Abstract
Although the phase III SUNLIGHT trial has demonstrated the survival benefit of the addition of bevacizumab (Bmab) to trifluridine/thymidine phosphorylase inhibitor (FTD/TPI), neutropenia, which frequently occurs during FDT/TPI + Bmab therapy, is a concern for clinicians. As TPI is excreted by the kidneys, the risk of adverse events is likely to be high in patients with an impaired renal function. This study aimed to investigate the relationship between renal impairment and the incidence of chemotherapy-induced neutropenia during FTD/TPI + Bmab therapy using real-world data. We retrospectively reviewed the medical records of 69 patients with metastatic colorectal cancer (mCRC) who were treated with FTD/TPI + Bmab for more than 28 days. Patients with renal impairment with an eGFR of 30–44 mL/min/1.73 m2 were defined as the G3b group. Seven patients (10.1%) were classified into the G3b group. Patients in the G3b group had an approximately 24% higher incidence of grade ≥ 3 neutropenia in comparison to others (71.4% vs. 46.8%), and the incidence of grade 4 neutropenia in the G3b group was significantly higher than that in others (42.9% vs. 9.7%, p = 0.042). The G3b group frequently developed grade ≥ 3 neutropenia within 30 days of the initiation of FTD/TPI + Bmab therapy. However, the duration required for neutrophil count to recover to ≥ 1500/mm3 and the treatment effects of the G3b group were comparable to those observed in other patients. Clinicians should pay extra attention to patients with a decreased renal function who are treated with FTD/TPI + Bmab therapy, but no special measures are required for patients with an eGFR ≥ 30 mL/min/1.73 m2 as no marked differences were observed in neutrophil count recovery.
Keywords: Colorectal cancer, Trifluridine/thymidine phosphorylase inhibitor, Bevacizumab, Neutropenia, Renal function
Subject terms: Gastroenterology, Oncology
Introduction
The phase III SUNLIGHT trial has demonstrated the survival benefit of the addition of bevacizumab (Bmab) to trifluridine/thymidine phosphorylase inhibitor (FTD/TPI)1, and FTD/TPI + Bmab therapy has established its position as a later-line treatment for metastatic colorectal cancer (mCRC). Relatively good treatment outcomes have been obtained even in patients who are refractory to standard regimens containing oxaliplatin or irinotecan. However, neutropenia, which frequently occurs during FTD/TPI + Bmab therapy, is a concern for clinicians2,3. FTD exerts its antitumor effect by causing DNA dysfunction, and TPI increases the bioavailability of FTD by specifically inhibiting thymidine phosphorylase, an enzyme that degrades FTD4–7. As TPI is excreted by the kidneys, its plasma concentration is likely to be high in patients with an impaired renal function, resulting in an increased risk of chemotherapy-induced neutropenia8–11. However, there is no need to reduce the initial dose if the creatinine clearance is ≥ 30 mL/min12. This study aimed to investigate the relationship between renal impairment and the incidence of chemotherapy-induced neutropenia during FTD/TPI + Bmab therapy using real-world data.
Patients and methods
Patients
We retrospectively reviewed the medical records of 69 patients with mCRC who were treated with FTD/TPI + Bmab for > 28 days at Osaka City University Hospital between January 2016 and December 2023. Patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 were excluded from this study. Patients were treated with FTD/TPI 35 mg/m2 orally twice a day on days 1–5 and 8–12 in a 28-day cycle, with Bmab 5 mg/kg administered by intravenous infusion every 2 weeks. Blood examinations were scheduled on days 1, 15, and 22, and added as needed.
This retrospective study was approved by the Ethics Committee of Osaka City University (approval number: 2020-026) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Methods
The eGFR was calculated using serum creatinine values, sex and age13. Patients with renal impairment with an eGFR of 30–44 mL/min/1.73 m2 were defined as the G3b group according to the GFR categories14. The incidence of grade ≥ 3 adverse events, including neutropenia, and treatment efficacy was compared between patients in the G3b group and others. Furthermore, we verified the timing of grade ≥ 3 neutropenia and the duration required for the neutrophil count to recover to ≥ 1500/mm3.
