ABSTRACT
Introduction
Lyme disease (LD) is caused by infection with the bacteria Borrelia burgdorferi sensu lato (Bb) through the bite of an infected Ixodes spp. tick. LD has emerged as a public and animal health issue in Canada, with human incidence increasing in part due to the expansion of Ixodes scapularis ticks and their vertebrate hosts. We sought to provide the first comprehensive summary of published tick and animal surveillance literature regarding LD in Canada to describe changes in LD over time.
Methods
We conducted a review to identify peer‐reviewed LD‐focused tick, mammal, and bird surveillance articles in three online databases between 1975 and 2023. Data on study characteristics, data collection years, and surveillance methods and findings were extracted. Descriptive statistics were reported.
Results
In total, 115 studies were included for review. Results showed an increase in published surveillance literature and changes in study approaches and their provincial distribution over time, coinciding with increased LD cases in Canada. Seventy‐four studies were published after 2014 when Canada's Federal Framework on Lyme Disease Act was introduced, and two‐thirds of these studies focused on tick surveillance only. Overall, 58% of studies involved surveillance in Ontario but increases in all other provinces were observed after 2009.
Conclusions
Observed changes in five decades of LD‐related tick and animal surveillance literature helps document the historical rapid spread of Ixodes and Bb across provinces. This can provide lessons for other regions that may transition from emerging to endemic status for LD in the coming years.
Keywords: Borrelia, Canada, Ixodes, Lyme, surveillance, tick
Summary.
This review provides the first historical summary of tick and animal surveillance research as it pertains to Lyme disease in Canada.
The rapid invasion of Ixodes ticks and Borrelia burgdorferi in Canada shows how Lyme disease became a growing One Health concern over the span of five decades.
The reviewed surveillance studies identify areas of Ixodes and Borrelia burgdorferi emergence and endemicity, which match the areas in Canada that are high risk for Lyme disease. This can be used to provide lessons for other regions, within and outside of Canada, that may transition from pre‐emerging to emerging to endemic status for Lyme disease in the coming years.
1. Introduction
Lyme disease (LD) is a tick‐borne disease (TBD) caused by infection with the bacterium Borrelia burgdorferi sensu lato (Bb) through the bite of an infected Ixodes species tick. LD has emerged as a public health threat in Canada, with the number of human cases increasing from a total of 140 reported annually between 1984 and 1990 to 2,525 in 2022 (“Consensus conference on Lyme disease,” 1991; PHAC 2024). While LD often follows an acute and mild clinical course, it can also result in more serious manifestations. In fact, recent data has shown that up to half of cases reported in Canada lead to more serious neurological, cardiac, or arthritic conditions, although under‐detection of milder cases may contribute to this high proportion (Murison et al. 2023).
In Canada, Bb is transmitted primarily by two Ixodes species: I. scapularis , the blacklegged tick (also called I. dammini prior to 1993) in the eastern and central parts of the country, and I. pacificus , the western blacklegged tick, in the west (Ogden et al. 2009; Oliver Jr. et al. 1993). The pathogen is maintained in nature via several small rodent and shrew species, most notably the white‐footed mouse ( Peromyscus leucopus ), which serve not only as hosts for ticks but as natural reservoir hosts for Bb (Anderson 1988; Levine, Wilson, and Spielman 1985). Larval Ixodes ticks feed on infected reservoir hosts, acquire the bacterium, and then, in the nymphal and adult stages, can transmit it to humans or animals (Apanaskevich and Oliver 2014). Tick dispersal throughout Canada is largely attributed to migratory birds. It has been estimated that they introduce 50–175 million blacklegged ticks across Canada each spring, facilitating local population establishment (Ogden et al. 2008). Additionally, large mammals such as white‐tailed deer ( Odocoileus virginianus ) can serve as local reproductive tick hosts, resulting in the expansion of established Ixodes populations in Canada (Bouchard et al. 2013; Bryan et al. 2011; Werden et al. 2014). To address the growing concern of LD in Canada, researchers and public health officials met at the Canadian Consensus Conference on Lyme Disease in 1991 to align on the epidemiology, epizootiology, clinical course, and laboratory diagnostics of LD (Canadian Medical Association Journal, 1991). Canada established LD as nationally notifiable in 2009, requiring reporting of human cases to the Public Health Agency of Canada (PHAC) (PHAC 2024). Following the national mandate, Canada enacted the Federal Framework on Lyme Disease Act in 2014, requiring the government to enhance LD surveillance, increase education and awareness, and establish best practices and guidelines to prevent and control its geographic spread (Canada 2017a).
The Federal Framework's approach to monitoring LD utilises One Health, defined by the World Health Organization as a “unifying approach to balance and optimize the health of people, animals, and the environment” (WHO 2017). One Health involves collaboration amongst the public health, veterinary, and environmental sectors with the understanding that the health of one affects the health of all. For TBDs specifically, one component of an integrated surveillance system utilising the One Health approach involves surveillance of ticks, their pathogens, and animal hosts that lead to disease emergence and spread (Braks et al. 2011). The Federal Framework set out to develop one such integrated system in places like parks, First Nation and ecological reserves, historical sites, and information from veterinarians. Importantly, the initiative provides critical data to produce risk maps and inform national prevention efforts against TBDs (Canada 2017b).
Canada utilises different field surveillance strategies to monitor the geographic distribution and abundance of vector tick populations and the prevalence of tick‐borne pathogens to inform TBD risk assessment. Passive surveillance involves the voluntary submission of sample specimens collected from host species by the public, which requires minimal resources to help establish a baseline distribution of ticks and their associated pathogens across vast geographies (Clow et al. 2019; Nsubuga et al. 2006). Active surveillance employs coordinated and more costly, resource‐intensive efforts to collect ticks from their natural habitat to estimate tick‐pathogen abundance and establish local tick populations (Jordan, Gable, and Egizi 2022). Examples of active surveillance include dragging and flagging for host‐seeking (“questing”) ticks and the collection of blood‐feeding ticks from their mammalian hosts via animal trapping (Clow et al. 2019; Sonenshine and Roe 1993). Surveillance on large mammals, including white‐tailed deer and domestic pets such as dogs ( Canis lupus familiaris ), and surveillance of migratory bird species and the ticks attached to them are other useful ways to understand tick presence and abundance and monitor geographic expansion of TBDs (Battaly and Fish 1993; Bouchard et al. 2013; Bryan et al. 2011; Werden et al. 2014).
Monitoring changes in the abundance and geographic reach of tick and key animal host species over time using a coupled active and passive surveillance strategy is a critical component of an integrated approach to TBD surveillance (Braks et al. 2011). The increase in human LD cases in Canada has followed the rapid northward geographic expansion of I. scapularis and its animal hosts since it was first identified in Ontario in the 1970s (Watson and Anderson 1976). Due to evolving ecological factors such as climate change, the expansion of I. scapularis , Bb, host species, and LD is expected to continue throughout Canada, emphasising the need for continued surveillance efforts to monitor spread (Bouchard et al. 2019; Canada 2023; Ogden 2014). A framework for adaptive surveillance of emerging tick‐borne zoonoses was developed using LD in Canada as a case study (Clow et al. 2019). The framework describes the most appropriate surveillance approaches to consider at different points of TBD emergence, including ecological studies and human disease monitoring in addition to tick and animal host surveillance. During the pre‐emergence phase, where the goal is to detect the introduction of ticks and their pathogens, passive tick surveillance and initial active tick surveillance at sentinel sites are the most suitable methods to use. Once ticks and their pathogens emerge, the focus should shift to identifying more precise locations of their established populations. For this, targeted active tick surveillance and collection of tick hosts and their serological data are suggested as the most appropriate methods (Clow et al. 2019).
The purpose of this scoping review is to provide the first summary of published tick and animal surveillance literature with respect to LD in Canada from 1975 to 2023. Furthermore, we aimed to describe how surveillance approaches and collection methods have evolved geographically and over time, helping to address and inform the public health threat of LD through a One Health lens.
2. Materials and Methods
This study utilised established scoping review methods, as described in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR) (Tricco et al. 2018). Analyses were conducted according to guidelines from a pre‐defined protocol, including clear eligibility criteria, search strategy, data collection and charting, and synthesis of results (Tricco et al. 2018).
2.1. Literature Search
A literature search was performed to identify peer‐reviewed tick, small and large mammal, and migratory bird surveillance articles in PubMed, Embase, and Web of Science published from 1 January 1975 through 6 November 2023 in the English or French languages. The starting point of 1975 was the year LD was first described in North America (CDC 1982). Search strings are provided in Appendix 1.
2.2. Eligibility Criteria
After removal of duplicates, titles and abstracts of remaining articles were evaluated for relevance by three researchers, independently. Full‐text article reviews were then conducted by two researchers independently to confirm study eligibility. Articles were included if they reported on one or more of the following: (1) tick surveillance on I. scapularis (including publications that referred to the species as I. dammini ) or I. pacificus (referred to as Ixodes here on) collected via active (flagging or dragging) or passive (from humans or animals, including citizen science) methods; (2) surveillance on Bb reservoir hosts or other species that play a significant role in the Bb‐tick lifecycle, like deer or migratory birds, collected via active (e.g., mammalian or bird trapping) or passive (e.g., hunting or collection of roadkill) methods; or (3) surveillance activities on other animal species that include serological testing of Bb, such as passive surveillance (e.g., serosurveys) on domesticated animals. Studies that did not occur in Canada or did not report the year(s) of data collection were excluded.
2.3. Data Extraction and Structure
Data from included studies were extracted into a data table in Microsoft Excel. Extracted data included: author(s); publication year; study collection year(s); province(s); surveillance method(s); collection method(s); species and number of tick(s) or animal(s) surveilled, tested for Bb, and tested positive for Bb; and diagnostic testing method type. For Ixodes, if tick life stages were specified in the articles, larvae were not counted.
For each study, two independent researchers categorised the study approach(es) based on the framework for adaptive surveillance of emerging tick‐borne zoonoses: passive tick surveillance, active tick surveillance—sentinel, active tick surveillance—targeted, and tick host (including domesticated or wild animals) collection or serological testing (Clow et al. 2019). A study that performed active tick surveillance was categorised into the “sentinel” approach if any active methods were performed at a site that was not known to have an established Ixodes population and the “targeted” approach if any active methods were performed at a site known to have an established Ixodes population.
2.4. Data Synthesis
To summarise results and identify how surveillance evolved across Canada and over time, particularly around key LD‐related legislation and policy changes, studies were grouped by relevant variables, including publication year ranges, study approaches, ticks and animals studied, and province. Studies could be included in more than one category for each grouping. Findings from these variables were synthesised and incorporated into a structured narrative review. Descriptive statistics, visualisations, and study synthesis were performed in Microsoft Excel and QGIS Desktop (version 3.32.1).
3. Results
3.1. Study Characteristics
The literature search retrieved 544 unique titles (Figure 1). After screening titles, abstracts, and full texts, a total of 115 (21%) articles met inclusion criteria for synthesis. A detailed table of the data extracted from the 115 reviewed articles can be found in Appendix 2.
FIGURE 1.
