Abstract
BACKGROUND:
Geographic atrophy (GA) is a form of advanced age-related macular degeneration (AMD) that can cause irreversible vision impairment and is responsible for approximately 20% of legal blindness in the United States. There is limited real-world evidence assessing health outcomes and health care resource use (HCRU) among individuals with GA.
OBJECTIVE:
To examine the progression from GA without subfoveal involvement (SFI) to GA with SFI, progression to irreversible blindness, and HCRU among older individuals with GA enrolled in Medicare Advantage Prescription Drug (MAPD) plans.
METHODS:
This retrospective study used claims data for MAPD-plan enrollees aged at least 65 years with an AMD diagnosis between 2018 and 2021. To assess progression of GA, development of blindness, and HCRU, propensity score matched cohorts of individuals with GA and without GA were identified and compared. For GA progression analysis, at least 12 months of follow-up was required, and patients were followed until the end of either follow-up or study period.
RESULTS:
Total 9,511 individuals with GA were matched 1:1 to individuals without GA. Among individuals with GA, initial diagnosis was primarily by an ophthalmologist (58.6%) followed by an optometrist (30.9%). The most common diagnostic imaging procedure at index was optical coherence tomography (53.0%). Mean follow-up time was 2.3 years. At index, 4,781 (50.3%) individuals had GA without SFI and 4,697 (49.4%) had GA with SFI. Among individuals with GA without SFI at index, 479 (10.2%) progressed to GA with SFI during the 12-month follow-up. Among individuals with GA without SFI at index, 173 (3.6%) developed irreversible blindness, compared to 312 (6.6%) of those with SFI, and 51 (0.5%) individuals without GA. Kaplan-Meier analysis indicated fastest progression to irreversible blindness among individuals with GA with SFI, followed by those without SFI (log-rank test P < 0.001). Both diagnosis of GA without SFI (hazard ratio [HR] [CI] = 6.77 [4.98-9.35], P < 0.001) and diagnosis of GA with SFI (HR [CI] = 12.59 [9.43-17.16], P < 0.001) were strongly associated with increased risk of developing irreversible blindness. Significant predictors of progression to GA with SFI were wet AMD at baseline (HR [CI] = 5.70 [4.63-6.99], P < 0.001), Elixhauser comorbidity score of 4-5 (HR [CI] = 1.46 [1.12-1.91], P = 0.006), and more than 5 (HR [CI] = 1.40 [1.02-1.89], P = 0.035).
CONCLUSIONS:
GA with or without SFI was associated with progression to irreversible blindness in an MAPD-plan population. Patients with GA with SFI progressed to irreversible blindness faster than patients with GA without SFI. With the recent approval of GA treatments, future research is needed to assess the impacts on disease progression, including blindness.
Plain language summary
Geographic atrophy (GA) is an eye disease. GA is the advanced form of dry age-related macular degeneration (AMD). It can cause permanent and irreversible vision loss. In this study, patients with GA were found to be at increased risk of going blind relative to people without GA. Furthermore, patients with GA who also had wet AMD, another advanced form of AMD, were more likely to have their disease progress than those who just had GA.
Implications for managed care pharmacy
According to this study, patients with GA have greater risk of becoming blind than those without GA. Those with comorbid wet AMD have an increased risk of progression to GA with subfoveal involvement. Differences were not observed between GA and non-GA on health care resource use, but patients with GA will use recently approved GA treatments to delay or prevent blindness. Further research is necessary to understand real-world treatment implications on health care resource use, disease progression, and longer-term outcomes.
Age-related macular degeneration (AMD) is a chronic, progressive degenerative disorder of the macula affecting older individuals and causes loss of central vision.1 AMD is one of the leading causes of visual impairment and blindness globally2 and can be classified broadly into the following 2 types: nonneovascular (dry) and neovascular (wet).1 Wet AMD is indicated by the development of choroidal neovascularization (ie, the growth of new blood vessels under the macula that could potentially leak). Dry AMD, the nonexudative form, accounts for the majority of all diagnosed cases of the disease.
Geographic atrophy (GA) is the advanced form of dry AMD and is characterized by the loss of cells in the macula resulting in atrophic lesions. On imaging, these areas of atrophy often resemble a map, hence the name GA. GA can cause irreversible loss of vision, especially once the fovea, the central point of the macula responsible for sharp central vision, is affected by the GA lesion. GA typically begins outside of the fovea (without subfoveal involvement [SFI]) and expands into the fovea (with SFI) where central visual acuity is most impacted by the progression.3 There are more than 1 million people in the United States who have GA in at least 1 eye.4 This disease causes progressive loss of the retinal pigment epithelium, photoreceptors, and underlying choriocapillaris, and is responsible for approximately 20% of legal blindness in North America.5
Therapies indicated for the treatment of GA have only recently received US Food and Drug Administration (FDA) approval. In February 2023, pegcetacoplan injection (Syfovre; Apellis Pharmaceuticals) was approved by the FDA for the treatment of GA, representing the first FDA approved medication with this indication.6 This drug is a complement inhibitor targeting components of the complement cascade, C3 and C3b, to slow the progression of GA lesion growth.7 The Syfovre phase 3 clinical trials included patients with GA lesions either with SFI or without SFI. Another treatment was approved by the FDA in August 2023: avacincaptad pegol intravitreal solution (Izervay; Astellas Pharma US, Inc.), a complement C5 inhibitor indicated for treating GA secondary to AMD.8,9 In contrast to the Syfovre trials, Izervay phase 3 clinical trials only included patients having GA lesions without SFI.
