Abstract
Coconut oil is eatable oil with many nutritional and cosmetic applications. In this investigation coconut oil was subjected to 0 to 5 L/min of ozone for 3 h and the chemical composition of both crude and ozonized oil was valued via Gas Chromatography-Mass Spectrometry (GC–MS). Some biological tests were done including antibacterial action versus Helicobacter pylori, anti-biofilm activity versus H. pylori, anti-hemolytic activity in the existence of H. pylori, anti-Alzheimer action, and cytotoxic effect towards A-413 cancer cell line to determine the activity of coconut oil and upon exposure to ozone. Fifteen compounds were detected in the coconut oil crude and ozonized oils where the fatty acid esters were the most common molecules in crude coconut oil, whereas alkenes were the most predominant compounds in ozonized coconut oil. A slight elevation of antibacterial action towards H. pylori from 23.0 ± 0.1 to 28.2 ± 0.5 mm was displayed upon exposure of the coconut oil to ozone. Both crude and ozonized coconut oil showed a bactericidal effect with MICs = 62.5 ± 0.1, 125.0 ± 0.2 µg/mL and MBCs = 15.62 ± 0.2, 31.25 0.2 µg/mL for crude and ozonized oil, respectively. A significant elevation in anti-biofilm activity was found upon using 25% of MBCs of ozonized oil relative to crude oil. A dramatic rise was observed in anti-hemolytic activity upon using 25 and 75% of MICs of ozonized oil relative to crude one. A notable elevation of anti-Alzheimer impact was evident upon exposing coconut oil to ozone. Besides, the cytotoxic impact towards A-431 cells was slightly increased after exposing the oil to ozone. The current results suggest a new technique to expose coconut oil to ozone to improve some of its in vitro pharmaceutical applications.
Keywords: Coconut, Ozone, H. pylori, Gas chromatography, Anti-hemolytic, Anti-Alzheimer
Introduction
Natural products have traditionally used as significant pharmaceuticals in a multiplicity of therapeutic topics (Qanash et al. 2022; Bakri et al. 2024). Examining various compounds libraries is seen to be a viable method for finding new candidates for the development of innovative medications (Alawlaqi et al. 2023; Alsalamah et al. 2023). Recent advancements in numerous scientific domains have prompted an upsurge of interest in natural product evaluation (Almehayawi et al. 2024; Qanash et al. 2024; Rao et al. 2024). These incorporate not only the use of bioinformatics research, genetics, and genomic mining, but also the exploration of novel environmental niches and development of new methodologies for extracting, investigating and modifying the chemical composition of substances for beneficial uses (Privalsky et al. 2021; Barth et al. 2024).
Helicobacter pylori has a significant effect on communities as well as the global economy. It is imperative that we develop a successful strategy to combat H. pylori to prevent possible associated conditions (Yahya et al. 2022; Qanash et al. 2023a). Natural products are desirable as compatible and substitute therapies because of their lesser harmful effects and less costs; natural substances have long been considered essential prospective for anti-H. pylori therapies (Al-Rajhi et al. 2023a, b).
Alzheimer’s is a degenerative condition that starts with minor memory impairment, impacting regions of the brain responsible for thinking, remembering, and language. This can significantly hinder an individual's capacity to perform everyday tasks (Tomášková et al. 2016). Although there is no known remedy for Alzheimer's, there are medications besides non-pharmacological therapies that may slow the disease's progress and alleviate its signs (Lima and Medeiros 2024). Several natural products were applied in management of Alzheimer's illness (Adedayo et al. 2020; Al-Rajhi et al. 2023a, b).
Oil of coconut, which is prepared from Cocos nucifera tree, is widely employed throughout many regions of the world for edible and commercial purposes. The traditional method of producing coconut oil in West Africa consists of grinding and compressing copra to release the oils (Boateng et al. 2016). This is carried out in sizable mills, and the oil is sold openly. The amazing nutritional and functional qualities of coconut oils can be used to the benefit of underdeveloped nations in Africa. For a very long time, West African communities have used coconut oil as a main source of dietary fat and as a medicine to treat various ailments (Yang et al. 2024). Many nations currently sell ozonized oil, although there is not much data accessible about its chemical composition, and biological properties. Characterization and classification of ozonized vegetable oils depend on an understanding of their physical and chemical characteristics (Ugazio et al. 2020; Radzimierska-Kaźmierczak et al. 2021). The ionization process is monitored and the efficacy of the ozonized vegetable oils is assessed using analytical techniques such as acidity and iodine levels (Díaz et al. 2006). GC–MS which has been applied to analyze both unsaturated and saturated fatty acids in oils from vegetables, is the most common method for analyzing fatty acids in organic substances (Al-Rajhi and Abdel Ghany 2023; Qanash et al. 2023b). In this study, the GC–MS testing was used to determine the constituents of the oils both before and after treatment by ozone. The current word was aimed to investigate the activities of the crude and ozonized coconut oils including anti-H. pylori, anti-biofilm, hemolytic activity, anti-Alzheimer, and cytotoxicity towards epidermoid carcinoma (A431) cell line.
Materials and methods
Ozonation of the coconut oil
Ozone gas was produced using an electrical barrier shock plasma reactor at faculty of the science Al-Azhar University. A 2.0 L drechsel container containing 1.0 L of coconut oil was submerged in a −4 °C chilling bath at the output of the plasma reactor. The ozone was bubbled in the coconut oil for 3 h at a rate of 0 to 5 L/minute, resulting in a semisolid form. The coconut oil was removed from the drechsel container after ozonation, transferred to an empty glass container, measured, and kept at 5 °C for storage (Elvis and Ekta 2011; Khalifa et al. 2022).
Gas Chromatography-mass spectrometry (GC–MS) for the crude and ozonized oil
GC–MS testing was done out through an auto-sampler (570-Thermo-fisher, USA), Rt-570 column (100.0 m × 0.26 mm × 0.21 µm; Thermo-Fisher, USA), and flame-ionization detector. Chromatography system (X-caliber data acquisition and software, Thermo-Fisher, USA) was implemented for details getting from the FID. Helium was applied as the transporter gas with rip administration (100:1). The Pre-run time out 10 min. The Equilibration time 0.5 min. 2 Ramps were done where, ramp 1 rate: 3°C/min ant its final temperature was 200 °C, while: ramp 2 rate:3°C/min. The Initial heat was 45°C ant its final temperature was 280 °C. The evaluations were performed out in systemized heat mode start at 100 to 280 °C and followed by isothermal for 14.0 min. The carrier flow 1.5 ml/min (Radzimierska-Kaźmierczak et al. 2021).
Antimicrobial action
To test the influence of both crude and ozonized coconut oil on H. pylori, a strain of H. pylori (ATCC 43504) was generously given by Al-Azhar University's Microbiology Department via prof. Tarek Abdelghany. Using the well agar diffusion procedure, the in vitro anti-H. pylori capabilities were done. In summary, 100 μL of H. pylori solution (1.0 × 108 CFUs/ml) was applied to Mueller Hinton agar dishes that had 10.0% sheep blood in them. Next, a 6 mm size circular cut was aseptically punched employing a sterile cork borer. Next, a volume (100 µL) of the samples at the specified dose was added to the well. Antibiotics namely amoxicillin (0.05 mg/mL) and clarithromycin (0.05 mg/mL) were utilized as positive controls, while DMSO served as the non-effective negative control. The dimension of the inhibitory area was tested following a 72-h incubation period at 36°C in a microaerophilic container with humid conditions (Santiago et al. 2022).