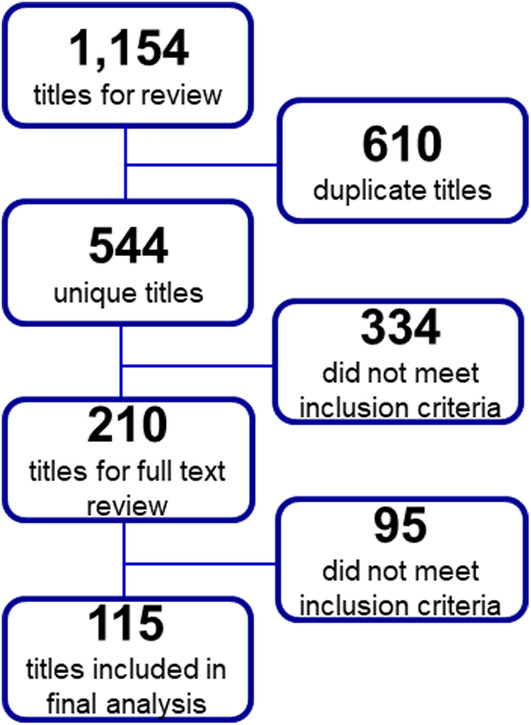
Flow diagram of the number of articles identified, screened, reviewed, and included for review. Articles were identified following searches in PubMed, Embase, and Web of Science. Figure includes the total number of articles at each step of the review process and the number of titles or articles excluded at each step.
Of the included studies, 91 (79%) were published after 2009, with 74 (64%) after 2014 (Figure 2). Only one study was published before 1990. Overall, 51% of studies present tick surveillance data only, 30% present animal surveillance data only, and 19% present data on both. More than 50% of the studies published through 2014 presented both tick and animal surveillance data compared with 15% after 2014. Approximately 24% of studies presented tick surveillance data only, compared with 66% after 2014. The percentage of studies presenting only animal surveillance data in 2014 and earlier compared with after 2014 was 24% and 19%, respectively.
FIGURE 2.
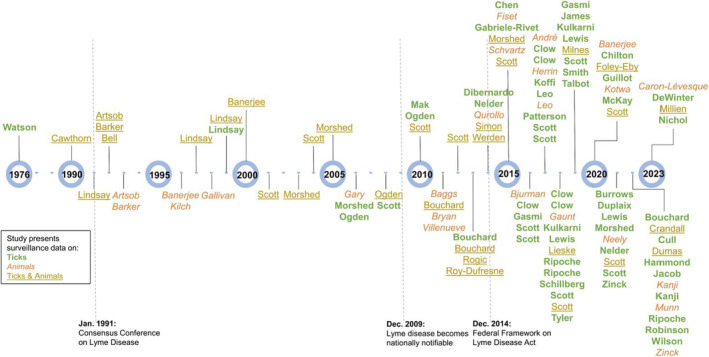
Timeline of reviewed tick and animal surveillance studies in Canada, by year of publication, 1976–2023. Included studies are ordered by last name of first author and publication year and also include type of surveillance data presented. Tick surveillance studies are displayed in green and are bolded, animal surveillance studies are displayed in orange and are italicised, and studies with surveillance on both ticks and animals are displayed in yellow and are underlined. The dates of the Consensus Conference on Lyme Disease, when Lyme disease became nationally notifiable, and when the Federal Framework on Lyme Disease Act was established are displayed, along with brief descriptions (André et al. 2017; Artsob et al. 1992; Artsob et al. 1993; Baggs et al. 2011; Banerjee et al. 1996; Banerjee et al. 2000; Banerjee et al. 2020; Barker et al. 1992; Barker et al. 1993; Bell et al. 1992; Bjurman et al. 2016; Bouchard et al. 2011; Bouchard, Beauchamp et al. 2013; Bouchard, Leighton et al. 2013; Bryan et al. 2011; Burrows et al. 2021; Caron‐Lévesque 2023; Cawthorn et al. 1990; Chen et al. 2015; Chilton et al. 2020; Clow et al. 2016; Clow, Finer, et al. 2018; Clow, Leighton et al. 2017; Clow, Ogden, et al. 2017; Clow, Ogden, et al. 2018; Crandall et al. 2022; Cull, 2022; DeWinter et al. 2023; Dibernardo et al. 2014; Dumas et al. 2022; Duplaix et al. 2021; Evason et al. 2019; Fiset et al. 2015; Foley‐Eby et al. 2020; Gabriele‐Rivet et al. 2015; Gallivan et al. 1998; Gary et al. 2006; Gasmi et al. 2016; Gasmi et al. 2019; Gaunt et al. 2018; Guillot et al. 2020; Guillot et al. 2022; Hammond‐Collins et al. 2022; Herrin et al. 2017; Jacob et al. 2022; James et al. 2019; Kanji et al. 2022; Klich et al. 1996; Koffi et al. 2017; Kotwa et al. 2020; Kulkarni et al. 2018; Kulkarni et al. 2019; Leo and Millien, 2017; Leo, Gonzalez, and Millien, 2017; Lewis et al. 2018; Lewis and Lloyd 2019; Lewis et al. 2021; Lieske and Lloyd 2018; Lindsay et al. 1991; Lindsay et al. 1997; Lindsay, Artsob et al. 1999; Lindsay, Mathison et al. 1999; Mak et al. 2010; McKay et al. 2020; Millien et al. 2023; Milnes et al. 2019; Morshed et al. 2003; Morshed et al. 2005; Morshed et al. 2006; Morshed et al. 2015; Morshed et al. 2021; Munn et al. 2022; Neely et al. 2021; Nelder et al. 2014; Nelder et al. 2021; Nichol et al. 2023; Ogden et al. 2006; Ogden et al. 2008; Ogden et al. 2010; Patterson et al. 2017; Qurollo et al. 2014; Ripoche et al. 2022; Ripoche, Gasmi et al. 2018; Ripoche, Lindsay et al. 2018; Robinson et al. 2022; Rogic et al. 2013; Roy‐Dufresne et al. 2013; Schillberg et al. 2018; Schvartz et al. 2015; Scott and Pesapane, 2021; Scott et al. 2001; Scott et al. 2004; Scott et al. 2008; Scott et al. 2010; Scott et al. 2012; Scott and Durden 2015; Scott et al. 2019; Scott et al. 2020; Scott, Anderson et al. 2016; Scott, Clark, et al. 2017; Scott, Clark, Foley, Anderson, et al. 2018; Scott, Clark, Foley, Bierman, and Durden, 2018; Scott, Foley et al. 2016; Scott, Foley, et al. 2017; Scott, Pascoe, et al. 2021; Simon et al. 2014; Smith et al. 2019; Talbot et al. 2019; Tyler et al. 2018; Villeneuve et al. 2011; Watson and Anderson, 1976; Werden et al. 2014; Wilson et al. 2022; Zinck and Lloyd, 2022; Zinck et al. 2021).
3.2. Surveillance Characteristics and Approaches
Of 90 (78%) studies that collected Ixodes by passive or active surveillance methods, 85 collected I. scapularis and 13 collected I. pacificus (Figure 3a,b). More than 20 species of rodents and shrews were collected across 25 (22%) studies (Figure 3a,d). The small mammals collected in the most studies were the white‐footed mouse ( Peromyscus leucopus ) in 18 studies, the deer mouse ( Peromyscus maniculatus ) in 11 studies, and the northern short‐tailed shrew ( Blarina brevicauda ) in 10 studies. Surveillance was performed on large mammals in 23 (20%) studies, 15 of which included surveillance on dogs ( Canis lupus familiaris ) (Figure 3a,c). There were 14 (12%) studies that included surveillance on bird species (Figure 3a). Over 150,000 Ixodes ticks, 12,000 small mammals, 960,000 large mammals, and 42,000 birds were collected and/or sampled across all studies (Figure 3).
FIGURE 3.
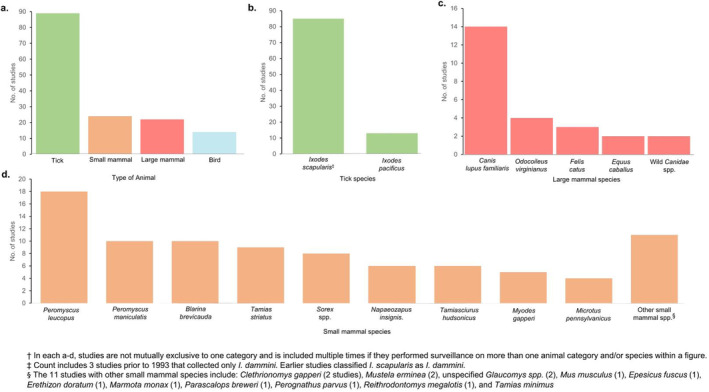
Number of reviewed studies that performed surveillance on ticks and different animal types and the species that were studied†. Bar graphs display the (a) number of reviewed studies performing surveillance on ticks, small mammals, large mammals, and birds; (b) the number of reviewed tick surveillance studies by Ixodes species collected; (c) the number of reviewed animal surveillance studies by species of large mammals collected; (d) the number of reviewed animal surveillance studies by species of small mammals collected.
Categorising the studies according to their surveillance approaches revealed 46 (40%) studies that performed passive tick surveillance and 57 studies that performed active tick surveillance, of which 33 (30%) and 25 (22%) conducted surveillance at sentinel sites and in targeted areas, respectively. One study conducted active tick surveillance in both site types. Fifty‐two (45%) studies collected tick hosts or host serological data (Figure 3).
3.3. Geographic Distribution of Studies and First Notification Year of Ixodes and Bb
Tick and tick host animal surveillance studies were conducted in all ten Canadian provinces. Ixodes were first identified in southeast Canada in Ontario (1972), Nova Scotia (1984), and Prince Edward Island (1989) (Table 1). By 2000, Ixodes had been identified in every province. All provinces detected Bb in Ixodes by 2003, except for Saskatchewan and Alberta, which detected it in 2009 and 2012, respectively. Ontario was the first province to detect Bb in any mammal in 1987, while other provinces did so beginning in 2008 (Table 1).
TABLE 1.
First detection of I . scapularis or I . pacificus , Borrelia burgdorferi (Bb) in I . scapularis or I . pacificus , and Bb in a mammal in each province across Canada amongst 115 reviewed surveillance articles.
| Province a | Year b | Reference |
|---|---|---|
| Ixodes c | ||
| ON | 1972 | Watson and Anderson (1976) |
| NS | 1984 | Bell, Specht, and Coombs (1992) |
| PEI | 1989 | Cawthorn, Horney, and Maloney (1990) |
| MN | 1990–2003 | Ogden, Trudel et al. (2006) |
| NB | 1990–2003 | Ogden, Trudel et al. (2006) |
| NL | 1990–2003 | Ogden, Trudel et al. (2006) |
| QC | 1990–2003 | Ogden, Trudel et al. (2006) |
| BC | 1993–2006 | Mak, Morshed, and Henry (2010) |
| AB | 1998 | Scott et al. (2001) |
| SK | 1998 | Lindsay et al. (1999) |
| Bb in ixodes c | ||
| ON | 1990 | Lindsay et al. (1991) |
| PEI | 1991 | Artsob et al. (1992) |
| MN | 1990–2003 | Ogden, Trudel et al. (2006) |
| NB | 1990–2003 | Ogden, Trudel et al. (2006) |
| NL | 1990–2003 | Ogden, Trudel et al. (2006) |
| QC | 1990–2003 | Ogden, Trudel et al. (2006) |
| NS | 2001 | Scott et al. (2001) |
| BC | 2003 | Morshed et al. (2005) |
| SK | 2009 | Scott, Anderson, and Durden (2012) |
| AB | 2012 | Dibernardo et al. (2014) |
| Bb in mammals c | ||
| ON | 1987 | Barker et al. (1993) |
| QC | 2007–2008 | Bouchard et al. (2011) |
| AB | 2008 | Villeneuve et al. (2011) |
| MN | 2008 | Villeneuve et al. (2011) |
| NB | 2008 | Villeneuve et al. (2011) |
| NS | 2008 | Villeneuve et al. (2011) |
| PEI | 2008 | Villeneuve et al. (2011) |
| SK | 2008 | Villeneuve et al. (2011) |
| BC | 2008–2012 | Qurollo et al. (2014) |
| NL | 2008–2015 | Evason et al. (2019) |
AB = Alberta, BC = British Columbia, MB = Manitoba, NB = New Brunswick, NL = Newfoundland & Labrador, NS = Nova Scotia, ON = Ontario, PEI = Prince Edward Island, QC = Quebec, SK = Saskatchewan.
