Abstract
Background/Aim
Reduction in skeletal muscle mass during chemotherapy is associated with poor outcomes. This study investigated the impact of changes in the psoas muscle index (PMI) on the prognosis of patients with unresectable colorectal liver metastases (CRLM) undergoing chemotherapy, including subgroup analyses based on the initial treatment response assessment.
Patients and Methods
We evaluated 47 patients with unresectable CRLM who underwent systematic chemotherapy and assessed changes in PMI to determine their prognosis.
Results
Changes in PMI were significantly associated with the presence or absence of primary tumor resection and the chemotherapeutic responses to first-line chemotherapy. The PMI reduction group was significantly associated with poor prognosis in both overall survival (OS) and progression-free survival (PFS) in patients with CRLM, and in both OS and PFS in the partial response (PR) group at the initial chemotherapy response assessment.
Conclusion
Skeletal muscle loss at chemotherapy initiation was significantly associated with poorer survival in patients with unresectable CRLM. Maintaining muscle mass could serve as a new indicator for identifying patients with a PR at the initial chemotherapy response assessment for prognosis. Personalized interventions should be investigated to determine whether they can improve muscle mass and lead to better clinical outcomes.
Keywords: Colorectal cancer, psoas muscle index, skeletal muscle mass, liver metastases, chemotherapy, systemic therapy, prognosis
Sarcopenia is a syndrome described by Rosenberg in 1989 (1), as an age-related decrease in muscle mass and is caused by various factors, including aging, inactivity, lifestyle habits, and chronic disease (2).
Sarcopenia is prevalent in 15-70% of patients with cancer and is associated with a decline in physical function, impaired quality of life, and difficulty in continuing treatment (3-6). Additionally, advances in sarcopenia assessment techniques have revealed close relationships between sarcopenia and survival in different solid tumors (3,5-7).
In patients with colorectal cancer (CRC), a low preoperative skeletal muscle index (SMI) or psoas muscle index (PMI) at the lumbar levels is associated with poor survival (7-9), and muscle mass loss during the first 1-2 years after diagnosis is related to overall and cancer-specific mortality in stages I-III (10).
Although a close relationship between baseline low muscle mass and poor prognosis has been reported in patients with CRC (7-9,11-13), other studies have suggested that in a cohort of patients receiving chemotherapy, baseline low muscle mass is not prognostic and that changes in muscle mass during treatment are more critical (14,15). These aspects, however, remain unelucidated.
The liver is the most common site of distant metastasis in CRC, with liver metastases present in 20% of patients at the initial tumor diagnosis and emerging later in an additional 25-50% of patients (16,17). Recent studies have reported that the 5-year survival rate after conversion treatment is 31-47% (18-22), demonstrating that the implementation of conversion treatment significantly affects the prognosis of patients with colorectal liver metastases. Moreover, most patients with unresectable CRC receive systemic chemotherapy. Among those who do not undergo conversion treatment despite initial tumor shrinkage, the majority eventually experience progressive disease (PD). Therefore, early prediction of prognosis, regardless of the initial response to systemic chemotherapy, is crucial.
This study aimed to determine whether muscle mass loss could serve as a prognostic factor in patients with liver-only metastases, and investigate the prognostic impact of early changes in the PMI during systemic chemotherapy and their association with clinicopathological factors.
Patients and Methods
A total of 52 patients with unresectable colorectal liver metastasis (CRLM) who received first-line systemic chemotherapy at Osaka Metropolitan University between January 2008 and December 2016 were retrospectively reviewed. The study was approved by the Osaka Metropolitan University Ethics Committee (Approval number: 4182). Written informed consent was obtained from all patients.
None of the patients had been definitively diagnosed with metastasis to other organs at the time of the liver metastasis. Synchronous CRLM was identified at the time of primary colorectal tumor diagnosis, while metachronous CRLM was defined as any CRLM identified after the primary colorectal tumor diagnosis (23). Five patients were excluded due to the absence of suitable computed tomography (CT) images and incomplete laboratory data, and finally, 47 patients were enrolled in the study. The chemotherapy agents administered for systemic therapy included a combination of 5-fluorouracil (5-FU) and oxaliplatin (mFOLFOX6) (24-26), a 5-FU and irinotecan (FOLFIRI) (27), Capecitabine + Oxaliplatin (CapeOx) (28), S-1+ Oxaliplatin (SOX) (29), and Capecitabine monotherapy (30).
