Abstract
Malaria, transmitted by mosquitoes infected with Plasmodium parasites, remains a significant health issue with global travel increasing the risk of imported malaria. This study investigates imported malaria cases in the Republic of Korea from 2009 to 2018 using data from the Korea National Infectious Disease Surveillance System. During this period, 601 imported cases were reported, with 82.4% male patients and a median age of 39.1 years. Most cases (76.5%) involved Korean residents returning from malaria-endemic areas, mainly Africa and Asia. Plasmodium falciparum (55.7%) and Plasmodium vivax (30.3%) were the predominant species. The annual percent change in incidence rate was 6.45%. Notably, 71.5% of the patients did not receive prophylactic chemotherapy, and 18% of those who did still developed malaria. Median diagnostic delays were 4 days for P. falciparum and 7 days for P. vivax. The case fatality rate was 2.3%, with all deaths occurring in travelers who contracted P. falciparum in Africa. This study emphasizes the ongoing risk of imported malaria in the ROK and highlights the need for better awareness and preventive measures among travelers. Enhancing surveillance and educating travelers on anti-malaria chemoprophylaxis are crucial.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84124-6.
Keywords: Imported malaria, Epidemiology, Plasmodium falciparum, Plasmodium vivax, Travel medicine
Subject terms: Disease prevention, Health policy
Introduction
Malaria is a preventable and treatable vector-borne disease caused by Plasmodium parasites and presents a significant global health concern. Of the five Plasmodium species that infect humans, P. falciparum and P. vivax are the most prevalent, with P. falciparum accounting for over 99% of estimated cases in Africa. Despite increases in malaria control efforts worldwide, the disease continues to affect a substantial portion of the world’s population. In 2022 alone, approximately 249 million infections were reported across 85 endemic countries, resulting in around 609,000 deaths, primarily concentrated in sub-Saharan Africa1.
The rise in global travel has led to an increased number of individuals visiting regions at high risk of malaria for various purposes such as trade, tourism, and employment2. An estimated 25–30 million people travel annually to malaria-endemic regions, resulting in approximately 30,000 travel-related malaria infections each year3,4. Europe accounts for approximately 70% of the global burden of imported malaria, followed by the United States with about 15%5,6.
In the Republic of Korea (ROK), approximately 600 malaria cases are reported annually5,6. According to the Korea Disease Control and Prevention Agency (KDCA), about 90% of these cases are indigenous P. vivax malaria, while the remaining cases are imported and primarily related to international travel5,7,8. Indigenous P. vivax malaria infections continue to occur mainly in border areas within 20 km of the Demilitarized Zone (DMZ), with fewer than 400 cases reported nationwide each year on average. More than 70% of these indigenous P. vivax malaria cases are reported among civilians throughout the year, but they occur most intensively from June to August. Due to the re-emergence of P. vivax malaria in 1993, the National Malaria Control Program (NMCP) has focused on managing indigenous P. vivax malaria7,9,10, which has led to a significant increase in the public’s awareness of the disease. However, the public’s awareness of imported malaria is still low, and the epidemiological characteristics of imported malaria in the ROK remain poorly understood. This study seeks to address this gap by analyzing imported malaria cases in the ROK between 2009 and 2018, utilizing nationwide surveillance data.
Materials and methods
Data collection
Malaria is a nationally notifiable disease in the ROK. A confirmed malaria case is classified when Plasmodium is identified through either microscopy on blood films or nucleic acid amplification tests (NAATs) conducted in a laboratory. If a malaria rapid diagnostic test (RDT) detects specific antigens (proteins) produced by malaria parasites that are present in the blood of infected individuals, they are classified as a suspected malaria case, and then blood smear microscopy or NAATs are performed to re-classify them as a confirmed case. All malaria case data can be accessed through the National Infectious Diseases Surveillance System (NIDSS) and the National Epidemiological Investigation System (NEIS)11.
We gathered records of imported malaria cases reported in ROK between 2009 and 2018 from the NIDSS and NEIS. The dataset included information on sex, age, residence, symptom onset, diagnosis and case registration dates, diagnostic method, death, nationality, travel history, chemoprophylaxis status, the Plasmodium species that caused the infection, and other relevant patient details. To estimate the number of the population at risk of imported malaria, foreign immigration statistics and data on the number of inbound and outbound travelers in Korea were obtained12. The list of countries with malaria risk was updated annually by referring to the World Malaria Report between 2009 and 2018.
Definition of imported malaria cases and diagnostic delay
Imported malaria was defined as an infection that was acquired in a malaria endemic area outside of ROK13. We assessed the Plasmodium species, incubation period, and history of international travel to a malaria-endemic area within the previous 2 years. We excluded: (i) cases with duplications, relapse, or recrudescence, (ii) indigenous cases, and (iii) induced cases.
