Abstract
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a major cause of liver-related morbidity and mortality, contributing to both cardiovascular and non-cardiovascular deaths. The Body Roundness Index (BRI) and Systemic Immune-Inflammation Index (SII) have emerged as predictors of adverse outcomes in metabolic diseases. This study investigates the association between BRI, SII, and mortality risk in MAFLD patients. A nationwide retrospective cohort study was conducted using data from the NHANES database (January 1999–December 2018), including patients diagnosed with MAFLD. BRI and SII were calculated at baseline. Cox proportional hazards models assessed the association between these indices and all-cause, cardiovascular, and non-cardiovascular mortality, adjusting for confounders. Among 12,435 participants diagnosed with MAFLD, 3,381 (27.2%) were classified into the low BRI and low SII group, 2,889 (23.2%) into the low BRI and high SII group, 2,802 (22.5%) into the high BRI and low SII group, and 3,363 (27.1%) into the high BRI and high SII group. Compared to the low BRI and low SII group, the high BRI and high SII group demonstrated significantly higher all-cause mortality, with an adjusted hazard ratio (HR) of 1.89. For cardiovascular mortality, the HR was 2.31, while for non-cardiovascular mortality, the HR was 1.78. The high BRI and high SII cohort exhibited the highest risk of all-cause mortality, cardiovascular mortality, and non-cardiovascular mortality. BRI and SII are independent predictors of mortality in MAFLD patients, and their combined use enhances risk stratification. Integrating these indices into clinical practice could improve personalized management strategies and outcomes in this high-risk population.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83324-4.
Keywords: MAFLD, Body roundness index, Systemic immune-inflammation index, Survival outcomes
Subject terms: Infection, Inflammation, Immunology, Biomarkers, Gastroenterology, Health care
Introduction
In 2020, there was a proposal to rename and redefine metabolic-associated fatty liver disease (MAFLD) to better characterize its underlying pathophysiology and associated metabolic abnormalities1. The global prevalence of MAFLD is approximately 38.8%2, and MAFLD is associated with increased all-cause mortality3, imposing a significant socioeconomic burden4. As the global obesity epidemic continues to rise, the prevalence of MAFLD is expected to increase, with projections suggesting that by 2030, approximately 25% of the global population will be affected5. The condition is not only linked to liver fibrosis, cirrhosis, and liver cancer but also plays a central role in the development of metabolic comorbidities, including type 2 diabetes and cardiovascular diseases, further compounding its impact on public health6.
Previous studies have established a strong link between obesity and metabolic-associated fatty liver disease (MAFLD)7,8. However, most research has focused on general obesity measures like body mass index (BMI), which fail to comprehensively assess abdominal fat distribution9. While waist circumference (WC) is commonly used to measure abdominal obesity, the body roundness index (BRI), introduced by Thomas et al. in 2013, offers a more accurate metric10. By incorporating both WC and height, BRI provides a better assessment of visceral and total body fat, addressing the limitations of traditional obesity indicators like BMI and WC11.
Research has demonstrated a strong link between MAFLD and inflammation, which plays a crucial role in elevating the risk of all-cause mortality, including cardiovascular and non-cardiovascular diseases12. MAFLD drives systemic inflammation by releasing inflammatory proteins such as interleukin-6, C-reactive protein, and tumor necrosis factor-alpha from hepatic steatosis13. These proteins contribute to disease progression by promoting immune responses and lipid accumulation. The systemic immune-inflammation (SII) index, a marker integrating neutrophils, platelets, and lymphocytes, has emerged as a reliable tool for assessing inflammation associated with MAFLD14. SII’s ability to capture both local immune responses and systemic inflammation makes it a valuable indicator for understanding the inflammatory burden imposed by MAFLD, offering insights into its impact on overall mortality15. Prior research has highlighted the connection between such immune-inflammatory markers and the risk and progression of liver conditions16, including viral hepatitis, cirrhosis, and hepatocellular carcinoma17,18. These findings suggest that SII could serve as a valuable tool for evaluating inflammation-related liver disease severity.
Numerous studies have established that obesity is marked by a chronic, low-grade inflammatory state, and the evaluation of circulating pro-inflammatory markers is critical for diagnosing and predicting outcomes in various chronic diseases19,20. However, no studies to date have examined the combined impact of obesity and inflammation on survival in individuals with MAFLD. In light of this, the present study aimed to investigate the influence of obesity, measured by the Body Roundness Index (BRI), and inflammation, assessed by the Systemic Immune Inflammation Index (SII), both independently and synergistically, on survival outcomes in patients with MAFLD.
Materials and methods
Study design and population
The study utilized data from the NHANES (National Health and Nutrition Examination Survey) database, administered by the Centers for Disease Control and Prevention (CDC) in the United States. This comprehensive program gathers information on individuals’ health and nutritional status through interviews, physical assessments, and laboratory analyses. Participants were chosen using a two-stage stratified cluster sampling method, based on population and housing census information. Data collection involved household interviews and standardized physical assessments. Prior to the survey, written informed consent was obtained from each participant, and secondary anonymized data were used for analysis. The study focused on data from the survey conducted between 1999 and 2018, during which participants underwent testing for Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD), Body Roundness Index, and Systemic Immune-Inflammation Index (SII). The NHANES database is publicly available at https://www.cdc.gov/nchs/nhanes/index.htm. A nationwide retrospective cohort study was conducted using data from the NHANES database, spanning from January 1999 to December 2018, including patients diagnosed with Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD) and having data on Body Roundness Index (BRI), Systemic Immune-Inflammation Index (SII), and relevant covariates (such as age, sex, BMI, smoking status, alcohol consumption, hypertension, diabetes mellitus, and hyperlipidemia). After excluding participants with missing data on BRI and SII, survival status, or key covariates, 12,435 participants were deemed eligible for the final analysis.
Assessment of metabolic associated fatty liver disease (MAFLD) status
In this study, hepatic steatosis was identified using the Fatty Liver Index (FLI), calculated as follows: FLI = 100*(eL /1 + eL), where L = 0.953×log(triglycerides/1) + 0.139×(BMI) + 0.718×log(GGT/1) + 0.053×(waist circumference) − 15.745.A Subjects were diagnosed with hepatic steatosis if their FLI score was ≥ 6021. A diagnosis of MAFLD was established when hepatic steatosis was present along with any of the following: overweight or obesity, diabetes, or metabolic dysfunction. Metabolic dysfunction was defined by the presence of at least two of the following: (1) waist circumference ≥ 102 cm in males or ≥ 88 cm in females, (2) hypertension, (3) hyperlipidemia (TG ≥ 1.70 mmol/L or under lipid-lowering treatment), (4) HDL-C < 1.0 mmol/L in men or < 1.3 mmol/L in women, (5) prediabetes, and (6) hsCRP > 2 mg/L22.
Patient bifurcation based on body roundness index (BRI)
The formula for calculating the Body Roundness Index (BRI) is as follows: BRI = 364.2 − 365.5 × 1−√ ((WC/(2π))2(0.5height)2)10. In this study, all included patients were categorized into a low BRI group and a high BRI group based on their BRI values, with a cutoff value of 5.954. Patients with a BRI higher than 5.954 were classified into the high BRI group, while those with a BRI lower than 5.954 were classified into the low BRI group.
Patient bifurcation based on systemic immune-inflammation index (SII)
SII was calculated as platelet count × neutrophil/lymphocyte ratio (P × N/L)23. In this study, all included patients were categorized into low SII and high SII groups based on a cutoff value of 482.4. Patients with SII values greater than 482.4 were placed in the high SII group, while those with values lower than 482.4 were categorized into the low SII group.
