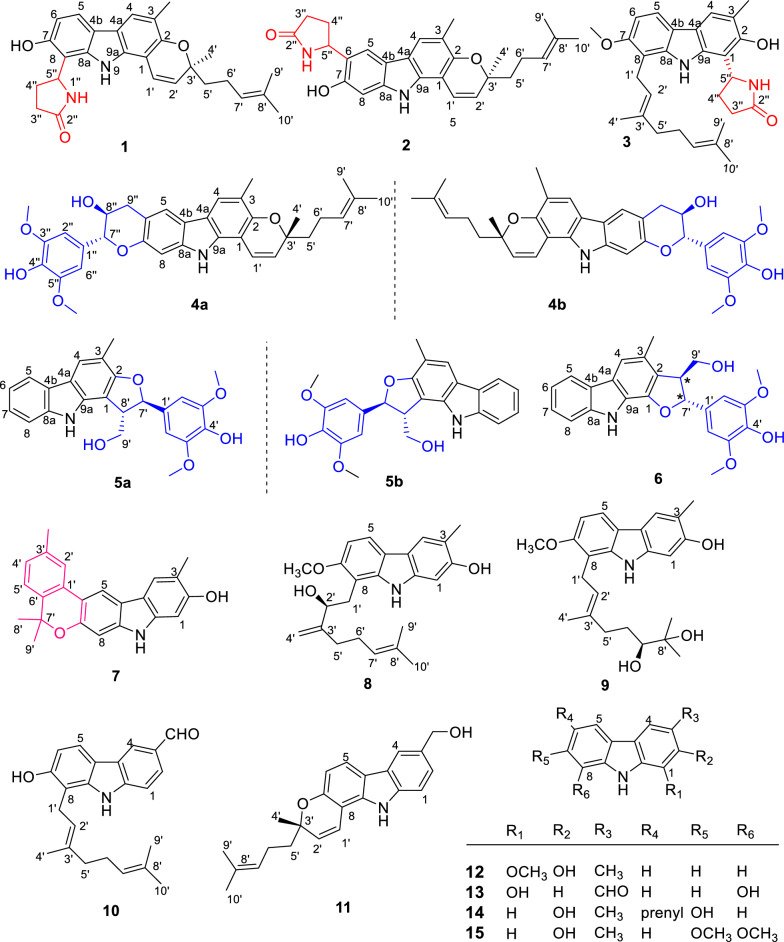
In order to discover more structurally novel and biologically active carbazole alkaloids from Murraya species [8, 11–13], an investigation was conducted on the ethanolic extract of the leaves and twigs of M. euchrestifolia to yield 15 novel carbazole alkaloids, designated as euchrestifolines A–O (1–15) (Fig. 1). Among them, euchrestifolines A–C (1–3) are pyrrolidone carbazoles obtained firstly from nature; euchrestifolines D–F (4–6) are carbazoles containing a rare phenylpropanyl, and euchrestifoline G (7) possesses a novel benzopyranocarbazole skeleton. Compounds 4–6 are three racemates, resolved by chiral-phase HPLC to afford their enantiomers. In this study, we reported the isolation process and structural illustration of 15 new carbazole alkaloids, and evaluated their potential effects on anti-ferroptosis in PC12 cells, their inhibition on nitric oxide (NO) production in RAW 264.7 macrophage cells, and their cytotoxicity against HepG2 cells.
Fig. 1.
Structures of compounds 1–15
Results and discussion
Structural explanation
Euchrestifoline A (1) was isolated in the form of a brown, non-crystalline solid, [α]25D + 20 (c 0.06, MeOH). The molecular formula C27H30N2O3 was established based on HRESIMS data (m/z 429.2169 [M − H]–, calcd for C27H29N2O3, 429.2178) and supported by 13C NMR findings. The UV spectrum showed peak absorptions at 221, 241, 296, and 312 nm, indicating the presence of a typical pyranocarbazole structure [14, 15]. The IR spectrum revealed absorption bands corresponding to hydroxy (3365 cm−1), carbonyl (1713 cm−1), olefinic, and aromatic (1648, 1612, 1517, and 1453 cm−1) functionalities.
The 1H NMR data (Table 1) presented a pair of ortho-coupled aromatic doublets [δH 6.76 (1H, d, J = 8.3 Hz, H-6), 7.66 (1H, d, J = 8.3 Hz, H-5)]. An aromatic singlet was observed at δH 7.57 (H-4), and a methyl group resonance appeared as a singlet at δH 2.28 (3-CH3). Additionally, signals related to 2,2-dimethyl-2H-pyran moiety were observed at δH 6.95 (1H, d, J = 9.8 Hz, H-1ʹ), 5.72 (1H, d, J = 9.8 Hz, H-2′), 1.42 (3H, s, H-4′), and 1.75 (2H, m, H-5ʹ), along with a set of prenyl signals at δH 2.18 (2H, m, H-6ʹ), 5.12 (1H, t, J = 7.4 Hz, H-7ʹ), 1.56 (3H, s, H-9ʹ), and 1.63 (3H, s, H-10ʹ).
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data of 1–6 (δH in ppm, J in Hz)
| No | 1a | 2a | 3a | 4b | 5a | 6a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | |
| 1 | 105.5, C | 105.3, C | 111.5, C | 104.4, C | 109.4, C | 145.1, C | ||||||
| 2 | 149.6, C | 149.4, C | 151.6, C | 149.4, C | 158.7, C | 122.4, C | ||||||
| 3 | 118.0, C | 117.5, C | 118.2, C | 118.3, C | 112.5, C | 126.4, C | ||||||
| 4 | 7.57, s | 120.7, CH | 7.60, s | 121.0, CH | 7.65, s | 120.6, CH | 7.54, s | 120.7, CH | 7.75, s | 121.4, CH | 7.44, s | 113.7, CH |
| 4a | 117.9, C | 117.8, C | 118.74, C | 116.5, C | 118.7, C | 127.1, C | ||||||
| 4b | 118.5, C | 117.2, C | 118.68, C | 119.0, C | 124.8, C | 124.2, C | ||||||
| 5 | 7.66, d (8.3) | 119.4, CH | 7.77, s | 116.8, CH | 7.73, d (8.5) | 117.8, CH | 7.60, s | 119.9, CH | 7.96, d (7.7) | 119.7, CH | 8.03, d (7.8) | 120.8, CH |
| 6 | 6.76, d (8.3) | 109.7, CH | 123.3, C | 6.86, d (8.5) | 105.4, CH | 112.7, C | 7.10, t (7.7) | 119.6, CH | 7.14, t (7.8) | 119.6, CH | ||
| 7 | 153.8, C | 153.4, C | 155.9, C | 152.2, C | 7.25, t (7.7) | 124.8, C | 7.36, t (7.8) | 126.2, C | ||||
| 8 | 111.0, C | 6.94, s | 97.6, CH | 112.3, C | 6.90, s | 97.7, CH | 7.44, d (7.7) | 111.6, CH | 7.51, d (8.8) | 112.1, CH | ||
| 9 | 9.63, br s | 10.00, br s | 8.79, br s | 7.75, br s | 9.84, br s | 10.26, br s | ||||||
| 8a | 140.3, C | 141.3, C | 141.0, C | 139.7, C | 140.9, C | 141.5, C | ||||||
| 9a | 136.1, C | 136.2, C | 138.7, C | 135.4, C | 136.8, C | 135.3, C | ||||||
| 1′ | 6.95, d (9.8) | 119.3, CH | 6.89, d (9.8) | 119.1, CH | 3.60, m | 24.3, CH2 | 6.62, d (9.8) | 117.7, CH | 133.4, C | 134.3, C | ||
| 2′ | 5.72, d (9.8) | 128.9, CH | 5.73, d (9.8) | 129.0, CH | 5.28, t (7.1) | 123.1, CH | 5.65, d (9.8) | 128.8, CH | 6.83, s | 104.8, CH | 6.74, s | 104.0, CH |
| 3′ | 78.6, C | 78.7, C | 136.6, C | 78.2, C | 148.8, C | 148.7, C | ||||||
| 4′ | 1.42, s | 26.1, CH3 | 1.43, s | 26.2, CH3 | 1.86, s | 16.5, CH3 | 1.44, s | 26.0, CH3 | 136.8, C | 136.4, C | ||
| 5′ | 1.75, m | 41.4, CH2 | 1.75, t (8.3) | 41.6, CH2 | 2.02, m | 40.4, CH2 | 1.76, t (8.4) | 40.9, CH2 | 148.8, C | 148.7, C | ||
| 6′ | 2.18, m | 23.5, CH2 | 2.18, m | 23.5, CH2 | 2.08, m | 27.5, CH2 | 2.16, m | 22.9, CH2 | 6.83, s | 104.8, CH | 6.74, s | 104.0, CH |
| 7′ | 5.12, t (7.4) | 125,2, CH | 5.14, m | 125.2, CH | 5.07, t (6.8) | 125,2, CH | 5.11, t (7.3) | 124.4, CH | 5.44, d (7.7) | 87.7, CH | 5.89, d (6.0) | 88.5, CH |
| 8′ | 131.9, C | 131.9, C | 131.7, C | 131.8, C | 3.88, m | 54.7, CH | 3.71, m | 55.6, CH | ||||
| 9′ | 1.56, s | 17.6, CH3 | 1.56, s | 17.6, CH3 | 1.54, s | 17.7, CH3 | 1.58, s | 17.7, CH3 |
4.01, m 4.07, m |
64.8 CH2 |
3.73, m 4.05, d (8.1) |
63.9, CH2 |
| 10′ | 1.63, s | 25.8, CH3 | 1.63, s | 25.8, CH3 | 1.58, s | 25.8, CH3 | 1.66, s | 25.8, CH3 | ||||
| 1′′ | 6.92, br s | 6.88, br s | 6.98, br s | 129.2, C | ||||||||
| 2′′ | 178.6, C | 177.9, C | 178.7, C | 6.74, s | 104.1, CH | |||||||
| 3′′ |
2.36, m 2.49, m |
28.1, CH2 | 2.28, m | 30.4, CH2 | 2.42, m | 31.8, CH2 | 147.4, C | |||||
| 4′′ |
2.37, m 2.47, m |
31.7, CH2 |
1,99, m 2.61, m |
30.5, CH2 |
2.22, m 2.53, m |
28.8, CH2 | 135.2, C | |||||
| 5′′ | 5.48, t (7.3) | 51.8, CH | 5.14, m | 53.6, CH | 5.53, t (7.9) | 52.5, CH | 147.4, C | |||||
| 6′′ | 6.74, s | 104.1, CH | ||||||||||
| 7′′ | 4.73, d (8.4) | 82.8, CH | ||||||||||
| 8′′ | 4.17, m | 68.9, CH | ||||||||||
| 9′′ |
3.09, dd (15.4, 9.4) 3.31, dd (15.4, 5.6) |
33.8, CH2 | ||||||||||
| 3-CH3 | 2.28, s | 16.2, CH3 | 2.29, s | 16.2, CH3 | 2.39, s | 17.1, CH3 | 2.32, s | 16.2, CH3 | 2.37, s | 15.8, CH3 | 2.44, s | 19.1, CH3 |
| 7-OCH3 | 3.89, s | 56.9, CH3 | ||||||||||
| 3′-OCH3 | 3.82, s | 56.8, CH3 | 3.73, s | 56.7, CH3 | ||||||||
| 3′′-OCH3 | 3.90, s | 56.9, CH3 | ||||||||||
| 5′-OCH3 | 3.82, s | 56.8, CH3 | 3.73, s | 56.7, CH3 | ||||||||