We reviewed data on diagnostic delay (DD), defined as the time between symptom onset and malaria diagnosis; patient delay (PD), defined as the time between symptom onset and the first medical facility visit or primary healthcare clinic; and medical diagnostic delay (MDD), defined as the time between the first medical facility visit or primary healthcare clinic and laboratory-confirmed malaria diagnosis14.
Incidence rate of imported malaria
The incidence rate of imported malaria was defined as the number of newly reported cases of imported malaria annually divided by the estimated number of the population at risk of imported malaria. The population at risk includes foreigners from malaria-risk countries who entered and stayed in the ROK for purposes other than tourism and Koreans returning from malaria-risk countries.
 |
To show the trend in the incidence rate of imported malaria, we estimated the annual percent change (APC). APC indicates how much the incidence of overseas imported malaria has changed over a specific period. If the APC is positive (+), it means that the incidence of overseas imported malaria is increasing. If the APC is negative (-), it means that the incidence of overseas imported malaria is decreasing. If the APC is 0, there is no change in the incidence of overseas imported malaria.
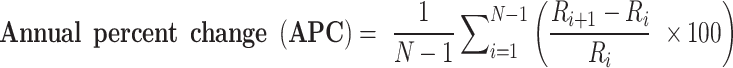 |
Ri: Incidence rate for year i, Ri+1: Incidence rate for year i + 1, N: Total number of years.
Statistical analyses
The general and epidemiological characteristics of imported malaria cases by Plasmodium species were examined using frequency analysis, analysis of variance (with post hoc testing), and chi-square tests. A regression model was used to explore the APC of the incidence rate of imported malaria during the study period. To compare clinical characteristics and outcomes between patients who died and those who did not, we used the Mann-Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. All analyses were performed using SPSS 26.0 (IBM Inc., USA). The level of statistical significance was set at 5%.
Ethical approval and ethical conduct of the study
We consulted the Public Institutional Review Board designated by the Ministry of Health and Welfare in the Republic of Korea to determine whether ethical clearance was required for this study. The board concluded that ethical clearance was not necessary.
Results
General characteristics
Between 2009 and 2018, a total of 601 confirmed cases of imported malaria were reported through the NIDSS in the ROK (Supplementary Fig. 1). Most of these patients were male, comprising 495 out of 601 cases (82.4%). The median age of the infected individuals was 39.1 years (interquartile range [IQR]: 28–49.5), with an age range of 4–79 years. The distribution of cases varied across age groups, with the highest proportion occurring in individuals in their 20s (25.8%), followed by those in their 40s (24.6%) and 30s (20.3%). Most cases (76.5%) were among Korean residents who had traveled to malaria endemic areas abroad (460 out of 601), and the remaining cases (23.5%) involved foreign nationals entrants to the ROK (141 out of 601). The majority of imported malaria cases originated from Africa, accounting for 414 cases (68.9%), followed by Asia with 179 cases (29.8%). The predominant Plasmodium species that caused imported malaria was P. falciparum (55.7%), followed by P. vivax (30.3%), P. ovale (2.8%), and P. malariae (2.0%). 49 cases (8.2%) could not be identified due to insufficient species data (Table 1).
Table 1.
General characteristics of imported malaria cases in the Republic of Korea (ROK), 2009–2018 (N = 601)