Response evaluations using computed tomography were performed every 8–10 weeks according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.115. If the treatment was discontinued due to deterioration of the general condition caused by the tumor burden, the response was considered progressive disease.
Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analyses
All statistical analyses were performed using the SPSS software package for Windows (SPSS, Chicago, IL, USA) and the EZR statistical software program version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). The significance of differences in the renal function and clinicopathological factors, adverse events, and treatment outcomes were analyzed using the chi-squared test, Fisher’s exact test, and Mann-Whitney U-test. The relationship between the frequency of neutropenia and the time course after the initiation of FTD + TPI + Bmab therapy in patients who developed neutropenia was analyzed using a Poisson regression model. The overall survival was defined as the interval between the date of initiation of FTD/TPI + Bmab and the date of death from any cause or the last follow-up examination. Progression-free survival was defined as the interval between the date of initiation of FTD/TPI + Bmab and the date of disease progression, death from any cause, or the last follow-up examination. An objective response was defined as complete or partial response. Disease control was defined as a complete or partial response or stable disease. Survival curves were estimated using the Kaplan–Meier method, and differences in survival curves were assessed using a log-rank test. P values of < 0.05 were considered to indicate statistical significance.
Results
Patient characteristics according to renal function are listed in Table 1, and adverse events according to renal function are listed in Tables 2 and 3. Seven patients (10.1%) were classified into the G3b group. Among the 69 patients enrolled in this study, grade ≥ 3 neutropenia was observed in 34 patients (49.3%), and grade 4 neutropenia was observed in 9 patients (13.0%). Patients in the G3b group were significantly older than other patients. The incidence of grade ≥ 3 neutropenia in the G3b group was approximately 24% higher than in other patients (71.4% vs. 46.8%), although no statistically significant difference was observed. Furthermore, the incidence of grade 4 neutropenia was significantly higher in the G3b group than that in other patients (42.9% vs. 9.7%, p = 0.042). To exclude the adverse effects of age on neutropenia, we examined the incidence of neutropenia in patients under 80 years old and found that the incidence of neutropenia was still higher in the G3b group than others (Gr. ≥3: 71.4% vs. 42.9%, p = 0.235; Gr.4: 43.4 vs. 11.3%, p = 0.062). In addition, the incidence of grade ≥ 3 thrombocytopenia was significantly higher in the G3b group than in other patients (42.9% vs. 4.8%, p = 0.012). Furthermore, although no statistically significant difference was observed in the incidence of grade 3 proteinuria, the incidence of proteinuria (3+) was higher in the G3b group than in the other patients (42.9% vs. 14.5%, p = 0.095).
Table 1.
Association between the renal function and clinicopathological factors -comparison of the clinicopathological factors between the G3b group and others-.
| Factors | Overall patient population (n = 69) | G3b group (n = 7) | Others (n = 62) | p-Value |
|---|---|---|---|---|
| Age (years) | ||||
| Median (range) | 69 (36–88) | 77 (58–77) | 68.5 (36–88) | 0.012* |
| Sex, n | ||||
| Male | 35 | 4 | 31 | |
| Female | 34 | 3 | 31 | 0.517 |
| Performance status, n | ||||
| 0, 1 | 65 | 7 | 58 | |
| ≥ 2 | 4 | 0 | 4 | 0.645 |
| Location of primary tumor, n | ||||
| Right side | 20 | 2 | 18 | |
| Left side | 49 | 5 | 44 | 0.675 |
| RAS status, n | ||||
| Wild type | 35 | 2 | 33 | |
| Mutant type | 32 | 5 | 27 | 0.178 |
| Unknown | 2 | 0 | 2 | |
| Number of metastatic organs, n | ||||
| 1 | 29 | 3 | 26 | |
| ≥ 2 | 40 | 4 | 36 | 0.632 |
| Serum CEA levels (ng/mL), n | ||||
| ≤ 5.0 | 13 | 0 | 13 | |
| > 5.0 | 56 | 7 | 49 | 0.215 |
CEA: Carcinoembryonic antigen.