Year indicates earliest study collection year detailed in the reviewed surveillance articles where Ixodes, Bb in Ixodes and Bb in a mammal. Where exact year of collection of first positive Ixodes or Bb‐positive specimen in Ixodes or mammal was not specified, the study collection start year was used.
Ixodes = I . scapularis or I. pacificus ; Bb = Borrelia burgdorferi . Detection of Bb includes identification by any testing technique (PCR, culture or antibody) in Ixodes or mammal collected via passive or active surveillance methods.
Overall, the provinces represented in the most studies were Ontario (58%), Quebec (35%), New Brunswick (21%), and Nova Scotia (19%) (Figure 4). All provinces appeared in studies with the following approaches: passive tick surveillance, active tick surveillance at sentinel sites, and tick host collection or serological testing. Only British Columbia, Manitoba, Ontario, Quebec, New Brunswick and Nova Scotia had one or more studies that performed active tick surveillance in targeted areas (Figure 4). Seventy‐nine percent of studies published through 2009 performed surveillance in Ontario compared with 55% of studies published after 2009. After 2009, the proportion of all studies that performed surveillance in the provinces to the east and west of Ontario increased (Figure 5).
FIGURE 4.
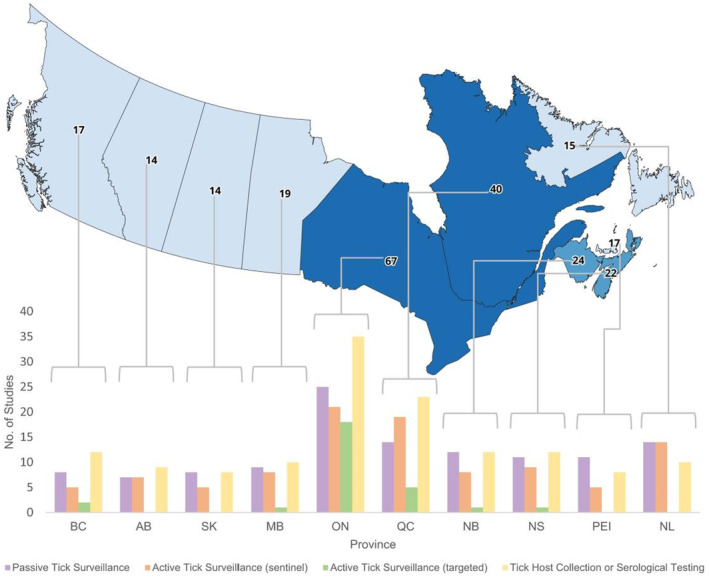
Map of Canada with the number of reviewed studies by province and bar graph showing the study approach(es) used in each reviewed study, by province. Map of 10 Canadian provinces shows the number of studies included in the review atop each province. Beneath the map is a multi‐bar graph that displays the number of studies that performed the four adaptive surveillance study approaches considered in each province. Provinces are ordered from west to east, beginning with British Columbia and ending with Newfoundland & Labrador. For each province, the study approaches are ordered left to right as: Passive Tick Surveillance, Active Tick Surveillance (sentinel), Active Tick Surveillance (targeted), Tick Host Collection or Serological Testing. BC = British Columbia, AB = Alberta, SK = Saskatchewan, MB = Manitoba, ON = Ontario, QC = Quebec, NB = New Brunswick, NS = Nova Scotia, PEI=Prince Edward Island, NL = Newfoundland & Labrador. Number of studies is not mutually exclusive, and a study is included more than once in the map if surveillance was performed in more than one province and more than one time in the multi‐bar graph if more than one study approach was taken within the study.
FIGURE 5.
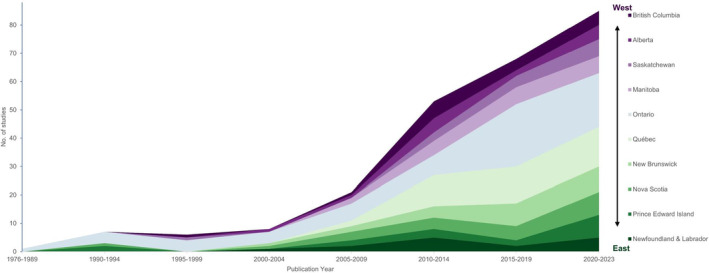
Count of included studies in each Canadian province, over time by publication year. Area chart with the count of reviewed studies with surveillance data in each province over time, by the study's publication year. Publication years are grouped. Provinces are stacked top to bottom from west to east, beginning with British Columbia and ending with Newfoundland & Labrador. Count of studies is not mutually exclusive, and a study is included more than once in the map if surveillance was performed in more than one province.
4. Discussion
In this review, we summarised the Ixodes and host animal surveillance literature as it relates to LD in Canada between 1975 and 2023. The increase in published literature and changes in both study approaches and provincial distribution over time illustrate the broad evolution of Bb and LD emergence within Canada over five decades. These observations coincide with increasing trends in annual reported human LD cases in Canada, specifically since 2009, when LD became nationally notifiable (PHAC 2024). Referring to parts of the framework for adaptive surveillance of emerging tick‐borne zoonoses (Clow et al. 2019), our review highlights when, where, and how different surveillance approaches were used, which can help guide future surveillance efforts as LD continues to grow as a public health concern across the country.
4.1. Tick and Animal Surveillance: The Past
The first identification of Ixodes in Canada occurred in 1972 when an I . scapularis tick was collected via passive surveillance from white‐tailed deer in Long Point, an area in southern Ontario along Lake Erie (Watson and Anderson 1976). Once Ixodes was found to have emerged in parts of Ontario, targeted active surveillance studies in these areas began to detect Bb in animal hosts like the white‐footed mouse and other rodents in the late 1980s and in Ixodes by the early 1990s (Barker et al. 1992; Lindsay et al. 1991). While Long Point was found to be the first endemic region for Ixodes and Bb in Canada, passive tick surveillance also detected them in Nova Scotia and Prince Edward Island between the late 1980s and early 1990s (Artsob et al. 1992; Bell, Specht, and Coombs 1992; Cawthorn, Horney, and Maloney 1990). These studies exemplify how passive and active tick surveillance, as well as surveillance of host species, can detect the emergence and spread of ticks and the pathogens they transmit.
Across the study years, all provinces had studies with passive tick surveillance, active tick surveillance at sentinel sites, and collection of tick host methods as ticks and Bb first emerged in Canada. However, we only saw studies with targeted active tick surveillance in British Columbia, Manitoba, Ontario, Quebec, New Brunswick, and Nova Scotia, which are the provinces Canada reports as having specific LD‐endemic or tick exposure risk areas (PHAC 2015). Ontario had the most studies overall and the most with targeted active tick surveillance approaches. This is unsurprising since Ontario was the first province found to have a LD‐endemic region, and there has been a more concerted effort to characterise the disease and its risks locally, aligning with the principles upon which the framework for adaptive surveillance was built (Clow et al. 2019).
At the Consensus Conference on Lyme Disease in 1991, public health officials established criteria for determining Ixodes‐endemic areas and also encouraged passive tick surveillance to identify newly emerging regions (Canadian Medical Association Journal 1991; PHAC 2015). In areas where Ixodes and Bb had already emerged, primarily in parts of Ontario, most studies focused on the expansion of ticks and their hosts, performing active tick surveillance in both sentinel and targeted areas, and collecting host species. In contrast, in areas without established Ixodes populations, we saw more studies with passive tick surveillance and active tick surveillance at sentinel sites, methods best suitable for detecting the introduction of ticks and their pathogens. For example, a government‐sponsored multi‐province passive surveillance program was set up to identify and test ticks submitted by the public (Ogden, Trudel et al. 2006). Through this program, Ixodes were identified in all provinces east of Saskatchewan. This was the first time in our review where Bb‐infected I. scapularis was identified in Manitoba, Quebec, New Brunswick, and Newfoundland & Labrador.
Additionally, some studies looked at the role that migratory birds play in tick dispersal. It is theorised that migratory birds and their ability to fly long distances introduced initial Bb‐infected ticks to Canada from the United States, where LD first emerged (Brewer 2000; Scott et al. 2001). Results from two of these studies were the first time Ixodes ticks were identified in Alberta and Bb‐infected Ixodes were identified in Nova Scotia and Saskatchewan (Scott, Anderson, and Durden 2012; Scott et al. 2001). By 2008, passive tick or sentinel active tick surveillance identified Ixodes and Bb in all provinces. While ticks are found in all provinces, it does not necessarily mean that they expand and establish endemic populations. In some areas, such as Newfoundland & Labrador, temperatures are generally too cold, even considering ongoing climate change, to support local tick populations, even though individual Ixodes were identified here via passive surveillance and migratory bird studies (Ogden, Maarouf et al. 2006).
Following LD becoming nationally notifiable in 2009 and the Federal Framework on Lyme Disease Act in 2014, which acknowledged the importance of tick surveillance as part of an integrated approach to LD, there was a noticeable shift in the amount and types of surveillance literature published. From this point, most studies performed only tick surveillance (active and/or passive) as the focus shifted from identifying tick, host, and Bb presence to determining specific tick risk areas and Bb prevalence. A recent meta‐analysis estimated Bb prevalence in I. scapularis nymphs across Canada from active and passive tick surveillance studies and found increasing prevalence of Bb in host‐seeking I. scapularis nymphs and adults over time (Kelly et al. 2024). Other studies conducted surveillance on larger mammalian hosts, like dogs (Bryan et al. 2011; Evason et al. 2019; Herrin et al. 2017). Estimating the prevalence of Bb in local domestic pets can serve as a proxy of risk to humans since pets, like humans, contract LD through an infected tick bite and spend time in the same local outdoor risk areas, like parks and yards, as their owners. The study approaches carried out during this period align well with those detailed in the framework for adaptive surveillance of emerging tick‐borne zoonoses for the disease emergence stage (Clow et al. 2019).
The changing surveillance landscape is even more evident when looking at the study approaches over time on the provincial level, providing insight into emergence and spread in different regions of the country. Since Canada stretches nearly 4700 miles with varying climates and areas with differing ecological factors, LD risk varies substantially, and therefore, study approaches adapt to different needs. Highlighting differences in surveillance methods on a provincial level provides more insight into LD spread than could be achieved through a national analysis.
