Abstract
Background
Double‐expressor lymphoma (DEL) has a poorer prognosis than other subtypes of diffuse large B‐cell lymphoma (DLBCL). This study is a multicenter, prospective, single‐arm, phase 2 clinical study initiated by investigators to evaluate the efficacy and safety of combined zanubrutinib with R‐CHOP, which includes rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for patients with DEL (stage II or more), as well as to explore factors related to efficacy preliminarily.
Methods
From November 2020 to July 2022, 48 newly diagnosed patients were enrolled. All patients received twice‐daily oral zanubrutinib (160 mg) for 6 months and standardized R‐CHOP regimen for six to eight cycles.
Results
The objective response rate (ORR) was 89.6%, with a complete response rate (CRR) of 83.3%. The median follow‐up was 29.3 months. The median progression‐free survival (PFS) and overall survival (OS) were not reached. The PFS and OS were 81.25% and 93.75% at 2 years, respectively. Grade ≥3 adverse events (AEs) were reported in 23 of 48 (47.9%) patients. Next‐generation sequencing (NGS) results of 33 patients showed that TP53, MYD88, and PIM1 were the most common mutated gene. Multivariate analysis revealed that BCL‐6 gene rearrangement was an adverse prognostic factor for both PFS (hazard ratio [HR], 0.247; 95% confidence article [CI], 0.068–0.9; p = .034) and OS (HR, 0.057; 95% CI, 0.006–0.591; p = .016), whereas the number of extranodal involvements also significantly influenced OS (HR, 15.12; 95% CI, 1.07–213.65; p = .044).
Conclusions
Zanubrutinib in combination with R‐CHOP is an effective option for DEL patients, and the toxicity of zanubrutinib is entirely acceptable for patients.
Keywords: BCL2, BTKi, double‐expressor lymphoma, MYC, zanubrutinib
Zanubrutinib in combination with R‐CHOP in double‐expressor lymphoma patients.
INTRODUCTION
The double‐expressor lymphoma (DEL) refers to the simultaneous expression of MYC and BCL2 protein in lymphoma tissue through immunohistochemical (IHC) staining, without the presence of MYC and BCL2 gene rearrangements or translocations. In 2016, the World Health Organization established cutoff values of positivity ≥40% for MYC and ≥50% for BCL2 by IHC for DEL. 1 DEL accounts for approximately 20%–30% of newly diagnosed diffuse large B‐cell lymphoma (DLBCL). 2 The standard frontline treatments for DLBCL are rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) protocols. Unfortunately, this protocol is associated with a poor prognosis in patients with DEL and the reported 5‐year overall survival (OS) was 39%. 3
For the above reasons, many attempts have also been made, such as adjusted chemotherapy intensity, first‐line autologous Hematopoietic Stem Cell Transplantation (auto‐HSCT), and combining with new targeted agents (R‐CHOP plus X). 4 , 5 , 6 Nevertheless, less efficacy and more toxic effects were found. For example, in the Phoenix study, an analysis of patients with high BCL2/MYC expression indicated that, compared to placebo plus R‐CHOP treatment, the combination of ibrutinib and R‐CHOP (IR‐CHOP) tended to improve progression‐free survival (PFS) in patients under 60 years old (p = .0381). 7 However, in patients 60 years and older, the IR‐CHOP was associated with increased toxicity, leading to a higher discontinuation rate and reduced efficacy.
As drug research advances, the second‐generation Bruton tyrosine kinase inhibitor (BTKi) acalabrutinib has demonstrated outstanding efficacy. In the ACCEPT trial, acalabrutinib combined with R‐CHOP (AR‐CHOP) was studied as a frontline treatment for DLBCL, achieving an objective response rate (ORR) of 95% and a metabolic complete response (mCR) of 82%. 8 The ongoing ESCALADE phase 3 trial is comparing the efficacy of AR‐CHOP versus R‐CHOP alone in untreated non‐GCB DLBCL patients. 9
Zanubrutinib is a second‐generation BTKi with higher target occupancy than ibrutinib and fewer off‐target effects. 10 Moreover, zanubrutinib may have better clinical efficacy, thereby increasing patient benefit.
The optimal treatment regimen for DEL patients has not yet been determined. In our phase 2 study, we aimed to evaluate the efficacy and safety of zanubrutinib combined with R‐CHOP (ZR‐CHOP) in patients with DEL (especially in elderly patients).
MATERIALS AND METHODS
Study design
This open‐label, multicenter, single‐arm, prospective phase 2 study aims to evaluate the efficacy and safety of ZR‐CHOP in newly diagnosed DEL patients. It was conducted at five hospitals (Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Qilu Hospital of Shandong University, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shengli Oilfield Central Hospital, and Jining First People's Hospital). All patients signed and dated a written informed consent form at the first screening visit. This study was approved by an ethics committee and adhered to the principles of the Helsinki Declaration for Good Clinical Practice and its subsequent amendments (ethical approval: SDZLEC2020‐129‐02). The institutional review boards and/or independent ethics committees reviewed and approved the protocol and informed consent forms.
Patients and eligibility
The study included patients diagnosed with de novo DEL from November 2020 to July 2022. The diagnosis was confirmed by IHC, which identified DEL: the possibility of MYC ≥40% and BCL‐2 >50%. Fluorescence in situ hybridization (FISH) testing was also conducted to exclude rearrangements and translocations of the two genes. Thirty‐three patients were tested with the same next‐generation sequencing (NGS), which was performed on genomic DNA isolated from formalin‐fixed paraffin‐embedded (FFPE) tumor samples using the NextSeq platform (Illumina, San Diego, California). Hybrid capture was employed to analyze the whole exonic sequences of 43 genes associated with DLBCL (ITPKB, JAK2, KMT2C, KMT2D, MEF2B, MFHAS1, MYC, MYD88, NOTCH1, NOTCH2, PAX5, PIM1, SGK1, SOCS1, STAT3, STAT6, TET2, TNFAIP3, TNFRSF14, TP53, XPO1, ARID1B, ATM, B2M, BCL10, BCL2, BCL6, BTG1, CARD11, CCND3, CD58, CD79A, CD79B, CDKN2A, PRDM1, CIITA, CREBBP, EP300, EPHA7, EZH2, FAS, GNA13, and IRF8). All variants were detected with >99% confidence based on allele frequency and amplicon coverage, with an average sequencing depth of coverage of >500 and an analytic sensitivity of 5%. A minimum variant allele frequency (VAF) of 5% was used to call single nucleotide variants.