| 5′′-OCH3 | 3.90, s | 56.9, CH3 | ||||||||||
aMeasured in acetone-d6
bMeasured in CDCl3
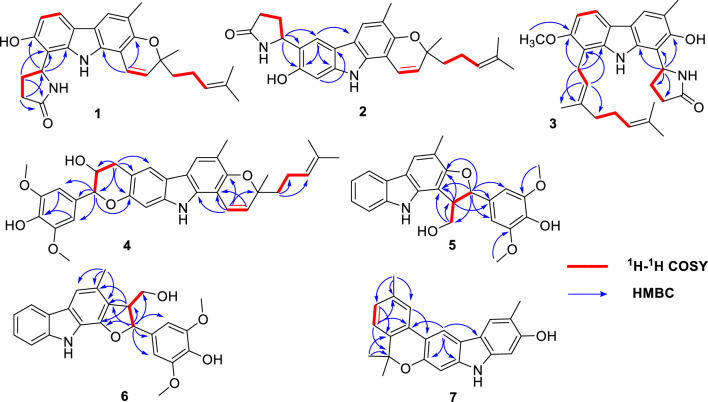
The 1H and 13C NMR data of compound 1 closely resembled those of mahanine [16], with a group of signals corresponding to an additional pyrrolidone unit appearing at δH 2.36 (1H, m, H-3ʹʹa), 2.37 (1H, m, H-4ʹʹa), 2.49 (1H, m, H-3ʹʹb), 2.47 (1H, m, H-4ʹʹb), 5.48 (1H, t, J = 7.3 Hz, H-5ʹʹ), and 6.92 (1H, brs, 1ʹʹ-NH), with carbon shifts at δC 28.1 (C-3ʹʹ), 31.7 (C-4ʹʹ), 51.8 (C-5ʹʹ), and 178.6 (C-2ʹʹ) [17]. The HMBC data (Fig. 2) revealed correlations between H-4ʹʹ and C-8, as well as between H-5ʹʹ and multiple carbons, namely C-7, C-8a, and C-8. These correlations strongly indicate that the pyrrolidone unit is linked to C-8 of pyranocarbazole. Compound 1 is the first reported natural carbazole alkaloid with a pyrrolidone unit.
Fig. 2.
Key HMBC and 1H-1H COSY correlations of 1–7
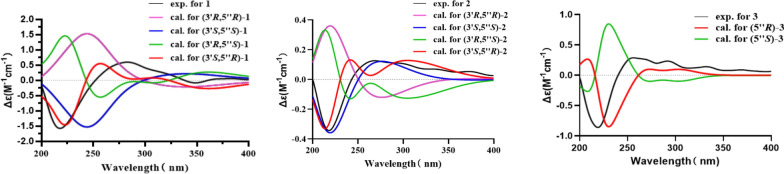
There are two chiral carbons in 1, thus, the ECD data of four possible configurations were calculated (Fig. 3), and from these data we found that the ECD curve of 1 was mainly contributed by the 3ʹS configuration (Fig. 3), also supported by the similar ECD data with (3ʹS)-mahanine (Fig. S13, Supporting Information), the biogenetic precursor of 1. However, due to the similarity in the trend of the calculated ECD curves for (3ʹS, 5ʹʹR) and (3ʹS, 5ʹʹS), it is difficult to give a definite answer to the conformation of C-5ʹʹ. Therefore, the quantum chemical calculations of the NMR data of (3ʹS, 5ʹʹR)-1 and (3ʹS, 5ʹʹS)-1 were performed. However, the results were still not satisfied to distinguish these two isomers (data not shown). Thus, only the 3ʹ configuration of 1 was defined as S, while the 5ʹʹ configuration was undetermined.
Fig. 3.
Experimental and calculated ECD spectra of compounds 1 (left), 2 (middle), and 3 (right)
Euchrestifoline B (2) was obtained as a brown, non-crystalline solid, [α]25D + 7 (c 0.09, MeOH). Its molecular formula, C27H30N2O3, matched that of compound 1, as confirmed by 13C NMR and HRESIMS data (m/z 429.2168 [M − H]–, calcd for C27H29N2O3, 429.2178). The NMR, UV, and IR characteristics are similar to those of 1, but a notable distinction was the change from a pair of ortho-coupled aromatic doublets in 1 to two aromatic singlets [δH 7.77 (H-5) and δH 6.94 (H-8)] in 2, indicating a shift in the pyrrolidone unit's attachment within the pyranocarbazole framework. The HMBC spectrum provided crucial correlations from H-5ʹʹ to C-5, C-6, and C-7 (Fig. 2). These correlations unambiguously positioned the pyrrolidone unit at the C-6 position. By comparing the calculated ECD curves of the four configurations of Euchrestifoline B (2) with its experimental curve (Fig. 3), the same question in 1 also existed in stereo configuration of 2, and thus only the 3ʹS configuration of 2 was determined.
Euchrestifoline C (3) was afforded as a brown, non-crystalline solid, [α]25D + 9 (c 0.12, MeOH). HRESIMS analysis revealed a molecular ion at m/z 445.2488 [M − H]– (calcd for C28H33N2O3, 445.2491), indicating that the molecular formula of 3 is C28H34N2O3. The NMR data of 3 (Table 1) bore resemblance to those of 1 and 2, with the key distinction being a geranyl moiety [δH 1.58 (H3-10ʹ), 1.54 (H3-9ʹ), 5.07 (H-7ʹ), 2.08 (H2-6ʹ), 2.02 (H2-5ʹ), 1.86 (H3-4ʹ), 5.28 (H-2ʹ), and 3.60 (H2-1ʹ)] replacing the 2-methyl-2-(4-methylpent-3-enyl)-2H-pyran moiety in 1 and 2. Additionally, the presence of a methoxy group in 3 was confirmed by NMR data (δH 3.89; δC 56.9). The HMBC correlations from the methoxy protons to C-7, from H-1ʹ to C-7/C-8a/C-8, and from H-2ʹ to C-8 (Fig. 2) indicated that the methoxy and the geranyl moieties are attached at C-7 and C-8, respectively. Similarly, the localization of the pyrrolidone unit at the C-1 position was deduced through the correlations observed between H-5ʹʹ and the carbon atoms C-1, C-2, and C-9a. The ECD spectrum computed for (5ʹʹR)-3 exhibited a favorable correspondence with the experimental data (Fig. 3), conclusively confirming the chiral configuration at the C-5ʹʹ position.
Euchrestifoline D (4) was isolated as a brown, non-crystalline solid with a specific rotation of [α]25D + 4 (c 0.08, MeOH). The molecular formula C34H37NO6 was confirmed by HRESIMS, which revealed a deprotonated molecular ion at m/z 554.2550 [M − H]− (calcd for C34H36NO6, 554.2543), and its 13C NMR data offered further support for the molecular formula. Comparison of the NMR data of 4 with those of mahanine [16], a set of ABX-coupled aromatic protons found in mahanine were substituted by two singlet signals at δH 7.60 (H-5) and δH 6.90 (H-8) in 4. Additionally, the 1H NMR spectrum displayed a 3,5-dimethoxy-4-hydroxyphenyl group [δH 6.74 (2H, s, H-2ʹʹ, H-6ʹʹ), 3.90 (6H, s, 3ʹʹ-OCH3, 5ʹʹ-OCH3)], alongside two methylene protons [δH 3.09 (1H, dd J = 15.4, 9.4 Hz, H-9ʹʹa), 3.31 (1H, dd J = 15.4, 5.6 Hz, H-9ʹʹb)], and two oxygenated methine protons [δH 4.17 (1H, m, H-8ʹʹ), 4.73 (1H, d J = 8.4 Hz, H-7ʹʹ)]. The 13C NMR data (Table 1) indicated a total of 34 distinct carbon signals, comprising 23 carbon signals from the mahanine unit, two from methoxy groups, six from aromatic carbons, and three from aliphatic carbons. By analyzing the 1H‒1H COSY relationships of H-9ʹʹ/H-8ʹʹ/H-7ʹʹ (Fig. 2) and their respective chemical shifts, a “–CH2–CHOH–CHR–O–” fragment was identified. The HMBC correlations of H-7ʹʹ with C-1ʹʹ/C-2ʹʹ/C-6ʹʹ/C-8ʹʹ/C-9ʹʹ, and of the methoxy protons (δH 3.90) with C-3ʹʹ/C-5ʹʹ (δC 147.4), confirmed the presence of a 4-hydroxy-3,5-dimethoxyphenylpropanyl unit in the structure. Given that 4 has 17 degrees of hydrogen deficiency, the existence of another ring was anticipated. In the HMBC spectrum, the correlations (Fig. 2) from H-9ʹʹ to C-5/C-6/C-7/C-7ʹʹ/C-8ʹʹ and from H-7ʹʹ to C-7 further established the attachment of the phenylpropanyl moiety to the mahanine unit via C-6 and C-7 to form a pyran ring. Ultimately, the 2D structure of 4 was characterized, marking it as the first phenylpropanyl-substituted pyranocarbazole to feature a new fused pyran ring.