Based on the RAS mutation status of the tumors (31,32), targeted biologics such as anti-vascular endothelial growth factor antibodies, such as bevacizumab, and anti-epidermal growth factor receptor antibodies, including panitumumab and cetuximab, were added to the aforementioned regimens. Adjustments to chemotherapy dosing or cessation were based on the doctor’s judgment and the patient’s condition.
Tumor response to chemotherapy was evaluated using CT imaging based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (33). Resectability of CRLM was assessed periodically, and curative liver resection was performed after discussions at joint conferences or cancer board meetings by multidisciplinary teams.
Conversion treatment was defined as converting initially unresectable liver metastases to resectable lesions after systemic chemotherapy, with the patient showing no evidence of disease following local radical treatment, including surgery and radiofrequency ablation (RFA). Six patients received adjuvant chemotherapy [e.g., 5-FU + folinate monotherapy or combinations, such as mFOLFOX6 or CapeOx (32)] following conversion therapy.
Patients were observed through physical examinations, serum tumor marker measurements, and CT scans. Treatment for recurrence was determined by factors, such as tumor size, location, growth rate, preceding therapies, and patient preferences and conditions. Eleven patients with metachronous liver metastases had previously received adjuvant therapy following primary tumor resection. Oxaliplatin doublet chemotherapy was administered in three cases, whereas oral anticancer agents were in eight cases.
Overall survival (OS) was measured from the date of treatment initiation until the date of death due to any cause or the last known follow-up for patients who were still alive. Progression-free survival (PFS) was defined as the time from treatment initiation to the date of disease progression, recurrence, death, or last contact with the patient. Patients were clinically followed up with physical examinations and blood tests. Demographic, pathological, and outcome data were collected for each patient.
Data collection. Clinical data were retrospectively obtained from the electronic patient databases. Data included baseline characteristics [sex, age, primary tumor location, chemotherapy exposure, evaluation of chemotherapy efficacy, information about treatment, Eastern Cooperative Oncology Group Performance Status (ECOG PS), serum albumin, C-reactive protein (CRP), and RAS status]; liver tumor variables (number of metastases, size of the largest metastases); disease recurrence; and date of death. To quantify the tumor burden in CRLM patients, we used the tumor burden score (TBS), developed by Sasaki et al. (34) as a prognostic marker. This was calculated as follows: TBS2=(maximum tumor diameter)2+ (number of liver lesions)2.
Psoas muscle index (PMI). We measured the cross-sectional area of the bilateral psoas muscles using manual tracing on CT images. The bilateral psoas muscle area (cm2) was calculated at the level of the navel from the CT images by a single investigator who was blinded to the patient outcomes. The PMI was calculated as follows: PMI=cross-sectional area of the bilateral psoas muscle/heights2 (cm2/m2) (35). We calculated the PMI (cm2/m2) at the initiation of first-line therapy (pre-PMI) and at the initial chemo-response assessment (post-PMI) [median duration: 2.43 months (1.23-4.23 months)]. We subtracted the pre-PMI from the post-PMI, divided it by the pre-PMI, and multiplied it by 100 to determine the change rate in PMI.
Statistical analysis. All patients were classified into two groups according to the median of each variable, except for the changes in PMI. Differences in categorical variables between groups were estimated using the chi-square test and Fisher’s exact test, where appropriate. Survival curves were constructed using the Kaplan–Meier method and compared using a log-rank test. The influence of each prognostic factor on patient survival was evaluated using Cox regression analysis. Multivariate logistic regression models were used to predict the factors influencing conversion treatment. Statistical significance was set at p<0.05. In multivariate analysis, p<0.05 was included as a factor. All analyses were performed using the JMP 14.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics. Forty-seven patients with unresectable CRLM underwent systemic chemotherapy after their diagnosis. Table I presents the patient characteristics. The study population included 32 men and 15 women, with a median patient age of 66 years (36-84 years) at the time of CRLM diagnosis. According to the RECIST (33), the chemotherapeutic responses at the initial chemotherapy response assessment for CRLM in patients were evaluated as follows: partial response (PR), n=25; stable disease (SD), n=16; and PD, n=6. Responses to the first-line systemic chemotherapy for CRLM in the patients were as follows: complete response (CR), n=2; PR, n=13; SD, n=7; and PD, n=25. The response and disease control rate were 32% and 47%, respectively.
Table I. Characteristics of patients with colorectal cancer liver metastasis.
ECOG PS: Eastern Cooperative Oncology Group Performance Status; RFA: radiofrequency ablation; CRP: C-reactive protein; TBS: tumor burden score; BMI: Body Mass Index; PMI: psoas muscle index.
