Abstract
The fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) is a major phytophagous pest that invaded China in late 2018, posing a serious threat to local agricultural production. Therefore, we investigated the effects of maize, soybean, and sweet potato on the growth, development, and reproduction of S. frugiperda under laboratory conditions. The developmental period of the egg-larval stage was significantly longer when S. frugiperda fed on sweet potato (28.94 days) compared to maize (16.19 days) and soybean (17.82 days). Sweet potato feeding significantly prolonged the pupal period, but this effect was not observed in the adult stage. Spodoptera frugiperda larvae fed on sweet potato had the lowest pupal weight (116.18 mg) and pupation rate (68.19%). The mean fecundity of females significantly differed among the plants, with egg production being highest for insects fed on maize (996.17 eggs) and lowest for those fed on sweet potato (319.28 eggs). Spodoptera frugiperda fed on sweet potato exhibited the smallest net reproductive rate (47.892), lowest intrinsic rate of increase (0.083 day−1), lowest finite rate of increase (1.086 day−1), and longest mean generation time (46.806 days). Overall, S. frugiperda can survive and complete its entire life cycle on all three host plants.
Keywords: Fall armyworm, Life table, Host plant, Development, Reproduction
Subject terms: Population dynamics, Agroecology
Introduction
The fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) is a notorious agricultural pest native to tropical and subtropical regions of the Americas1,2. It is a highly polyphagous herbivore that reportedly can consume more than 350 plant species from 76 plant families, causing serious damage to economically important cultivated crops such as maize, wheat, rice, sorghum, cotton, tobacco, millet, beans, vegetables, and pasture grasses3–7. In early 2016, this invasive pest was officially reported for the first time in West and Central Africa8. Subsequently, it rapidly spread to more than 80 countries worldwide9. Unfortunately, by the end of 2018, S. frugiperda was discovered in Yunnan Province, China, and it rapidly expanded its range to other regions. By October 2021, the pest had invaded 27 provinces (municipalities and autonomous regions), such as Guangdong, Guangxi, Fujian, Sichuan, Guizhou, Shanxi, Gansu, Hainan, Hebei, and Taiwan10–13, damaging more than 1.32 million hectares and posing a grave threat to Chinese agricultural production and ecological security.
Based on host plant preferences, mating behavior, and pheromone compositions, S. frugiperda is classified into two morphologically identical strains: the corn strain and the rice strain14,15. As the names suggest, the corn strain prefers maize, sorghum, and cotton, whereas the rice strain prefers rice, millet, and grasses. According to previous studies, the S. frugiperda population that invaded China is likely a peculiar “corn strain” originating from the hybrid offspring of a corn strain male and a rice strain female16,17. Notably, the host plant range of this special “corn strain” likely expanded with its spread. It is widely known that differences in host plants can influence the development and population dynamics of phytophagous insects18. For instance, compared with S. frugiperda fed on maize and wheat, those fed on tomato and cotton had longer larval developmental stages, lower larval survival, and lower fecundity19. Similarly, the larval developmental duration and mean fecundity of S. frugiperda fed on pepper, tomato, and eggplant were significantly different from those fed on maize6. However, studies have found that S. frugiperda fed on various host plants can complete development and reproduction. Based on these findings, we speculate that this invasive pest is likely to cause significant economic losses among food and fiber crops. Therefore, analyzing the potential risk posed by S. frugiperda to different host plants, especially cultivated crops, is essential.
Maize, soybean, and sweet potato are important economic crops for grain, oil, and feed in China, and their collective planting area accounted for 35.59% of the country’s total agricultural crop sown area in 2022. Because of the overlap of the growing regions for these crops, pests that prefer different host plants can cause trans-boundary harm. For example, Spodoptera litura (Lepidoptera: Noctuidae), a major pest of soybean, has caused damage to maize20, and Ostrinia nubilalis (Lepidoptera: Pyralidae), a principal pest of maize, can also feed on soybean21. S. frugiperda is an invasive pest that prefers to feed on members of the grass family. However, whether this new corn strain of S. frugiperda is likely to cause trans-boundary damage to soybean and sweet potato is a key area of concern. Furthermore, the effects of soybean and sweet potato feeding on the biological characteristics of S. frugiperda, such as developmental duration, survival rate, pupation rate, emergence rate, and fecundity, are not yet fully understood in the Chinese populations.
In this study, we investigated the growth attributes, developmental cycle, and reproduction of S. frugiperda fed on soybean and sweet potato in comparison to maize under laboratory conditions. The age–stage, two-sex life table method was applied to obtain the life table parameters of S. frugiperda. Our results should help clarify the growth attributes and developmental cycle of S. frugiperda in its expanded range, facilitating the implementation of effective management programs for this invasive pest.
Materials and methods
Host plants
Seeds of maize (variety: Jingnuo 10) and soybean (cultivar: Zhonghuang 57) were purchased from the market. Sweet potato seedlings (cultivar: 19 − 7) were donated by the Institute of Biotechnology, Guizhou Academy of Agricultural Sciences. The three host plants were planted in the experimental field of Kaili University without pesticide exposure. Healthy and undamaged plant leaves were collected for testing.
