Abstract
Hypertension is one of the most serious chronic diseases. This study will focus on the systemic antihypertensive mechanisms of 5,7-dihydroxyflavone from in silico simulations to in vivo validations. In-silico studies were applied by network pharmacology, molecular docking, and molecular dynamic simulation. Based on the information of network pharmacology, 5,7-dihydroxyflavone could act on several different blood pressure regulating pathways, molecular docking results confirmed it might direct binding on the active pocket of eNOS, and the average molecular distance between 5,7-dihydroxyflavone -eNOS is less than 0.4 Å by molecular dynamic simulation. The in vivo studies were carried by SHRs oral administrated with 10 mg/kg 5,7-dihydroxyflavone that could alleviate hypertensive symptoms within 30 min, but if SHRs pretreated with L-NAME (10 mg/kg, an eNOS inhibitor) can erase the anti-hypertensive effects of 5,7-dihydroxyflavone, but no affected by aminoguanidine pretreatment (100 mg/kg, the selective antagonist of iNOS). Furthermore, oral administration of 5,7-dihydroxyflavone does not affect the heart rate and pulse pressure difference in SHR rats. In conclusion, the effects of 5,7-dihydroxyflavone on blood pressure regulation may act on eNOS as an agonist to achieve its acute antihypertensive effects. These acute antihypertensive effects suggest that 5,7-dihydroxyflavone has the potential to be a candidate medication for urgently lowering blood pressure requirements without posing hypertensive risks.
Keywords: Hypertension, Molecular docking, eNOS, In vivo
Subject terms: Biochemistry, Cardiovascular diseases
Introduction
Hypertension is a serious medical condition characterized by systolic blood pressure exceeding 140 mmHg or diastolic blood pressure exceeding 80 mmHg. According to the World Health Organization (WHO) newsroom website, an estimated 1.28 billion adults aged 30–79 years worldwide suffer from hypertension (https://www.who.int/news-room/fact-sheets/detail/hypertension). The burden of hypertension is particularly severe in low- and middle-income countries or developing nations due to unhealthy lifestyles, dietary habits, and undiagnosed health conditions. High blood pressure increases cardiac workload and significantly raises the risk of cardiovascular, cerebrovascular, and renal diseases. It is also the leading cardiovascular risk factor for mortality 1. Approximately 90% of cases of essential hypertension lack identifiable etiology. Therefore, ongoing research aims to elucidate mechanisms for reducing blood pressure to normal levels. Traditional therapies for hypertension target various mechanisms, including angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), diuretics, β-blockers, calcium channel blockers, and vasodilators such as nitroglycerin. Clinical trials underscore the crucial role of vasodilative effects in therapeutic treatment. However, traditional medications often have limitations due to adverse reactions, highlighting the importance of discovering novel antihypertensive drugs.
The natural compound 5,7-dihydroxyflavone (chrysin) (CID: 5281607), also known as 5,7-dihydroxyflavone, is a flavone found in honey, propolis, and various plants 2. Many flavonoids have been shown to possess pharmacological effects of antioxidant, anti-apoptotic, and anti-inflammatory, and are used in various medical treatments 3–6. Traditional medications containing chrysin as the primary active ingredient have been used in numerous countries and regions 7. Recent studies suggest that chrysin has potential as an antihypertensive agent to decrease blood pressure 8,9, although other reports have shown contradictory results 10. Furthermore, chrysin has been shown to possess antioxidant, anti-inflammatory, antidiabetic, and antiallergic properties 11. Evidence also indicates that chrysin can stimulate the release of nitric oxide (NO), possibly mediated via PI3-kinase and independent of calcium 12. A recent study demonstrated that chrysin increases the protein expression of endothelial nitric oxide synthase (eNOS) while decreasing inducible nitric oxide synthase (iNOS) protein expression 13. Other studies have shown that chrysin relaxes contractions induced by noradrenaline in isolated endothelium-intact rat aortic rings by upregulating NO content. Therefore, this vasorelaxation induced by chrysin is eNOS-dependent 14.
Considering this evidence, the objectives of the current study were to investigate the antihypertensive effects of oral chrysin mediated by nitric oxide (NO) and to elucidate the underlying mechanisms contributing to its antihypertensive effect. These findings lay the groundwork for exploring additional possibilities in the development of chrysin for hypertension treatment. Specifically, we investigated the signaling pathway of chrysin in attenuating hypertension status through in silico analysis and molecular docking simulations. Our findings identified a direct interaction between chrysin and endothelial nitric oxide synthase (eNOS) and aimed to verify its efficiency and efficacy in modulating hypertensive symptoms using an in vivo spontaneously hypertensive rat (SHR) model.
Materials and methods
Bioinformatics analysis with BATMAN-TCM
BATMAN-TCM is a recognized bioinformatics analysis tool designed to explore the molecular mechanisms of traditional Chinese medicine. It was employed to investigate the potential signaling pathway of chrysin that may lower blood pressure. The PubChem CID and InChI of chrysin were submitted to the online analytical system, and subsequently, the website enumerated a comprehensive list of potential target proteins. Among these, the KEGG oxytocin signaling pathway was suggested as a potential mechanism related to hypertension. This revelation offers a valuable research direction, particularly as eNOS emerges as a key target protein in the regulation of blood pressure within the oxytocin signaling pathway. Therefore, our focus is on examining the protein expression of these target proteins during the chrysin-induced blood pressure-lowering phase, which can corroborate the actual signaling pathway responsible for chrysin’s blood pressure-lowering effects.
Molecular docking
Whether the candidate ingredients initially screened can be used as a drug depends on a rigorous filtering program. After conducting a literature review, 29 bioactive molecules were selected for further analysis. The source of 3D structure receptor molecules was retrieved from the RCSB PDB (https://www.rcsb.org/), the 3D structure of chrysin was downloaded from the PubChem database website (https://pubchem.ncbi.nlm.nih.gov/). The molecular docking between chrysin and target proteins was performed using the Discovery Studio (version 2019) and GOLD. The results revealed that the optimal binding conformational structures of the receptor–ligand pair were chrysin (CID: 5281607) and eNOS (PDB ID: 3NOS) 15. The sequences and structures of eNOS was downloaded from UniProt (https://www.uniprot.org/). Utilized the AlphaFold Sever (https://alphafoldserver.com/) to create the 3D structure of eNOS via input the sequence. The 3D structure of eNOS generated using AlphaFold3 was used for molecular docking with chrysin, followed by visualization and analysis of the docking conformations and binding positions.