Progression of GA may take place over a prolonged period; however, limited evidence exists assessing changes in the use of health care services during the period of initial identification and diagnosis. In the Age-Related Eye Disease (AREDS) study, among individuals with median age of 71 years, the median time to development of central GA after any GA diagnosis was found to be 2.5 years.3 In a recent study among patients aged at least 50 years, based on data from the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry, 12.8% of study eyes that had GA without SFI at baseline progressed to GA with SFI at the end of 2 years of follow-up.10 Prior to the availability of treatment, a claims analysis assessing a commercial population concluded patients with GA have significantly higher health care resource use (HCRU) and spending in the first year after diagnosis; however, there is little contemporary research on GA progression among Medicare Advantage Prescription Drug (MAPD) plan enrollees.11,12
To assess outcomes among MAPD-plan enrollees with GA, we examined progression from GA without SFI to GA with SFI, progression to irreversible blindness, incidence of depression, incidence of fall-related injuries, and HCRU.
Methods
DATA SOURCE
This retrospective study was conducted using the Humana Research Database that contains health care administrative claims data for MAPD and commercial health plan enrollees. For this study, member enrollment data, medical claims, and pharmacy claims for older individuals enrolled in MAPD plans were examined. Claims data included information regarding physician visits, outpatient visits, emergency department (ED) visits, and inpatient hospitalizations. Pharmacy claims data included detailed information for each prescription fill.
STUDY POPULATION AND STUDY DESIGN
This study was a longitudinal historical cohort study that examined outcomes among propensity score (PS)−matched GA and non-GA cohorts. The GA cohort included individuals enrolled in MAPD plans with at least 1 medical claim with a diagnosis code for GA (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes: GA without SFI [Right eye = H35.3113, Left eye = H35.3123, Bilateral GA = H35.3133]; GA with SFI [Right eye = H35.3114, Left eye = H35.3124, Bilateral = H35.3134]) (Supplementary Table 1 (336.3KB, pdf) ) in any position during the identification period, July 1, 2018, to June 30, 2021 (Figure 1). Date of the first claim with the diagnosis of GA was set as the index date. Study participants were required to be aged at least 65 years on index date, with continuous enrollment in medical and pharmacy benefits for 12 months during the pre-index period and for at least 12 months during the follow-up period. Follow-up was continued beyond 12 months for individuals with longer enrollment, until the end of the study period (June 30, 2022) or end of enrollment. Individuals with a diagnosis of GA during the pre-index period were excluded. Similarly, individuals who had at least 1 claim with a diagnosis of certain ophthalmic conditions (visual impairment or blindness, glaucoma, diabetic retinopathy, and cataract) during the pre-index period were excluded; however, study participants could have wet AMD.
FIGURE 1.
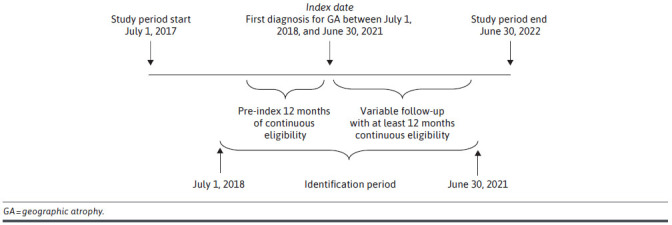
Study Diagram
The non-GA cohort included MAPD-plan enrollees without a diagnosis of GA during the study period. The non-GA cohort was randomly assigned index dates such that the distribution of the index dates in non-GA cohort reflected the distribution of index dates in the matched GA cohort. To be included in the non-GA cohort, individuals were required to be aged 65 years or older on index date, with at least 1 physician office visit during the 30 days before the index date, and 12 months of pre-index and post-index continuous enrollment. A variable follow-up period was allowed beyond 12 months until the end of the study period or end of enrollment. Individuals with at least 1 claim with a diagnosis of ophthalmic conditions (visual impairment or blindness, glaucoma, diabetic retinopathy, and cataract) during the pre-index period were excluded as these conditions could lead to blindness, thereby confounding the results.