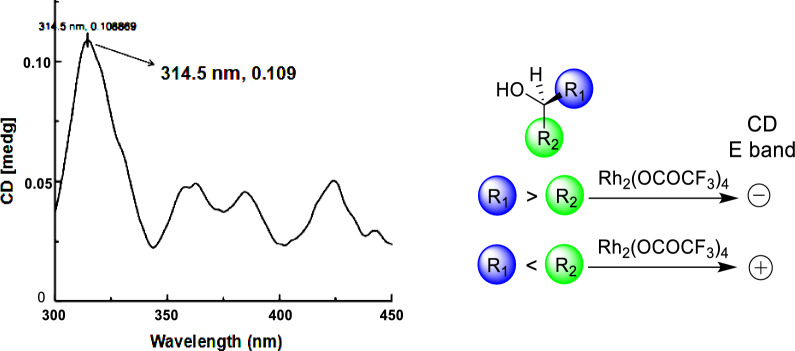
Compound 4 was characterized as a racemic mixture, with its specific rotation value being nearly zero. Furthermore, the ECD spectrum exhibited minimal Cotton effects, also indicating the presence of a racemic composition. Subsequently, it was separated into enantiomers, 4a and 4b using chiral-phase HPLC with a mobile phase of n-hexane–isopropanol (70:30, v/v), in a ratio of approximately 1:1 (see Fig. S2, Supporting Information). The specific rotations of compounds 4a and 4b were completely opposite (4a: + 27; 4b: − 27) as were their Cotton effects (Fig. S2, Supporting Information). The trans-configuration of H-7ʹʹ relative to H-8ʹʹ was inferred from the larger coupling constant, JH-7ʹʹ‒H-8ʹʹ (8.4 Hz) [18, 19]. The (8ʹʹS) absolute configuration of 4a was validated by the observation of a pronounced positive Cotton effect at 314 nm in the ECD spectrum of its Rh2(OCOCF3)4 complex, dissolved in CH2Cl2 (Fig. 4) [20]. The stereochemistry at the C-3ʹ position in 4a was determined by comparing the theoretical ECD curves of (3ʹR,7ʹʹR,8ʹʹS-4) and (3ʹS,7ʹʹS,8ʹʹR-4) with the experimental data, as shown in Fig. S2 of Supporting Information. Thus, the absolute configuration of (+)-euchrestifoline D (4a) was designated as (3ʹR,7ʹʹR,8ʹʹS), while (−)-euchrestifoline D (4b) was defined as (3ʹS,7ʹʹS,8ʹʹR).
Fig. 4.
The Rh2(OCOCF3)4 induced CD spectrum of compound 4a in CHCl3
Euchrestifoline E (5) was a brown, non-crystalline solid, with a molecular formula of C24H23NO5 determined from its HRESIMS data (m/z 404.1491 [M − H]−, calcd for C24H22NO5, 404.1498) and 13C NMR data. In the 1H NMR data (Table 1), characteristic signals were observed for ortho-disubstituted phenyl protons [δH 7.96 (H-5), 7.10 (H-6), 7.25 (H-7), 7.44 (H-8)] alongside an aromatic singlet at δH 7.75 (H-4) and a methyl singlet at δH 2.37 (3-CH3) attributed to the carbazole nucleus. The remaining 1H NMR signals were found to be similar to those in the phenylpropanyl group observed in 4. Through analysis of the 1H‒1H COSY correlations and the chemical shifts of H-7ʹ (δH 5.44), H-8ʹ (δH 3.88), and H-9ʹ (δH 4.01, 4.07), a “‒OCH2‒CHR1‒CHR2‒O‒” fragment was constructed. HMBC correlations (Fig. 2) from H-7ʹ to C-1/C-2/C-1ʹ/C-2ʹ/C-6ʹ/C-8ʹ/C-9ʹ, from H-8ʹ to C-1/C-2/C-1ʹ/C-7ʹ/C-9ʹ, and from H2-9ʹ to C-1/C-7ʹ/C-8ʹ suggested a “C-8ʹ‒C-1, C-7ʹ‒O‒C-2” linkage, forming a furan ring at C-1/C-2 of carbazole. This aligned with the hydrogen deficiency index of 14 of 5. Consequently, compound 5, a phenylpropanyl-substituted carbazole alkaloid, was characterized as described.
Similar to compound 4, 5 also existed as a racemate, and compounds 5a and 5b were separated by chiral-phase HPLC, displaying opposite specific rotations and Cotton effects (Fig. S3, Supporting Information). The configuration of H-7ʹ relative to H-8ʹ was determined to be trans based on their larger coupling constant, JH-7ʹ‒H-8ʹ (7.7 Hz) [18, 19], supported by NOE interactions observed between H-7ʹ and H-9ʹ. Subsequent to a comparison of the calculated and experimental ECD spectra of 5a and 5b (Fig. S3, Supporting Information), their absolute configurations were deduced as (7′R,8′S) and (7′S,8′R), respectively.
Euchrestifoline F (6) was obtained as a brown oil. The HRESIMS identified a molecular ion at m/z 404.1490 [M − H]− (calcd for C24H22NO5, 404.1498), confirming the molecular formula of C24H23NO5, which was the same as that of 5. Assessment of UV, IR, and NMR data revealed structural similarities between 6 and 5. However, the key difference resides in the manner of attachment of the furan ring to the carbazole group. The HMBC analysis indicated C-8ʹ‒C-2 and C-7ʹ‒O‒C-1 linkages for 6, derived from correlations of H-7ʹ to C-1/C-2, and of H-8′ to C-1/C-2/C-3 (Fig. 2). Thus, the 2D structure of 6 was articulated as shown.
A trans-position of H-7ʹ and H-8ʹ was concluded based on their coupling constant, JH-7ʹ‒H-8ʹ (6.0 Hz) [18, 19], along with the observed NOE correlation between H-7ʹ and H2-9ʹ. Similar to compounds 4 and 5, the zero specific rotation and chiral-phase HPLC analysis revealed that compound 6 is also a racemic mixture, with a pair of enantiomers 6a and 6b in a ratio of approximately 1:1 (Fig. S56, Supporting Information). Unfortunately, due to the small amount remaining, compound 6 was not subjected to preparative separation.
Euchrestifoline G (7) was obtained as a brown, non-crystalline solid. Its molecular formula was confirmed as C23H21NO2 by analysis of a deprotonated molecular ion detected at m/z 342.1492 [M − H]− (calcd for C23H20NO2, 342.1494) in the HRESIMS, along with the 13C NMR data. Its 1H NMR data (Table 2) revealed distinct signals characteristic of a carbazole structure, including four aromatic singlets [δH 6.80 (H-1), 7.77 (H-4), 8.24 (H-5), and 6.91 (H-8)], one methyl singlet [δH 2.41 (3-CH3)], and an active hydrogen proton [δH 7.76 (H-9)]. Additionally, ABX coupled phenyl signals were observed at δH 7.69 (H-2ʹ), 7.08 (H-4ʹ), and 7.16 (H-5ʹ), alongside three methyl singlets [δH 2.45 (3ʹ-CH3), 1.64 (H3-8ʹ and H3-9ʹ)]. The 13C NMR data (Table 2) displayed 23 carbon resonances that included four methyl carbons, one sp3 quaternary carbon, and 18 aromatic carbons, indicating the presence of three benzene rings in compound 7.
Table 2.