| Category | Total | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | |||||||||||
| Gender | ||||||||||||
| Male | 495 | (82.4) | 14 | 33 | 51 | 39 | 55 | 71 | 63 | 55 | 56 | 58 |
| Female | 106 | (17.6) | 6 | 10 | 11 | 11 | 4 | 8 | 8 | 13 | 20 | 15 |
| Age (median, range) | 39.1 | (28.0–49.5) | ||||||||||
| < 20 | 26 | (4.3) | 1 | 1 | 8 | 1 | 2 | 3 | 2 | 2 | 3 | 3 |
| 20–29 | 155 | (25.8) | 6 | 14 | 18 | 15 | 17 | 10 | 18 | 19 | 20 | 18 |
| 30–39 | 122 | (20.3) | 2 | 12 | 5 | 13 | 14 | 20 | 20 | 11 | 8 | 17 |
| 40–49 | 148 | (24.6) | 2 | 8 | 12 | 16 | 13 | 29 | 14 | 18 | 19 | 17 |
| 50–59 | 113 | (18.8) | 9 | 6 | 15 | 2 | 9 | 13 | 15 | 13 | 20 | 11 |
| ≥ 60 | 37 | (6.2) | 0 | 2 | 4 | 3 | 4 | 4 | 2 | 5 | 6 | 7 |
| Nationality | ||||||||||||
| Korean | 460 | (76.5) | 18 | 37 | 53 | 34 | 46 | 63 | 48 | 48 | 64 | 49 |
| Non-Korean | 141 | (23.5) | 2 | 6 | 9 | 16 | 13 | 16 | 23 | 20 | 12 | 24 |
| Visiting country | ||||||||||||
| Africa | 414 | (68.9) | 13 | 23 | 37 | 24 | 44 | 58 | 56 | 52 | 67 | 40 |
| Asia | 179 | (29.8) | 7 | 20 | 25 | 23 | 13 | 20 | 14 | 15 | 9 | 33 |
| Oceania | 6 | (1.0) | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 0 |
| America | 2 | (0.3) | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Death | ||||||||||||
| Yes | 14 | (2.3) | 0 | 0 | 0 | 0 | 2 | 5 | 0 | 1 | 3 | 3 |
| No | 587 | (97.7) | 20 | 43 | 62 | 50 | 57 | 74 | 71 | 67 | 73 | 70 |
| Plasmodium species | ||||||||||||
| P. falciparum | 335 | (55.7) | 8 | 20 | 35 | 17 | 31 | 52 | 45 | 38 | 53 | 36 |
| P. vivax | 182 | (30.3) | 4 | 15 | 21 | 28 | 13 | 20 | 18 | 17 | 12 | 34 |
| P. ovale | 17 | (2.8) | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 5 | 5 | 1 |
| P. malariae | 12 | (2.0) | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 6 | 2 |
| Mixed infection§ | 6 | (1.0) | 0 | 0 | 1 | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| Unclassified | 49 | (8.2) | 7 | 6 | 5 | 4 | 10 | 3 | 6 | 8 | 0 | 0 |
| Total | 601 | (100.0) | 20 | 43 | 62 | 50 | 59 | 79 | 71 | 68 | 76 | 73 |
*IQR, interquartile range
§The P. vivax and P. falciparum correspond to 5 cases; the P. falciparum and P. ovale correspond to 1 case
Region of travel
Between 2009 and 2018, patients with imported malaria in the ROK arrived from 50 different countries, including 32 in Africa, 15 in Asia, 2 in Oceania, and 1 in the Americas. The majority of P. falciparum cases originated from Equatorial Guinea, Ghana, Uganda, Nigeria, and Sierra Leone. 9 cases of P. falciparum malaria were reported from India and Cambodia. Plasmodium vivax cases primarily originated from Asian countries, including Pakistan, India, the Philippines, and Cambodia (Supplementary Table 1, Supplementary Fig. 2), and were primarily associated with travel to Southeast Asia (44.5%), South Asia (31.3%), and Western Africa (10.4%). Patients infected with P. falciparum predominantly traveled to Western Africa (37.3%), followed by Central (30.1%) and Eastern Africa (14.3%). Most reported cases of P. ovale and P. malariae were linked to travel in Africa (Table 2).
Table 2.
Epidemiological and clinical characteristics of imported malaria cases by Plasmodium species in the ROK, 2009–2018 (N = 601).
| Category | P.falciparum | P.vivax | P. ovale | P. malariae | Mixed infectio*n | Unclassified | X 2 /F | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||
| Gender | ||||||||||||||
| Male | 278 | (83.0) | 148 | (81.3) | 13 | (76.5) | 10 | (83.3) | 5 | (83.3) | 41 | (83.7) | 0.672 | 0.984 |
| Female | 57 | (17.0) | 34 | (18.7) | 4 | (23.5) | 2 | (16.7) | 1 | (16.7) | 8 | (16.3) | ||
| Visiting risk area | ||||||||||||||
| Africa | ||||||||||||||