Previous studies have identified sex-based differences in PMI among patients with cancer (35). The median of pre-treatment PMI was 7.41 cm2/m2 (3.79 cm2/m2-11.54 cm2/m2) for men and 5.11 cm2/m2 (2.95 cm2/m2-6.86 cm2/m2) for women. Using these results, we determined the cutoff values for the pretreatment PMI for men and women separately and for each type of cancer.
The proposed cutoff value for sarcopenia is the average PMI of healthy people -2SD (6.36 cm2/m2 for men, 3.92 cm2/m2 for women) (36). Based on these criteria, nine men and four women in this cohort were diagnosed with sarcopenia.
The number of patients with >5% skeletal muscle loss was 29. The mean rate of change in the PMI was 10.66% (range=–21.73%-39.13%). We set a 5% reduction in PMI as the cutoff value based on a report that patients with unresectable CRC having >5% skeletal muscle reduction had poor prognosis (14). Patients were categorized into PMI reduction and non-reduction groups according to the cutoff value.
Correlation between the change in the PMI and clinico-pathological characteristics. Next, we assessed the association between changes in PMI and clinicopathological characteristics of patients with CRLM (Table II). Regarding clinical and pathological features, PMI reduction group was significantly associated with the presence or absence of colectomy (p=0.0031), chemotherapeutic response to first-line therapy (p=0.0063), higher CRP levels (p=0.030), and >5% body weight loss (p=0.014). Although a significant relationship was observed between the change in PMI and body weight, 40% of the patients had a decrease in muscle mass, even though their body weight did not decrease, indicating that muscle mass loss cannot always be detected solely by monitoring weight changes.
Table II. Correlation between clinicopathological variables and psoas muscle index (PMI) reduction in patients with colorectal cancer liver metastasis.
*Statistically significant. ECOG PS: Eastern Cooperative Oncology Group Performance Status; CRP: C-reactive protein; TBS: tumor burden score; BMI: Body Mass Index; PMI: psoas muscle index.