BASELINE MEASURES
All baseline measures were evaluated during the 12 months before index GA diagnosis, including index date. Demographic characteristics measured at baseline included age, sex, race, region, population density, low-income subsidy status, and Medicaid/Medicare dual eligibility. Comorbidity burden and severity among the matched cohorts was evaluated using Deyo-Charlson comorbidity index (DCI) and Elixhauser comorbidities. Additionally, presence of comorbidities like wet AMD, depression, and anxiety disorders at baseline and during follow-up were also assessed. The DCI score is based on 17 categories of comorbidities to calculate a score that reflects the cumulative increased likelihood of 1-year mortality.13 The Elixhauser comorbidity score uses 31 categories of ICD-9 and ICD-10 diagnosis codes to calculate a score that is associated with hospital charges, length of stay, and mortality.14,15 The Elixhauser score is unweighted for each of the comorbidities and can range from 0 to 31.
The specialty of the GA diagnosing provider was determined using National Provider Identifiers, and diagnostic imaging procedures used for confirming GA diagnosis were identified using Current Procedural Terminology codes (Supplementary Table 2 (336.3KB, pdf) ).
STUDY OUTCOMES
The primary outcome for GA vs non-GA comparison was progression to irreversible blindness. Irreversible blindness was defined as any patient with a diagnosis code for blindness (ICD-10-CM = H54.0, H54.1, H54.4, H54.8) in the claims database. Another primary outcome of interest was progression to GA with SFI, which only applied to those with GA but without SFI at index. Progression of GA was defined among patients with GA without SFI in both eyes at index by a subsequent code for GA with SFI during the study period. GA progression was not studied at individual eye level as several inconsistencies were found in the eye-level coding of GA. Additionally, fall-related injuries, depression, anxiety, and HCRU were assessed during follow-up. These outcomes were measured using ICD-10-CM codes (Supplementary Table 1 (336.3KB, pdf) ) observed on medical claims during the follow-up period. Crude incidence rate (IR) per 1,000 person years and 95% CIs were calculated for depression, anxiety disorders, and fall-related injuries. Individuals with prevalent depression or anxiety during the baseline period were excluded from the IR calculations, and these rates reflect the rates of new diagnosis of these conditions. Use of antivascular endothelial growth factor (anti-VEGF) medications, laser therapy, antidepressants, and other medications were also assessed.
STATISTICAL ANALYSIS
To mitigate selection bias and adjust for baseline differences between GA and non-GA cohorts, the PS-matching method was used. The PSs were estimated for each patient by modeling the probability of being diagnosed with GA. The logistic regression model used for calculating PS included baseline demographic characteristics, selected medical comorbidities, and baseline HCRU (Supplementary Table 3 (336.3KB, pdf) ). Individuals with and without GA were matched 1:1 using a greedy matching algorithm for PS matching.16 To assess the balance between the matched cohorts on variables used for matching, the standardized differences for each variable were evaluated both before and after the matching (Supplementary Table 3 (336.3KB, pdf) ).
Descriptive statistics (percentages, means, medians, SDs) were used to describe baseline demographic/clinical characteristics. Chi-square tests or Fischer’s exact tests were used for comparisons of frequencies between the cohorts, and Wilcoxon–Mann-Whitney tests were used for continuous variables. Kaplan-Meier methods were implemented to estimate median survival time for time to event outcomes (progression from GA without SFI to GA with SFI, progression to irreversible blindness from index date, and time to first fall-related injury from the index date). A log-rank test was performed for testing the difference in proportion of patients with the outcomes of interest across the various strata. A Cox proportional hazard model including all individuals without evidence of irreversible blindness at index was used to assess factors associated with progression to irreversible blindness. Likewise, a Cox proportional hazard model was used to assess factors associated with progression to SFI among the subgroup of individuals with GA with no evidence of SFI. Proportional hazards were assessed to understand the association between baseline characteristics and the outcomes. The independent variables included in the model were age, sex, race, population density (urban, suburban, and rural), Elixhauser comorbidity score, wet AMD, pre-index anti-VEGF use, and HCRU (inpatient stays, ED visits, and telehealth visits). Analyses were performed using SAS Enterprise Guide v8.3.
Results
BASELINE CHARACTERISTICS
A total of 9,519 individuals with a diagnosis of GA were identified, and a total of 9,511 individuals with and without GA were successfully matched (Figure 2). Standardized differences associated with all baseline variables included in the PS match were less than 0.10 after matching. For a complete list of the variables used and the standardized differences before and after matching, refer to Supplementary Table 3 (336.3KB, pdf) . The mean [SD] age in the matched cohorts was 82.3 [7.4] vs 82.3 [7.6] years for GA and non-GA cohorts, respectively (P = 0.638, standardized difference = 0.01). The majority of individuals were female, White race, living in the South region of the United States, and living in urban locations (see Table 1). DCI score and Elixhauser condition count were similar among the GA and non-GA cohorts. The proportion of patients with wet AMD during the pre-index period was higher among individuals with GA (1,806 [19.0%] vs 366 [3.9%], GA and non-GA cohort, respectively, P < 0.001). GA was most commonly diagnosed during an encounter with an ophthalmologist (5,574 [58.6%]), followed by optometrists (2,935 [30.9%]). A total 73.3% of patients received at least 1 GA-related imaging procedure on the index date. The most common diagnostic imaging procedure was optical coherence tomography (5,042 [53.0%]), followed by fundus photography (2,039 [21.4%]) and fluorescein angiography (600 [6.3%]). The mean (SD) follow-up duration in days was 847 (320) vs 845 (321), for GA and non-GA cohort, respectively (P = 0.655).
