Abstract
Background
Development of ventilator-associated pneumonia (VAP) is attributed to the microaspiration of pooled secretions around the cuff of airway devices. Despite the emphasis on the use of endotracheal tubes (ET) with subglottic secretion (SS) drainage ports to prevent VAP, the quality of the evidence for this recommendation remains moderate. This prospective observational study analyzed microbiological concordance between SS and endotracheal aspirate (ETA) cultures to generate further evidence in this regard.
Materials and methods
Paired samples (SS and ETA) of 100 consenting patients admitted to intensive care unit (ICU) were sent on day 1, 4, and 7 to the microbiology laboratory where they were transcultured and species identification was performed. The SS and ETA were considered concordant or discordant based on isolated organisms and antibiotic sensitivity profile. Clinical surveillance for VAP was done according to CDC criteria during the first week of ventilation.
Results
For a total of 197 paired samples, the overall concordance of SS and ETA cultures was 71.5%, with day-wise concordances of 68, 76.2, and 73.5% for D1, D4, and D7, respectively. Gram-negatives bacteria were the most frequently isolated, with 125 (31.7%) samples reporting A. baumannii. Amongst 18 patients clinically diagnosed with VAP during the first week of MV, the concordance between SS and ETA was 73.5%, and day-wise concordance was 77.2, 72.2, and 76.9% on D1, D4, and D7, respectively.
Conclusion
A fairly high microbiological concordance was observed in SS and ETA samples obtained from patients with invasive airway devices, and similar concordance was found in patients developing VAP during the first week of ventilation.
How to cite this article
Panigrahi P, Ganesh V, Angrup A, Sahni N, Biswal M, Yaddanapudi L. Microbiological Concordance of Subglottic Secretion and Tracheal Aspirate Cultures of Critically Ill Patients with Invasive Airway Devices: A Prospective Observational Study. Indian J Crit Care Med 2024;28(12):1139–1146.
Keywords: Critically ill patients, Intensive care unit, Mechanical ventilation, Nosocomial infection, Observational study, Respiratory infections, Ventilator associated pneumonia
Highlights
The study provides a preliminary proof of microbiological concordance between subglottic secretions and tracheal aspirate. It generates evidence for the use of invasive airway devices with subglottic secretions to prevent ventilator-associated pneumonia (VAP). The subglottic secretions can also be a potential source for VAP surveillance.
Introduction
Ventilator-associated pneumonia has been found in about 9–27% of intubated critically ill patients, and is an important nosocomial infection.1,2 The proposed mechanisms for the development of VAP are microaspiration of collected secretions surrounding the endotracheal (ET) or tracheostomy tube (TT) cuff, which contaminate the lower airways. As the cuff of airway devices does not prevent the passage of subglottic secretions (SS) into the lower airways completely, the suction of SS can aid in VAP prevention.3
Various recommendations such as from Infectious Diseases Society of America (IDSA), Society of Healthcare Epidemiology (SHEA), and Association for Professionals in Infection Control and Epidemiology (APIC) suggest the use of airway devices with drainage ports for SS for VAP prevention.4 However, the data regarding beneficial effects of tubes with SS drainage ports on length of stay in intensive care units (ICUs), total duration of MV, or mortality is not robust.5–10 Hence, the quality of evidence for this recommendation remains moderate.
A proof of the microbiological concordance of SS and endotracheal aspirate (ETA) could generate further evidence in this regard. The cultures of SS could also be a potential alternative to ETA and bronchioalveolar lavage (BAL) for microbiological surveillance of VAP.11 With this background, this study was conducted with the primary objective of analyze microbiological concordance between the cultures of SS and ETA in patients requiring mechanical ventilation using invasive airway devices. The secondary objective was to conduct VAP surveillance in the first week in patients requiring mechanical ventilation.
Materials and Methods
Study Design
This was a 1-year (April 2022–March 2023) single-center, prospective, observational, cohort study.
Study Setting
The study was done in the medical ICU of our tertiary care academic hospital in North India.
Study Approval
After written informed consent from their family, this study was performed according to the Declaration of Helsinki. Formal approval was sought from our ethics committee of our institute, via approval number IEC- INT/2022/MD/90, dated March 4th, 2022. The study commenced after the registration of the study protocol in the Clinical Trial Registry of India (Number: CTRI/2022/04/041592).
