Abstract
The purpose of this study was to investigate the effect of different times return to activity on tendon healing after Double Kessler method suture in rats with Achilles tendon rupture. The left Achilles tendon of 80 10-week-old rats was repaired. The rats were randomly divided into 4 groups: non-fixed group, fixed one week group, fixed two weeks group and fixed three weeks group. In the fourth week, all rats were trained on a treadmill for one hour a day at a speed of 10 m/min. Complications were recorded during the study period and passive ankle motion was measured after the rats were euthanized. The healing and adhesion of tendons were evaluated by anatomy, biomechanics, histology and immunohistochemistry. The earlier return to activity after surgery, the higher the quality of tendon healing and the less adhesion will occur. There was no difference in complication rate among the four groups (P< 0.05). There was one case of tendon re-rupture in the non-fixed group and one case in the fixed one week group. The passive range of motion, biomechanical properties, histological evaluation and immunohistochemical results of the ankle in non-fixed group were better than those in the other three groups, while those in fixed three weeks group were worse than those in the other three groups (P< 0.05). The passive ankle range of motion, count of fibroblasts, biomechanical results, and immunohistochemical results showed no statistic significant difference between the fixed one week group and fixed two weeks group (P> 0.05). Early return to activity with strong sutures is advantageous for tendon injuries. With the advanced of return to activity time, the healing strength of tendon increased and the degree of adhesion decreased four weeks after surgery.
Keywords: Tendon, Adhesion, Healing, Return to activity, Rat study
Subject terms: Immunohistochemistry, Trauma
Introduction
With the increase of age and the popularization of sports, tendon injury has gradually become an important factor affecting people’s health1,2. Tendon is a special connective tissue characterized by low cell and blood vessel density, resulting in challenging healing processes3. The tendon healing process can be categorized into endogenous and exogenous healing based on the origin of cells involved4. The process of exogenous healing involves the proliferation of fibroblasts around the tendon, which then grow into the broken end of the tendon and ultimately form scar tissue5. As a result, exogenous healing inevitably leads to tendon adhesion6. Therefore, how to promote tendon healing and reduce the formation of scar adhesion is a problem that needs to be solved in clinical work.
A number of recent studies have shown that early activity can increase the strength of tendon healing7–9. Silva Barreto et al., using micro- and nanostructure specific X-ray tomography in a rat model, found delayed and more disorganized regeneration of tendon fibers in fully immobilized rats10. In a study by Zhi Li et al., dynamic tensile stress was found to promote tendon healing through the integrin/FAK/ERK signaling pathway, tendon healing length and failure load were significantly lower in postoperatively fixed mice than in non-fixed mice11. In addition, early return to activity is thought to reduce adhesion and increase joint motion12,13. However, a recent meta-analysis has shown that returning to activity immediately after surgery significantly increases the risk of tendon re-rupture14. In clinical practice, surgeons prefer to stick with conservative fixed timing to avoid medical disputes. These factors make it difficult for patients with tendon rupture in clinical work to return to activity early.
We hypothesized that early return of activity with a low incidence of tendon re-rupture could be achieved with strong suturing. The rat Achilles tendon injury model is widely utilized in the investigation of tendon injuries, providing a convenient and effective approach to comprehend the mechanisms and developmental patterns of such injuries15. The utilization of animal models for investigating tendon injuries allows for the control of injury type and the development of consistent surgical and rehabilitation protocols. Therefore, this study intends to apply relatively strong suture mode in the rat Achilles tendon rupture model to explore the influence of different time to return to activity on tendon healing.
Materials and methods
Study design and surgical procedure
This study has been approved by the Medical Ethical Committee of the Hebei Medical University Third Hospital and is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. Eighty 10-week-old male Sprague-Dawley rats (weight 300–350 g) provided by Shandong Hengrong Biotechnology Co. LTD. were used in this study. The rats were kept in separate cages at a controlled temperature (21°± 2℃) under a 12-h light-dark cycle with ad libitum access to food and water. Rats were acclimated to the new environment for 7 days before starting the experiment.
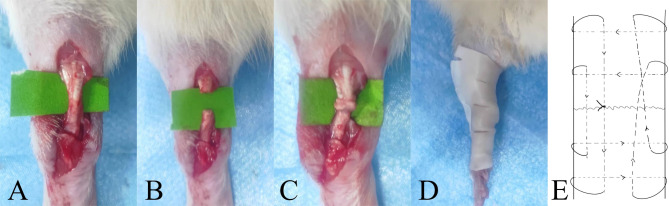
The rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg). Cefazolin sodium (10 mg/kg) is administered internally to prevent infection. Disinfect left leg with povidone-iodine solution after shaving. Incise the skin about 2 cm along the long axis, expose the Achilles tendon and blunt transection it at the midpoint. The ends of the Achilles tendon were sutured together using the Double Kessler method (Prolene 4–0). After disinfection again and cleaning the wound, use 1 − 0 thread to suture the skin incision (Fig. 1).
Fig. 1.
Reconstruction of ruptured Achilles tendon of rats. (A) Cut the skin about 2cm to expose the rat’s Achilles tendon. (B) Blunt transected tendon with knife back. (C) The Double Kessler method was used to anastomose the severed end of the tendon. (D) After surgery, the ankle joint was fixed in plantarflexion with a polymer splint. (E) Diagram of Double Kessler suture method.