1H (500 MHz) and 13C (125 MHz) NMR data of 7–11 (δH in ppm, J in Hz)
| No | 7a | 8a | 9b | 10a | 11a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | |
| 1 | 6.80, s | 96.8, CH | 6.77, s | 97.1, CH | 6.93, s | 97.4, CH | 7.46, d (8.3) | 110.8, CH | 7.38, d (8.0) | 110.6, CH |
| 2 | 152.5, C | 152.4, C | 154.7, C | 7.89, d (8.3) | 126.5, CH | 7.35, d (8.0) | 124.4, CH | |||
| 3 | 116.3, C | 116.1, C | 117.3, C | 129.4, C | 132.6, C | |||||
| 4 | 7.77, s | 121.3, CH | 7.66, s | 121.2, CH | 7.65, s | 121.5, CH | 8.48, s | 123.0, CH | 7.94, s | 118.7, CH |
| 4a | 117.8, C | 118.1, C | 117.6, C | 124.4, C | 124.3, C | |||||
| 4b | 118.7, C | 118.2, C | 119.1, C | 117.3, C | 117.5, C | |||||
| 5 | 8.24, s | 113.6, CH | 7.73, d (8.4) | 117.9, CH | 7.70, d (8.4) | 117.5, CH | 7.83, d (8.3) | 119.3, CH | 7.77, d (8.4) | 120.7, CH |
| 6 | 116.1, C | 6.81, d (8.4) | 104.1, CH | 6.82, d (8.4) | 105.0, CH | 6.83, d (8.3) | 110.7, CH | 6.74, d (8.4) | 110.0, CH | |
| 7 | 151.6, C | 155.4, C | 155.6, C | 153.3, C | 152.1, C | |||||
| 8 | 6.91, s | 99.3, CH | 109.4, C | 112.3, C | 109.2, C | 104.8, C | ||||
| 9 | 7.76, br s | 8.48, br s | 9.55, br s | 8.29, br s | 7.96, br s | |||||
| 8a | 140.9, C | 141.8, C | 141.0, C | 140.9, C | 136.8, C | |||||
| 9a | 139.7, C | 140.2, C | 141.4, C | 143.6, C | 139.3, C | |||||
| 1′ | 129.9, C |
2.91, dd (14.3, 8.4) 3.34, dd (14.3, 2.5) |
32.4, CH2 | 3.61, d (6.6) | 24.6, CH2 | 3.65, d (7.0) | 24.4, CH2 | 6.66, d (9.8) | 117.3, CH | |
| 2′ | 7.69, br s | 122.8, CH | 4.41, dd (8.4, 2.5) | 76.1, CH | 5.36, d (6.6) | 123.4, CH | 5.39, t (7.0) | 121.2, CH | 5.68, d (9.8) | 129.0, CH |
| 3′ | 137.4, C | 152.3, C | 136.3, C | 139.2, C | 78.6, C | |||||
| 4′ | 7.08, d (7.9) | 127.6, CH | 4.91, s; 5.13, s | 108.9, CH2 | 1.83, s | 16.5, CH3 | 1.91, s | 16.7, CH3 | 1.46, s | 26.2, CH3 |
| 5′ | 7.16, d (7.9) | 123.2, CH | 2.23, m | 32.6, CH2 | 2.01, m; 2.35, m | 37.8, CH2 | 2.10‒2.15, m | 39.8, CH2 | 1.76, m | 41.0, CH2 |
| 6′ | 136.5, C | 2.23, m | 26.8, CH2 | 1.31, m; 1.67, m | 30.8, CH2 | 2.10‒2.15, m | 26.6, CH2 | 2.17, m | 22.9, CH2 | |
| 7′ | 77.8, C | 5.18, t (6.6) | 124.2, CH | 3.23, d (10.9) | 78.6, CH | 5.06, m | 123.8, CH | 5.11, t (7.5) | 124.2, CH | |
| 8′ | 1.64, s | 27.7, CH3 | 132.1, C | 72.8, C | 132.3, C | 131.9, C | ||||
| 9′ | 1.64, s | 27.7, CH3 | 1.64, s | 17.9, CH3 | 1.08, s | 25.2, CH3 | 1.59, s | 17.9, CH3 | 1.58, s | 17.8, CH3 |
| 10′ | 1.71, s | 25.9, CH3 | 1.08, s | 25.9, CH3 | 1.64, s | 25.8, CH3 | 1.66, s | 25.8, CH3 | ||
| 3-CH3 | 2.41, s | 16.3, CH3 | 2.38, s | 16.3, CH3 | 2.32, s | 16.7, CH3 | ||||
| 3′-CH3 | 2.45, s | 21.6, CH3 | ||||||||
| 3-CHO | 10.07, s | 192.2, CH | ||||||||
| 3-CH2OH | 4.83, s | 66.3, CH2 | ||||||||
| 7-OCH3 | 3.90, s | 56.5, CH3 | 3.87, s | 56.9, CH3 | ||||||
aMeasured in CDCl3
bMeasured in acetone-d6
In HMBC spectrum, the correlations (Fig. 2) from 3ʹ-CH3 to C-2ʹ/C-3ʹ/C-4ʹ and from 8ʹ-CH3/9ʹ-CH3 to C-6ʹ (δC 136.5) and C-7ʹ (δC 77.8) suggested that a methyl group was attached to C-3ʹ and an isopropyl group was presented at C-6ʹ of the phenyl segment. Furthermore, there were HMBC correlations between H-5 and C-1ʹ, and H-2ʹ and C-6, suggesting a linkage between the phenyl group and the carbazole core via C-1ʹ–C-6. Additionally, the formation of a pyran ring was inferred due to a C-7ʹ–O–C-7 linkage, identified by the hydrogen deficiency index of 14 for 7 and the lack of a proton at H-7. Thus, the structure of euchrestifoline G (7), a novel benzopyranocarbazole alkaloid, was characterized as depicted.
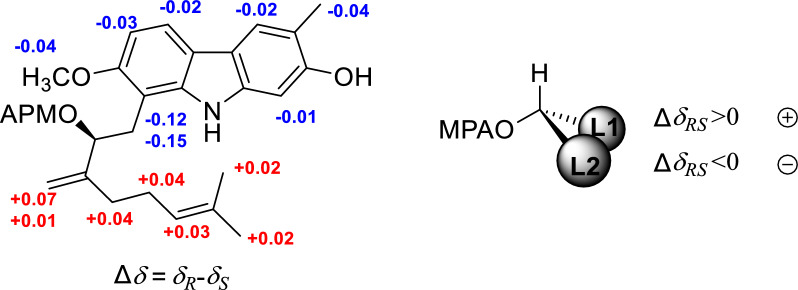
Euchrestifoline H (8) was similarly obtained as a brown, non-crystalline solid, [α]25D + 11 (c 0.14, MeOH). Its molecular formula was determined to be C24H29NO3, confirmed by a quasimolecular ion at m/z 378.2072 [M − H]− (calcd for C24H28NO3, 378.2069). Analysis of the UV, IR, and NMR data (Table 2) revealed that the structure of 8 closely resembled that of euchrestine B [21]. The distinction lied in the oxidation of H-2′ of the geranyl group in euchrestine B to a hydroxy group in 8, alongside a shift of the olefinic double bond from C-2ʹ–C-3′ to C-3ʹ–C-4′ [δH 4.41 (H-2ʹ), 4.91 (H-4ʹa), 5.13 (H-4ʹb); δC 76.1 (C-2ʹ), 152.3 (C-3ʹ), 108.9 (C-4ʹ)]. The HMBC correlations from H-1ʹ to C-7/C-8/C-8a/C-2ʹ/C-3ʹ, from H-2ʹ to C-8/C-1ʹ/C-4ʹ/C-5ʹ, and from H-4ʹ to C-2ʹ/C-5ʹ (Fig. S1, Supporting Information) further supported this conclusion. The (2ʹS) absolute configuration was established through Mosher ester analysis (Fig. 5) [22]. Ultimately, the structure of euchrestifoline H (8) was confirmed as illustrated.
Fig. 5.
Δδ = δR-δS values obtained from the 1H NMR data of the MPA esters of 8
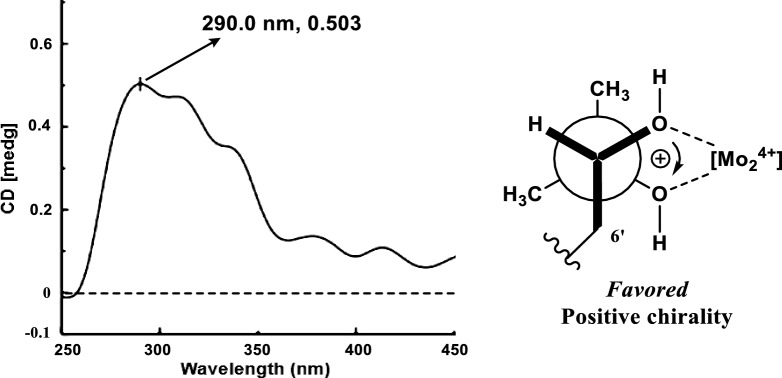
Euchrestifoline I (9) was also obtained as a brown, non-crystalline solid, [α]25D + 15 (c 0.14, MeOH). Its molecular formula was defined as C24H31NO4 based on a deprotonated molecular ion at m/z 396.2167 [M − H]– (calcd for C24H30NO4, 396.2175) in the negative-ion HRESIMS and 13C NMR data. Comparison of NMR data of 9 (Table 2) with those of euchrestine B [21] indicated an oxidation of the double bond between C-7ʹ and C-8ʹ in euchrestine B to a dihydroxy group [δH 3.23 (H-7ʹ); δC 78.6 (C-7ʹ), 72.8 (C-8ʹ)] in 9. The 2D configuration of 9 was established as depicted, supported by relevant HMBC correlations (Fig. S1, Supporting Information). In the ECD spectrum of its Mo2(OAc)4 complex in DMSO, a significant positive Cotton effect was observed at 290 nm, from which the absolute configuration of 9 was deduced to be 7ʹS (Fig. 6) [23].
Fig. 6.
Mo2(OAc)4-induced CD spectrum for 9 and Newman projection of the diol moiety of 9, with the helicity rule applied
Euchrestifoline J (10) was isolated as a brown, non-crystalline solid. The HRESIMS analysis presented a deprotonated molecular ion at m/z 346.1807 [M − H]− (calcd for C23H24NO2, 346.1807), which corresponded to a molecular formula of C23H25NO2, corroborated by the 13C NMR data. The NMR characteristics of compound 10 (Table 2) closely mirrored those of euchrestine C [24], with notable differences such as the absence of a 2-OH group and the substitution of a 3-CH3 with a 3-CHO group (δH 10.07; δC 192.2). The structure of euchrestifoline J was thereby confirmed alongside the HMBC correlations (Fig. S1, Supporting Information).