Evaluation for minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC)
The micro-dilution broth procedure was applied to test MIC of the specimens being studied by employing nutritional broth for bacterial. To find their final levels, which varied from 0.98 to 1000 µg/mL, the specimens under inquiry were diluted twice. 200 µl of the specimen dilutions under examination in broth medium were added to each well of the 96-well micro-titrate plate for preparing it. Following the generation of the inoculum using fresh bacterial cultures that met the turbidity standards of the 1.0 McFarland standard, 2.0 µL of sterile 0.9% NaCl was put to each well to achieve level of 3.0 × 106 CFU/ml. H. pylori was then incubated for 72 h at 36°C. The minimum inhibitory concentration (MICs) were detected by visually measuring the specimens’ levels at which the standard strain's growth was completely inhibited. On each microplate, there was a positive control (an inoculum without the investigated specimens) and a negative control (tested specimens without an inoculum) (Huang et al. 2021).
MBC was estimated by sub-culturing 100 ml of the bacterial culture onto Mueller–Hinton agar developed with 10% blood plates/ well with total growth suppression, from the final positive, and from the development control. Following a 72-h incubation period at 35°C, the MBC was found to be the lowest concentration of specimens that did not promote microbial growth. To ascertain whether the tested had a bactericidal or bacteriostatic action that prevented microbial growth, the MBC/MIC ratios were computed (Huang et al. 2021).
Anti-biofilm action
The samples' impact on the development of biofilm was judged using 96-well polystyrene flat bottom dishes. By adding 280 µL of freshly seeded trypticase soy yeast broth (TSY) to every single well of a microplate, a ultimate level of 106 CFU/mL was ascertained. Then, the microplate was cultivated in MBC at the 75, 50, and 25% sub-lethal levels that were already established. As controls, wells with medium and those with just alcohol and no specimens were utilized. For 48 h, dishes were kept at 36°C. Following the discarding of the supernatant, each well's free-floating cells were thoroughly cleaned using sterile distilled water. The biofilm that had progressed was colored for fifteen minutes at room temperature operating a water-based solution of 0.10% crystal violet after the dishes were allowed to air dry for thirty minutes. Following the incubation procedure, the extra color was again eliminated by rinsing with sterile distilled water a further three times. Afterwards 15 min of incubation and the incorporation of 250 µL of 96% ethanol to the wells in order to ultimately destroy the dye attached to the cells, absorbance was measured at 560 nm using a microplate reader (Jeong et al. 2021).
Anti-hemolytic activity
To measure the hemolysis activity of samples treated with bacteria in sub-MIC (25% and 50% of MIC). After adjusting the cultures of tested bacteria to an OD600 of 0.40, they were centrifuged at 22,000×g for 15 min, using a 25%, 50%, and 75% MIC besides untreated cultures. The fresh erythrocyte suspension (2.0%) in 0.9 mL saline was mixed with 500 µL of supernatants, and the mixture was incubated for two hours at 35°C. The mixture was then rotated at 12,000×g for fifteen minutes at 5°C. A negative control of un-hemolyzed erythrocytes was formed by incubating erythrocytes in LB broth (Sigma, USA) under the same conditions as the positive control, which was produced via addition of 0.1% Sodium Dodecyl sulphate to an erythrocyte suspension. Measurement of absorbance at 550 nm was operated to gauge hemoglobin production (Mogrovejo et al. 2020).
Testing for anti-Alzheimer impact
Ten mg/10 ml of each component were mixed in a solution of phosphate buffer (pH 7.5), resulting in 100 µg/mL at the end. Earlier every test, the stock solutions were diluted by a 20 mM sodium phosphate buffer solution (pH 7.6) to a range of concentrations. The DTNB-phosphate-ethanol reagent: adding 50 mL of 0.1 mM phosphate buffer (pH 7.6) to 12.7 mg of DTNB dissolved in 120 mL of 96% ethanol. Ten microliters of the tested specimens in 0.3% DMSO, seventy-nine microliters of 20 mM sodium phosphate buffer (pH 7.6), and one microliter of the enzyme solution (final concentrations of 0.196 to 100 µg/ml for the compounds studied, and 0.3 units/mL for BChE) were combined and kept for fifteen minutes. After adding 10 μL of the substrate solution—final butyrylthiocholine iodide level: 4 mM—to the combination, it was incubated for 30 min. By putting 900 μL of DTNB-phosphate-ethanol, the process was stopped. The absorbance was determined using a reader for microplates at 410 nm (Cai et al. 2019).
Anti-proliferative activity
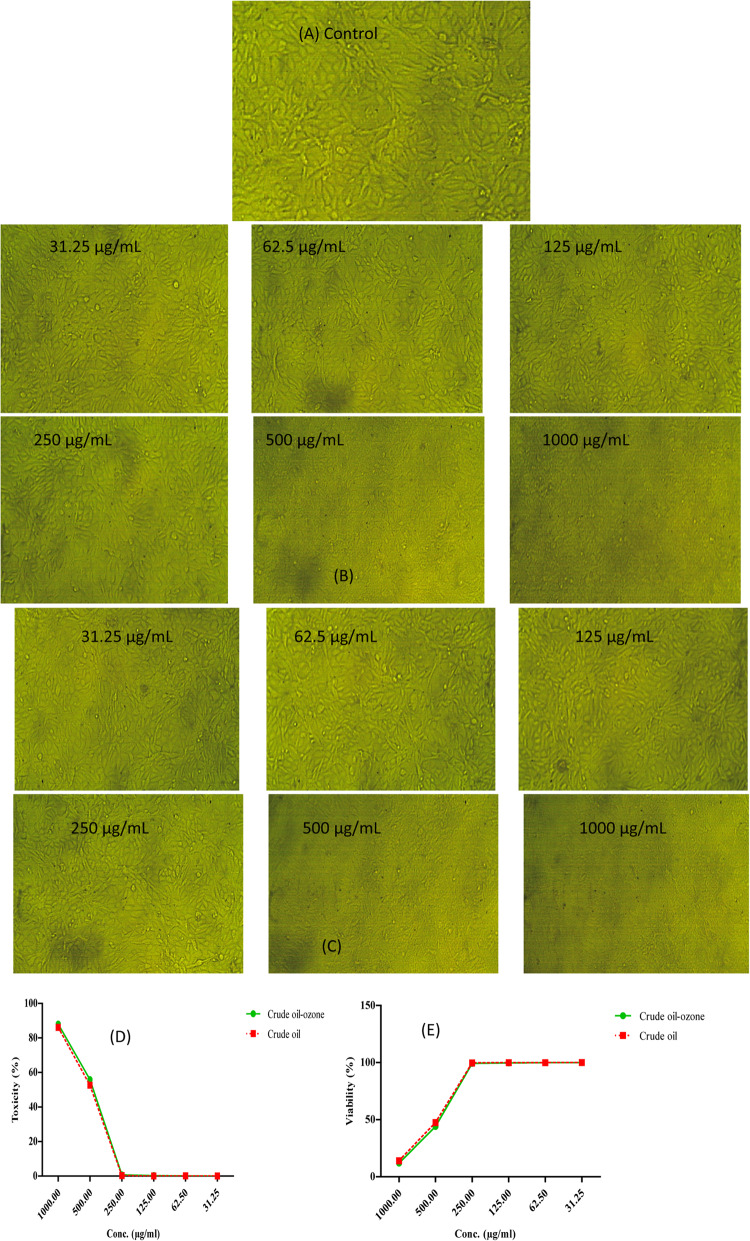
The cytotoxic impact of ozonized and crude oil on A-431 cells (cell type: epithelial; disease: epidermoid carcinoma) was detected by MTT procedure after the oil was dissolved in DMSO. Using conventional levels, the result is a blue color whose values directly connected with the quantity of living cells. At 560 nm, the absorbance was measured using a computerized microplate analyzer (Tecan Life Science Infinite F50, USA). The cells were put at 35°C for a further 24 h after the samples in levels from 1000 to 31.25 µg/mL was added after 24 h of attachment till merging. 100 µL of MTT solution (5.0 mg/mL) was incorporated after the fresh medium was supplied, and it was left at 35°C for four hours. A microscope (OMAX, USA) connected with a CCD camera to observe the cells (Examinati et al. 2008).