Treatment
The patients received zanubrutinib (160 mg by mouth, twice daily) for 6 months and rituximab (375 mg/m2, day 0), cyclophosphamide (750 mg/m2, day 1), doxorubicin (50 mg/m2, day 1), vincristine (1.4 mg/m2, day 1), and prednisone (100 mg, days 1–5) in repeated 21‐day cycles for six to eight cycles. 11 , 12 , 13
Efficacy and safety assessment
The study's primary end points are PFS and OS. Secondary end points include complete response rate (CRR), ORR (complete response [CR] plus partial response [PR]), and adverse events (AEs). Exploratory end points investigate the impact of clinical characteristics, biological markers, genetic abnormalities, and other prognostic factors on treatment efficacy and survival. Patients will undergo positron emission tomography and contrast computed tomography (PET‐CT) evaluation before treatment, after four and eight cycles of treatment, and will have efficacy assessment with enhanced CT scans of the neck, chest, abdomen, and pelvis at two and six cycles as a substitute for PET‐CT. Treatment responses were evaluated according to Lugano criteria. 14 The investigator assessed the severity of AEs reported during the study. When applicable, AEs were assessed and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0 guidelines.
Statistical analysis
PFS was defined as the time from the date of diagnosis to the date of disease progression/relapse, death, or last follow‐up. OS was calculated as the time from diagnosis to the day of death due to any cause or last follow‐up. Survival was calculated with the Kaplan–Meier method, and the significance between subgroups was calculated using the log‐rank test. In univariate and multivariate analysis, the Cox proportional hazards model was used to identify the factors that were significantly associated with PFS or OS. Variables with statistical significance (p < .05) were included in the multivariate analysis. Descriptive statistics for continuous data included mean, median, maximum, and minimum values. Frequency counts and percentages were used for categorical data. This study used SPSS 26 statistical analysis software for statistical calculations. Survival curves and heat maps were generated using R software. p < 0.05 indicates the statistical significance of the differences. The sample size was estimated that 2‐year PFS rate for the treatment in this study will be 70%, compared to the 50% in the historical control. If 50 subjects (15 events) are enrolled within 12 months, and the analysis of 2‐year PFS rate is conducted 12 months after the last patient is enrolled, with an expected dropout rate of 20%, it will have 85% power that the lower limit of the 90% confidence interval for 2‐year PFS rate will be higher than the historical control of 50%.
RESULTS
Patients and baseline characteristics
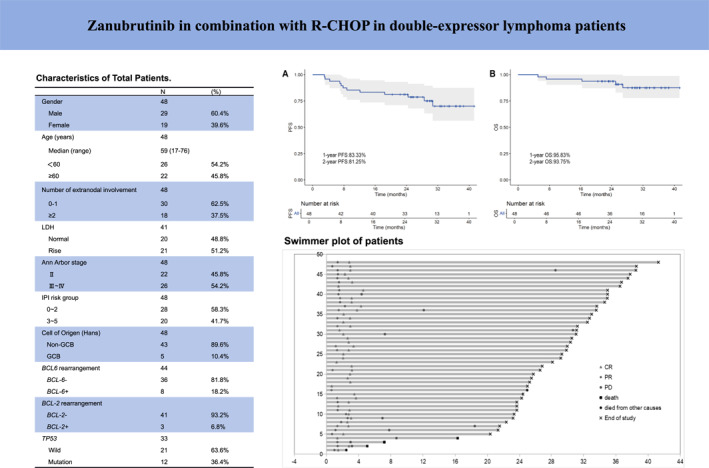
From November 2020 to July 2022, 48 patients were enrolled in the study (38, six, two, one, and one patient from Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Qilu Hospital of Shandong University, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Shengli Oilfield Central Hospital, and Jining First People's Hospital, respectively) and detailed in Figure 1. The demographic and baseline characteristics of these patients are shown in Table 1. Of the patients enrolled, 29 were male and 19 were female. The median age was 59 years (range, 17–76). We recruited only patients with stages II, III, and IV. The majority of them had predominantly non‐GCB typing (89.6%). Additionally, 33 patients underwent NGS testing for TP53, with 12 patients showing TP53 mutation. Forty‐four patients tested for BCL‐6 FISH assay, and eight (18.2%) patients had BCL‐6 rearrangement.
FIGURE 1.
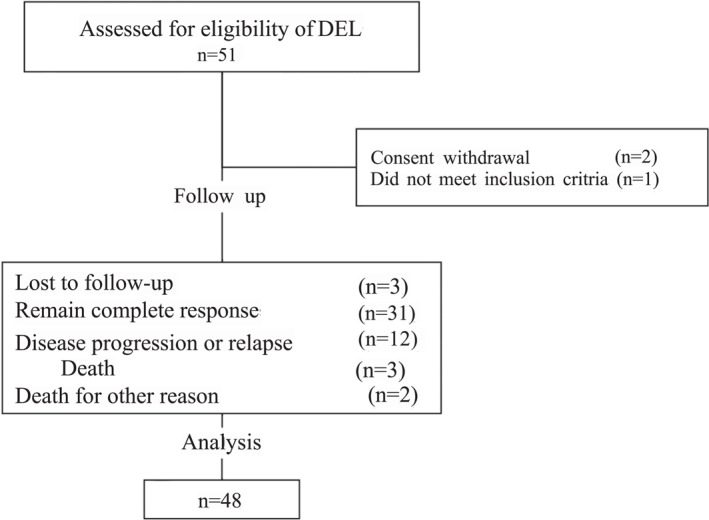
The CONSORT diagram “Death” in the diagram means patients died for disease progression or relapse. CONSORT indicates Consolidated Standards of Reporting Trials.
TABLE 1.
Baseline characteristics of total patients.
| No. | % | |
|---|---|---|
| Gender | 48 | |
| Male | 29 | 60.4 |
| Female | 19 | 39.6 |
| Age (years) | 48 | |
| Median (range) | 59 (17–76) | |
| <60 | 26 | 54.2 |
| ≥60 | 22 | 45.8 |
| No. of extranodal involvement | 48 | |
| 0‐1 | 30 | 62.5 |
| ≥2 | 18 | 37.5 |
| LDH | 41 | |
| Normal | 20 | 48.8 |
| Rise | 21 | 51.2 |
| Ann Arbor stage | 48 | |
| II | 22 | 45.8 |
| III–IV | 26 | 54.2 |
| IPI risk group | 48 | |
| 0–2 | 28 | 58.3 |
| 3–5 | 20 | 41.7 |
| Cell of origin (Hans) | 48 | |
| Non‐GCB | 43 | 89.6 |
| GCB | 5 | 10.4 |
| BCL6 rearrangement | 44 | |
| BCL‐6– | 36 | 81.8 |
| BCL‐6+ | 8 | 18.2 |
| BCL‐2 rearrangement | 44 | |
| BCL‐2– | 41 | 93.2 |
| BCL‐2+ | 3 | 6.8 |
| TP53 | 33 | |
| Wild | 21 | 63.6 |
| Mutation | 12 | 36.4 |
Abbreviations: BCL‐6+, BCL‐6 rearrangement positive; BCL‐2+, BCL‐2 rearrangement positive; GCB, germinal center B‐cell type; IPI, International Prognostic Index; LDH, lactate dehydrogenase; non‐GCB, nongerminal center B‐cell type.