The rats were randomly divided into 4 groups: non-fixed (NF) group, fixed one week (F-1 W) group, fixed two weeks (F-2 W) group and fixed three weeks (F-3 W) group. Each group consisted of 20 rats. Polymer splints were used to fix the ankle joint in plantarflexion except in the NF group. Subsequently, the rats were kept in cages for three weeks. The F-2 W and F-3 W groups had their splints changed weekly until the end of fixation. In the fourth week, all rats were trained on a treadmill for one hour a day at a speed of 10 m/min9. For the F-3 W group of rats, treadmill training was scheduled to begin the day after the splints was removed. The complications of rats were observed and recorded daily. The rats were euthanized after seven days of treadmill training by injecting potassium chloride (1.5 mg/kg) under deep anesthesia.
Gross observation
The healing of the Achilles tendon was evaluated according to Tang’s grading method (n = 19–20/group)16. The specific classification is as follows: (i) No adhesions: There are no adhesions around the tendon, but some granulation tissue may be present; (ii) Membranous adhesions: only a few membranous adhesions have no effect on tendon gliding; (iii) Loose adhesions: These are thin, loose, soft fibers and tendons that are easily separated; (iv) Moderately dense adhesions: moderate texture with some tendon mobility; (v) Severe extensive adhesion: poor mobility and no boundary between the tendon and the peritendinous tissue.
Passive ankle motion
The lower limb of the rats were dissected and fixed on the operating table to keep the knee joint extended. Tie a suture 1 cm away from the rat’s ankle joint. After ensuring that the suture is tight, pull the ankle joint with different weights of blocks. The weight was increased from 15 g, 25 g, to 35 g, and the dorsiflexion and plantarflexion were measured once each. A digital camera was used to take pictures and record the range of motion of the ankle (n = 19–20/group).
Biomechanical analysis
The adherent tissue around the Achilles tendon is separated, secure both ends of the tendon using a specialized aluminum fixture with sandpaper (n = 9–10/group). The whole construct was then mounted onto the biomechanical testing machine (Electroforce 3230, US). The tensile test was conducted using a device set at a speed of 0.1 mm/sec and an initial load of 0.2 N17. The maximum load (N) and stiffness (N/mm) values at the breaking point of the Achilles tendons were recorded. Stiffness values were calculated by dividing the maximum load values by the amount of elongation at the breaking point of the Achilles tendons.
Histological staining
Tendon tissue samples were fixed using 10% neutral formaldehyde solution and kept in 5% formic acid (n = 10/ group). Following histopathological preparation processes, the specimens were embedded in paraffin blocks and sectioned. The sections were stained with hematoxylin and eosin (H&E) (Abcam, Cambridge, UK) and Masson’s trichrome (BIOGNOST, Zagreb, Crotia). The number, morphology and collagen arrangement of fibroblasts were observed under a microscope. Six well-stained fields were randomly selected under a 200-fold light microscope, and the number of fibroblasts in each field was calculated using Image Pro Plus 6.0, and the results were analyzed. Bonar’s semi-quantitative score grading scale were used for evaluation.
Bonar’s scale includes the analysis of the following components: (i) tenocytes, (ii) ground substance, (iii) collagen, and (iv) vascularity. Each variable was scored on a 4-point scale of 0–3 as follows: 0, normal; 1, slightly abnormal; 2, abnormal; and 3, markedly abnormal. The samples were assessed for the presence of significant abnormality, with a total score ranging from 0 (normal tendon) to 12 (most severe abnormality)18.
Immunohistochemistry
The same tissue specimens from histology were utilized (n = 10/group). The paraffin block is longitudinally sectioned, and stained with TGF-β1, anti-type I collagen, and anti-type III collagen antibodies (Wuhan Yunkron, China). After dewaxing in xylene, the sections were dehydrated with ethanol. They were then incubated with 0.5% trypsin at 37 °C for 15 min and endogenous peroxidase activity was inhibited using hydrogen peroxide. Blocking serum was applied for 1 h, followed by incubation with primary antibodies at 4 °C overnight. The sections were then treated with the antimouse biotin-streptavidin hydrogen peroxidase secondary antibody. DAB staining solution was applied, observed under microscope for 2–5 min until the cell base color turned brown, and then restained with hematoxylin staining solution for 10s. Image Pro Plus 6.0 was used for quantitative analysis. The integrated option density (IOD) and area value of each image are measured, and then the mean density (MD = IOD/area) is calculated.
Statistical analyses
We performed all statistical analyses using the Statistical Package for Social Sciences (SPSS) 26.0 (IBM Corporation, Armonk, New York, USA). In descriptive analysis, means and standard deviations were used for continuous variables and frequencies as well as percentages were used for categorical variables. One-way ANOVA analysis of variance was used for comparison multiple groups, and the LSD-t test was used for pairwise comparisons. P < 0.05 was considered to indicate a statistically significant difference.
Results
Postoperative complications
A total of 5 splints loss during fixation, including 2 cases in the F-1 W group, 2 cases in the F-2 W group, and 1 case in the F-3 W group. All splint loss occurred 5 to 9 days after surgery. We reinstalled the splint within 24 h after it was lost. One case of skin necrosis in the F-3 W group occurred on the seventh day after surgery. There were 4 cases of incisional infection, 2 cases in NF group and 1 case each in F-2 W and F-3 W group. No rat deaths occurred during the experiment. There was no difference in complication rate among the groups (P> 0.05) (Table 1).
Table 1.