Molecular dynamics simulation
To further assess the stability of chrysin–eNOS (3NOS) protein complexes resulting from molecular docking, the key docked complexes were subjected to molecular dynamics (MD) simulations. MD simulations provide a more comprehensive and fundamental molecular analysis of ligand recognition, enhancing our understanding of the interactions. The ligand–protein complex calculations were conducted using the CHARMM force field within Discovery Studio 2019. Prior to the MD simulation module, the ligand–protein complexes from molecular docking underwent a solvation process in Discovery Studio 2019. The solvent model was set as explicit with periodic boundaries. The standard dynamic cascade protocol was followed with default settings, except for the simulation time of the production step, which was set to 100 ns (temperature 300 K, time step 2 fs). Additionally, the SHAKE algorithm was enabled in the advanced settings to constrain bond lengths, improving the numerical stability of the simulation. To analyze the stability of the system over time, the RMSD (root mean square deviation) was calculated using the Analyze Trajectory tool. This metric provides a measure of the deviation of atomic positions from their initial configuration, allowing us to assess the conformational changes during the simulation. The RMSD changes were then visualized using a chart line plot. Finally, RMSD mappings were generated from the output of the MD simulation, providing a spatial representation of the conformational variations within the protein–ligand complex. These mappings offer insights into the regions of the complex that exhibit the most significant structural changes, crucial for understanding the stability and functionality of the chrysin–eNOS interaction.
Animals and treatments
All the methods were performed in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animal experiments were approved by Xiamen University Laboratory animals center Ethics Committee and the “Guide for the Care and Use of Laboratory Animals” of Xiamen Medical College was followed (Approved protocol ID SYXK 2018–0009, Aug. 24, 2021). Seven-week-old male spontaneously hypertensive rats (SHRs), weighing 250–280 g, were purchased from WUSHI animals (Hangzhou) Biotechnology Co. These rats were housed in IVCs at the Xiamen Medical College Laboratory Animal Center with a controlled environment maintained at 25 °C with a 12-h light/dark cycle and provided with standard diet and water ad libitum. The rats were randomly assigned to 4 groups, each comprising at least six rats: control group, 2.5 mg/kg chrysin group, 5 mg/kg chrysin group, 10 mg/kg chrysin group, 20 mg/kg chrysin group, l-NAME group, l-NAME-chrysin group, aminoguanidine group, aminoguanidine-chrysin group, Benazepril group, Benazepril-chrysin group.
Drugs and reagents
The chrysin (98%) was purchased from Xi’an Yunyue Biotechnology Co., Ltd. (Xi’an, China). NG-nitro-L-arginine methyl ester (L-NAME) and aminoguanidine were purchased from Sigma-Aldrich, while dimethyl sulfoxide (DMSO) and carboxymethylcellulose sodium (CMC-Na) were purchased from Xilong Scientific Co., Ltd. (Xilong, China). As a vehicle for oral administration of chrysin in animals, 1% CMC-Na was used.
Dissolution and infusion of drugs
Chrysin was dissolved in a 1% carboxymethylcellulose sodium (CMC-Na) solution. Due to its insolubility in water, both olive oil and 1% CMC-Na were evaluated as potential solvents. The findings demonstrated that 1% CMC-Na was more effective than olive oil as a solvent for chrysin. Consequently, chrysin was dissolved in 1% CMC-Na at concentrations of 2.5 mg/kg, 5 mg/ml, 10 mg/ml, and 20 mg/ml. L-NAME was dissolved in 0.9% normal saline at a concentration of 10 mg/ml, while aminoguanidine was dissolved in 0.9% normal saline at 100 mg/ml. Heparin was dissolved in 0.9% normal saline at a concentration of 200 IU. The chrysin solution was administered via gastric gavage needle at a volume of 1 ml/kg, adjusted according to the body weight of spontaneously hypertensive rats (SHRs). After arterial cannulation, blood pressure data were allowed to stabilize for 10 min before drug administration commenced. Each treatment group consisted of a minimum of six randomly selected rats for experimentation.
Blood pressure measurements
One week after acclimatizing in individually ventilated cages (IVCs) at the laboratory animal center, spontaneously hypertensive rats (SHRs) were randomly assigned to various drug treatment groups, each comprising at least six rats. Before the experimental procedures, rats were anesthetized with a 5% isoflurane–oxygen mixture for 4 min to induce anesthesia, followed by maintenance with 2% isoflurane administered via a facial oxygen mask. The method for rat anesthesia is carried out according to the current protocol 16. Throughout surgery and blood pressure recordings, body temperature was monitored using an electric anal thermometer and maintained at 37 ± 0.5 ℃ with an electric blanket.
Arterial blood pressure was recorded via the femoral artery. The femoral artery was cannulated with a PE-50 catheter, which was filled with heparin saline (200 IU) and connected to a blood pressure transducer (TM1909201). Blood pressure signals were recorded using an HF-12 recording system (Taimeng, China) and BL-420N software (Taimeng, China) for over 70 min per animal. Recorded parameters included systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), pulse pressure (PP), and heart rate (HR). Chrysin solutions or vehicle were administered via gastric gavage using a syringe with a volume of 1 ml/kg.
In experiments involving L-NAME (10 mg/ml, a selective eNOS inhibitor) and aminoguanidine (100 mg/ml, a selective iNOS inhibitor) to explore different NOS pathways, intraperitoneal administration of NOS inhibitors occurred 30 min prior to femoral artery cannulation, also at a volume of 1 ml/kg.
To demonstrate the effects of chrysin co-infused with the ACE inhibitor benazepril, the antihypertensive effect of benazepril was first verified. After converting the human dosage to the corresponding rat dosage, the dose of benazepril for SHR rats was determined to be 4.5 mg/kg. The effects of oral administration of 4.5 mg/kg benazepril on SHR blood pressure were recorded for 60 min, and the results were presented based on changes in the mean blood pressure of the SHR rats. In verifying the effects of chrysin co-infused with the ACE inhibitor benazepril, SHR rats were first administered 4.5 mg/kg benazepril via oral gavage. After 90 min, the blood pressure data of the SHR rats were recorded via the BL420 system, and the rats were administered with vehicle and chrysin based on their group assignments. The control group was treated with vehicle (1% CMC-Na), while the benazepril-chrysin group was treated with 10 mg/kg chrysin.