Participant grouping
Based on the above definitions, participants were divided into the following four groups according to their BRI (Body Roundness Index) and SII (Systemic Inflammation Index) levels: Group 1 had low BRI and low SII, Group 2 had low BRI but high SII, Group 3 had high BRI but low SII, and Group 4 had high BRI and high SII. This classification aims to assess the impact of different BRI and SII levels on study outcomes, shedding light on their potential roles in patient health.
Covariates
The survey questionnaire collected a comprehensive range of participant data, including age, sex, ethnicity, marital status, family income, Body Mass Index (BMI), smoking status, alcohol consumption, hypertension status, diabetes mellitus (DM), and hyperlipidemia. Hypertension was defined as having a systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or being on antihypertensive medication. Smoking status was categorized into three groups: non-smokers (individuals who had never smoked or had smoked fewer than 100 cigarettes in their lifetime), former smokers (those who had smoked at least 100 cigarettes but had stopped), and current smokers (those who had smoked at least 100 cigarettes in their lifetime and reported smoking within the past 30 days). Alcohol consumption was divided into four categories: never drinkers (those who had consumed fewer than 12 alcoholic drinks in their lifetime), former drinkers (individuals who had consumed more than 12 drinks but had abstained for at least one year), and current drinkers (further classified as mild, moderate, or heavy drinkers based on daily consumption and binge drinking episodes). Educational level was classified into five categories: College Graduate or above, some college or Associate’s Degree, high school graduate/GED or equivalent, 9-11th grade, and less than 9th grade. Family income was categorized into three levels: high income, moderate income, and low income. For the purposes of this study, diabetes mellitus (DM) was defined by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L), a 2-hour plasma glucose level ≥ 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test, or an HbA1c level ≥ 6.5%, or a previous diagnosis of diabetes with ongoing treatment. Hyperlipidemia was defined by total cholesterol ≥ 240 mg/dL (6.2 mmol/L), low-density lipoprotein cholesterol (LDL-C) ≥ 160 mg/dL (4.1 mmol/L) or ≥ 130 mg/dL (3.4 mmol/L) in high-risk individuals, triglycerides (TG) ≥ 150 mg/dL (1.7 mmol/L), or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL (1.0 mmol/L) for men or < 50 mg/dL (1.3 mmol/L) for women.
Study endpoint and outcomes
The main outcomes investigated in this study comprised mortality rates associated with all causes, cardiovascular conditions, and non-cardiovascular conditions. Vital status data were sourced from the National Death Index, provided by the National Center for Health Statistics (NCHS), encompassing comprehensive records up to December 2018.
Statistical analysis
R version 3.4.0 was used to perform the data analysis (R Foundation for Statistical Computing, Vienna, Austria; available at http://www.R-project.org). Continuous variables are expressed as means ± standard deviations, while categorical variables are presented as frequencies with weighted percentages. The Cox proportional hazards model was employed to estimate the hazard ratio (HR) and 95% confidence interval (CI) for mortality based on the grouping of Body Roundness Index (BRI) and Systemic Immune-Inflammation Index (SII) data. The crude model included only the primary grouping, while Model 1 was adjusted for age and sex. Model 2 was further adjusted for age, sex, ethnicity, marital status, and family income. Model 3 additionally incorporated adjustments for BMI, smoking status, alcohol use, hypertension, diabetes mellitus (DM), and hyperlipidemia. To ensure the reliability of our results, sensitivity analyses were conducted by excluding participants with a follow-up time of less than 24 months or those younger than 45 years. To further explore the associations within specific populations, subgroup analyses were conducted based on sex and age groups (20–60 years and 60–85 years), allowing for a more detailed examination of the interactions between BRI and SII across different demographics.
Results
Baseline characteristics
Among the 12,435 participants, all were diagnosed with MAFLD (Metabolic Associated Fatty Liver Disease), 3,381 were classified into the low BRI and low SII group, 2,889 into the low BRI and high SII group, 2,802 into the high BRI but low SII group, and 3,363 into the high BRI and high SII group. Table 1 outlines the baseline characteristics of participants across the four groups. Participants in the high BRI and high SII group had significantly higher rates of hypertension (56.86% vs. 38.23% vs. 54.23% vs. 38.01%), diabetes mellitus (27.57% vs. 12.32% vs. 27.45% vs. 11.81%), and hyperlipidemia (84.28% vs. 84.08% vs. 83.62% vs. 81.11%) compared to the other groups. The baseline characteristics of the included patients are shown in Table 1.
Table 1.
Baseline characteristics of participants with metabolic associated fatty liver disease (MAFLD) stratified by body roundness index (BRI) and systemic immune-inflammation index (SII).
| Variables | Total | High SII and High BRI | High SII and Low BRI | Low SII and High BRI | Low SII and Low BRI | P value |
|---|---|---|---|---|---|---|
| Age | 49.24(0.23) | 50.56(0.39) | 47.64(0.39) | 52.21(0.43) | 47.28(0.34) | < 0.0001 |
| BRI | 6.29(0.03) | 8.10(0.05) | 4.88(0.02) | 7.73(0.05) | 4.79(0.02) | < 0.0001 |
| SII | 562.68(4.12) | 779.25(7.57) | 754.63(7.20) | 347.33(2.38) | 340.28(2.07) | < 0.0001 |
| Sex | < 0.0001 | |||||
| Female | 5850(44.69) | 2159(61.79) | 1169(39.29) | 1549(52.75) | 973(27.50) | |
| Male | 6585(55.31) | 1204(38.21) | 1720(60.71) | 1253(47.25) | 2408(72.50) | |