A summary of complications in each group.
| Groups | Skin necrosis | splint loss | Incisional infection | Tendon re-rupture |
|---|---|---|---|---|
| NF (n = 20) | 0 | 0 | 2 | 1 |
| F-1 W (n = 20) | 0 | 2 | 0 | 1 |
| F-2 W (n = 20) | 0 | 2 | 1 | 0 |
| F-3 W (n = 20) | 1 | 1 | 1 | 0 |
Gross observation
The tendon was examined for re-rupture after euthanasia. In the NF group and the F-1 W group, there was one case of re-rupture of the Achilles tendon each. In the F-1 W group, the rat that experienced re-rupture also had the splint fall off. Both rats that experienced re-rupture were not included in any further experiments. By assessing the degree of tendon adhesion, it was observed that the degree of adhesion in each group gradually increased with the extension of splint fixation time. The adhesion degree of F-2 W was significantly higher than that of NF and F-1 W groups (P<0.05). Additionally, the adhesion degree of F-3 W group was significantly higher than that of the other three groups (P<0.005) (Table 2).
Table 2.
Statistical results of tendon adhesion grading.
| Group | n | Grading of tendon adhesion | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| NF | 19 | 8 | 8 | 3 | 0 | 0 |
| F-1 W | 19 | 6 | 8 | 5 | 0 | 0 |
| F-2 W | 20 | 2 | 5 | 11 | 2 | 0 |
| F-3 W | 20 | 0 | 2 | 8 | 7 | 3 |
Passive ankle motion
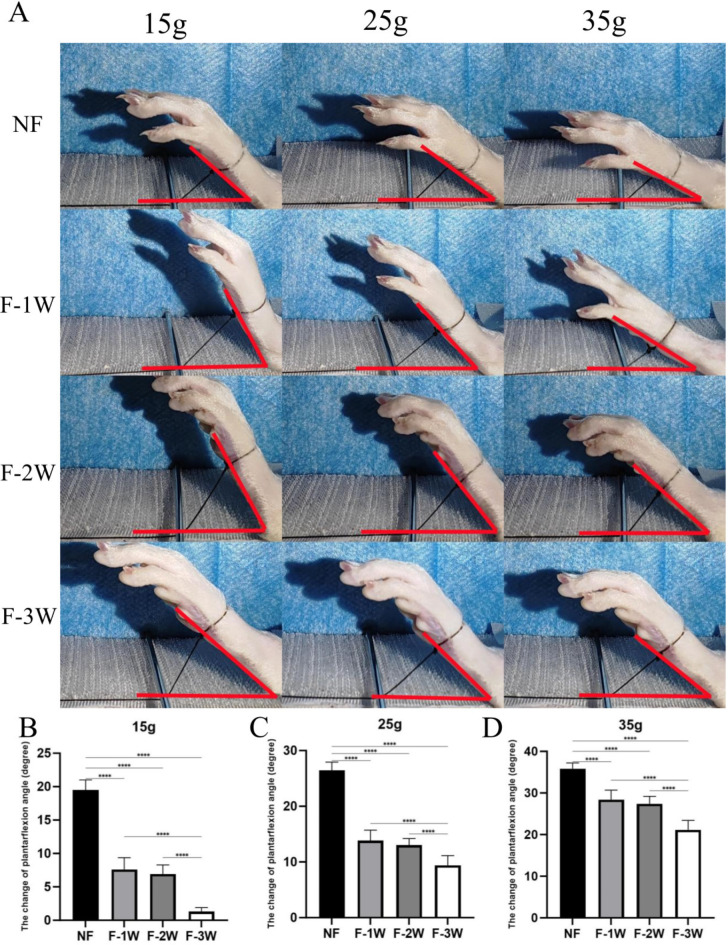
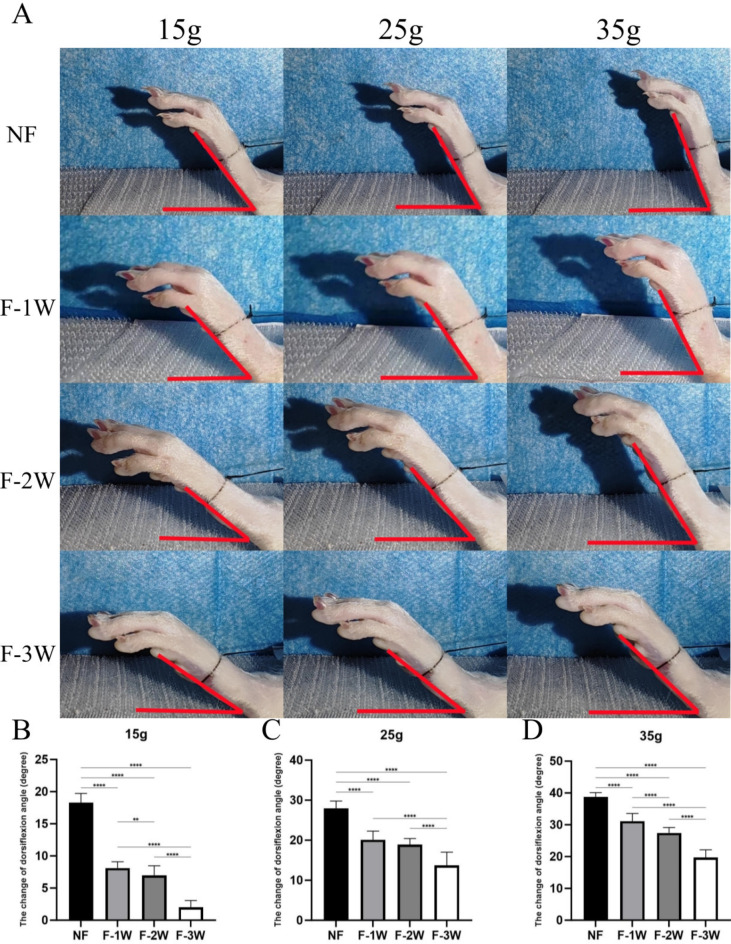
The plantarflexion (Fig. 2) and dorsiflexion (Fig. 3) motion of NF group were significantly better than those of the other three groups under different weights (P<0.001). The plantarflexion and dorsiflexion motion of F-3 W group were significantly lower than those of the other three groups under different weights (P<0.001). Particularly, the ankle joint of the F-3 W group showed nearly no movement at a weight of 15 g. Although there was no statistical difference between F-1 W and F-2 W dorsiflexion range of motion (P>0.05), overall ankle motion decreased with longer braking time.