FIGURE 2.
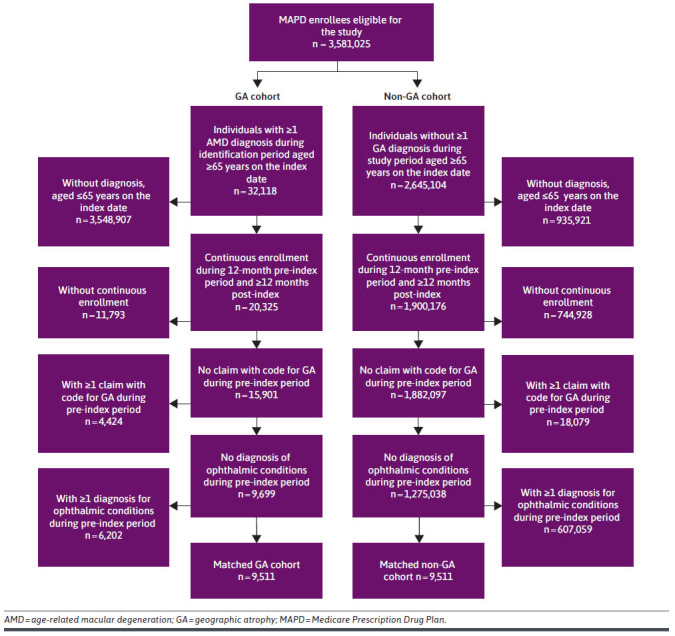
Attrition Diagram
TABLE 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | GA cohort | Non-GA cohort | P value | Standardized difference |
|---|---|---|---|---|
| n | 9,511 | 9,511 | ||
| Demographic characteristics | ||||
| Age on index date, mean (SD) | 82.27 (±7.4) | 82.34 (±7.6) | 0.638 | 0.009 |
| Sex, n (%) | 0.263 | 0.016 | ||
| Female | 6,136 (64.5) | 6,062 (63.7) | ||
| Male | 3,375 (35.5) | 3,449 (36.3) | ||
| Race, n (%) | 0.853 | |||
| White | 8,886 (93.4) | 8,860 (93.2) | 0.011 | |
| Black | 192 (2.0) | 204 (2.1) | 0.009 | |
| Other | 248 (2.6) | 262 (2.8) | 0.009 | |
| Unknown | 185 (1.9) | 185 (1.9) | 0.000 | |
| Geographic location, n (%) | 0.457 | |||
| Northeast | 299 (3.1) | 336 (3.5) | 0.022 | |
| Mid-West | 2,366 (24.9) | 2,387 (25.1) | 0.005 | |
| South | 5,472 (57.5) | 5,410 (56.9) | 0.013 | |
| West | 1,374 (14.4) | 1,378 (14.5) | 0.001 | |
| Population density, n (%) | 0.515 | |||
| Urban | 5,566 (58.5) | 5,483 (57.6) | 0.018 | |
| Suburban | 2,559 (26.9) | 2,633 (27.7) | 0.017 | |
| Rural | 1,170 (12.3) | 1,192 (12.5) | 0.007 | |
| Unknown | 216 (2.3) | 203 (2.1) | 0.009 | |
| Low-income subsidy status, n (%) | 2,121 (22.3) | 2,156 (22.7) | 0.543 | 0.009 |
| Dual eligibility status, n (%) | 1,659 (17.4) | 1,641 (17.3) | 0.730 | 0.005 |
| Clinical characteristics | ||||
| GA without subfoveal involvement at index, n (%) | 4,781 (50.3) | — | — | — |
| GA with subfoveal involvement at index, n (%) | 4,697 (49.4) | — | — | — |
| Irreversible blindness at index, n (%)a | 33 (0.3) | — | — | — |
| DCCI, mean (SD)b,c | 1.8 (2.0) | 1.9 (2.0) | 0.001 | — |
| Elixhauser comorbidity score, mean (SD)b,c | 3.3 (2.8) | 3.3 (2.7) | 0.001 | — |
| Wet AMD, n (%)b | 1,806 (19) | 366 (3.9) | <0.0001 | — |
| Depression, n (%)b | 1,896 (19.9) | 1,962 (20.6) | 0.234 | — |
| Anxiety disorders, n (%)b | 1,807 (19) | 1,817 (19.1) | 0.854 | — |
| Diagnostic characteristics | ||||
| GA diagnosing provider specialty, n (%)b | ||||
| Ophthalmologist | 5,574 (58.6) | — | — | — |
| Optometrist | 2,935 (30.9) | — | — | — |
| Primary care provider | 243 (2.6) | — | — | — |
| Other | 327 (3.4) | — | — | — |
| Unknown | 432 (4.5) | — | — | — |
| GA diagnostic procedure received on index date, n (%)b | ||||
| Any of the below procedures | 6,968 (73.3) | — | — | — |
| OCT | 5,042 (53.0) | — | — | — |
| FP | 2,039 (21.4) | — | — | — |
| FA | 600 (6.3) | — | — | — |
| ICG | <11 (<0.1) | — | — | — |
| FA & ICG (at same time) | 97 (1.0) | — | — | — |
| Length of follow-up | ||||
| Follow-up duration (days), mean (SD)b | 847 (320) | 845 (321) | 0.655 | — |
a Note that 33 patients with diagnosis of irreversible blindness at index were not included in some analyses.