| Eastern | 48 | (14.3) | 11 | (6.0) | 3 | (17.6) | 4 | (33.3) | 2 | (33.3) | 8 | (16.3) | 383.48 | 0.000 |
| Western | 125 | (37.3) | 19 | (10.4) | 3 | (17.6) | 3 | (25.0) | 1 | (16.7) | 7 | (14.3) | ||
| Southern | 26 | (7.8) | 2 | (1.1) | 1 | (5.9) | 0 | 0.0 | 0 | 0.0 | 2 | (4.1) | ||
| Northern | 18 | (5.4) | 1 | (0.5) | 1 | (5.9) | 0 | 0.0 | 0 | 0.0 | 1 | (2.0) | ||
| Central | 101 | (30.1) | 4 | (2.2) | 6 | (35.3) | 5 | (41.7) | 3 | (50.0) | 8 | (16.3) | ||
| Asia | ||||||||||||||
| East | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Western | 1 | (0.3) | 1 | (0.5) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| South | 7 | (2.1) | 57 | (31.3) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 12 | (24.5) | ||
| Southeast | 8 | (2.4) | 81 | (44.5) | 3 | (17.6) | 0 | 0.0 | 0 | 0.0 | 10 | (20.4) | ||
| South America | 1 | (0.3) | 1 | (0.5) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Oceania | 0 | 0.0 | 5 | (2.7) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | (2.0) | ||
| Chemoprophylaxis | ||||||||||||||
| No | 239 | (71.8) | 139 | (76.4) | 8 | (47.1) | 7 | (58.3) | 5 | (83.3) | 32 | (65.3) | 26.57 | 0.003 |
| Yes | 70 | (21.0) | 19 | (10.4) | 7 | (41.2) | 4 | (33.3) | 1 | (16.7) | 7 | (14.3) | ||
| Non-response | 26 | (7.2) | 24 | (13.2) | 2 | (11.8) | 1 | (8.3) | 0 | 0.0 | 10 | (20.4) | ||
| Symptom | ||||||||||||||
| Fever | 306 | (92.2) | 175 | (96.2) | 17 | (100.0) | 10 | (83.3) | 5 | (83.3) | 46 | (93.9) | ||
| Chill | 213 | (64.2) | 123 | (67.6) | 11 | (64.7) | 10 | (83.3) | 3 | (50.0) | 31 | (63.3) | ||
| Headache | 173 | (52.1) | 96 | (52.7) | 9 | (52.9) | 6 | (50.0) | 2 | (33.3) | 25 | (51.0) | ||
| Malaise | 148 | (44.6) | 75 | (41.2) | 7 | (41.2) | 6 | (50.0) | 3 | (50.0) | 24 | (49.0) | ||
| Sweating | 109 | (32.8) | 52 | (28.6) | 5 | (29.4) | 5 | (41.7) | 0 | 0.0 | 13 | (26.5) | ||
| Diarrhea | 32 | (9.6) | 13 | (7.1) | 1 | (5.9) | 0 | 0.0 | 0 | 0.0 | 8 | (16.3) | ||
*The P. vivax and P. falciparum correspond to 5 cases; the P. falciparum and P. ovale correspond to 1 case.
Clinical symptoms
The most prevalent symptom at malaria diagnosis was fever, occurring in 93.5% of cases, followed by chills (65.4%), headache (52.0%), malaise (44.0%), and sweating (30.8%). Diarrhea was reported in approximately 9.0% of patients. These symptoms were consistent across different Plasmodium species (Table 2).
Incidence rate of imported malaria in the ROK
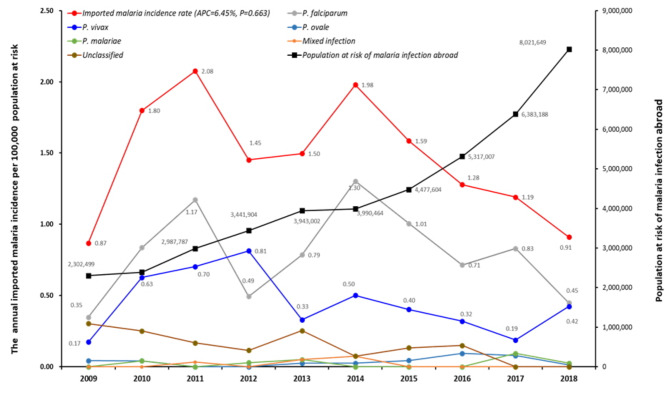
Figure 1 illustrates the annual trend in imported malaria incidence in the ROK, categorized by Plasmodium species. From 2009 to 2011, the incidence rate steadily increased, peaking in 2011. Although it decreased in 2012, it rose again in 2014. After 2015, the incidence rate slightly declined but remained higher than that in earlier years. The APC was 6.45%, indicating that incidence rates increased by an average of 6.45% each year. The annual differences in incidence rates were statistically significant (Χ2 = 44.72, P < 0.000). However, despite the overall increase, a slight downward trend observed over time, although this trend was not statistically significant (F = 0.8509, P = 0.383).
Fig. 1.
Annual imported malaria incidence rate per 100,000 risk population and number of risk population.
Prophylactic chemotherapy
Of the patients with P. falciparum malaria, 72% did not receive prophylactic chemotherapy and 21% did. Of the patients with P. vivax malaria, 76% did not receive prophylactic chemotherapy and 10% did (Table 2). However, the completeness of prophylactic chemotherapy and the appropriateness of the prescribed medications were not verified.