| eth1 | < 0.0001 | |||||
| Mexican American | 2556( 9.18) | 695( 8.77) | 588( 8.30) | 612(10.32) | 661( 9.55) | |
| Non-Hispanic Black | 2369(10.05) | 551( 9.19) | 366( 6.24) | 688(14.05) | 764(11.42) | |
| Non-Hispanic White | 5684(70.16) | 1687(71.78) | 1513(74.80) | 1126(66.64) | 1358(66.95) | |
| Other Hispanic | 1094( 5.68) | 300(6.11) | 233(5.48) | 256(4.95) | 305(6.01) | |
| Other Race - Including Multi-Racial | 732( 4.92) | 130(4.14) | 189(5.19) | 120(4.05) | 293(6.06) | |
| Edu | < 0.0001 | |||||
| Above high school | 5863(55.72) | 1523(53.46) | 1388(55.43) | 1240(53.89) | 1712(59.48) | |
| High school or equivalent | 2973(25.60) | 800(26.11) | 749(27.83) | 648(24.67) | 776(23.74) | |
| Under high school | 3599(18.68) | 1040(20.43) | 752(16.74) | 914(21.44) | 893(16.78) | |
| Family_income | < 0.0001 | |||||
| High | 3775(41.90) | 879(37.39) | 997(45.64) | 711(37.47) | 1188(46.00) | |
| Low | 3753(20.52) | 1151(24.14) | 784(18.15) | 970(23.90) | 848(16.79) | |
| Medium | 4907(37.58) | 1333(38.47) | 1108(36.21) | 1121(38.62) | 1345(37.21) | |
| Marital | < 0.0001 | |||||
| Married | 8045(68.33) | 2053(64.83) | 1923(69.08) | 1718(65.95) | 2351(72.72) | |
| Other | 4390(31.67) | 1310(35.17) | 966(30.92) | 1084(34.05) | 1030(27.28) | |
| BMI | < 0.0001 | |||||
| <23.9 | 385( 2.78) | 4(0.06) | 183(5.20) | 0(0.00) | 198(5.19) | |
| >=28 | 8994(73.20) | 3190(96.72) | 1389(51.73) | 2689(96.74) | 1726(53.29) | |
| 24 ~ 27.9 | 3056(24.02) | 169( 3.22) | 1317(43.07) | 113( 3.26) | 1457(41.51) | |
| Alcohol.user | < 0.0001 | |||||
| Former | 2544(17.59) | 799(21.56) | 509(15.26) | 673(20.97) | 563(13.47) | |
| Heavy | 2528(20.84) | 590(17.89) | 681(23.97) | 485(17.02) | 772(23.59) | |
| Mild | 4000(35.45) | 984(32.34) | 976(36.65) | 824(33.11) | 1216(39.00) | |
| Moderate | 1648(15.10) | 427(14.58) | 406(15.58) | 363(15.62) | 452(14.77) | |
| Never | 1715(11.03) | 563(13.64) | 317( 8.54) | 457(13.27) | 378( 9.17) | |
| Smoke | < 0.0001 | |||||
| Former | 3569(29.00) | 994(30.76) | 805(27.73) | 810(29.70) | 960(27.99) | |
| Never | 6392(50.64) | 1746(49.93) | 1370(47.29) | 1536(53.89) | 1740(51.99) | |
| Now | 2474(20.36) | 623(19.30) | 714(24.98) | 456(16.41) | 681(20.02) | |
| Hypertension | < 0.0001 | |||||
| No | 6246(53.66) | 1426(43.14) | 1672(61.77) | 1162(45.77) | 1986(61.99) | |
| Yes | 6189(46.34) | 1937(56.86) | 1217(38.23) | 1640(54.23) | 1395(38.01) | |
| DM | < 0.0001 | |||||
| No | 9437(80.72) | 2325(72.43) | 2418(87.68) | 1874(72.55) | 2820(88.19) | |
| Yes | 2998(19.28) | 1038(27.57) | 471(12.32) | 928(27.45) | 561(11.81) | |
| Hyperlipidemia | 0.04 | |||||
| No | 2033(16.79) | 507(15.72) | 450(15.92) | 450(16.38) | 626(18.89) | |
| Yes | 10,402(83.21) | 2856(84.28) | 2439(84.08) | 2352(83.62) | 2755(81.11) | |
| CVD | < 0.0001 | |||||
| No | 10,867(89.49) | 2852(86.77) | 2580(91.56) | 2363(86.28) | 3072(92.53) | |
| Yes | 1567(10.51) | 511(13.23) | 309( 8.44) | 438(13.72) | 309( 7.47) | |
| Mortstat | < 0.0001 | |||||
| 0 | 10,452(88.01) | 2724(85.21) | 2397(87.53) | 2340(87.46) | 2991(91.49) | |
| 1 | 1983(11.99) | 639(14.79) | 492(12.47) | 462(12.54) | 390( 8.51) | |
Impact of BRI levels on survival in MAFLD patients
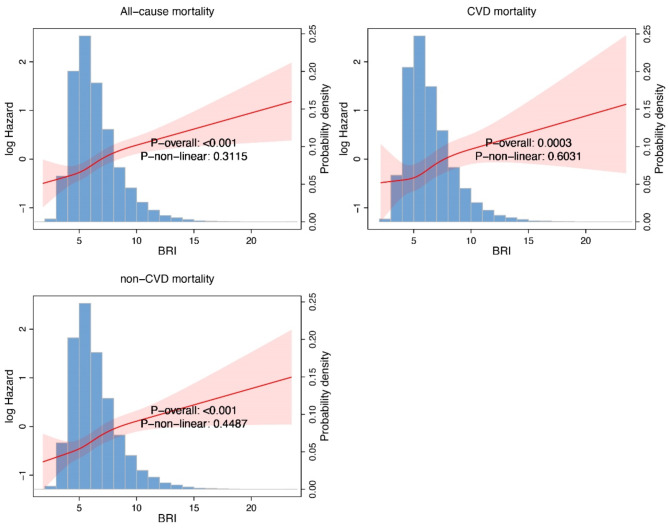
Compared to the low BRI group, participants with high BRI consistently demonstrated worse outcomes across all mortality categories. Overall, a higher BRI is associated with worse survival outcomes, as reflected in all-cause, cardiovascular, and non-cardiovascular mortality (Fig. 1).
Fig. 1.
The relationship between high and low body roundness index (BRI) and all-cause mortality, cardiovascular mortality (CVD), and non-cardiovascular mortality (non-CVD).
For all-cause mortality, the high BRI group had significantly higher risks in all models: crude model (HR: 1.63, 95% CI: 1.46–1.82, P < 0.0001), model 1 (HR: 1.28, 95% CI: 1.14–1.44, P < 0.0001), model 2 (HR: 1.22, 95% CI: 1.09–1.37, P < 0.001), and model 3 (HR: 1.29, 95% CI: 1.11–1.51, P = 0.001). Similarly, the high BRI group exhibited significantly worse cardiovascular mortality, with consistent findings across models: crude model (HR: 1.88, 95% CI: 1.56–2.27, P < 0.0001), model 1 (HR: 1.44, 95% CI: 1.17–1.76, P < 0.001), model 2 (HR: 1.37, 95% CI: 1.12–1.67, P = 0.002), and model 3 (HR: 1.33, 95% CI: 1.04–1.70, P = 0.02). For non-cardiovascular mortality, the trend was equally pronounced, with the high BRI group showing elevated risks across all models: crude model (HR: 1.56, 95% CI: 1.37–1.79, P < 0.0001), model 1 (HR: 1.23, 95% CI: 1.07–1.41, P = 0.003), model 2 (HR: 1.17, 95% CI: 1.02–1.34, P = 0.03), and model 3 (HR: 1.29, 95% CI: 1.06–1.56, P = 0.01). These findings consistently highlight the association between higher BRI and increased mortality risk across all categories (Tables 2, 3 and 4).
Table 2.
Impact of high and low BRI on all-cause mortality.
| BRIQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| nhs ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Q1 | Ref | Ref | Ref | Ref | ||||
| Q2 | 1.63(1.46,1.82) | < 0.0001 | 1.28(1.14,1.44) | < 0.0001 | 1.22(1.09,1.37) | < 0.001 | 1.29(1.11,1.51) | 0.001 |
BRIQ.
Crudel model: BRIQ.
Model 1: BRIQ, age, sex.
Model 2: BRIQ, age, sex, eth1, Family_income, edu, marital.
Model 3: BRIQ, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia.
Table 3.
Impact of high and low BRI on cardiovascular mortality.
| BRIQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| subset(nhs, ucod_leading %in% c(heart, Cerebrovascular, no)) ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Q1 | Ref | Ref | Ref | Ref | ||||
| Q2 | 1.88(1.56,2.27) | < 0.0001 | 1.44(1.17,1.76) | < 0.001 | 1.37(1.12,1.67) | 0.002 | 1.33(1.04,1.70) | 0.02 |
BRIQ.
Crudel model: BRIQ.
Model 1: BRIQ, age, sex.
Model 2: BRIQ, age, sex, eth1, Family_income, edu, marital.
Model 3: BRIQ, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia.
Table 4.