Methods
As per our standard clinical practice in our ICU, immediately upon admission to the ICU, the already in-situ invasive airway devices, whether ETT or TT, were replaced with the ones with drainage ports for SS if the patients were hemodynamically stable and did not have a high oxygen and/or PEEP requirement. The airway devices were changed for clinical indication upon admission to the ICU either due to the inappropriate size of the device, device blockage or the lack of a subglottic suction port. The cuff pressure was monitored at least twice a day and was maintained between 25 and 30 cm H2O. All patients were enrolled in the study on day 1 of ICU admission. Patients without the need for an invasive airway device and those with a history of upper airway disorders were excluded.
Data Collection
The demographic data (age, gender, admission details), comorbidities, diagnosis at the time of admission, acute physiology and chronic health evaluation (APACHE II) score, number of days of intubation or tracheostomy prior to ICU admission, and hospitalization days prior to ICU admission were recorded.
On days 1, 4, and 7 (D1, D4, and D7) of admission, both ETA and secretions suctioned from the SS port were sent for microbiological analysis. The ETA was collected in a sterile mucus extractor with an appropriate-size suction catheter and labeled as ETA. The manual aspiration of SS was attempted using a syringe (5 mL) after cleaning the external port with 70% isopropyl alcohol. The aspirated sample was collected in a sterile container and labeled as SS. The retrieved samples were sent for further processing to the microbiology laboratory immediately.
The samples were trans cultured in the microbiological laboratory. They were vortexed for 60 seconds, and gram staining was done for quality assessment of the samples. It was considered acceptable if the ETA contained <10 squamous epithelial cells per low power field in gram stain. Cultures were performed on blood agar and McConkey agar using standard techniques. Matrix-assisted laser desorption/ionization time-of-flight (MALDI) mass spectrometry was used for species identification, and the Vitek 2 system was used to test in vitro susceptibility.
After the results of the culture sensitivity, SS and ETA were classified as concordant if both samples displayed the same microbiological profile on a paired set of collected samples, that is, both cultures showed the same microorganism with similar susceptibility to antimicrobial agents or both were negative for any growth. The ETA cultures were considered the reference to which the SS was compared. The concordance in samples was calculated as a percentage, taking the total number of processed samples as the denominator.
Culture samples were considered to be discordant when both had different microbiological profiles on a paired set of collected samples, that is, species of microorganism retrieved in one sample was absent in the other or the susceptibility to antimicrobial agents did not match. If one sample cultured more than one organism and the other sample cultured only one of those organisms, the samples were still considered discordant.
VAP Surveillance
Centre for Disease Control (CDC) criteria was used to carry out VAP surveillance during the first week of the ICU stay, and day of the development of VAP was noted.12 The microbiological concordance for SS and ETA cultures for patients developing VAP during the first week of mechanical ventilation was evaluated separately, and a descriptive analysis of the characteristics of patients developing VAP was done.
Follow-up
All patients, whether they developed VAP or not, were followed up for clinical outcomes like days of VAP development, days of MV, days of ICU stay, and discharge/mortality.
Results
Statistical Analysis
A total of one hundred patients were recruited over the period of 1 year and both SS and ETA samples were collected and analyzed for all patients on days 1, 4, and 7. Descriptive analysis was carried out for demographic and other characteristics. The concordant and discordant samples were calculated as a percentage of the total number of paired samples collected. Clinical outcomes like days of MV, days of ICU stay, and discharge/mortality were compared between patients not developing VAP and those developing VAP using the Mann–Whitney U test and the Chi-square test.