Survival/efficacy
At the end of four treatment cycles, PET‐CT examination for efficacy evaluation revealed that 37 (77.1%) patients achieved CR, and 10 (20.8%) patients achieved PR. ORR reached 97.9% and CRR reached 77.1%. After completing eight cycles of treatment, our efficacy evaluation indicated that 40 patients (83.3%) achieved CR, three patients (6.3%) achieved PR, and three patients (6.3%) had progressive disease (PD). The ORR and CRR were 89.6% and 83.3%, respectively.
As of the follow‐up conducted until March 29, 2024, the median follow‐up was 29.3 (2.7–43.3) months. Three patients were lost to follow‐up. Thirty‐one patients (64.6%) remain in CR, with a total of 12 patients (25.0%) experiencing PD, of which three patients died due to PD after six cycles of treatment. One patient died of cardiovascular disease at the end of the second cycle of treatment at a nonresearch clinical center, and an autopsy was not performed, so it cannot be ruled out whether the death was caused by sudden death due to AEs. Additionally, one patient died because of the COVID‐19 infection after eight cycles of treatment and his efficacy evaluation is CR. The median PFS and OS were not reached. The PFS and OS were 81.25% and 93.75% at 2 years, respectively. The PFS and OS curves are shown in Figure 2A,B. All exhibited PR or CR at the first assessment Figure 3.
FIGURE 2.
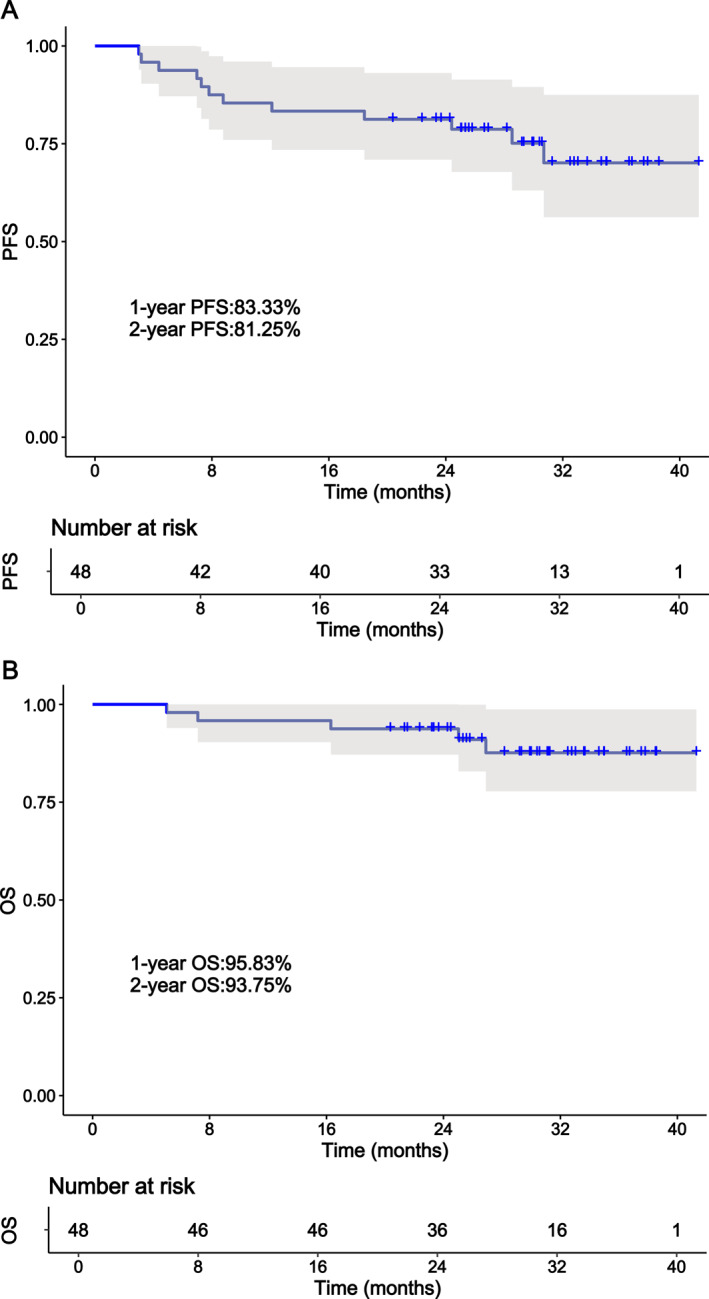
The PFS and OS of DEL patients treated with ZR‐CHOP. (A) Kaplan–Meier survival curve for PFS. (B) Kaplan–Meier survival curve for OS. DEL indicates double‐expressor lymphoma; OS, overall survival; PFS, progression‐free survival; ZR‐CHOP, zanubrutinib in combination with R‐CHOP.
FIGURE 3.
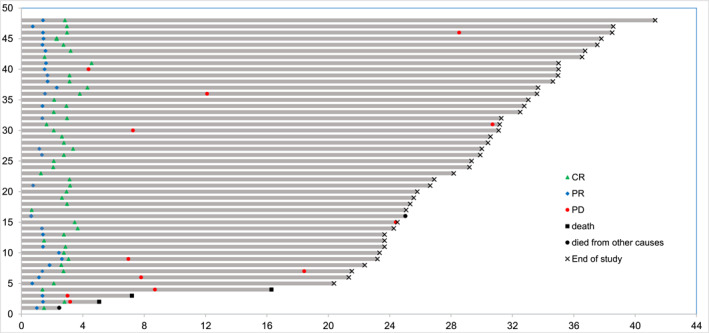
Swimmer plot of patients enrolled in the trial. Each row represents a patient and the length of each bar represents the time from treatment initiation. CR indicates complete response; PD, progressive disease; PR, partial response.