G3b group: patients with an estimated glomerular filtration rate (eGFR) 30–44 mL/min/1.73 m2; Others: patients with an eGFR ≥ 45 mL/min/1.73 m2.
*: p < 0.05.
Table 2.
Relationship between stage of renal impairment and incidence of neutropenia.
| Incidence of neutropenia | ||||
|---|---|---|---|---|
| Stage of renal impairment (eGFR) | Number of cases | Grade ≥ 3 | Grade 3 | Grade 4 |
| Grade 1 (≥ 90 mL/min/1.73 m2) | 11 | 45.5% | 36.4% | 9.1% |
| Grade 2 (60–89 mL/min/1.73 m2) | 39 | 53.8% | 43.6% | 10.3% |
| Grade 3a (45–59 mL/min/1.73 m2) | 12 | 25.0% | 16.7% | 8.3% |
| Grade 3b (30–44 mL/min/1.73 m2) | 7 | 71.4% | 28.6% | 42.9% |
eGFR: estimated glomerular filtration rate.
Table 3.
Comparison of adverse events between the G3b group and others.
| Overall cohort (n = 69) | G3b group (n = 7) | Others (n = 62) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse events | Grade ≥ 3 | Grade 3 | Grade 4 | Grade ≥ 3 | Grade 3 | Grade 4 | Grade ≥ 3 | Grade 3 | Grade 4 | p-Value |
| Hematological | ||||||||||
| Neutropenia, n (%) | 34 (49.3) | 25 (36.2) | 9 (13.0) | 5 (71.4) | 2 (28.6) | 3 (42.9) | 29 (46.8) | 23 (37.1) | 6 (9.7) | 0.259/>0.999/0.042* |
| Anemia, n (%) | 7 (10.1) | 5 (7.2) | 2 (2.9) | 2 (28.6) | 1 (14.3) | 1 (14.3) | 5 (8.1) | 4 (6.5) | 1 (1.6) | 0.145/0.424/0.194 |
| Thrombocytopenia, n (%) | 6 (8.7) | 5 (7.2) | 1 (1.4) | 3 (42.9) | 2 (28.6) | 1 (14.3) | 3 (4.8) | 3 (4.8) | 0 (0) | 0.012*/0.077/0.101 |
| Non-hematological | ||||||||||
| Nausea, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA/NA/NA |
| Anorexia, n (%) | 1 (1.4) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) | 1 (1.6) | 0 (0) | > 0.999/>0.999/NA |
| Diarrhea, n (%) | 1 (1.4) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) | 1 (1.6) | 0 (0) | > 0.999/>0.999/NA |
| Fatigue, n (%) | 2 (2.9) | 2 (2.9) | 0 (0) | 1 (14.3) | 1 (14.3) | 0 (0) | 1 (1.6) | 1 (1.6) | 0 (0) | 0.194/0.194/NA |
| Hypertension, n (%) | 1 (1.4) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) | 1 (1.6) | 0 (0) | > 0.999/>0.999/NA |
| Proteinuria, n (%) | 3 (4.3) | 3 (4.3) | 1 (14.3) | 1 (14.3) | 2 (3.2) | 2 (3.2) | 0.278/0.278 | |||
| Edema, n (%) | 2 (2.9) | 2 (2.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3.2) | 2 (3.2) | 0 (0) | > 0.999/>0.999/NA |
NA: not applicable.
G3b group: patients with an estimated glomerular filtration rate (eGFR) 30–44 mL/min/1.73 m2; Others: patients with an eGFR ≥ 45 mL/min/1.73 m2.
*: p < 0.05.