Diagnostic delay
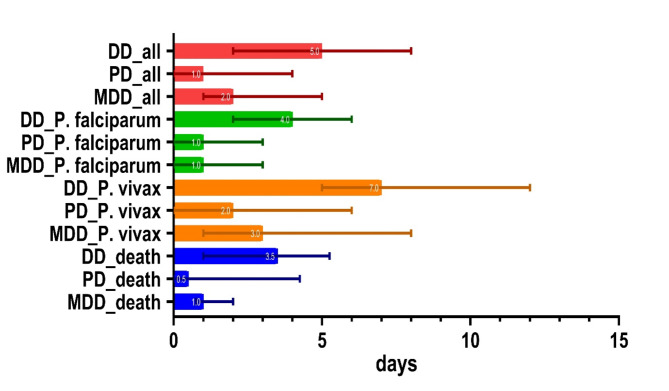
The median DD for P. falciparum malaria was 4 days (IQR 2–6), with a median PD and MDD of 1 day each (IQR 0–3). For P. vivax malaria, the median DD was 7 days (IQR 5–12), with median PD and MDD of 2 (IQR 0–6) and 3 days (IQR 1–8), respectively (Fig. 2).
Fig. 2.
Diagnostic delay in total cases by Plasmodium species and in deceased cases. Numbers in graphs represent median values, lines represent interquartile ranges. DD: diagnostic delay, PD: patient delay, MDD: medical diagnostic delay.
Mortality
Over the span of the 10-year period, 14 deaths were documented, yielding a fatality rate of 2.2%; 93% of the deceased were male. All fatalities were residents of the ROK who contracted P. falciparum malaria while in Africa, for a P. falciparum fatality rate of 4.18%. Deceased patients had a higher median age compared with survivors (56 vs. 39 years, P = 0.004). The median DD for fatal cases was 3.5 days (IQR 1.0–5.3), with a median PD and MDD of 0.5 days (IQR 0–4.3) and 1 day (IQR 0.8–2.0), respectively (Table 3; Fig. 2).
Table 3.
Characteristics of imported malaria deaths in the ROK, 2009–2018.
| Alive (N = 587) | Death (N = 14) | p-value | |
|---|---|---|---|
| Male gender, n(%) | 482 (82.1) | 13 (92.9) | 0.483 |
| Age, median [IQR] | 39 [28—49] | 56 [39.5–61.5] | 0.004 |
| < 20, n(%) | 25 (4.3) | 1 (7.1) | |
| 20–29, n(%) | 153 (26.1) | 2 (14.3) | |
| 30–39, n(%) | 122 (20.8) | 0 (0) | |
| 40–49, n(%) | 145 (24.7) | 3 (21.4) | |
| 50–59, n(%) | 110 (18.7) | 3 (21.4) | |
| > 59, n(%) | 32 (5.5) | 5 (35.7) | 0.001 |
| Nationality | 0.049 | ||
| Korean, n(%) | 446 (76.0) | 14 (100.0) | |
| Non-Korean, n(%) | 141 (24.0) | 0 (0) | |
| Visiting country | 0.032 | ||
| Africa, n(%) | 400 (68.1) | 14 (100.0) | |
| Asia, n(%) | 179 (30.5) | 0 (0) | |
| Oceania, n(%) | 6 (1.0) | 0 (0) | |
| America, n(%) | 2 (0.3) | 0 (0) | |
| Plasmodium species | 0.040 | ||
| P. falciparum, n(%) | 321 (54.7) | 14 (100.0) | |
| P. vivax, n(%) | 182 (31.0) | 0 (0) | |
| P. ovale, n(%) | 17 (2.9) | 0 (0) | |
| P. malariae, n(%) | 12 (2.0) | 0 (0) | |
| Mixed infection, n(%) | 6 (1.0) | 0 (0) | |
| Unclassified, n(%) | 49 (8.3) | 0 (0) | |
| Chemoprophylaxis | 0.162 | ||
| No, n(%) | 421 (72.0) | 9 (64.3) | |
| Yes, n(%) | 103 (17.6) | 5 (35.7) | |
| Non-response, n(%) | 63 (10.7) | 0 (0) | |
| Diagnostic delay, days§ | |||
| DD, median [IQR] | 5 [2–8] | 3.5 [1—5.25] | 0.050 |
| PD, median [IQR] | 1 [0—4] | 0.5 [0—4.25] | 0.548 |
| MDD, median [IQR] | 2 [1–5] | 1 [0.75—2] | 0.152 |
*IQR interquartile rang.
§Diagnostic delay (DD): the time between the onset of symptoms and the diagnosis of malaria; Patient delay (PD): the time between the onset of symptoms and first attending a medical facility; Medical diagnostic delay (MD): the time between first attending a medical facility and the diagnosis of malaria.