Electrocardiogram measurements
SHR rats were induced anesthesia by 5% isoflurane and maintained with 2% isoflurane throughout the experimental procedure. The SHR rats were randomly divided into two groups: a Control group (0.5% CMC-Na) and a Chrysin group receiving oral chrysin at a dose of 10 mg/kg. Initially, the rats were anesthetized as described earlier and secured onto a platform. Following this, acupuncture needles were inserted subcutaneously into the limbs to monitor and record the electrocardiogram (ECG) using the BL-420N system while under 2% isoflurane anesthesia. Used the biological information recording system to record the electrocardiogram (ECG) of rats at 10 min, 20 min, and 30 min after oral administration.
Statistical analysis
The results are presented as mean ± SD. Statistical analysis was performed with non-paired t-test. A p value less than 0.05 is considered statistically significant.
Results
In silico analysis and simulations of chrysin on antihypertensive targets
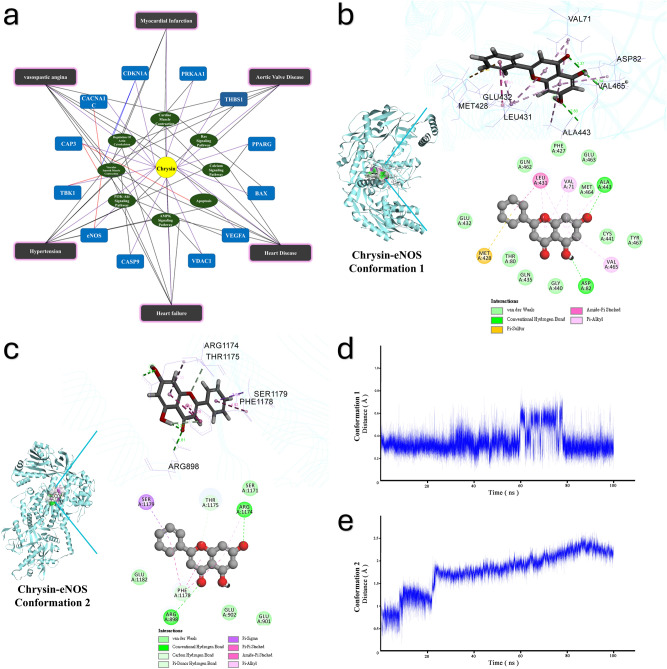
Based on BATMAN-TCM guidance on essential potential pathways influenced by chrysin, the results of network pharmacology illustrated restructuring of the antihypertensive KEGG pathway, and potential targets are shown in a network pharmacological diagram (Fig. 1a). The visual blue disease icons denote potential diseases regulated by chrysin. This suggests that the PI3K/AKT signaling pathway is a candidate therapeutic mechanism, with hypertension predicted as a potential disease. Based on these predictions, validations were conducted through molecular docking simulations using Discovery Studio 2019 to simulate potential interactions between chrysin and each target protein. The PI3K/AKT/eNOS pathway plays a significant role in the oxytocin signaling mechanism, with eNOS as the primary target protein in this cascade. Consequently, our focus shifted to direct evidence of the interaction between chrysin and eNOS, utilizing in silico experiments to explore these interactions. Docking results revealed a notable finding: chrysin exhibited high affinity for eNOS, forming hydrogen bonds with ASP82 and ALA443, achieving the highest LibDock score among active pockets. Additionally, it interacted via van der Waals forces with GLU432, THR80, GLN435, GLY440, CYS441, TYR467, MET464, GLU463, PHE427, and GLN462 (Fig. 1b) (conformation 1). Moreover, in another conformation, the docking position of chrysin with eNOS differed from the previous one, forming interactions with the amino acid residues ARG898, ARG1174, THR1175, PHE1178, and SER1779 on eNOS (Fig. 1c) (conformation 2). This conformational pattern suggests that chrysin acts as an agonist of eNOS and could potentially serve as an ectopic regulatory candidate. In summary, these findings indicate that chrysin exhibits strong affinity for eNOS and may function as an agonist, highlighting its potential therapeutic implications.
Fig. 1.
The network pharmacological analysis of chrysin-target interactions. (a) There are five kinds of labels including Chrysin compound (rotundity, yellow), drug targets (rectangle, blue), KEGG pathways (oval, green), OMIM disease (rectangle, grey). A view of the chrysin effects and actives. (b) The 2D and 3D structure diagram of conformation 1 Chrysin-eNOS complex, the black labels are binding sites. (c) The 2D and 3D structure diagram of conformation 2 Chrysin-eNOS complex, the black labels are binding sites. (d) The molecular dynamic simulation RMSD value for 100 ns of conformation 1 Chrysin-eNOS complex. (e) The molecular dynamic simulation RMSD value for 100 ns of conformation 2 Chrysin-eNOS complex.
Results of MD simulation confirmed the effects of chrysin on eNOS
The stability of binding interaction between chrysin and eNOS was validated with molecular dynamic simulation. The RMSD value is the molecular distance between the eNOS and chrysin in the complex as the index binding stability. The conformation 1 RMSD of the chrysin–eNOS complex remained stable throughout the 100 ns simulation period, but with a significant deviation in 60–80 ns (Fig. 1d), while the conformation 2 complex exhibited the stability during the 100 ns of the simulation (Fig. 1e). Specifically, the average RMSD value of conformation 2 chrysin–eNOS was about 1.84926 Å, and it remained below 2 Å throughout the whole 100 ns simulation. The results suggested that conformation 2 of chrysin-eNOS exhibited the stable interactions.