Insect rearing
The initial population of S. frugiperda larvae was obtained from maize fields at Gechong Village, Kaili City, Guizhou Province, China, in June 2022. The larvae were transferred to an artificial climate incubator at 25℃ ± 1℃ and 70% ± 5% relative humidity (RH) under a 16-h/8-h light/dark photoperiod in the laboratory6,12. To maintain culturing conditions, the insects were reared on each of the aforementioned plants for four consecutive generations. The fifth-generation eggs were then used to perform the following experiments.
Life table study
At least 200 eggs laid within 6 h were collected from each plant and placed on a new leaf in each plastic Petri dish (8 × 1.5 cm2). After hatching, the neonates were randomly selected and transferred individually to a 12-hole hyaline culture plate (12.5 × 8.5 × 2.3 cm3; single hole: 2.3 × 1.7 cm2). Two 12-hole hyaline culture plates with fresh leaves were prepared for each plant, and five replications were performed for each plant. Culture plates with fresh maize, soybean, and sweet potato leaves were replaced every 24 h to avoid contamination by microorganisms. Until the third instar, individual larvae were transferred from the culture plates to a 500-mL plastic cup and provided fresh leaves daily to ensure adequate nutrition. The incubation times of the eggs, developmental periods, and survival rates of larvae were recorded daily. Furthermore, we quantified the pupal weight on the second day after pupation. Following the eclosion of S. frugiperda, one female and one male reared on the same host were paired in a 500-mL plastic cup lined with fresh leaves of the same host for oviposition. All adults were fed a 10% (v/v) honey solution. Subsequently, parameters such as adult lifespan, number of eggs laid per female, pre-oviposition period, and egg-laying period were recorded. The aforementioned experiments were conducted under constant conditions of 25℃ ± 1℃, 70% ± 5% RH, and a 16-h/8-h photoperiod.
Life table data analysis
Based on the age–stage, two-sex life table principle22and the method described by Chi23, the life history raw data of all individuals were recorded and pooled, and the computer program TWOSEX-MSChart24,25 was used to calculate all life table statistics. Thus, we obtained population and life table parameters for each experiment, including the age–stage-specific fecundity (fxj, x is age and j is stage); age-specific survival rate (lx); age–stage-specific survival rate (sxj); age-specific fecundity (mx); age–stage-specific life expectancy (exj); age–stage-specific reproductive value (vxj); adult pre-oviposition period of female adults (APOP); total pre-oviposition period of females counted from birth (TPOP); oviposition period (OP); and the net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T).
Statistical analysis
All experimental raw data were recorded using Microsoft Excel 2007 (Microsoft, Redmond, WA, USA). Life table parameters were analyzed using TWOSEX-MSChart, and means and standard errors (SEs) for all parameters were estimated using the bootstrap method with 100,000 replicates. Differences among the various treatments were assessed using the paired bootstrap test at a significance level of P < 0.05. Pupal weight and pupation rate were tested via one-way analysis of variance using SPSS 13.0 (IBM Inc., Armonk, NY, USA), with significant differences determined using the Duncan’s multiple range test. Graphs were generated using OriginPro 2023 (OriginLab Corp., Northampton, MA, USA).
Results
Effect of different hosts on the developmental period of S. frugiperda
The effects of the three tested plants on the developmental period of S. frugiperda are presented in Table 1. S. frugiperda completed its life cycle on all tested plants. The egg stage was longest on sweet potato (2.58 days, P < 0.05). The first–sixth instar larvae that fed on sweet potato had a longer developmental duration than those fed on maize and soybean (both P < 0.05). In total, the developmental period of the egg-larval stage was shortest on maize (16.19 days) and longest on sweet potato (28.94 days), indicating that S. frugiperda had significantly reduced development on maize. The prepupal period was significantly shorter for S. frugiperda fed on maize (1.81 days) and soybean (1.55 days) than for those fed on sweet potato (2.22 days, both P < 0.05). The pupal stage was 1.95 days longer for sweet potato feeding compared to maize and 1.8 days longer compared to soybean feeding. The pre-adult stage was significantly longer for insects fed on sweet potato than for those fed on maize and soybean (both P < 0.05). There were no significant differences in the adult stage among the tested plants.
Table 1.
Developmental period and total longevity of S. frugiperda fed on three tested plants.
