Abstract
Transcription factors (TFs), including steroidogenic factor-1 (SF-1), T-box transcription factor (TPIT) and pituitary transcription factor–1 (PIT-1), play a pivotal role in the cytodifferentiation of adenohypophysis. However, the impact of TFs on the growth patterns of nonfunctioning pituitary adenomas (NFPAs) remains unclear. This study aims to investigate the correlation between the expression of TFs and NFPAs growth patterns. Preoperative MRI in 171 patients who underwent surgery for nonfunctioning pituitary macroadenomas were analyzed to determine tumor growth patterns. Immunohistochemical staining for transcription factors PIT-1, TPIT, and SF-1 was done on all samples. Extrasellar growth was divided into three principal directions: infrasellar, suprasellar and lateral cavernous sinus invasion (CSI). Suprasellar extension was defined as tumor extension superior to the tuberculum sellae-dorsum sellae line, inferior extension as invasion through the sellar floor into the sphenoid sinus or clivus and CSI as Knosp grading score of 3 ~ 4. Statistical analysis to compare the groups was conducted using the Fisher’s exact test and t-test. TPIT-expressing tumors were more likely to exhibit combined infrasellar extension (55.17 vs 17.70%, p < 0.0001), as well as isolated infrasellar extension (18.97 vs 0%, p < 0.0001) compared to SF-1-expressing tumors. Conversely, SF-1-expressing tumors were more likely to exhibit combined suprasellar extension (92.92 vs 77.59%, p = 0.0061), as well as isolated suprasellar extension (75.22 vs 41.38%, p < 0.0001). TPIT-expressing tumors had a significantly higher CSI invasion (55.17 vs 35.40%, p = 0.0148). The mean maximal tumor diameter in TPIT and SF-1 macroadenomas was similar (28 vs 26 mm, p = 0.1213). The expression of TFs affects the extrasellar growth pattern of NFPAs. TPIT tumors exhibit a higher propensity for bone invasion and CSI, while SF-1 tumors tend to extend into the suprasellar region. Isolated infrasellar extension is specific to TPIT tumors and can serve as a radiologic sign to distinguish between TPIT tumors and SF-1 tumors.
Keywords: Nonfunctioning pituitary adenomas, PitNET, Invasion, Sellar region, MR image, Transcription factors
Subject terms: Endocrinology, Pathogenesis
Introduction
Nonfunctioning pituitary adenomas (NFPAs), described as nonfunctioning pituitary neuroendocrine tumors (PitNETs) in the 5th edition of the WHO Classification of Endocrine and Neuroendocrine Tumors, constituting 22 ~ 54% of all pituitary adenomas1,2. Usually, NFPAs show compression symptoms, such as headaches and vision deficits due to its larger size. The classification of NFPAs subtypes relies on immunohistochemical analysis of transcription factors (TFs) and anterior pituitary hormones3,4. The most common subtype is steroidogenic factor-1 (SF-1) expressing tumor (constituting 70 ~ 75% of NFPAs), followed by T-box transcription factor (TPIT) expressing tumor (20 ~ 30% of NFPAs)5,6. SF-1 and TPIT are responsible for the development of gonadotroph cells and corticotroph cells, respectively7–9. Typically, NFPAs expressing SF-1 are diagnosed as silent gonadotroph adenomas (SGAs), while those expressing TPIT are identified as silent corticotroph adenomas (SCAs)3,10.
With over 95% of NFPAs exhibiting extrasellar extension11, achieving total resection of tumors remains a challenge for surgeons in the era of endoscopic transsphenoidal surgery12,13. Understanding the growth patterns of NFPAs is crucial for optimizing surgical planning. It has been reported that different pathological types of pituitary tumors show distinct growth and invasion patterns14, such as growth hormone (GH) adenomas, which frequently invade the sphenoid sinus and clivus15–18. However, few studies have focused on the extrasellar growth pattern of the two major subtypes of NFPAs. In this study, we aimed to analyze extrasellar growth patterns of NFPAs based on the MRI and expression of TFs to enhance our understanding of tumor growth propensities.