Impact of high and low BRI on non-cardiovascular mortality.
| BRIQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| subset(nhs, !ucod_leading %in% c(heart, Cerebrovascular)) ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Q1 | Ref | Ref | Ref | Ref | ||||
| Q2 | 1.56(1.37,1.79) | < 0.0001 | 1.23(1.07,1.41) | 0.003 | 1.17(1.02,1.34) | 0.03 | 1.29(1.06,1.56) | 0.01 |
BRIQ.
Crudel model: BRIQ.
Model 1: BRIQ, age, sex.
Model 2: BRIQ, age, sex, eth1, Family_income, edu, marital.
Model 3: BRIQ, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia.
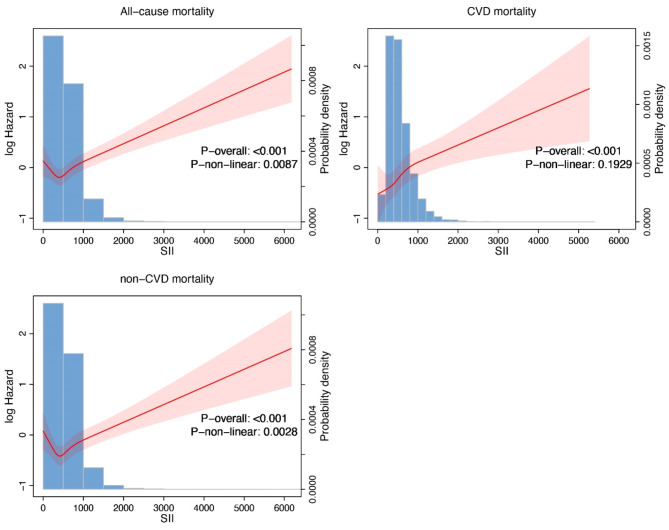
Impact of SII levels on survival in MAFLD patients
Compared to the low SII group, individuals with high SII demonstrated worse outcomes across all mortality categories. Overall, a higher SII is associated with worse survival outcomes, as reflected in all-cause, cardiovascular, and non-cardiovascular mortality (Fig. 2). For all-cause mortality, the high SII group consistently showed higher risks in all models: crude model (HR: 1.21, 95% CI: 1.07–1.36, P = 0.002), model 1 (HR: 1.22, 95% CI: 1.10–1.36, P < 0.001), model 2 (HR: 1.21, 95% CI: 1.09–1.35, P < 0.001), and model 3 (HR: 1.19, 95% CI: 1.07–1.32, P = 0.002). For cardiovascular mortality, the high SII group also exhibited significantly higher risks across all models: crude model (HR: 1.35, 95% CI: 1.09–1.67, P = 0.01), model 1 (HR: 1.40, 95% CI: 1.13–1.74, P = 0.002), model 2 (HR: 1.43, 95% CI: 1.15–1.77, P = 0.001), and model 3 (HR: 1.44, 95% CI: 1.16–1.79, P < 0.001). Similarly, for non-cardiovascular mortality, high SII was associated with elevated risks: crude model (HR: 1.16, 95% CI: 1.01–1.33, P = 0.04), model 1 (HR: 1.19, 95% CI: 1.05–1.35, P = 0.01), model 2 (HR: 1.18, 95% CI: 1.05–1.34, P = 0.01), and model 3 (HR: 1.15, 95% CI: 1.01–1.30, P = 0.03). These results indicate that higher SII is consistently linked to worse mortality outcomes across all categories (Tables 5, 6 and 7).
Fig. 2.
The relationship between high and low systemic immune-inflammation index (SII) and all-cause mortality, cardiovascular mortality (CVD), and non-cardiovascular mortality (non-CVD).
Table 5.
Impact of high and low SII on all-cause mortality.
| nhs ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Q1 | Ref | Ref | Ref | Ref | ||||
| Q2 | 1.21(1.07,1.36) | 0.002 | 1.22(1.10,1.36) | < 0.001 | 1.21(1.09,1.35) | < 0.001 | 1.19(1.07,1.32) | 0.002 |
SIIQ.
Crudel model: SIIQ.
Model 1: SIIQ, age, sex.
Model 2: SIIQ, age, sex, eth1, Family_income, edu, marital.
Model 3: SIIQ, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia.
Table 6.
Impact of high and low SII on cardiovascular mortality.
| SIIQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| subset(nhs, ucod_leading %in% c(heart, Cerebrovascular, no)) ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Q1 | Ref | Ref | Ref | Ref | ||||
| Q2 | 1.35(1.09,1.67) | 0.01 | 1.40(1.13,1.74) | 0.002 | 1.43(1.15,1.77) | 0.001 | 1.44(1.16,1.79) | < 0.001 |
SIIQ.
Crudel model: SIIQ.
Model 1: SIIQ, age, sex.
Model 2: SIIQ, age, sex, eth1, Family_income, edu, marital.
Model 3: SIIQ, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia.
Table 7.
Impact of high and low SIII on non-cardiovascular mortality.
| SIIQ | ||||||||
|---|---|---|---|---|---|---|---|---|
| subset(nhs, !ucod_leading %in% c(heart, Cerebrovascular)) ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Q1 | Ref | ref | Ref | Ref | ||||
| Q2 | 1.16(1.01,1.33) | 0.04 | 1.19(1.05,1.35) | 0.01 | 1.18(1.05,1.34) | 0.01 | 1.15(1.01,1.30) | 0.03 |
SIIQ.
Crudel model: SIIQ.
Model 1: SIIQ, age, sex.
Model 2: SIIQ, age, sex, eth1, Family_income, edu, marital.
Model 3: SIIQ, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia.
All-cause, cardiovascular, and non-cardiovascular mortality according to BRI and SII grouping
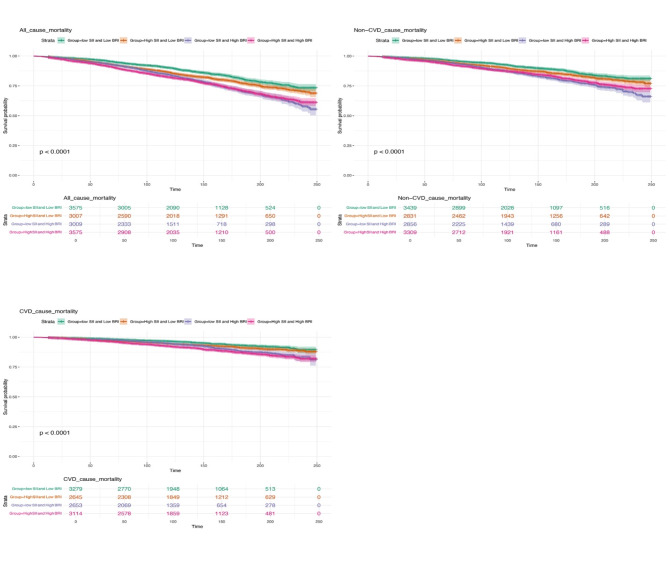
We found that participants with both high BRI and high SII had the poorest survival rates. Kaplan-Meier survival analysis revealed a clear descending pattern: the group with low BRI and low SII had the highest survival rates, followed by those with high SII and low BRI, then those with low SII and high BRI (These results are presented in Fig. 1). The group with both high BRI and high SII consistently demonstrated the lowest survival rates across all categories. In all models, patients with high BRI alone, high SII alone, or both high BRI and high SII were associated with a higher risk of all-cause mortality compared to those with low BRI and low SII. In the fully adjusted model, compared to the low BRI and low SII group, individuals with high BRI and low SII had a 24% higher risk of all-cause mortality (HR: 1.24; 95% CI: 1.06–1.46, P = 0.01), those with high SII and low BRI had a 73% higher risk (HR: 1.73; 95% CI: 1.46–2.05, P < 0.0001), and those with both high BRI and high SII had an 89% higher risk (HR: 1.89; 95% CI: 1.62–2.21, P < 0.0001). The observed trend in survival rates across these categories was statistically significant, with a p-value of < 0.0001, indicating a robust correlation between higher BRI and SII levels and increased mortality risk (The results mentioned above are presented in Table 8).