Euchrestifoline K (11) was similarly purified as a brown, non-crystalline solid. Its molecular formula, C23H25NO2, was supported by HRESIMS revealing a deprotonated molecular ion at m/z 346.1802 [M − H]− (calcd for C23H24NO2, 346.1807). The NMR data (Table 2) exhibited high similarities to the structure of mahanimbicine [25], with the distinction that a methyl singlet in mahanimbicine was replaced by a hydroxymethyl group (δH 4.83, δC 66.3) in 11. Additional 2D NMR investigations further confirmed the structure of euchrestifoline K as depicted. By comparison with (3ʹS)-pyrayafoline D [26], the (3ʹS) absolute configuration of 11 was established by its corresponding optical rotation and similar ECD curve (Fig. S95, Supporting Information).
Euchrestifoline L (12) was isolated as a brown, non-crystalline solid. The HRESIMS revealed a deprotonated molecular ion at m/z 226.0867 [M − H]− (calcd for C14H12NO2, 226.0868), which matched the calculated formula for C14H13NO2. A comparison of its NMR data (Table 3) with that of 2-hydroxy-3-methylcarbazole [27] indicated that a methoxy group (δH 4.01) in compound 12 substituted the H-1 proton of 2-hydroxy-3-methylcarbazole. This substitution was also supported by HMBC correlations (Fig. S1, Supporting Information) from the methoxy protons to C-1 (δC 131.3). Consequently, euchrestifoline L (12) was elucidated as 2-hydroxy-1-methoxy-3-methylcarbazole.
Table 3.
1H (500 MHz) and 13C (125 MHz) NMR data of 12–15 (δH in ppm, J in Hz)
| No | 12a | 13b | 14b | 15b | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | |
| 1 | 131.3, C | 144.6, C | 6.85, s | 97.0, CH | 6.96, s | 97.4, CH | ||
| 2 | 145.4, C | 7.42, s | 108.6, CH | 154.0, C | 154.9, C | |||
| 3 | 118.0, C | 131.3, C | 116.8, C | 117.5, C | ||||
| 4 | 7.57, s | 117.1, CH | 8.22, s | 119.3, CH | 7.60, s | 121.1, CH | 7.66, s | 121.7, CH |
| 4a | 118.1, C | 125.8, C | 117.5, C | 117.5, C | ||||
| 4b | 124.2, C | 126.4, C | 117.5, C | 120.7, C | ||||
| 5 | 7.94, d (7.7) | 119.7, CH | 7.70, d (7.7) | 112.7, CH | 7.60, s | 120.1, CH | 7.56, d (8.4) | 114.7, CH |
| 6 | 7.20, t (7.7) | 119.8, CH | 7.11, t (7.7) | 121.8, CH | 120.7, C | 6.84, d (8.4) | 106.9, CH | |
| 7 | 7.33, t (7.7) | 124.7, C | 6.96, d (7.7) | 112.1, CH | 153.6, C | 150.2, C | ||
| 8 | 7.40, d (7.7) | 110.7, C | 144.4, C | 6.86, s | 97.1, CH | 135.0, C | ||
| 9 | 7.94, br s | 10.46, br s | 9.82, br s | |||||
| 8a | 139.8, C | 130.8, C | 140.6, C | 135.1, C | ||||
| 9a | 131.1, C | 134.5, C | 140.4, C | 141.3, C | ||||
| 1′ | 3.43, d (7.4) | 29.5, CH2 | ||||||
| 2′ | 5.44, t (7.4) | 125.1, CH | ||||||
| 3′ | 131.5, C | |||||||
| 4′ | 1.74, s | 26.0, CH3 | ||||||
| 5′ | 1.76, s | 17.9, CH3 | ||||||
| 3-CH3 | 2.43, s | 16.3, CH3 | 2.31, s | 16.7, CH3 | 2.32, s | 16.7, CH3 | ||
| 3-CHO | 10.01, s | 191.8, CH | ||||||
| 1-OCH3 | 4.01, s | 60.9, CH3 | ||||||
| 7-OCH3 | 3.90, s | 60.7, CH3 | ||||||
| 8-OCH3 | 3.92, s | 57.3, CH3 | ||||||
aMeasured in CDCl3
bMeasured in acetone-d6
Euchrestifoline M (13), also a brown, non-crystalline solid, was identified with a molecular formula of C13H9NO3 based on the data from 13C NMR and HRESIMS (m/z 226.0507 [M − H]−, calcd for C13H8NO3, 226.0504). A comparison of the MS and NMR data for 13 (Table 3) with those of O-demethylmurrayanine [28] revealed that 13 has a mass increase of 16 Da and ortho-disubstituted phenyl protons in O-demethylmurrayanine shifts to ortho-trisubstituted phenyl signals at δH 7.70 (H-5), 7.11 (H-6), and 6.96 (H-7) in 13, indicating the presence of an additional hydroxy group. Based on the corresponding HMBC correlations (Fig. S1, Supporting Information), the hydroxy group was determined to be at C-8. Therefore, euchrestifoline M (13) was characterized as 1,8-dihydroxy-3-formylcarbazole.
The molecular formula of euchrestifoline N (14) was determined to be C18H19NO2 based on analysis of the 13C NMR and the negative-ion HRESIMS data (m/z 280.1337 [M − H]−, calcd for C18H18NO2, 280.1337). The NMR data of 14 (Table 3) displayed similarities to those of euchrestine A [24], with the exception that the prenyl group shifted from C-8 to C-6, as inferred from the presence of two aromatic singlets [δH 7.60, 6.86] in 14 and supported by HMBC correlations (Fig. S1, Supporting Information). Thus, the structure of euchrestifoline N (14) was designated as 2,7-dihydroxy-3-methyl-6-prenylcarbazole.
Euchrestifoline O (15) was isolated as a brown, non-crystalline solid. Its HRESIMS data revealed a deprotonated molecular ion at m/z 226.0507 [M − H]− (calcd for C13H8NO3, 226.0504), consistent with the molecular formula of C13H9NO3, further confirmed by the 13C NMR data. The NMR characteristics of 15 showed strong resemblance to 2-hydroxy-3-methylcarbazole [27], with the notable addition of two methoxy groups (δH 3.92, 3.90) in 15. The presence of ortho-coupled aromatic doublets [δH 6.84 (H-6), 7.56 (H-5)] in the 1H NMR spectrum, along with HMBC correlations, identified these two methoxy groups at C-7 and C-8, respectively (Fig. S1, Supporting Information). Therefore, the structure of euchrestifoline O (15) was identified as 2-hydroxy-7,8-dimethoxy-3-methylcarbazole.
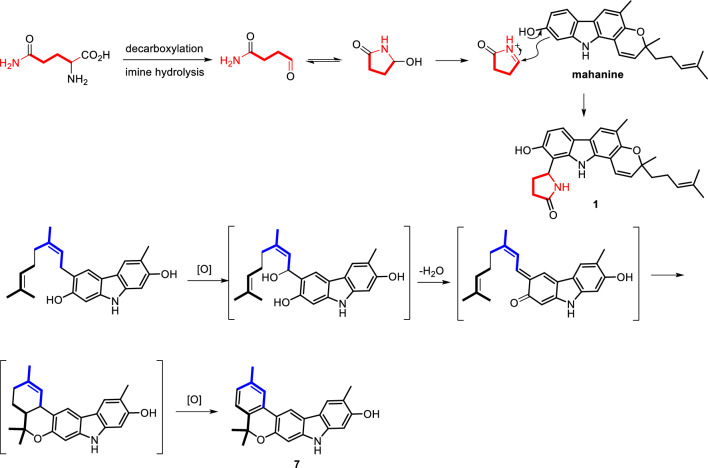
Among the new compounds, 1–3 represent a distinctive group of carbazole alkaloids incorporating a pyrrolidone unit. In Scheme 1, the proposed biosynthetic pathways for compounds 1 and 7 are illustrated. It is theorized that compound 1 is derived from mahanine, a well-known carbazole alkaloid found in Murraya species, in conjunction with a pyrrolidone iminium ion moiety that is generated from glutamine through decarboxylation, imine hydrolysis, and subsequent cyclization. Additionally, as depicted in Scheme 1, a new benzopyranocarbazole alkaloid structure, represented by compound 7, is believed to be formed via an intramolecular hetero-Diels–Alder reaction between the in situ generated ortho-quinomethide and an adjacent double bond, followed by oxidative aromatization.
Scheme 1.
Putative biosynthetic pathways for 1 and 7
Investigation of NO inhibitory activity
Taking into account the well-established anti-inflammatory and pain-relieving properties of M. euchrestifolia, compounds 1–15 were assessed for their capacity to inhibit NO production stimulated by LPS in RAW 264.7 cells. As summarized in Table 4, compounds 4a, 4b, 6, and 11–14 exhibited strong inhibitory effects, with IC50 values below 20 μM. Meanwhile, compounds 1, 5a, 5b, 8–10, and 15 showed moderate inhibitory activity, with their IC50 values in the range of 21.6 to 32.5 μM. It is worth noting that the position of the pyrrolidone substituent may impact anti-inflammatory activity (for instance, comparing compounds 1 and 2), while isomerization appeared to have a minimal effect on activity (e.g., 4a compared to 4b and 5a compared to 5b). No isolates presented significant cytotoxicity at 50 μM.
Table 4.