4.2. Tick and Animal Surveillance: The Present
Understanding adaptive surveillance for emerging TBDs helps monitor areas where LD is reaching endemicity and predict where it may spread next. Due to climate change and related ecological factors, the reach and distribution of Ixodes, their mammalian hosts, and eventually LD incidence is likely to expand and increase in Canada (Bouchard et al. 2019; Kotchi et al. 2021; Ogden 2014). Such changes have already been observed and described in the US, emphasising the need for continued surveillance to monitor further expansion (Eisen and Eisen 2023). A recent report published by Environment and Climate Change Canada showed that 2022 was 1.2°C above the average temperature and that much of northern Canada, including parts of British Columbia, Quebec, and most of the eastern maritime provinces—many areas where LD is not yet endemic—experienced average temperatures significantly above baseline (2023). Similar to how I. scapularis and Bb spread northward from the US into Ontario upon first emerging in Canada, continued temperature increases are predicted to increase the range and activity period of Ixodes and their hosts, exposing previously naïve humans to tick populations (Nelder et al. 2018; Tardy et al. 2023).
Over the last decade, evidence of this expansion has been reported by PHAC. The incidence of human LD cases has increased nationally from 1.0 per 100,000 population in 2012 to 4.0 per 100,000 in 2018 and 6.5 per 100,000 in 2022, mostly attributed to increased incidence in the provinces known to have endemic regions (PHAC 2024). However, the proportion of cases from Ontario, Quebec, and Nova Scotia, provinces with the most‐known risk areas, decreases slightly each year: 96.7% of cases in 2018 were from these provinces compared with 94.7% in 2022 (PHAC 2023, 2024). More cases have been reported in provinces that were historically considered lower risk, such as Manitoba. While some cases can be associated with travel to existing LD‐endemic areas, the steady increase of cases from these provinces in addition to increased incidence in provinces already known to have endemic areas suggests that risk areas for LD are expanding.
4.3. Tick and Animal Surveillance: The Future
While statutory human surveillance can identify increases in LD incidence, the continued surveillance of ticks and animal hosts will be critical to detect emergence and endemicity in new areas. For example, since 2009, LD incidence rates have been highest in Nova Scotia and Ontario, which are two provinces where Ixodes and Bb were first identified and detected in Canada (PHAC 2024). This suggests the importance of an integrated surveillance approach for LD and shows that animal surveillance is a useful first step since it can help indicate where the disease will emerge and spread and allow public health professionals to disseminate important knowledge and prevention information to the public before disease rates increase in these areas. (Braks et al. 2011; PHAC 2024). Passive and active tick surveillance at sentinel sites will be essential to first detect Ixodes and Bb in new areas. One research group in Canada has already proposed criteria for selecting sentinel sites for active tick surveillance where LD and other TBDs may emerge next, based on human population, risk of disease due to the presence of Bb‐transmitting Ixodes ticks, and ecological suitability for Ixodes ticks (Guillot et al. 2024). Coordinated tick surveillance efforts using data from passive and active tick surveillance methods from PHAC, provincial and local public health, and citizen science have been conducted since 2019 to describe the recent and changing distribution of ticks and their pathogens across Canada (Wilson et al. 2023; Wilson et al. 2022). When new regions become endemic for Ixodes and Bb, active targeted tick surveillance and surveillance of animal hosts will be able to monitor their densities and overall disease risk in these areas. These surveillance approaches also benefit the monitoring of other tick‐borne pathogens. For example, in addition to Bb, Ixodes can transmit the pathogens causing anaplasmosis ( Anaplasma phagocytophilum ) and babesiosis (Babesia microti) in humans, and cases of these diseases have begun to be identified in Canada over the last 15 years (Allehebi et al. 2022; Parkins et al. 2009; Yang, Smith, and Battad 2021).
4.4. Limitations
Our review is subject to some limitations. While comprehensive, our research is limited by the studies available in the three databases searched. This particularly affects the earlier years of our study period before the rapid digitization of scientific literature. This also means our search also did not include grey literature. Several provinces conduct local tick surveillance, and while some reviewed articles include these data, any tick surveillance data not published in peer‐reviewed literature were not included in this review. Additionally, the variability in methods and sparsity of data within included studies impeded us from drawing comparisons over time. For example, Bb testing methods varied across studies and time periods, and while we excluded tick larvae from the counts, when possible, we were unable to remove them when studies did not provide a breakdown by tick life stage. We also excluded any study that used an identical data set to another included study to avoid duplication of tick and animal counts; however, we may still have included some duplicative data. While the data and nature of the publications have limitations, they did not impede our ability to identify major themes in the surveillance literature over time and how it reflects the landscape of LD in Canada.
Our categorisation of surveillance approaches as described by the framework for adaptive surveillance of emerging tick‐borne zoonoses is subjective and was determined by the way surveillance methods were described in the methods sections of included studies and the independent researchers' interpretation of them (Clow et al. 2019). For example, “sentinel” and “targeted” surveillance is an ideal way to describe active tick surveillance activities to identify risk in a stepwise approach, but these terms were not always used in the articles. Additionally, the magnitude of studies categorised into these groups does not simply equate to an area with emergence or endemicity. For example, PHAC considers all of Nova Scotia as a risk area, but we only identified one study that performed targeted active tick surveillance there. The benefits of performing active tick surveillance in Nova Scotia might be limited if Bb‐infected Ixodes are widespread across the entire province. The existence of fewer targeted studies does not necessarily mean that the province has no endemic areas. However, these categorisations are still useful to estimate risk areas, as evidenced by their presence in some provinces like Ontario compared with their absence in Alberta and Saskatchewan.
Lastly, adaptive surveillance incorporates other types of research, including ecological investigations and human disease surveillance. This review's scope highlighted the history of tick and animal surveillance methods in Canada only, but it would be important to consider how other research methods within adaptive surveillance frameworks can inform how LD will change and spread in the coming years. Future research should consider the complementary role of different types of surveillance and research to improve our understanding of LD as a One Health issue and drive public health decision‐making.
5. Conclusions
This is the first historical review of tick and animal surveillance studies for LD in Canada and can help document the historical rapid invasion of Ixodes and Bb across provinces. It can also provide lessons for other countries, which may have regions transitioning from emerging to endemic status for LD, about the most useful surveillance methods for monitoring TBDs.
Author Contributions
Alexander Davidson: conceptualization, data curation, synthesis, methodology, supervision, data visualisation, and writing – original draft, review and editing. Patrick H. Kelly: supervision, conceptualization, data curation; synthesis, methodology, and writing – review and editing. Julie Davis: data curation, synthesis, and writing – review and editing. Maria Major: writing – review and editing. Jennifer C. Moïsi: supervision, writing – review and editing. James H. Stark: funding acquisition, supervision, and writing – review and editing.
Ethics Statement
The authors have nothing to report.
Consent
Authors consent to publishing data and images included in this manuscript.
Conflicts of Interest
A.D., P.H.K., M.M., J.C.M., and J.H.S. are employees of Pfizer and may hold shares and/or stock options in the company. The other authors have no potential conflict of interest to disclose.
Supporting information
AppenSearch strings used in PubMed, Web of Science, and Embase for scoping review of tick and animal surveillance studies for Lyme disease in Canada
Appendix 2. Chronological list of Lyme disease‐related tick and animal surveillance studies in Canada included in synthesis, by study start year, published 1976‐2023. Reviewed studies are ordered based on the year surveillance began (from earliest to latest), total collection period (number of years), and year of publication. For each study, the province(s) where the study took place and the tick or animal type collected (Ixodes [ I. scapularis and/or I. pacificus ], small mammal, large mammal, bird) are shaded. When provided in the studies, numbers of Ixodes or animal hosts collected are documented and Bb testing (by any testing method) status indicated. For each province, the first study to detect Ixodes, Bb in Ixodes, and Bb in a mammal (by study collection year) is noted.
Funding: This analysis was supported and jointly funded by Valneva and Pfizer as part of their co‐development of a Lyme Disease vaccine.
Data Availability Statement
The authors have nothing to report.
References
- Allehebi, Z. O. , Khan F. M., Robbins M., et al. 2022. “Lyme Disease, Anaplasmosis, and Babesiosis, Atlantic Canada.” Emerging Infectious Diseases 28, no. 6: 1292–1294. 10.3201/eid2806.220443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. F. 1988. “Mammalian and Avian Reservoirs for Borrelia burgdorferi .” Annals of the New York Academy of Sciences 539: 180–191. 10.1111/j.1749-6632.1988.tb31852.x. [DOI] [PubMed] [Google Scholar]
- André, A. , Mouton A., Millien V., and Michaux J.. 2017. “Liver Microbiome of Peromyscus leucopus , a Key Reservoir Host Species for Emerging Infectious Diseases in North America.” Infection, Genetics and Evolution 52: 10–18. 10.1016/j.meegid.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Apanaskevich, A. , and Oliver J. J.. 2014. “Life Cycles and Natural History of Ticks.” In Biology of Ticks, edited by Sonenshine D. and Roe R., vol. 1. New York: Oxford University Press. [Google Scholar]
- Artsob, H. , Barker I. K., Fister R., et al. 1993. “Serological Studies on the Infection of Dogs in Ontario With Borrelia burgdorferi , the Etiological Agent of Lyme Disease.” Canadian Veterinary Journal 34, no. 9: 543–548. [PMC free article] [PubMed] [Google Scholar]
- Artsob, H. , Garvie M., Cawthorn R. J., et al. 1992. “Isolation of the Lyme Disease Spirochete, Borrelia burgdorferi , From Ixodes dammini (Acari: Ixodidae) Collected on Prince Edward Island Canada.” Journal of Medical Entomology 29, no. 6: 1063–1066. 10.1093/jmedent/29.6.1063. [DOI] [PubMed] [Google Scholar]
- Baggs, E. M. , Stack S. H., Finney‐Crawley J. R., and Simon N. P.. 2011. “ Peromyscus maniculatus , A Possible Reservoir Host of Borrelia garinii From the Gannet Islands Off Newfoundland and Labrador.” Journal of Parasitology 97, no. 5: 792–794. 10.1645/GE-2548.1. [DOI] [PubMed] [Google Scholar]
- Banerjee, A. , Baid K., Byron T., et al. 2020. “Seroprevalence in Bats and Detection of Borrelia burgdorferi in Bat Ectoparasites.” Microorganisms 8, no. 3: 440. 10.3390/microorganisms8030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, S. , Stephen C., Fernando K., Coffey S., and Dong M.. 1996. “Evaluation of Dogs as Sero‐Indicators of the Geographic Distribution of Lyme Borreliosis in British Columbia.” Canadian Veterinary Journal 37, no. 3: 168–169. [PMC free article] [PubMed] [Google Scholar]
- Banerjee, S. N. , Banerjee M., Fernando K., Scott J. D., Mann R., and Morshed M. G.. 2000. “Presence of Spirochete Causing Lyme Disease, Borrelia burgdorferi , in the Blacklegged Tick, Ixodes scapularis , in Southern Ontario.” Canadian Medical Association Journal 162, no. 11: 1567–1569. [PMC free article] [PubMed] [Google Scholar]
- Barker, I. K. , Lindsay L. R., Campbell G. D., Surgeoner G. A., and McEwen S. A.. 1993. “The Groundhog Tick Ixodes cookei (Acari: ixodidae): A Poor Potential Vector of Lyme Borreliosis.” Journal of Wildlife Diseases 29, no. 3: 416–422. 10.7589/0090-3558-29.3.416. [DOI] [PubMed] [Google Scholar]
- Barker, I. K. , Surgeoner G. A., Artsob H., et al. 1992. “Distribution of the Lyme Disease Vector, Ixodes dammini (Acari: Ixodidae) and Isolation of Borrelia burgdorferi in Ontario Canada.” Journal of Medical Entomology 29, no. 6: 1011–1022. 10.1093/jmedent/29.6.1011. [DOI] [PubMed] [Google Scholar]
- Battaly, G. R. , and Fish D.. 1993. “Relative Importance of Bird Species as Hosts for Immature Ixodes dammini (Acari: Ixodidae) in a Suburban Residential Landscape of Southern New York State.” Journal of Medical Entomology 30, no. 4: 740–747. 10.1093/jmedent/30.4.740. [DOI] [PubMed] [Google Scholar]
- Bell, C. R. , Specht H. B., and Coombs B. A.. 1992. “The Search for Ixodes Dammini and Borrelia burgdorferi in Nova Scotia.” Canadian Journal of Infectious Diseases 3, no. 5: 224–230. 10.1155/1992/242635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjurman, N. K. , Bradet G., and Lloyd V. K.. 2016. “Lyme Disease Risk in Dogs in New Brunswick.” Canadian Veterinary Journal 57, no. 9: 981–984. [PMC free article] [PubMed] [Google Scholar]
- Bouchard, C. , Beauchamp G., Leighton P. A., Lindsay R., Belanger D., and Ogden N. H.. 2013. “Does High Biodiversity Reduce the Risk of Lyme Disease Invasion?” Parasites & Vectors 6: 195. 10.1186/1756-3305-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard, C. , Beauchamp G., Nguon S., et al. 2011. “Associations Between Ixodes scapularis Ticks and Small Mammal Hosts in a Newly Endemic Zone in Southeastern Canada: Implications for Borrelia burgdorferi Transmission.” Ticks and Tick‐borne Diseases 2, no. 4: 183–190. 10.1016/j.ttbdis.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Bouchard, C. , Dibernardo A., Koffi J., Wood H., Leighton P. A., and Lindsay L. R.. 2019. “N Increased Risk of Tick‐Borne Diseases With Climate and Environmental Changes.” Canada Communicable Disease Report 45, no. 4: 83–89. 10.14745/ccdr.v45i04a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard, C. , Leighton P. A., Beauchamp G., et al. 2013. “Harvested White‐Tailed Deer as Sentinel Hosts for Early Establishing Ixodes scapularis Populations and Risk From Vector‐Borne Zoonoses in Southeastern Canada.” Journal of Medical Entomology 50, no. 2: 384–393. 10.1603/me12093. [DOI] [PubMed] [Google Scholar]
- Braks, M. , van der Giessen J., Kretzschmar M., et al. 2011. “Towards an Integrated Approach in Surveillance of Vector‐Borne Diseases in Europe.” Parasites & Vectors 4: 192. 10.1186/1756-3305-4-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, D. 2000. “Canadian Atlas of Bird Banding. Volume 1, Doves, Cuckoos, and Hummingbirds Through Passerines, 1921–1995. E. a. C. C. Canada.”