| Parameters | n | Maize | n | Soybean | n | Sweet potato |
|---|---|---|---|---|---|---|
| Egg stage (days) | 120 | 2.34 ± 0.04b | 120 | 2.38 ± 0.05b | 120 | 2.58 ± 0.05a |
| First instar (days) | 118 | 1.95 ± 0.07c | 97 | 2.37 ± 0.08b | 120 | 4.58 ± 0.09a |
| Second instar (days) | 111 | 1.70 ± 0.06b | 87 | 1.97 ± 0.06b | 120 | 3.38 ± 0.07a |
| Third instar (days) | 107 | 1.53 ± 0.05b | 86 | 1.80 ± 0.07b | 120 | 2.57 ± 0.05a |
| Fourth instar (days) | 103 | 2.10 ± 0.06b | 84 | 2.11 ± 0.07b | 119 | 3.14 ± 0.08a |
| Fifth instar (days) | 101 | 2.86 ± 0.07b | 81 | 2.74 ± 0.08b | 103 | 4.82 ± 0.08a |
| Sixth instar (days) | 100 | 4.36 ± 0.10b | 79 | 4.30 ± 0.10b | 68 | 7.72 ± 0.14a |
| Egg-larva (days) | 100 | 16.19 ± 0.19b | 79 | 17.82 ± 0.19b | 68 | 28.94 ± 0.26a |
| Prepupa (days) | 88 | 1.81 ± 0.08b | 60 | 1.55 ± 0.07b | 46 | 2.22 ± 0.06a |
| Pupa (days) | 73 | 8.56 ± 0.13b | 48 | 8.71 ± 0.13b | 37 | 10.51 ± 0.14a |
| Pre-adult (days) | 73 | 27.21 ± 0.29b | 48 | 27.90 ± 0.29b | 37 | 41.27 ± 0.44a |
| Adult (days) | 73 | 8.16 ± 0.23a | 48 | 8.62 ± 0.27a | 37 | 8.03 ± 0.33a |
| Total longevity (days) | 73 | 35.37 ± 0.38b | 48 | 36.52 ± 0.43b | 37 | 49.30 ± 0.54a |
Note: Values (mean ± SE) followed by different lowercase letters within the same row were significantly different at P < 0.05 when analyzed using the paired bootstrap test.
Effect of different hosts on the pupal weight and pupation rate of S. frugiperda
The pupal weight and pupation rate of S. frugiperda fed on three host plants are presented in Fig. 1. In particular, the pupal weight of S. frugiperda fed on sweet potato was 116.18 mg, which was significantly lower than that of insects fed on maize (159.14 mg) and soybean (142.49 mg; both P < 0.05). The pupation rate of S. frugiperda fed on maize was significantly higher than that of insects fed on soybean and sweet potato (both P < 0.05).
Fig. 1.
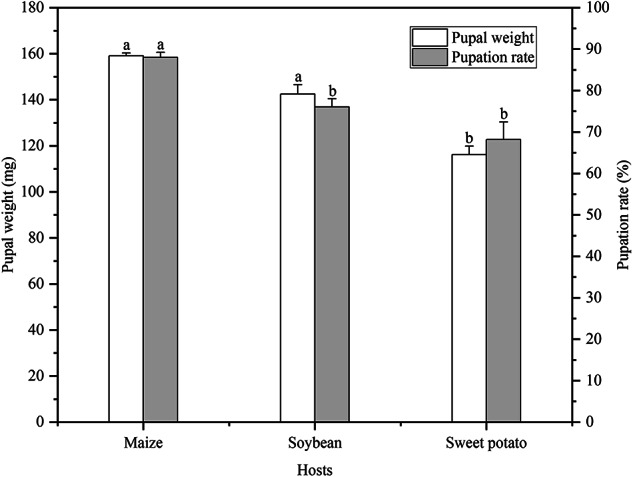
Effect of maize, soybean, and sweet potato feeding on the pupal weight and pupation rate of S. frugiperda. The data are presented as the mean ± SE. Different lowercase letters above the error bars indicate a significant difference by Duncan’s multiple range test (P < 0.05).
Effect of different hosts on the reproductive parameters of S. frugiperda
The reproductive parameters of S. frugiperda varied depending on the host plants (Table 2). APOP for S. frugiperda females fed on maize was 1.73 days, which was significantly shorter than that recorded on soybean (P < 0.05) but similar to that recorded on sweet potato. TPOP for S. frugiperda females fed on sweet potato was 43.33 days, which was 14.38 and 12.68 days longer than that for insects fed on maize and soybean, respectively. No significant differences were observed in OP (P > 0.05). The mean fecundity was highest on maize (996.17 eggs), followed by soybean (671.96 eggs, P < 0.05) and sweet potato (319.28 eggs, P < 0.05).
Table 2.
Reproductive parameters of S. frugiperda fed on three plants.
| Parameters | n | Maize | n | Soybean | n | Sweet potato |
|---|---|---|---|---|---|---|
| Pre-oviposition | 40 | 1.73 ± 0.09b | 26 | 2.35 ± 0.12a | 18 | 2.22 ± 0.17ab |
| Total pre-oviposition | 40 | 28.95 ± 0.42b | 26 | 30.65 ± 0.44b | 18 | 43.33 ± 0.64a |
| Oviposition period (days) | 40 | 7.00 ± 0.19a | 26 | 7.00 ± 0.18a | 18 | 6.06 ± 0.15a |
| Fecundity (eggs/female) | 40 | 996.17 ± 15.96a | 26 | 671.96 ± 16.93b | 18 | 319.28 ± 10.84c |
Note: Values (mean ± SE) followed by different lowercase letters within the same row were significantly different at P < 0.05 when analyzed using the paired bootstrap test.