Table 8.
Impact of BRI and SII combinations on all-cause mortality.
| nhs ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Low SII and Low BRI | Ref | Ref | Ref | Ref | ||||
| High SII and Low BRI | 1.24(1.06,1.46) | 0.01 | 1.25(1.08,1.46) | 0.003 | 1.24(1.05,1.45) | 0.01 | 1.19(1.01,1.41) | 0.03 |
| Low SII and High BRI | 1.73(1.46,2.05) | < 0.0001 | 1.32(1.12,1.57) | 0.001 | 1.25(1.04,1.48) | 0.01 | 1.30(1.07,1.58) | 0.01 |
| High SII and High BRI | 1.89(1.62,2.21) | < 0.0001 | 1.54(1.32,1.79) | < 0.0001 | 1.47(1.26,1.70) | < 0.0001 | 1.52(1.28,1.81) | < 0.0001 |
| p for trend(character2integer) | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
Group.
Crudel model: Group.
Model 1: Group, age, sex.
Model 2: Group, age, sex, eth1, Family_income, edu, marital.
Model 3: Group, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia, CVD.
In the fully adjusted model, compared to the low BRI and low SII group, individuals with high BRI and low SII had a 17% higher risk of cardiovascular death (HR: 1.17; 95% CI: 0.86–1.59, P = 0.31), those with high SII and low BRI had a 67% higher risk (HR: 1.67; 95% CI: 1.23–2.27, P < 0.001), and those with both high BRI and high SII had a 131% higher risk (HR: 2.31; 95% CI: 1.79–2.96, P < 0.0001). The trend in cardiovascular mortality risk was significant (p < 0.0001), highlighting a strong association between higher BRI and SII levels and increased risk of cardiovascular death (The results mentioned above are presented in Table 9).
Table 9.
Impact of BRI and SII combinations on cardiovascular mortality.
| Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| subset(nhs, ucod_leading %in% c(heart, Cerebrovascular, no)) ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Low SII and low BRI | Ref | Ref | Ref | Ref | ||||
| High SII and low BRI | 1.17(0.86,1.59) | 0.31 | 1.23(0.91,1.68) | 0.18 | 1.23(0.90,1.68) | 0.18 | 1.26(0.93,1.70) | 0.14 |
| Low SII and high BRI | 1.67(1.23,2.27) | < 0.001 | 1.26(0.92,1.72) | 0.15 | 1.17(0.85,1.60) | 0.34 | 1.14(0.79,1.64) | 0.48 |
| High SII and high BRI | 2.31(1.79,2.96) | < 0.0001 | 1.86(1.43,2.43) | < 0.0001 | 1.81(1.39,2.36) | < 0.0001 | 1.81(1.34,2.44) | < 0.001 |
| p for trend(character2integer) | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
Group.
Crudel model: Group.
Model 1: Group, age, sex.
Model 2: Group, age, sex, eth1, Family_income, edu, marital.
Model 3: Group, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia, CVD.
In the fully adjusted model, compared to the low BRI and low SII group, individuals with high BRI and low SII had a 28% higher risk of non-cardiovascular death (HR: 1.28; 95% CI: 1.08–1.53, P = 0.01), those with high SII and low BRI had an 80% higher risk (HR: 1.80; 95% CI: 1.48–2.19, P < 0.0001), and those with both high BRI and high SII had a 78% higher risk (HR: 1.78; 95% CI: 1.48–2.15, P < 0.0001). The trend in non-cardiovascular mortality remained statistically significant with p < 0.001. (The results mentioned above are presented in Table 10).
Table 10.
Impact of BRI and SII combinations on non-cardiovascular mortality.
| Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| subset(nhs, ucod_leading %in% c(heart, Cerebrovascular, no)) ~ ~ permth_int, mortstat | Crude model | Model 1 | Model 2 | Model 3 | ||||
| Character | 95%CI | P | 95%CI | P | 95%CI | P | 95%CI | P |
| Low SII and low BRI | Ref | Ref | Ref | Ref | ||||
| High SII and low BRI | 1.17(0.86,1.59) | 0.31 | 1.23(0.91,1.68) | 0.18 | 1.23(0.90,1.68) | 0.18 | 1.26(0.93,1.70) | 0.14 |
| Low SII and high BRI | 1.67(1.23,2.27) | < 0.001 | 1.26(0.92,1.72) | 0.15 | 1.17(0.85,1.60) | 0.34 | 1.14(0.79,1.64) | 0.48 |
| High SII and high BRI | 2.31(1.79,2.96) | < 0.0001 | 1.86(1.43,2.43) | < 0.0001 | 1.81(1.39,2.36) | < 0.0001 | 1.81(1.34,2.44) | < 0.001 |
| p for trend(character2integer) | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
Group.
Crudel model: Group.
Model 1: Group, age, sex.
Model 2: Group, age, sex, eth1, Family_income, edu, marital.
Model 3: Group, age, sex, eth1, marital, Family_income, edu, BMI, smoke, alcohol.user, Hypertension, DM, Hyperlipidemia, CVD.
These findings underscore the critical impact of both BRI and SII on mortality risk in MAFLD patients. Specifically, higher BRI, SII, or both are associated with significantly increased risks of all-cause, cardiovascular, and non-cardiovascular mortality. Notably, individuals with both high BRI and high SII face the highest risks across all mortality categories. Furthermore, the poorest outcomes associated with both high BRI and high SII suggest that there may be an underlying relationship between these two metrics and the physiological state of the body. A Kaplan-Meier survival curve stratified by Body Roundness Index (BRI) and Systemic Immune-Inflammation Index (SII) has been plotted to visually demonstrate our research findings regarding the relationship between BRI, SII, and survival (Fig. 3).
Fig. 3.
Kaplan-Meier survival curves stratified by body roundness index (BRI) and systemic immune-inflammation index (SII). Participants were divided into four groups: low BRI/low SII, high BRI/low SII, low BRI/high SII, and high BRI/high SII.
Subgroup analyses
The results show that both high SII and high BRI are associated with significantly increased risks across sexes and age groups. In males, those with high SII and high BRI had a hazard ratio (HR) of 2.262 (95% CI: 1.771, 2.888, p < 0.0001), while low SII and high BRI were also linked to an increased risk (HR: 1.947, 95% CI: 1.538, 2.466, p < 0.0001). Similarly, in females, high SII and high BRI had an HR of 1.579 (95% CI: 1.188, 2.099, p = 0.002), and low SII and high BRI had an HR of 1.472 (95% CI: 1.067, 2.031, p = 0.019).