- Bryan, H. D. , Paquet P. C., Ellis J. A., Goji N., Gouix M., and Smits J. E.. 2011. “Exposure to Infectious Agents in Dogs in Remote Coastal British Columbia: Possible Sentinels of Diseases in Wildlife and Humans.” Canadian Journal of Veterinary Research‐Revue Canadienne De Recherche Veterinaire 75: 11–17. [PMC free article] [PubMed] [Google Scholar]
- Burrows, H. , Talbot B., McKay R., et al. 2021. “A Multi‐Year Assessment of Blacklegged Tick ( Ixodes scapularis ) Population Establishment and Lyme Disease Risk Areas in Ottawa, Canada, 2017‐2019.” PLoS One 16, no. 2: e0246484. 10.1371/journal.pone.0246484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada, G. o. 2017a. “Federal Framework on Lyme Disease Act S.C. 2014, c. 37.” https://laws‐lois.justice.gc.ca/eng/acts/F‐7.35/FullText.html.
- Canada, G. o. 2017b. Lyme Disease in Canada: A Federal Framework. Ottawa, Ontario: Government of Canada.Canadian Medical Association Journal . https://www.canada.ca/en/public‐health/services/publications/diseases‐conditions/lyme‐disease‐canada‐federal‐framework.html. [Google Scholar]
- Canada, G. o. 2023. “Canadian Environmental Sustainability Indicators: Temperature Change in Canada.” https://www.canada.ca/en/environment‐climate‐change/services/environmental‐indicators/temperature‐change.html.
- Canadian Medical Association Journal. 1991. “Consensus conference on Lyme disease.” Canadian Medical Association Journal 144, no. 12: 1627–1632. [PMC free article] [PubMed] [Google Scholar]
- Caron‐Lévesque, M. C. 2023. “Of Mice, Ticks, and Fleas: Host Behaviour and Co‐Occurring Parasites.” Canadian Journal of Zoology 101: 210–521. 10.1139/cjz-2022-0107. [DOI] [Google Scholar]
- Cawthorn, R. J. , Horney B. S., and Maloney R.. 1990. “Lyme Disease Vector, Ixodes dammini , (The Northern Deer Tick) Identified in Prince Edward Island.” Canadian Veterinary Journal 31: 220. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control . 1982. “Lyme Disease.” Morbidity and Mortality Weekly Report 31, no. 27: 367–368. [PubMed] [Google Scholar]
- Chen, D. W. , Belanger P., Moore K., Peterson M., and Cunningham J.. 2015. “Analyzing the Correlation Between Deer Habitat and the Component of the Risk for Lyme Disease in Eastern Ontario, Canada: A GIS‐Based Approach.” ISPRS International Journal of Geo‐Information 4: 105–123. 10.3390/ijgi4010105. [DOI] [Google Scholar]
- Chilton, N. B. , Curry P. S., Lindsay L. R., Rochon K., Lysyk T. J., and Dergousoff S. J.. 2020. “Passive and Active Surveillance for Ixodes scapularis (Acari: Ixodidae) in Saskatchewan, Canada.” Journal of Medical Entomology 57, no. 1: 156–163. 10.1093/jme/tjz155. [DOI] [PubMed] [Google Scholar]
- Clow, K. M. , Finer R., Lumsden G., and Jardine C. M.. 2018. “Assessing the Repeatability of Tick Dragging as a Method for Ixodes scapularis Surveillance.” Vector Borne and Zoonotic Diseases 18, no. 11: 628–631. 10.1089/vbz.2018.2301. [DOI] [PubMed] [Google Scholar]
- Clow, K. M. , Leighton P. A., Ogden N. H., et al. 2017. “Northward Range Expansion of Ixodes scapularis Evident Over a Short Timescale in Ontario, Canada.” PLoS One 12, no. 12: e0189393. 10.1371/journal.pone.0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow, K. M. , Leighton P. A., Pearl D. L., and Jardine C. M.. 2019. “A Framework for Adaptive Surveillance of Emerging Tick‐Borne Zoonoses.” One Health 7: 100083. 10.1016/j.onehlt.2019.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow, K. M. , Ogden N. H., Lindsay L. R., Michel P., Pearl D. L., and Jardine C. M.. 2016. “Distribution of Ticks and the Risk of Lyme Disease and Other Tick‐Borne Pathogens of Public Health Significance in Ontario, Canada.” Vector Borne and Zoonotic Diseases 16, no. 4: 215–222. 10.1089/vbz.2015.1890. [DOI] [PubMed] [Google Scholar]
- Clow, K. M. , Ogden N. H., Lindsay L. R., Michel P., Pearl D. L., and Jardine C. M.. 2017. “The Influence of Abiotic and Biotic Factors on the Invasion of Ixodes scapularis in Ontario, Canada.” Ticks Tick Borne Dis 8, no. 4: 554–563. 10.1016/j.ttbdis.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Clow, K. M. , Ogden N. H., Lindsay L. R., et al. 2018. “A Field‐Based indicator for Determining the Likelihood of Ixodes scapularis Establishment at Sites in Ontario Canada.” PLOS ONE 13, no. 2: e0193524. 10.1371/journal.pone.0193524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall, K. E. , Kerr J. T., and Millien V.. 2022. “Emerging Tick‐Borne Pathogens in Central Canada: Recent Detections of Babesia Odocoilei and Rickettsia rickettsii .” Vector Borne and Zoonotic Diseases 22, no. 11: 535–544. 10.1089/vbz.2022.0036. [DOI] [PubMed] [Google Scholar]
- Cull, B. 2022. “Monitoring Trends in Distribution and Seasonality of Medically Important Ticks in North America Using Online Crowdsourced Records from iNaturalist.” Insects 13, no. 5: 404. 10.3390/insects13050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWinter, S. , Bauman C., Peregrine A., Weese J. S., and Clow K. M.. 2023. “Assessing the Spatial and Temporal Patterns and Risk Factors for Acquisition of Ixodes Spp. by Companion Animals Across Canada.” Ticks Tick Borne Dis 14, no. 2: 102089. 10.1016/j.ttbdis.2022.102089. [DOI] [PubMed] [Google Scholar]
- Dibernardo, A. , Cote T., Ogden N. H., and Lindsay L. R.. 2014. “The Prevalence of Borrelia miyamotoi Infection, and Co‐Infections With Other Borrelia spp. in Ixodes scapularis Ticks Collected in Canada.” Parasites & Vectors 7: 183. 10.1186/1756-3305-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, A. , Bouchard C., Dibernardo A., et al. 2022. “Transmission Patterns of Tick‐Borne Pathogens Among Birds and Rodents in a Forested Park in Southeastern Canada.” PLoS One 17, no. 4: e0266527. 10.1371/journal.pone.0266527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplaix, L. , Wagner V., Gasmi S., et al. 2021. “Exposure to Tick‐Borne Pathogens in Cats and Dogs Infested With Ixodes scapularis in Quebec: An 8‐Year Surveillance Study.” Frontiers in Veterinary Science 8: 696815. 10.3389/fvets.2021.696815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen, L. , and Eisen R. J.. 2023. “Changes in the Geographic Distribution of the Blacklegged Tick, Ixodes scapularis , in the United States.” Ticks Tick Borne Dis 14, no. 6: 102233. 10.1016/j.ttbdis.2023.102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evason, M. , Stull J. W., Pearl D. L., et al. 2019. “Prevalence of Borrelia burgdorferi , Anaplasma spp., Ehrlichia spp. and Dirofilaria Immitis in Canadian Dogs, 2008 to 2015: A Repeat Cross‐Sectional Study.” Parasites & Vectors 12, no. 1: 64. 10.1186/s13071-019-3299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset, J. , Tessier N., Millien V., and Lapointe F. J.. 2015. “Phylogeographic Structure of the White‐Footed Mouse and the Deer Mouse, Two Lyme Disease Reservoir Hosts in Quebec.” PLoS One 10, no. 12: e0144112. 10.1371/journal.pone.0144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley‐Eby, A. H. , Savidge C., and Lloyd V. K.. 2020. “ Ixodes scapularis Ticks and Borrelia burgdorferi on Prince Edward Island: Passive Tick Surveillance and Canine Seroprevalence.” Canadian Veterinary Journal 61, no. 10: 1107–1110. [PMC free article] [PubMed] [Google Scholar]
- Gabriele‐Rivet, V. , Arsenault J., Badcock J., et al. 2015. “Different Ecological Niches for Ticks of Public Health Significance in Canada.” PLoS One 10, no. 7: e0131282. 10.1371/journal.pone.0131282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan, G. J. , Barker I. K., Artsob H., Magnarelli L. A., Robinson J. T., and Voigt D. R.. 1998. “Serologic Survey for Antibodies to Borrelia burgdorferi in White‐Tailed Deer in Ontario.” Journal of Wildlife Diseases 34, no. 2: 411–414. 10.7589/0090-3558-34.2.411. [DOI] [PubMed] [Google Scholar]
- Gary, A. T. , Webb J. A., Hegarty B. C., and Breitschwerdt E. B.. 2006. “The Low Seroprevalence of Tick‐Transmitted Agents of Disease in Dogs From Southern Ontario and Quebec.” Canadian Veterinary Journal 47, no. 12: 1194–1200. [PMC free article] [PubMed] [Google Scholar]