Statistical testing
Each experiment has been run three times, and the outcomes are shown as the average ±SD. The t-test was performed to evaluate the difference between means using Graph Pad Prism V5 (San Diego, CA, USA) software. Findings with p < 0.05 were referred to substantial change.
Results
Various molecules in crude and ozonized coconut oil using GC–MS
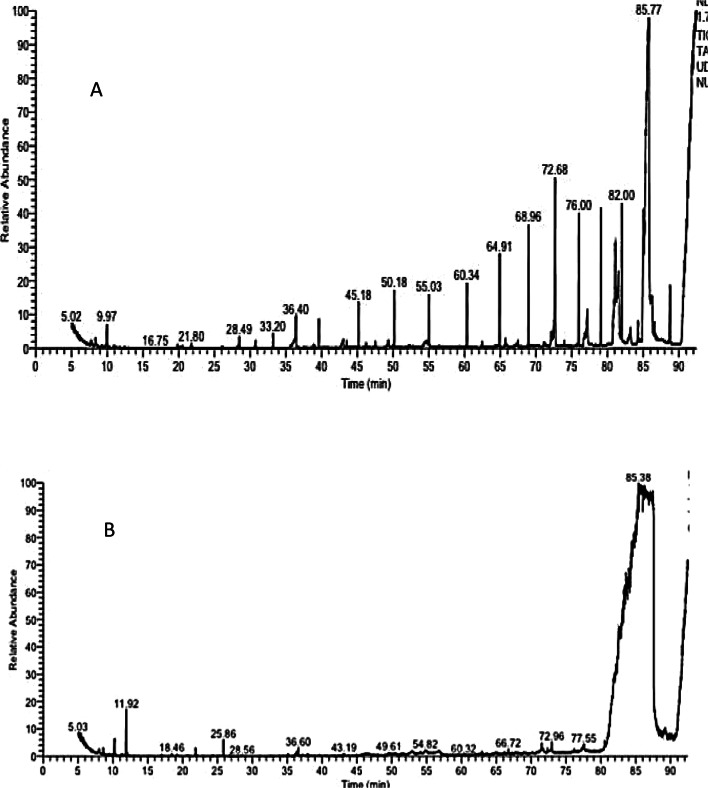
The investigation of crude coconut oil by GC–MS shown the occurrence of 15 compounds which consisted of seven fatty acids esters including: 2-(Decanoyloxy) propane-1,3-diyl dioctanoate, Glyceryl trilaurate, 1-(hydroxymethyl)-1,2-ethanediyl ester, Decanoic acid, 2-[(1-oxooctyl)oxy]-1,3-propanediyl ester, Decanoic acid, 1-[[(1-oxooctyl)oxy]methyl]-1,2-ethanediyl ester, 2-Lauroylgylcerol and Glyceryl 2-caprate dicarpylate; (Three alkenes) which were: Cyclohexane, nitro, Cyclohexane, bromo and 1-Butene, 2,3,3-trimethyl; (one alkene hydrocarbon) which was: Tetradecane; (One fatty acid) which was: Dodecanoic acid, (One pytosterol) which was ç-Sitosterol, (one alcohol) which was: 1,5-Heptadien-4-one, 3,3,6-trimethyl, (one glyceride) which was: 1-Dodecanoyl-3 myristoylglycerol as shown in (Fig. 1a, Table 1). While, testing ozonized coconut oil by GC–MS revealed the existence of fifteen compounds as well which were: (Four Alkene) which were: Pentane, 2,2-dimethyl, Cyclohexane, nitro, 1,8-Cineole 1,8-Epoxy-p-menthane cajeputol 1,8-epoxy-p-menthane, Hexadecane; (one hydroxyl-dicarboxylic acid) which were: D-(-)-Citramalic acid; (Three fatty acid ester) which were: Decanoic acid, 1-[[(1-oxooctyl)oxy]methyl]-1,2-ethanediyl ester, Glyceryl trilaurate and 9-Octadecenoic acid (Z)-, oxiranylmethyl ester; (two fatty acids) which were: Dodecanoic acid, Tetradecanoic acid; (two methane monoterpenoids) which were: Terpinen-4-ol, Alpha-terpinyl acetate; (one terpenoid) which was caryophyllene oxide; (one α, β-unsaturated aldehyde) which was: Cinnamaldehyde, (E)-; and (one diarylmethane) which was: p-Cresol, 2,2'-methylenebis[6-tert-butyl-as depicted in (Fig. 1b, Table 1).
Fig. 1.
GC- MS showing separation of various compounds in (A) Crude coconut oil, (B) Ozonized coconut oil
Table 1.