Fig. 2.
The passive plantarflexion activity of ankle in four groups (NF group, F-1W group, F-2W group and F-3W group) under different weights was observed. (A) Measurement of ankle plantarflexion movement Angle in rats. (B-D) Comparison of plantarflexion motion of each group under different weights (15g, 25g, 35g). *P 0.05, **P 0.01, ***P 0.001, **** P 0.0001.
Fig. 3.
The passive dorsiflexion activity of ankle in four groups (NF group, F-1 W group, F-2 W group and F-3 W group) under different weights was observed. (A) Measurement of ankle dorsiflexion movement Angle in rats. (B-D) Comparison of dorsiflexion motion of each group under different weights (15 g, 25 g, 35 g). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Biomechanical evaluation
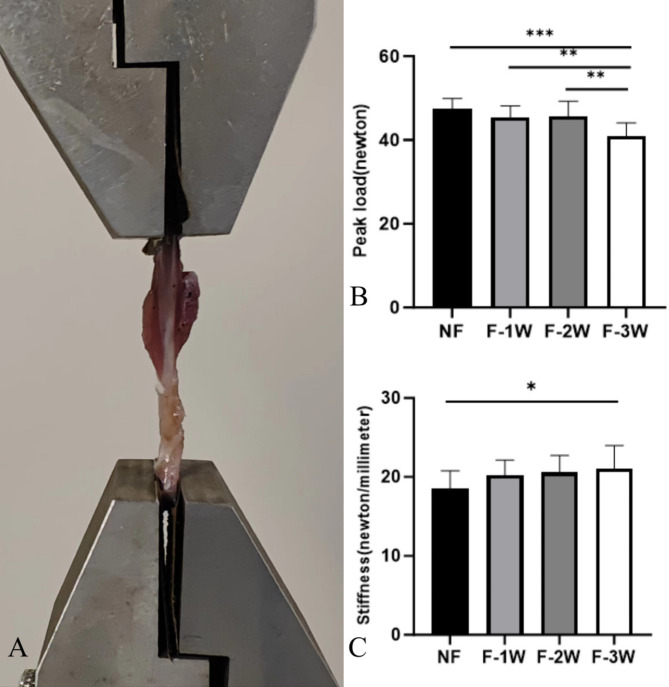
In comparison of peak load among the groups, the peak load of the F-3 W group was significantly lower than the other three groups (P<0.01). The stiffness of the F-3 W group was 21.09 ± 2.91 N/mm, which was significantly greater than that of the NF group at 18.56 ± 2.22 N/mm (P<0.05). The peak load and stiffness of NF group, F-1 W group and F-2 W group were not statistically significant (Fig. 4).
Fig. 4.
Comparison of biomechanical properties of tendons in each group. (A) Specialized aluminum clamps and fixation of rat Achilles tendon. (B) Comparison of the peak load of tendons in each group. (C) Comparison of tendon stiffness in each group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Histological observation
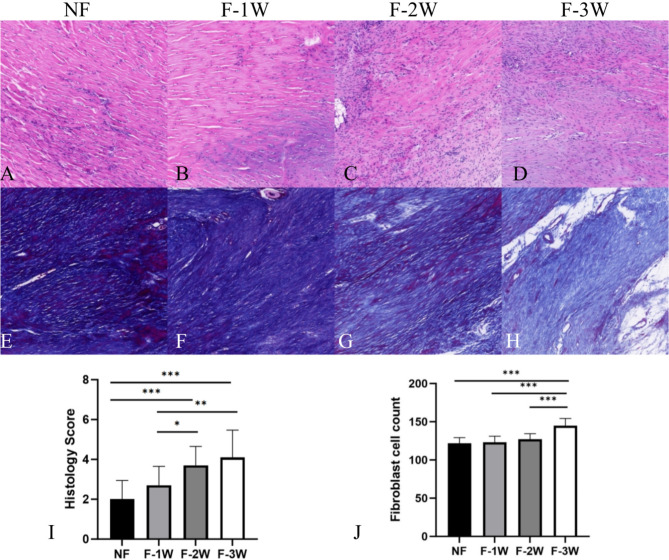
HE staining revealed that the regenerated collagen fibers in the F-3 W group exhibited lower density and organization compared to the other three groups. Furthermore, the F-3 W group displayed heightened vascularization and accumulation of inflammatory cells. Additionally, Masson’s trichrome staining indicated a decreased collagen fiber density in the F-3 W group relative to the other three groups. Image Pro Plus 6.0 image analysis software showed that the number of fibroblasts in the F-3 W group was significantly higher than in the other groups (P<0.001). Bonar score was used to quantitatively evaluate the quality of regenerated tissue, and the scores of NF and F-1 W groups were significantly lower than those of the other two groups (P < 0.05) (Fig. 5).
Fig. 5.
