Abstract
The study was to explore the efficacy and safety of sivelestat (SV) in the treatment of severe acute pancreatitis (SAP) with systemic inflammatory response syndrome (SIRS). A total of 102 SAP patients diagnosed and treated in the Emergency Intensive Care Unit of the First Affiliated Hospital of Zhengzhou University from January 2021 to August 2024 were selected. The changes of disease outcome, hospital stays and mortality were compared between the two groups. A total of 102 patients were recruited to control group (n = 56) or SV group (n = 46) according to whether SV was applied or not. There was no significant difference in baseline data at admission between the two groups. After 1 week of treatment, all the indexes in both groups improved. The duration of ventilator use (p = 0.0400) and ICU stays (p = 0.0495) in SV group was shorter than that in control group, but there was no significant difference in mortality between the two groups. Although SV did not reduce the mortality of patients with SAP, it reduced the length of ventilator use and ICU stay.
Keywords: Sivelestat, Severe acute pancreatitis, Systemic inflammatory response syndrome
Subject terms: Drug safety, Pancreatic disease
Introduction
Severe acute pancreatitis (SAP) is a cascade inflammatory disease caused by local lesions of the pancreas, with acute onset, severe illness and high mortality1. SAP is a common critical illness in clinical emergencies, and the persistence of systemic inflammatory response syndrome (SIRS) and organ dysfunction are important determinants of the severity of the condition. Inhibiting inflammatory response and protecting organ function are very important to improve the prognosis of patients. Sivelestat (SV) is a selective human neutrophil elastase inhibitor (HNEI) for the treatment of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) with SIRS2–4. The present study found that SV may also play an important role in ameliorating SAP and its associated lung and kidney damage5–8. The Chinese experts’ consensus on clinical application of Sivelestat Sodium, published in 2022, recommended that SV should be considered for early use in patients with acute pancreatitis (AP) combined with SIRS based on standard treatment9. But high-quality clinical evidence is lacking.
Our study retrospectively analyzed 102 patients with SAP, aiming to explore the efficacy and safety of SV in the treatment of SAP combined with SIRS, and to provide a certain basis for clinical treatment.
Methods
Study design and participants
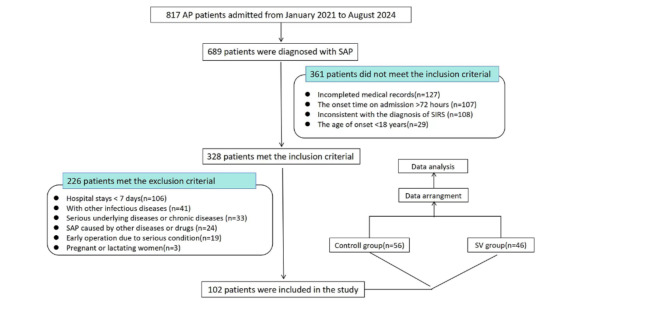
A total of 102 SAP patients diagnosed and treated in the Emergency Intensive Care Unit of the First Affiliated Hospital of Zhengzhou University from January 2021 to August 2024 were selected, including 69 males and 33 females, aged 18–72 years, who were all admitted within 72 h after onset. The inclusion and exclusion criteria and study process were shown in Fig. 1. The study did not affect patients’ treatment. Data from all patients was used for study purposes only. This study has been approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and all methods were performed in accordance with the guidelines and regulations. Informed consent was obtained from all study participants or their legal guardians.
Fig. 1.
Flow chart of the study. AP, acute pancreatitis; SAP, severe acute pancreatitis; SIRS, systemic inflammatory response syndrome; SV, sivelestat.
Relevant definitions and standards
Acute kidney injury(AKI): Serum creatinine (Scr) increased ≥ 26.4µM/L, or ≥ 50% from baseline within 48 h, and/or urine volume < 0.5mL / (kg· h) for six hours10. AKI recovery: Serum creatinine returned to a standard Risk level below risk, Injury, Failure, Loss, and End-stage renal disease (RIFLE) classification within seven days of onset of AKI, or within 1.5 times the baseline Scr level, or without renal replacement therapy11,12. The diagnostic criteria for SIRS were two or more of the following clinical manifestations13: (1) Heart rates > 90 beats/min; (2) Body temperature < 36 ℃ or > 38 ℃; (3) White blood cells (WBC) count < 4 × 109/L or > 12 × 109/L; (4) Breaths > 20 times/min or arterial carbon dioxide pressure < 30 mmHg. Absence of bowel sounds: Abdominal auscultation lasted 3–5 min without hearing bowel sounds.