Univariate and multivariate analysis of survival
Cox univariate and multivariate models concerning the PFS and OS of the patients were performed as summarized in Tables 2 and 3. We performed a univariate analysis on the impacts of age, gender, number of extranodal involvement, lactate dehydrogenase (LDH), stage, International Prognostic Index (IPI), cell of origin, BCL‐6, TP53, and Eastern Cooperative Oncology Group (ECOG) for PFS and OS. Multivariate analysis revealed that BCL‐6 gene rearrangement was an adverse prognostic factor for both PFS (HR, 0.247; 95% CI, 0.068–0.9; p = .034) and OS (HR, 0.057; 95% CI, 0.006–0.591; p = .016), whereas the number of extranodal involvements also significantly influenced OS (HR, 15.12; 95% CI,1.07–213.65; p = .044). Factors associated with PFS and OS are shown in Figures 4A–F.
TABLE 2.
Univariate and multivariate analysis for PFS.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Gender | 1.698 (0.544–5.298) | .362 | ||
| Age (<60; ≥60), years | 0.832 (0.263–2.636) | .755 | ||
| No. of extranodal involvement (0–1; ≥2) | 6.519 (1.747–24.325) | .005 | 5.6 (0.807–39.179) | .081 |
| LDH (normal; rise) | 1.922 (0.561–6.587) | .298 | ||
| Ann Arbor stage (II; III–IV) | 10.734 (1.385–83.192) | .023 | 2.66 (0.21–33.73) | .450 |
| IPI risk group (0–2; 3–5) | 2.88 (0.864–9.6) | .085 | ||
| Cell of origin (Hans) non‐GCB; GCB | 0.696 (0.09–5.4) | .729 | ||
| BCL‐6 rearrangement | 0.289 (0.084–0.997) | .049 | 0.247 (0.068–0.9) | .034 |
| TP53 mutation | 0.24 (0.03–1.97) | .18 | ||
| ECOG | 1.308 (0.541–3.163) | .551 | ||
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B‐cell type; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; non‐GCB, nongerminal center B‐cell type; PFS, progression‐free survival.
TABLE 3.
Univariate and multivariate analysis for OS.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Gender | 0.356 (0.04–3.187) | .356 | ||
| Age (<60; ≥60), years | 5.276 (0.589–47.254) | .137 | ||
| No. of extranodal involvement (0–1; ≥2) | 9.097 (1.00–82.694) | .05 | 15.12 (1.07–213.65) | .044 |
| LDH (normal; rise) | 77.779 (0.056–108516) | .239 | ||
| Ann Arbor stage (II; III–IV) | 3.8 (0.431–34.665) | .227 | ||
| IPI risk group (0–2; 3–5) | 6.1 (0.691–55.426) | .103 | ||
| Cell of origin (Hans) non‐GCB; GCB | 1.678 (0.185–15.21) | .646 | ||
| BCL‐6 rearrangement | 0.113 (0.019–0.686) | .018 | 0.057 (0.006–0.591) | .016 |
| TP53 mutation | 0.519 (0.057–4.713) | .56 | ||
| ECOG | 2.25 (0.711–7.1) | .167 | ||
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B‐cell type; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; non‐GCB, nongerminal center B‐cell type; PFS, progression‐free survival.
FIGURE 4.
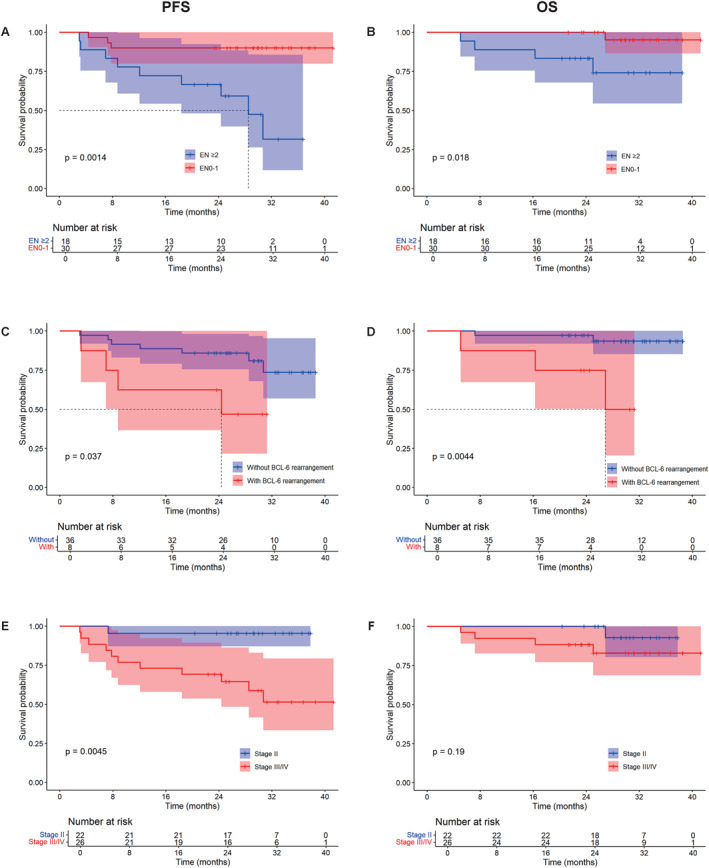
PFS and OS of DEL patients received ZR‐CHOP. Prognostic effector of EN (A and B), BCL‐6 gene rearrangement (C and D), and Ann Arbor stage (E and F) for PFS and OS. Abbreviations: DEL indicates double‐expressor lymphoma; EN, number of extranodal involvements; OS, overall survival; PFS, progression‐free survival; ZR‐CHOP, zanubrutinib in combination with R‐CHOP.
Biomarker substudy
We created a heatmap for 33 patients who were tested for the same genes. The results showed that the genes with the highest mutation rates were TP53 (36%), MYD88 (33%), and PIM1 (33%). Among the patients with TP53 mutations, four had TP53 oncogenic gene mutations, with only one patient showing PD at the sixth cycle (mutation site: NM_000546.844C>T [p.R282W] exon 8, mutation frequency: 45.50%). The remaining three patients maintained CR at the end of the last follow‐up. All seven patients with possible oncogenic mutations maintained CR at the end of follow‐up. Of the nine patients with MYD88 oncogenic gene mutations, one experienced PD (mutation site: NM_002468.4.794T>C [p.L265P] exon 5). Seven patients maintained CR at the end of follow‐up. One patient died of COVID‐19 (mutation frequency of 14.69%). The heatmap is shown in Figure 5.
FIGURE 5.