The HE and Masson staining results and quantitation. (A-D) HE staining microscopic observation of each group. (E-H) Masson staining microscopic observation of each group. (I) Comparison of the count of fibroblasts in each group. (J) Comparison of the histology score in each group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Immunohistochemistry
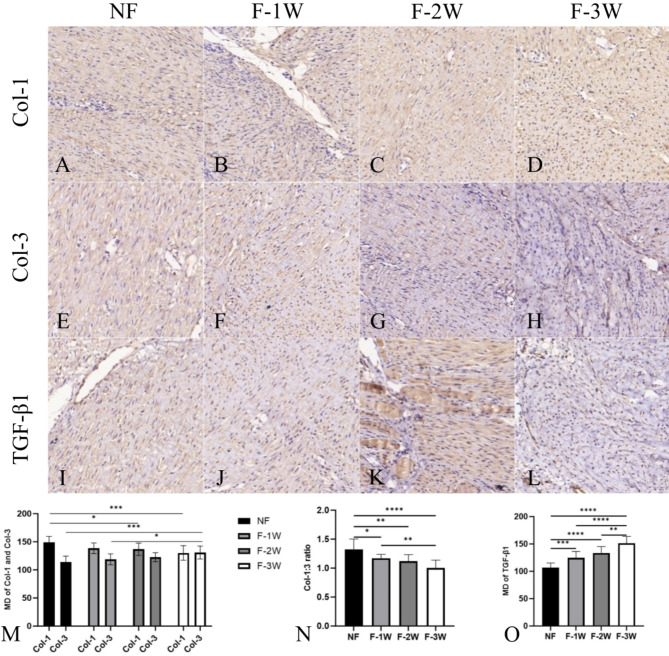
Col-1 expression in NF group was higher than that in F-2 W and F-3 W groups (P < 0.05), and Col-3 expression in F-3 W group was higher than that in NF and F-1 W groups (P < 0.05). The ratio of Col-1:3 in the NF group was higher than in the other three groups (P < 0.05), and the ratio of Col-1:3 in the F-1 W group was higher than in the F-3 W group (P < 0.05). The expression of TGF-β1 was lower in the NF group than in the other three groups, while the expression of TGF-β1 was higher in the F-3 W group than in the other three groups (P < 0.05). It is worth noting that there was no statistically significant difference in the immunohistochemical results between F-1 W and F-2 W (P > 0.05) (Fig. 6).
Fig. 6.
The immunohistochemical results and quantitation of Col-1, Col-3 and TGF-β1. (A-D) Light microscope images with immunohistochemical for Col-1 staining. (E-F) Light microscope images with immunohistochemical for Col-3 staining. (I-L) Light microscope images with immunohistochemical for TGF-β1 staining. (M) Quantitative analysis of Col-1 and Col-3 expression. (N) Comparison of Col-1:3 ratio in each group. (O) Quantitative analysis of TGF-β1 expression. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Discussion
This study investigated the effects of different time to return to activity on the tendon healing of a rat Achilles tendon injury model. Through various experimental methods, we found that as the time to return to activity advanced, the strength of the tendon increased while the degree of adhesion decreased. In clinical practice, it is more beneficial for patients to use strong suture to withstand earlier rehabilitation exercises.
Early functional exercise can elongate and relax the external connective tissue, reduce the contact between the anastomosis and the surrounding tissue, inhibit the growth of scar tissue, and prevent external adhesion19. Additionally, mechanical stress stimulation can promote cell proliferation and tendon differentiation, thereby enhancing the strength of tendon healing20. However, in a study by Godbout et al., immediate post-operative exercise appeared to result in a decrease in tendon mechanical properties21. In this study, although the duration of active movement varied among the groups of rats, this was done to ensure that all rats had the same four-week period for tendon healing. This is inevitable in the case that the immobilization time is the variable and the healing time is consistent, and it can be regarded as another manifestation of different immobilization times. Therefore, we controlled the treadmill training time of all rats at the fourth week after surgery. Through this method, we avoided the re-rupture of the Achilles tendon caused by early high-intensity exercise in some groups, thus ensuring the authenticity of the study results.
To safely remove the splint earlier, two measures need to be considered. The first is the strength of the tendon suture, and the second is the speed and quality of tendon healing. For suture strength, the most important factor is the number of sutures crossing the tendon ends, but excessive suturing can affect the blood supply of the tendon22,23. In this study, we used the double Kessler method to support early postoperative activity, and the number of stitches across the broken end is twice that of the traditional Kessler suture method. The incidence of tendon re-rupture under strong suture is much lower than that reported in clinic1,24. In addition, the method of sewing is also crucial. For patients, suture methods such as “Modified Lim”, “Tsai”, “Tsuge”, etc. may be able to withstand different intensity of rehabilitation exercises25,26. However, the influence of different suture methods on tendon healing and its strength still needs to be further studied.
In recent years, most of the research on improving the quality of tendon healing focuses on two directions. One approach is autologous transplantation, including bone marrow concentrate, platelet products, and fat, which utilizes the rich growth factors to promote tendon healing17,27,28. Another approach is local drug delivery, including Aspirin, Metformin, and even Sildenafil, to control inflammatory responses and improve the quality of tendon healing29–31. These methods have shown promising results in animal experiments, but they are currently less commonly used in clinical practice. We need to find a reliable measure among the many interventions that can enable most patients to safely and earlier return to activity, which will be our focus for further research.