Methods
We conducted a retrospective analysis of all patients who underwent endoscopic pituitary adenoma resection at The First Affiliated Hospital of Sun Yat-sen University between January 2021 and February 2023. All patients underwent 3.0 T MRI examination of sellar region with and without contrast before operation. Immunohistochemical staining for the three lineage-based TFs PIT-1 (Anti-Pit1, rabbit, Abcam), TPIT (Anti-Tpit antibody, mouse, Abcam) and SF-1 (Anti-Steroidogenic Factor 1, rabbit, Abcam) was done on all samples by pathologist. Tumors were classified according to the 5th WHO CNS endocrine classification guidelines and were clustered according to their adenohypophysis cell lineage4. The inclusion criteria comprised: (1) A diagnosis of NFPA according to the preoperative clinical manifestations. (2) Positive postoperative immunohistochemical staining for SF-1 or TPIT alone. (3) Presence of primary pituitary macroadenoma (tumor larger than 1 cm in diameter). The exclusion criteria comprised: (1) Positive postoperative immunohistochemical staining for PIT-1. (2) Positive costaining for transcription factors (such as SF-1&TPIT). (3) Recurrent tumor.
MR images were evaluated for extrasellar extension.Two neurosurgeons (Weijie Su and Jia Yang) independently performed imaging assessments. The extrasellar extension region was categorized into three parts: infrasellar, suprasellar, and cavernous sinus. Suprasellar extension was defined as tumor growth beyond the tuberculum sellae-posterior clinoid line in the sagittal plane18. Infrasellar extension was confirmed when the tumor clearly breached the sellar floor into the sphenoid sinus17 and the sellar floor destruction was confirmed intraoperatively. Cavernous sinus invasion (CSI) was categorized as Knosp score 3 ~ 419. In the vertical direction, tumors were defined as isolated suprasellar or infrasellar extension when they extended only suprasellar or infrasellar regions. The word “any” and “combined” mean suprasellar or infrasellar extension, including extension in both directions. (Fig. 1).
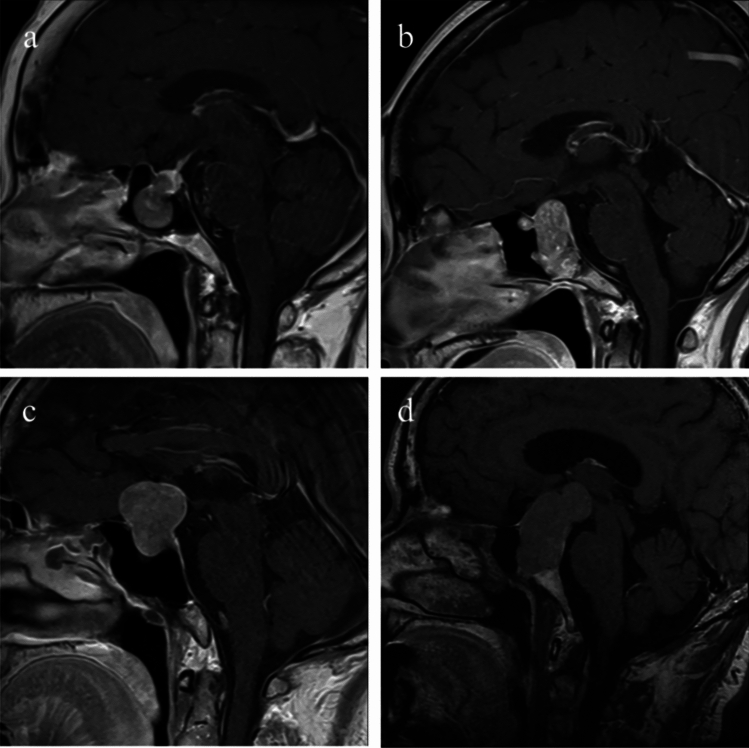
Fig. 1.
Examples of extrasellar growth patterns for TPIT and SF-1 tumors. Isolated infrasellar invasion (a) and mainly infrasellar invasion (b) of TPIT tumors. Isolated suprasellar extension (c) and mainly suprasellar extension (d) of SF-1 tumors.
Furthermore, we performed geometric measurements of tumor growth patterns according to the practice in previous literature15,18,20. The posterior extension line of the sphenoid plane (dotted line A’) was used as the upper edge of the sella turcica. A line 10 mm below A’ (dotted line B’) was taken as the floor. The distance of suprasellar extension(A-A’), infrasellar extension(B-B’) and the propensity of suprasellar extension((A-A’)-(B-B’) were calculated (Fig. 2).