Intervention and follow‑up
The control group received fasting, gastrointestinal decompression, fluid rehydration, spasmolysis, anti-inflammatory, inhibition of gastric acid and pancreatic fluid secretion, electrolyte balance and other symptomatic treatments. SV treatment group was additionally injected with SV 4.8 mg/kg/d by intravenous micropump on the basis of conventional symptomatic treatment.
After selecting the enrolled patients, we reviewed their medical records and examination results and collected patients’ data. The changes of clinical indexes (such as breaths, heart rates, urine volume per hour, oxygenation index, etc.) and serological indexes (serum amylase, lipase, Scr, blood urea nitrogen (BUN), IL-6, IL-10, TNF-α, procalcitonin (PCT) and white blood cells (WBC)) were observed in each group upon admission and one week after treatment. The duration of ventilator usage and continuous renal replacement therapy (CRRT) use, the length of intensive Care Unit (ICU) stay and the number of patients died were recorded.
Statistical analysis
All clinical data were statistically analyzed using GraphPad Prism 10.4.0 software. When comparing measurement data, normality test was carried out first. Measurement data conformed to normal distribution were presented as the means ± SD. If the variance was homogenous, the independent sample t test was used; if not, the mann-whitney test was used. Unpaired t test was used for inter-group comparison, and paired t test was used for intra-group comparison before and after treatment. Data with skewed distribution were expressed as the median (quartile) [M (QL, QU)] and tested for non-parametric rank sum. The counting data were expressed as m/n (%), and the χ2 test, adjusted χ2 test or Fisher exact test were used for comparison between groups. p < 0.05 indicated a statistically significant difference.
Results
Participant characteristics
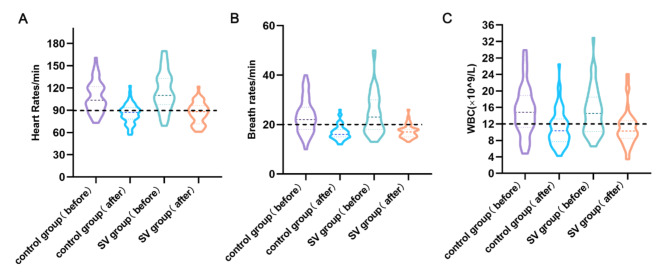
A total of 102 patients with SAP were included in this study, including 56 in the control group and 46 in the SV group. There were no significant differences in gender, age, acute physiology and chronic health evaluation II (APACHE II), CT severity index (CTSI) and clinical and laboratory indicators between the two groups at admission (Table 1). The mean breath rates of both groups were greater than 20 times/min (p < 0.05), heart rates of three quarters digits greater than 100 beats/min (p < 0.05) (Fig. 2). The baseline data of the two groups of patients at admission were basically similar and comparable.
Table 1.
Baseline characteristics of patients.
| Variable | Control group (n = 56) | SV group (n = 46) |
P value |
|---|---|---|---|
| Sex (male/female) | 39/17 | 30/16 | 0.6746 |
| Age (years) | 42(32,57) | 39(32,49) | 0.2465 |
| APACHE II score | 8(6,11) | 8(5,13) | 0.5760 |
| CTSI | 6(5,8) | 6(5,7) | 0.9195 |
| Heart rates(/min) | 108 ± 21 | 116 ± 26 | 0.1303 |
| Body temperature (℃) | 36.9(36.6,37.5) | 36.9(36.7,37.9) | 0.7536 |
| Breath rates(/min) | 22(18,27) | 23(18,30) | 0.8083 |
| Serum lipase (IU/ml) | 470.7(251.0,706.5) | 596.2(373.5,914.6) | 0.1147 |
| Serum amylase (IU/ml) | 429.5(156.3,828.0) | 594.0(364.8,1047.0) | 0.0706 |
| WBC count (×109/L) | 14.84(11.19,18.88) | 14.52(10.17,18.49) | 0.7570 |
| PCT (ng/L) | 1.965(0.875,6.765) | 4.355(1.227,8.740) | 0.0679 |
| IL-6 (pg/ml) | 100.50(67.76,254.60) | 173.40(63.21,282.10) | 0.3845 |
| IL-10 (pg/ml) | 4.79(3.13,7.61) | 5.21(3.75,10.44) | 0.1754 |
| TNF-α (pg/ml) | 2.89(1.49,4.67) | 2.86(2.09,4.38) | 0.6675 |
| Urine volume (ml/h) | 90(44,110) | 77(21,122) | 0.4946 |
| Scr (µmol/L) | 82.0(59.3,191.8) | 99.0(73.8,150.0) | 0.0973 |
| BUN (mmol/L) | 6.20(4.15,9.32) | 6.05(4.91,11.0) | 0.5721 |
| Proportion of AKI patients | 21/56(37.50%) | 22/46(47.83%) | 0.4710 |
| Oxygenation index | 244 ± 73 | 233 ± 86 | 0.4921 |
| Proportion of patients without bowel sounds | 23/56(41.07%) | 24/46(52.17%) | 0.3197 |
APACHE II, acute physiology and chronic health evaluation II; CTSI, CT severity index; WBC, white blood cell; PCT, procalcitonin; Scr, serum creatinine; BUN, blood urea nitrogen; AKI, acute kidney injury. Measurement data conformed to normal distribution are presented as the means ± SD, and the skewness distribution with median (quartile). Counting data is expressed as m/n (%). P < 0.05 indicates a statistically significant difference. Significant values are in italics.