For individuals aged 20–60, high SII and high BRI were linked to a higher risk (HR: 1.446, 95% CI: 1.041, 2.008, p = 0.028), while low SII and high BRI also showed an increased risk (HR: 1.527, 95% CI: 1.026, 2.271, p = 0.037). In the 60–85 age group, high SII and high BRI were associated with a significantly increased risk (HR: 1.600, 95% CI: 1.254, 2.041, p < 0.001), and low SII and high BRI similarly showed a higher risk (HR: 1.345, 95% CI: 1.028, 1.760, p = 0.031) (The results of these subgroup analyses are presented in Supplementary Table 1).
Sensitivity analyses
Sensitivity analysis was performed by excluding participants with follow-up times of less than 24 months to mitigate potential biases or data inaccuracies associated with shorter follow-up periods. In the fully adjusted model, individuals with high BRI and low SII had a 19% higher risk of all-cause mortality (HR: 1.19; 95% CI: 1.01–1.41, P = 0.03), those with high SII and low BRI had a 30% higher risk (HR: 1.30; 95% CI: 1.07–1.58, P = 0.01), and those with both high BRI and high SII exhibited a 52% higher risk (HR: 1.52; 95% CI: 1.28–1.81, P < 0.0001). For cardiovascular death, the risk was 26% higher for individuals with high BRI and low SII (HR: 1.26; 95% CI: 0.93–1.70, P = 0.14), 14% higher for those with high SII and low BRI (HR: 1.14; 95% CI: 0.79–1.64, P = 0.48), and 81% higher for those with both high BRI and high SII (HR: 1.81; 95% CI: 1.34–2.44, P < 0.001). Regarding non-cardiovascular death, individuals with high BRI and low SII had a 22% higher risk (HR: 1.22; 95% CI: 1.02–1.47, P = 0.03), those with high SII and low BRI had a 38% higher risk (HR: 1.38; 95% CI: 1.10–1.73, P = 0.01), and those with both high BRI and high SII faced a 48% higher risk (HR: 1.48; 95% CI: 1.19–1.84, P < 0.001). These results highlight that while both high BRI and high SII are associated with increased mortality risks, their combined presence leads to the highest risk, suggesting an interaction between these two indicators in predicting mortality outcomes (The results are presented in Supplementary Table 2).
Sensitivity analysis was conducted by excluding patients younger than 45 years to minimize potential bias from younger age groups. In the fully adjusted model, compared to the low BRI and low SII group, individuals with high BRI and low SII had a 23% higher risk of all-cause mortality (HR: 1.23; 95% CI: 1.04–1.45, P = 0.02), those with high SII and low BRI had a 33% higher risk (HR: 1.33; 95% CI: 1.07–1.64, P = 0.01), and those with both high BRI and high SII exhibited a 48% higher risk (HR: 1.48; 95% CI: 1.23–1.76, P < 0.0001). For cardiovascular death, the risk was 35% higher for individuals with high BRI and low SII (HR: 1.35; 95% CI: 0.98–1.85, P = 0.07), 18% higher for those with high SII and low BRI (HR: 1.18; 95% CI: 0.82–1.70, P = 0.38), and 79% higher for those with both high BRI and high SII (HR: 1.79; 95% CI: 1.34–2.40, P < 0.0001). For non-cardiovascular death, individuals with high BRI and low SII had a 23% higher risk (HR: 1.23; 95% CI: 1.02–1.48, P = 0.03), those with high SII and low BRI had a 40% higher risk (HR: 1.40; 95% CI: 1.09–1.78, P = 0.01), and those with both high BRI and high SII had a 41% higher risk (HR: 1.41; 95% CI: 1.11–1.79, P = 0.004). These results highlight that while both high BRI and high SII are associated with increased mortality risks, their combined presence leads to the highest risk, suggesting an interaction between these two indicators in predicting mortality outcomes. (The results are presented in Supplementary Table 3).
These findings reinforce that the combination of high BRI and SII poses the greatest mortality risk, underscoring the potential interaction between these two indicators. Furthermore, the consistency of these sensitivity analysis results with prior findings supports the robustness of the overall conclusions.
Discussion
Our study explored the relationship between obesity, as measured by the Body Roundness Index (BRI), systemic inflammation assessed by the Systemic Immune-Inflammation Index (SII), and survival outcomes in patients with metabolic-associated fatty liver disease (MAFLD). Our findings indicate that both elevated BRI and SII are independently associated with worse survival outcomes, including all-cause, cardiovascular, and non-cardiovascular mortality. Specifically, participants with high BRI exhibited a significantly increased risk of all-cause mortality (HR: 1.63, 95% CI: 1.46–1.82), cardiovascular mortality (HR: 1.88, 95% CI: 1.56–2.27), and non-cardiovascular mortality (HR: 1.56, 95% CI: 1.37–1.79). Similarly, high SII levels were associated with elevated risks of all-cause mortality (HR: 1.21, 95% CI: 1.07–1.36), cardiovascular mortality (HR: 1.35, 95% CI: 1.09–1.67), and non-cardiovascular mortality (HR: 1.16, 95% CI: 1.01–1.33). Notably, patients with both high BRI and high SII had the highest mortality risks, with HRs of 1.89 (95% CI: 1.62–2.21) for all-cause mortality, 2.31 (95% CI: 1.79–2.96) for cardiovascular mortality, and 1.78 (95% CI: 1.48–2.15) for non-cardiovascular mortality.
The role of obesity in MAFLD has been well-established, with excess visceral fat contributing to the disease through mechanisms such as insulin resistance and dyslipidemia. Traditional metrics like BMI may not fully capture visceral fat distribution, whereas BRI, which integrates waist circumference and height, provides a more accurate assessment24. Our study demonstrates that high BRI is a strong predictor of increased mortality in MAFLD patients, emphasizing the importance of precise obesity indices in evaluating health risks. Studies have shown that WC often provides better risk prediction for obesity-associated diseases compared to BMI. For instance, WC was found to be more effective than BMI in predicting metabolic syndrome in patients with T2DM25. Additionally, a prospective cohort study indicated that increased WC could lead to elevated blood pressure even without an increase in BMI26. Although WC has demonstrated strong performance in diagnosing MAFLD27, it still falls short of capturing the full scope of visceral fat distribution. The BRI’s calculation of body roundness, which incorporates both waist circumference and height, provides a superior index for capturing visceral fat and its associated risks, offering a more nuanced understanding of obesity’s impact on health.
Several mechanisms may explain the relationship between BRI and MAFLD11. Abnormal lipid accumulation induces stress in the endoplasmic reticulum and oxidative stress, which in turn impairs pancreatic β-cell function. This impairment decreases both insulin sensitivity and secretion, leading to chronic hyperglycemia28. Moreover, increased visceral fat reduces the production of protective adipokine adiponectin and elevates pro-inflammatory cytokines like tumor necrosis factor and interleukin 6, worsening insulin resistance29. In obesity, insufficient suppression of lipolysis raises liver fatty acid levels, leading to lipid accumulation, reduced liver insulin sensitivity, increased hepatic gluconeogenesis, and impaired glucose regulation30.