In the clinical study of tendon rupture, the benefits of early activity are undeniable32,33. In a prospective randomized controlled trial conducted by Deng et al., early mobilization not only improved the early functional outcomes of the ankle joint, but also resulted in earlier hospital discharge and return to work for patients7. In a clinical study with over a decade of follow-up, the Leppilahti score of the early activity group was still higher than that of the control group in the late stage34. However, there is still controversy over whether early rehabilitation activities will increase the incidence of re-rupture. Despite a wealth of studies showing the benefits of early activity, a recent Meta-analysis still supports the idea that immediate activity after Achilles tendon repair may increase the risk of re-rupture14. This is also the reason for considering stronger suture methods in this study.
According to a study by Aoto Sato et al.35 on the chicken flexor tendon, immobilization for more than 3 weeks would result in irreversible adhesion of the tendon. In this study, all rats were subjected to treadmill exercise at the end of the fourth week. The passive ankle range of motion, count of fibroblasts, biomechanical results, and immunohistochemical results were not different between the F-1 W and F-2 W groups. It may be that functional exercise improves some of the results. Nevertheless, the experimental results of the F-3 W group were significantly different from those of the other three groups, reflecting not only that prolonged immobilization can affect tendon healing and increase adhesion, but also that such effects may require a longer rehabilitation exercise or even cause irreversible functional loss. Taking all these factors into consideration, we believe that tendon rupture should be combined with a strong suture to enable the patient to return to activity within two weeks after surgery.
Healing of tendon injury can be divided into three stages: inflammation, proliferation, and remodeling36. During the proliferative phase, the synthesis of Col-3 reaches its peak and constitutes the main component of the extracellular matrix37. However, the arrangement of Col-3 is disordered, its mechanical properties are poor, and it can inhibit the growth of collagen fiber diameter, which may be the reason for the decline in the biomechanical performance of tendon healing38. Therefore, the Col-1:3 ratio is an important indicator for judging the quality of healing. In this study, the difference in the ratio of Col1:3 between the groups was more significant than comparing Col-1 or Col-3 alone.
TGF-β1 is recognized as one of the most potent profibrogenic factors during the tendon healing process. For tendon injuries, changes in TGF-β1 exhibit a pattern of initial increase followed by decrease. Most studies support that TGF-β1 reaches its peak in 2 weeks and returns to normal around 4 weeks39,40. Additionally, it plays a multifunctional role in regulating all three stages of tendon healing41. During the inflammatory phase, activated platelets release cytokines, particularly TGF-β1, which rapidly recruit inflammatory cells to the injury site and accelerate angiogenesis in an autocrine or paracrine manner42,43. The proliferation stage is characterized by a significant increase in fibrotic scar tissue and peak cell numbers in the repair area44. TGF-β1 is closely associated with this phase as it strongly promotes fibrotic scar formation and controls various cell behaviors. During the remodeling stage, TGF-β1 can accelerate the remodeling process through collagen synthesis rather than degrading scar tissue45. In this study, the expression of TGF-β1 in F-3 W was significantly higher than that in other groups. This suggests that one of the mechanisms by which early return to activity improves tendon healing quality may be that TGF-β1 expression returns to normal more quickly. We boldly hypothesize that the sustained high expression of TGF-β1 in rats with long-term immobilization is a compensation for poor tendon healing, but it also promotes exogenous tendon healing, leading to adhesion around the tendon. This conclusion can provide insight for future studies on tendon healing mechanisms.
There are still several limitations in this study. First, this study only examined the tendon performance at 4 weeks after surgery, which means that the function of the tendon may still improve further with an extended follow-up period. Second, rats cannot develop a gradual rehabilitation program like humans do, taking into account that the contralateral limb may have some degree of compensation, even if they are trained on a treadmill with fixed exercises, which cannot guarantee that the weight-bearing and activity of the operated limb are consistent among different groups of rats. Finally, although the use of rat models to study tendon injury has become quite established, further clinical studies are needed to validate the accuracy of the results.
Conclusion
In the four groups of rats, the NF group had the best biomechanical performance and ankle passive range of motion, as well as the least degree of adhesion. Meanwhile, the healing quality of the tendons in the F-3 W group was significantly lower than that of the other three groups. This suggests that early return to activity under strong tendon sutures is more beneficial for improving patient outcomes. After a comprehensive observation of four groups of rats, it was found that as the time of return to activity was advanced, the tensile strength of the repaired tendon in rats increased and the degree of adhesion was reduced at 4 weeks postoperatively. These results indicate that it is necessary for patients with tendon injuries to explore safe and early methods of early return to activity.
Author contributions
LDK conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. JL, THG and CFL completed animal experiments and collected experimental data. XYS, MF and YPY conducted an initial analysis of the experimental results. YQZ and BZ coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. CFL wrote the first draft of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by Hebei Provincial Health Commission (funding number 20230676).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study has been approved by the Medical Ethical Committee of the Hebei Medical University Third Hospital and is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Consent for publication
The authors would like to declare that the study is original research that has not been published elsewhere and is not under consideration by another journal. All the authors have approved the enclosed manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lingde Kong and Bing Zhang contributed equally to this work.