Effect of different hosts on the survival rate of S. frugiperda
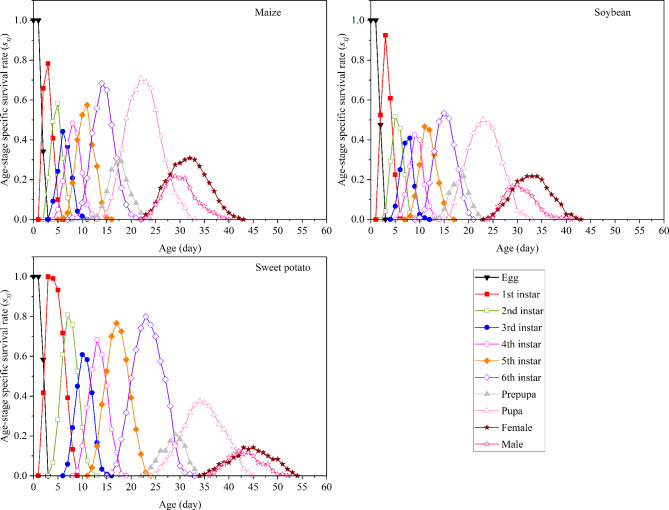
sxj indicates the probability that a newborn individual will survive to age x and stage j (Fig. 2). sxj of S. frugiperda eggs exceeded 0.90 on all three tested host plants. From egg development to sixth instar larvae, sxj was highest for S. frugiperda fed on sweet potato (0.80), exceeding the values for insects fed on maize and soybean by 0.1167 and 0.2667, respectively. sxj of pupa was considerably higher on maize (0.7083) than on soybean (0.50) and sweet potato (0.3750). For the adult stage, sxj of females and males fed on maize (0.3083 and 0.2167, respectively) were higher than those of insects fed on soybean (0.2167 and 0.1750, respectively) and sweet potato (0.1417 and 0.1250, respectively).
Fig. 2.
Age-stage specific survival rate (sxj) of S. frugiperda fed on three plants.
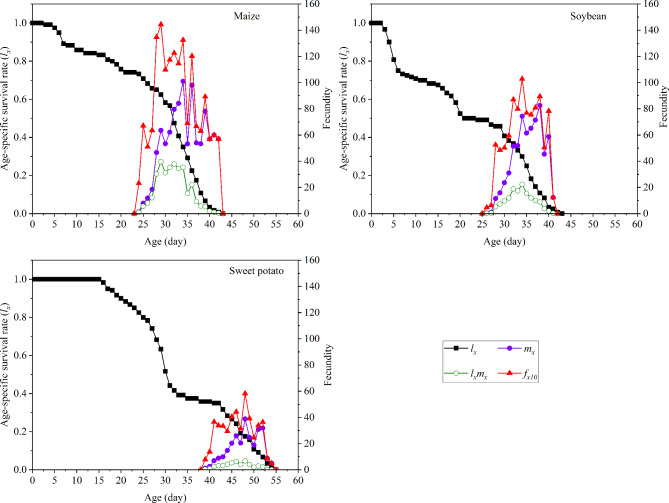
The lx curves of S. frugiperda fed on different host plants exhibited a downward trend over time (Fig. 3). The lx curve of S. frugiperda fed on maize slowly decreased, reaching 0.70 by day 25. The lx curve of S. frugiperda fed on soybean rapidly decreased, and the survival rate decreased by > 0.3 between days 3 and 10. Surprisingly, the lx curve for S. frugiperda fed on sweet potato declined rapidly starting on day 16.
Fig. 3.
Age-specific survival rate (lx) and fecundity of S. frugiperda fed on three plants.
Effect of different hosts on the fecundity of S. frugiperda
The larval diet significantly affected fx10, mx, and population age-specific maternity (lxmx) in S. frugiperda (Fig. 3). In general, fx10, mx, and lxmx of S. frugiperda fed on all three tested host plants increased initially before decreasing. fx10 of females fed on maize, soybean, and sweet potato peaked on days 29, 34, and 48, respectively, with mean fecundities of 144.36, 102.81, and 58.21 eggs, respectively, for insects fed on these plants.
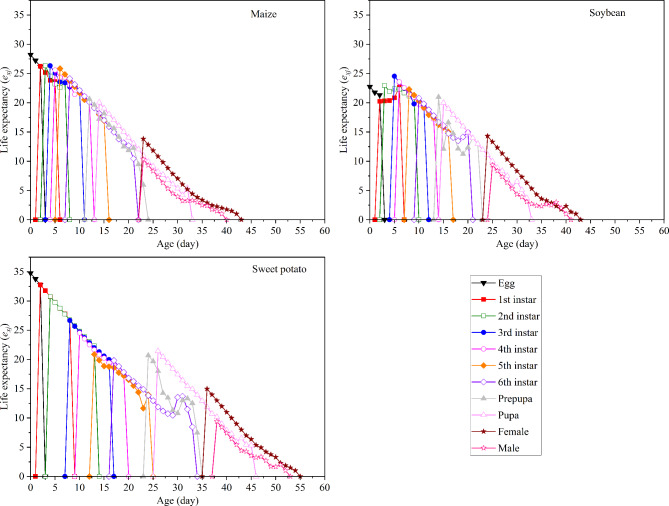
Effect of different hosts on the life expectancy of S. frugiperda
The exj of S. frugiperda fed on three host plants is presented in Fig. 4. The results indicated that the e01 values for S. frugiperda fed on maize, soybean, and sweet potato were 28.22, 22.75, and 34.77 days, respectively. Further, exj declined over time for insects fed on all three plants (Fig. 4).