Effect of chrysin on blood pressure and HR in SHR
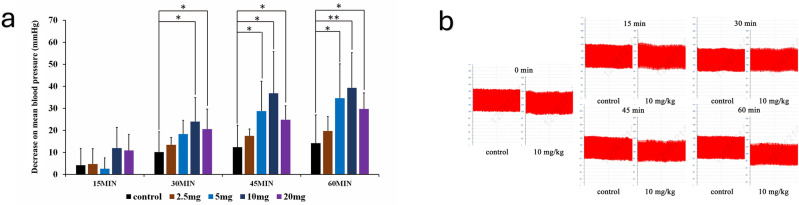
The antihypertensive effect of chrysin on spontaneously hypertensive rats (SHRs) was evaluated by administering various doses of chrysin (0, 2.5, 5, 10, 20 mg/ml dissolved in 1% CMC-Na) via gastric gavage, followed by blood pressure measurements taken over 1 h under anesthesia induced by 2% isoflurane (Fig. 2a,b). The mean ± SD from Fig. 2 is presented in Supplementary Table S1. The results indicated that in the control group treated with inhaled isoflurane anesthesia, there was a trend of slight gradual decrease in both blood pressure and heart rate up to 45 min post anesthesia, but these changes were not statistically significant. When compared with pretreatment levels using paired t-tests, there were no significant differences in blood pressure within the first 15 min after oral administration of chrysin at doses below 5 mg/kg. However, doses greater than 10 mg/kg showed significant reductions in blood pressure, though not significantly different from the control group by 15 min post treatment. By 30 min post treatment, doses above 10 mg/kg demonstrated significant attenuation of arterial pressure compared to the control group. The maximal blood pressure-lowering effect was observed after 45 min and persisted for more than 1 h. Significant blood pressure reduction was also observed in the group treated with 5 mg/kg chrysin, but this effect appeared later, not until 45 min post treatment. There was no further increase in antihypertensive effects observed with chrysin doses greater than 10 mg/kg. Additionally, chrysin treatments did not induce significant changes in heart rate or pulse pressure at doses of 2.5 mg/kg, 5 mg/kg, 10 mg/kg, or 20 mg/kg. These results suggest that acute antihypertensive effects of chrysin can be observed within 30 min at doses exceeding 10 mg/kg, with 10 mg/kg identified as potentially the most effective dose for reducing blood pressure in SHR rats.
Fig. 2.
The anti-hypertensive effects of chrysin by different dose. (a) The attenuation of mean blood pressure by different dose of chrysin (0 mg/kg, 2.5 mg/kg, 5 mg/kg, 20 mg/kg) within post-treated 60 min. Data are shown as mean ± SEM, n = 6. (b) The anti-hypertensive effects on Blood pressure regulation by 10 mg/kg dose of chrysin in different moments.
Effect of chrysin co-infused with L-NAME and aminoguanidine on BP and HR in SHR
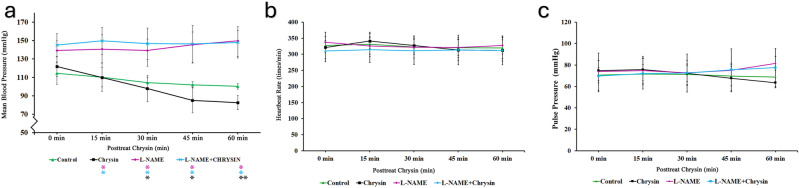
To validate the antihypertensive effects of chrysin via the endothelial NOS pathway, spontaneously hypertensive rats (SHRs) were orally administered with the selective eNOS inhibitor L-NAME (10 mg/kg in DDW) or vehicle control (1 ml/kg DDW) as pretreatment. Compared to the vehicle control group, SHRs pretreated with 10 mg/kg L-NAME for 30 min showed a significant increase in mean blood pressure (MBP) (P < 0.05), while heart rate remained unaffected (Fig. 3a–c). Furthermore, SHRs pretreated with 10 mg/kg L-NAME for 30 min followed by chrysin (10 mg/kg) via gastric gavage did not exhibit significant blood pressure-lowering effects compared to the vehicle control (Table 1). This suggests that the antihypertensive effect of chrysin (10 mg/kg) was attenuated in the presence of L-NAME, indicating the involvement of the endothelial NOS pathway in mediating chrysin’s blood pressure-lowering effects. Conversely, when SHRs were pretreated with 100 mg/kg aminoguanidine (a selective iNOS inhibitor), the antihypertensive effect of chrysin (10 mg/kg) was not significantly inhibited. This suggests that inhibition of inducible NOS (iNOS) did not interfere with chrysin’s ability to lower blood pressure, further supporting the involvement of eNOS specifically in mediating chrysin’s effects. These findings indicate that chrysin’s antihypertensive effects are mediated, at least in part, through the endothelial NOS pathway, as evidenced by the lack of efficacy in the presence of the eNOS inhibitor L-NAME, but not affected by inhibition of iNOS with aminoguanidine.
Fig. 3.
The effects of oral chrysin and the eNOS inhibitor (L-NAME). (a) The effects on mean blood pressure by chrysin only (10 mg/kg), L-NAME only (10 mg/kg) and combine treatment with pretreated L-NAME before oral chrysin administration. (b) The effects on Heartbeat rate by chrysin only, L-NAME only, and combine treatment. (c) The effects on pulse pressure by chrysin only, L-NAME only, and combine treatment. Data are shown as mean ± SEM, n = 6.
Table 1.
Blood pressure-lowering effects of chrysin and different inhibitors compared to the vehicle control.