Discussion
We analyzed national surveillance data of imported malaria in the ROK between 2009 and 2018 to identify its epidemiological characteristics. The incidence rates of imported malaria remained consistent throughout the study period. The majority of cases were observed in ROK men (over 70%), predominantly in their 20s and 40s. This trend aligns with findings from other studies15–20.
In this study, we were unable to assess the specific reasons for travel to malaria-risk areas. Overseas travel from the ROK to tropical regions is increasing, although the reasons for this trend remain unclear. Possible factors include social changes leading to more trips to tropical areas and a preference among younger travelers for high-risk travel, such as remote adventures, over business travel21.
Approximately 76% of imported malaria cases were Korean, which is similar to the proportion of imported malaria cases in Japan17. However, this contrasts with findings from Europe and North America, where a high proportion of malaria cases are among those visiting friends and relatives16,18,19,22,23. This discrepancy may be attributed to differences in population composition between Europe and the ROK. In 2020, foreign residents (including workers, immigrants, students, and Koreans with foreign nationality) constituted approximately 4.1% of Korea’s population (51.84 million), with their numbers steadily increasing. Among these, Chinese nationals (44.0%) were the largest group, followed by Vietnamese (10.4%) and Thai (8.9%), with less than 0.95% from Africa.
The primary regions from which P. falciparum was imported into the ROK were West Africa, followed by Central Africa, whereas imported P. vivax cases were mainly contracted in Southeast Asia. Among all patients diagnosed with imported malaria, 71.5% (430/601) did not receive anti-malaria chemoprophylaxis. A comparison between those who received chemoprophylaxis and those who did not revealed that chemoprophylaxis was more likely to be administered to older travelers, Koreans, and those traveling to Africa (Supplementary Table 2). The incidence rate of imported malaria cases reported in the ROK from 2009 to 2018 increased by an average of 6.45% annually over the past decade. APC (Annual Percent Change) is a metric that quantifies the rate of change in incidence over a specific period, and in this case, it shows a consistent upward trend. As illustrated in Fig. 1, while there appears to be a temporary decline in incidence rates during specific periods, the analysis using APC reveals a statistically significant average annual increase of 6.45%. These findings indicate a continuous rise in imported malaria cases, underscoring the need for sustained efforts in prevention and management. To reduce the incidence of imported malaria, it is crucial to increase efforts to educate travelers to malaria-endemic areas about the risk of malaria and the importance of chemoprophylaxis22,24–26.
Approximately 18% (108/601) of patients took anti-malaria chemoprophylaxis and still developed malaria. However, we were unable to confirm whether these patients adhered to their prescribed medication regimen or whether they were given the appropriate medication considering drug resistance among Plasmodium species in the destination country. Therefore, it is not possible to conclusively determine the efficacy of chemoprophylaxis in these cases.
The median DD for P. falciparum and P. vivax was four and 7 days, respectively. The median PD and MDD were 1 day each for P. falciparum, and 2 and 3 days, respectively, for P. vivax. This suggests that travelers returning from Africa, where P. falciparum is predominantly endemic, are more likely to seek healthcare service promptly if they develop relevant symptoms, and healthcare providers are more likely to suspect malaria in these patients. While P. vivax malaria is rarely severe enough to be fatal, it can be transmitted domestically27–31, necessitating prompt diagnosis and treatment. For autochthonous P. vivax malaria in the ROK, the median DD is reported to be approximately 5 days, with a median PD of around 3 days and a median MDD of less than 1 day32. The slightly longer DD observed for imported P. vivax cases in our study (7 days) may be due to differences in healthcare-seeking behavior or lower suspicion of malaria among healthcare providers when treating returning travelers. These observations underscore the importance of raising awareness and enhancing education on imported malaria to reduce diagnostic delays. Over the span of 10 years, 14 patients died from imported malaria. All fatalities were Koreans who contracted P. falciparum after traveling to Africa. Interestingly, in our study, the median DD and PD for fatal cases were shorter than those for non-fatal cases. This observation contrasts with previous studies14,33–35 and may suggest that the severity of symptoms in fatal cases prompted quicker medical attention and diagnosis. However, given the small number of fatal cases (n = 14), this finding should be interpreted cautiously. The limited sample size may restrict the generalizability of our results and make direct comparisons with other studies challenging. Educating individuals planning to travel to Africa about the potential risk of P. falciparum infection and the possibility of developing severe, potentially fatal malaria is essential. Travelers should be informed about the necessity of taking preventive measures against mosquito bites, including chemoprophylaxis, and of seeking immediate medical attention if malaria symptoms manifest36–38.