- Gasmi, S. , Ogden N. H., Leighton P. A., Lindsay L. R., and Thivierge K.. 2016. “Analysis of the Human Population Bitten by Ixodes scapularis Ticks in Quebec, Canada: Increasing Risk of Lyme Disease.” Ticks Tick Borne Disease 7, no. 6: 1075–1081. 10.1016/j.ttbdis.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Gasmi, S. , Ogden N. H., Ripoche M., et al. 2019. “Detection of Municipalities At‐Risk of Lyme Disease Using Passive Surveillance of Ixodes scapularis as an Early Signal: A Province‐Specific indicator in Canada.” PLoS One 14, no. 2: e0212637. 10.1371/journal.pone.0212637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt, M. C. , Carr A. P., and Taylor S. M.. 2018. “Serological Survey of Canine Vector‐Borne Diseases in Saskatchewan, Canada.” Canadian Veterinary Journal 59, no. 10: 1109–1111. [PMC free article] [PubMed] [Google Scholar]
- Guillot, C. , Aenishaenslin C., Acheson E. S., Koffi J., Bouchard C., and Leighton P. A.. 2024. “Spatial Multi‐Criteria Decision Analysis for the Selection of Sentinel Regions in Tick‐Borne Disease Surveillance.” BMC Public Health 24, no. 1: 294. 10.1186/s12889-024-17684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot, C. , Badcock J., Clow K., et al. 2020. “Sentinel Surveillance of Lyme Disease Risk in Canada, 2019: Results From the First Year of the Canadian Lyme Sentinel Network (CaLSeN).” Canada Communicable Disease Report 46, no. 10: 354–361. 10.14745/ccdr.v46i10a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot, C. , Bouchard C., Buhler K., et al. 2022. “Sentinel Surveillance Contributes to Tracking Lyme Disease Spatiotemporal Risk Trends in Southern Quebec, Canada.” Pathogens 11, no. 5: 531. 10.3390/pathogens11050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond‐Collins, K. , Tremblay M., Milord F., et al. 2022. “An Ecological Approach to Predict Areas With Established Populations of Ixodes scapularis in Quebec, Canada.” Ticks Tick Borne Disease 13, no. 6: 102040. 10.1016/j.ttbdis.2022.102040. [DOI] [PubMed] [Google Scholar]
- Herrin, B. H. , Peregrine A. S., Goring J., Beall M. J., and Little S. E.. 2017. “Canine Infection With Borrelia burgdorferi , Dirofilaria Immitis, Anaplasma spp. and Ehrlichia spp. in Canada, 2013‐2014.” Parasites & Vectors 10, no. 1: 244. 10.1186/s13071-017-2184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, A. E. , Weese J. S., Rosseau J., and Clow K. M.. 2022. “Spatial Patterns of Borrelia burgdorferi , Borrelia miyamotoi and Anaplasma phagocytophilum Detected in Ixodes Spp. Ticks From Canadian Companion Animals, 2019‐2020.” Zoonoses and Public Health 69, no. 8: 944–955. 10.1111/zph.12992. [DOI] [PubMed] [Google Scholar]
- James, C. A. , Pearl D. L., Lindsay L. R., Peregrine A. S., and Jardine C. M.. 2019. “Risk Factors Associated With the Carriage of Ixodes scapularis Relative to Other Tick Species in a Population of Pet Dogs From Southeastern Ontario, Canada.” Ticks and Tick‐Borne Diseases 10, no. 2: 290–298. 10.1016/j.ttbdis.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Jordan, R. A. , Gable S., and Egizi A.. 2022. “Relevance of Spatial and Temporal Trends in Nymphal Tick Density and Infection Prevalence for Public Health and Surveillance Practice in Long‐Term Endemic Areas: A Case Study in Monmouth County, NJ.” Journal of Medical Entomology 59, no. 4: 1451–1466. 10.1093/jme/tjac073. [DOI] [PubMed] [Google Scholar]
- Kanji, J. N. , Isaac A., Gregson D., et al. 2022. “Epidemiology of Ticks Submitted From Human Hosts in Alberta, Canada (2000‐2019).” Emerging Microbes & Infections 11, no. 1: 284–292. 10.1080/22221751.2022.2027217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, P. H. , Tan Y., Yan Q., et al. 2024. “ Borrelia burgdorferi Sensu Lato Prevalence in Ixodes scapularis From Canada: A Thirty‐Year Summary and meta‐Analysis (1990‐2020).” Acta Tropica 56: 107268. 10.1016/j.actatropica.2024.107268. [DOI] [PubMed] [Google Scholar]
- Klich, M. , Lankester M. W., and Wu K. W.. 1996. “Spring Migratory Birds (Aves) Extend the Northern Occurrence of Blacklegged Tick (Acari:Ixodidae).” Journal of Medical Entomology 33, no. 4: 581–585. 10.1093/jmedent/33.4.581. [DOI] [PubMed] [Google Scholar]
- Koffi, J. K. , Savage J., Thivierge K., et al. 2017. “Evaluating the Submission of Digital Images as a Method of Surveillance for Ixodes scapularis Ticks.” Parasitology 144, no. 7: 877–883. 10.1017/S0031182017000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchi, S. O. , Bouchard C., Brazeau S., and Ogden N. H.. 2021. “Earth Observation‐Informed Risk Maps of the Lyme Disease Vector in Central and Eastern Canada.” Remote Sensing 13, no. 3. 10.3390/rs13030524. [DOI] [Google Scholar]
- Kotwa, J. D. , Jardine C. M., Pearl D. L., Berke O., Mercer N. J., and Peregrine A. S.. 2020. “Evaluation of the SNAP(R) 4Dx(R) Plus Test for the Detection of Dirofilaria Immitis Antigen and Characterization of Exposure to Tick‐Borne Pathogens in Wild Canids in Southern Ontario.” Veterinary Parasitology 283: 109176. 10.1016/j.vetpar.2020.109176. [DOI] [PubMed] [Google Scholar]
- Kulkarni, M. , Kryuchkov R., Statculescu A., et al. 2018. “ Ixodes scapularis Tick Distribution and Infection Rates in Ottawa, Ontario, 2017.” Canada Communicable Disease Report 44, no. 10: 237–242. 10.14745/ccdr.v44i10a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, M. A. , Narula I., Slatculescu A. M., and Russell C.. 2019. “Lyme Disease Emergence After Invasion of the Blacklegged Tick, Ixodes scapularis , Ontario, Canada, 2010‐2016.” Emerging Infectious Diseases 25, no. 2: 328–332. 10.3201/eid2502.180771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo, S. S. , Gonzalez A., and Millien V.. 2017. “The Genetic Signature of Range Expansion in a Disease Vector‐The Black‐Legged Tick.” Journal of Heredity 108, no. 2: 176–183. 10.1093/jhered/esw073. [DOI] [PubMed] [Google Scholar]
- Leo, S. S. T. , and Millien V.. 2017. “Microsatellite Markers Reveal Low Frequency of Natural Hybridization Between the White‐Footed Mouse ( Peromyscus leucopus ) and Deer Mouse ( Peromyscus maniculatus ) in Southern Quebec, Canada.” Genome 60, no. 5: 454–463. 10.1139/gen-2016-0163. [DOI] [PubMed] [Google Scholar]
- Levine, J. F. , Wilson M. L., and Spielman A.. 1985. “Mice as Reservoirs of the Lyme Disease Spirochete.” American Journal of Tropical Medicine and Hygiene 34, no. 2: 355–360. 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- Lewis, J. , Boudreau C. R., Patterson J. W., Bradet‐Legris J., and Lloyd V. K.. 2018. “Citizen Science and Community Engagement in Tick Surveillance‐A Canadian Case Study.” Healthcare (Basel) 6, no. 1: 22. 10.3390/healthcare6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J. , Kirby A. M., Harris K. D., et al. 2021. “Monitoring Risk: Tick and Borrelia burgdorferi Public Participatory Surveillance in the Canadian Maritimes, 2012‐2020.” Pathogens 10, no. 10: 1284. 10.3390/pathogens10101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J. , and Lloyd V. K.. 2019. “Identification of Borrelia Bissettii in Ixodes scapularis Ticks From New Brunswick, Canada.” Canadian Journal of Microbiology 65, no. 2: 155–161. 10.1139/cjm-2018-0376. [DOI] [PubMed] [Google Scholar]
- Lieske, D. J. , and Lloyd V. K.. 2018. “Combining Public Participatory Surveillance and Occupancy Modelling to Predict the Distributional Response of Ixodes scapularis to Climate Change.” Ticks Tick Borne Disease 9, no. 3: 695–706. 10.1016/j.ttbdis.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Lindsay, L. R. , Barker I. K., Surgeoner G. A., McEwen S. A., and Campbell G. D.. 1997. “Duration of Borrelia burgdorferi Infectivity in White‐Footed Mice for the Tick Vector Ixodes scapularis Under Laboratory and Field Conditions in Ontario.” Journal of Wildlife Diseases 33, no. 4: 766–775. 10.7589/0090-3558-33.4.766. [DOI] [PubMed] [Google Scholar]
- Lindsay, L. R. , Barker I. K., Surgeoner G. A., McEwen S. A., Elliott L. A., and Kolar J.. 1991. “Apparent Incompetence of Dermacentor variabilis (Acari: Ixodidae) and Fleas (Insecta: Siphonaptera) as Vectors of Borrelia burgdorferi in an Ixodes dammini Endemic Area of Ontario Canada.” Journal of Medical Entomology 28, no. 5: 750–753. 10.1093/jmedent/28.5.750. [DOI] [PubMed] [Google Scholar]
- Lindsay, L. R. , Mathison S. W., Barker I. K., McEwen S. A., and Surgeoner G. A.. 1999. “Abundance of Ixodes scapularis (Acari: Ixodidae) Larvae and Nymphs in Relation to Host Density and Habitat on Long Point Ontario.” Journal of Medical Entomology 36, no. 3: 243–254. 10.1093/jmedent/36.3.243. [DOI] [PubMed] [Google Scholar]