Fig.2.
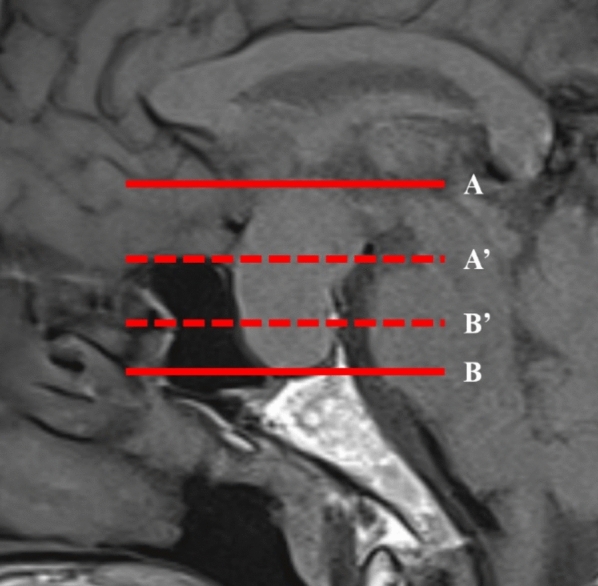
Geometry measurement of the tumor extrasellar extension in the vertical direction on MRI. The posterior extension line of the sphenoid plane (dotted line A’) was used as the upper edge of the sella turcica. A line 10 mm below A’ (dotted line B’) was taken as the floor.
GraphPad software (version 9.0) was used to analyze the data. Descriptive analysis was used to summarize the baseline characteristics of the patients. Quantitative data were expressed as mean ± standard deviation (SD), qualitative data were expressed as the number of cases and percentage. Unpaired two-tailed Student’s t test was used to test the significance of continuous variables between the two groups, and unpaired two-tailed Welch’s t test was used if the variance of the two populations was not equal. The Kolmogorov–Smirnov test was used to assess the distribution of the data, and if the parameters did not fit the normal distribution, the unpaired Mann–Whitney U test was used. Categorical variables were compared using Pearson’s chi-square test or Yates continuity correction. Statistical significance was defined as p < 0.05. This study was approved by the Ethics Committee for Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat-sen University (reference number: 2024–265). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all patients.
Results
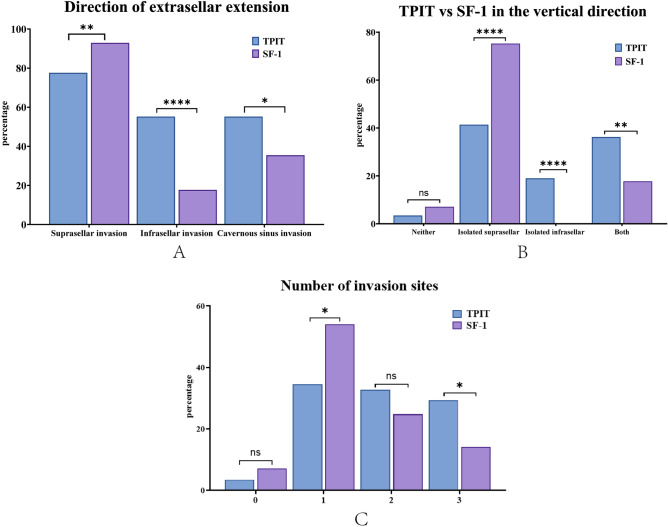
In our consecutive cohort, a total of 171 patients who underwent endoscopic resection of pituitary macroadenomas were included in the study. 103 patients were excluded, including 79 with tumors expressing PIT-1, 5 with tumors expressing multiple transcription factors, and 19 with recurrence. There were 113 patients with SF-1-expressing NFPAs and 58 patients with TPIT-expressing NFPAs. The proportion of female patients in the TPIT-expressing NFPAs cohort was significantly higher than in SF-1 tumors (81.03 vs 27.43%, p < 0.0001, Table1). TPIT-expressing patients were younger than SF-1-expressing patients (48.67 ± 12.31 vs 53.90 ± 12.96, p = 0.0120, Table 1). No significant difference in size was observed between the two tumor subtypes (28 vs 26 mm, p = 0.1213). Tumors expressing SF-1 and TPIT showed distinct growth patterns (Fig. 1). SF-1-expressing tumors demonstrated a higher propensity for suprasellar extention, both isolated (75.22 vs 41.38%, p < 0.0001, Table 1) and combined (92.92 vs 77.59%, p = 0.0061, Table 1). TPIT- expressing tumors were more prone to infrasellar extention, both isolated (18.97 vs 0.00%, p < 0.0001, Table 1) and combined (55.17 vs 17.70%, p < 0.0001, Table 1). TPIT tumors were more likely to invade the cavernous sinus (55.17 vs 35.40%, p = 0.0148, Table 1) and expand to multiple regions (62.07 vs 38.94%, p = 0.0058, Table 1) than SF-1. (Fig. 3).