This study is subject to the limitations inherent to a retrospective study design. Firstly, there is a possibility of underreporting of imported malaria cases. However, this is unlikely to be significant because reporting a malaria diagnosis is mandatory in Korea. Secondly, incomplete data, such as the purpose of travel, whether anti-malaria chemoprophylaxis was fully taken, or whether the appropriate medication was prescribed, prevented more detailed analysis. Furthermore, data on foreign immigration and the number of inbound and outbound travelers in Korea were employed to estimate the population at risk of malaria infection. However, it should be noted that this estimation may not accurately reflect the actual population at risk. Despite these limitations, our report offers a comprehensive overview of malaria imported to the ROK.
In conclusion, this study represents the first large-scale analysis describing the characteristics of imported malaria cases reported in the ROK between 2009 and 2018. Imported malaria cases are on the rise, and travelers infected with P. falciparum malaria have died despite early diagnosis. Therefore, it is imperative to educate the public and promote travel clinics so that travelers visiting malaria-endemic areas, particularly those with P. falciparum malaria, can receive chemoprophylaxis through pre-travel consultations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
BHJeon: Conceptualization, Study design, Data collection, Analysis, Statistical analysis, Interpretation of results, Writing-original draft, Writing & review. JALee: Conceptualization, Study design, Analysis, Statistical analysis, Interpretation of results, Writing-original draft, Writing & review. SYLee: Interpretation of results, Writing & review. SELee: Interpretation of results, Writing & review. JSYeom: Conceptualization, Interpretation of results, Writing & review.
Funding
No specific grant from funding agencies in the public, commercial, or non-profit sectors was obtained for this research.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Byoung Hak Jeon and Jung Ah Lee contributed equally to this work.
References
- 1.Global Malaria Programme. World Malaria Report 2023 283 (WHO, 2023).
- 2.Huang, Z. & Tatem, A. J. Global malaria connectivity through air travel. Malar. J.12, 269. 10.1186/1475-2875-12-269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kain, K. C. & Keystone, J. S. Malaria in travelers. Epidemiology, disease, and prevention. Infect. Dis. Clin. N. Am.12, 267–284. 10.1016/S0891-5520(05)70005-2 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Loutan, L. Malaria: still a threat to travellers. Int. J. Antimicrob. Agents. 21, 158–163. 10.1016/S0924-8579(02)00367-9 (2003). [DOI] [PubMed] [Google Scholar]
- 5.KDCA. 2024 Malaria Management Guideline in ROK (Korea Disease Control Prevention Agency, 2024). (In Korean).
- 6.KDCA. Annual Report on the Notified Infectious Diseases in Korea. Korea Disease Control Prevention Agency, Cheongju-si, 2024 (2023). (In Korean)
- 7.Jeon, B. H., Park, K. E., Kwon, J. R. & Cho, E. H. Epidemiological characteristics of reported malaria cases in South Korea in 2018. Public. Health Wkly. Rep.12, 599–605 (2019). [Google Scholar]
- 8.Kim, H., Lee, S., Shin, N. R. & Hwang, K. Status of Malaria and diagnosis rate in the Republic of Korea, 2018–2022. Public. Health Wkly. Rep.16, 852–866. 10.56786/PHWR.2023.16.26.3 (2023). [Google Scholar]
- 9.Whang, C. H. Induced malaria in Korea. Yonsei Med. J.4, 51–57. 10.3349/ymj.1963.4.1.51 (1963). [DOI] [PubMed] [Google Scholar]
- 10.Park, J. W. et al. Vivax malaria: a continuing health threat to the Republic of Korea. Am. J. Trop. Med. Hyg.69, 159–167. 10.4269/ajtmh.2003.69.159 (2003). [PubMed] [Google Scholar]
- 11.Park, S. & Cho, E. National infectious diseases surveillance data of South Korea. Epidemiol. Health36, 4. 10.4178/EPIH/E2014030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korea Tourism Organization. Tourism & Statistics (2024). https://datalab.visitkorea.or.kr/datalab/portal/ts/getEntcnyFrgnCustFormEng.do
- 13.Global Malaria Programme (GMP). Malaria Surveillance, Monitoring & Evaluation: A Reference Manual208 (World Health Organization, 2018).