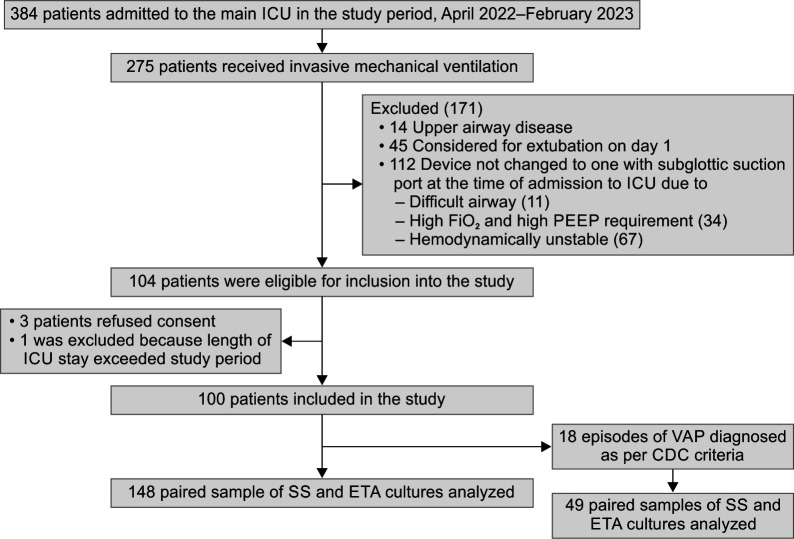
Out of 104 patients recruited from total admissions of 384 during the study period, 4 were excluded (Fig. 1). The overall median age of patients was 30 years (21.25–50) and the median APACHE II score was 20 (15–26). The median age of patients developing VAP (N = 18) was 34.5 years (18.25–49) and median APACHE II score was 22 (16–27) (Table 1).
Fig. 1.
Study flow chart depicting patient recruitment and number of samples analyzed
Table 1.
Demographic and other characteristics
| Characteristic | OverallN = 100 (100%)a | No VAPN = 82 (82%)a | VAPN = 18 (18%)a | Standardized differenceb |
|---|---|---|---|---|
| Age in years | −0.08 (–0.59, 0.43) | |||
| Mean (SD) | 35.89 (17.95) | 35.60 (17.08) | 37.22 (22.02) | |
| Median (IQR) | 30.00 (21.75, 50.00) | 30.00 (22.25, 49.75) | 34.50 (18.25, 49.00) | |
| Range | 11.00, 80.00 | 11.00, 74.00 | 13.00, 80.00 | |
| Gender | 0.44 (–0.07, 0.96) | |||
| Female | 45.00 (45.00%) | 40.00 (48.78%) | 5.00 (27.78%) | |
| Male | 55.00 (55.00%) | 42.00 (51.22%) | 13.00 (72.22%) | |
| APACHE II score | −0.18 (–0.69, 0.33) | |||
| Median (IQR) | 20.00 (15.00, 26.00) | 19.00 (15.00, 25.00) | 22.00 (16.00, 27.00) | |
| Range | 10.00, 36.00 | 10.00, 36.00 | 12.00, 31.00 | |
| Diagnostic category | 1.2 (0.65, 1.7) | |||
| Acute febrile illness | 4.00 (4.00%) | 4.00 (4.88%) | 0.00 (0.00%) | |
| Hepatology | 4.00 (4.00%) | 4.00 (4.88%) | 0.00 (0.00%) | |
| Infectious | 5.00 (5.00%) | 4.00 (4.88%) | 1.00 (5.56%) | |
| Nephrology | 9.00 (9.00%) | 7.00 (8.54%) | 2.00 (11.11%) | |
| Neurology | 10.00 (10.00%) | 9.00 (10.98%) | 1.00 (5.56%) | |
| Neuromuscular | 9.00 (9.00%) | 8.00 (9.76%) | 1.00 (5.56%) | |
| Obstetrics | 14.00 (14.00%) | 13.00 (15.85%) | 1.00 (5.56%) | |
| Others | 11.00 (11.00%) | 9.00 (10.98%) | 2.00 (11.11%) | |
| Poisoning | 21.00 (21.00%) | 16.00 (19.51%) | 5.00 (27.78%) | |
| Post-surgery | 7.00 (7.00%) | 7.00 (8.54%) | 0.00 (0.00%) | |
| Respiratory | 6.00 (6.00%) | 1.00 (1.22%) | 5.00 (27.78%) | |
| Hospitalization days prior to ICU admission | −0.11 (–0.62, 0.40) | |||
| Mean (SD) | 2.94 (2.12) | 2.90 (2.19) | 3.11 (1.81) | |
| Median (IQR) | 3.00 (1.00, 4.00) | 3.00 (1.00, 3.00) | 3.00 (1.25, 4.00) | |
| Range | 1.00, 15.00 | 1.00, 15.00 | 1.00, 7.00 | |
| Intubation days prior to ICU | −0.50 (–1.0, 0.01) | |||
| Mean (SD) | 0.64 (1.00) | 0.54 (0.86) | 1.11 (1.41) | |
| Median (IQR) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.50 (0.00, 2.00) | |
| Range | 0.00, 4.00 | 0.00, 4.00 | 0.00, 4.00 | |
aData as n (%) unless mentioned otherwise; bStandardized difference and () 95% confidence intervals; APACHE, acute physiology and chronic health evaluation II
Microbiological Concordance
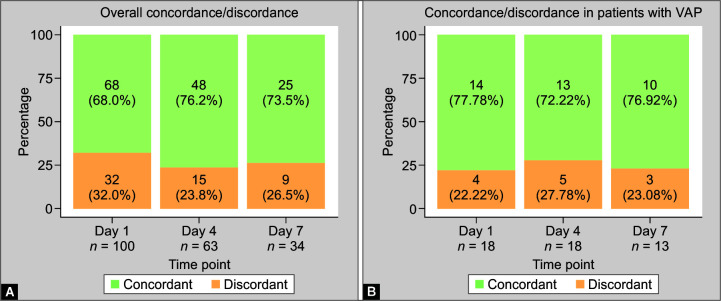
The total number of paired samples of SS and ETA was 197, or a, total of 394 samples. The microbiological concordance between the SS and ETA culture samples was 71.5% (141 paired samples). The concordance in samples collected on day 1 was 68% (68/100 paired samples), on day 4 it was 76.2% (48/68 paired samples), and on day 7, it was 73.5% (25/34 paired samples).