Fig. 4.
Age-stage specific life expectancy (exj) of S. frugiperda fed on three host plants.
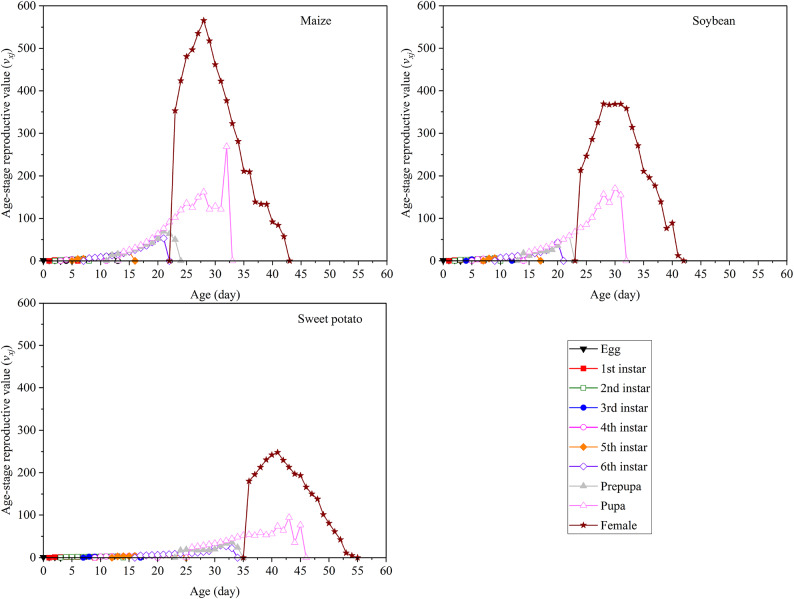
Effect of different hosts on reproduction in S. frugiperda
The different host plants markedly affected vxj in S. frugiperda (Fig. 5). At age zero (v01), the vxj values of S. frugiperda fed on maize, soybean, and sweet potato were 1.20, 1.16, and 1.09, respectively. vxj gradually increased with successive developmental stages, peaking after the adults laid eggs. The highest reproductive values for S. frugiperda fed on maize, soybean, and sweet potato were observed at 28, 31, and 41 days, reaching 565.6, 368.6, and 248.2 eggs, respectively.
Fig. 5.
Age-stage specific reproductive value (vxj) of S. frugiperda fed on three plants.
Effect of different hosts on the life table parameters of S. frugiperda
Significant differences were observed among the life table parameters of S. frugiperda fed on the three host plants (Table 3). R0 of S. frugiperda fed on maize (332.058) was significantly higher than that of insects fed on soybean (145.592) and sweet potato (47.892, both P < 0.05). The differences in r and λ were significant for S. frugiperda fed on the three tested host plants (P < 0.05). More importantly, r and λ were > 0 and > 1, respectively, indicating that S. frugiperda could survive on all three host plants. Conversely, T was highest for S. frugiperda fed on sweet potato (46.806 days), followed by soybean (33.958 days) and maize (31.766 days).
Table 3.
Life table parameters of S. frugiperda fed on different host plants.
| Hosts | Parameters | |||
|---|---|---|---|---|
| Net reproductive rate (R0) | Intrinsic rate of increase (r, day−1) | Finite rate of increase (λ, day−1) | Mean generation time (T, day) | |
| Maize | 332.058 ± 43.273a | 0.183 ± 0.005a | 1.201 ± 0.006a | 31.766 ± 0.399b |
| Soybean | 145.592 ± 25.518b | 0.147 ± 0.006b | 1.158 ± 0.007b | 33.958 ± 0.426b |
| Sweet potato | 47.892 ± 10.560c | 0.083 ± 0.005c | 1.086 ± 0.006c | 46.806 ± 0.647a |
Note: Values (mean ± SE) followed by different lowercase letters within the same column were significantly different at P < 0.05 when analyzed using the paired bootstrap test.
Discussion
It is known that phytophagous insects prefer the most suitable host plants for feeding. The primary reason is that a preferred host plant can best meet the water and nutritional requirements necessary for insect growth and development18,26. Hence, biological indicators of insects, such as a shorter developmental period, higher survival rate, and greater reproductive capacity, are often used to measure the adaptability of insects to host plants27. For example, Guo et al.28 reported that S. frugiperda larvae fed on maize exhibited a shorter larval developmental time, higher survival rate, greater longevity, and a higher number of eggs per female than those fed on potato and tobacco. Zhang et al.29 studied the feeding adaptability of S. frugiperda on different maize varieties (three special and three common varieties) and found significant differences in larval developmental duration, pupal weight, and fecundity among the different maize varieties, with S. frugiperdafed on common maize varieties showing better adaptability. According to previous studies28,29, the adaptability of invasive pests differs both among various host plants and among different varieties of the same host plants. In this study, we empirically verified the effects of different host plants on S. frugiperda development. The results clearly indicated that S. frugiperda could complete its developmental cycle on maize, soybean, and sweet potato; however, the three tested plants significantly affected larval development. The duration of the larval stage was shortest and the survival rate highest when S. frugiperda fed on maize. Conversely, when the pest fed on soybean, they experienced a prolonged larval period and a lower survival rate. These results align with previously reported findings of a longer larval development time and lower survival rate for S. frugiperdafed on soybean compared to maize4,13,19,30,31. During the test, we found also that soybean leaves with thinner mesophyll lose water faster than maize and sweet potato leaves with thicker mesophyll, which might be responsible for the lower survival rate on soybean plants. On the other hand, The differences in the developmental duration and survival rate of S. frugiperda may be attributed to the chemical characteristics of plant species26. Generally, the extension of the larval stage and increased mortality result from reduced feed intake due to one or more inhibitors in host plants, nutritional inadequacies of the host plants32, or the production of defense metabolites that impair the growth and development of insects33,34.