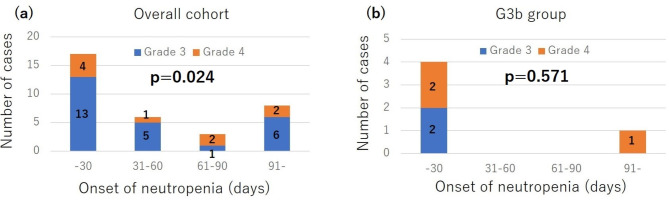
Regarding the onset of neutropenia, of the 34 patients who developed grade ≥ 3 neutropenia, 17 (50%) developed neutropenia within 30 days after the initiation of FTD/TPI + Bmab therapy, and 23 (67.6%) developed neutropenia within 60 days after the initiation of FTD/TPI + Bmab therapy (Fig. 1a). When analyzing the relationship between the frequency of neutropenia and the time course after initiation of FTD + TPI + Bmab therapy in patients who developed grade ≥ 3 neutropenia, neutropenia was found to significantly occur within 30 days after the initiation of FTD + TPI + Bmab therapy in the overall cohorts (p = 0.024). In an analysis limited to the G3b group, of the 5 patients who developed grade ≥ 3 neutropenia, 4 patients (80%) developed grade ≥ 3 neutropenia, and 2 patients (40%) developed grade 4 neutropenia within 30 days after initiation of FTD/TPI + Bmab therapy (Fig. 1b). The incidence of grade 4 neutropenia within 30 days after the initiation of FTD/TPI + Bmab therapy was significantly higher in the G3b group than that in the other patients (28.6% vs. 3.2%, p = 0.048). Among the 34 patients with grade ≥ 3 neutropenia, there were no differences in the duration required for neutrophil count to recover to ≥ 1500/mm3 or higher between the G3b group and the other patients (Table 4).
Fig. 1.
Onset of first grade 3/4 neutropenia in the overall cohort and G3b group, who developed grade ≥ 3 neutropenia.
Table 4.
The duration required for neutrophil count to recover to ≥ 1500/mm3 according to the renal function among 34 cases with grade ≥ 3 neutropenia.
| G3b group (n = 5) | Others (n = 29) | p-Value | |
|---|---|---|---|
| The duration required for the neutrophil count to recover to ≥ 1500/mm3 (days) | |||
| Median (range) | 13 (6–14) | 14 (2-112) | 0.232 |
G3b group: patients with an estimated glomerular filtration rate (eGFR) 30–44 mL/min/1.73 m2; Others: patients with an eGFR ≥ 45 mL/min/1.73 m2.
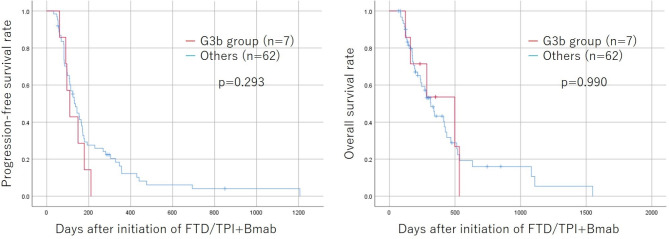
Treatment effects, such as response rate, disease control rate, progression-free survival, and overall survival, of the G3b group were comparable to those of other patients (Table 5; Fig. 2).
Table 5.
Comparison of the objective response rate/disease control rate according to the renal function.
| Response | G3b group (n = 7) | Others (n = 62) | p-Value |
|---|---|---|---|
| Complete response, n | 0 | 0 | |
| Partial response, n | 0 | 3 | |
| Stable disease, n | 3 | 18 | |
| Progressive disease, n | 4 | 14 | |
| Response rate | 0% | 9.70% | 0.513 |
| Disease control rate | 42.9% | 51.6% | 0.483 |
G3b group: patients with an estimated glomerular filtration rate (eGFR) 30–44 mL/min/1.73 m2; Others: patients with an eGFR ≥ 45 mL/min/1.73 m2.
Fig. 2.
Kaplan-Meier survival curves for progression-free and overall survival according to the renal function. The survival time between the G3b group and other patients did not differ to a statistically significant extent. FTD/TPI + Bmab; Trifluridine/thymidine phosphorylase inhibitor + bevacizumab.
Discussion
This study revealed that the risk of neutropenia increases as the renal function declines during FTD/TPI + Bev therapy for mCRC. To our knowledge, this is the first study to examine the relationship between renal impairment and chemotherapy-induced neutropenia, specifically for FTD/TPI + Bmab therapy. Unlike clinical trials, in which enrolled patients were in relatively good condition, this study used real-world data, which included patients with renal impairment. Therefore, the results of this study provide useful information for daily clinical practice.