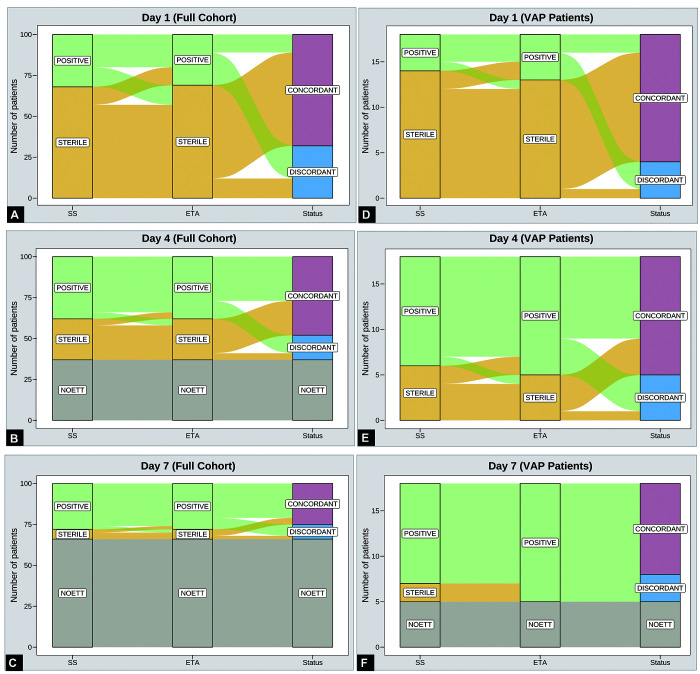
Amongst 18 patients (total of 49 paired samples) considered to have VAP during surveillance in first week of MV, the microbiological concordance between SS and ETA culture samples was 73.5% (36 of the 49 paired samples). On day 1, the concordance was 77.7% (14/18), on day 2, it was 72.2% (13/18) and on day 7, it was 76.9% (10/13) (Fig. 2). The samples reporting sterility were higher on days 1 and day 4 than on day 7 (83.8% and 43.75% vs 16%, respectively) (Fig. 3).
Figs 2A and B.
Microbiological concordance of SS and ETA samples across different time points, that is, day 1, 4, and 7. (A) Overall concordance on day 1, 4, and 7; (B) Concordance of samples from patients with VAP
Figs 3A to F.