The characteristics in the pupal stage reflect the fitness of larvae to a specific host plant. Pupal weight and pupation rate are critical indicators of an insect’s physiological condition and serve as the mirror for its overall nutritional status and health during the larval stage35,36. In the present study, we found that both pupal weight and pupation rate were lower for larvae fed on sweet potato than for those fed on maize and soybean. Our results were consistent with those reported by Silva et al.31 and Chen et al.4. A plausible explanation for these findings may be the reduced intake of host plants by the larvae, leading to less efficient food assimilation and, consequently, lower pupal weight and pupation rate37,38. Furthermore, pupal weight is also an important predictor of an insect’s ability to withstand environmental stressors and complete its development successfully. Heavier pupae, with their greater energy reserves, are generally more likely to survive to adulthood and have a higher probability of successful mating and oviposition39,40.
The host plant can affect the growth and development of herbivorous insects as well as the reproductive capacity of adult insects26,31–43. In the present study, the OP did not significantly differ among insects fed on maize, sweet potato, and soybean, which contradicts the findings of Xu et al.44. However, S. frugiperda larvae fed on sweet potato and soybean laid significantly fewer eggs per female than those fed on maize. Similarly, a previous study found that S. frugiperda fed on wheat and barley had lower fecundity than insects fed on maize and faba beans45. Furthermore, a positive correlation was observed between pupal weight and fecundity in S. frugiperda, as also found in Mythimna separata (Lepidoptera: Noctuidae) and Spodoptera littoralis (Lepidoptera: Noctuidae)46,47.
The age–stage, two-sex life table effectively evaluates the fitness of insect populations and can be used to predict the characteristics of pest populations23,48. Life table parameters such as R0, r, λ, and T are important indicators of the effects of host plants on insect population performance49, and these variables differ according to the host plant6,50,51. In this study, we found that r was lowest for S. frugiperda fed on sweet potato (0.083 day−1), followed by soybean (0.147 day−1) and maize (0.183 day−1). The trend for λ was similar to that for r. Based on life table theory, r > 0 and λ> 1 indicate that the host plant is suitable for the growth and development of insects52, as clearly supported by our results. R0 is an equally vital index of population growth51. Our results indicated that R0 was significantly higher for S. frugiperda fed on maize (332.058) than for insects fed on soybean (145.592) and sweet potato (47.892), suggesting that the three plant species were vulnerable to S. frugiperda damage.
Our results demonstrated that maize was more suitable for the growth, survival, and reproduction of S. frugiperda than soybean and sweet potato. However, when S. frugiperda larvae become excessively dense or their preferred hosts are scarce or undernourished in the field, the pests may move to other host plants, such as soybean and sweet potato2,53. In China, the maize–soybean/sweet potato intercropping system is widely adopted, allowing S. frugiperda larvae to transfer from maize to soybean or sweet potato, thereby damaging all of these crops. Therefore, it is necessary to strongly monitor the population dynamics of S. frugiperda in the field. Certainly, we know well that monitoring alone is insufficient to address the potential threat posed by this pest. Based on our study’s results and the current understanding of S. frugiperda’s biology and ecology, we propose the following integrated pest management (IPM) strategies to complement regular monitoring. First, utilize or breed crop varieties that exhibit resistance to S. frugiperda. Second, implement crop rotation and diversification practices to disrupt the pest’s life cycle and reduce its population density. Third, enhance the use of natural enemies, such as parasitoids, predators, and pathogens, which can help keep S. frugiperda populations in check. Fourth, when necessary, apply selective pesticides with caution to reduce the damage of this pest. Finally, continue research into the pest’s behavior, host range, and management options, including the development of novel control methods and the improvement of existing ones.
Conclusion
This study investigated the effects of three host plants—maize, soybean, and sweet potato—on the growth, survival, and reproduction of S. frugiperda. S. frugiperda was able to survive and complete its entire life cycle on all three plants, although the performance of insect populations varied significantly among the hosts. Based on the results obtained from the experiments, we concluded that S. frugiperda is likely to cause harm to alternative plants, such as soybean and sweet potato, in the field.
Author contributions
Z.W. and D.C.J. conceived and designed the experiments. Z.W., H.Z., J.T.H., and X.L. performed the experiments. Z.W. and D.C.J. analyzed the data and wrote the paper. All authors have read and approved the manuscript for publication.