Table 1.
Comparison of TPIT and SF-1 tumor extension patterns.
| Variable | TPIT | SF-1 | p value |
|---|---|---|---|
| Number of patients | 58 | 113 | |
| Female | 47(81.03) | 31(27.43) | < 0.0001 |
| Age(year) | 48.67 ± 12.31 | 53.90 ± 12.96 | 0.0120 |
| Mean tumor diameter(mm) | 28 | 26 | ns |
| Extrasellar extension | 56(96.55) | 105(92.92) | ns |
| Suprasellar extension | |||
| Any | 45(77.59) | 105(92.92) | 0.0061 |
| Isolated | 24(41.38) | 85(75.22) | < 0.0001 |
| Infrasellar extension | |||
| Any | 32(55.17) | 20(17.70) | < 0.0001 |
| Isolated | 11(18.97) | 0(0) | < 0.0001 |
| Cavernous sinus invasion | |||
| Any | 32(55.17) | 40(35.40) | 0.0148 |
| Isolated | 0(0) | 0(0) | ns |
| Multiple regions of extension | 36(62.07) | 44(38.94) | 0.0058 |
Statistical significance was defined as p < 0.05.
Fig.3.
Comparison of TPIT and SF-1 tumor extension patterns. TPIT tumors tend to extend to the infrasellar region and cavernous sinus, whereas SF-1 tumors invade the suprasellar region (A,B). TPIT tumors were more likely to expand to multiple regions (C).
Geometry measurement indicated that the extent of the suprasellar extension (A-A’) between TPIT-expressing tumor and SF-1-expressing tumor is similar (9.86 ± 8.09 mm vs 10.47 ± 6.23 mm, p = 0.590; Table 2). The distance of inferior extension (B-B’) of TPIT tumors was significantly longer than SF-1 tumors (8.28 ± 8.76 mm vs 5.24 ± 5.82 mm, p = 0.008; Table 2). The tendency of superior extention[(A-A')-(B-B')] was more distinct in SF-1 tumors than TPIT tumors (5.23 ± 7.66 mm vs 1.59 ± 13.9 mm, p = 0.029; Table 2).
Table 2.
Geometrical variables measurement on MRI.
| Variable | Total | TPIT | SF-1 | p value |
|---|---|---|---|---|
| A-A' (mm) | 10.26 ± 6.90 | 9.86 ± 8.09 | 10.47 ± 6.23 | 0.590 |
| B-B' (mm) | 6.27 ± 7.08 | 8.28 ± 8.76 | 5.24 ± 5.82 | 0.008 |
| (A-A')-(B-B')(mm) | 3.99 ± 10.32 | 1.59 ± 13.9 | 5.23 ± 7.66 | 0.029 |
Discussion
In this large group of patients treated surgically for NFPAs, we investigated the impact of TFs expression on the tumor extrasellar growth patterns. Our results showed SF-1 tumors and TPIT tumors exibiting distinct extrasellar growth patterns. This not only reveals the biological significance of TFs in the growth of pituitary tumors, but also contributes to the establishment of radiomics models in predicting the pathological types of tumor.