Fig. 2.
Heart rates (A), breath rates (B), blood WBC (C) level and their changes at admission (before) and one week after treatment (after) in both groups. WBC, white blood cell.
The characteristics of the two groups before and after treatment
After one week of treatment, the heart rates, breaths, serum amylase, serum lipase, IL-6, IL-8, TNF-α, WBC, PCT of the two groups were significantly decreased compared with that before treatment (Table 2), indicating that pancreatitis and SIRS were improved. After treatment, urine volume per hour in both groups increased significantly, Scr and BUN decreased, suggesting improved renal function (Table 2). Oxygenation index was also higher than before treatment, indicating respiratory function improved (Table 2). The results showed that the treatment was effective in both groups.
Table 2.
Comparison of the characteristics of the two groups before and after treatment.
| Variable | Control group (n = 56) |
P值 | SV group (n = 46) |
P value | ||
|---|---|---|---|---|---|---|
| Before | After 1 week of treatment | Before | After 1 week of treatment | |||
| Heart rates(/min) | 108 ± 21 | 85 ± 13 | < 0.0001 | 116 ± 26 | 86 ± 14 | < 0.0001 |
| Body temperature (℃) | 36.9(36.6,37.5) | 36.8(36.7,37.2) | 0.6874 | 36.9(36.7,37.9) | 36.9(36.7,37.2) | 0.3196 |
| Breath rates(/min) | 22(18,27) | 16(15,18) | < 0.0001 | 23(18,30) | 17(15,18) | < 0.0001 |
| Serum lipase (IU/ml) | 470.7(251.0,706.5) | 58.6(30.1,90.8) | < 0.0001 | 596.2(373.5,914.6) | 51.3(31.2,84.4) | < 0.0001 |
| Serum amylase (IU/ml) | 429.5(156.3,828.0) | 41.5(28.3,68.0) | < 0.0001 | 594.0(364.8,1047.0) | 44.5(31.0,72.0) | < 0.0001 |
|
WBC count (×109/L) |
14.84(11.19,18.88) | 10.33(7.70,13.23) | 0.0016 | 14.52(10.17,18.49) | 10.27(8.50,12.77) | 0.0138 |
| PCT (ng/L) | 1.965(0.875,6.765) | 0.785(0.328,1.749) | < 0.0001 | 4.355(1.227,8.740) | 0.984(0.363,2.487) | < 0.0001 |
| IL-6 (pg/ml) | 100.50(67.76,254.60) | 48.01(21.37,101.4) | < 0.0001 | 173.40(63.21,282.10) | 36.87(19.06,82.28) | < 0.0001 |
| IL-10 (pg/ml) | 4.79(3.13,7.61) | 2.63(1.60,4.72) | < 0.0001 | 5.21(3.75,10.44) | 2.62(1.55,4.78) | < 0.0001 |
| TNF-α (pg/ml) | 2.89(1.49,4.67) | 1.78(1.15,2.76) | 0.0053 | 2.86(2.09,4.38) | 1.52(1.06,2.55) | < 0.0001 |
| Urine volume (ml/h) | 90(44,110) | 107(84,160) | 0.0008 | 77(21,122) | 120(81,151) | < 0.0001 |
| Scr (µmol/L) | 82.0(59.3,191.8) | 65.5(51.0,89.0) | < 0.0001 | 99.0(73.8,150.0) | 79.0(58.8,97.5) | < 0.0001 |
| BUN (mmol/L) | 6.20(4.15,9.32) | 3.66(2.78,5.29) | < 0.0001 | 6.05(4.91,11.00) | 3.85(3.143,6.00) | < 0.0001 |
| Oxygenation index | 244 ± 73 | 287 ± 72 | 0.0011 | 233 ± 86 | 290 ± 91 | < 0.0001 |
CTSI, CT severity index; WBC, white blood cell; PCT, procalcitonin; Scr, serum creatinine; BUN, blood urea nitrogen. Measurement data conformed to normal distribution are presented as the means ± SD, and the skewness distribution with median (quartile). Counting data is expressed as m/n (%). P < 0.05 indicates a statistically significant difference. Significant values are in italics.