Different separated compounds in crude and ozonized coconut oils
| RT | Peak name | Molecular weight | Molecular formula | Peak area % | *RT (O3) | Peak name | Molecular weight | Molecular Formula | Peak area % |
|---|---|---|---|---|---|---|---|---|---|
| 5.02 | 1-Butene, 2,3,3-trimethyl- | 98 | C7H14 | 2.055 | 5.03 | Pentane, 2,2-dimethyl | 100 | C7H16 | 0.944 |
| 9.97 | Cyclohexane, nitro- | 129 | C6H11NO2 | 2.096 | 8.60 | D-(-)-Citramalic acid | 148 | C5H8O5 | 0.826 |
| 16.75 | 1,5-Heptadien-4-one, 3,3,6-trimethyl | 152 | C10H16O | 0.610 | 10.24 | Cyclohexane, nitro- | 129 | C6H11NO2 | 1.7936 |
| 21.80 | Cyclohexane, bromo | 162 | C6H11Br | 0.814 | 11.92 | 1,8-Cineole 1,8-Epoxy-p-menthane cajeputol 1,8-epoxy-p-menthane | 154 | C10H18O | 4.956 |
| 28.49 | Tetradecane | 198 | C14H30 | 0.814 | 18.46 | Terpinen-4-ol | 154 | C10H18O | 0.472 |
| 36.40 | Dodecanoic acid | 200 | C12H24O2 | 2.442 | 21.87 | Cinnamaldehyde, (E)- | 132 | C9H8O | 0.826 |
| 80.80 | Glyceryl 2-caprate dicarpylate | 498 | C29H54O6 | 0.936 | 25.86 | Alpha-terpinyl acetate | 196 | C12H20O2 | 1.475 |
| 81.10 | Glyceryl trilaurate | 638 | C39H74O6 | 10.177 | 35.10 | Caryophyllene oxide | 220 | C15H24O | 0.236 |
| 81.58 | 2-Lauroylgylcerol | 484 | C29H56O5 | 12.823 | 36.4 | Dodecanoic acid | 200 | C12H24O2 | 0.472 |
| 84.29 | ç-Sitosterol | 414 | C29H50O | 1.933 | 36.6 | Hexadecane | 226 | C16H34 | 0.5428 |
| 85.34 | Decanoic acid, 2-[(1-oxooctyl)oxy]-1,3-propanediyl ester | 526 | C31H58O6 | 3.460 | 43.19 | Tetradecanoic acid | 228 | C14H28O2 | 0.177 |
| 85.78 | Decanoic acid, 1-[[(1-oxooctyl)oxy]methyl]-1,2-ethanediyl ester | 526 | C31H58O6 | 45.552 | 49.61 | Decanoic acid, 1-[[(1-oxooctyl)oxy]methyl]-1,2-ethanediyl ester | 526 | C31H58O6 | 0.3422 |
| 86.26 | 1-Dodecanoyl-3-myristoylglycerol | 484 | C29H56O5 | 4.070 | 54.58 | Glyceryl trilaurate | 638 | C39H74O6 | 1.4986 |
| 90.72 | 1-(hydroxymethyl)-1,2-ethanediyl ester | 581 | C37H72O4 | 0.610 | 60.32 | p-Cresol, 2,2'-methylenebis[6-tert-butyl- | 340 | C23H32O2 | 0.3422 |
| 91.23 | 2-(Decanoyloxy)propane-1,3-diyl dioctanoate | 493 | C29H54O6 | 11.601 | 64.83 | 9-Octadecenoic acid (Z)-, oxiranyl-methyl ester | 338 | C21H38O3 | 85.196 |
*RT, Retention time
Assessment of the impact of ozone on anti-H. pylori of coconut oil
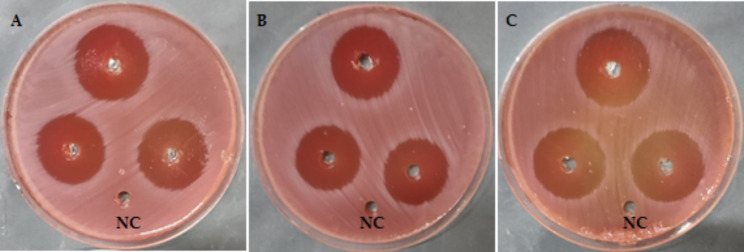
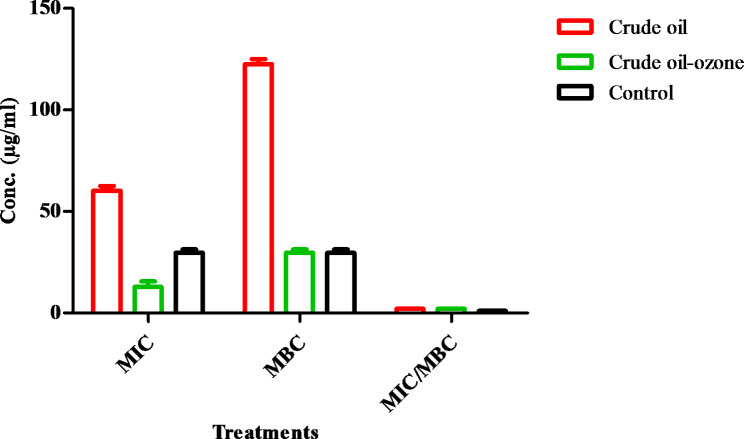
The antibacterial activity of standard drugs, crude coconut oil, and ozonated coconut oil against H. pylori was evaluated. The inhibition zone of standard drugs versus H. pylori was 24.3 ± 0.6 mm, whereas the crude coconut oil showed slightly lower anti-H. pylori with inhabitation diameter of 23.0 ± 0.1 mm. Finally, exposure of coconut oil to ozone increased the anti- H. pylori inhibition zone to 28.2 ± 0.5 mm as depicted in (Fig. 2). The MIC and MBC values of the standard drug detected as 31.25 µg/mL revealed its bactericidal impact versus H. pylori. Besides, the MIC value of crude coconut oil was 62.5 µg/mL and its MBC versus H. pylori was 125 µg/mL. On the other hand, the MIC value of ozonized coconut oil was 15.62 µg/mL, and its MBC was 31.25 µg/mL revealing its bactericidal action of both crude and ozonized coconut oil as shown in (Table 2, Fig. 3).
Fig. 2.
Antimicrobial action of (A) Standard drug; (B) Crude coconut oil; and (c) Ozonized coconut oil towards H. pylori (Data are illustrated as means ± SD)
Table 2.
Anti-H. pylori with MIC and MBC results of crude and ozonized coconut oil. (The outcomes were tabulated as means ± SD); The MBC/MIC of the specimens ≤ 4 proposed their bactericidal impact towards H. pylori
| Treatments | Inhibition area (mm) | MIC (µg/mL) | MBC (µg/mL) | MIC/MBC Index |
|---|---|---|---|---|
| Standard drug* | 24.3 ± 0.6 | 31.25 ± 0.1 | 31.25 ± 0.1 | 1 |
| Crude oil | 23.0 ± 0.1 | 62.5 ± 0.1 | 125.0 ± 0.2 | 2 |
| Ozonized oil | 28.2 ± 0.5 | 15.62 ± 0.2 | 31.25 ± 0.1 | 2 |
*Standard drug (0.05 mg/ml clarithromycin), Index > 4 showed their bacteriostatic action
Fig. 3.
Comparison of MIC, MBC and MIC/MBC upon using crude and ozonized oil of coconut oil relative to control (clarithromycin) (outcomes are drawn as means ± SD)
Evaluation of the role of ozone on anti-biofilm action of coconut oil
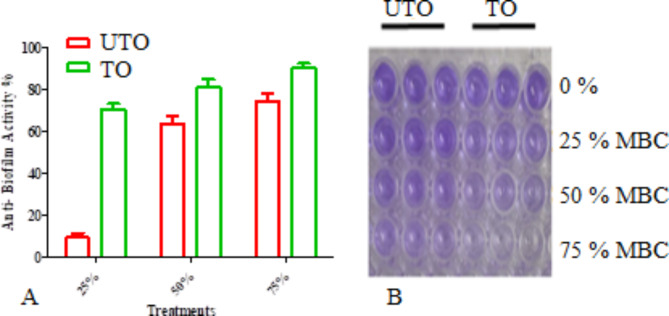
Anti-biofilm impact activity (%) of cured and ozonized coconut oil towards H. pylori were examined using (25, 50 and 75%) of MBC. There was a significant difference of anti-biofilm activity of cured and ozonized coconut oil (P≤0.05) upon using 25% of MBC towards H. pylori. While, upon using 50% of MBC of crude and ozonized coconut showed activities = 67% and 84% respectively (Fig. 4). Lastly, using 75% of MBC of crude and ozonized coconut had activities = 78 and 92% consecutively as shown also in (Fig. 4).
Fig. 4.
Anti-biofilm action (%) towards H. pylori upon applying 25, 50 and 75% of MBC of crude (UTO) and ozonized (TO) coconut oil (results are illustrated as means ± SD) (A) with the stained plate
Detection of the effect of ozone on anti-hemolytic activity
The released hemoglobin was evaluated upon using 25, 50 and 75% of MIC of crude and ozonized coconut oil was relative to the positive control. There was substantial difference (P≤0.05) of activity upon using 25 and 75% crude and ozonized coconut oil. While, a slight difference of activity could be seen upon using 50% of crude and ozonized coconut oil which were 5.4±0.2 and 4.9±0.5% consecutively as shown in (Fig. 5).