Previous clinical trials have demonstrated that the incidence of chemotherapy-induced neutropenia is as high as 33–38%, even with FTD/TPI monotherapy16,17. Furthermore, the addition of Bmab to FTD/TPI therapy further increases the incidence of chemotherapy-induced neutropenia18–20. No statistically significant difference in the incidence of chemotherapy-induced neutropenia was observed between the impaired renal function group and others because the incidence of chemotherapy-induced neutropenia was high regardless of the renal function. However, the incidence of grade ≥ 3 neutropenia was approximately 24% higher in patients with renal impairment than in other cases. Furthermore, the incidence of grade 4 neutropenia was significantly higher in patients with an impaired renal function than in other cases. Based on these findings, it is necessary to pay more attention to the occurrence of neutropenia in patients with renal impairment.
Determining cutoff values for stratifying renal dysfunction is difficult. The results of this study showed that the incidence of grade ≥ 3 neutropenia was higher in patients with G3b stage renal impairment than in those with other stages of impairment. Therefore, the G3b group was designated as having an impaired renal function. There have been several reports on the relationship between renal dysfunction and neutropenia after FTD/TPI monotherapy. Falcone et al. reported that in cases with a creatinine clearance of 30–59 ml/min, the incidence of grade ≥ 3 adverse events increased, and dose reduction was necessary in 23.9% of cases9. Yoshida et al. reported that the incidence of adverse events is increased in patients with an eGFR < 50 mL/min/1.73 m210. Shiroyama et al. reported that moderate to severe renal dysfunction with an eGFR < 60 mL/min/1.73 m2 is a risk factor for grade ≥ 3 neutropenia11. Based on the above findings, moderate renal dysfunction may be an indicator of risk factors for neutropenia. Furthermore, although whether or not increased blood concentrations have a direct effect on increased incidence of adverse events is unclear, the “107 Study” showed that the Cmax and area under the blood concentration time curve (AUC) of FTD and TPI were considerably increased in patients with moderate renal impairment21. Even though mild renal dysfunction may affect neutropenia, owing to the small number of cases, it was difficult to perform a more detailed analysis of renal function. In addition, although a reduced dosage is recommended for patients with renal impairment with an eGFR < 30 mL/min/1.73 m2, no criteria for dose reduction have been established, so such patients were excluded from this study.
Among patients who developed grade ≥ 3 neutropenia, the first neutropenia episode developed within 30 days in 50% of cases and within 60 days in 67.6% of cases. This is consistent with previous reports that FTD/TPI therapy tends to cause neutropenia soon after initiation8. Clinicians must keep in mind that FTD/TPI + Bmab therapy is likely to cause neutropenia relatively early after the initiation of treatment. Furthermore, it should be noted that in patients with an impaired renal function, not only grade 3 but also grade 4 neutropenia frequently occur within 30 days after administration. As stated in the guide for the appropriate use of medication and previous report8,21, it is important to conduct blood examinations on days 1, 15, and 22.
Although neutropenia occurred more frequently in patients with an impaired renal function, there were no significant differences in the time required for neutrophil count recovery (≥ 1500/mm3) between the impaired renal function group and other patients. Therefore, special measures against chemotherapy-induced neutropenia may not be necessary, even in patients with an impaired renal function. We were able to continue treatment even after grade 3 or higher neutropenia in patients with an impaired renal function. In addition, as in previous reports on FTD/TPI monotherapy, no difference was observed in the therapeutic effects based on renal function11. Therefore, FTD/TPI + Bmab therapy is a useful treatment, even for patients with impaired renal function, as long as adverse events are carefully monitored. However, if neutropenia occurs repeatedly, it may be better to reduce the dose upon restart, even with grade 3 neutropenia.
The present study is associated with several limitations. First, this was a non-randomized retrospective study with a limited sample size in a single institution. There may be some subjects for whom statistically significant differences were not observed owing to the small sample size of the patients in the group with an impaired renal function. Second, this study used eGFR, which approximates creatinine clearance and is easier to calculate than creatinine clearance, to evaluate renal function. It is appropriate to use creatinine clearance to accurately assess the renal function because there may be a small difference between eGFR and creatinine clearance in elderly patients22.