Alluvial plot showing distribution of sterile and culture positive concordant and discordant samples on day 1, 4, and 7. (A) Samples on day 1 (full cohort); (B) Samples on day 4 (full cohort); (C) Samples on day 7 (full cohort); (D) Samples on day 1 (only VAP patients); (E) Samples on day 4 (only VAP patients); (F) Samples on day 7 (only VAP patients)
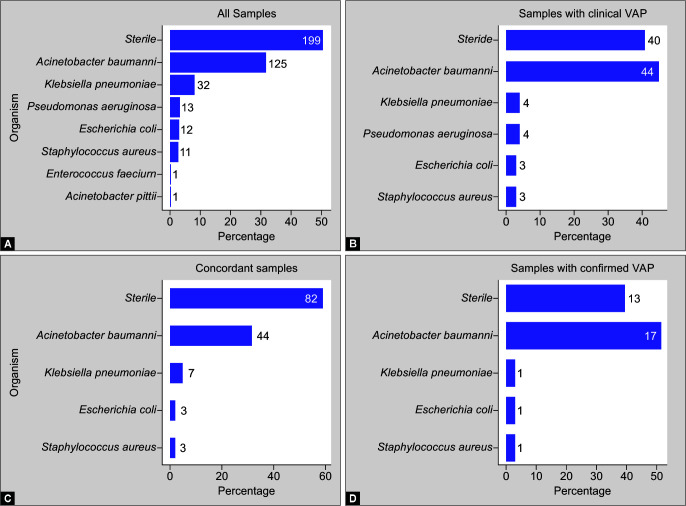
Out of a total of 394 samples, the majority of the samples (50.5%) were sterile and in remaining samples, A. baumannii was the most frequently encountered organism (n = 125, 31.7% samples). Amongst 141 paired concordant samples, 82 (58.1%) samples were sterile, with A. baumannii as the most frequent microorganism isolated in 44 (31.2%) samples. In 33 paired concordant samples obtained for confirmed VAP cases, 13 (39.3%) of the samples were sterile and 17 (51.7%) of the samples isolated A. baumannii (Fig. 4).
Figs 4A to D.
Microbiological profile of samples. (A) All samples; (B) Samples from patients with clinical VAP; (C) Concordant samples; (D) Samples from patients with confirmed VAP
Clinical Outcomes
The clinical outcomes in terms of days of mechanical ventilation, days of ICU stay, median day of development of VAP, number of patients discharged from ICU, and mortality are depicted in Table 2.
Table 2.
Clinical outcomes
| Outcome | OverallN = 100 (100%)a,b | No VAPN = 82 (82%)a,b | VAPN = 18 (18%)a,b | p-valuec |
|---|---|---|---|---|
| Days of mechanical ventilation median (IQR) | 6.00 (4.00, 13.00) (6.5, 10) | 6.00 (4.00, 7.75) (5.0, 7.5) | 15.50 (10.25, 19.75) (12, 19) | <0.001 |
| Days of ICU stay median (IQR) | 10.00 (5.00, 17.00) (9.5, 13) | 8.00 (5.00, 13.00) (7.5, 11) | 21.00 (16.00, 26.00) (16, 26) | <0.001 |
| Day of VAP development median (IQR) | 4.00 (4.00, 5.00) (4.0, 4.5) | NA | 4.00 (4.00, 5.00) (4.0, 4.5) | NA |
| Final outcome | 0.4 | |||
| Death | 30.00 (30.00%) (21%, 40%) | 26.00 (31.71%) (22%, 43%) | 4.00 (22.22%) (7.4%, 48%) | |
| Discharged | 70.00 (70.00%) (60%, 79%) | 56.00 (68.29%) (57%, 78%) | 14.00 (77.78%) (52%, 93%) | |
aData as n (%) or median (IQR); b() 95% confidence intervals; cMann–Whitney U test or Chi-square test
Discussion
In this prospective, observational study, 197 paired samples (SS and ETA) taken at fixed time intervals (D1, D4, and D7 of ICU admission) showed an overall microbiological concordance of 71.5% (141/197). Eighteen patients who developed VAP during the first week also showed microbiological concordance of 75.5% (35/49) in SS and ETA samples.
Among the mechanisms proposed for the development of VAP in intubated patients, namely, aspiration of secretions of the oropharynx during intubation, trauma and mechanical forces that compromise mucosal integrity and mucociliary clearance, microaspiration through the ETT cuff crevices and biofilm formation with bacterial colonization inside the ETT lumen, the phenomenon of microaspiration of the secretions surrounding the cuff has been given a lot of emphasis.3,13,14 Tanzarella et al. demonstrated the presence of enzyme alpha amylase (level >1685 IU/L) in 55% of tracheal aspirate samples, thereby suggesting microaspiration of oropharyngeal secretions despite ensuring an adequate endotracheal tube cuff pressure between 25 and 30 cm H2O. The median volume of subglottic secretions was about 31 mL.15 Our study established microbiological concordance between the secretions that are pooled near the cuff and the secretions obtained from the lower respiratory tract.