Funding
This study was funded by the Training Project of High-Level Innovative Talent in Guizhou Province (QTT[2023]202004), the Qiandongnan Basic Research Planning Project (QKHB[2021]07), the Quality Improvement Project of the University from the Guizhou Education Department (202227), the Planning Research Topics of Kaili University (KUKT[2023]5), and the Innovative Talent Team Program from the Guizhou Education Department (201326).
Data availability
The data presented in this study are availability on request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Tables.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sparks, A. N. A review of the biology of the fall armyworm. Fla. Entomol.62, 82–87 (1979). [Google Scholar]
- 2.Tay, W. T., Meagher, R. L. Jr, Czepak, C. & Groot, A. T. Spodoptera frugiperda: ecology, evolution, and management options of an invasive species. Annu. Rev. Entomol.68, 299–317 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Montezano, D. G. et al. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol.26, 286–300 (2018). [Google Scholar]
- 4.Chen, Y. et al. Performance of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on six host plants: potential risks to mid-high latitude crops in China. J. Agr Sci.12, 16–27 (2020). [Google Scholar]
- 5.Wan, J. et al. Biology, invasion and management of the agricultural invader: fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agr. 20, 646–663 (2021). [Google Scholar]
- 6.Wu, L. H. et al. Fitness of fall armyworm, Spodoptera frugiperda to three solanaceous vegetables. J. Integr. Agr. 20, 755–763 (2021). [Google Scholar]
- 7.Nandhini, D., Deshmukh, S. S., Satish, K. M., Kalleshwaraswamy, C. M. & Sannathimmappa, H. G. Host plant feeding and ovipositional preferences of Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions. J. Entomol. Sci.59, 133–141 (2024). [Google Scholar]
- 8.Goergen, G., Kumar, P. L., Sankung, S. B., Togola, A. & Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One. 11, e0165632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyckhuys, K. A. G. et al. Global scientific progress and shortfalls in biological control of the fall armyworm Spodoptera frugiperda. Biol. Control. 191, 105460 (2024). [Google Scholar]
- 10.Jiang, Y. Y. et al. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant. Prot.45, 10–19 (2019). [Google Scholar]
- 11.Chen, W. H., Itza, B., Kafle, L. & Chang, T. Y. Life table study of fall armyworm (Spodoptera frugiperda) (Lepidoptera: Noctuidae) on three host plants under laboratory conditions. Insects14, 329 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, Z., Zhu, H., Huang, J., Jin, D. C. & Yang, H. Feeding preference and fitness of Spodoptera frugiperda on rice and its relationship with biochemical substance contents of leaves. China Plant. Prot.49, 21–28 (2023). (in Chinese). [Google Scholar]
- 13.Tao, W. C. et al. Effects of the host plants of the maize-based intercropping systems on the growth, development and preference of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects15, 26 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashley, D. P. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex? Ann. Entomol. Soc. Am.79, 898–904 (1986). [Google Scholar]
- 15.Dumas, P. et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant varitwos: Two host strains or two distinct species? Genetica143, 305–316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juarez, M. L. et al. Population structure of Spodoptera frugiperda maize and rice host forms in South America: are they host strains? Entomol. Exp. Appl.152, 182–199 (2014). [Google Scholar]
- 17.Zhang, L. et al. Molecular characterization analysis of fall armyworm populations in China. China Plant. Prot.45, 20–27 (2019). (in Chinese). [Google Scholar]
- 18.Wetzel, W. C., Kharouba, H. M., Robinson, M., Holyoak, M. & Karban, R. Variability in plant nutrients reduces insect herbivore performance. Nature539, 425–427 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Wang, W. W. et al. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects11, 639 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punithivali, M., Sharma, A. & Rajkumar, B. M. Seasonality of the common cutworm, Spodoptera litura in a soybean ecosystem. Phytoparasitica42, 213–222 (2014). [Google Scholar]
- 21.Ivas, A. & Muresanu, F. Researches on the monitoring of the most frequent pests from maize and soybean crops in the conditions at ARDS Turda. Agriculture70, 265–272 (2013). [Google Scholar]
- 22.Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985). [Google Scholar]
- 23.Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol.17, 26–34 (1988). [Google Scholar]
- 24.Chi, H. et al. TWOSEX-MSChart: the key tool for life table research and education. Entomol. Gen.42, 845–849 (2022). [Google Scholar]
- 25.Chi, H. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. (2023). http://140.120.197.174/Ecology/download/TWOSEX-MSChart.rar