- Lindsay, R. , Artsob H., Galloway T., and Horsman G.. 1999. “Vector of Lyme Borreliosis, Ixodes scapularis , Identified in Saskatchewan.” Canada Communicable Disease Report 25, no. 9: 81–83. [PubMed] [Google Scholar]
- Mak, S. , Morshed M., and Henry B.. 2010. “Ecological Niche Modeling of Lyme Disease in British Columbia Canada.” Journal of Medical Entomology 47, no. 1: 99–105. 10.1603/033.047.0114. [DOI] [PubMed] [Google Scholar]
- McKay, R. , Talbot B., Slatculescu A., Stone A., and Kulkarni M. A.. 2020. “Woodchip Borders at the Forest Ecotone as an Environmental Control Measure to Reduce Questing Tick Density Along Recreational Trails in Ottawa Canada.” Ticks Tick Borne Disease 11, no. 2: 101361. 10.1016/j.ttbdis.2019.101361. [DOI] [PubMed] [Google Scholar]
- Millien, V. , Leo S. S. T., Turney S., and Gonzalez A.. 2023. “It's About Time: Small Mammal Communities and Lyme Disease Emergence.” Scientific Reports 13, no. 1: 14513. 10.1038/s41598-023-41901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes, E. L. , Thornton G., Leveille A. N., et al. 2019. “Babesia Odocoilei and Zoonotic Pathogens Identified From Ixodes scapularis Ticks in Southern Ontario Canada.” Ticks Tick Borne Diseases 10, no. 3: 670–676. 10.1016/j.ttbdis.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Morshed, M. G. , Lee M. K., Boyd E., et al. 2021. “Passive Tick Surveillance and Detection of Borrelia Species in Ticks From British Columbia, Canada: 2002‐2018.” Vector Borne and Zoonotic Diseases 21, no. 7: 490–497. 10.1089/vbz.2020.2743. [DOI] [PubMed] [Google Scholar]
- Morshed, M. G. , Scott J. D., Fernando K., et al. 2005. “Migratory Songbirds Disperse Ticks Across Canada, and First Isolation of the Lyme Disease Spirochete, Borrelia burgdorferi , From the Avian Tick, Ixodes auritulus .” Journal of Parasitology 91, no. 4: 780–790. 10.1645/GE-3437.1. [DOI] [PubMed] [Google Scholar]
- Morshed, M. G. , Scott J. D., Fernando K., et al. 2006. “Distribution and Characterization of Borrelia burgdorferi Isolates From Ixodes Scapularis and Presence in Mammalian Hosts in Ontario Canada.” Journal of Medical Entomology 43, no. 4: 762–773. 10.1603/0022-2585(2006)43[762:DACOBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Morshed, M. G. , Scott J. D., Fernando K., Mann R. B., and Durden L. A.. 2003. “Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park Ontario.” Journal of Medical Entomology 40, no. 1: 91–94. 10.1603/0022-2585-40.1.91. [DOI] [PubMed] [Google Scholar]
- Morshed, M. G. , Lee M. K., Man S., et al. 2015. “Surveillance for Borrelia burgdorferi in Ixodes Ticks and Small Rodents in British Columbia.” Vector Borne and Zoonotic Diseases 15, no. 11: 701–705. 10.1089/vbz.2015.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, D. L. , Lindsay L. R., Dibernardo A., and Nocera J. J.. 2022. “Ruffed Grouse Do Not Exhibit High Potential for Reservoir Competency of Common Tick‐Borne Pathogens.” Wildlife Society Bulletin 46: e1380. 10.1002/wsb.1380. [DOI] [Google Scholar]
- Murison, K. , Wilson C. H., Clow K. M., et al. 2023. “Epidemiology and Clinical Manifestations of Reported Lyme Disease Cases: Data From the Canadian Lyme Disease Enhanced Surveillance System.” PLoS One 18, no. 12: e0295909. 10.1371/journal.pone.0295909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, M. , Arroyo L. G., Jardine C., et al. 2021. “Seroprevalence and Evaluation of Risk Factors Associated With Seropositivity for Borrelia burgdorferi in Ontario Horses.” Equine Veterinary Journal 53, no. 2: 331–338. 10.1111/evj.13317. [DOI] [PubMed] [Google Scholar]
- Nelder, M. P. , Russell C., Lindsay L. R., et al. 2014. “Population‐Based Passive Tick Surveillance and Detection of Expanding Foci of Blacklegged Ticks Ixodes Scapularis and the Lyme Disease Agent Borrelia burgdorferi in Ontario Canada.” PLOS ONE 9, no. 8: e105358. 10.1371/journal.pone.0105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder, M. P. , Russell C. B., Dibernardo A., et al. 2021. “Monitoring the Patterns of Submission and Presence of Tick‐Borne Pathogens in Ixodes scapularis Collected From Humans and Companion Animals in Ontario, Canada (2011‐2017).” Parasites & Vectors 14, no. 1: 260. 10.1186/s13071-021-04750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder, M. P. , Wijayasri S., Russell C. B., et al. 2018. “The Continued Rise of Lyme Disease in Ontario, Canada: 2017.” Canada Communicable Disease Report 44, no. 10: 231–236. 10.14745/ccdr.v44i10a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol, G. K. , Weese J. S., and Clow K. M.. 2023. “Isolation and Multilocus Sequence Typing of Borrelia burgdorferi From Ixodes scapularis Collected From Dogs in Ontario Canada.” BMC Research Notes 16, no. 1: 43. 10.1186/s13104-023-06315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsubuga, P. , White M., Thacker D., et al. 2006. “Public Health Surveillance: A Tool for Targeting and Monitoring Interventions.” In Disease Control Priorities in Developing Countries, edited by DT J., JG B., and AR M., 2nd ed. Washington DC: International Bank for Reconstruction and Development / The World Bank. https://www.ncbi.nlm.nih.gov/books/NBK11770/. [PubMed] [Google Scholar]
- Ogden, N. H. 2014. “Lyme Disease and Climate Change. <Go to ISI>://WOS:000355487300011.”
- Ogden, N. H. , Bouchard C., Kurtenbach K., et al. 2010. “Active and Passive Surveillance and Phylogenetic Analysis of Borrelia burgdorferi Elucidate the Process of Lyme Disease Risk Emergence in Canada.” Environmental Health Perspectives 118, no. 7: 909–914. 10.1289/ehp.0901766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden, N. H. , Lindsay L. R., Hanincova K., et al. 2008. “Role of Migratory Birds in Introduction and Range Expansion of Ixodes scapularis Ticks and of Borrelia Burgdorferi and Anaplasma phagocytophilum in Canada.” Applied and Environmental Microbiology 74, no. 6: 1780–1790. 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden, N. H. , Lindsay L. R., Morshed M., Sockett P. N., and Artsob H.. 2009. “The Emergence of Lyme Disease in Canada.” Canadian Medical Association Journal 180, no. 12: 1221–1224. 10.1503/cmaj.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden, N. H. , Maarouf A., Barker I. K., et al. 2006. “Climate Change and the Potential for Range Expansion of the Lyme Disease Vector Ixodes scapularis in Canada.” International Journal for Parasitology 36, no. 1: 63–70. 10.1016/j.ijpara.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Ogden, N. H. , Trudel L., Artsob H., et al. 2006. “ Ixodes scapularis Ticks Collected by Passive Surveillance in Canada: Analysis of Geographic Distribution and Infection With Lyme Borreliosis Agent Borrelia burgdorferi .” Journal of Medical Entomology 43, no. 3: 600–609. 10.1603/0022-2585(2006)43[600:Istcbp]2.0.Co;2. [DOI] [PubMed] [Google Scholar]
- Oliver, J. H., Jr. , Owsley M. R., Hutcheson H. J., et al. 1993. “Conspecificity of the Ticks Ixodes Scapularis and I . dammini (Acari: Ixodidae).” Journal of Medical Entomology 30, no. 1: 54–63. 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- Parkins, M. D. , Church D. L., Jiang X. Y., and Gregson D. B.. 2009. “Human Granulocytic Anaplasmosis: First Reported Case in Canada.” Canadian Journal of Infectious Diseases and Medical Microbiology 20, no. 3: e100–e102. 10.1155/2009/124173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, J. D. , McIntyre A. M., Lloyd K. C., and Lloyd V. K.. 2017. “Evidence for Genetic Hybridization Between Ixodes Scapularis and Ixodes cookei .” Canadian Journal of Zoology 95: 527–537. 10.1139/cjz-2016-0134. [DOI] [Google Scholar]
- PHAC . 2015. Surveillance of Lyme Disease. Ottawa, Ontario: Public Health Agency of Canada. https://www.canada.ca/en/public‐health/services/diseases/lyme‐disease/surveillance‐lyme‐disease.html#a2. [Google Scholar]
- PHAC . 2023. “Lyme Disease Surveillance in Canada: Annual Edition 2018.” https://www.canada.ca/en/public‐health/services/publications/diseases‐conditions/surveillance‐lyme‐disease‐annual‐edition‐2018.html.
- PHAC . 2024. “Lyme Disease in Canada Surveillance Report: Annual Edition 2022.” https://www.canada.ca/en/public‐health/services/publications/diseases‐conditions/lyme‐disease‐surveillance‐canada‐annual‐edition‐2022.html.