Fig. 5.
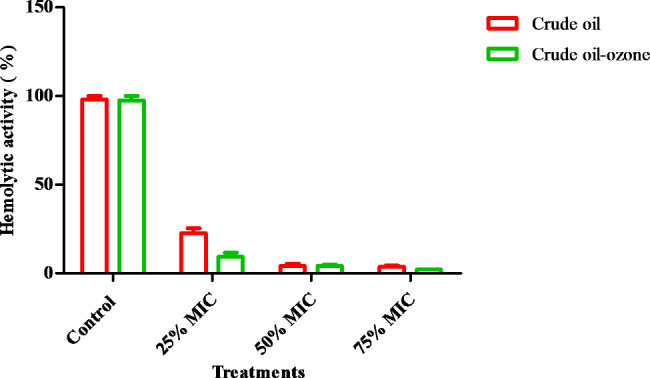
Anti-hemolytic action upon applying 25, 50 and 75% of MIC of crude and ozonized coconut oil (results are illustrated as means ± SD)
Assessment of the impact of ozone on anti-Alzheimer action
The butylcholinesterase (BuChE) inhibition activity of both crude coconut oil and upon exposure to ozone was determined. There was a substantial rise (P≤0.05) in the action after exposing the coconut oil to ozone, Where IC50 for crude, ozonized and standard were 9.33±0.3, 2.59±0.2, and 0.51±0.1 µg/mL respectively (Figure 6).
Fig. 6.
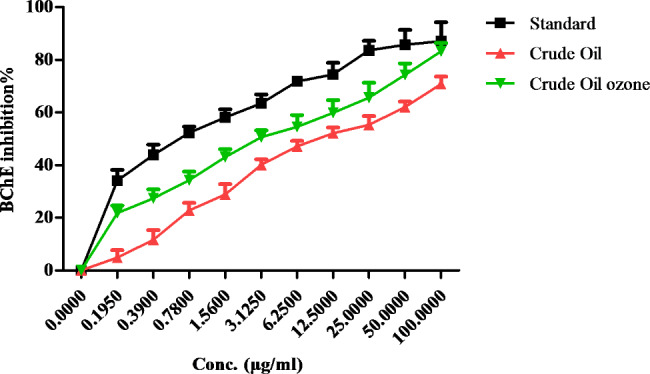
Anti-Alzheimer of crude and ozonized coconut oil compared to rivastigmine as a standard drug (results are illustrated as means ± SD)
3.6 Determination of the role of ozone on anti-proliferative activity
The cytotoxic impacts of crude and ozonized coconut oil were tested using MTT assay towards A431 cells and it could be seen that upon using different concentrations from 1000 to 31.25 of the oil to ozone slightly elevate its cytotoxic impact where IC50= 484.96 ± 5.4 and 467.11 ± 4.15 µg/mL for crude and ozonized oil respectively as shown in Table 3 and Fig. 7.
Table 3.
Determination of cytotoxic effect of different coconut oil and after exposing to O3 on A431 cells (Data are tabulated as means ± SD)
| Treatment | Dose (µg/mL) | Mean O.D | ± SE | Viability % | Toxicity % |
|---|---|---|---|---|---|
| Ozonized | 1000 | 0.088667 | 0.002333 | 11.97 | 88.03 |
| 500 | 0.326667 | 0.00441 | 44.08 | 55.92 | |
| 250 | 0.735667 | 0.001453 | 99.28 | 0.72 | |
| 125 | 0.739 | 0.000577 | 99.73 | 0.27 | |
| 62.5 | 0.740333 | 0.000333 | 99.91 | 0.09 | |
| 31.25 | 0.740667 | 0.001202 | 99.96 | 0.04 | |
| 0.0 | 0.741 | 0.000577 | 100 | 0 | |
| IC50 | 467.11 ± 4.15 µg/mL | ||||
| Control | 1000 | 0.088667 | 0.002333 | 11.97 | 88.03 |
| 500 | 0.326667 | 0.00441 | 44.08 | 55.92 | |
| 250 | 0.735667 | 0.001453 | 99.28 | 0.72 | |
| 125 | 0.739 | 0.000577 | 99.73 | 0.27 | |
| 62.5 | 0.740333 | 0.000333 | 99.91 | 0.09 | |
| 31.25 | 0.740667 | 0.001202 | 99.96 | 0.04 | |
| 0.0 | 0.741 | 0.000577 | 100 | 0.0 | |
| IC50 | 484.96 ± 5.4 µg/mL | ||||
Fig. 7.
(A) A regular monolayer A431 control cells; (B) A431 cells treated by various levels of crude coconut oil and (C) A431 cells subjected to different concentrations of ozonized coconut oil. (D) Comparing between impact of crude coconut oil and after exposing to O3 on toxicity % towards A-431 cells at different levels of oils. (E) Comparing between impact of crude coconut oil and after exposing to O3 on viability % of A431- cells at different concentrations of oils
Discussion
Ozonized oils are gaining more attention from scientists and being used in medical centers as a result of the ongoing search for potent biomedical therapies that can treat illnesses with manageable negative consequences (Pietrocola et al. 2018). Vegetable oils contain unsaturated triacylglycerides that may combine with ozone which consider a potent oxidizing agent (Boland-Nazar et al. 2016). At ambient temperature, all of the ozonides produced through vegetable oil ozonation are liquid or semisolid, and it is necessary to determine whether or not the quality of them will be sufficient for use under typical conditions for preservation (Cirlini et al. 2012; Moureu et al. 2016).
In the our study, ozonation has been done using a designated system to produce a flow rate of ozone of 0 to 5 L/minute of ozone and the crude coconut oil has been treated through this protocol. According to many reports, a number of factors affect the general efficacy of ozonated derivatives, including: (i) the kind and caliber of ozone producers; (ii) the ozonation circumstances, including reactor type and duration, substance kind and quantity, existence of water and/or catalysts; and (iii) the ozonizer's effectiveness, including output O3 level, oxygen supply, and oxygen transporter (Napolitano et al. 2004; Kim et al. 2009; Jacinto et al. 2023). From the published investigation, the oxygen for medical purposes is quite effective in ozonation of sunflower oil (Díaz et al. 2012). Longer ozonation reaction durations are associated with larger degrees of unsaturation in the interaction between ozone and carbon–carbon double bonds (Moureu et al. 2015). This aspect undoubtedly affects the ozonation kinetics as a sequence of the source of the green oils and the chemical structure of their fatty acids (De Almeida et al. 2016).
In the present study exposure to crude coconut oil to ozone led to alteration of the chemical composition of the oil which contained seven fatty acids esters, three alkenes, one alkene hydrocarbon, one fatty acid, one glyceride in crude cocnut oil to ozonized oil which contained four Alkene, one hydroxyl-dicarboxylic acid, three fatty acid ester, two fatty acid, two methane monoterpenoids, one terpenoid and one α, β-unsaturated aldehyde and one diarylmethane. Using GC-MS investigations of the volatile portion of oil exposed to ozone, researchers examined the breakdown of fatty acid ozonides; they discovered degradation products include furyl derivatives, saturated and unsaturated aldehydes, and carboxylic acids. However, it is important to keep in mind that glyceryl oil-based ozonides can be impacted by light and/or humidity, which can facilitate a number of disintegration processes, the main one leading to the creation of aldehydes (Sega et al. 2010; Guerra et al. 2015; Ozturk et al. 2017).