- 26.Awmack, C. S. & Leather, S. R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol.47, 817–844 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Moreau, J., Benrey, B. & Thiéry, D. Grape variety affects larval performance and also female reproductive performance of the European grapevine moth lobesia botrana (Lepidoptera: Tortricidae). B Entomol. Res.96, 205–212 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Guo, J. F. et al. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: potential risks to potato and tobacco crops. Insect Sci.28, 602–610 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Zhang, Q. Y. et al. Oviposition preference and age-stage, two-sex life table analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different maize varieties. Insects14, 413 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias, A. S. et al. Bioecology of Spodoptera frugiperda (Smith, 1757) in different cover crops. Biosci. J.32, 337–345 (2016). [Google Scholar]
- 31.da Silva, D. M. et al. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Sci. Agr. 74, 18–31 (2017). [Google Scholar]
- 32.Peruca, R. D. et al. Impacts of soybean-induced defenses on Spodoptera frugiperda (Lepidoptera: Noctuidae) development. Arthropod-Plant Inte. 12, 257–266 (2018). [Google Scholar]
- 33.Isman, M. Insect antifeedants. Pestic Outlook. 13, 152–157 (2002). [Google Scholar]
- 34.Schoonhoven, L. M., van Loon, J. J. A. & Dicke, M. Insect-plant Biology (Oxford University Press, 2005). [Google Scholar]
- 35.Eduardo, M. S. et al. Pupal emergence pattern in cactophilic Drosophila and the effect of host plants. Insect Sci.25, 1108–1118 (2018). [DOI] [PubMed] [Google Scholar]
- 36.He, L. M. et al. Larval diet affects development and reproduction of east Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agr. 20, 736–744 (2021). [Google Scholar]
- 37.Slansky, J. & Scriber, J. Food consumption and utilization in Comprehensive Insect Physiology, Biochemistry, and Pharmacology (ed Kerkut, G. A.) (1985). & Gilbert, L. I.) 87–163 (Pergamon. [Google Scholar]
- 38.Giongo, A. M. M. V., Freitas, J. D., Freitas, S. D. L. & Silva, M. F. G. F. Growth and nutritional physiology of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on Meliaceae fractions. Rev. Colomb Entomol.41, 33–40 (2015). [Google Scholar]
- 39.Wu, K. J. & Li, M. H. Nutritional ecology of the cotton bollworm, Heliothis armigera (Hübner): life tables of the population on the artificial diets with different protein levels. Acta Entomol. Sin. 36, 21–28 (1993). (in Chinese). [Google Scholar]
- 40.Soto, E. M. et al. Pupal emergence pattern in cactophilic Drosophila and the effect of host plants. Insect Sci.25, 1108–1118 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Jabraeil, R., Bahram, N. & Seyed, A. H. Comparative performance of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on various host plants. J. Pest Sci.87, 29–37 (2014). [Google Scholar]
- 42.Blair, W. C. et al. Does host plant quality constrain the performance of the Parthenium beetle Zygogramma Bicolorata? Biol. Control. 139, 104078 (2019). [Google Scholar]
- 43.Gong, Z. J. et al. Life table study of Spodoptera frugiperda at different wheat stages and the effect of larval population density on wheat yield. Pest Manag Sci.79, 4057–4065 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Xu, L. N. et al. Effects of maize-soybean intercropping on the growth, development and reproduction of fall armyworm Spodoptera frugiperda. J. Plant. Prot.50, 642–650 (2023). (in Chinese). [Google Scholar]
- 45.Gebretsadik, K. G. et al. Population growth of fall armyworm, Spodoptera frugiperda fed on cereal and pulse host plants cultivated in Yunnan Province, China. Plants12, 950 (2023). [DOI] [PMC free article] [PubMed]
- 46.Huang, Q. et al. A comparative study on growth, development and reproduction of Mythimna Separata in four host plants. China Plant. Prot.38, 5–10 (2018). (in Chinese). [Google Scholar]
- 47.Hemmati, S. A., Shishehbor, P. & Stelinski, L. L. Life table parameters and digestive enzyme activity of Spodoptera Littoralis (Boisd) (Lepidoptera: Noctuidae) on selected legume cultivars. Insects13, 661 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi, M. Z., Fu, J. W., Li, J. Y., Chi, H. & You, M. S. Projection of insect population dynamics with age-stage, two-sex life table and its application in pest management. Acta Entomol. Sin. 66, 255–266 (2023). (in Chinese). [Google Scholar]
- 49.Atlihan, R., Kasap, İ., Özgökçe, M. S., Polat-Akköprü, E. & Chi, H. Population growth of Dysaphis pyri (Hemiptera: Aphididae) on different pear cultivars with discussion on curve fitting in life table studies. J. Econ. Entomol.110, 1890–1898 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Alami, S., Naseri, B., Golizadeh, A. & Razmjou, J. Age-stage, two-sex life table of the tomato looper, Chrysodeixis chalcites (Lepidoptera: Noctuidae), on different bean cultivars. Arthropod-Plant Inte. 8, 475–484 (2014). [Google Scholar]
- 51.Ahmed, M. A. et al. Oviposition preference and two-sex life table of Plutella xylostella and its association with defensive enzymes in three Brassicaceae crops. Crop Prot.151, 105816 (2022). [Google Scholar]
- 52.Chi, H., Fu, J. W. & You, M. S. Age-stage, two-sex life table and its application in population ecology and integrated pest management. Acta Entomol. Sin. 62, 255–262 (2019). (in Chinese). [Google Scholar]
- 53.Bai, Y. W., Li, X. S., La, B. P. C. & Sun, L. J. Risk warning of fall armyworm invading soybean in China. J. Plant. Prot.47, 729–734 (2020). (in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are availability on request from the corresponding author.