References
- 1.Xue, R. et al. Current clinical opinion on surgical approaches and rehabilitation of hand flexor tendon injury-a questionnaire study. Front. Med. Technol.6, 1269861. 10.3389/fmedt.2024.1269861 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashjian, R. Z. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin. Sports Med.31, 589–604. 10.1016/j.csm.2012.07.001 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Alkhabbaz, O., Bibi, Y., Marikh, M. & Clearfield, D. A. Platelet releasate and extracorporeal shock Wave Therapy (ESWT) for treatment of a partial Supraspinatus tear in an adolescent baseball player. Cureus16, e61057. 10.7759/cureus.61057 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie, X. et al. Janus membranes Patch achieves high-quality Tendon Repair: inhibiting Exogenous Healing and promoting endogenous Healing. Nano Lett.24, 4300–4309. 10.1021/acs.nanolett.4c00818 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Deng, J. et al. Uni-directional release of ibuprofen from an asymmetric fibrous membrane enables effective peritendinous anti-adhesion. J. Controlled Release: Official J. Controlled Release Soc.10.1016/j.jconrel.2024.06.046 (2024). [DOI] [PubMed] [Google Scholar]
- 6.Liu, J. et al. Unidirectional gene delivery electrospun fibrous membrane via charge repulsion for tendon repair. Bioactive Mater.37, 191–205. 10.1016/j.bioactmat.2024.03.008 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng, Z. et al. Outcomes of early versus late functional weight-bearing after the acute Achilles tendon rupture repair with minimally invasive surgery: a randomized controlled trial. Arch. Orthop. Trauma Surg.143, 2047–2053. 10.1007/s00402-022-04535-w (2023). [DOI] [PubMed] [Google Scholar]
- 8.Won Lee, K., Bae, J. Y., Ho, B. C., Kim, J. H. & Seo, D. K. Immediate Weightbearing and Ankle Motion Exercise after Acute Achilles Tendon rupture repair. J. foot Ankle Surgery: Official Publication Am. Coll. Foot Ankle Surg.61, 604–608. 10.1053/j.jfas.2021.10.021 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Freedman, B. R. et al. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J. Orthop. Research: Official Publication Orthop. Res. Soc.34, 2172–2180. 10.1002/jor.23253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva Barreto, I. et al. Micro- and nanostructure specific X-ray tomography reveals less matrix formation and altered collagen organization following reduced loading during Achilles tendon healing. Acta Biomater.174, 245–257. 10.1016/j.actbio.2023.12.015 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Li, Z. et al. Dynamic tensile stress promotes regeneration of Achilles Tendon in a panda Rope Bridge technique mice Model. Ann. Biomed. Eng.51, 2735–2748. 10.1007/s10439-023-03320-z (2023). [DOI] [PubMed] [Google Scholar]
- 12.Yin, S. & Sun, X. Analysis of the effects of Early Rehabilitation Treatment conducted by nurses on the Prevention of Tendon Adhesion after Finger Flexor Tendon rupture: a Randomized Clinical Trial. Int. J. Clin. Pract.2022 (8284646). 10.1155/2022/8284646 (2022). [DOI] [PMC free article] [PubMed]
- 13.Abdolrazaghi, H., Ramin, M. & Molaei, H. Comparison the range of Motion following early Versus late active mobilization after repairing surgery on Flexor Tendon Injury in the zone II: a Randomized Clinical Trial. World J. Plast. Surg.12, 29–33. 10.52547/wjps.12.2.29 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, R. et al. Immediate mobilization after repair of Achilles tendon rupture may increase the incidence of re-rupture: a systematic review and meta-analysis of randomized controlled trials. Int. J. Surg. (London England). 110, 3888–3899. 10.1097/js9.0000000000001305 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, H. et al. Malvidin attenuates trauma-induced heterotopic ossification of tendon in rats by targeting Rheb for degradation via the ubiquitin-proteasome pathway. J. Cell. Mol. Med.28, e18349. 10.1111/jcmm.18349 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu, L. M. et al. Up-regulation of CREB-1 regulates tendon adhesion in the injury tendon healing through the CREB-1/TGF-β3 signaling pathway. BMC Musculoskelet. Disord.24, 325. 10.1186/s12891-023-06425-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oztermeli, A. & Okyay, M. F. An immunohistochemical and biomechanical evaluation of the effect of fat graft on tendon healing. J. Orthop. Surg.31, 10225536231220839. 10.1177/10225536231220839 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Genç, E., Yüksel, S., Çağlar, A., Beytemur, O. & Güleç, M. A. Comparison on effects of platelet-rich plasma versus autologous conditioned serum on Achilles tendon healing in a rat model. Acta Orthop. Traumatol. Turc.54, 438–444. 10.5152/j.aott.2020.18498 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent, D. et al. Relationship between tendon structure, stiffness, gait patterns and patient reported outcomes during the early stages of recovery after an Achilles tendon rupture. Sci. Rep.10, 20757. 10.1038/s41598-020-77691-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich-Zagonel, F., Aspenberg, P. & Eliasson, P. Dexamethasone enhances Achilles Tendon Healing in an animal Injury Model, and the effects are Dependent on Dose, Administration Time, and mechanical loading stimulation. Am. J. Sports Med.50, 1306–1316. 10.1177/03635465221077101 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godbout, C., Ang, O. & Frenette, J. Early voluntary exercise does not promote healing in a rat model of Achilles tendon injury. J. Appl. Physiol. (Bethesda Md. : 1985). 101, 1720–1726. 10.1152/japplphysiol.00301.2006 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Chang, Y. J., Duffy, D. J. & Moore, G. E. Investigation of the effects of two-, four-, six- and eight-strand suture repairs on the biomechanical properties of canine gastrocnemius tenorrhaphy constructs. Am. J. Vet. Res.82, 948–954. 10.2460/ajvr.20.11.0199 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Vlajcic, Z. et al. Biomechanical trial of modified flexor tendon sutures: an in vitro study. J. Plast. Surg. Hand Surg.46, 222–228. 10.3109/2000656x.2012.686916 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Choi, J. Y., Choo, S. K., Kim, B. H. & Suh, J. S. Conservative treatment outcome for Achilles tendon re-rupture occurring in the subacute phase after primary repair. Arch. Orthop. Trauma Surg.144, 1055–1063. 10.1007/s00402-023-05161-w (2024). [DOI] [PubMed] [Google Scholar]