In this investigation exposing coconut oil to ozone led to a slight increase in anti-H. pylori impact. Many studies illustrated the possibility of using crude coconut oil as adjuvant therapy in animals infected with H. pylori and developed gastric ulcer [Selvarajah et al. 2015; Meng et al. 2019). It has been shown that adding ozone can improve the qualities of oils and its antimicrobial actions (Martínez et al. 2005). Ozone's stronger oxidizing qualities, which cause bacterial outer structures to break down, and become more permeable and allow ozone to enter (Zeng and Lu et al. 2018; Borges et al. 2017).
The exposing of coconut oil to ozone led to intensify the anti-biofilm role of the oil towards H. pylori. Unique and potent antimicrobial medications for the management of biofilms have been appeared in response to the growing crisis of antibiotic resistance and formation of biofilms of bacteria. Through the application of oxidizing chemicals that are effective towards microorganisms without causing antibiotic resistance, new and exciting methods for treating biofilms have emerged. Ozoneated derivatives, which are produced when ozone reacts with unsaturated substances like oil, offer a wide range of uses because of the high volatility of ozone as a gas (Cao et al. 2017; Song et al. 2018; Ng et al. 2024).
Some studies reported the anti-hemolytic potential of virgin coconut oil (Hmidani et al. 2021; Utarı et al. 2022). In this work ozonized coconut oil had a better anti-hemolytic pattern through prevention of oxidative impact to erythrocytes. It has been reported that the human body's erythrocyte cells are the ones most frequently used in medication transfer. Activated oxygen species is primarily promoted by hemoglobin and polyunsaturated fatty acids, which are oxidant active transporter molecules and erythrocyte-targeting agents. A number of conditions, including, oxidative medications, excess transition metals, and impairments in erythrocyte antioxidant coordination, may contribute to hemolysis, which is caused by oxidative damage to the lipids and proteins that make up the erythrocyte membrane (Hamidi and Tajerzadeh 2003; Ebrahimzadeh et al. 2009; Afsar et al. 2016).
The mental state of humans in areas critical to cognition and behavior—functions weakened in Alzheimer's disease (Tomášková et al. 2016). BChE is primarily expressed in white matter, glia, and different populations of neurons. There is neither a treatment for AD, a neurodegenerative disease that causes dementia. The use of coconut oil to avoid or manage Alzheimer's disease is not well-supported by scientific research. However, some research indicates that include coconut oil in daily food may worsen the symptoms of certain illnesses (Chatterjee et al. 2020; Thawkar and Kaur 2024). The present work illustrated the in vitro impact of coconut oil as anti-Alzheimer agent which has been improved upon exposure of the oil to ozone. Many studies showed the anticancer impact of coconut oil (Verma et al. 2019; Alotaibi et al. 2023). In this work the exposing of coconut oil to ozone improve its anticancer activity towards A431 cells. In accordance with a previous report by Al-Rajhi and Abdel Ghany (2023) that showed treatment of oils with ozone enhance its anticancer impact. Finallly this investigation showed that exposing coconut oil to a flow rate of ozone of 0 to 5 L/minute for 3 hours alter its chemical composition and leading to enhance its antibacterial and biofilm capability versus H. pyloi, as well as improve its anti-hemolytic activity, anti-Alzheimer impact as well as it cytotoxic effect towards A431 cells to be applied and verification using animal studies. Future studies should focus on seperating active constituents of coconut oil after treatment by ozone along with studying the ultrastructure of H. pyloi to understand the action mechanisms of active constituents. Furthermore more than one cancer cell lines must be investigated to confirm the anticancer activity.
Acknowledgements
The authors gratefully acknowledge technical and financial support provided by King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Author contributions
Conceptualization, methodology M.S.A. and M.H.A.; formal analysis, investigation M.K.T., S.K.A., A.A.A., F.A.A., D.A.B, H.M.A.; writing—original draft preparation, writing—review and editing, M.S.W., S.A. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
All data that support the findings of this study are available within the article.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammed S. Almuhayawi, Email: msalmuhayawi@kau.edu.sa
Samy Selim, Email: sabdulsalam@ju.edu.sa.
References
- Adedayo BC, Oyeleye SI, Okeke BM, Oboh G (2020) Anti-cholinesterase and antioxidant properties of alkaloid and phenolic-rich extracts from pawpaw (Carica papaya) leaf: a comparative study. Flavour Fragr J 36:47–54 [Google Scholar]
- Afsar T, Razak S, Khan MR, Mawash S, Almajwal A, Shabir M, Haq IU (2016) Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R. Parker aerial parts. BMC Complement Altern Med 16:258. 10.1186/s12906-016-1240-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawlaqi MM, Aisha MH, Tarek MA, Ganash M, Moawad H (2023) Evaluation of biomedical applications for linseed extract: antimicrobial, antioxidant, anti-diabetic, and anti-inflammatory activities in vitro. J Funct Biomater 14(6):300. 10.3390/jfb14060300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almehayawi MS, Almuhayawi MS, Abo El-Fadl SR, Nagshabandi MK, Tarabulsi MK, Selim S, Abdelghany TM (2024) Evaluating the anti-yeast, anti-diabetic, wound healing activities of Moringa oleifera extracted at different conditions of pressure via supercritical fluid extraction. BioResources 19(3):55. 10.15376/biores.19.3.5961-5977 [Google Scholar]
- Alotaibi HF, Khafagy ES, Abu Lila AS, Alotaibe HF, Elbehairi SE, Alanazi AS, Alfaifi MY, Alamoudi JA, Alamrani SS, Mokhtar FA (2023) Anticancer potentials of metformin loaded coconut oil nanoemulsion on MCF-7, HepG2 and HCT-116 cell lines. Artif Cells Nanomed Biotechnol 51(1):419–427. 10.1080/21691401.2023.2246145 [DOI] [PubMed] [Google Scholar]
- Al-Rajhi AMH, Qanash H, Almashjary MN, Hazzazi MS, Felemban HR, Abdelghany TM (2023a) Anti-Helicobacter pylori, antioxidant, antidiabetic, and anti-Alzheimer’s activities of laurel leaf extract treated by moist heat and molecular docking of its flavonoid constituent, naringenin, against acetylcholinesterase and butyrylcholinesterase. Life 13(7):1512. 10.3390/life13071512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rajhi AMH, Abdel Ghany TM (2023) Nanoemulsions of some edible oils and their antimicrobial, antioxidant, and anti-hemolytic activities. BioResources 18(1):1465–1481. 10.15376/biores.18.1.1465-1481 [Google Scholar]