| 0 min | 15 min | 30 min | 45 min | 60 min | |
|---|---|---|---|---|---|
| Mean blood pressure (mmHg) | |||||
| Control | 114 ± 11.7 | 110 ± 10.5 | 104 ± 5.6 | 102 ± 3.2 | 100 ± 3.0 |
| 10 mg/kg | 121 ± 10.5 | 109 ± 15.0 | 97 ± 14.2 | 85 ± 13.3 | 82 ± 7.7 |
| L-NAME (10 mg/kg) | 140 ± 10.3 | 145 ± 15.6 | 141 ± 12.2 | 145 ± 13.5 | 147 ± 11.4 |
| L-NAME (10 mg/kg) + Chrysin (10 mg/kg) | 143 ± 12.4 | 149 ± 14.5 | 146 ± 17.0 | 147 ± 19.9 | 149 ± 17.1 |
| Aminoguanidine (100 mg/kg) + chrysin (10 mg/kg) | 127 ± 24.6 | 110 ± 10.9 | 103 ± 10.8 | 97 ± 12.8 | 94 ± 13.6 |
| Systolic blood pressure (mmHg) | |||||
| Control | 156 ± 7.9 | 152 ± 13.5 | 145 ± 9.4 | 143 ± 7.7 | 141 ± 6.6 |
| 10 mg/kg | 163 ± 16.1 | 150 ± 19.8 | 142 ± 14.2 | 126 ± 18.8 | 120 ± 8.7 |
| L-NAME (10 mg/kg) | 178 ± 11.6 | 185 ± 21.4 | 181 ± 16.9 | 185 ± 15.6 | 192 ± 13.3 |
| L-NAME (10 mg/kg) + Chrysin (10 mg/kg) | 181 ± 18.1 | 188 ± 21.3 | 185 ± 24.9 | 185 ± 26.7 | 189 ± 25.1 |
| Aminoguanidine (100 mg/kg) + chrysin (10 mg/kg) | 166 ± 27.7 | 150 ± 15.5 | 143 ± 16.2 | 136 ± 16.0 | 133 ± 18.7 |
| Diastolic blood pressure (mmHg) | |||||
| Control | 85 ± 13.3 | 80 ± 8.9 | 74 ± 5.5 | 73 ± 4.3 | 72 ± 4.1 |
| 10 mg/kg | 90 ± 6.2 | 77 ± 9.2 | 69 ± 12.2 | 59 ± 9.2 | 58 ± 6.8 |
| L-NAME (10 mg/kg) | 104 ± 14.3 | 110 ± 12.6 | 107 ± 11.4 | 110 ± 13.7 | 110 ± 12.9 |
| L-NAME (10 mg/kg) + Chrysin (10 mg/kg) | 110 ± 10.4 | 116 ± 12.2 | 112 ± 14.8 | 109 ± 16.0 | 111 ± 12.9 |
| Aminoguanidine (100 mg/kg) + chrysin (10 mg/kg) | 93 ± 21.7 | 79 ± 7.2 | 70 ± 9.5 | 65 ± 11.9 | 64 ± 10.6 |
| Pluse pressure (mmHg) | |||||
| Control | 70 ± 7.1 | 71 ± 9.0 | 71 ± 9.6 | 69 ± 9.1 | 68 ± 9.0 |
| 10 mg/kg | 74 ± 9.3 | 75 ± 10.3 | 72 ± 7.6 | 67 ± 12.7 | 64 ± 4.7 |
| L-NAME (10 mg/kg) | 73 ± 17.4 | 74 ± 13.4 | 72 ± 11.7 | 75 ± 6.6 | 81 ± 6.3 |
| L-NAME (10 mg/kg) + Chrysin (10 mg/kg) | 69 ± 14.7 | 72 ± 14.5 | 72 ± 17.7 | 75 ± 19.5 | 77 ± 17.9 |
| Aminoguanidine (100 mg/kg) + chrysin (10 mg/kg) | 73 ± 6.2 | 71 ± 8.0 | 73 ± 11.9 | 70 ± 11.6 | 67 ± 13.6 |
| Heart beat rate (BPM) | |||||
| Control | 327 ± 41.3 | 331 ± 37.6 | 322 ± 34.0 | 320 ± 31.6 | 319 ± 32.3 |
| 10 mg/kg | 321 ± 16.9 | 341 ± 23.0 | 327 ± 22.1 | 313 ± 32.3 | 312 ± 32.5 |
| L-NAME (10 mg/kg) | 337 ± 17.4 | 325 ± 25.0 | 322 ± 23.4 | 321 ± 25.4 | 327 ± 26.4 |
| L-NAME (10 mg/kg) + Chrysin (10 mg/kg) | 310 ± 33.2 | 314 ± 39.7 | 311 ± 42.7 | 313 ± 45.8 | 312 ± 44.7 |
| Aminoguanidine (100 mg/kg) + chrysin (10 mg/kg) | 334 ± 27.8 | 318 ± 41.6 | 309 ± 35.5 | 301 ± 30.8 | 310 ± 38 |
Effect of chrysin co-infused with ACE inhibitor-Benazepril on BP
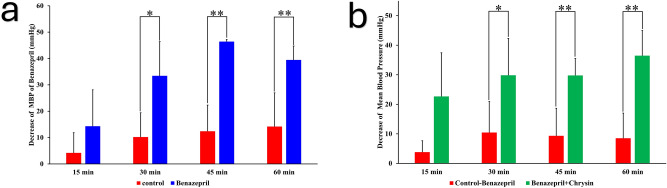
To demonstrate the antihypertensive effects of chrysin via the RAAS pathway, SHR rats were orally administered with the ACE inhibitor Benazepril (4.5 mg/kg in DDW) and recorded the BP data during 1 h. The results suggested that Benazepril attenuate the MBP significantly in 30 min (P < 0.05) and reach to the maximum antihypertensive effect in 45 min (P < 0.01). Later on, the MBP exhibited an upward trend, and the results were presented in Fig. 4a. The SHR rats were orally administered with Benazepril (4.5 mg/kg in DDW) as pretreatment for 90 min. Subsequently, the SHR rats were treated with vehicle control or 10 mg/kg chrysin. Afterward, blood pressure data were recorded for the following 60 min. The results indicated that under pretreatment with benazepril, chrysin demonstrated an additional antihypertensive effect, reaching its peak at 45 min (P < 0.01) (Fig. 4b). The results verified that the antihypertensive effects of chrysin are not entirely mediated through the RAAS pathway.
Fig. 4.
The effects of oral chrysin and the ACE inhibitor (Benazepril). (a) The effects on mean blood pressure by oral Benazepril (4.5 mg/kg) administration. (b) The attenuation of mean blood pressure by 10 mg/kg chrysin in 60 min with pretreatment of Benazepril (4.5 mg/kg). Data are shown as mean ± SEM, n = 6.