The characteristics between the two groups after one week of treatment
We found no significant differences in blood pressure, heart rates, breaths, serum lipase, serum amylase, IL-6, IL-10, TNF-α, urine volume per hour, Scr, BUN and oxygenation index in SV group after one week of treatment compared with the control group. The proportion of patients with restored renal function (77.27% vs. 42.86%,p = 0.0305) and bowel sounds (62.50% vs. 30.43%,p = 0.0415) were both higher (Table 3). It was suggested that SV treatment may be more beneficial to the recovery of AKI and intestinal function in SAP patients. However, no significant advantage was seen in the improvement of pancreatic inflammation.
Table 3.
Comparison of characteristics between the two groups after one week of treatment.
| Variable | Control group (n = 56) |
SV group (n = 46) |
P value |
|---|---|---|---|
| Heart rates(/min) | 85 ± 13 | 86 ± 14 | 0.7187 |
| Body temperature (℃) | 36.8(36.7,37.2) | 36.9(36.7,37.2) | 0.8525 |
| Breath rates(/min) | 16(15,18) | 17(15,18) | 0.2337 |
| Serum lipase (IU/ml) | 58.6(30.1,90.8) | 51.3(31.2,84.4) | 0.5607 |
| Serum amylase (IU/ml) | 41.5(28.3,68.0) | 44.5(31.0,72.0) | 0.8532 |
| WBC count (×109/L) | 10.33(7.70,13.23) | 10.27(8.50,12.77) | 0.9759 |
| PCT (ng/L) | 0.785(0.328,1.749) | 0.984(0.363,2.487) | 0.6965 |
| IL-6 (pg/ml) | 48.01(21.37,101.4) | 36.87(19.06,82.28) | 0.2366 |
| IL-10 (pg/ml) | 2.63(1.60,4.72) | 2.62(1.55,4.78) | 0.9063 |
| TNF-α (pg/ml) | 1.78(1.15,2.76) | 1.52(1.06,2.55) | 0.2834 |
| Urine volume (ml/h) | 107(84,160) | 120(81,151) | 0.5974 |
| Scr (µmol/L) | 65.5(51.0,89.0) | 79.0(58.8,97.5) | 0.0531 |
| BUN (mmol/L) | 3.66(2.78,5.29) | 3.85(3.143,6.00) | 0.5813 |
|
Proportion of patients recovering from AKI |
9/21(42.86%) | 17/22(77.27%) | 0.0305 |
|
The proportion of patients with restored bowel sounds |
7/23(30.43%) | 15/24(62.50%) | 0.0415 |
| Oxygenation index | 287 ± 72 | 290 ± 91 | 0.8309 |
WBC, white blood cell; PCT, procalcitonin; Scr, serum creatinine; BUN, blood urea nitrogen; AKI, acute kidney injury; CRRT, continuous renal replacement therapy. Measurement data conformed to normal distribution are presented as the means ± SD, and the skewness distribution with median (quartile). Counting data is expressed as m/n (%). P < 0.05 indicates a statistically significant difference. Significant values are in italics.
The comparison of treatment course and outcome between two groups
There were no statistically significant differences in CRRT and ventilator utilization rate between the two groups during treatment, and there were no statistically significant differences in final mortality (Table 4). However, the duration of ventilator use in SV group was shorter than that in control group (10(7,16) vs. 7(6,10),p = 0.0400), and as was the length of ICU stay (13(7,19) vs. 9(7,13),p = 0.0495) (Table 4).
