Abstract
Background/Aim
The albumin-bilirubin (ALBI) grade is an assessment tool for hepatic function and prognosis in patients with hepatocellular carcinoma (HCC). However, its significance in patients with non-small cell lung cancer (NSCLC) treated with an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) remains unclear. We retrospectively investigated the relationship between pre-treatment ALBI grade and hepatotoxicity and treatment efficacy in patients with NSCLC receiving EGFR-TKIs.
Patients and Methods
We analyzed data from 182 patients with NSCLC treated with EGFR-TKIs. Patients were categorized into ALBI grades 1/2a and 2b/3 groups. We examined the association between ALBI grade, hepatotoxicity, and time to treatment failure (TTF) using univariate and multivariate analyses.
Results
In the univariate Kaplan-Meier analysis, ALBI grade was not associated with hepatotoxicity (log-rank p=0.56). This finding was consistent with the multivariate analysis of patients treated with gefitinib and erlotinib (n=158). However, In the univariate Kaplan-Meier analysis, the median TTF for the ALBI grade 1/2a group was 10.6 months, compared to 5.8 months for the ALBI grade 2b/3 group (hazard ratio=1.66, 95% confidence interval=1.19-2.33, p=0.003). Multivariate analysis confirmed that ALBI grade 2b/3 (hazard ratio=1.64, 95% confidence interval=1.16-2.30, p<0.01) was independently associated with shortened TTF.
Conclusion
Pretreatment ALBI grade classification can predict efficacy in patients with NSCLC treated with EGFR-TKIs.
Keywords: Non-small cell lung cancer, epidermal growth factor receptor, tyrosine kinase inhibitors, albumin-bilirubin grade, hepatotoxicity, time to treatment failure
Globally, lung cancer is the leading cause of cancer-related mortality, with non-small cell lung cancer (NSCLC) accounting for more than 85% of all lung cancer cases. Epidermal growth factor receptor (EGFR) mutations have been reported in approximately 45% (ranging from 21% to 68%) of Japanese patients with adenocarcinomas (1). The development of EGFR tyrosine kinase inhibitors (TKIs) has significantly improved the prognosis of patients with NSCLC with EGFR mutations (2). According to the Japanese Lung Cancer Society Guidelines for stage IV NSCLC, osimertinib is recommended for patients with a superior performance status (PS). In contrast, gefitinib and erlotinib are recommended as first-line therapies for elderly patients and those with inferior PS who have NSCLC with exon 19 deletions or L858R point mutations in exon 21 of the EGFR gene (3-5).
Adverse events (AEs) associated with EGFR-TKIs include rash, diarrhea, hepatotoxicity, and interstitial lung disease (ILD) (2,6,7). Some patients discontinue EGFR-TKI treatment due to these AEs. The most common reasons for discontinuation are skin toxicity, ILD, and hepatotoxicity (8). Hepatotoxicity of grade ≥3, as defined by the Common Terminology Criteria for Adverse Events (CTCAE), was observed in 18.0% of patients treated with gefitinib and 5.4% of patients treated with erlotinib (8). While hepatotoxicity has been reported with second- and third-generation TKIs (such as afatinib, dacomitinib, and osimertinib), it occurs less frequently than with first-generation TKIs (gefitinib and erlotinib) (6,7,9). There have been a few previous reports on factors associated with the risk of hepatotoxicity due to gefitinib and erlotinib. Common factors associated with hepatotoxicity for both gefitinib and erlotinib include liver metastases, exon 19 deletion mutations in EGFR, concomitant use of acid-suppressing medications (AS) or cytochrome P450 (CYP) 3A4 inducers, and the presence of hepatitis virus. Additional factors identified include age <65 years and a body mass index (BMI) ≥25 kg/m2 for gefitinib and age ≥65 years for erlotinib. It has been reported that the concomitant use of AS with erlotinib decreases the risk of hepatotoxicity (10-14). Therefore, further evidence is required to predict hepatotoxicity better.