Bello et al. observed 81% (170/211) microbiological concordance between SS and ETA by sending surveillance cultures of SS and ETA twice-weekly. Even in patients developing VAP, 73% microbiological concordance was reported between SS and BAL isolates. Similar to our study, gram-negative organisms were frequently identified, with A. baumannii being the most common (31.9% vs 18%).11
We selected the sampling time points of D1, D4, and D7 to obtain samples from the same airway device, as the majority of patients are either extubated or tracheostomized between 7 and 10 days in our practice. The samples sent on the first day, day 4, and day 7 showed almost similar concordance (68, 76.2, and 73.5%, respectively). The samples reporting sterility were higher on day 1 and 4 than day 7 (83.8% and 43.75% vs 16%, respectively). The possible microaspiration of SS to ETA, which continued over time, led to fewer sterile samples on day 7. However, all of these patients did not develop VAP according to CDC criteria. The overall microbiological concordance of SS and ETA in 18 patients who developed VAP was also high (75.5%), and it was similar between samples collected on days 1, 4, and 7 (77.7, 72.2, and 76.9% respectively). Among the initially discordant samples in VAP patients, 3/4th changed to concordant samples by day 4, and all these patients satisfied the CDC VAP clinical criteria on days 3 and 4 and not on day 1. However, our sample size of only 18 VAP cases is too small to draw the conclusion that the microaspiration of secretions surrounding the cuff colonizing the lower airway is the only phenomenon taking place.
The partial incongruity in SS and ETA in the remaining samples can be explained by the possibility of other sources of development of VAP. For example, regurgitation of gastric contents due to increased gastrooesophageal reflux due to the presence of nasogastric tubes, or the introduction of microorganisms that may happen due to any possible breach in sterility during endotracheal suction.3
The clinical implication of demonstrating a high microbiological concordance between SS and ETA would be the use of ETT with SS drainage ports in all ICU patients, along with suction of subglottic secretions. The recent strategies laid out by the SHEA, IDSA, and APIC to prevent VAP in adult patients emphasize the use of endotracheal tubes (ET) with SS drainage ports, especially if the endotracheal tube is expected to remain in-situ for more than 48 hours. However, due to conflicting and insufficient data on the duration of MV or mortality, the quality of evidence for the recommendation of ETT with sub-glottic suction port remains moderate, only to be practiced in addition to the WHO bundle of care to prevent VAP. Also, the non-availability of ETT with sub-glottic in smaller sizes (below 6.0 internal diameter) precludes the use of these tubes in children below 10 years of age.4
Majority of literature supporting the use of ET with SS drainage ports is available in three meta-analyses by Caroff et al., Muscedere et al., and Pozuelo-Carrascosa et al.1,5,6 While Muscedere et al. suggested an overall risk ratio (RR) of 0.55, 95% confidence interval (CI), 0.46–0.66 for incidence of VAP with the use of tubes with SS drainage ports as compared to conventional ETTs, Caroff et al. demonstrated a lower VAP rate (RR of 0.58, 95% CI, 0.51–0.67) with the use of ETT with SS drainage ports. In addition, while Muscedere et al. suggested reduced days of MV (–1.08 days, 95% CI, –2.04 to –0.12) and decreased length of ICU stay (–152 days, 95% CI, –2.94 to –0.11) with the use of tubes with SS drainage ports, Caroff et al. did not demonstrate any such benefit.1,5 A meta-analysis by Pozuelo-Carrascosa et al. concluded a significant reduction in the incidence of VAP (RR 0.56, 95% CI, 0.48–0.63) and mortality (RR 0.88, 95% CI, 0.80–0.97) with the use of ETT with SS drainage port, though there was no difference in the duration of MV, ICU or hospital length of stay.6
Amongst the other studies, Juneja et al. found a decreases in the incidence of VAP with the use of ETT with SS drainage when compared to the use of closed suction alone (23.9 vs 15.7 per 1000 ventilator days). Also, the combined practice of using ETT with SS drainage with the use of closed suction catheters resulted in an even lower VAP incidence (14.3 per 1000 ventilator days). However, no significant difference could be appreciated in clinical outcomes like duration of MV, length of ICU, and hospital stay or mortality.8