Relationship between the change in the PMI and side effects. Using the Common Terminology Criteria for AEs v.4.0, the Japanese Clinical Oncology Group version and side effects from treatment initiation to initial chemo-response assessment were estimated. Although not statistically significant, decreased appetite (p=0.082) and stomatitis (p=0.082) were associated with the PMI reduction group (Table III). These results suggest that the side effects of chemotherapy may influence changes in skeletal muscle.
Table III. Correlation between side effects and psoas muscle index (PMI) reduction in patients with colorectal cancer liver metastasis.
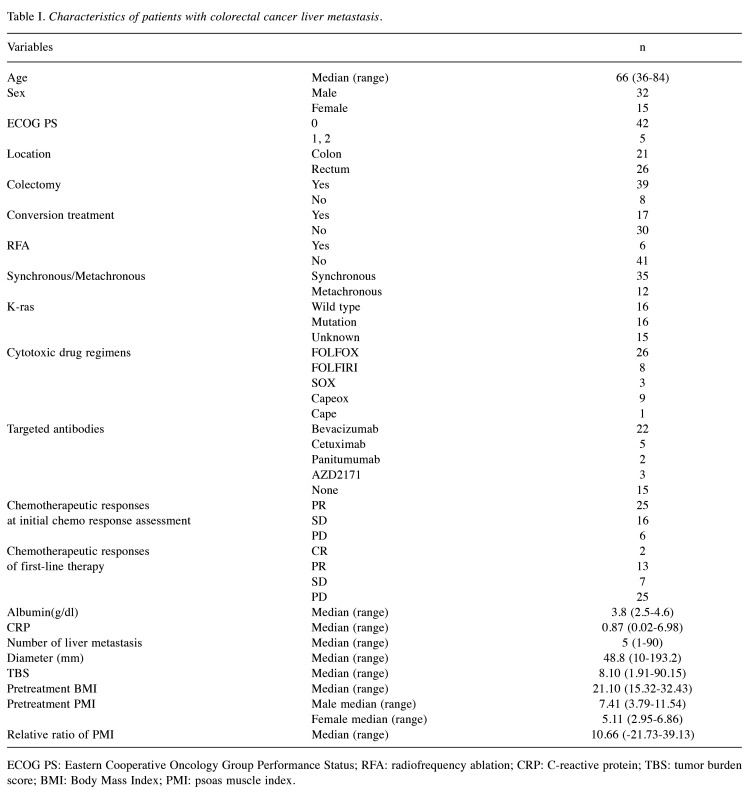
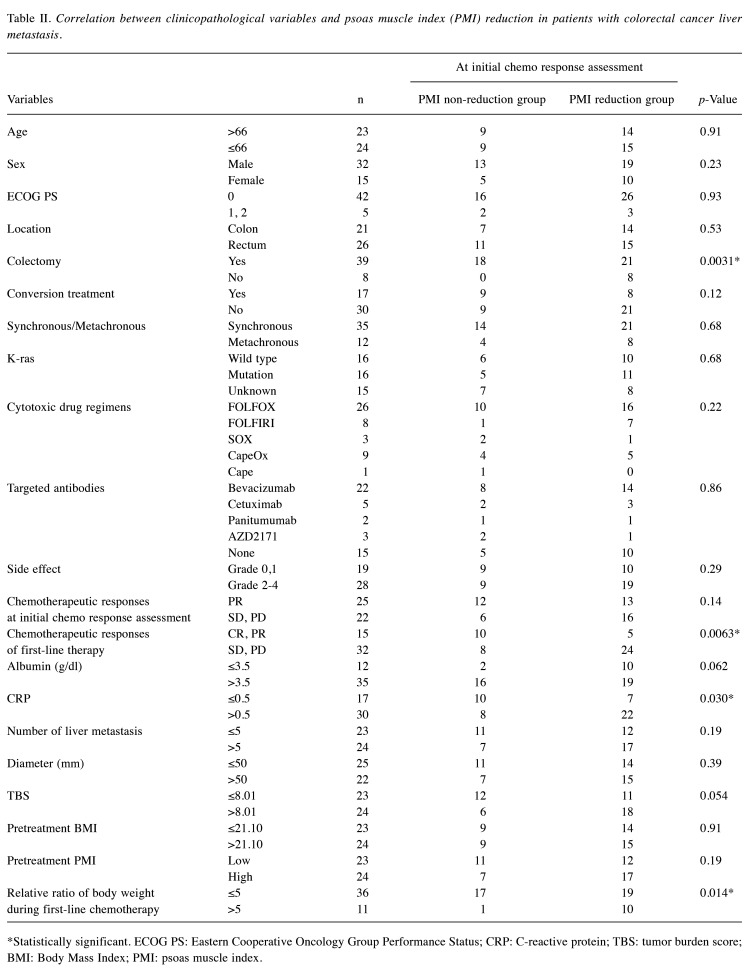
Prognostic significance of the change in the PMI in patients with CRLM. We next investigated how the change in PMI affected the prognosis of patients with CRLM. The PMI reduction group had a significantly poorer prognosis than the PMI non-reduction group in terms of both OS (log-rank test, p<0.001; Figure 1A) and PFS (log-rank test, p=0.033; Figure 1B).
Figure 1.
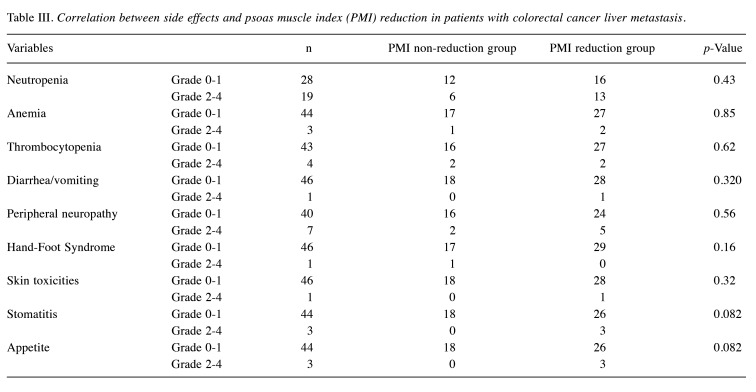
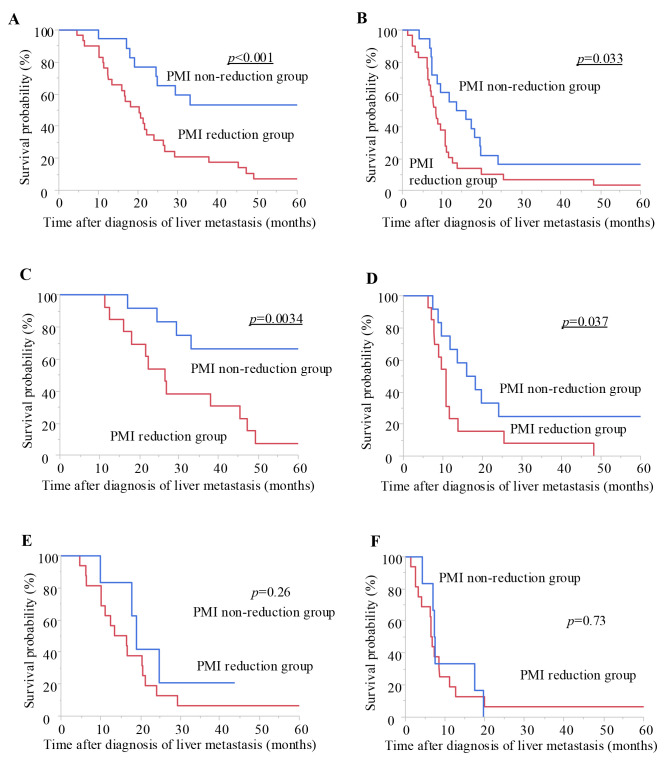
Prognostic impact of the change in psoas muscle index (PMI) on overall survival (OS) and progression-free survival (PFS) in patients with colorectal liver metastasis (CRLM). We conducted a Kaplan–Meier survival analysis, subdividing patients into the PMI reduction group (>5%) and the non-reduction group (≤5%). In patients with CRLM, the PMI reduction group had a significantly poorer prognosis compared with the non-reduction group in terms of OS (log-rank test, p<0.001, A). With respect to PFS, the PMI reduction group also had a significantly poorer prognosis (p=0.033, B). Although there was no significant correlation between the change in PMI and survival or disease progression in stable disease (SD)/progressive disease (PD) group at initial efficacy assessment (OS: E, PFS: F), the PMI reduction group had a significantly poorer prognosis compared to the non-reduction group in terms of both OS (log-rank test, p=0.0034, C) and PFS (p=0.037, D) in the partial response (PR) group at initial efficacy assessment.
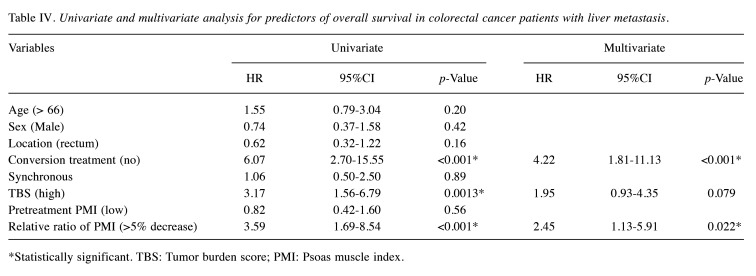
Univariate analyses revealed that a >5% decrease in PMI (p<0.001), high TBS (p=0.0013), and conversion treatment (p<0.001) were significantly associated with OS (Table IV). In multivariate analysis, a >5% decrease in PMI (p=0.022) and conversion treatment (p<0.001) were independently associated with OS (Table IV).