The albumin-bilirubin (ALBI) score/grade was proposed as an assessment tool for hepatic function in patients with hepatocellular carcinoma (HCC) (15). It is based on the objective indices of serum albumin (Alb) and total bilirubin (T-Bil). The ALBI score is simple and objective and offers discriminatory advantages over the Child-Pugh Score. Detailed stratification using the modified ALBI (mALBI) score can accurately predict the prognosis of patients with HCC (16). Although not widely well known, the ALBI score may be associated with drug-induced liver injury (17,18). Pretreatment ALBI score has been linked to hepatotoxicity in patients with metastatic colorectal cancer treated with regorafenib (19). However, the relationship between ALBI grade and the hepatotoxicity of EGFR-TKI treatment in NSCLC has not been fully elucidated. Recent studies have shown that the ALBI score is closely related to oncological outcomes in some malignancies. For instance, a preoperative ALBI score is associated with the discontinuation of S-1 adjuvant monotherapy and overall survival in patients with gastric cancer (20,21). Preoperative ALBI score is also a prognostic factor for esophageal cancer and hepatocellular carcinoma patients after surgery (22,23). The pretreatment ALBI score is associated with the prognosis of patients with metastatic colorectal cancer treated with regorafenib (19). Some studies have also reported that the ALBI score is linked to the prognosis of NSCLC. The pretreatment ALBI grade can predict the efficacy of immune checkpoint inhibitors (ICIs) in NSCLC (24). Additionally, the preoperative ALBI grade is an independent prognostic factor in surgically resected NSCLC (25). However, the association between ALBI grade and the efficacy of EGFR-TKI treatment in NSCLC has not been fully elucidated.
The present study aimed to evaluate the clinical impact of the pretreatment ALBI score in patients with NSCLC who received EGFR-TKIs. We retrospectively investigated the association between pretreatment ALBI grade and the incidence of hepatotoxicity, as well as time to treatment failure (TTF).
Patients and Methods
Patients. This study was approved by the Institutional Review Board Kanazawa Medical Center (R03-069), Handa City Hospital (2021-002), and the National Center for Geriatrics and Gerontology (No.21TB13). Informed consent was obtained in the form of opt-out. Between January 2010 and June 2020, we conducted a retrospective study of patients with NSCLC treated with EGFR-TKIs, including gefitinib, erlotinib, afatinib, osimertinib, and dacomitinib. We excluded patients who did not have ALBI scores, as well as those whose aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels results were not recorded before the administration of TKIs. Additionally, patients with elevated AST/ALT levels of grade ≥2, as assessed by the Common Terminology Criteria for Adverse Events (CTCAE) version 5, before TKI administration, were excluded. Patients who started treatment outside the observation period or at another institution were also excluded.
Data collection. Patient characteristics and laboratory test data at the start of EGFR-TKI treatment were obtained from electronic medical records. The following data were collected prior to treatment: sex, age, BMI, smoking history, presence of hepatitis B and C viruses, presence of liver metastasis, type of EGFR mutation, laboratory test results, and concomitant medications. The age cutoff was defined based on previous reports (10,11). The BMI at the start of treatment was calculated as weight (kg) divided by height (m) squared. Patients were stratified into the following BMI categories, as defined by the World Health Organization and prior studies (26,27): underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5-25 kg/m2), and overweight (BMI ≥25 kg/m2). Laboratory data collected before TKI treatment included Alb, T-Bil, AST, ALT, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and serum creatinine. Creatinine clearance (Ccr) was calculated using serum creatinine and the Cockcroft-Gault formula. Concomitant medications included AS agents and CYP3A4 inducers, based on a previous report (12). The AS agents included cimetidine, famotidine, lafutidine, nizatidine, ranitidine, omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole, and bonoprazan. The CYP3A4 inducers included bosentan, carbamazepine, dexamethasone, efavirenz, ethosuximide, etravirine, fosphenytoin, modafinil, nafcillin, oxcarbazepine, phenobarbital, phenytoin, prednisolone, primidone, rifabutin, and rifampicin.