Various activity screening of 1–15
| Compd | IC50 (μM)a | EC50 (µM) | |
|---|---|---|---|
| NO inhibition | Cytotoxicity | Anti-ferroptosis | |
| 1 | 27.7 ± 1.9 | 18.5 ± 2.6 | 1.21 ± 0.09 |
| 2 | > 50 | 30.5 ± 0.8 | 0.46 ± 0.06 |
| 3 | > 50 | 34.0 ± 2.7 | 0.24 ± 0.05 |
| 4a | 18.6 ± 1.1 | 2.6 ± 0.5 | 2.20 ± 0.18 |
| 4b | 16.0 ± 0.1 | 15.0 ± 2.1 | |
| 5a | 21.7 ± 1.2 | 3.4 ± 0.7 | 0.42 ± 0.04 |
| 5b | 24.6 ± 1.3 | 3.4 ± 0.6 | |
| 6 | 13.0 ± 1.7 | 1.2 ± 1.1 | 0.19 ± 0.03 |
| 7 | > 50 | 15.9 ± 2.4 | 9.18 ± 0.40 |
| 8 | 32.5 ± 0.7 | 2.7 ± 0.2 | 0.23 ± 0.06 |
| 9 | 21.6 ± 3.2 | 25.7 ± 2.0 | 0.04 ± 0.01 |
| 10 | 23.0 ± 0.9 | 30.0 ± 2.9 | 17.09 ± 4.09 |
| 11 | 19.0 ± 2.8 | > 50 | 0.15 ± 0.08 |
| 12 | 16.0 ± 2.1 | > 50 | 4.23 ± 0.26 |
| 13 | 12.7 ± 0.8 | > 50 | 1.18 ± 0.17 |
| 14 | 19.7 ± 0.1 | 41.4 ± 2.0 | 0.63 ± 0.05 |
| 15 | 26.3 ± 1.9 | > 50 | 4.91 ± 0.14 |
| Dexamethasoneb | 10.1 ± 0.4 | ||
| Taxolb | 0.032 ± 0.014 | ||
| Ferrostatin-1b | 1.33 ± 0.16 | ||
aIC50 values are presented as mean ± SD (n = 3)
bPositive control
Evaluations of cytotoxic activity
The cytotoxic effects of the isolated carbazole alkaloids were evaluated, drawing from findings in the literature [29]. The data presented in Table 4 illustrated the cytotoxic effects of compounds 1–10 and 14 on HepG2 cells, with IC50 values ranging from 1.2 to 41.4 μM. Notably, compounds 4a, 5a, 5b, 6, and 8 exhibited considerable cytotoxic effects on HepG2 cells, each with IC50 values below 4.0 μM. The presence of a phenylpropanyl substituent appears to enhance the cytotoxicity of these carbazole derivatives, as all phenylpropanyl-substituted carbazoles (4–6) demonstrated superior cytotoxic abilities compared to other carbazole compounds. For the phenylpropanyl-substituted pyranocarbazole, isomerization may significantly influence cytotoxicity, as seen from 4a versus 4b.
Evaluations of anti-ferroptosis effects
Carbazole alkaloids have been reported to present potent neuro-protection activities [30], and recently anti-ferroptosis has been disclosed to be an important pathway for neuroprotection [31], thus these compounds were assessed for their anti-ferroptosis effects against erastin-induced ferroptosis in PC12 cells. As summarized in Table 4, all compounds provided notable protection, exhibiting EC50 values spanning from 0.04 to 17.09 μM, most surpassing the positive control, ferrostatin-1 (EC50: 1.33 μM). Compound 9 exhibited the highest potency, with an EC50 of 40 nM, suggesting that the ortho-dihydroxy group on the geranyl derivative is crucial for its activity. Conversely, the presence of an aldehyde group or a rigid ring structure, such as a benzopyranocarbazole, appears to diminish activity, as illustrated by compounds 10 (EC50: 17.09 μM) and 7 (EC50: 9.18 μM).
Materials and methods
General experimental procedures
The reagents and instruments used for isolation, purification and structural elucidation of compounds were in accordance with those used in the literature [26]. The analytical grade solvents were utilized for CC, while those of chromatography grade for HPLC.
Plant materials
The dried leaves and twigs of the plant were collected in May 2016 in Jingxi County, Guangxi Province of China. Prof. P.-F. Tu, a co-author of this study identified them as Murraya euchrestifolia Hayata. A reference sample, designated as No. DYJLX201605, was deposited in the Herbarium of Modern Research Center for Traditional Chinese Medicine, Peking University.
Extraction and isolation processes
Air-dried leaves and twigs of M. euchrestifolia (10 kg) were pulverised and extracted with 95% ethanol (100 L) thrice, and each for 2 h. The dried extract (450 g) was obtained under reduced pressure, and then, it was dissolved in water and extracted with CH2Cl2 to give an extract of 150 g. This extract was processed through silica gel CC, employing a stepwise elution of petroleum ether-acetone (10:1, 5:1, 1:1, and 0:1, v/v) to yield six distinct fractions (Frs. 1–6).
Fr. 4 (42 g) was treated with Sephadex LH-20 CC eluting with CH2Cl2–MeOH (1:1, v/v) and five subfractions, Frs. 4a–4e, were obtained. Fr. 4d was divided into seven subfractions (Frs. 4d1–4d7) by ODS CC (gradient MeOH–H2O, 50:50–100:0, v/v). Fr. 4d3 underwent purification via semi-preparative HPLC with MeCN–H2O (47:53, v/v), eluting at 3 mL/min to obtain 12 (4.0 mg, tR 14.2 min). Fr. 4d4 was subjected to a similar semi-preparative HPLC process with a different eluent composition of MeCN–H2O (75:25, v/v, 3 mL/min) to give 8 (2.3 mg, tR 7.1 min) and 10 (3.1 mg, tR 9.2 min).
Fr. 5 (35 g) was purified by Sephadex LH-20 CC (CH2Cl2–MeOH, 1:1, v/v) to yield Frs. 5a–5f. Fr. 5b underwent a gradient elution process utilizing MCI CC with a methanol–water solvent system ranging from 30:70 to 100:0 (v/v), which led to the acquisition of six subfractions (Frs. 5b1–5b6). Fr. 5b5 was further purified by using MeCN–H2O (45:55, v/v, 3 mL/min) as eluent on a semi-preparative HPLC to afford compounds 15 (3.0 mg, tR 6.2 min) and 7 (3.2 mg, tR 13.8 min). Fractions (5c1–5c5) were obtained from subfraction 5c by ODS CC, utilizing a gradient elution of methanol and water (v/v) from 50:50 to 100:0. Compounds 3 (3.4 mg, tR 9.2 min) and 4 (3.7 mg, tR 12.6 min) were purified from Fr. 5c2 by semi-preparative HPLC, employing a solvent system of acetonitrile and water in 80:20 at a flow rate of 3 mL/min. Fr. 5c3 was further purified with a mobile phase of MeCN–H2O (45:55, v/v, 3 mL/min) to obtain 5 (2.3 mg, tR 6.9 min), 6 (2.4 mg, tR 7.7 min), and 9 (3.4 mg, tR 8.3 min). Fr. 5c5 was further treated with semi-preparative HPLC (MeCN–H2O, 55:45, v/v) at a flow rate of 3 mL/min to obtain 11 (1.6 mg, tR 4.0 min), 13 (3.2 mg, tR 4.7 min), and 14 (3.7 mg, tR 5.1 min).
Fr. 6 (19 g) was treated with Sephadex LH-20 under the same conditions as Fr. 4 and Fr. 5 to obtain six subfractions, 6a–6f. By ODS CC, subfraction 6c was treated with MeOH–H2O gradient elution (30:70–100:0, v/v) to obtain six fractions (6c1–6c6). Based on semi-preparative HPLC, Frs. 6c4 and 6c6 were purified with different gradients of MeCN–H2O (60:40 and 70:30, respectively, v/v, 3 mL/min) to obtain 1 (3.0 mg, tR 12.2 min) and 2 (1.5 mg, tR 14.0 min), respectively. A Chiralpak AD-H column was used on the semi-preparative HPLC for the enantioseparation of 4a/4b and 5a/5b. Under the chromatographic conditions of n-hexane–iPrOH (70:30, v/v, 1 mL/min) and 238 nm detection wavelength, compounds 4a (1.6 mg, tR 20.7 min) and 4b (1.7 mg, tR 24.5 min), and 5a (1.0 mg, tR 13.3 min) and 5b (1.1 mg, tR 15.7 min) were obtained, respectively.
Euchrestifoline A (1): brown, non-crystalline solid; [α]25D + 20 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 221 (4.39), 241 (4.40), 296 (4.16), 312 (3.94) nm; ECD (MeOH) λmax (Δε) 217 (− 0.68), 282 (+ 0.26) nm; IR (KBr) νmax 3365, 2976, 2931, 2154, 1713, 1648, 1612, 1517, 1453, 1367, 1252, 1164, 1032, 1019, 929, 857, 762, 578 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 429.2169 [M − H]− (calcd for C27H29N2O3, 429.2178).
Euchrestifoline B (2): brown, non-crystalline solid; [α]25D + 7 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 225 (4.28), 240 (4.33), 297 (4.05) nm; ECD (MeOH) λmax (Δε) 220 (− 0.84), 256 (+ 0.29) nm; IR (KBr) νmax 3364, 2975, 2929, 2154, 1713, 1517, 1367, 1253, 1163, 1021, 9230, 767, 576 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 429.2168 [M − H]− (calcd for C27H29N2O3, 429.2178).
Euchrestifoline C (3): brown, non-crystalline solid; [α]25D + 9 (c 0.12, MeOH); UV (MeOH) λmax (log ε) 218 (4.21), 239 (4.27), 264 (4.05), 309 (3.83) nm; ECD (MeOH) λmax (Δε) 220 (− 0.62), 268 (+ 0.23) nm; IR (KBr) νmax 3376, 2970, 2922, 2859, 1737, 1722, 1616, 1457, 1367, 1217, 1176, 1052, 1032, 1018, 884, 578 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 445.2488 [M − H]− (calcd for C28H33N2O3, 445.2491).
Euchrestifoline D (4): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 209 (4.39), 241 (4.34), 299 (4.03), 357 (3.46) nm; IR (KBr) νmax 3385, 2922, 2852, 1700, 1618, 1464, 1300, 1273, 1153,1025, 835, 575 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 554.2550 [M − H]− (calcd for C34H36NO6, 554.2543).