- 14.Bastaki, H., Carter, J., Marston, L., Cassell, J. & Rait, G. Time delays in the diagnosis and treatment of malaria in non-endemic countries: a systematic review. Travel Med. Infect. Dis.21, 21–27. 10.1016/j.tmaid.2017.12.002 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Mischlinger, J. et al. Imported malaria in countries where malaria is not endemic: a comparison of semi-immune and nonimmune travelers. Clin. Microbiol. Rev.33, e00104–e00119. 10.1128/cmr.00104-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andriopoulos, P., Economopoulou, A., Spanakos, G. & Assimakopoulos, G. A local outbreak of autochthonous Plasmodium Vivax malaria in Laconia, Greece-a re-emerging infection in the southern borders of Europe? Int. J. Infect. Dis.17, e125–e128. 10.1016/j.ijid.2012.09.009 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Kanayama, A. et al. Epidemiology of Imported Malaria cases in Japan, 2006–2014: a sentinel traveler Surveillance Approach. Am. J. Trop. Med. Hyg.97, 1532–1539. 10.4269/AJTMH.17-0171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domínguez García, M. et al. Imported malaria cases: the connection with the European ex-colonies. Malar. J.18, 397. 10.1186/S12936-019-3042-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mace, K. E., Arguin, P. M., Lucchi, N. W. & Tan, K. R. Malaria surveillance - United States, 2016. MMWR Surveill Summ.68, 1–40. 10.15585/mmwr.ss6805a1 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Yu, T. et al. Epidemiological characteristics of imported malaria in Shandong Province, China, from 2012 to 2017. Sci. Rep.10, 1–8. 10.1038/s41598-020-64593-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeom, J. S. Survey on Korean Travelers’ Insight and Prevention Behavior on Malaria and Other Imported Infectious Diseases (Korea Disease Control and Prevention Agency (KDCA), 2015). (In Korean).
- 22.Broderick, C. et al. Clinical, geographical, and temporal risk factors associated with presentation and outcome of vivax malaria imported into the United Kingdom over 27 years: observational study. BMJ350, h1703. 10.1136/bmj.h1703 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UK Health Security Agency. Malaria in the UK: Annual Report (2023). https://www.gov.uk/government/publications/malaria-in-the-uk-annual-report/malaria-imported-into-the-uk-2021
- 24.Tatem, A. J. et al. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect. Dis.17, 98–107. 10.1016/S1473-3099(16)30326-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohail, A. et al. Imported malaria into Australia: surveillance insights and opportunities. J. Travel Med.31, taad164. 10.1093/jtm/taad164 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturrock, H. J. W., Roberts, K. W., Wegbreit, J., Ohrt, C. & Gosling, R. D. Tackling imported malaria: an elimination endgame. Am. J. Trop. Med. Hyg.93, 139–144. 10.4269/ajtmh.14-0256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whang, C. H. Biological observations on Anopheline mosquitos in Korea, with special reference to Anopheles (Anopheles) Sinensis Wiedman. Yonsei Med. J.3, 39–50. 10.3349/ymj.1962.3.1.39 (1962). [Google Scholar]
- 28.Jeong, K. Y. et al. Population dynamics of five anopheles species of the Hyrcanus group in northern Gyeonggi-Do. Korea Korean J. Parasitol.48, 351–353. 10.3347/kjp.2010.48.4.351 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ubalee, R. et al. Vector competence of Anopheles kleini and Anopheles sinensis (Diptera: Culicidae) from the Republic of Korea to vivax malaria-infected blood from patients from Thailand. J. Med. Entomol. 53, 1425–1432. (2016). 10.1093/jme/tjw109 [DOI] [PubMed]
- 30.Park, S. Y. et al. Severe vivax malaria in the Republic of Korea during the period 2000 to 2016. Travel Med. Infect. Dis.30, 108–113. 10.1016/j.tmaid.2019.04.013 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Ubalee, R. et al. Vector competence and the susceptibility of Anopheles pullus and Anopheles belenrae to Plasmodium Vivax-infected blood from Thai patients. J. Med. Entomol.59, 1047–1052. 10.1093/jme/tjab226 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Kan, H., Kwon, J., Park, S., Kim, H. & Park, S. Characteristics of reported malaria cases, 2020. Public. Health Wkly. Rep.14, 1023–1035 (2021). [Google Scholar]
- 33.Checkley, A. M. et al. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ344, e2116. 10.1136/bmj.e2116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman, R. D., Parise, M. E., Barber, A. M. & Steketee, R. W. Malaria-related deaths among U.S. travelers, 1963–2001. Ann. Intern. Med.141, 547–555. 10.7326/0003-4819-141-7-200410050-00012 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Christen, D., Steffen, R. & Schlagenhauf, P. Deaths caused by malaria in Switzerland 1988–2002. Am. J. Trop. Med. Hyg.75, 1188–1194 (2006). [PubMed] [Google Scholar]
- 36.Seringe, E. et al. Severe imported Plasmodium Falciparum malaria, France, 1996–2003. Emerg. Infect. Dis.17, 807–813. 10.3201/eid1705.101527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy, A. E., Morgan, C., Prematunge, C. & Geduld, J. Severe malaria in Canada, 2001–2013. Malar. J.14, 1–8. 10.1186/s12936-015-0638-y (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White, N. J. Severe malaria. Malar. J.21, 1–17. 10.1186/s12936-022-04301-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.