Effect of oral chrysin on electrocardiogram
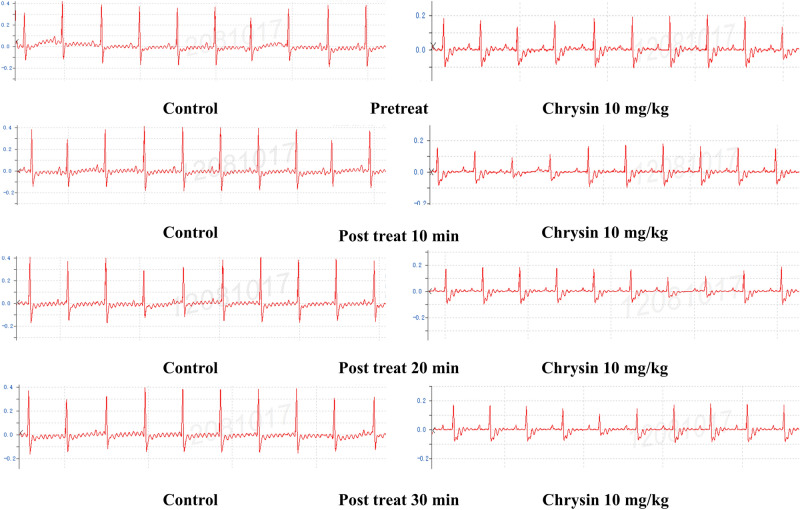
To investigate whether oral chrysin induces arrhythmias, the electrocardiograms (ECGs) of rats were measured at 10 min, 20 min, and 30 min after oral administration. Surprisingly, compared to the control group, the ECG data revealed that oral chrysin did not cause any significant changes in the ECGs of the rats (Fig. 5).
Fig. 5.
The effects of control group (oral 0.5% CMC-Na) and the oral chrysin (10 mg/kg) on ECG.
Discussion
Pharmacological studies have shown that the oral bioavailability of chrysin is extremely low (< 1%). In a pharmacokinetic study on adult male SD rats, the oral bioavailability (F%) of chrysin was found to be 1%, and most of the chrysin detected in plasma was in the form of glucuronide 17. This may help explain why chrysin is highly effective in vitro, but its efficacy in in vivo studies is less promising. The efficacy and safety of chrysin for human health have been partially evaluated. In some clinical studies, chrysin was administered orally either as a purified compound in capsule form or as an herbal extract. The maximum dosage was 625 mg/day for a duration of 4 weeks 18. The measurement of blood pressure in rats in a conscious state using non-invasive methods may indeed induce stress responses, which can affect the accuracy of the results 19. In contrast, performing invasive blood pressure measurements under anesthesia primarily benefits from the reduction of stress on the results 20. Anesthesia effectively alleviates any anxiety or discomfort that rats might experience during the measurement process, thereby providing more stable and consistent physiological data 21. Under these conditions, blood pressure measurements can more accurately reflect the physiological state of the rats, avoiding fluctuations caused by stress. Furthermore, anesthesia allows researchers to perform precise operations and monitoring more easily, thereby enhancing the reproducibility and reliability of the experiments 22.
In clinical practice, pharmacological management of hypertension targets five major pathways: calcium channels, β-adrenergic receptors, nitric oxide, diuretics, and the renin–angiotensin–aldosterone system (RAAS) 23. Medications that block calcium channels or β-adrenergic receptors can induce rapid antihypertensive effects but may decrease cardiac output and heart rate 24. Inhibiting RAAS using angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or diuretics provides slower but sustained antihypertensive effects 25. Our study results demonstrate the rapid antihypertensive effects of chrysin in attenuating symptoms in spontaneously hypertensive rats (SHRs), without affecting pulse pressure or heart rate.
Alterations in the neuroendocrine system by the sympathetic nervous system can directly impact cardiac output and heart rate, affecting blood pressure, particularly heart rate. Additionally, the sympathetic nervous system influences heart rate and cardiac contractility, thereby altering cardiac output. Furthermore, pulse pressure differences largely reflect variations in cardiac output. Based on our results, chrysin did not significantly alter heart rate or pulse pressure. Therefore, this further demonstrates that the antihypertensive effects of chrysin are not mediated through the neuroendocrine system.
Heart output and heart rate are influenced by alterations in calcium channels 26. Blocking calcium channel proteins can lower blood pressure, making calcium channel blockers a well-established mechanism for developing antihypertensive drugs. Based on in vivo experiments, oral chrysin significantly reduced mean blood pressure (MBP), systolic blood pressure (SBP), and diastolic blood pressure (DBP) but did not affect pulse pressure or heart rate. This suggests that chrysin’s regulation of blood pressure is not achieved through the calcium channel molecular mechanism.
An in vitro study suggested that chrysin induces vasodilation in aortas from spontaneously hypertensive rats (SHRs), with the endothelium playing a crucial role in this vasorelaxant effect 27. This study demonstrated that pretreatment with L-NAME (an eNOS inhibitor) completely abolishes the antihypertensive effects of chrysin in SHR. Nitric oxide (NO) serves as a critical signaling molecule within blood vessels. All nitric oxide synthase (NOS) enzymes bind to calmodulin and contain heme. Endothelial NOS (eNOS) is primarily expressed in endothelial cells, where it maintains blood vessel dilation, regulates blood pressure, and exerts various Vaso-protective and anti-atherosclerotic effects 28. NO release can occur via the PI3K/PTEN/Akt/eNOS pathway. eNOS is a pivotal player in hypertension treatment due to its vasodilatory function and is downstream of Akt in the PI3K/Akt/eNOS pathway, which is crucial for signal transduction in endothelial cells. In this cascade, PI3K promotes the conversion of PIP2 to PIP3, thereby enhancing Akt and eNOS activity to increase NO levels. Consistent with this mechanism, recent experiments measuring NO release in endothelial cells have reported that chrysin enhances NO production 29. Both IP3R and NOS are regulated by calcium ions and calmodulin, which can significantly affect heart rate. Current research indicates that treatment with chrysin does not affect heart rate or pulse pressure, suggesting that chrysin does not exert its effects through calcium channels in SHRs. However, pretreatment with the NO inhibitor L-NAME blocks the antihypertensive effects of chrysin, indicating that chrysin’s blood pressure-lowering effects may be mediated directly through eNOS.
The main mechanism of benazepril is inhibiting the activity of ACE, plays a crucial role in the RAAS. Benazepril prevents the conversion of angiotensin I to angiotensin II, leading to vasodilation and thereby reducing peripheral vascular resistance 30. The current study suggested that chrysin attenuates the blood pressure with the pretreatment of benazepril. This indicated that the antihypertensive effects of chrysin were not entirely mediated through the RAAS pathway.