b Not included in matching.
c Aggregate score not used in matching, but individual comorbidities were used in matching.
AMD = age-related macular degeneration; DCCI = Deyo-Charlson Comorbidity Index; FA = fluorescein angiography; FP = fundus photography; GA = geographic atrophy; ICG = indocyanine-green angiography; OCT = optical coherence tomography.
During the study period, there was no treatment approved for GA. In assessing therapies used to treat other ocular conditions, there was higher use of laser therapy among individuals with GA (464 [4.9%] vs 169 [1.8%]; GA and non-GA cohort, respectively, P < 0.001) (Supplementary Table 4 (336.3KB, pdf) ). Use of anti-VEGF agents, which are indicated for treating wet AMD, was also higher among individuals with GA (bevacizumab = 961 [10.1%] vs 159 [1.7%], P < 0.001; aflibercept = 455 [4.8%] vs 109 [1.1%], P < 0.001; ranibizumab = 215 [2.3%] vs 53 [0.6%], P < 0.001).
CLINICAL COMORBIDITIES AND OUTCOMES
The prevalence of wet AMD observed during the follow-up period was higher among individuals with GA compared with the non-GA cohort (2,805 [29.5%] vs 392 [4.1%], respectively; P < 0.0001) (Supplementary Table 5 (336.3KB, pdf) ). A high proportion of patients had GA with SFI at index in 1 or both eyes (n = 4,697, 49.4%). Hence, the progression to GA with SFI during follow-up period was assessed among a subgroup of patients with GA but without SFI at index in both the eyes (n = 4,781, 50.3%). Progression to GA with SFI was observed in 479 (10.2%) of these individuals (see Table 2). The strongest predictors of progression to SFI were wet AMD (hazard ratio [HR] [CI] = 5.70 [4.63-6.99], P < 0.001), Elixhauser scores of 4-5 (HR [CI] = 1.46 [1.12-1.91], P = 0.006), and an Elixhauser score of more than 5 (HR [CI] = 1.39 [1.02-1.89], P = 0.035, Supplementary Table 6 (336.3KB, pdf) ).
TABLE 2.
Clinical Conditions During Follow-Up Period
| Clinical outcome | GA cohort | Non-GA cohort | P value |
|---|---|---|---|
| Comorbidities of interest, n (%) | |||
| Wet AMD | 2,805 (29.5) | 392 (4.1) | <0.0001 |
| Mental health conditions (depression or anxiety) | 2,274 (23.4) | 2,144 (22.0) | 0.023 |
| Depression | 2,123 (22.3) | 2,145 (22.6) | 0.702 |
| Anxiety disorders | 1,948 (20.5) | 1,865 (19.6) | 0.133 |
| Clinical outcomes, n (%) | |||
| Progression to GA with SFIa | 479 (10.2) | — | — |
| Progression to blindnessb | 485 (5.1) | 51 (0.5) | 0.266 |
| GA without SFI at indexa | 173 (3.6) | — | — |
| GA with SFI at indexc | 312 (6.6) | — | — |
| Fall-related injuriesd | 1,393 (14.7) | 1,303 (13.7) | 0.052 |
| Crude incidence rate (per 1,000 person years) | |||
| Fall-related injuries (CI) | 74.7 (70.8-78.7) | 68.8 (65.1-72.6) | NS |
| Depression (CI) | 86.6 (82.1-91.3) | 77.3 (73.0-81.7) | 0.004 |
| Anxiety disorders (CI) | 84.5 (80.1-89.1) | 78.9 (74.6-83.4) | NS |
a Calculated among 4,781 individuals with GA without evidence of SFI at baseline.
b Calculated among 9,478 individuals in the GA cohort and 9,511 individuals in the non-GA cohort without evidence of blindness at baseline.
c Calculated among 4,697 patients with GA with SFI at baseline.
d Calculated among 9,511 individuals in the GA cohort and 9,511 individuals in the non-GA cohort.