The growth morphology of pituitary tumors is closely linked to the surgical resection rate21–24. To achieve the goal of total resecting NFPAs, a comprehensive understanding of tumor growth patterns is imperative prior to surgery. Previous studies have reported that certain anatomical and pathological factors can influence the extrasellar growth patterns of pituitary tumors18,25–27. This study mainly focused on the role of TFs in influencing the growth patterns of NFPAs. To better characterize the biological characteristics of NFPA subtypes, we compared the two most prevalent NFPA subtypes using the latest classification methods. In our series, TPIT tumors were more aggressive than SF-1 tumors, characterized by easy extension to multiple extrasellar regions. Notablely, TPIT tumors and SF-1 tumors exhibited similar sizes in our cohort, eliminating the possibility that inconsistent tumor sizes contribute to different invasion patterns.
According to the 2022 edition of the WHO classification of pituitary tumors, SCAs can be diagnosed by demonstrating immunopositivity for T-PIT with ACTH immunopositivity or immunonegativity. With the use of TFs immunohistochemical staining as a routine pathological diagnostic method, the prevalence of SCA is increasing, while null-cell PitNET are becoming increasingly rare, accounting for only 1% of PitNET5,10. SCAs were hypothesized to exhibit more aggressive behavior and worse long-term outcomes compared with other NFPAs28–30. Several studies differentiated patients with SCAs and non-SCAs preoperatively using noninvasive radiomics and clinical scale to help make appropriate treatment strategies3,28,31–35. However, these studies grouped all non-SCAs together without further discrimination, ignoring the heterogeneity of NFPAs. Meanwhile, they did not delve into the impact of transcription factors on NFPAs. A previous study showed that PIT-1 tumors were more likely to invade the cavernous sinus than TPIT and SF-1tumors, but no difference was found between TPIT and SF-1 tumors36. This study was limited by small sample size. Another study has demonstrated that the expression of additional TFs in tumor cells predicts more aggressive behavior37. Our study was the first to explore the invasiveness of NFPAs expressing single TF based on a large sample.
In the vertical direction, we observed TPIT tumors exhibit a higher propensity for bone invasion and thus enter the sphenoid sinus, while SF-1 tumors were more likely to extend into the suprasellar region. Importantly, isolated infrasellar extension is specific to TPIT tumors and can serve as a radiologic sign to distinguish between these two tumors preoperatively. To quantify this difference, we attempted geometric measurements on MRI. The geometric measurement results also support this difference. This distinction was not consistently observed in similar previous studies5,28, perhaps potentially due to variations in TF expression assessment, its inclusion of pituitary microadenomas and recurrent adenomas maybe with different growth locations38 and inadequate discrimination between different NFPAs subtypes. In lateral direction, TPIT tumors were more likely to invade the cavernous sinus, aligning with previous findings14,34. In addition, a higher incidence of TPIT tumors in females and younger patients was observed, consistent with previous studies5,28, indicating that TPIT tumors are strongly related to sex and age.
The growth patterns of these two tumor subtypes suggest differences in their ability to invade bone and dura. SF-1 tumors exhibit poor bone destruction ability, leading them to extend into the suprasellar region through the sellar septum foramen. Similarly, their limited ability to invade the dura makes them less likely to invade the cavernous sinus compared to TPIT tumors. Notably, the GH adenomas in the previous study showed infrasellar tendendcy similar to the TPIT tumors in the our study17. However, it remains unclear whether TPIT tumors present with thickening of the soft tissue of the diaphragma sellae while enlarging the sellar space, as seen in GH adenomas. Alternatively, the biological characteristics of TPIT tumors may render them more susceptible to invading surrounding dural and bone structures, possibly due to the expression of degrading enzymes like matrix metalloproteinases39. This susceptibility might also be linked to factors promoting tumor bone metastasis40. In the future, whole-genome sequencing of pituitary tumors with large sample size is necessary to screen for genes related to bone destruction. In this way, we can further clarify the mechanism of TPIT tumors that are more likely to cause sellar floor bone destruction and find new therapeutic target options for refractory NFPAs.
There are several limitations in our study. First, it is a single-center retrospective study with a relatively small sample size. This may cause sample bias and limit the reproducibility of the results. Second, the manual geometric measurement methods for tumor morphology lack diversity and has the potential to create bias. In the later study, we will compare the tumor invasion confirmed intraoperatively by neurosurgeons with preoperative MRI performance to determine the reliability of imaging assessments. Thrid, we did not analyze the effects of tumor growth patterns on clinical symptoms and prognosis. Resection and recurrence rates of tumors will be included in future studies. Additionally, future investigations should explore the biological characteristics of NFPAs and involve larger multicenter studies with comprehensive assessments to provide a more nuanced understanding of these complex tumors.