Conclusion
FTD/TPI + Bmab therapy carries a high risk of developing severe neutropenia within 30 days of initiation, especially in patients with a decreased renal function. Therefore, FTD/TPI + Bmab therapy requires careful follow-up, depending on the renal function.
Acknowledgements
We thank Dr. Brian Quinn, who provided medical writing services on behalf of JMC Ltd.We thank Dr. Ryota Kawai and Dr. Ayumi Shintani who provided their statistical expertise.
Author contributions
MS designed the study, performed the statistical analysis and draft the manuscript. HT, HK, TF and SK collected the clinical data and revised the manuscript critically. KM designed the study and critically reviewed the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prager, G. W. et al. SUNLIGHT investigators. Trifluridine-tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N Engl. J. Med.388, 1657–1667 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer, P. et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol.21, 412–420 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Kuboki, Y. et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol.18, 1172–1181 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Emura, T., Suzuki, N., Fujioka, A., Ohshimo, H. & Fukushima, M. Potentiation of the antitumor activity of alpha, alpha, alpha-trifluorothymidine by the co-administration of an inhibitor of thymidine phosphorylase at a suitable molar ratio in vivo. Int. J. Oncol.27, 449–455 (2005). [PubMed] [Google Scholar]
- 5.Temmink, O. H., Emura, T., de Bruin, M., Fukushima, M. & Peters, G. J. Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci.98, 779–789 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka, N. et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol. Rep.32, 2319–2326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakamoto, K. et al. Crucial roles of thymidine kinase 1 and deoxyUTPase in incorporating the antineoplastic nucleosides trifluridine and 2’-deoxy-5-fluorouridine into DNA. Int. J. Oncol.46, 2327–2334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshino, T. et al. Post-marketing surveillance study of trifluridine/tipiracil in patients with metastatic colorectal cancer. Jpn J. Clin. Oncol.51, 700–706 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Falcone, A. et al. Integrated safety summary for trifluridine/tipiracil (TAS-102). Anticancer Drugs. 29, 89–96 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Yoshida, N. et al. Effects and risk factors of TAS-102 in real-world patients with metastatic colorectal cancer, EROTAS-R study. Int. J. Clin. Oncol.28, 1378–1387 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Shiroyama, M. et al. Renal impairment as a risk factor for trifluridine/tipiracil-induced adverse events in metastatic colorectal cancer patients from the REGOTAS study. Sci. Rep.13, 17931 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, J. J. & Chu, E. Adherence, dosing, and managing toxicities with Trifluridine/Tipiracil (TAS-102). Clin. Colorectal Cancer. 16, 85–92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese Society of Nephrology. Guideline, Evidence-based Practice Guideline for the Treatment of CKD. [cited 2024 May 2]. Available from https://jsn.or.jp/en/guideline/guideline.php
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int.105, S117–S314 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Mayer, R. J. et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl. J. Med.372, 1909–1919 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Moriwaki, T. et al. Propensity Score Analysis of Regorafenib Versus Trifluridine/Tipiracil in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapy (REGOTAS): A Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study. Oncologist. 23, 7–15 (2018). [DOI] [PMC free article] [PubMed]
- 18.Chida, K. et al. Efficacy and safety of trifluridine/tipiracil plus bevacizumab and trifluridine/tipiracil or regorafenib monotherapy for chemorefractory metastatic colorectal cancer: a retrospective study. Ther. Adv. Med. Oncol.13, 17588359211009143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii, H. et al. Bevacizumab in Combination with TAS-102 improves clinical outcomes in patients with refractory metastatic colorectal Cancer: a retrospective study. Oncologist25, e469–e476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotani, D. et al. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer. 19, 1253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifluridine/tipiracil. (Lonsurf) Prescribing Information Guide (Taiho Pharmaceutical Co., Ltd., 2024). (in Japanese).
- 22.Kikuchi, T. et al. Applicability of the Japanese equation for estimating glomerular filtration rate in patients with advanced-stage thoracic cancer. Respir Investig. 54, 479–483 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.