- Al-Rajhi AMH, Qanash H, Bazaid AS, Binsaleh NK, Abdelghany TM (2023b) Pharmacological evaluation of Acacia nilotica flower extract against Helicobacter pylori and human hepatocellular carcinoma in vitro and in silico. J Funct Biomater 14:237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsalamah SA, Alghonaim MI, Jusstaniah M, Abdelghany TM (2023) Proteins Life 13(9):1839. 10.3390/life13091839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri MM, Alghonaim MI, Alsalamah SA, Yahya RO, Ismail KS, Abdelghany TM (2024) Impact of moist heat on phytochemical constituents, anti-Helicobacter pylori, antioxidant, anti-diabetic, hemolytic and healing properties of rosemary plant extract in vitro. Waste Biomass Valor 15:4965–4979. 10.1007/s12649-024-02490-8 [Google Scholar]
- Barth SA, Preussger D, Pietschmann J, Feßler AT, Heller M, Herbst W, Schnee C, Schwarz S, Kloss F, Berens C, Heller M, Herbst W, Menge C (2024) In vitro antibacterial activity of microbial natural products against bacterial pathogens of veterinary and zoonotic relevance. Antibiotics 13:135. 10.3390/antibiotics13020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng L, Ansong R, Owusu WB, Steiner-Asiedu M (2016) Coconut oil and palm oil’s role in nutrition, health and national development: a review. Ghana Med J 50(3):189–196 [PMC free article] [PubMed] [Google Scholar]
- Boland-Nazar NS, Eslamirad Z, Sarmadian H, Ghasemikhah R (2016) An in vitro evaluation of ozonized organic extra-virgin olive oil on Giardia lamblia cysts Jundishapur. J Microbiol 9:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges GÁ, Elias ST, da Silva SMM, Magalhães PO, Macedo SB, Ribeiro APD, Guerra ENS (2017) In vitro evaluation of wound healing and antimicrobial potential of ozone therapy. J Cranio-Maxillofac Surg 45:364–370 [DOI] [PubMed] [Google Scholar]
- Cai Y, Zhou S, Stewart MJ, Zheng F, Zhan CG (2019) Dimerization of human butyrylcholinesterase expressed in bacterium for development of a thermally stable bioscavenger of organophosphorus compounds. Chem Biol Interact 310:108756. 10.1016/jcbi2019108756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Spilker T, Fan Y, Kalikin LM, Ciotti S, Lipuma JJ, Makidon PE, Wilkinson JE, Baker JR, Wang SH (2017) Nanoemulsion is an effective antimicrobial for methicillin-resistant Staphylococcus aureus in infected wounds. Nanomedicine 12:1177–1185 [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Fernando M, Fernando B, Dias CB, Shah T, Silva R, Williams S, Pedrini S, Hillebrandt H, Goozee K, Barin E, Sohrabi HR, Garg M, Cunnane S, Martins RN (2020) Potential of coconut oil and medium chain triglycerides in the prevention and treatment of Alzheimer’s disease. Mech Ageing Dev 186:111209. 10.1016/jmad2020111209 [DOI] [PubMed] [Google Scholar]
- Cirlini M, Caligiani A, Palla G, de Ascentiis A, Tortini P (2012) Stability Studies of ozonized sunflower oil and enriched cosmetics with a dedicated peroxide value determination. Ozone: Sci Eng 34(4):293–299 [Google Scholar]
- De Almeida, NR; Beatriz, A; de Arruda, EJ; de Lima, DP; de Oliveira, LCS; Micheletti, AC (2016) Ozonized vegetable oils: production, chemical characterization and therapeutic potential in vegetable oil: properties, uses and benefits; Nova Science Publishers, Hauppauge: New York, NY, USA, pp 129–160
- Díaz FM, Hernández R, Martínez G, Vidal G, Gómez M, Fernández H (2006) Comparative study of ozonized olive oil and ozonized sunflower oil. J Braz Chem Soc. 10.1590/S0103-50532006000200026 [Google Scholar]
- Díaz MF, Sánchez Y, Gómez M, Hernández F, Da C, Veloso MC, De P, Pereira PA, Mangrich AS, De Andrade JB (2012) Physicochemical characteristics of ozonated sunflower oils obtained by different procedures. Grasas Y Aceites 63:466–474
- Ebrahimzadeh M, Nabavi S, Nabavi S (2009) Antioxidant activities of methanol extract of Sambucus Ebulus L Flower. Pak J Biol Sci 12(5):447. 10.3923/pjbs2009447450 [DOI] [PubMed] [Google Scholar]
- Elvis AM, Ekta JS (2011) Ozone therapy: a clinical review. J Nat Sci Biol Med 2:66–70. 10.4103/0976-966882319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examinati RRIN, Wulandari AP, Putri Huspa DH, Andayaningsih P (2008) Cytotoxicity of aromatic compound from an endophytic fungus, Cladosporium Sp. En-s01. Int J Curr Pharm Sci 10:10–12. 10.22159/ijcpr2018v10i630964 [Google Scholar]
- Guerra BP, Poznyak T, Chairez I, Brito AM (2015) Correlation of structural characterization and viscosity measurements with total unsaturation: an effective method for controlling ozonation in the preparation of ozonated grape seed and sunflower oils. Eur J Lipid Sci Technol 117:988–998 [Google Scholar]
- Hamidi M, Tajerzadeh H (2003) Carrier erythrocytes: an overview. Drug Deliv 10(1):9–20. 10.1080/713840329 [DOI] [PubMed] [Google Scholar]
- Hmidani A, Bouhlali EdT, Ajebli M, Khouya T, Benlyas M, Alem C (2021) In vitro investigation of antioxidant and antihemolytic activities of three Lamiaceae species from Morocco. Beni-Suef Univ J Basic Appl Sci 10:27. 10.1186/s43088-021-00116-9 [Google Scholar]
- Huang X, Liu Y, Lin Z, Wu B, Nong G, Chen Y, Lu Y, Ji X, Zhou X, Suo B, Chen Q, Wei J (2021) Minimum inhibitory concentrations of commonly used antibiotics against Helicobacter pylori: A multicenter study in South China. PLoS ONE 16(9):e0256225. 10.1371/journalpone0256225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto GS, Benvenutti L, Santin JR, de Freitas RA, Bella Cruz A, Corrêa R, Malheiros A, Klein-Junior LC, Bresolin MB (2023) The effect of ozone dosage of sunflower and olive oils on their biological activities and chemical properties. Ozone: Sci Eng 46(3):197–216. 10.1080/0191951220232266734 [Google Scholar]
- Jeong YJ, Kim HE, Han SJ, Choi JS (2021) Antibacterial and anti-biofilm activities of cinnamon essential oil nanoemulsion against multi-species oral biofilms. Sci Rep 11:5911. 10.1038/s41598-021-85375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa K, Barghoth M, Desouky S, Elsayed H, Roushdy M (2022) Highly efficiently inactivation of microbial pathogens using advanced ozone generator unit as an eco- friendly promising strategy. Al-Azhar J Pharm Sci 66(2):193–207. 10.21608/ajps2022269252 [Google Scholar]
- Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, Park YM (2009) Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci 24(3):368–374. 10.3346/jkms2009243368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima E, Medeiros J (2024) Terpenes as potential anti-Alzheimer’s disease agents. Appl Sci 14:3898. 10.3390/app14093898 [Google Scholar]
- Martínez SG, Al-Dalain SM, Menéndez S, Re L, Giuliani A, Candelario JE, Álvarez H, Fernández-Montequín JI, León OS (2005) Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharm 523:151–161 [DOI] [PubMed] [Google Scholar]
- Meng J, Chen T, Zhao Y, Lu S, Yu H, Chang Y, Chen D (2019) Study of the mechanism of anti-ulcer effects of virgin coconut oil on gastric ulcer-induced rat model. Arch Med Sci 15(5):1329–1335. 10.5114/aoms201876943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogrovejo DC, Perini L, Gostinčar C, Sepčić K, Turk M, Ambrožič-Avguštin J, Brill FHH, Gunde-Cimerman N (2020) Prevalence of antimicrobial resistance and hemolytic phenotypes in culturable arctic bacteria. Front Microbiol 11:570. 10.3389/fmicb202000570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moureu S, Violleau F, Ali Haimoud-Lekhal D, Calmon A (2016) Influence of storage temperature on the composition and the antibacterial activity of ozonized sunflower oil. Ozone: Sci Eng 38:143–149 [Google Scholar]