Table IV. Univariate and multivariate analysis for predictors of overall survival in colorectal cancer patients with liver metastasis.
*Statistically significant. TBS: Tumor burden score; PMI: Psoas muscle index.
Next, we performed a subgroup analysis by categorizing the patients into PR and SD/PD group based on the initial chemotherapy response assessment results. Although there was no significant correlation between the change in PMI and survival or progression of disease in the SD/PD group (Figure 1E and F), the PMI reduction group had a significantly poorer prognosis than the PMI non-reduction group in terms of both the OS and PFS in the PR group (log-rank test, p=0.0034 and p=0.037, respectively; Figure 1C and D). In the PR group, PMI reduction was suggested to have a potential impact on the prognosis at the initial chemotherapy response assessment.
Discussion
Several studies have highlighted the importance of pretreatment muscle mass in patients (7-9,11). However, in patients with CRC undergoing chemotherapy, several studies have indicated that loss of muscle mass during chemotherapy, rather than pretreatment muscle mass, is associated with prognosis (14,15). Furthermore, studies on the relationship between changes in skeletal muscle mass and clinico-pathological factors are limited, and no subgroup analyses based on initial chemotherapy response assessments have been conducted for CRLM. The mechanism linking skeletal muscle loss to prognosis in patients with CRLM remains unclear.
We investigated the clinical significance of changes in skeletal muscle mass from liver metastasis diagnosis to the initial chemotherapy response assessment in patients with CRLM and made several novel discoveries. First, changes in skeletal muscle mass were significantly associated with body weight loss, primary tumor resection status, CRP levels, and responses to first-line chemotherapy. Second, appetite loss and stomatitis, observed as side effects of anticancer drugs in patients with CRLM, tended to lead to a decreased skeletal muscle mass. Finally, a reduction in muscle mass was a more reliable indicator of poor OS in patients with CRLM and was significantly associated with poor prognosis for both OS and PFS in the PR group at the initial chemotherapy response assessment.