AMD = age-related macular degeneration; GA = geographic atrophy; NS = not significant; SFI = subfoveal involvement.
For assessing progression to irreversible blindness, 9,478 patients from the GA cohort and 9,511 from the non-GA cohort were included; a total of 33 patients with GA were excluded because of having a diagnosis of irreversible blindness at index. A total of 173 (3.6%) individuals with GA without SFI at index, 312 (6.6%) with GA with SFI at index, and 51 (0.5%) individuals without GA had claims-based evidence of irreversible blindness during follow-up. Figure 3 presents the Kaplan-Meier curves for progression to irreversible blindness, indicating a statistically significant difference between the cohorts (log-rank test P < 0.0001). The strongest predictors of progression to blindness were presence of GA with SFI at index (HR [CI] = 12.59 [9.43-17.16], P < 0.001) and GA without SFI at index (HR [CI] = 6.77 [4.98-9.35], P < 0.001) (Supplementary Table 7 (336.3KB, pdf) ).
FIGURE 3.
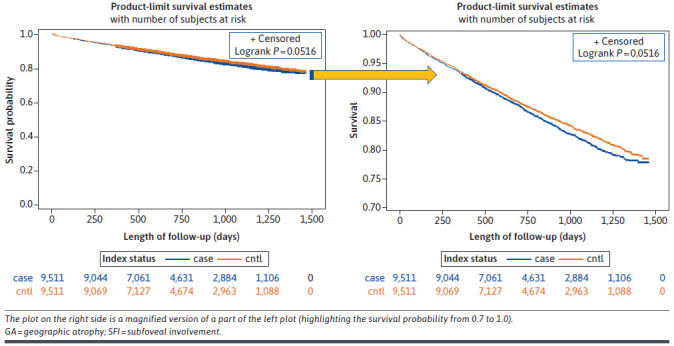
Kaplan-Meier Curves Comparing Blindness Between the GA and Non-GA Cohorts
There was no statistically significant difference in IR per 1,000 person years for prevalence of depression and anxiety disorders during follow-up in the 2 cohorts (see Table 2); however, new diagnosis of depression was higher among individuals with GA than in the non-GA cohort (86.6 [82.1-91.3] vs 77.3 [73.0-81.7], P = 0.004). No statistically significant differences were observed in the IRs for fall-related injuries and anxiety disorders between the matched cohorts (Table 2).
There was no statistically significant difference between the 2 cohorts in the use of health care resources like inpatient stays, length of stay, ED visits, and telehealth services, except for higher number of outpatient visits in individuals with GA (mean [SD] = 18.3 [15.9] vs 16.7 [15.9]; GA vs non-GA cohort, respectively, P < 0.001) (Supplementary Table 8 (336.3KB, pdf) ).
Discussion
AMD is one of the leading causes of blindness in the United States and the leading cause of blindness among White individuals.2,17 In our study, the average age of patients with GA was 82.3 years, approximately 64% were female, and about 93% identified as White race.
In a past study using commercially available claims data, it was reported that approximately 40% of all patients with GA had wet AMD at the diagnosis of GA.11 The mean age of patients in that study was 69 years, and both incident and prevalent patients with GA were included, the latter of which may include individuals with more advanced disease at study entry. That study did not exclude patients with certain ophthalmic conditions during the pre-index period (visual impairment or blindness, glaucoma, diabetic retinopathy, and cataract), unlike the current study. In our study, about 30% of the GA cohort had evidence of wet AMD during the follow-up period, which was slightly lower than the aforementioned study. The prevalence of co-occurring wet AMD in our study may be a result of inclusion of only incident patients with GA, and exclusion of a significant number of patients with aforementioned ophthalmic conditions during the pre-index period. However, our study affirms that wet AMD and GA are frequently co-occurring in patients with AMD. These patients may require treatment with both novel GA therapies and anti-VEGF therapy at the same time because GA and wet AMD have differing pathophysiology.
Additionally, we observed approximately 10% of patients progressed from GA without SFI to GA with SFI during the follow-up period. The average follow-up period in the current study was 2.3 years. In the AREDS study, which was a prospective, randomized, interventional trial for treatments of AMD, the median survival time for progression to central GA was found to be 2.5 years (ie, the probability of progression was 0.50 at 2.5 years).3 Although the duration of follow-up available in the current study was relatively prolonged for a longitudinal study based on administrative claims data, follow-up in the AREDS study continued for up to a decade and allowed examination of the fundus at regular intervals during follow-up. The IRIS registry also observed higher rates of progression to GA with SFI, which suggests that coding inconsistencies in real-world databases may impact the rate of GA progression seen in this study.