- 25.Chang, M. K., Acharyya, S., Lim, Z. Y. & Tay, S. C. Clinical outcomes of Zone 2 Flexor Tendon repairs using the modified Lim/Tsai Technique. J. hand Surg. Asian-Pacific Volume. 24, 83–88. 10.1142/s2424835519500152 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Koehler, S. V., Sauerbier, M., Terzis, A. & Rupture Rate Functional Outcome and Patient Satisfaction after Primary Flexor Tendon Repair with the Modified 4-Strand Core Suture Technique by Tsuge and Using the Arthrex FiberLoop(®) with Early Motion Rehabilitation. J. Clin. Med.1010.3390/jcm10194538 (2021). [DOI] [PMC free article] [PubMed]
- 27.Gissi, C. et al. Extracellular vesicles from rat-bone-marrow mesenchymal stromal/stem cells improve tendon repair in rat Achilles tendon injury model in dose-dependent manner: a pilot study. PloS One. 15, e0229914. 10.1371/journal.pone.0229914 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu, T. Y. et al. Platelet-Rich plasma Releasate promotes early healing in Tendon after Acute Injury. Orthop. J. Sports Med.9, 2325967121990377. 10.1177/2325967121990377 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Y. et al. Aspirin inhibits inflammation and scar formation in the injury tendon healing through regulating JNK/STAT-3 signalling pathway. Cell Prolif.52, e12650. 10.1111/cpr.12650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurt, V., Guner, S., Kayacan, A. M. & Eronat, O. The effect of Sildenafil, a phosphodiesterase-5 inhibitor, on tendon healing: an experimental study in rat model of achilles tendon injury. Arch. Orthop. Trauma Surg.144, 1107–1115. 10.1007/s00402-023-05178-1 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Li, J. Metformin suppressed tendon injury-induced adhesion via hydrogel-nanoparticle sustained-release system. Int. J. Pharm.642, 123190. 10.1016/j.ijpharm.2023.123190 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Čretnik, A. & Košir, R. Prospective randomized comparison of functional bracing versus rigid immobilization with early weightbearing after modified percutaneous achilles tendon repair under local anesthesia. Foot (Edinburgh Scotland). 60, 102124. 10.1016/j.foot.2024.102124 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Hoeffner, R. et al. Tendon elongation and function after delayed or standard loading of surgically repaired Achilles Tendon ruptures: a Randomized Controlled Trial. Am. J. Sports Med.52, 1022–1031. 10.1177/03635465241227178 (2024). [DOI] [PubMed] [Google Scholar]
- 34.Lantto, I. et al. Early functional treatment versus cast immobilization in tension after achilles rupture repair: results of a prospective randomized trial with 10 or more years of follow-up. Am. J. Sports Med.43, 2302–2309. 10.1177/0363546515591267 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Satou, N., Yagi, M., Yoshida, K. & Shiba, N. Morphological changes in Flexor Tendon Adhesion following early Exercise after Tendon Repair. Kurume Med. J.67, 23–29. 10.2739/kurumemedj.MS671008 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Millar, N. L., Murrell, G. A. & McInnes, I. B. Inflammatory mechanisms in tendinopathy - towards translation. Nat. Rev. Rheumatol.13, 110–122. 10.1038/nrrheum.2016.213 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Almeida, L. H. et al. Acta Ortopedica Brasileira24, 11–15, doi:10.1590/1413-785220162401146706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, S. et al. Intratendon delivery of leukocyte-rich platelet-rich plasma at early stage promotes tendon repair in a rabbit Achilles tendinopathy model. J. Tissue Eng. Regen. Med.14, 452–463. 10.1002/term.3006 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Würgler-Hauri, C. C., Dourte, L. M., Baradet, T. C., Williams, G. R. & Soslowsky, L. J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J. Shoulder Elbow Surg.16, 198–203. 10.1016/j.jse.2007.04.003 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berglund, M., Reno, C., Hart, D. A. & Wiig, M. Patterns of mRNA expression for matrix molecules and growth factors in flexor tendon injury: differences in the regulation between tendon and tendon sheath. J. Hand. Surg.31, 1279–1287. 10.1016/j.jhsa.2006.06.011 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Klass, B. R., Rolfe, K. J. & Grobbelaar, A. O. In vitro flexor tendon cell response to TGF-beta1: a gene expression study. J. Hand. Surg.34, 495–503. 10.1016/j.jhsa.2008.10.032 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Graham, J. G., Wang, M. L., Rivlin, M. & Beredjiklian, P. K. Biologic and mechanical aspects of tendon fibrosis after injury and repair. Connect. Tissue Res.60, 10–20. 10.1080/03008207.2018.1512979 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Lipman, K., Wang, C., Ting, K., Soo, C. & Zheng, Z. Tendinopathy: injury, repair, and current exploration. Drug. Des. Devel. Ther.12, 591–603. 10.2147/dddt.S154660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connizzo, B. K. et al. The detrimental effects of systemic Ibuprofen delivery on tendon healing are time-dependent. Clin. Orthop. Relat. Res.472, 2433–2439. 10.1007/s11999-013-3258-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols, A. E. C., Best, K. T. & Loiselle, A. E. The cellular basis of fibrotic tendon healing: challenges and opportunities. Translational Research: J. Lab. Clin. Med.209, 156–168. 10.1016/j.trsl.2019.02.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.