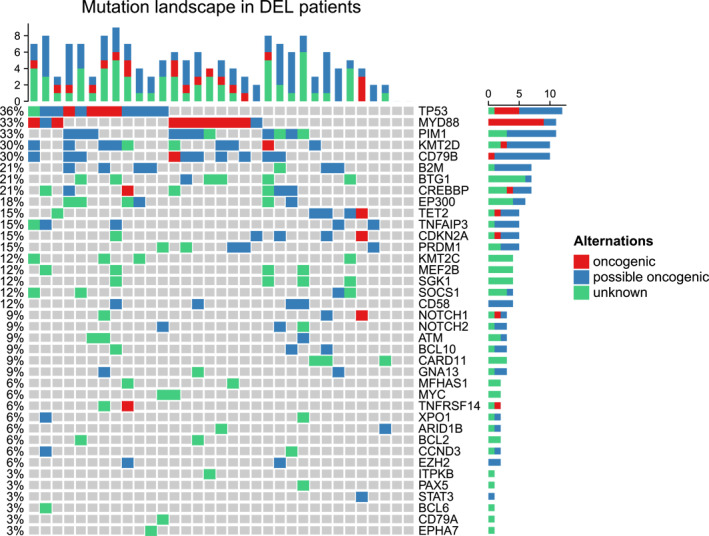
Mutation plot for 33 double‐expressor lymphoma cases. Red represents oncogenic, blue represents possible oncogenic, and green represents unknown.
STAT3 (HR, 31.49; 95% CI, 1.97–503.58, p = 0.015) and PAX5 (HR, 15.49; 95% CI, 1.40–170.89; p = .025) mutation associated with poor PFS. Moreover, mutation of NOTCH1 (HR, 7.12; 95% CI, 1.16–43.36; p = .033), CDKN2A (HR, 10.62; 95% CI, 1.76–63.98; p = .01), IRF8 (HR, 15.49; 95% CI, 1.4–170.89; p = .025), and BCL10 (HR, 6.98; 95% CI, 1.15–42.06; p = .034) were found to be associated with poor OS. However, no significant correlation was observed between TP53 mutations and either PFS (HR, 0.24; 95% CI, 0.03–1.97; p = .18) or OS (HR, 0.519; 95% CI, 0.057–4.713; p = .56) in the 33 patients who underwent TP53 gene testing.
Because only 33 cases underwent NGS testing, the 15 patients did not undergo NGS testing due to insufficient material, and we must exclude the differences between patients who did not undergo testing and those who did. First, there was no significant difference in the sources of the two groups, seven of the 15 patients came from Shandong Cancer Hospital, whereas the other eight were from other research centers. Second, a demographic analysis showed that the overall proportions of gender, age (<60; ≥60), LDH (normal; elevated), Ann Arbor stage (II; III–IV), and IPI risk group (0–2; 3–5) were similar between the two groups (Table S1). However, regarding the number of extranodal involvement, patients who did not undergo NGS had a lower percentage of those with two or more extranodal sites (42.4% vs. 26.7%). Additionally, 100% of the patients without NGS were identified as non‐GCB. In terms of survival analysis, there were no statistically significant differences in PFS (p = .792) and OS (p = .122) between NGS‐tested and non‐NGS‐tested patients (Figures S1).
Additionally, 44 patients underwent FISH testing for BCL‐2, BCL‐6, and MYC genes. We found a total of eight patients with BCL‐6 rearrangement. Among the 12 patients with PD, four patients were BCL‐6 rearrangement. Among the three patients who died due to the disease, one patient had BCL‐6 rearrangement, one had both BCL‐6 rearrangement and TP53 mutation, and another had TP53 mutation. Univariate and multivariate analyses showed that BCL‐6 rearrangement was an adverse prognostic factor for PFS and OS in patients with DEL (p < .05).
Toxicity and safety
Treatment was well tolerated with no dose reductions or interruptions, and the most common grade ≥3 adverse events were neutropenia (18; 37.5%), pulmonary infection (11; 22.9%), febrile neutropenia (three; 6.3%), anemia (four; 8.3%), and thrombocytopenia (four; 8.3%). All the details of the AEs are summarized in Table 4.
TABLE 4.
AEs in DEL patients receiving ZR‐CHOP.
| AEs | Patients with events, No. (%) | |
|---|---|---|
| All grades | Grade 3–4 | |
| Fever | 12 (25.0) | 0 |
| Febrile neutropenia | 3 (6.3) | 3 (6.3) |
| Gastritis | 1 (2.1) | 0 |
| GERD | 6 (12.5) | 0 |
| Dyspepsia | 9 (18.8) | 0 |
| Oral mucositis | 9 (18.8) | 0 |
| Edema of the limbs | 2 (4.2) | 0 |
| Fatigue | 19 (39.6) | 0 |
| Pulmonary infection | 16 (33.3) | 11 (22.9) |
| Upper respiratory tract infection | 2 (4.2) | 0 |
| Skin Infection | 2 (4.2) | 0 |
| Increased alanine aminotransferase | 11 (22.9) | 0 |
| Increased aspartate transferase | 7 (14.6) | 0 |
| Anemia | 25 (52.1) | 4 (8.3) |
| Neutropenia | 34 (70.8) | 18 (37.5) |
| Thrombocytopenia | 11 (22.9) | 4 (8.3) |
Abbreviations: AEs, adverse events; DEL, double‐expressor lymphoma; GERD, gastroesophageal reflux; ZR‐CHOP, zanubrutinib in combination with R‐CHOP.
DISCUSSION
DEL was characterized by the overexpression of MYC and BCL‐2 proteins, which is unrelated to gene rearrangements. It primarily originates from nongerminal center B‐cell type (non‐GCB) and is highly aggressive. Patients with DEL have a poor prognosis with the standard R‐CHOP treatment regimen.
Until recently, little prospective study has been made to improve the prognosis of patients with DEL. Several retrospective studies have found no benefit with the intensified DA‐EPOCH‐R regimen. 4 , 5 Similarly, in the context of chemotherapy followed by autologous transplantation, the SWOG 9704 study and the DLCL04 study showed a tendency to improve the prognosis of DEL patients, but the small sample size remains a limitation. 15 , 16
Some other studies explore the effects of the R‐CHOP regimen combined with other treatments. The POLARIX study aimed to compare the 2‐year PFS of R‐CHOP with that of Pola‐R‐CHP in patients with intermediate‐risk or high‐risk DLBCL (IPI ≥2) who had not received prior therapy. Subgroup analyses of DEL patients showed improved PFS for patients treated with Pola‐R‐CHP regimen than those with R‐CHOP, 17 but no confirmation study has been made and the cost of Pola imposes a financial burden on patients. In a randomized, double‐blind, placebo‐controlled, multicenter phase 3 clinical trial (DEB study), the efficacy and safety of chidamide, a histone deacetylase inhibitor that promotes apoptosis in tumor cells by directly downregulating MYC and BCL2 proteins and upregulates CD20 expression to increase tumor cell sensitivity to rituximab, combined with R‐CHOP were compared to R‐CHOP in treatment‐naive patients with DEL patients. 18 , 19 Interim results indicated that chidamide combined with R‐CHOP significantly improved the CRR in DEL patients compared to the standard R‐CHOP regimen (73.0% vs. 61.8%). Additionally, 24‐month event‐free survival also showed a clear trend of benefit (58.9% vs. 46.2%). Chidamide combined with R‐CHOP is approved as a new regimen for DEL in China, however, the 2‐year EFS is still unsatisfactory. 20 The present study using ZR‐CHOP achieved CRR of 83.3% and 2‐year PFS of 81.25%, which is relatively higher than any other studies reported up to now.