Table 4.
The comparison of treatment course and outcome between two groups.
| Variable | Control group (n = 56) |
SV group (n = 46) |
P value |
|---|---|---|---|
| Proportion of patients treated with CRRT | 15/56(26.79%) | 14/46(30.43%) | 0.8258 |
| Proportion of AKI patients treated with CRRT | 15/21(71.43%) | 14/22(61.64%) | 0.7470 |
| Days of CRRT | 8(7,15) | 8(6,11) | 0.3004 |
| Proportion of patients on ventilators | 18/56(32.14%) | 15/46(32.61%) | 0.9999 |
| Days of ventilator use | 10(7,16) | 7(6,10) | 0.0400 |
| Mortality ratio | 11/56(19.64%) | 6/46(13.04%) | 0.4323 |
| ICU stay | 13(7,19) | 9(7,13) | 0.0495 |
AKI, acute kidney injury; CRRT, continuous renal replacement therapy; ICU, intensive Care Unit. Measurement data conformed to normal distribution are presented as the means ± SD, and the skewness distribution with median (quartile). Counting data is expressed as m/n (%). P < 0.05 indicates a statistically significant difference. Significant values are in italics.
Discussion
AP is a disease that causes acute inflammation of the pancreas due to the activation of pancreatic enzymes by multiple triggers, which can lead to local damage and systemic inflammatory reactions. Gallstones and heavy alcohol consumption are the two most common causes14. According to the severity, AP patients can be divided into three types: mild acute pancreatitis (MAP), moderate severe acute pancreatitis (MASP) and SAP15. SAP accounts for a small proportion of AP, but it is often combined with MODS, leading to severe illness and high fatality rate. A retrospective multiple center study found that MAP, MSAP and SAP accounted for 73.4%, 13.5% and 13.1%, but the case fatality rate was 0.3%, 3.1% and 14.3%, respectively16. A national multiple center investigation in China found that the overall case fatality rate of acute pancreatitis was up to 4.6%, with 73.9% of deaths occurring within two weeks after admission. In 1743 patients (28.0%) diagnosed with SAP, the in-hospital fatality rate was 15.6%17. Although mortality rates have gradually decreased as intensive care has improved, SAP still has a high mortality rate18,19. Seeking more effective treatment plan and promoting the rapid recovery of organ injury can shorten the course of disease and hospital stay, but also can reduce the pain of patients and family economic burden.
SV is a highly specific HNEI that acts by inhibiting neutrophil elastase (NE) activity and has beneficial effects on a variety of conditions caused by acute inflammation. It is currently the only drug approved worldwide for the treatment of ALI/ARDS20–22. Recent studies have found that the powerful anti-inflammatory ability of SV may also have a better inhibitory effect on the inflammatory response in other organs23–26.
Blood NE activity was significantly increased in SAP patients, especially those with respiratory failure27. Necropsy of patients with acute necrotizing pancreatitis complicated with multiple organ failure showed increased neutrophil infiltration and significantly increased NE expression in necrotic pancreatic tissue28. These results suggest that SV as a NE inhibitor may be a potentially effective drug for the treatment of SAP. However, there is a lack of study results on large samples.
We retrospectively analyzed 71 patients with SAP, 29 of whom received SV in addition to conventional treatment, and the remaining 42 patients received conventional symptomatic supportive treatment. Previous studies have found that IL-6, IL-10 and TNF-α are important biomarkers in the progression of SAP and correlated with disease severity29–33. Therefore, these indicators were included in our study to reflect the disease progression of SAP. The results showed that there were no statistically significant differences in heart rates, breath rates, oxygenation index, body temperature, IL-6, IL-8, TNF-α, WBC, PCT, the rate of CRRT and ventilator utilization the two groups after one week of treatment, which were all improved compared with admission. In addition, there was no difference in the mean length of hospital stays and mortality between the two groups. SV showed no significant advantage in the treatment of SAP and SIRS.
The ratio of AKI recovery in SV group was higher than that in control group after one week of treatment, which may be related to the improvement of AKI kidney inflammation by SV. Li et al. found that SV could reduce the iNOS overexpression in the kidney of sepsis rats, inhibit the activation of Akt signaling pathway, and reduce inflammation, thus reducing the AKI caused by sepsis5. SV also significantly reduced Scr and urine KIM-1 levels in extracorporeal shock wave lithotripsy (ESWL) treated rats, and inhibited renal tissue inflammation and interstitial damage34. These results suggested that SV may play a role in the recovery of kidney injury.