Even Tomaszek et al. found a reduced incidence of VAP (9.6% vs 19.1%), especially early-onset VAP when comparing the combined the use of ETT with SS drainage and continuous cuff pressure monitoring techniques to the use of conventional ETT. However, there was no effect on days of MV, length of ICU stay, or mortality.9
In a review of 4 systematic reviews (44 randomized controlled trials and 10,193 patients), Suclupe et al. found a significant effect of the use of SS drainage on the incidence of VAP. The odds ratio of the favorable effect of using tubes with SS drainage ranged from 0.48 (95% CI, 0.38–0.60) to 0.55 (95% CI, 0.46–0.66).10
We practiced intermittent subglottic suction every 4th hourly, while Bello et al. used continuous subglottic suction technique with a gap of 4 hours to facilitate sampling of SS.11 A systematic review showed no difference in the incidence of VAP (RR 0.83, 95% CI, 0.61–1.13), time to occurrence of VAP (RR 2.73, 95% CI, –0.39–5.85), duration of MV (RR -0.89, 95% CI, –2.72–0.94), length of ICU stay (RR 3.98, 95% CI, –4.44–12.41) or mortality (RR 0.80, 95% CI, 0.48–1.31) when comparing the continuous suction and intermittent suction of ET with SS drainage.13 Seguin et al. attempted to analyze the effect of continuous or intermittent subglottic suctioning on tracheal mucosa tear and did not find any significant difference with the use of either technique.14
While we compared SS and ETA even in patients developing VAP, Bello et al. obtained BAL samples for comparison with SS.11 In our clinical practice, we routinely do not take BAL samples for VAP surveillance or for patients developing VAP. According to 2016 clinical practice guidelines by IDSA and the American Thoracic Society (ATS), non-invasive techniques of sampling can be reliably used to diagnose VAP. There is no evidence that invasive methods of microbiological surveillance improve clinical outcomes, in terms of mortality rate, duration of MV or length of ICU stay. Additionally, it increases the cost as well as the potential risk due to the invasive nature of the procedure.16
Though our study provided good microbiological concordance between SS and ETA, we did experience difficulty in aspirating subglottic secretions. Adi et al. described the use of airway ultrasound as a non-invasive and safe tool to detect the collection of subglottic secretions, with a positive predictive value of 93.5% and a negative predictive value of 94.7%.17 The use of airway ultrasound to detect subglottic secretions could ease the aspiration of collected secretions.
Secondly, the concordance of samples was based on the organism and its antibiotic sensitivity profile. However, genotyping of the organism obtained in both samples would be required to establish concordance with greater reliability. Lastly, it was a single-seater study and the results of the study cannot be generalized due to the different patient populations and different clinical practices in other ICUs and/or centers. Robust evidence generated from a multicentric study with a larger cohort is needed to validate the results of this study.
Conclusion
A fairly high microbiological concordance was observed in SS and ETA samples obtained from critically ill intubated patients, and similar concordance was found in patients developing VAP during the first week of mechanical ventilation.
Clinical Significance
The preliminary proof of microbiological concordance from our study can guide studies that can help assess the reliability of the SS antibiogram to facilitate clinical decision-making for antimicrobial prescription and stewardship. Subglottic secretions cultures may serve as an easier to implement alternative option for microbiological surveillance during MV. However, before considering SS for VAP diagnosis, appropriate criteria should be standardized to ensure that the sample being processed is relevant, and the subsequent report is meaningful. Subglottic secretions needs to be further explored as a viable replacement for ETA, especially in cases where it is difficult to get the ETA (example, high FiO2 and PEEP requirements). Future studies with larger sample sizes may explore treatment based on SS sensitivity patterns in cases where the ETA is sterile, but the clinical picture is highly suggestive of VAP.
Authors Contribution
PP: Data collection, data curation, investigation, writing-original draft; VG: Data curation, formal analysis, visualization, writing-review and editing; AA: Methodology, investigation, validation, writing-review and editing; NS: Conceptualization, methodology, investigation, data curation, project administration, writing-review and editing; MB: Methodology, validation, writing, review and editing; LNY: Conceptualization, methodology, validation, project administration, supervision, writing-review and editing.