Another investigation is a randomized phase 2/3 study on treating previously untreated DEL patients with R‐CHOP with or without the BCL‐2 inhibitor venetoclax. The results showed no statistical difference in estimated 12‐month PFS and OS between the two groups (PFS: HR, 0.98; 95% CI, 0.48–2.01; p = .95 and OS: HR, 1.27; 95% CI, 0.57–2.79; p = .56). The addition of venetoclax to R‐CHOP led to increased toxicity (Alliance A051701). 21
The Phoenix study found that IR‐CHOP improved EFS and OS in DEL subgroup patients younger than 60 years. However, no improvement was observed in patients 60 years older, due to the increased AEs and consequently insufficient use of both ibrutinib and chemotherapy. 22
Zanubrutinib is a next‐generation small‐molecule BTKi with less toxicity due to less off‐target effects on EGFR, TEC, ITK, and other kinases. 23 Currently, it has been approved for the treatment of mantle cell lymphoma, adult chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and Waldenström macroglobulinemia (WM). Consequently, an increasing number of studies are investigating the efficacy of ZR‐CHOP for various forms of malignant lymphoma. For instance, a study on the ZR‐CHOP regimen for newly diagnosed non‐GCB DLBCL patients with multiple extranodal involvement reported an ORR of 91.7%, a CRR of 79.2%, an estimated 2‐year PFS of 83.1%, and a 2‐year OS of 87.1%. 24 Additionally, a study involving untreated DLBCL patients with specific gene expression demonstrated an ORR of 90%, a CRR of 76%, a 2‐year PFS of 74%, a 2‐year OS of 87%, and a complete metabolic response (CMR) of 82% (MCD type). 25 The research on intravascular large B‐cell lymphoma (IVBCL) reported a CRR of 12 of 13. All these studies achieved high response rates and showed overall favorable safety profiles, with significant hematological toxicity primarily manifesting as neutropenia (66.7%; 5 of 55; 15 of 23). Notably, in the study involving newly diagnosed non‐GCB DLBCL patients with multiple extranodal involvements, two patients experienced atrial fibrillation, whereas no occurrences were reported in other studies. 26
However, no prospective study has been reported on DEL. That is why this study was initiated. As expected, ZR‐CHOP got a CRR of 83.3% and a 2‐year PFS of 81.25% after almost 30 months of follow‐up. Our results confirmed that no significant differences in PFS (HR, 0.832; 95% CI,0.263‐2.636; p = 0.755) and OS (HR, 5.276; 95% CI, 0.589–47.254; p = .137) across age groups. Compared to the ibrutinib group in the Phoenix study, zanubrutinib demonstrated better safety (all‐grade adverse events: 77.55% vs. 100%; grade ≥3 adverse events: 47.91% vs. 89.9%) and no patients discontinued due to adverse events.
Nevertheless, phase 3 trials comparing zanubrutinib monotherapy to ibrutinib monotherapy in relapsed/refractory CLL and WM revealed that neutropenia occurred more frequently in the zanubrutinib arms. 27 Notably, the incidence of ≥3 grade neutropenia in our study was significantly lower than that observed in the PHOENIX study. This phenomenon may be influenced by various factors. 22 First, differences in patient baseline characteristics may play an important role. In the PHOENIX study, the overall incidence of bone marrow involvement in the IR‐CHOP group was 11.9%, with 12.5% in patients under 60 years old 22 ; in our study, the overall incidence of bone marrow involvement was 8.3% (four of 48), which may contribute to the lower incidence of neutropenia in our study. Second, in the ALPINE study, although neutropenia was more common in the zanubrutinib group (16%) compared to the ibrutinib group (13.9%), 27 the analysis of the Chinese subgroup in ALPINE showed that the incidence of neutropenia ≥3 grade was higher in the ibrutinib group (30.2%) than in the zanubrutinib group (24.4%). 28 Third, age may also be a contributing factor, in the PHOENIX study, 62.8% of patients in the IR‐CHOP group were ≥60 years old, whereas in our study, the proportion of patients ≥60 years old was 45.8%. Additionally, compared to the ALPINE and PHOENIX studies, our study has a smaller patient population, which presents certain limitations. 22 , 27 Finally, although the study protocol did not mandate preventive measures for neutropenia, researchers at each study center used granulocyte colony‐stimulating factor (G‐CSF) for proactive secondary prevention at their discretion. This may lead to a lower incidence of neutropenia in our study.
The mechanism of BTKi on DEL remains unclear. It is supposed that the B‐cell receptor (BCR) and nuclear factor–κB (NF‐κB) signaling transduction are involved in the pathogenesis of DLBCL. 29 , 30 The activated NF‐κB promotes the expression of BCL‐2 and MYC. BTKi blocked the BCR and NF‐κB pathway, reducing the expression of the two proteins and overcoming their influence.
Based on the FISH results of 44 patients, our study found that the presence of BCL‐6 rearrangement was a poor prognostic factor for DEL patients, both in univariate and multivariate analyses. BCL‐6 rearrangement patients are often associated with high expression of the BCL‐6 protein. In previous studies, the expression level of BCL‐6 is significantly correlated with the OS and PFS of DLBCL patients (p < .05). 31 , 32 Typically, positive expression of BCL‐6 is associated with better OS (p = .02). 33 Studies show that BCL‐6–/MYC+ expressing patients have poorer PFS than those expressing BCL‐6+/MYC+ (p < .05). 34 However, in the DLBCL patients with high expression of MYC and BCL‐2 proteins, additional BCL‐6 rearrangement causes poor outcomes. The detailed mechanisms underlying this discrepancy require further study.
TP53 is a critical oncogene, with a mutation rate as high as 21% in patients with primary DLBCL. Patients with TP53 expression greater than 50% are not responsive to traditional R‐CHOP regimens. 35 TP53 mutation is an independent marker of poor prognosis in DLBCL patients. 36 , 37 Various previous treatments have failed to alter the adverse prognostic effects of TP53 mutations, even with CAR‐T–cell therapy. 38 In our trial, NGS was performed on 33 patients, 12 of whom had TP53 mutations. The patients with TP53 mutations have relatively poor outcomes versus those with wild‐type TP53, but the difference was not significant (p > .05). This could be attributed to the small sample size.