Muscle mass loss is multifactorial, caused by reduced nutritional intake, metabolic changes induced by tumors, and oncological treatments (37,38). In this study, PMI reduction was associated with the presence or absence of a primary tumor, CRP level, body weight loss, and appetite loss. Appetite loss, attributed to the side effects of anticancer drugs and cancer itself, is thought to contribute to muscle weakness. Furthermore, loss of appetite can lead to weight loss. Gastrointestinal tumors may also cause abdominal pain and other related symptoms.
Reports indicate that fat and muscle do not necessarily change at the same rate as Body Mass Index (BMI) during cancer progression (39-42). Our data also indicated that in 40% of cases, muscle mass decreased despite the absence of weight loss. This finding suggests that weight loss and muscle mass are not strongly associated and that weight loss alone may not reliably predict muscle mass reduction. Reduction in skeletal muscle mass without weight or appetite loss may stem from metabolic changes caused by cancer cells.
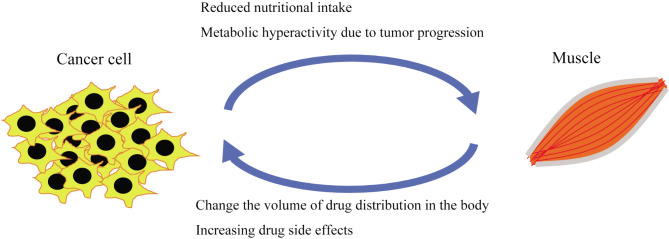
Muscle loss is associated with adverse clinical outcomes, including physical dysfunction, decreased quality of life, reduced treatment tolerance, and ultimately shortened survival (40,43). It may also alter drug distribution in the body, potentially increasing drug exposure (42). Increased drug side effects that require treatment interruption or dose reduction can compromise efficacy and ultimately reduce survival (42,44-46). As illustrated in Figure 2, cancer progression likely perpetuates this vicious cycle by interacting with muscle mass.
Figure 2.
Muscle mass loss is caused by reduced nutritional intake and metabolic hyperactivity due to the tumor. Maintaining skeletal muscle mass may help prevent changes in pharmacokinetics and reduce the risk of increased toxicity. Chemotherapy can reduce tumor burden, thereby preventing muscle mass loss and preserving muscle mass. By maintaining muscle mass and disrupting the vicious cycle, it is believed that we can secure treatment efficacy and enhance the potential for conversion therapy.
Muscle mass reduction was associated with a poor OS and PFS and was a more reliable indicator of poor OS in patients with CRLM. Our study found no association between pretreatment PMI and survival in patients with unresectable CRLM, consistent with earlier findings showing no association between pretreatment sarcopenia and prognosis in patients with rectal cancer (47). These results emphasize the importance of monitoring PMI changes during preoperative treatment. Similarly, Miyamoto et al. (14) reported that changes in PMI are important. They reported that achieving tumor control with effective chemotherapy may reverse the catabolic processes that cause cachexia. Changes in PMI, easily assessed using routine CT scans, provide valuable prognostic information beyond BMI and pretreatment PMI.
Furthermore, measuring changes in PMI during initial response assessment enables early detection of muscle loss, making it a highly useful prognostic factor. Notably, in cases achieving PR at the initial efficacy assessment, a decrease in muscle mass became a prognostic factor. Subgroup analysis revealed that patients in the PMI reduction group had significantly worse OS and PFS, even among those classified as PR, during the initial chemotherapy response assessment. While a poor prognosis is expected for patients with no initial response, patients who achieved a PR were expected to have a relatively good prognosis; however, those with muscle weakness had a poorer prognosis. This prognostic factor can serve as an early indicator of the need for chemotherapy when first-line treatment efficacy wanes. It can also guide decisions on adjuvant therapy, even after conversion has been achieved. Early PMI changes at the initiation of chemotherapy could be a highly useful prognostic clinical marker.
Disrupting the vicious cycle, as shown in Figure 2, is crucial for preserving muscle mass. Maintaining muscle mass allows for chemotherapy administration, which reduces the tumor size and, in turn, helps maintain muscle mass. A comprehensive approach that includes multiple interventions, such as pharmacotherapy, nutritional therapy, exercise, and psychosocial interventions, is essential to prevent muscle atrophy (48-51). Our analysis also identified that gastrointestinal side effects of chemotherapy were associated with muscle weakness, highlighting the importance of managing these adverse effects.