Systemic inflammation is a pivotal contributor to the pathogenesis and progression of MAFLD. In this context, the systemic immune-inflammation index (SII) has emerged as a valuable tool, integrating neutrophil, platelet, and lymphocyte counts to provide a comprehensive measure of both inflammation and immune response. Our findings demonstrate that elevated SII is significantly correlated with increased mortality in MAFLD patients, and this correlation persists independently of obesity, underscoring the critical role of inflammation in exacerbating disease severity. Previous research has substantiated the relationship between SII and both NAFLD and cardiovascular disease (CVD) mortality. For instance, Liu et al. identified a strong positive association between the natural logarithm of SII (ln(SII)) and NAFLD risk, confirming a linear relationship between ln(SII) and NAFLD in a cohort of 10,821 adults from NHANES data16. Moreover, several large-scale cohort studies have demonstrated that elevated SII independently increases the risk of CVD mortality31,32. Wang et al.’s study corroborates these findings, showing similar associations between elevated SII and poor MAFLD outcomes. Furthermore, the study’s sensitivity analysis in male subgroups affirmed the robustness of the positive correlation between SII and MAFLD, even when accounting for potential variations in SII categorizations15. Additonally, SII has also been validated as a diagnostic marker in various inflammatory digestive diseases33.
SII reflects inflammatory responses driven by lymphocytes and granulocytes, which release pro-inflammatory factors such as TNF-α, IFN-γ, IL-2, IL-6, and IL-134. These mediators trigger oxidative stress, leading to cellular damage through excessive ROS production, which further exacerbates inflammation and impairs liver function35. In particular, hepatocytes in MAFLD release pro-inflammatory mediators such as reactive oxygen species (ROS), C-reactive protein (CRP), and interleukin-6 (IL-6), which exacerbate liver inflammation and contribute to systemic inflammatory responses36. This inflammation not only drives liver damage but also perpetuates the cycle of injury, further mobilizing inflammatory cells to the liver37.Elevated SII causes oxidative stress that damages hepatocytes, increasing ALT levels, a key marker of liver inflammation. Inflammatory factors like TNF-α and IL-6 also promote insulin resistance (IR), reducing insulin’s ability to inhibit ALT. Additionally, inflammation promotes lipid accumulation in liver cells and disrupts insulin signaling, further driving MAFLD progression through increased lipid retention38. Recent studies emphasize the significant impact of systemic inflammation instigated by MAFLD on endothelial damage, inflammatory cell activation, and smooth muscle cell proliferation, which can directly intertwine with atherosclerosis. Advanced methodologies such as single-cell RNA sequencing and high-dimensional multi-omics have substantially enriched our understanding of immune cell subpopulation heterogeneity within the liver. These innovative approaches have shed light on emerging inflammatory mechanisms, including notable macrophage heterogeneity, involvement of auto-aggressive T cells, the role of unconventional T cells, and interactions between platelets and immune cells39. This growing body of evidence highlights the intricate relationship between systemic inflammation, MAFLD, and cardiovascular complications, which further emphasizes the critical role of adipose tissue in mediating these processes.”
Moreover, the synergistic impact of high BRI and high SII on survival outcomes was particularly noteworthy. Participants with both high BRI and high SII had the worst survival outcomes, reinforcing the combined role of obesity and systemic inflammation in MAFLD prognosis. This finding aligns with the growing understanding that metabolic dysfunction and inflammation are closely linked in the pathophysiology of MAFLD. Obesity promotes a chronic low-grade inflammatory state, which exacerbates liver damage and contributes to the progression of MAFLD to more severe conditions such as cirrhosis and hepatocellular carcinoma. Studies have demonstrated that with weight gain, the number of adipocytes increases, and enlarged adipocytes or adipose tissue release pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and MCP-121. Additionally, obesity alters the homeostatic functions of immune cells within adipose tissue, contributing to complications like diabetes and dyslipidemia40. Consequently, it is essential to consider varying ranges of BRI and inflammation levels when evaluating the relationship between obesity and inflammatory biomarkers. Zhou et al.‘s study also demonstrated that levels of platelets, neutrophils, monocytes, SII, and SIRI were significantly higher in the obesity group compared to the non-obesity group20. As weight increases, the proliferation and hypertrophy of adipocytes trigger the activation and recruitment of innate immune cells, fostering a proinflammatory adipose tissue microenvironment41. White adipose tissue (WAT) serves as both a fat storage depot and an endocrine organ that secretes adipokines and cytokines involved in metabolic and inflammatory processes42. In obesity, WAT undergoes a phenotypic switch, with inflamed adipocytes and immune cell infiltration, leading to the release of proinflammatory cytokines35. This chronic low-grade inflammation, or “metaflammation,” contributes to insulin resistance and metabolic dysfunction. Visceral adipose tissue (VAT) in particular shows more intense inflammation than subcutaneous adipose tissue (SAT), with higher macrophage infiltration and greater hypertrophy43. Within this environment, elevated inflammatory and metabolic demands lead to changes in proinflammatory cytokines, adipokines, free fatty acids, and other markers, ultimately disrupting organ and tissue homeostasis44. These results underscore the potential value of incorporating both BRI and SII as complementary tools in risk stratification for MAFLD patients, enabling better prediction of clinical outcomes and informing more targeted therapeutic strategies. Future research should explore the underlying mechanisms connecting BRI, SII, and mortality, as well as assess the potential benefits of interventions aimed at reducing BRI and SII levels in this patient population.
To enhance the clinical relevance of our findings, it is important to consider interventions that could modify BRI and SII in MAFLD patients. Lifestyle changes, such as weight loss and regular exercise, are key strategies for reducing both adiposity and systemic inflammation. Weight loss has been shown to decrease visceral fat and inflammatory markers, improving both BRI and SII. Exercise also reduces pro-inflammatory cytokines and enhances immune function. Additionally, pharmacological treatments like metformin and anti-inflammatory agents such as TNF-α inhibitors may help target the metabolic and inflammatory pathways contributing to elevated BRI and SII. These interventions, if integrated into clinical practice, could improve patient outcomes by lowering the mortality risks associated with these indices. Further research is needed to assess the combined impact of these treatments on long-term outcomes in MAFLD patients. In clinical practice, the prognostic value of BRI and SII may be influenced by comorbidities such as diabetes, hypertension, and dyslipidemia, which are common in MAFLD patients. These conditions can modulate the relationship between adiposity, inflammation, and patient outcomes. Diabetes, for example, exacerbates systemic inflammation and endothelial dysfunction, potentially enhancing the adverse effects of elevated BRI and SII. Hypertension, by contributing to vascular damage, may further amplify their impact on cardiovascular outcomes. Dyslipidemia, linked to both increased adiposity and inflammation, complicates the pathophysiology of MAFLD. Systemic inflammation has also been shown to promote atherosclerosis, increasing CHD risk in these patients. Elevated SII and SIRI indexes are associated with poor cardiovascular outcomes and may serve as biomarkers for predicting CHD development. Therefore, while BRI and SII are valuable prognostic markers, the presence of comorbidities should be considered for a more nuanced interpretation. Future studies exploring the interaction between these factors could provide insights for tailored management in MAFLD patients45.
Our study holds several unique advantages. First, it is one of the few cohort investigations to assess the long-term impact of both BRI and SII on mortality outcomes in patients with MAFLD. This large-scale cohort study utilized a substantial and diverse population sample, enhancing the generalizability and statistical power of the findings. By examining all-cause, cardiovascular, and non-cardiovascular mortality, our study offers a comprehensive evaluation of the prognostic value of BRI and SII in this population. Additionally, the study adjusted for multiple potential confounding factors through robust multivariate models, further strengthening the validity and reliability of the results.