(+)-Euchrestifoline D (4a): [α]25D + 27 (c 0.01, MeOH); ECD (MeOH) λmax (Δε) 221 (‒0.88), 247 (+ 1.08), 289 (‒0.35), 330 (+ 0.29) nm.
(−)-Euchrestifoline D (4b): [α]25D − 27 (c 0.01, MeOH); ECD (MeOH) λmax (Δε) 222 (+ 1.77), 252 (− 0.57), 231 (+ 0.66), 330 (− 0.13) nm.
Euchrestifoline E (5): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 209 (4.34), 241 (4.28), 256 (4.10), 306 (3.80) nm; IR (KBr) νmax 3413, 3004, 2917, 2849, 1713, 1422, 1362, 1222, 1029, 530 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 404.1491 [M − H]− (calcd for C24H22NO5, 404.1498).
(+)-Euchrestifoline E (5a): [α]25D + 46 (c 0.05, MeOH); ECD (MeOH) λmax (Δε) 201 (‒17.09), 252 (+ 6.57), 231 (+ 2.27) nm, 245 (+ 7.21), 304 (+ 3.44) nm.
(−)-Euchrestifoline E (5b): [α]25D − 46 (c 0.07, MeOH); ECD (MeOH) λmax (Δε) 200 (+ 13.60), 252 (− 7.03), 231 (− 2.75) nm, 245 (− 5.20), 304 (− 2.44) nm.
Euchrestifoline F (6): brown oil; UV (MeOH) λmax (log ε) 208 (4.26), 248 (4.24), 294 (3.74) nm; ECD (MeOH) λmax (Δε) 216 (− 2.32) nm; IR (KBr) νmax 3385, 2922, 2851, 1706, 1613, 1517, 1222, 1160, 1021, 529 cm–1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 404.1490 [M − H]− (calcd for C24H22NO5, 404.1498).
Euchrestifoline G (7): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 208 (4.12), 244 (4.15), 306 (4.16), 358 (3.76) nm; IR (KBr) νmax 3375, 2921, 2852, 1706, 1613, 1454, 1294, 1221, 1154, 1122, 1018, 578 cm–1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 342.1492 [M − H]− (calcd for C23H20NO2, 342.1494).
Euchrestifoline H (8): brown, non-crystalline solid; [α]25D + 11 (c 0.14, MeOH); UV (MeOH) λmax (log ε) 214 (4.23), 238 (4.29), 265 (4.07), 309 (3.88) nm; ECD (MeOH) λmax (Δε) 216 (− 0.16) nm; IR (KBr) νmax 3386, 2920, 2851, 2154, 1714, 1613, 1452, 1383, 1366, 1162, 1138, 1020, 578 cm–1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 378.2072 [M − H]− (calcd for C24H28NO3, 378.2069).
Euchrestifoline I (9): brown, non-crystalline solid; [α]25D + 15 (c 0.14, MeOH); UV (MeOH) λmax (log ε) 213 (4.31), 238 (4.40), 309 (3.92) nm; ECD (MeOH) λmax (Δε) 211 (− 1.33), 255 (0.37) nm; IR (KBr) νmax 3381, 2923, 2852, 1705, 1617, 1454, 1262, 1223, 1162, 1021, 577 cm–1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 396.2167 [M − H]− (calcd for C24H30NO4, 396.2175).
Euchrestifoline J (10): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 205 (3.90), 242 (3.99), 293 (4.07) nm; IR (KBr) νmax 3356, 2970, 2920, 2851, 1737, 1722, 1366, 1228, 1216, 1038, 1025, 577 cm–1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 346.1807 [M − H]− (calcd for C23H24NO2, 346.1807).
Euchrestifoline K (11): brown, non-crystalline solid; [α]25D − 12 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 239 (4.26), 288 (4.13) nm; ECD (MeOH) λmax (Δε) 229 (− 0.81) nm; IR (KBr) νmax 3420, 2921, 1706, 1611, 1453, 1163, 1021, 579, 448 cm–1; 1H and 13C NMR data, see Table 2; HRESIMS m/z 346.1802 [M − H]− (calcd for C23H24NO2, 346.1807).
Euchrestifoline L (12): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 216 (3.93), 238 (4.04), 300 (3.65) nm; IR (KBr) νmax 3385, 2919, 2850, 1704, 1637, 1614, 1463, 1316, 1198, 1074, 1019, 1007, 742 cm–1; 1H and 13C NMR data, see Table 3; HRESIMS m/z 226.0867 [M − H]− (calcd for C14H12NO2, 226.0868).
Euchrestifoline M (13): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 208 (3.81), 236 (4.02), 253 (3.87), 271 (3.95), 291 (3.66), 340 (3.55) nm; IR (KBr) νmax 3363, 2973, 2925, 1744, 1710, 1514, 1367, 1253, 1161, 1023, 901, 856, 579 cm–1; 1H and 13C NMR data, see Table 3; HRESIMS m/z 226.0507 [M − H]− (calcd for C13H8NO3, 226.0504).
Euchrestifoline N (14): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 212 (4.07), 236 (4.21), 267 (3.83), 314 (3.76), 329 (3.75) nm; IR (KBr) νmax 3401, 2965, 2917, 1700, 1622, 1469, 1294, 1207, 1140, 1007, 873, 830, 464 cm–1; 1H and 13C NMR data, see Table 3; HRESIMS m/z 280.1337 [M − H]− (calcd for C18H18NO2, 280.1337).
Euchrestifoline O (15): brown, non-crystalline solid; UV (MeOH) λmax (log ε) 212 (4.03), 236 (4.20), 258 (3.90), 309 (3.69), 330 (3.46) nm; IR (KBr) νmax 3385, 2924, 2853, 1701, 1621, 1513, 1466, 1366, 1274, 1162, 1039, 1008, 579 cm–1; 1H and 13C NMR data, see Table 3; HRESIMS m/z 226.0507 [M − H]− (calcd for C13H8NO3, 226.0504).
ECD calculations
The stereochemistry of 1–5 was preliminarily determined based on their NOE correlations and relevant coupling constants. Subsequently, Sybyl-X 2.0 software was utilized for their stochastic conformational search within a 6 kcal/mol energy window with the MMFF94s force field. The geometry was optimized using DFT at the B3LYP/6-31G(d) computational level. TDDFT ECD calculations of 1–5 were performed at either B3LYP/6-31+G(d) or B3LYP/6-311+G(d) level with the PCM (methanol). The ECD spectra were synthesized by fitting all conformational results according to the Boltzmann-calculated contribution in SpecDis v1.51 software with 0.3 eV as the half-bandwidth [32]. The calculated ECD spectra of the relevant diastereomers and enantiomers of 1–5 were directly compared with their experimental ECD spectra. The calculation software is Gaussian 09 [33].
Preparation of the (R)- and (S)-MPA esters of 8
Weighed 1.0 mg of compound 8 and completely dissolved in 0.5 mL of CDCl3. Subsequently, a series of reagents were introduced in sequence: 4-dimethylaminopyridine (0.5 mg), dicyclohexylcarbodiimide (2 mg), and (R)-(+)-α-methyl-α-(trifluoromethyl)-phenylacetyl (MPA) (1.0 mg), and stirred vigorously at room temperature for 16 h to ensure the reaction was complete. Following this, the reaction products were isolated using semipreparative HPLC, with a MeCN-H2O solvent ratio of 70:30 (v/v) at a flow rate of 1.0 mL/min, which allowed for the collection of the (R)-MPA ester (8r) at 12.5 min. Following a similar procedure, the reaction of compound 8 (1.0 mg) with (S)-MPA led to the acquisition of the (S)-MPA ester (8s), also under the identical HPLC conditions, with the retention time of 12.8 min.
Anti-inflammatory activity assay
RAW 264.7 cells line was sourced from Peking Union Medical College (Beijing, China). The procedures for cell cultivation, experimental techniques, and the subsequent analysis and interpretation of data adhere to the methods previously detailed [34]. The positive control was dexamethasone.
Cytotoxicity assay
The cytotoxicity assay was performed in HepG2 cells sourced from Peking Union Medical College (Beijing, P.R. China). The assessment of cytotoxicity was performed utilizing the MTT assay. The experimental manipulations and data analysis were carried out with the protocols reported in the literature [35], and taxol was adopted as a positive control.
Anti-ferroptosis in PC12 cell
PC12 cells were inoculated into 96-well microplates at a concentration of 1 × 104/well and treated with a concentration of 2 μM erastin to induce ferroptosis. The isolates were then added to the cells. After 24 h, the culture medium was removed, and 0.5 g/L MTT was added and incubated in an incubator for 4 h. After addition of DMSO, the optical density was recorded using a microplate spectrophotometer at 570 nm.
Conclusions
In summary, the chemical study of M. euchrestifolia resulted in the identification of 15 novel carbazole alkaloids labelled as euchrestifolines A–O. In a series of activity screens, these compounds showed different biological activities. Especially, compounds 2, 3, 5, 6, 8, 9, 11, and 14 exhibited neuroprotective effects superior to that of the positive control ferrostatin-1 against erastin-induced ferroptosis in PC12 cells, with EC50 values below 1 μM. Moreover, compounds 4a, 4b, 6, and 11–14 showed inhibition of LPS-induced NO production in RAW 264.7 cells with IC50 values spanning from 12.7 to 19.7 μM. For cytotoxicity, the IC50 values of compounds 4a, 5a, 5b, 6, and 8 were below 4.0 μM in HepG2 cells. These results deepen our understanding of the chemical and bioactivity diversity of carbazole alkaloids from Murraya species, and their significant anti-ferroptosis effects suggest a promising future in neuroprotection.