In our study, we observed the risk of developing irreversible blindness double if a patient had GA with SFI at index compared with patients having GA without SFI at index. In the published literature, there are reports that the presence of wet AMD in the fellow eye is associated with a higher progression rate for GA.18,19 Although coding limitations precluded an eye-level analysis to determine whether patients had GA and wet AMD in the same eye or fellow eye, our study estimated that among the patients with GA without SFI at index, co-occurring wet AMD increased the risk of progression to GA with SFI by 5.7 times, compared with those without wet AMD at baseline. Given that no treatments have existed for GA until recently, there has likely been little incentive for consistent and accurate diagnosis coding by specialists, which might, to some extent, result in potential underreporting of GA progression in claims data. Park et al suggested this might be due to providers revising their diagnostic coding from nonexudative to exudative AMD when the patient develops choroidal neovascularization resulting in the removal of nonexudative AMD from the chart.12 Subsequently, if the patient develops GA, the provider may not redocument the diagnosis of nonexudative AMD. As GA has been shown to be underreported in medical records, the availability of a treatment for this disease may motivate improved reporting and coding of this condition.12
Another factor associated with increased progression from GA without SFI to GA with SFI was an Elixhauser comorbidity score of 4 or more. The Elixhauser score is made up of a total of 31 comorbidities; however, none of the comorbidities are ophthalmic conditions. A higher score indicates greater comorbidity burden and could be characteristic of an aging population. The only factor found to be associated with reducing the risk of progression to GA with SFI was residence in an urban location, which may be a proxy for health care access.
In this study, the prevalence of depression at baseline was observed in one-fifth or more of the study sample (ranged 19.9%-22.6%). Furthermore, the incidence of new onset depression during the follow-up period was greater in GA cohort than non-GA cohort (86.6 vs 77.3 per 1,000 person years, P = 0.004). These results align with the findings of Cimarolli et al,20 which concluded that depression was common among patients with advanced macular degeneration, with one-third reporting clinically significant depressive symptomology. Additionally, approximately 20% of patients with GA in the current study had evidence of an anxiety disorder during the follow-up period, which aligns with a systematic literature review based on prior research that found the prevalence estimate of anxiety disorders among individuals with AMD between 9.6% and 30.1%.21
In the current study, approximately 17% of patients with GA with wet AMD received one of the anti-VEGF agents during the follow-up period. As we observed co-occurring GA and wet AMD increased the risk for GA disease progression, treating these patients for both GA and wet AMD may be crucial. We also found that 30.9% of patients were diagnosed by optometrists, and 58.6% of the patients with GA were diagnosed by ophthalmologists. A higher proportion of patients in our study were diagnosed by optometrists compared with the IRIS registry, in which only 6.3% of patients with GA in both eyes were seen by an optometrist.10 In the IRIS registry, it was also observed that patients with fellow eye wet AMD were also more likely to be seen by a retina specialist, probably related to anti-VEGF treatment and associated monitoring, suggesting a gap for patients with GA without AMD who may benefit from the care of a retina specialist.10 Increasing the awareness of GA among optometrists, including the existence of GA co-occurring with wet AMD, may increase referrals for possible treatment by a retina specialist leading to more positive outcomes for patients.
LIMITATIONS
This study is limited by the fact the study subjects included individuals enrolled in the MAPD plan, aged at least 65 years, and as such, the results cannot be generalized to other populations. Individuals with claims for other ophthalmic comorbidities were excluded from this analysis in an effort to minimize confounding. Given that many patients with GA also have these conditions, this may limit generalizability of the results. For the assessment of progression to GA with SFI, we included only patients with GA without SFI at index and did not examine progression at the individual eye level because of the coding inconsistencies found within the claims.
Mainly 2 types of inconsistencies were observed in the claims data: (1) biologically implausible temporal sequences of codes were observed for multiple combinations of unilateral and bilateral diagnoses related to various stages of AMD, implying reversal of AMD whereas AMD is irreversible; and (2) multiple claims with the same date that contained inconsistent diagnoses (ie, for the same patient on the same date, different claims containing codes for the same eye or eyes that indicated multiple different disease severities, and/or different claims indicating the condition to be both unilateral and bilateral on the same date). As such, progression may be underestimated in our study.
We assessed health care utilization only during the first 12 months of follow-up. However, over a longer follow-up period, we may see differences in health care spending because of GA. In this analysis, we could not distinguish between ophthalmologists and retina specialists, as they all show as ophthalmologists in the claims data. Furthermore, limitations common to studies using administrative claims data apply. These include lack of certain information in the database (eg, changes in visual acuity over time) and error in claims coding. No causal inference can be ascertained from this study, as it is an observational study using retrospective claims data. Because this study used data from a single health plan and focused on a senior population enrolled in the MAPD plan, the results may not be generalizable to other settings. Any comparisons of GA with SFI to GA without SFI should be approached with caution because the study was designed to compare GA vs non-GA. GA with SFI and GA without SFI subgroups were not PS matched, so any comparisons would be subject to bias because of lack of control of confounders. GA with SFI and GA without SFI subgroups were not described separately in the manuscript so readers cannot assess potential differences on baseline measures.