In silico simulations have shown that chrysin exhibits a high affinity for eNOS by forming several hydrogen bonds, suggesting a potential new allosteric active site within the eNOS conformation. The binding position of conformation 1 chrysin-eNOS is structurally similar to the activation of eNOS at the interaction site TYR83 reported previously 31, but chrysin does not form an interaction on the TYR83 amino acid residue in current study. However, in conformation 2 chrysin-eNOS, chrysin form an interaction in SER1179 and the interactions of this conformation was stable in the molecular simulation. Previous reports had shown that eNOS undergoes agonist-induced phosphorylation at SER1179 site 32, which coincides with the docking results. Molecular dynamics simulations of the eNOS–chrysin complex indicate stable interactions throughout the RMSD simulation, suggesting that chrysin and eNOS may form stable complexes. These findings provide further evidence for chrysin’s potential as an eNOS agonist, contributing to its antihypertensive effects. Further studies investigating the allosteric sites of eNOS are necessary to elucidate the molecular target and complex conformation of chrysin, potentially through crystallography and X-ray analysis.
The in vivo results of this study demonstrated the acute antihypertensive effects of chrysin in spontaneously hypertensive rats (SHRs), which were observed in a dose-dependent manner following oral administration. A sub-chronic oral administration study of chrysin showed potent antihypertensive and antioxidant properties in L-NAME-induced hypertensive rats 33. Despite proposing vasorelaxant actions, chronic daily administration of chrysin in SHRs did not sustain its antihypertensive effects over time 34. In this study, through testing with different doses, we found that the antihypertensive effect of chrysin is dose-dependent, with 10 mg/kg being the optimal dose for chrysin’s antihypertensive effect. Previous reports have suggested that 25 mg/kg 9 can be used as a dose to lower blood pressure. Our results, which are lower than this dose, demonstrate that chrysin can exert its blood pressure-lowering effect at a smaller dose.
The differing effects of chrysin in different hypertensive models are likely attributed to its activation of eNOS. However, this activation did not result in persistent blood pressure-lowering effects over extended periods of oral administration, possibly due to factors such as daily dosage, method or timing of blood pressure measurement, or specific characteristics of the SHR model. Importantly, while the blood pressure-lowering effect of chrysin was insignificant in normotensive animals, it was significant in hypertensive animals.
Furthermore, our research has revealed that chrysin can bind strongly to the MLCK binding site, suggesting its potential as an MLCK inhibitor. Myosin light-chain kinase (MLCK) plays a crucial role in regulating events of smooth muscle relaxation and contraction 35,36. Supporting our hypothesis, a previous study demonstrated the relaxant effects of chrysin on rat aortic smooth muscle 37.
Therefore, chrysin may block MLCK from catalyzing the phosphorylation of MLC20, which reduces the motive force generated by MLC20 phosphorylation and ultimately regulates the cycling of myosin cross-bridges along actin filaments. Consequently, chrysin has the potential to inhibit MLCK, thereby effectively suppressing smooth muscle contraction. However, further studies are necessary to fully elucidate the exact molecular mechanisms involved.
Based on in silico and in vivo evidence, the rapid antihypertensive effects of chrysin may depend on its action as an eNOS agonist, promoting endogenous nitric oxide (NO) production and resulting in blood vessel dilation. These acute antihypertensive effects suggest that chrysin has the potential to be a candidate medication for urgently lowering blood pressure requirements without posing hypertensive risks.
Supplementary Information
Acknowledgements
The authors would like to thank the participants who participate in the study project and especially Jingqi Xu, Jie Cai and Jingru Lin for relevant contributions to this project.
Abbreviations
- ACE
Angiotensin-converting enzyme
- ARBs
Angiotensin receptor blockers
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- eNOS
Endothelial nitric oxide synthase
- iNOS
Inducible nitric oxide synthase
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- OMIM
Online Mendelian Inheritance in Man
- MD
Molecular dynamics
- L-NAME
NG-nitro-l-arginine methyl ester
- DMSO
Dimethyl sulfoxide
- CMC-Na
Carboxymethylcellulose sodium
- IVCs
Individually ventilated cages
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- MLCK
Myosin light-chain kinase
Author contributions
All authors contributed to the study conception and design. Zi-Han Shen: Writing – original draft, Validation, Project administration, Methodology. Tongjie Ye: Software, Formal analysis, Data curation. Xiaolin Lu: Resources, Supervision, Visualization. Baozhen Chen: Validation, Methodology. Congchao Wan: Project administration, Data curation. Ting-Hsu Chen: Software. Shuang Lin, Jia-Xin Ye: Supervision. Liping Xie: Resources, Conceptualization. Yaw-Syan Fu: Writing – review and editing, Resources. The first draft of the manuscript was written by Zi-Han Shen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Xiamen Medical College Grant [Grant Numbers K2019-03]; Xiamen Medical College, Xiamen, Fujian, China.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All the methods were performed in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animal experiments were approved by Xiamen University Laboratory animals center Ethics Committee and the “Guide for the Care and Use of Laboratory Animals” of Xiamen Medical College was followed (Approved protocol ID SYXK 2018–0009, Aug. 24, 2021).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84259-6.