Definition of ALBI grade. The ALBI score was calculated using the following formula: 0.66×log10[T-Bil (mg/dl)×1.71]-0.085×10×[Alb (g/dl)]. ALBI grades were defined as follows: grade 1 (score ≤−2.60), grade 2a (score >−2.60 and <−2.27), grade 2b (score ≥−2.27 and ≤−1.39), and grade 3 (score >−1.39).
Assessment of hepatotoxicity and efficacy. Hepatotoxicity was defined as an increase in either AST or ALT levels to grade 2 or the use of hepatoprotective drugs prescribed by a physician. Following a previous report on the onset of hepatotoxicity, we monitored AST and ALT levels for 180 days from the start of treatment (13). TTF was defined as the period from the first dose of EGFR-TKIs to the last dose or death.
Statistical analysis. Chi-square tests or Fisher’s exact tests were used to compare the categorical variables. The Mann-Whitney U-test or Student’s t-test was used to compare the continuous variables. The onset time of hepatotoxicity and TTF were estimated using the Kaplan-Meier method, with differences analyzed using the log-rank test. For univariate and multivariate analyses of factors related to hepatotoxicity or TTF-, Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95%CIs). In the multivariate analysis, factors deemed to have high clinical importance based on previous reports were included using forced entry methods. Statistical significance was set at p<0.05. Statistical analyses were performed using SPSS Statistics, version 27 (IBM Japan Ltd., Tokyo, Japan).
Result