Study limitations. First, this was a retrospective observational study with a short follow-up period, conducted at a single institution with a small sample size. This may have introduced potential bias. Second, data on patients’ intake and calories consumed per day during systematic chemotherapy were not collected. While we focused on PMI, other methods of muscle evaluation, such as bioelectrical impedance analysis, were not used due to the retrospective design. Larger, multicenter prospective trials are needed to validate these findings.
Conclusion
Early PMI reduction at chemotherapy initiation is significantly associated with poor survival in patients with CRLM. Maintaining muscle mass could serve as a novel prognostic indicator for identifying PR patients with better outcomes. Further studies should investigate whether personalized interventions may attenuate or improve muscle mass during treatment and lead to improvements in clinical outcomes such as survival.
Conflicts of Interest
Drs. Kusunoki, Fukuoka, Sugimoto, Tsujio, Yonemitsu, Seki, Kasashima, Shibutani and Maeda have no conflicts of interest or financial ties to disclose in relation to this study.
Authors’ Contributions
Yukina Kusunoki and Tatsunari Fukuoka designed the study, performed the statistical analysis, and drafted the manuscript. Atsushi Sugimoto, Gen Tsujio, Ken Yonemitsu, Yuki Seki, Hiroaki Kasashima and Masatsune Shibutani collected the clinical data and revised the manuscript critically. Kiyoshi Maeda designed the study and critically reviewed the manuscript. All Authors read and approved the final manuscript.
Acknowledgements
The Authors thank Editage (https://www.editage.jp) for the English language editing.
Funding
This study has no funding sources.
References
- 1.Rosenberg IH. Sarcopenia: Origins and clinical relevance. J Nutr. 1997;127(5):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med. 1980;69(4):491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199–211. doi: 10.1017/s002966511500419x. [DOI] [PubMed] [Google Scholar]
- 5.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547. doi: 10.1200/jco.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 6.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Yin C, Narumi A, Omura Y, Ide S, Kitajima T, Fujikawa H, Yasuda H, Hiro J, Yoshiyama S, Kobayashi M, Araki T, McMillan DC, Miki C, Kusunoki M. Clinical impact of muscle quantity and quality in colorectal cancer patients: a propensity score matching analysis. JPEN J Parenter Enteral Nutr. 2018;42(8):1322–1333. doi: 10.1002/jpen.1171. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida M, Watanabe M, Baba H. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–2668. doi: 10.1245/s10434-014-4281-6. [DOI] [PubMed] [Google Scholar]
- 9.Ojima Y, Harano M, Sumitani D, Okajima M. Impact of preoperative skeletal muscle mass and quality on the survival of elderly patients after curative resection of colorectal cancer. J Anus Rectum Colon. 2019;3(4):143–151. doi: 10.23922/jarc.2018-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, Kroenke CH, Castillo A, Kwan ML, Prado CM. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: a population-based cohort study (C-SCANS) J Cachexia Sarcopenia Muscle. 2018;9(4):664–672. doi: 10.1002/jcsm.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TCK, Ijzermans JNM. Body composition and outcome in patients undergoing resection of colorectal liver metastases19. Br J Surg. 2012;99(4):550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 12.Nagata T, Nakase Y, Nakamura K, Sougawa A, Mochiduki S, Kitai S, Inaba S. Prognostic impact of a nutritional index including muscle volume in stage 4 colorectal cancer. In Vivo. 2016;30(6):885–892. doi: 10.21873/invivo.11009. [DOI] [PubMed] [Google Scholar]
- 13.Wagner D, Karitnig R, Wienerroither V, Hau HM, Lederer A, Sucher R, Kornprat P. Sarcopenic obesity promotes recurrence in patients undergoing resection for colorectal liver metastases (CRLM) Anticancer Res. 2024;44(5):2177–2183. doi: 10.21873/anticanres.17024. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe M, Baba H. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One. 2015;10(6):e0129742. doi: 10.1371/journal.pone.0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurk SA, Peeters PHM, Dorresteijn B, de Jong PA, Jourdan M, Creemers GM, Erdkamp FLG, de Jongh FE, Kint PAM, Poppema BJ, Radema SA, Simkens LHJ, Tanis BC, Tjin-A-Ton MLR, Van Der Velden A, Punt CJA, Koopman M, May AM. Loss of skeletal muscle index and survival in patients with metastatic colorectal cancer: Secondary analysis of the phase 3 CAIRO3 trial. Cancer Med. 2020;9(3):1033–1043. doi: 10.1002/cam4.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg. 1989;210(2):127–138. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D, First BEAT investigators Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 18.Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, D’Angelica M. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14(3):1151–1160. doi: 10.1245/s10434-006-9068-y. [DOI] [PubMed] [Google Scholar]