Among the patients who refractory or relapsed by the follow‐up date, most had three or more mutated genes, suggesting that the overall accumulation of various mutated genes may be a factor contributing to poorer prognosis.
Our study has several limitations compared to other studies. First, the sample size is small, and a larger sample size study is required. Second, our study is a single‐arm phase 2 trial, the comparison above mentioned is indirect and based on different studies. Despite these disadvantages, ZR‐CHOP demonstrated an excellent response rate and 2‐year PFS and OS in DEL patients with stage II or late disease. Additionally, the regimen is well tolerated, especially for patients ≥60 years old, who did not benefit from IR‐CHOP in the Phoenix study. These findings encourage us to initiate the multicenter, phase 3 clinical study, and the underlying mechanisms of BTKi on DEL are under investigation, as well as the prognostic factors.
AUTHOR CONTRIBUTIONS
Xia Yin: Data curation; validation; writing–original draft; investigation; visualization; formal analysis; and writing–review and editing. Qiang He: Conceptualization; data curation; writing–review and editing; funding acquisition; supervision; and methodology. Dan Liu: Data curation; formal analysis; methodology; and writing–review and editing. Linna Xie: Data curation and writing–review and editing. Hui Wang: Data curation and writing–review and editing. Chunyan Chen: Data curation and writing–review and editing. Chuanli Zhao: Data curation and writing–review and editing. Ningning Shan: Data curation and writing–review and editing. Shanshan Shi: Data curation and writing–review and editing. Haichen Wei: Data curation and writing–review and editing. Ji Ma: Data curation and writing–review and editing. Ke Lu: Data curation and writing–review and editing. Liang Wang: Data curation and writing–review and editing. Yan Wang: Data curation and writing–review and editing. Lijie Xing: Data curation; conceptualization; methodology; writing–review and editing; visualization; and supervision. Zengjun Li: Resources; project administration; writing–review and editing; funding acquisition; methodology; conceptualization; and supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supplementary Material S1
Figure S1
Table S1
ACKNOWLEDGMENTS
The authors thank the patients who participated in the study. This research was supported by the Start‐up Fund of Shandong Cancer Hospital (2020‐129‐02), the Natural Science Foundation of Shandong Province (ZR2021MH072), the National Natural Science Foundation of China (82200224), the China Postdoctoral Science Foundation (2023M732124), and Shandong Excellent Youth Science Fund Project (Overseas) (2023HWYQ‐115).
Yin X, He Q, Liu D, et al. Zanubrutinib plus R‐CHOP for the treatment of newly diagnosed double‐expressor lymphoma: a phase 2 clinical study. Cancer. 2025;e35697. doi: 10.1002/cncr.35697
Xia Yin and Qiang He contributed equally to this article.
Contributor Information
Lijie Xing, Email: xiaopiao423@126.com.
Zengjun Li, Email: zengjunli@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443‐459. doi: 10.3322/caac.21357 [DOI] [PubMed] [Google Scholar]
- 2. Riedell PA, Smith SM. Double hit and double expressors in lymphoma: definition and treatment. Cancer. 2018;124(24):4622‐4632. doi: 10.1002/cncr.31646 [DOI] [PubMed] [Google Scholar]
- 3. Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452‐3459. doi: 10.1200/JCO.2011.41.0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Angelo CR, Hanel W, Chen Y, et al. Impact of initial chemotherapy regimen on outcomes for patients with double‐expressor lymphoma: a multi‐center analysis. Hematol Oncol. 2021;39(4):473‐482. doi: 10.1002/hon.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Othman T, Penaloza J, Zhang S, et al. R‐CHOP Vs DA‐EPOCH‐R for double‐expressor lymphoma: a University of California Hematologic Malignancies Consortium retrospective analysis. Clin Lymphoma Myeloma Leuk. 2022;22(10):e947‐e957. doi: 10.1016/j.clml.2022.06.013 [DOI] [PubMed] [Google Scholar]
- 6. Davies AJ, Barrans S, Stanton L, et al. Differential efficacy from the addition of bortezomib to R‐CHOP in diffuse large B‐cell lymphoma according to the molecular subgroup in the REMoDL‐B study with a 5‐year follow‐up. J Clin Oncol. 2023;41(15):2718‐2723. doi: 10.1200/JCO.23.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu J, Hong X, Song YQ, et al. Ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone in patients with previously untreated non‐germinal centre B‐cell‐like diffuse large B‐cell lymphoma: a Chinese subgroup analysis of the phase III PHOENIX trial. EJHaem. 2022;3(4):1154‐1164. doi: 10.1002/jha2.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies A, Barrans S, Burton C, et al. ACCEPT ‐ combining acalabrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R‐CHOP) for diffuse large B‐cell lymphoma (DLBCL): study protocol for a phase Ib/II open‐label non‐randomised clinical trial. F1000Res. 2020;9:941. doi: 10.12688/f1000research.22318.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sehn LH, Kahl BS, Matasar MJ, et al. ESCALADE: A phase 3 study of acalabrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) for patients ≤65y with untreated non‐germinal center B‐cell–like (non‐GCB) diffuse large B‐cell lymphoma (DLBCL). J Clin Oncol. 2021;39(suppl 15):TPS7572. doi: 10.1200/JCO.2021.39.15_suppl.TPS7572 [DOI] [Google Scholar]
- 10. Guo Y, Liu Y, Hu N, et al. Discovery of zanubrutinib (BGB‐3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J Med Chem. 2019;62(17):7923‐7940. doi: 10.1021/acs.jmedchem.9b00687 [DOI] [PubMed] [Google Scholar]
- 11. Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single‐arm, multicenter study. J Hematol Oncol. 2020;13(1):48. doi: 10.1186/s13045-020-00884-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song Y, Zhou K, Zou D, et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long‐term efficacy and safety results from a phase 2 study. Blood. 2022;139(21):3148‐3158. doi: 10.1182/blood.2021014162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma J, Zhu J, Shen z, et al. Guidelines of Chinese Society of Clinical Oncology (CSCO) lymphoid malignancies. 2022. https://meeting.csco.org.cn/pdf/web/viewer.html?file=/Upload/Periodical/20230111024217.pdf