Supplementary Information
Acknowledgements
We express our gratitude to the National Natural Science Foundation of China (NSFC) for their financial support through grant numbers 81973199, 82173949, U23A20514, 81773864, and 81473106. Additionally, we acknowledge the funding provided by the Key Research and Development Project of Shandong Province (2021CXGC010507). Thanks to Mr. Cao Fei from Hebei University for his help in quantum chemical calculations.
Author contributions
CYM isolated and purified compounds and identified them; CNK assisted in the isolation experiments and wrote the original manuscript. ZSS performed a portion of the pharmacological experiments. DM was responsible for the verification and optimization of the manuscript and the delivery of the manuscript. LHZ and ZMB provided assistance in processes such as extraction and isolation and structural characterisation. ZKW and TPF guided the experiments, JY supervised, acquired the funding, and revised the manuscript. All authors above reviewed this manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare that they have no financial conflicts of interest in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Yue-Mei Chen and Nan-Kai Cao have contributed equally to this work.
References
- 1.Editorial Committee of Flora of China. Flora of China, Science Press. Beijing; 1997. p. 139−151.
- 2.Ji XD, Pu QL, Yang GZ. The chemical constituents of essential oil from Murraya euchrestifolia Hayata. Acta Pharm Sin. 1983;18:626–9. [PubMed] [Google Scholar]
- 3.Wu TS, Wang ML, Wu PL, Furukawa H. Carbazole alkaloids from the leaves of Murraya euchrestifolia. Phytochemistry. 1996;41:1433–5. 10.1016/0031-9422(95)00794-6. [Google Scholar]
- 4.Wu TS, Wang ML, Wu PL, Jong TT. Two carbazole alkaloids from leaves of Murraya euchrestifolia. Phytochemistry. 1995;40:1817–9. 10.1016/0031-9422(95)00447-F. [Google Scholar]
- 5.Furukawa H, Wu TS, Kuoh CS. Structures of murrafoline-B and -C, new binary carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull. 1985;33:2611–3. [Google Scholar]
- 6.Furukawa H, Ito C, Wu TS, Mcphail AT. Structural elucidation of murrafolines, six novel binary carbazole alkaloids isolated from Murraya euchrestifolia. Chem Pharm Bull. 1993;41:1249–54. [Google Scholar]
- 7.Knölker HJ, Reddy KR. Isolation and synthesis of biologically active carbazole alkaloids. Chem Rev. 2002;102:4303–428. 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]
- 8.Lv HN, Wen R, Zhou Y, Zeng KW, Li J, Guo XY, et al. Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J Nat Prod. 2015;78(10):2432–9. 10.1021/acs.jnatprod.5b00527. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt AW, Reddy KR, Knölker H. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem Rev. 2012;112:3193–328. 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- 10.Nandy BC, Gupta AK, Mittal A, Vyas V. Carbazole: it's biological activity. J Pharm Biomed. 2014;3:42–8. [Google Scholar]
- 11.Ma XL, Cao NK, Zhang C, Guo XY, Zhao MB, Tu PF, et al. Cytotoxic carbazole alkaloid derivatives from the leaves and stems of Murraya microphylla. Fitoterapia. 2018;127:334–40. 10.1016/j.fitote.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Lv HN, Wen R, Zhou Y, Shi ML, Zeng KW, Xia F, et al. Murradiate and murradiol, two structurally unique heterodimers of carbazole-monoterpene and carbazole-phenylethanol from Murraya tetramera. Phytochem Lett. 2016;15:113–5. 10.1016/j.phytol.2015.12.002. [Google Scholar]
- 13.Zhou Y, Lv HN, Wang WG, Tu PF, Jiang Y. Flavonoids and anthraquinones from Murraya tetramera, C. C. Huang (Rutaceae). Biochem Syst Ecol. 2014;57:78–80. 10.1016/j.bse.2014.07.016. [Google Scholar]
- 14.Uvarani C, Sankaran M, Jaivel N, Chandraprakash K, Ata A, Mohan PS. Palathurai, bioactive dimeric carbazole alkaloids from Murraya koenigii. J Nat Prod. 2013;76:993–1000. 10.1021/np300464t. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty DP. Progress in the chemistry of organic natural products. New York: Springer; 1977. p. 299. [Google Scholar]
- 16.Ramsewak RS, Nair MG, Strasburg GM, DeWitt DL, Nitiss JL. Biologically active carbazole alkaloids from Murraya koenigii. J Agr Food Chem. 1999;47:444–7. 10.1021/jf9805808. [DOI] [PubMed] [Google Scholar]
- 17.Liu WY, Zhang WD, Chen HS, Gu ZB, Li TZ, Zhou Y. Pyrrole alkaloids from Bolbostemma Paniculatum. J Asian Nat Prod Res. 2003;5:159–63. 10.1080/1028602031000066861. [DOI] [PubMed] [Google Scholar]
- 18.Gruner KK, Hopfmann T, Matsumoto K, Jäger A, Katsuki T, Knölker H. Efficient iron-mediated approach to pyrano[3,2-a]carbazole alkaloids–first total syntheses of O-methylmurrayamine A and 7-methoxymurrayacine, first asymmetric synthesis and assignment of the absolute configuration of (–)-trans-dihydroxygirinimbine. Org Biomol Chem. 2011;9:2057–61. 10.1039/C0OB01088J. [DOI] [PubMed] [Google Scholar]
- 19.Knölker HJ, Hofmann C. Transition metal complexes in organic synthesis, part 33. Molybdenum-mediated total synthesis of girinimbine, murrayacine, and dihydroxygirinimbine. Tetrahedron Lett. 1996;37:7947–50. 10.1016/0040-4039(96)01830-8. [Google Scholar]
- 20.Xia GY, Wang M, Chen LX, Ding LQ, Qiu F. Application of dirhodium reagent Rh2(OCOCF3)4 to the determination of the absolute configurations of secondary and tertiary alcohols. J Int Pharm Res. 2015;42:726–33. 10.13220/j.cnki.jipr.2015.06.006. [Google Scholar]
- 21.Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem. 2001;49:5589–94. 10.1021/jf010621r. [DOI] [PubMed] [Google Scholar]
- 22.Hoye TR, Jeffrey CS, Shao F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat Protoc. 2007;2:2451–8. 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- 23.Tao QQ, Ma K, Yang YL, Wang K, Chen BS, Huang Y, et al. Bioactive sesquiterpenes from the edible mushroom Flammulina velutipes and their biosynthetic pathway confirmed by genome analysis and chemical evidence. J Org Chem. 2016;81:9867–77. 10.1021/acs.joc.6b01971. [DOI] [PubMed] [Google Scholar]
- 24.Ito C, Nakagawa M, Wu TS, Furukawa H. New carbazole alkaloids from Murraya euchrestifolia. Chem Pharm Bull. 1991;39:2525–8. 10.1248/cpb.39.2525. [Google Scholar]
- 25.Gassner C, Hesse R, Schmidt AW, Knölker H. Total synthesis of the cyclic monoterpenoid pyrano [3,2-a] carbazole alkaloids derived from 2-hydroxy-6-methylcarbazole. Org Biomol Chem. 2014;12:6490–9. 10.1039/C4OB01151A. [DOI] [PubMed] [Google Scholar]
- 26.Chen YM, Cao NK, Lv HN, Yuan JQ, Guo XY, et al. Anti-inflammatory and cytotoxic carbazole alkaloids from Murraya kwangsiensis. Phytochemistry. 2020;170: 112186. 10.1016/j.phytochem.2019.112186. [DOI] [PubMed] [Google Scholar]
- 27.Dhara K, Mandal T, Das J, Dash J. Synthesis of carbazole alkaloids by ring-closing metathesis and ring rearrangement–aromatization. Angew Chem Int Edit. 2015;54:15831–5. 10.1002/anie.201508746. [DOI] [PubMed] [Google Scholar]
- 28.Bringmann G, Tasler S, Endress H, Peters K, Peters E. Synthesis of mukonine and seven further 1-oxygenated carbazole alkaloids. Synthesis-Stuttgart. 1998;10:1501–5. 10.1055/s-1998-2184. [Google Scholar]
- 29.Ito C, Itoigawa M, Nakao K, Murata T, Tsuboi M, Kaneda N, et al. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine. 2006;13:359–65. 10.1016/j.phymed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Li CH, Zhou Y, Tu PF, Zeng KW, Jiang Y. Natural carbazole alkaloid murrayafoline A displays potent anti-neuroinflammatory effect by directly targeting transcription factor Sp1 in LPS-induced microglial cells. Bioorg Chem. 2022;129: 106178. 10.1016/j.bioorg.2022.106178. [DOI] [PubMed] [Google Scholar]
- 31.Tan QY, Wu DY, Lin YT, Ai HP, Xu J, Zhou HB, et al. Identifying eleven new ferroptosis inhibitors as neuroprotective agents from FDA-approved drugs. Bioorg Chem. 2024;146: 107261. 10.1016/j.bioorg.2024.107261. [DOI] [PubMed] [Google Scholar]
- 32.Fan K, Zhang LC, Tan BY, Njateng GSS, Qin ML, Guo RR, et al. Antimicrobial indole alkaloids from Tabernaemontana corymbosa. Chin J Nat Med. 2023;21:146–53. 10.1016/S1875-5364(23)60393-0. [DOI] [PubMed] [Google Scholar]
- 33.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009.
- 34.Chen D, Xu ZR, Chai XY, Zeng KW, Jia YX, Bi D, et al. Nine 2-(2-phenylethyl) chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 cells. Eur J Org Chem. 2012;2012:5389–97. 10.1002/ejoc.201200725. [Google Scholar]
- 35.Ma K, Wang JS, Luo J, Yang MH, Kong LY. Tabercarpamines A-J, apoptosis-inducing indole alkaloids from the leaves of Tabernaemontana corymbosa. J Nat Prod. 2014;77:1156–63. 10.1021/np401098y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.