Conclusions
In this analysis of patients newly diagnosed with GA, GA was associated with development of blindness, and the greatest rate of progression to blindness was observed for individuals having GA with SFI. Wet AMD was a commonly occurring comorbidity in patients with GA and was the strongest predictor of progression to GA with SFI among patients who had GA without SFI at baseline. With the recent approval of treatments for GA, it is critical that managed care plans are aware of the burden of this disease, rates of progression, and potential outcomes in the absence of treatment for their enrollees. Future real-world evidence assessing the impact of therapies on disease progression, HCRU, and spending are needed.
ACKNOWLEDGMENTS
The authors want to acknowledge contributions of Roger Luo and Darcie Sharp in the designing of this study and interpretation of the results. The authors also want to acknowledge Dr Mary Costantino, PhD, employee of Humana Healthcare Research, Inc., for her support in writing and reviewing this manuscript.
Funding Statement
This study was funded by Apellis Pharmaceuticals. Drs Borns and Broderick are employees at Apellis Pharmaceuticals and are shareholders of Apellis Pharmaceuticals.
REFERENCES
- 1.Thomas CJ, Mirza RG, Gill MK. Age-related macular degeneration. Med Clin North Am. 2021;105(3):473-91. doi:10.1016/j.mcna.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019. Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-60. doi:10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindblad AS, Lloyd PC, Clemons TE, et al. ; Age-Related Eye Disease Study Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168-74. doi:10.1001/archophthalmol.2009.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rein DB, Wittenborn JS, Burke-Conte Z, et al. Prevalence of age-related macular degeneration in the US in 2019. JAMA Ophthalmol. 2022;140(12):1202-8. doi:10.1001/jamaophthalmol.2022.4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BrightFocus Foundation. Geographic atrophy. Accessed October 10, 2022. https://www.brightfocus.org/macular/geographic-atrophy
- 6.U.S. Food & Drug Administration. NDA approval of Syfovre (pegcetacoplan injection). Accessed September 15, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/217171Orig1s000ltr.pdf
- 7.ClinicalTrials Arena. Syfovre (pegcetacoplan) for the treatment of geographic atrophy, USA. Accessed September 9, 2023. https://www.clinicaltrialsarena.com/projects/syfovre-pegcetacoplan-treatment-geographic-atrophy-usa/
- 8.U.S. Food & Drug Administration. Novel drug approvals for 2023. Accessed January 10, 2024. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023
- 9.ClinicalTrials Arena. Izervay for the treatment of GA, US. Accessed November 10, 2023. https://www.clinicaltrialsarena.com/projects/izervay-ga-treatment-us/
- 10.Rahimy E, Khan MA, Ho AC, et al. Progression of geographic atrophy: Retrospective analysis of patients from the IRIS® Registry (Intelligent Research in Sight). Ophthalmol Sci. 2023;3(4):100318. doi:10.1016/j.xops.2023.100318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim A, Devine B, Campbell J, Shirneshan E, Zhao C, Bansal A. Healthcare resource utilization and costs in patients with geographic atrophy secondary to age-related macular degeneration. Clin Ophthalmol. 2021;15:2643-51. doi:10.2147/OPTH.S307603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JG, Chen XD, Clontz M, Begaj T, Runner MM, Wolfe JD. Coding of geographic atrophy and exudative age-related macular degeneration. Ophthalmol Retina. 2023;7(7):644-5. doi:10.1016/j.oret.2023.03.011 [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-9. doi:10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-9. doi:10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 16.Greifer N, Stuart EA. Matching methods for confounder adjustment: An addition to the epidemiologist’s toolbox. Epidemiol Rev. 2022;43(1):118-29. doi:10.1093/epirev/mxab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Congdon N, O’Colmain B, Klaver CC, et al. ; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-85. doi:10.1001/archopht.122.4.477 [DOI] [PubMed] [Google Scholar]
- 18.Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-90. doi:10.1016/j.ophtha.2017.08.038 [DOI] [PubMed] [Google Scholar]
- 19.Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. ; FAM Study Group. Progression of age-related geographic atrophy: Role of the fellow eye. Invest Ophthalmol Vis Sci. 2011;52(9):6552-7. doi:10.1167/iovs.11-7298 [DOI] [PubMed] [Google Scholar]
- 20.Cimarolli VR, Casten RJ, Rovner BW, Heyl V, Sörensen S, Horowitz A. Anxiety and depression in patients with advanced macular degeneration: Current perspectives. Clin Ophthalmol. 2015;10:55-63. doi:10.2147/OPTH.S80489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson SR, Mallen CD, Gouldstone MB, Yarham R, Mansell G. The prevalence of anxiety and depression in people with age-related macular degeneration: A systematic review of observational study data. BMC Ophthalmol. 2014;14(1):78. doi:10.1186/1471-2415-14-78 [DOI] [PMC free article] [PubMed] [Google Scholar]