- 14. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059‐3068. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiappella A, Martelli M, Angelucci E, et al. Rituximab‐dose‐dense chemotherapy with or without high‐dose chemotherapy plus autologous stem‐cell transplantation in high‐risk diffuse large B‐cell lymphoma (DLCL04): final results of a multicentre, open‐label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18(8):1076‐1088. doi: 10.1016/S1470-2045(17)30444-8 [DOI] [PubMed] [Google Scholar]
- 16. Puvvada SD, Stiff PJ, Leblanc M, et al. Outcomes of MYC‐associated lymphomas after R‐CHOP with and without consolidative autologous stem cell transplant: subset analysis of randomized trial intergroup SWOG S9704. Br J Haematol. 2016;174(5):686‐691. doi: 10.1111/bjh.14100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B‐cell lymphoma. N Engl J Med. 2022;386(4):351‐363. doi: 10.1056/NEJMoa2115304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao S, Guo J, Zhao Y, et al. Chidamide, a novel histone deacetylase inhibitor, inhibits the viability of MDS and AML cells by suppressing JAK2/STAT3 signaling. Am J Transl Res. 2016;8(7):3169‐3178. [PMC free article] [PubMed] [Google Scholar]
- 19. Guan XW, Wang HQ, Ban WW, et al. Novel HDAC inhibitor chidamide synergizes with rituximab to inhibit diffuse large B‐cell lymphoma tumour growth by upregulating CD20. Cell Death Dis. 2020;11(1):20. doi: 10.1038/s41419-019-2210-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao W, Zhu J, Song Y, et al. Tucidinostat plus R‐CHOP in previously untreated diffuse large B‐cell lymphoma with double expression of MYC and BCL2: an interim analysis from the phase III DEB study. J Clin Oncol. 2024;42(suppl 17):LBA7003. doi: 10.1200/JCO.2024.42.17_suppl.LBA7003 [DOI] [Google Scholar]
- 21. Abramson JS, Geyer SM, Pederson LD, et al. Randomized phase II/III study of R‐CHOP +/‐ venetoclax in previously untreated MYC/BCL2 double expressor diffuse large B cell lymphoma (DLBCL): Alliance A051701. J Clin Oncol. 2024;42(suppl 16):7012. doi: 10.1200/JCO.2024.42.16_suppl.7012 [DOI] [Google Scholar]
- 22. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non‐germinal center B‐cell diffuse large B‐cell lymphoma. J Clin Oncol. 2019;37(15):1285‐1295. doi: 10.1200/JCO.18.02403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B‐cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851‐859. doi: 10.1182/blood.2019001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geng H, Li J, Zhang Y, et al. Zanubrutinib combined with R‐CHOP regimen in the treatment of untreated non‐germinal center B‐cell subtype diffuse large B‐cell lymphoma with multiple extranodal involvement [PB3028]. Poster presented at: 29th European Hematology Association Congress; June 13–16, 2024; Madrid, Spain.
- 25. Zhang Q, Jiang S, Chen G, et al. The phase II study of zanubrutinib combined with R‐CHOP in previously untreated diffuse large B‐cell lymphoma (DLBCL) patients with specific gene‐expression [P1218]. Poster presented at: 29th European Hematology Association Congress; June 13–16, 2024; Madrid, Spain.
- 26. Zhang Y, Chen C, Zhao D, et al. P1185: a prospective single‐center phase 2 study of zanubrutinib plus R‐CHOP in treat‐naïve intravascular large B cell lymphoma. Hemasphere. 2023;7(suppl):e1662573. doi: 10.1097/01.HS9.0000971636.16625.73 [DOI] [Google Scholar]
- 27. Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319‐332. doi: 10.1056/NEJMoa2211582 [DOI] [PubMed] [Google Scholar]
- 28. Zhou K, Wang T, Pan L, et al. Improved efficacy and safety of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) in China: a subgroup of ALPINE. Ann Hematol. 2024;103(10):4183‐4191. doi: 10.1007/s00277-024-05823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B‐cell lymphoma. N Engl J Med. 2018;378(15):1396‐1407. doi: 10.1056/NEJMoa1801445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turturro F. Constitutive NF‐κB activation underlines major mechanism of drug resistance in relapsed refractory diffuse large B cell lymphoma. BioMed Res Int. 2015;2015:484537. doi: 10.1155/2015/484537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen WT, Yao HX, Wu CM, Liu D, Tang RM. [Value of MYC, BCL‐2 and BCL‐6 for evaluation of prognosis in patients with diffuse large B cell lymphoma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(2):452‐457. doi: 10.19746/j.cnki.issn.1009-2137.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 32. Liang X, Wang J, Bai W, Sun R. [Expression of CD68, cyclin D1 and rearrangement of BCL‐6 gene are adverse prognostic factors in diffuse large B‐cell lymphoma]. Zhonghua Bing Li Xue Za Zhi. 2015;44(8):559‐564. [PubMed] [Google Scholar]
- 33. Bodoor K, Matalka I, Hayajneh R, Haddad Y, Gharaibeh W. Evaluation of BCL‐6, CD10, CD138 and MUM‐1 expression in diffuse large B‐cell lymphoma patients: CD138 is a marker of poor prognosis. Asian Pac J Cancer Prev. 2012;13(7):3037‐3046. doi: 10.7314/apjcp.2012.13.7.3037 [DOI] [PubMed] [Google Scholar]
- 34. Li L, Zhang X, Zhang T, et al. Prognostic significance of BCL‐2 and BCL‐6 expression in MYC‐positive DLBCL. Clin Lymphoma Myeloma Leuk. 2018;18(10):e381‐e389. doi: 10.1016/j.clml.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 35. Stefancikova L, Moulis M, Fabian P, et al. Prognostic impact of p53 aberrations for R‐CHOP‐treated patients with diffuse large B‐cell lymphoma. Int J Oncol. 2011;39(6):1413‐1420. doi: 10.3892/ijo.2011.1170 [DOI] [PubMed] [Google Scholar]
- 36. Jiang S, Qin Y, Jiang H, et al. Molecular profiling of Chinese R‐CHOP treated DLBCL patients: identifying a high‐risk subgroup. Int J Cancer. 2020;147(9):2611‐2620. doi: 10.1002/ijc.33049 [DOI] [PubMed] [Google Scholar]
- 37. Xu‐Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B‐cell lymphoma patients treated with R‐CHOP: report from an International DLBCL Rituximab‐CHOP Consortium program study. Blood. 2012;120(19):3986‐3996. doi: 10.1182/blood-2012-05-433334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Porpaczy E, Wohlfarth P, Königsbrügge O, et al. Influence of TP53 mutation on survival of diffuse large B‐cell lymphoma in the CAR T‐cell era. Cancers (Basel). 2021;13(22):5592. doi: 10.3390/cancers13225592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Figure S1
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