References
- 1.Yang, L. et al. Vascular VPO1 expression is related to the endothelial dysfunction in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun.439, 511–516. 10.1016/j.bbrc.2013.09.012 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Mani, R. & Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry145, 187–196. 10.1016/j.phytochem.2017.09.016 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Saqib, U. et al. Polypharmacology or promiscuity? Structural interactions of resveratrol with its bandwagon of targets. Front. Pharmacol.9, 1201. 10.3389/fphar.2018.01201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakatsu, R. et al. The combination of venetoclax and quercetin exerts a cytotoxic effect on acute myeloid leukemia. Sci. Rep.14, 26418. 10.1038/s41598-024-78221-9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreczmer, B., Dyba, B., Barbasz, A. & Rudolphi-Szydło, E. Curcumin’s membrane localization and disruptive effects on cellular processes—insights from neuroblastoma, leukemic cells, and Langmuir monolayers. Sci. Rep.14, 16636. 10.1038/s41598-024-67713-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu, H. et al. Exploring the mechanism of Jinlida granules against type 2 diabetes mellitus by an integrative pharmacology strategy. Sci. Rep.14, 10286. 10.1038/s41598-024-61011-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tie, Y. et al. Multi-omic analysis revealed the therapeutic mechanisms of Alpinia oxyphylla fructus water extract against bladder overactivity in spontaneously hypertensive rats. Phytomedicine123, 155154. 10.1016/j.phymed.2023.155154 (2024). [DOI] [PubMed] [Google Scholar]
- 8.El-Nahhal, Y. & El-Nahhal, I. Cardiotoxicity of some pesticides and their amelioration. Environ. Sci. Pollut. Res.28, 44726–44754. 10.1007/s11356-021-14999-9 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Veerappan, R. & Malarvili, T. Chrysin pretreatment improves angiotensin system, cGMP concentration in L-NAME induced hypertensive rats. Indian J. Clin. Biochem.34, 288–295. 10.1007/s12291-018-0761-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tew, W. Y. et al. Evaluation of vasodilatory effect and antihypertensive effect of chrysin through in vitro and sub-chronic in vivo study. Biomed. Pharmacother.157, 114020. 10.1016/j.biopha.2022.114020 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Naz, S. et al. Chrysin: Pharmacological and therapeutic properties. Life Sci.235, 116797. 10.1016/j.lfs.2019.116797 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Villar, I. C. et al. Endothelial nitric oxide production stimulated by the bioflavonoid chrysin in rat isolated aorta. Planta Med.71, 829–834. 10.1055/s-2005-871296 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Kseibati, M. O., Sharawy, M. H. & Salem, H. A. Chrysin mitigates bleomycin-induced pulmonary fibrosis in rats through regulating inflammation, oxidative stress, and hypoxia. Int. Immunopharmacol.89, 107011. 10.1016/j.intimp.2020.107011 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Duarte, J. et al. Vasorelaxant effects of the bioflavonoid chrysin in isolated rat aorta. Planta Med.67, 567–569. 10.1055/s-2001-16492 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Garcin, E. D. et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol.4, 700–707. 10.1038/nchembio.115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh, S. S. & Narver, H. L. Mouse and rat anesthesia and analgesia. Curr. Protoc.4, e995. 10.1002/cpz1.995 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noh, K. et al. Pharmacokinetic interaction of chrysin with caffeine in rats. Biomol. Ther. (Seoul)24, 446–452. 10.4062/biomolther.2015.197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, S. et al. Developing nutritional component chrysin as a therapeutic agent: Bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed. Pharmacother.142, 112080. 10.1016/j.biopha.2021.112080 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatem, A. et al. Stress-induced effects on cardiovascular function in rats. J. Psychosom. Res.62, 123–130. 10.1016/j.jpsychores.2006.09.004 (2007). [Google Scholar]
- 20.Fischer, M. et al. The impact of measurement methods on blood pressure in rats: Stress considerations. J. Psychosom. Res.74, 467–473. 10.1016/j.jpsychores.2013.01.001 (2013). [Google Scholar]
- 21.Kikuchi, S. et al. Behavioral stress and its effects on blood pressure measurements in rodents. Neuroscience288, 189–196. 10.1016/j.neuroscience.2015.03.001 (2015). [Google Scholar]
- 22.Bowers, A. A. et al. Anesthesia and cardiovascular monitoring: Enhancing data reliability in rodent studies. Prog. Neuropsychopharmacol. Biol. Psychiatry35, 327–334. 10.1016/j.pnpbp.2011.02.007 (2011). [Google Scholar]
- 23.Ghatage, T., Goyal, S. G., Dhar, A. & Bhat, A. Novel therapeutics for the treatment of hypertension and its associated complications: Peptide- and nonpeptide-based strategies. Hypertens. Res.44, 740–755. 10.1038/s41440-021-00643-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah, K., Seeley, S., Schulz, C., Fisher, J. & Gururaja Rao, S. Calcium channels in the heart: Disease states and drugs. Cells11, 943. 10.3390/cells11060943 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaman, M. A., Oparil, S. & Calhoun, D. A. Drugs targeting the renin-angiotensin-aldosterone system. Nat. Rev. Drug Discov.1, 621–636. 10.1038/nrd873 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Goto, K. & Kitazono, T. Chloride ions, vascular function and hypertension. Biomedicines10, 2316. 10.3390/biomedicines10092316 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Förstermann, U. & Sessa, W. C. Nitric oxide synthases: Regulation and function. Eur. Heart J.33, 829–837. 10.1093/eurheartj/ehr304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Q. Q., Cheng, N., Yi, W. B., Peng, S. M. & Zou, X. Q. Synthesis, nitric oxide release, and α-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorg. Med. Chem.22, 1515–1521. 10.1016/j.bmc.2014.01.038 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Ihara, E. & MacDonald, J. A. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can. J. Physiol. Pharmacol.85, 79–87. 10.1139/y06-103 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Balfour, J. A. & Goa, K. L. Benazepril. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in hypertension and congestive heart failure. Drugs42, 511–539. 10.2165/00003495-199142030-00008 (1991). [DOI] [PubMed]
- 31.Ruan, L. et al. Calcineurin-mediated dephosphorylation of eNOS at serine 116 affects eNOS enzymatic activity indirectly by facilitating c-Src binding and tyrosine 83 phosphorylation. Vasc. Pharmacol.59, 27–35. 10.1016/j.vph.2013.05.004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez, E., Kou, R., Lin, A. J., Golan, D. E. & Michel, T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J. Biol. Chem.277, 39554–39560. 10.1074/jbc.M207299200 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Sellers, J. R., Spudich, J. A. & Sheetz, M. P. Light chain phosphorylation regulates the movementof smooth muscle myosin on actin filaments. J. Cell Biol.101, 1897–1902. 10.1083/jcb.101.5.1897 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrera, M. D., Zarzuelo, A., Jiménez, J., Marhuenda, E. & Duarte, J. Effects of flavonoids on rat aortic smooth muscle contractility: Structure-activity relationships. Gen. Pharmacol.27, 273–277. 10.1016/0306-3623(95)02010-1 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Liu, Y. et al. The role of nitric oxide in cardiovascular disease: Implications for therapy. Eur. J. Pharmacol.774, 90–99. 10.1016/j.ejphar.2016.01.003 (2016). [Google Scholar]
- 36.Sharma, P. et al. The impact of oxidative stress in cardiovascular disease. Heart Fail. Rev.24, 175–183. 10.1007/s10741-019-09785-6 (2019). [Google Scholar]
- 37.Zhao, S. et al. Regulation of blood pressure through neurogenic and vascular mechanisms. Hypertens. Res.42, 1683–1691. 10.1038/s41440-019-0300-7 (2019).31316170 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.