Conclusion
In conclusion, elevated Body Roundness Index (BRI) and Systemic Immune-Inflammation Index (SII) significantly increase the risk of all-cause, cardiovascular, and non-cardiovascular mortality in patients with MAFLD. Notably, individuals with both high BRI and high SII exhibited the highest risks, suggesting a synergistic interaction between these two indicators. The combined assessment of BRI and SII offers valuable insights into risk stratification, highlighting their potential role in guiding clinical management and improving patient outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Qingyue Zeng: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Writing – original draft (lead); Writing – review & editing (lead). Di Zeng: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Writing – review & editing (equal). Shuangqing Li: Investigation (equal); Writing – review & editing (equal); Methodology (lead); Supervision (equal). Jiong Lu: Supervision (lead); Writing – review & editing (lead).
Funding
This study did not receive any funding.
Data availability
All data generated or analyzed during this study are included in this published article. All figures in our study are original and have not been previously published.
Declarations
Competing interests
The authors declare no competing interests.
Ethics declaration
This study utilized data from the National Health and Nutrition Examination Survey (NHANES), a publicly available dataset provided by the Centers for Disease Control and Prevention (CDC). All data were anonymized prior to analysis, and NHANES participants provided informed consent at the time of data collection. The NHANES protocol is reviewed and approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and all procedures are conducted in accordance with the Declaration of Helsinki.
Consent for publication
All authors unanimously agreed to submit this manuscript for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Di Zeng and Qingyue Zeng have contributed equally to this work.
Contributor Information
Jiong Lu, Email: lujiong@scu.edu.cn.
Nansheng Cheng, Email: nanshengcheng@yeah.net.
References
- 1.Cen, C., Fan, Z., Ding, X., Tu, X. & Liu, Y. Associations between metabolic dysfunction-associated fatty liver disease, chronic kidney disease, and abdominal obesity: A national retrospective cohort study. Sci. Rep.14 (1), 12645 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, K. E. et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: A meta-analysis and systematic review of 10 739 607 individuals. J. Clin. Endocrinol. Metab.107 (9), 2691–2700 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Kim, D. et al. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J. Hepatol.75 (6), 1284–1291 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Li, S. et al. Identifying the most critical behavioral lifestyles associated with MAFLD: Evidence from the NHANES 2017–2020. Front. Endocrinol. (Lausanne)15, 1375374 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi, Z. et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol.15 (1), 11–20 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Bae, S. D. W., George, J. & Qiao, L. From MAFLD to hepatocellular carcinoma and everything in between. Chin. Med. J. (Engl). 135 (5), 547–556 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado, M. V., Cortez-Pinto, H. & NAFLD MAFLD and obesity: Brothers in arms? Nat. Rev. Gastroenterol. Hepatol.20 (2), 67–68 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Després, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature444 (7121), 881–887 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Müller, M. J. et al. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev.13 (Suppl 2), 6–13 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Thomas, D. M. et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring)21 (11), 2264–2271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu, L., Xiao, Z., Fan, B., Li, L. & Sun, G. Association of body roundness index with diabetes and prediabetes in US adults from NHANES 2007–2018: A cross-sectional study. Lipids Health Dis.23 (1), 252 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou, X. D. et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol.21 (1), 270 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu, D. F. & Chen, B. The relationship between the systemic immune inflammation index and the nonalcoholic fatty liver disease in American adolescents. BMC Gastroenterol.24 (1), 233 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao, E., Cheng, Y., Yu, C., Li, H. & Fan, X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann. Med.55 (1), 2197652 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, W., Guo, X. L., Qiu, X. P., Yu, Y. J. & Tu, M. Systemic immune-inflammation index mediates the association between metabolic dysfunction-associated fatty liver disease and sub-clinical carotid atherosclerosis: A mediation analysis. Front. Endocrinol. (Lausanne). 15, 1406793 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, K. et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front. Immunol.15, 1337241 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren, A. et al. Systemic immune-inflammation index is a prognostic predictor in patients with intrahepatic cholangiocarcinoma undergoing liver transplantation. Mediat. Inflamm.2021, 6656996 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motomura, T. et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J. Hepatol.58 (1), 58–64 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Haybar, H., Pezeshki, S. M. S. & Saki, N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp. Mol. Pathol.110, 104267 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Zhou, Y., Wang, Y., Wu, T., Zhang, A. & Li, Y. Association between obesity and systemic immune inflammation index, systemic inflammation response index among US adults: A population-based analysis. Lipids Health Dis.23 (1), 245 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed, B., Sultana, R. & Greene, M. W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 137, 111315 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol.73 (1), 202–209 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Zhang, H., Lin, J., Huang, Y. & Chen, Y. The systemic immune-inflammation index as an independent predictor of survival in patients with locally advanced esophageal squamous cell carcinoma undergoing neoadjuvant radiotherapy. J. Inflamm. Res.17, 4575–4586 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian, X., Ding, N., Su, Y. & Qin, J. Comparison of obesity-related indicators for nonalcoholic fatty liver disease diagnosed by transient elastography. Turk. J. Gastroenterol.34 (10), 1078–1087 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, H. et al. Optimal obesity- and lipid-related indices for predicting metabolic syndrome in chronic kidney disease patients with and without type 2 diabetes Mellitus in China. Nutrients14(7) (2022). [DOI] [PMC free article] [PubMed]
- 26.Wang, Y. et al. Waist circumference change is associated with blood pressure change independent of BMI change. Obesity (Silver Spring)28 (1), 146–153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng, H. et al. Prediction of MAFLD and NAFLD using different screening indexes: A cross-sectional study in U.S. adults. Front. Endocrinol. (Lausanne)14, 1083032 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner, R. et al. Metabolic implications of pancreatic fat accumulation. Nat. Rev. Endocrinol.18 (1), 43–54 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Ohashi, K., Shibata, R., Murohara, T. & Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab.25 (7), 348–355 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature510 (7503), 84–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao, Y. et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: Results from NHANES. Front. Immunol.14, 1087345 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, M. et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: The Dongfeng-Tongji cohort study. Atherosclerosis323, 20–29 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Tiucă, O. M. et al. Predictive performances of blood-count-derived inflammatory markers for liver fibrosis severity in Psoriasis Vulgaris. Int. J. Mol. Sci.24(23) (2023). [DOI] [PMC free article] [PubMed]
- 34.Xu, P. et al. Usefulness of the systemic inflammation response index and the systemic immune inflammation index in predicting restenosis after stent implantation. J. Inflamm. Res.17, 4941–4955 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotamisligil, G. S. Inflammation and metabolic disorders. Nature444 (7121), 860–867 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Olveira, A. et al. The essential role of IL-17 as the pathogenetic link between Psoriasis and metabolic-Associated fatty liver disease. Life (Basel)13(2) (2023). [DOI] [PMC free article] [PubMed]
- 37.Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology52 (5), 1836–1846 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Qi, L. et al. Adipocyte inflammation is the primary driver of hepatic insulin resistance in a human iPSC-based microphysiological system. Nat. Commun.15 (1), 7991 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peiseler, M. et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J. Hepatol.77 (4), 1136–1160 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Schleh, M. W. et al. Metaflammation in obesity and its therapeutic targeting. Sci. Transl. Med.15 (723), eadf9382 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell178 (3), 686–98e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahima, R. S. & Lazar, M. A. Adipokines and the peripheral and neural control of energy balance. Mol. Endocrinol.22 (5), 1023–1031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harman-Boehm, I. et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab.92 (6), 2240–2247 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell161 (1), 146–160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong, W. et al. A combined analysis of TyG index, SII index, and SIRI index: Positive association with CHD risk and coronary atherosclerosis severity in patients with NAFLD. Front. Endocrinol. (Lausanne)14, 1281839 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. All figures in our study are original and have not been previously published.