Conclusions
In a consecutive cohort of 58 TPIT-expressing and 113 SF-1-expressing NFPAs, TPIT-expressing tumors are more likely to invade bone and enter the sphenoid sinus, whereas SF-1 tumors tend to grow suprasellar. Importantly, isolated infrasellar extension is specific to TPIT tumors and can serve as a radiologic sign to distinguish between these two tumors. In addition, TPIT tumors are prone to invade the cavernous sinus and extend to multiple extrasellar regions. The results enhances our understanding of the role TFs play in the biological behavior of NFPAs. It also aids in the establishment of radiomics models predicting the pathological types of tumor and provides insights for selecting new therapeutic targets.
Abbreviations
- NFPAs
Nonfunctioning pituitary adenomas
- TFs
Transcription factors
- SCA
Silent corticotroph adenomas
- SGA
Silent gonadotroph adenomas
- PIT-1
Pituitary transcription factor–1
- SF-1
Steroidogenic factor–1
- TPIT
T-box transcription factor
- GH
Growth hormone
- CSI
Cavernous sinus invasion
- PitNET
Pituitary neuroendocrine tumor
Author contributions
Jiakun Xu, Shaolin Zhang and Xixi Li contributed to the conception of the study; Jiakun Xu, Shaolin Zhang, Weijie Su and Jia Yang performed the experiment; Jiakun Xu, Shaolin Zhang and Lixuan Yang contributed significantly to analysis and manuscript preparation; Jiakun Xu, Shaolin Zhang and Xixi Li performed the data analyses and wrote the manuscript; Lixuan Yang and Xixi Li helped perform the analysis with constructive discussions. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82073049).
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethic committee approval has been taken.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiakun Xu and Shaolin Zhang contributed equally to this work.
References
- 1.Ntali, G. & Wass, J. A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary21, 111–118 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Batista, R. L. et al. Nonfunctioning pituitary adenoma recurrence and its relationship with sex, size, and hormonal immunohistochemical profile. World Neurosurg.120, e241–e246 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Vuong, H. G. & Dunn, I. F. The clinicopathological features and prognosis of silent corticotroph tumors: an updated systematic review and meta-analysis. Endocrine82, 527–535 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Asa, S. L., Mete, O., Perry, A. & Osamura, R. Y. Overview of the 2022 WHO classification of pituitary tumors. Endocr. Pathol.33, 6–26 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Jiang, S. et al. Clinical profiles of silent corticotroph adenomas compared with silent gonadotroph adenomas after adopting the 2017 WHO pituitary classification system. Pituitary24, 564–573 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shlomo, A. & Cooper, O. Silent corticotroph adenomas. Pituitary21, 183–193 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Melmed, S. et al. Clinical biology of the pituitary adenoma. Endocr. Rev.43, 1003–1037 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mete, O., Cintosun, A., Pressman, I. & Asa, S. L. Epidemiology and biomarker profile of pituitary adenohypophysial tumors. Modern Pathol.31, 900–909 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Nishioka, H. et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr. Pathol.26, 349–355 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Drummond, J., Roncaroli, F., Grossman, A. B. & Korbonits, M. Clinical and pathological aspects of silent pituitary adenomas. J. Clin. Endocrinol. Metab.104, 2473–2489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerges, M. M. et al. Long-term outcomes after endoscopic endonasal surgery for nonfunctioning pituitary macroadenomas. J. Neurosurg.134, 535–546 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Makarenko, S., Alzahrani, I., Karsy, M., Deopujari, C. & Couldwell, W. T. Outcomes and surgical nuances in management of giant pituitary adenomas: a review of 108 cases in the endoscopic era. J. Neurosurg.137, 635–646 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Micko, A. et al. Treatment strategies for giant pituitary adenomas in the era of endoscopic transsphenoidal surgery: a multicenter series. J. Neurosurg.136, 776–785 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Nishioka, H., Inoshita, N., Sano, T., Fukuhara, N. & Yamada, S. Correlation between histological subtypes and MRI findings in clinically nonfunctioning pituitary adenomas. Endocr. Pathol.23, 151–156 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara, A. et al. Comparison of growth hormone–producing and non–growth hormone–producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology228, 533–538 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Zada, G., Lin, N. & Laws, E. R. Patterns of extrasellar extension in growth hormone–secreting and nonfunctional pituitary