- 19.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer. Cancer. 2007;109(4):718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 20.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis. Ann Surg. 2009;250(3):440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 21.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Gigot JF, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210(5):755–764. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Morris EJA, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, Cottier B, Poston G. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97(7):1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat Rev. 2015;41(9):729–741. doi: 10.1016/j.ctrv.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/jco.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 25.Cheeseman SL, Joel SP, Chester JD, Wilson G, Dent JT, Richards FJ, Seymour MT. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer. 2002;87(4):393–399. doi: 10.1038/sj.bjc.6600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, André T, Bennamoun M, Mabro M, Artru P, Carola E, Flesch M, Dupuis O, Colin P, Larsen AK, Afchain P, Tournigand C, Louvet C, de Gramont A. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 study. J Clin Oncol. 2009;27(34):5727–5733. doi: 10.1200/jco.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 27.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 28.Díaz-Rubio E, Evans TR, Tabemero J, Cassidy J, Sastre J, Eatock M, Bisset D, Regueiro P, Baselga J. Capecitabine (Xeloda®) in combination with oxaliplatin: a phase I, dose-escalation study in patients with advanced or metastatic solid tumors. Ann Oncol. 2002;13(4):558–565. doi: 10.1093/annonc/mdf065. [DOI] [PubMed] [Google Scholar]
- 29.Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, Chung IJ, Cho SH, Lee KH, Shin SJ, Kang HJ, Shin DB, Jo SJ, Lee JW. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: a randomised, non-inferiority phase 3 trial. Lancet Oncol. 2012;13(11):1125–1132. doi: 10.1016/s1470-2045(12)70363-7. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, Mckendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz J, Thompson P, Vieitez JM, Weitzel C, Harper P. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19(21):4097–4106. doi: 10.1200/jco.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 31.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25 Suppl. 2014;25:iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 32.Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM. The Tumor Burden Score: a new “Metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267(1):132–141. doi: 10.1097/sla.0000000000002064. [DOI] [PubMed] [Google Scholar]
- 35.Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K, Uemoto S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157(6):1088–1098. doi: 10.1016/j.surg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, Inagaki N, Uemoto S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11-12):1200–1205. doi: 10.1016/j.nut.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Oshima T. The latest treatments for cancer cachexia: an overview. Anticancer Res. 2023;43(2):511–521. doi: 10.21873/anticanres.16188. [DOI] [PubMed] [Google Scholar]
- 38.Lavalle S, Valerio MR, Masiello E, Gebbia V, Scandurra G. Unveiling the intricate dance: how cancer orchestrates muscle wasting and sarcopenia. In Vivo. 2024;38(4):1520–1529. doi: 10.21873/invivo.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. doi: 10.1139/h08-075. [DOI] [PubMed] [Google Scholar]
- 40.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/s1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 41.Baracos V, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29(suppl_2):ii1–ii9. doi: 10.1093/annonc/mdx810. [DOI] [PubMed] [Google Scholar]
- 42.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/s1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 43.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, Mollevi C, Senesse P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5(4):607–616. doi: 10.1002/cam4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, Xiao J, Castillo AL, Caan BJ. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer. 2017;123(24):4868–4877. doi: 10.1002/cncr.30950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66(4):583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 47.Fukuoka T, Maeda K, Nagahara H, Shibutani M, Iseki Y, Matsutani S, Hirakawa K, Ohira M. Change in PMI during neoadjuvant therapy is a predictive prognostic marker in rectal cancer. Anticancer Res. 2019;39(9):5157–5163. doi: 10.21873/anticanres.13711. [DOI] [PubMed] [Google Scholar]
- 48.Bland KA, Harrison M, Zopf EM, Sousa MS, Currow DC, Ely M, Agar M, Butcher BE, Vaughan V, Dowd A, Martin P. Quality of life and symptom burden improve in patients attending a multidisciplinary clinical service for cancer cachexia: a retrospective observational review. J Pain Symptom Manage. 2021;62(3):e164–e176. doi: 10.1016/j.jpainsymman.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 49.Borg JJ, Anker SD, Rosano G, Serracino-Inglott A, Strasser F. Multimodal management as requirement for the clinical use of anticachexia drugs - a regulatory and a clinical perspective. Curr Opin Support Palliat Care. 2015;9(4):333–345. doi: 10.1097/spc.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 50.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 51.Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, Buonaccorso L, de van der Schueren MAE, Baldwin C, Chasen M, Ripamonti CI, ESMO Guidelines Committee. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines(I) ESMO Open. 2021;6(3):100092. doi: 10.1016/j.esmoop.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]