Previous studies have shown that SV could significantly reduce pathological abnormalities of lung and pancreas as well as biochemical abnormalities in rat models with taurochate-induced acute pancreatitis7,8. Our results found that although there was no difference in the proportion of patients using ventilator between the two groups, the SV group had shorter ventilator use and reduced patient suffering.
In addition, we found that bowel sounds seemed to recover better in the SV group after one week treatment. SV might improve intestinal motility in SAP patients. This may attribute to that SV can improve the intestinal microbial and metabolic disorders caused by sepsis35. However, this phenomenon is influenced by subjective factors and there is no relevant study at present. The validity of the results needs more data to verify.
Limitation
Our study preliminarily observed the safety and efficacy of SV in SAP combined with SIRS. Due to the small sample size and retrospective analysis, our study had some limitations, which may affect the determination of results. In addition, there are few studies related to SV in AP treatment, and our study lacks sufficient theoretical basis, and a larger sample size randomized controlled study is needed to verify it. Considering the retrospective nature of the study, there is a high potential for selection bias and confounding factors. We plan to address these issues in future iterations of the study through prospective study designs.
Conclusion
SV had a good safety in the treatment of SAP combined with SIRS, which could shorten the time of ventilator use in patients with respiratory failure and promote the recovery of renal function and intestinal function. Although SV did not reduce the mortality of patients with SAP, it reduced the length of ICU stay.
Acknowledgements
Thanks to the support of Henan Medical Key Laboratory of Emergency and Trauma Research and Henan Emergency and Trauma Medicine Engineering Research Center.
Author contributions
Jiafeng Xie and Ruyi Lei wrote the main manuscript. Zhiqiang Zhu and Changju Zhu decided the research direction. Yulei Gu and Ruyi Lei provided the fundings.Jiafeng Xie, Hui Pei and Luanluan Zhang did data analysis. Jiafeng Xie, Yanhui Huang, Yepeng Zhang, Jingrong Liu and Yanan Zi prepared figures and tables. All authors reviewed the manuscript.
Funding
This study was supported by programs from the National Natural Science Foundation of China (Grant no. 81902008), Key scientific research projects of colleges and universities in Henan Province (Grant no. 22A320052), and Henan Provincial Science and Technology Research Project (Grant no. LHGJ20190210).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Changju Zhu, Email: zhuchangju98@163.com.
Zhiqiang Zhu, Email: 13623810089@126.com.
References
- 1.Forsmark, C. E., Vege, S. S., Wilcox, C. M. & Acute Pancreatitis New. Engl. J. Med.375, 1972–1981 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Aikawa, N. et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol. Ther.24, 549–554 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Tamakuma, S. et al. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol. Ther.17, 271–279 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Iba, T. et al. Pretreatment of sivelestat sodium hydrate improves the lung microcirculation and alveolar damage in lipopolysaccharide-induced acute lung inflammation in hamsters. Shock26, 95–98 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Li, G. et al. The neutrophil elastase inhibitor, sivelestat, attenuates sepsis-related kidney injury in rats. Int. J. Mol. Med.38, 767–775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, H. et al. Renoprotective activity of sivelestat in severe acute pancreatitis in rats. Exp. Ther. Med.6, 29–32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, H. H., Tang, A. M., Chen, L. & Zhou, M. T. Potential of sivelestat in protection against severe acute pancreatitis-associated lung injury in rats. Exp. Lung Res.38, 445–452 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Wang, H. et al. Combined effects of sivelestat and resveratrol on severe acute pancreatitis-associated lung injury in rats. Exp. Lung Res.40, 288–297 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Sun, T. & Zhang, H. Chinese experts’ consensus on clinical application of Sivelestat Sodium. Chin. Res. Hosp.9, 9–13 (2022). [Google Scholar]
- 10.Thomas, M. E. et al. The definition of acute kidney injury and its use in practice. Kidney Int.87, 62–73 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Chawla, L. S. et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat. Rev. Nephrol.13, 241–257 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Bellomo, R., Ronco, C., Kellum, J. A., Mehta, R. L. & Palevsky, P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) Group. CRIT CARE. 8, R204-R212. (2004). [DOI] [PMC free article] [PubMed]
- 13.Singer, M. et al. The Third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama-J Am. Med. Assoc.315, 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lankisch, P. G., Apte, M., Banks, P. A. & Acute pancreatitis Lancet. 386, 85–96. (2015). [DOI] [PubMed] [Google Scholar]
- 15.Banks, P. A. et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. GUT62, 102–111 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Berger, Z. et al. Acute pancreatitis in Chile: a multicenter study on epidemiology, etiology and clinical outcome. Retrospective Anal. Clin. Files Pancreatol.. 20, 637–643 (2020). [DOI] [PubMed] [Google Scholar]
- 17.National Associated Group for acute pancreatitis. Etiology and mortality of acute pancreatitis in China: analysis of 6223 clinical cases. Yixianbingxue (06), 321–325. (2006).