macroadenomas. Neurosurg. Focus29, 4 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Pangal, D. J. et al. Growth hormone secreting pituitary adenomas show distinct extrasellar extension patterns compared to nonfunctional pituitary adenomas. Pituitary25, 480–485 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Monsalves, E. et al. Growth patterns of pituitary adenomas and histopathological correlates. J. Clin. Endocrinol. Metab.99, 1330–1338 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Knosp, E., Steiner, E., Kitz, K. & Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery33, 610–618 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Bakhtiar, Y. et al. Geometric survey on magnetic resonance imaging of growth hormone producing pituitary adenoma. Pituitary17, 142–149 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Xiao, L. et al. Subclassification of knosp grade 4 pituitary adenoma: Bringing insights into the significance of tumor growth pathways. Neurosurgery92, 213–222 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Serra, C. et al. Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir.160, 2255–2262 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Sarkar, S., Corrales, C. E., Laws, E. R. & Smith, T. R. Morphological classification of pituitary tumors with suprasellar extension. Neurosurgery94, 1183–1190 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Micko, A. S. G. et al. Dumbbell-shaped pituitary adenomas: prognostic factors for prediction of tumor nondescent of the supradiaphragmal component from a multicenter series. J. Neurosurg.137, 609–617 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan, V. R. et al. Sphenoid sinus anatomy and suprasellar extension of pituitary tumors. J. Neurosurg.119, 669–674 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Sarkar, S., Chacko, A. G. & Chacko, G. Clinicopathological correlates of extrasellar growth patterns in pituitary adenomas. J. Clin. Neurosci.22, 1173–1177 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Hayashi, Y. et al. Directional regulation of extrasellar extension by sellar dura integrity and intrasphenoidal septation in pituitary adenomas. World Neurosurg.122, e130–e138 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Strickland, B. A. et al. Silent corticotroph pituitary adenomas: clinical characteristics, long-term outcomes, and management of disease recurrence. J. Neurosurg.135, 1706–1713 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Ioachimescu, A. G. et al. Silent corticotroph adenomas: Emory University cohort and comparison with ACTH-negative nonfunctioning pituitary adenomas. Neurosurgery71, 296–303 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Cho, H. Y. et al. Silent corticotroph adenomas have unique recurrence characteristics compared with other nonfunctioning pituitary adenomas. Clin. Endocrinol. (Oxf.)72, 648–653 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Wang, H. et al. Radiomics model and clinical scale for the preoperative diagnosis of silent corticotroph adenomas. J. Endocrinol. Invest.46, 1843–1854 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Rui, W. et al. Radiomics analysis allows for precise prediction of silent corticotroph adenoma among non-functioning pituitary adenomas. Eur. Radiol.32, 1570–1578 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Kim, D. et al. Clinical parameters to distinguish silent corticotroph adenomas from other nonfunctioning pituitary adenomas. World Neurosurg.115, e464–e471 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Huang, L. et al. Clinical course and unique features of silent corticotroph adenomas. World Neurosurg.161, e274–e281 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Goyal-Honavar, A. et al. A clinicoradiological analysis of silent corticotroph adenomas after the introduction of pituitary-specific transcription factors. Acta Neurochir.163, 3143–3154 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Asmaro, K. et al. Cytodifferentiation of pituitary tumors influences pathogenesis and cavernous sinus invasion. J. Neurosurg.69, 118 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Micko, A. et al. Expression of additional transcription factors is of prognostic value for aggressive behavior of pituitary adenomas. J. Neurosurg.134, 1139–1146 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Negm, H. M. et al. Reoperative endoscopic endonasal surgery for residual or recurrent pituitary adenomas. J. Neurosurg.127, 397–408 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Gong, J. et al. Matrix metalloproteinase-9, a potential biological marker in invasive pituitary adenomas. Pituitary11, 37–48 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui, J. A. et al. GDF15 promotes prostate cancer bone metastasis and colonization through osteoblastic CCL2 and RANKL activation. Bone Res.10.1038/s41413-021-00178-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.