- Moureu S, Violleau F, Ali Haimoud-Lekhal D, Calmon A (2015) Ozonation of sunflower oils: Impact of experimental conditions on the composition and the antibacterial activity of ozonized oils. Chem Phys Lipids 186:79–85 [DOI] [PubMed] [Google Scholar]
- Napolitano A, Panzella L, Savarese M, Sacchi R, Giudicianni I, Paolillo L, D′Ischia M (2004) Acid-induced structural modifications of unsaturated fatty acids and phenolic olive oil constituents by nitrite ions: a chemical assessment. Chem Res Toxicol 17(10): 1329–1337. 10.1021/tx049880b [DOI] [PubMed]
- Ng YM, Sockalingam SNM, Shafiei Z, Zakaria ASI, Mahyuddin A, Rahman MA (2024) Biological activities of virgin coconut and virgin olive oil mixture against oral primary colonizers: an in vitro study. J Contemp Dent Pract 25(3):260–266. 10.5005/jp-journals-10024-3645 [DOI] [PubMed] [Google Scholar]
- Ozturk B, Kurtoglu T, Durmaz S, Kozaci LD, Abacigil F, Ertugrul B, Erel O (2017) The effects of ozone on bacterial growth and thiol-disulphide homeostasis in vascular graft infection caused by MRSA in rats. Acta Cir Bras 32:219–228 [DOI] [PubMed] [Google Scholar]
- Pietrocola G, Ceci M, Preda F, Poggio C, Colombo M (2018) Evaluation of the antibacterial activity of a new ozonized olive oil against oral and periodontal pathogens. J Clin Exp Dent 10:e1103–e1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky TM, Soohoo AM, Wang J, Walsh CT, Wright GD, Gordon EM, Gray NS, Khosla C (2021) Prospects for antibacterial discovery and development. J Am Chem Soc 143(50):21127–21142. 10.1021/jacs1c10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qanash H, Alotaibi K, Aldarhami A, Bazaid AS, Ganash M, Saeedi NH, Abdelghany TA (2023a) Effectiveness of oil-based nanoemulsions with molecular docking of its antimicrobial potential. BioResources 18(1):1554. 10.15376/biores1811554-1576 [Google Scholar]
- Qanash H, Al-Rajhi AMH, Almashjary MN, Basabrain AA, Hazzazi MS, Abdelghany TM (2023b) Inhibitory potential of rutin and rutin nano-crystals against Helicobacter pylori, colon cancer, hemolysis and Butyrylcholinesterase in vitro and in silico. Appl Biol Chem 66:79. 10.1186/s13765-023-00832-z [Google Scholar]
- Qanash H, El-Fadl SRA, Binsaleh NK, Aljahdali IA, Altayar MA, Khalel AF, Sulaiman AA, Alghonaim MI, Abdelghany TM (2024) Ecofriendly extraction approach of Moringa peregrina biomass and their biological activities in vitro. Biomass Convers Bioref. 5:55. 10.1007/s13399-024-05916-4 [Google Scholar]
- Qanash H, Yahya R, Bakri M M, Bazaid AS, Qanash S, Shater AF, Abdelghany TM (2022) Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci Rep12, article 5914. 101038/s41598-022-09993-1 [DOI] [PMC free article] [PubMed]
- Radzimierska-Kaźmierczak M, Śmigielski K, Sikora M, Nowak A, Plucińska A, Kunicka-Styczyńska A, Czarnecka-Chrebelska KH (2021) Olive oil with ozone-modified properties and its application. Molecules 26(11):3074. 10.3390/molecules26113074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao YL, Pai MM, Krishnaprasad PR, Pai MV, Murlimanju BV, Mohan A, Prabhu LV, Vadgaonkar R (2024) Virgin coconut oil - its methods of extraction, properties and clinical usage: a review. Clin Ter 175(2):83–91. 10.7417/CT20245037 [DOI] [PubMed] [Google Scholar]
- Santiago MB, Leandro LF, Rosa RB, Silva MV, Teixeira SC, Servato JPS, Ambrósio SR, Veneziani RCS, Aldana-Mejía JA, Bastos JK, Martins CHG (2022) brazilian red propolis presents promising anti-H pylori Activity in in vitro and in vivo assays with the ability to modulate the immune response. Molecules 27(21):7310. 10.3390/molecules27217310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sega A, Zanardi I, Chiasserini L, Gabbrielli A, Bocci V, Travagli V (2010) Properties of sesame oil by detailed 1H and 13C NMR assignments before and after ozonation and their correlation with iodine value, peroxide value, and viscosity measurements. Chem Phys Lipids 163(2):148–156. 10.1016/jchemphyslip200910010 [DOI] [PubMed] [Google Scholar]
- Selvarajah M, Ahmad Z, Zakaria ZA, Chiong HS, Yong YK, Long K, Hakim MN (2015) Comparative investigation into the anti-ulcer activity of virgin coconut oil and coconut oil in pylorous ligated animal model. Cell Med 5(4):281–286. 10.5667/TANG20150030 [Google Scholar]
- Song M, Zeng Q, Xiang Y, Gao L, Huang J, Huang J, Wu K, Lu J (2018) The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol Med Rep 17:2449–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawkar BS, Kaur G (2024) Betanin combined with virgin coconut oil inhibits neuroinflammation in aluminum chloride-induced toxicity in rats by regulating NLRP3 inflammasome. J Tradit Complement Med 14(3):287–299. 10.1016/jjtcme202311001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomášková H, Kühnová J, Kuča K (2016) Ageing and alzheimer disease-system dynamics model prediction. Ceska a Slovenska Farmacie: Casopis Ceske Farmaceuticke Spolecnosti a Slovenske Farmaceuticke Spolecnosti 65(3):99–103 [PubMed] [Google Scholar]
- Ugazio E, Tullio V, Binello A, Tagliapietra S, Dosio F (2020) Ozonated oils as antimicrobial systems in topical applications their characterization, current applications, and advances in improved delivery techniques. Molecules 25:334. 10.3390/molecules25020334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utarı AU, Djabir YY, Palinggi BP (2022) A Combination of virgin coconut oil and extra virgin olive oil elicits superior protection against doxorubicin cardiotoxicity in rats. Turk J Pharm Sci 19(2):138–144. 10.4274/tjpsgalenos202137998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Naik S, Nanda P, Banerjee S, Naik S, Ghosh A (2019) In Vitro anticancer activity of virgin coconut oil and its fractions in liver and oral cancer cells. Anticancer Agents Med Chem 19(18):2223–2230. 10.2174/1871520619666191021160752 [DOI] [PubMed] [Google Scholar]
- Yahya R, Al-Rajhi AMH, Alzaid SZ, Al Abboud MA, Almuhayawi MS, Al Jaouni SK, Selim S, Ismail KS, Abdelghany TM (2022) Molecular docking and efficacy of Aloe vera gel based on chitosan nanoparticles against Helicobacter pylori and its antioxidant and anti-inflammatory activities. Polymers 14(15):2994. 10.3390/polym14152994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Nguyen VA, Nulu NPC, Kalaipandian S, Beveridge FC, Biddle J, Young A, Adkins SW (2024) Towards pathogen-free coconut germplasm exchange. Plants (Basel) 13(13):1809. 10.3390/plants13131809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Lu J (2018) Mechanisms of action involved in ozone-therapy in skin diseases. Int Immunopharmacol 56:235–241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available within the article.