- 18.Laterre, P. F. & Collienne, C. Improving the management of severe acute pancreatitis: the new guidelines from the French Society of Anaesthesia and Intensive Care Medicine. Anaesth. Crit. Care Pa.41, 101103 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Oland, G. L. & Hines, O. J. New guidelines for the treatment of severe acute pancreatitis. Hepatobil Surg. Nutr.11, 913–916 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maki, C. et al. Evaluation of appropriate indications for the use of sivelestat sodium in acute respiratory distress syndrome: A retrospective cohort study. Acute Med. Surg.7, e471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, X. et al. Efficacy, safety, and pharmacoeconomics of sivelestat sodium in the treatment of septic acute respiratory distress syndrome: a retrospective cohort study. Ann. Palliat. Med.10, 11910–11917 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Lewis, S. R., Pritchard, M. W., Thomas, C. M. & Smith, A. F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Db Syst. Rev.7, D4477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie, H. et al. Sivelestat alleviates atherosclerosis by improving intestinal barrier function and reducing Endotoxemia. Front. Pharmacol.13, 838688 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhukov, A. S., Khairutdinov, V. R., Samtsov, A. V., Krasavin, M. & Garabadzhiu, A. V. Preclinical efficacy investigation of human neutrophil elastase inhibitor sivelestat in animal model of psoriasis. Skin. Health Dis.2, e90 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayasam, A. et al. Viral mimetic triggers cerebral arteriopathy in juvenile brain via neutrophil elastase and NETosis. J. Cerebr Blood F Met.41, 3171–3186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, G. et al. Neutrophil elastase induces chondrocyte apoptosis and facilitates the occurrence of osteoarthritis via caspase signaling pathway. Front. Pharmacol.12, 666162 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novovic, S. et al. Activity of neutrophil elastase reflects the progression of acute pancreatitis. Scand. J. Clin. Lab. Inv. 73, 485–493 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Bilyy, R. et al. Neutrophil Extracellular traps Form a barrier between necrotic and viable areas in Acute Abdominal inflammation. Front. Immunol.7, 424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, Z. et al. Prevention of severe Acute Pancreatitis with Cyclooxygenase-2 inhibitors: a Randomized Controlled Clinical Trial. Am. J. Gastroenterol.115, 473–480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao, S. A. & Kunte, A. R. Interleukin-6: an early predictive marker for severity of Acute Pancreatitis. Indian J. Crit. Care M. 21, 424–428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, J., Niu, J. & Yang, J. Interleukin-6, interleukin-8 and interleukin-10 in estimating the severity of acute pancreatitis: an updated meta-analysis. Hepatogastroenterology61, 215–220 (2014). [PubMed] [Google Scholar]
- 32.Staubli, S. M., Oertli, D. & Nebiker, C. A. Laboratory markers predicting severity of acute pancreatitis. Crit. Rev. Cl. Lab. Sci.52, 273–283 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Cho, I. R., Do, M. Y., Han, S. Y., Jang, S. I. & Cho, J. H. Comparison of Interleukin-6, C-Reactive protein, Procalcitonin, and the computed tomography severity index for early prediction of severity of Acute Pancreatitis. GUT Liver. 17, 629–637 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colakerol, A. et al. Tissue neutrophil elastase contributes to extracorporeal shock wave lithotripsy-induced kidney damage and the neutrophil elastase inhibitor, sivelestat, attenuates kidney damage with gratifying immunohistopathological and biochemical findings: an experimental study. Urolithiasis50, 103–112 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Sun, Y. et al. Positive effects of Neutrophil elastase inhibitor (Sivelestat) on gut microbiome and metabolite profiles of septic rats. Front. Cell. Infect. Mi. 12, 818391 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.