- Qurollo, B. A. , Chandrashekar R., Hegarty B. C., et al. 2014. “A Serological Survey of Tick‐Borne Pathogens in Dogs in North America and the Caribbean as Assessed by Anaplasma phagocytophilum , A . platys , Ehrlichia canis , E . chaffeensis , E. Ewingii, and Borrelia burgdorferi Species‐Specific Peptides.” Infection Ecology & Epidemiology 4. 10.3402/iee.v4.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche, M. , Bouchard C., Irace‐Cima A., Leighton P., and Thivierge K.. 2022. “Current and Future Distribution of Ixodes scapularis Ticks in Quebec: Field Validation of a Predictive Model.” PLoS One 17, no. 2: e0263243. 10.1371/journal.pone.0263243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche, M. , Gasmi S., Adam‐Poupart A., et al. 2018. “Passive Tick Surveillance Provides an Accurate Early Signal of Emerging Lyme Disease Risk and Human Cases in Southern Canada.” Journal of Medical Entomology 55, no. 4: 1016–1026. 10.1093/jme/tjy030. [DOI] [PubMed] [Google Scholar]
- Ripoche, M. , Lindsay L. R., Ludwig A., Ogden N. H., Thivierge K., and Leighton P. A.. 2018. “Multi‐Scale Clustering of Lyme Disease Risk at the Expanding Leading Edge of the Range of Ixodes scapularis in Canada.” International Journal of Environmental Research and Public Health 15, no. 4: 603. 10.3390/ijerph15040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, E. L. , Jardine C. M., Koffi J. K., et al. 2022. “Range Expansion of Ixodes Scapularis and Borrelia burgdorferi in Ontario, Canada, From 2017 to 2019.” Vector Borne and Zoonotic Diseases 22, no. 7: 361–369. 10.1089/vbz.2022.0015. [DOI] [PubMed] [Google Scholar]
- Rogic, A. , Tessier N., Legendre P., Lapointe F. J., and Millien V.. 2013. “Genetic Structure of the White‐Footed Mouse in the Context of the Emergence of Lyme Disease in Southern Quebec.” Ecology and Evolution 3, no. 7: 2075–2088. 10.1002/ece3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy‐Dufresne, E. , Logan T., Simon J. A., Chmura G. L., and Millien V.. 2013. “Poleward Expansion of the White‐Footed Mouse ( Peromyscus leucopus ) Under Climate Change: Implications for the Spread of Lyme Disease.” PLoS One 8, no. 11: e80724. 10.1371/journal.pone.0080724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillberg, E. , Lunny D., Lindsay L. R., et al. 2018. “Distribution of Ixodes scapularis in Northwestern Ontario: Results From Active and Passive Surveillance Activities in the Northwestern Health Unit Catchment Area.” International Journal of Environmental Research and Public Health 15, no. 10. 10.3390/ijerph15102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz, G. , Epp T., Burgess H. J., Chilton N. B., and Lohmann K. L.. 2015. “Comparison Between Available Serologic Tests for Detecting Antibodies Against Anaplasma phagocytophilum and Borrelia burgdorferi in Horses in Canada.” Journal of Veterinary Diagnostic Investigation 27, no. 4: 540–546. 10.1177/1040638715587548. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Anderson J. F., and Durden L. A.. 2012. “Widespread Dispersal of Borrelia burgdorferi ‐Infected Ticks Collected From Songbirds Across Canada.” Journal of Parasitology 98, no. 1: 49–59. 10.1645/ge-2874.1. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Anderson J. F., Durden L. A., Smith M. L., Manord J. M., and Clark K. L.. 2016. “Ticks parasitizing gallinaceous birds in Canada and First Record of Borrelia burgdorferi‐infected Ixodes pacificus (Acari: Ixodidae) from California Quail.” Systematic & Applied Acarology 21, no. 1: 1. 10.11158/saa.21.1.1. [DOI] [Google Scholar]
- Scott, J. D. , Clark K. L., Coble N. M., and Ballantyne T. R.. 2019. “Detection and Transstadial Passage of Babesia Species and Borrelia burgdorferi Sensu Lato in Ticks Collected from Avian and Mammalian Hosts in Canada.” Healthcare (Basel, Switzerland) 7, no. 4: 155. 10.3390/healthcare7040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , Clark K. L., Foley J. E., Anderson J. F., Bierman B. C., and Durden L. A.. 2018. “Extensive Distribution of the Lyme Disease Bacterium, Borrelia burgdorferi Sensu Lato, in Multiple Tick Species Parasitizing Avian and Mammalian Hosts Across Canada.” Healthcare (Basel) 6, no. 4: 131. 10.3390/healthcare6040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , Clark K. L., Foley J. E., et al. 2017. “Detection of Borrelia Genomospecies 2 in Ixodes spinipalpis Ticks Collected From a Rabbit in Canada.” Journal of Parasitology 103, no. 1: 38–46. 10.1645/16-127. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Clark K. L., Foley J. E., Bierman B. C., and Durden L. A.. 2018. “Far‐Reaching Dispersal of Borrelia burgdorferi Sensu Lato‐Infected Blacklegged Ticks by Migratory Songbirds in Canada.” Healthcare (Basel) 6, no. 3: 89. 10.3390/healthcare6030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , and Durden L. A.. 2015. “New Records of the Lyme Disease Bacterium in Ticks Collected from Songbirds in Central and Eastern Canada.” International Journal of Acarology 41, no. 4: 241–249. 10.1080/01647954.2015.1038301. [DOI] [Google Scholar]
- Scott, J. D. , Fernando K., Banerjee S. N., et al. 2001. “Birds Disperse Ixodid (Acari: Ixodidae) and Borrelia burgdorferi ‐Infected Ticks in Canada.” Journal of Medical Entomology 38, no. 4: 493–500. 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Fernando K., Durden L. A., and Morshed M. G.. 2004. “Lyme Disease Spirochete, Borrelia burgdorferi , Endemic in Epicenter at Turkey Point Ontario.” Journal of Medical Entomology 41, no. 2: 226–230. 10.1603/0022-2585-41.2.226. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Foley J. E., Anderson J. F., Clark K. L., and Durden L. A.. 2017. “Detection of Lyme Disease Bacterium, Borrelia burgdorferi Sensu Lato, in Blacklegged Ticks Collected in the Grand River Valley, Ontario Canada.” International Journal of Medical Sciences 14, no. 2: 150–158. 10.7150/ijms.17763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , Foley J. E., Clark K. L., et al. 2016. “Established Population of Blacklegged Ticks With High Infection Prevalence for the Lyme Disease Bacterium, Borrelia burgdorferi Sensu Lato, on Corkscrew Island, Kenora District Ontario.” International Journal of Medical Sciences 13, no. 11: 881–891. 10.7150/ijms.16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , Lee M. K., Fernando K., et al. 2010. “Detection of Lyme Disease Spirochete, Borrelia burgdorferi Sensu Lato, Including Three Novel Genotypes in Ticks (Acari: Ixodidae) Collected From Songbirds (Passeriformes) Across Canada.” Journal of Vector Ecology 35, no. 1: 124–139. 10.1111/j.1948-7134.2010.00038.x. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Lee M. K., Fernando K., Jorgensen D. R., Durden L. A., and Morshed M. G.. 2008. “Rapid Introduction of Lyme Disease Spirochete, Borrelia burgdorferi Sensu Stricto, in Ixodes scapularis (Acari: Ixodidae) Established at Turkey Point Provincial Park, Ontario Canada.” Journal of Vector Ecology 33, no. 1: 64–69. 10.3376/1081-1710(2008)33[64:RIOLDS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Scott, J. D. , Pascoe E. L., Sajid M. S., and Foley J. E.. 2020. “Monitoring of Nesting Songbirds Detects Established Population of Blacklegged Ticks and Associated Lyme Disease Endemic Area in Canada.” Healthcare (Basel) 8, no. 1: 59. 10.3390/healthcare8010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , Pascoe E. L., Sajid M. S., and Foley J. E.. 2021. “Detection of Babesia Odocoilei in Ixodes scapularis Ticks Collected in Southern Ontario, Canada.” Pathogens 10, no. 3: 327. 10.3390/pathogens10030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. D. , and Pesapane R. R.. 2021. “Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi Sensu Lato, and Hepatozoon canis in Ixodes scapularis Ticks Collected in Eastern Canada.” Pathogens (Basel, Switzerland) 10, no. 10: 1265. 10.3390/pathogens10101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, J. A. , Marrotte R. R., Desrosiers N., et al. 2014. “Climate Change and Habitat Fragmentation Drive the Occurrence of Borrelia burgdorferi , the Agent of Lyme Disease, at the Northeastern Limit of Its Distribution.” Evolutionary Applications 7, no. 7: 750–764. 10.1111/eva.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. A. , Oesterle P. T., Jardine C. M., et al. 2019. “Tick Infestations of Wildlife and Companion Animals in Ontario, Canada, With Detection of Human Pathogens in Ixodes scapularis Ticks.” Ticks Tick Borne Dis 10, no. 1: 72–76. 10.1016/j.ttbdis.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Sonenshine, D. , and Roe R.. 1993. Biology of Ticks. Vol. 2. New York: Oxford University Press. [Google Scholar]
- Talbot, B. , Slatculescu A., Thickstun C. R., et al. 2019. “Landscape Determinants of Density of Blacklegged Ticks, Vectors of Lyme Disease, at the Northern Edge of Their Distribution in Canada.” Scientific Reports 9, no. 1: 16652. 10.1038/s41598-019-50858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy, O. , Acheson E. S., Bouchard C., et al. 2023. “Mechanistic Movement Models to Predict Geographic Range Expansions of Ticks and Tick‐Borne Pathogens: Case Studies With Ixodes Scapularis and Amblyomma americanum in Eastern North America.” Ticks Tick Borne Diseases 14, no. 4: 102161. 10.1016/j.ttbdis.2023.102161. [DOI] [PubMed] [Google Scholar]
- Tricco, A. C. , Lillie E., Zarin W., et al. 2018. “PRISMA Extension for Scoping Reviews (PRISMA‐ScR): Checklist and Explanation.” Annals of Internal Medicine 169, no. 7: 467–473. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- Tyler, S. , Tyson S., Dibernardo A., et al. 2018. “Whole Genome Sequencing and Phylogenetic Analysis of Strains of the Agent of Lyme Disease Borrelia burgdorferi From Canadian Emergence Zones.” Scientific Reports 8, no. 1: 10552. 10.1038/s41598-018-28908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve, A. , Goring J., Marcotte L., and Overvelde S.. 2011. “Seroprevalence of Borrelia burgdorferi , Anaplasma phagocytophilum , Ehrlichia canis, and Dirofilaria Immitis Among Dogs in Canada.” Canadian Veterinary Journal 52, no. 5: 527–530. [PMC free article] [PubMed] [Google Scholar]
- Watson, T. G. , and Anderson R. C.. 1976. “ Ixodes scapularis Say on White‐Tailed Deer ( Odocoileus virginianus ) From Long Point, Ontario.” Journal of Wildlife Diseases 12: 66–71. [DOI] [PubMed] [Google Scholar]
- Werden, L. , Barker I. K., Bowman J., et al. 2014. “Geography, Deer, and Host Biodiversity Shape the Pattern of Lyme Disease Emergence in the Thousand Islands Archipelago of Ontario Canada.” PLOS ONE 9, no. 1: e85640. 10.1371/journal.pone.0085640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2017. One Health. Geneva: World Health Organization. https://www.who.int/news‐room/questions‐and‐answers/item/one‐health. [Google Scholar]
- Wilson, C. , Gasmi S., Bourgeois A. C., et al. 2023. “Surveillance for Ixodes Scapularis and Ixodes pacificus Ticks and Their Associated Pathogens in Canada, 2020.” Canada Communicable Disease Report 49, no. 6: 288–298. 10.14745/ccdr.v49i06a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, C. H. , Gasmi S., Bourgeois A. C., et al. 2022. “Surveillance for Ixodes Scapularis and Ixodes pacificus Ticks and Their Associated Pathogens in Canada, 2019.” Canada Communicable Disease Report 48, no. 5: 208–218. 10.14745/ccdr.v48i05a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Smith C., and Battad A.. 2021. “Babesia Microti Acquired in Canada.” Canadian Medical Association Journal 193, no. 31: E1213–E1217. 10.1503/cmaj.201983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck, C. B. , and Lloyd V. K.. 2022. “Borrelia Burgdorferi and Borrelia miyamotoi in Atlantic Canadian Wildlife.” PLoS One 17, no. 1: e0262229. 10.1371/journal.pone.0262229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck, C. B. , Priest J. M., Shutler D., Boudreau M., and Lloyd V. K.. 2021. “Detection of Borrelia spp., Ehrlichia canis , Anaplasma Phagocytophilum, and Dirofilaria Immitis in Eastern Coyotes ( Canis latrans ) in Nova Scotia, Canada.” Journal of Wildlife Diseases 57, no. 3: 678–682. 10.7589/JWD-D-20-00188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppenSearch strings used in PubMed, Web of Science, and Embase for scoping review of tick and animal surveillance studies for Lyme disease in Canada
Appendix 2. Chronological list of Lyme disease‐related tick and animal surveillance studies in Canada included in synthesis, by study start year, published 1976‐2023. Reviewed studies are ordered based on the year surveillance began (from earliest to latest), total collection period (number of years), and year of publication. For each study, the province(s) where the study took place and the tick or animal type collected (Ixodes [ I. scapularis and/or I. pacificus ], small mammal, large mammal, bird) are shaded. When provided in the studies, numbers of Ixodes or animal hosts collected are documented and Bb testing (by any testing method) status indicated. For each province, the first study to detect Ixodes, Bb in Ixodes, and Bb in a mammal (by study collection year) is noted.
Data Availability Statement
The authors have nothing to report.