Orcid
Pritam Panigrahi https://orcid.org/0000-0002-1334-4406
Venkata Ganesh https://orcid.org/0000-0001-8265-5198
Archana Angrup https://orcid.org/0000-0002-9051-3007
Neeru Sahni https://orcid.org/0000-0002-8506-8076
Manisha Biswal https://orcid.org/0000-0003-2016-3678
Lakshminarayana Yaddanapudi https://orcid.org/0000-0002-9822-0217
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Caroff DA, Li L, Muscedere J, Klompas M. Subglottic secretion drainage and objective outcomes: A systematic review and meta-analysis. Crit Care Med. 2016;44(4):830–840. doi: 10.1097/CCM.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Hunter JD. Ventilator associated pneumonia. Postgrad Med J. 2006;82(965):172–178. doi: 10.1136/pgmj.2005.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klompas M, Branson R, Cawcutt K, Crist M, Eichenwald EC, Greene LR, et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and non-ventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2022;43(6):687–713. doi: 10.1017/ice.2022.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. Crit Care Med. 2011;39(8):1985–1991. doi: 10.1097/CCM.0b013e318218a4d9. [DOI] [PubMed] [Google Scholar]
- 6.Pozuelo-Carrascosa DP, Herraiz-Adillo A, Alvarez-Bueno C, Anon JM, Martinez-Vizcaino V, Cavero-Redondo I. Subglottic secretion drainage for preventing ventilator-associated pneumonia: An overview of systematic reviews and an updated meta-analysis. Eur Respir Rev. 2020;29(155):190107. doi: 10.1183/16000617.0107-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deem S, Treggiari MM. New endotracheal tubes designed to prevent ventilator associated pneumonia: Do they make a difference? Respir Care. 2010;55(8):1046–1058. https://rc.rcjournal.com/content/respcare/55/8/1046.full.pdf Available from: [PubMed] [Google Scholar]
- 8.Juneja D, Javeri Y, Singh O, Nasa P, Pandey R, Uniyal B. Comparing influence of intermittent subglottic secretions drainage with/without closed suction systems on the incidence of ventilator associated pneumonia. Indian J Crit Care Med. 2011;15(3):168–172. doi: 10.4103/0972-5229.84902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomaszek L, Pawlik J, Mazurek H, Medrzycka-Dabrowska W. Automatic continuous control of cuff pressure and subglottic secretion suction used together to prevent pneumonia in ventilated patients: A retrospective and prospective cohort study. J Clin Med. 2011;10(21):4952. doi: 10.3390/jcm10214952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suclupe S, Bustillos PEP, Bracchiglione J, Requeijo C, Salas-Gama K, Sola I, et al. Effectiveness of nonpharmacological interventions to prevent adverse events in the intensive care unit: A review of systematic reviews. Aust Crit care. 2023;36(5):902–914. doi: 10.1016/j.aucc.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Bello G, Bisanti A, Giammatteo V, Montini L, Eleuteri D, Fiori B, et al. Microbiologic surveillance through subglottic secretion cultures during invasive mechanical ventilation: A prospective observational study. J Crit Care. 2020;59:42–48. doi: 10.1016/j.jcrc.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 12.National Healthcare Safety Network (NHSN) Pneumonia (ventilator-associated [VAP] and non-ventilator-associated pneumonia [PNEU]) event. 2024. [online]https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf Available from: [Last accessed November, 2024] [Google Scholar]
- 13.Wen Z, Zhang H, Ding J, Wang Z, Shen M. Continuous versus intermittent subglottic secretion drainage to prevent ventilator-associated pneumonia: A systematic review. Crit Care Nurse. 2017;37(5):e10–e17. doi: 10.4037/ccn2017940. [DOI] [PubMed] [Google Scholar]
- 14.Seguin P, Perrichet H, Pabic EL, Launey Y, Tiercin M, Corre R, et al. Effect of continuous versus intermittent subglottic suctioning on tracheal mucosa by the Mallinckrodt TaperGuard Evac oral tracheal tube in intensive care unit ventilated patients: A prospective randomized study. Indian J Crit Care Med. 2018;22(1):1–4. doi: 10.4103/ijccm.IJCCM_350_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanzarella ES, Lombardi G, Baroni S, Sarlo F, Cutuli SL, Carelli S, et al. Use of an innovative cuff pressure control and subglottic secretions drainage system in COVID-19 ARDS patients undergoing pronation. Crit Care. 2022;26(1):338. doi: 10.1186/s13054-022-04225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adi O, Fong CP, Sallehuddin RM, Ahmad AH, Sum KM, Yusof ZM, et al. Airway ultrasound to detect subglottic secretion above endotracheal tube cuff. Ultrasound J. 2023;15(1):23. doi: 10.1186/s13089-023-00318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]