Patient characteristics. A total of 246 patients received EGFR-TKIs during the study period. We excluded patients who started treatment outside the observation period or at another institution (n=26), those whose AST/ALT levels and ALBI scores were not recorded before TKI administration (n=34), and those with elevated AST or ALT levels above grade 2 before TKI administration (n=2). Consequently, the data from 184 patients treated with TKIs were analyzed (Figure 1). Patient characteristics are summarized in Table I. The median age of the patients was 72 years (range=36-94 years). Of the 184 patients, 69 (37.5%) were male, 96 (52.2%) had a history of smoking, 22 (12.0%) had a history of hepatitis virus infection, and 25 (13.6%) had liver metastasis. Additionally, 78 patients (42.4%) had an exon 19 deletion, while 67 patients (36.4%) had an L858R point mutation in exon 21. The distribution of patients treated with different EGFR-TKIs was as follows: gefitinib (101 patients, 54.9%), erlotinib (57 patients, 31.0%), osimertinib (18 patients, 9.8%), afatinib (eight patients, 4.3%), and dacomitinib (zero patients, 0%).
Figure 1.
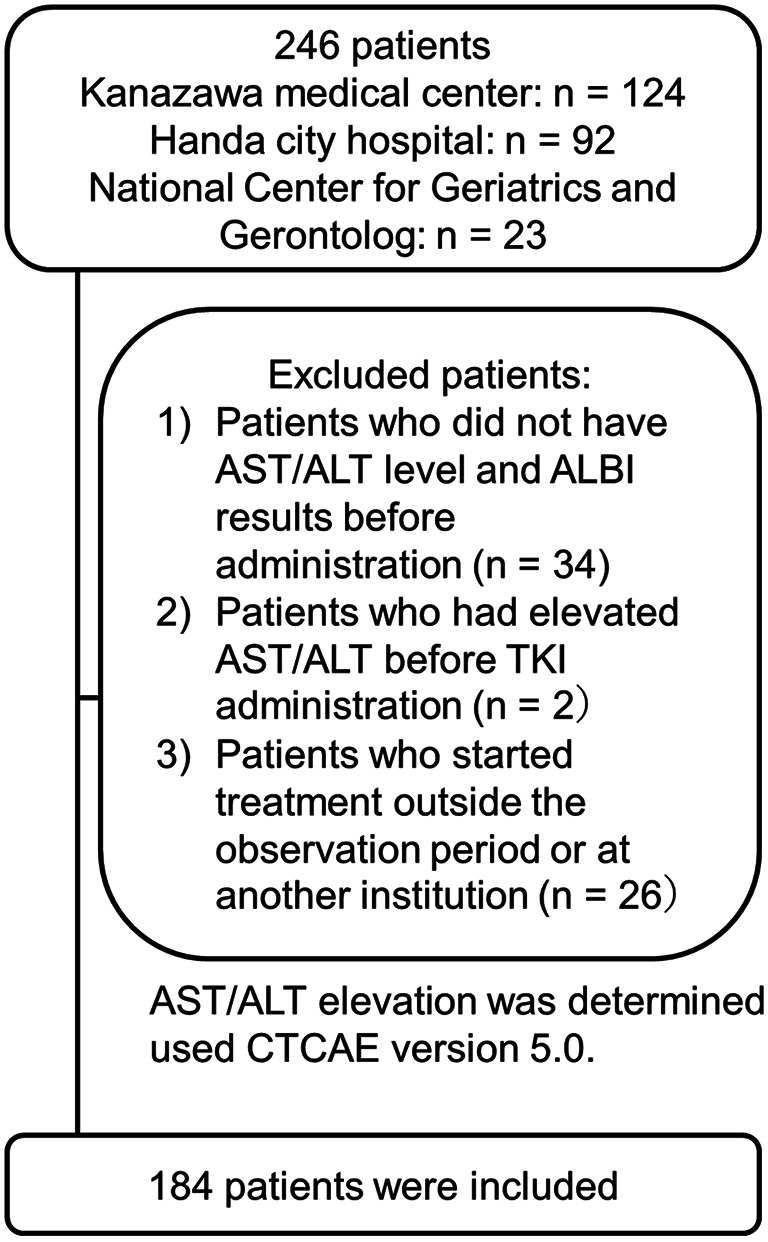
Flow chart of study participants.
Table I. Patients’ background characteristics (n=184).
BMI: Body mass index; EGFR: epidermal growth factor receptor; TKIs: tyrosine kinase inhibitors; ALBI grade: albumin-bilirubin grade.
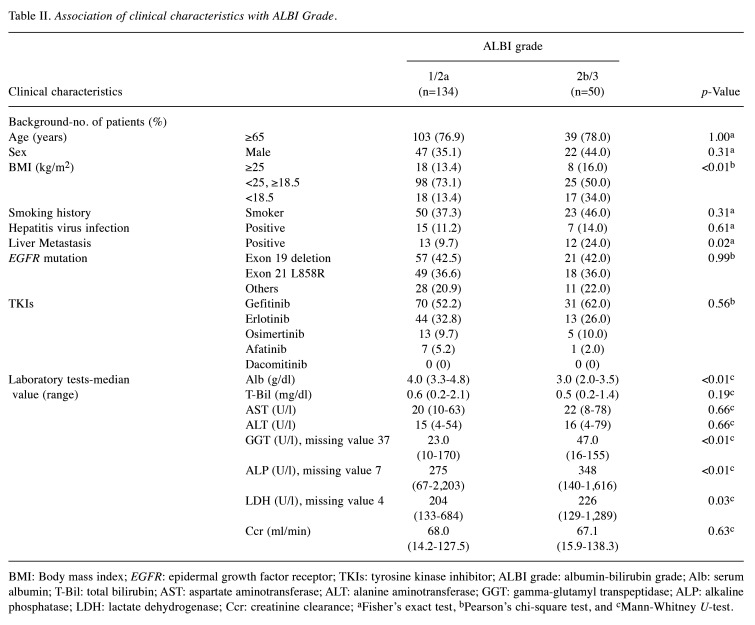
Clinical characteristics associated with ALBI grade. There were 92 patients (50.0%) with ALBI grade 1, 42 (22.8%) with grade 2a, 42 (22.8%) with grade 2b, and eight (4.3%) with grade 3. Based on a previous study, we divided the patients into two groups: ALBI grade 1/2a and ALBI grade 2b/3 (24). We analyzed the association between clinical characteristics and ALBI grade (Table II). Analysis of the patients’ characteristics revealed that ALBI grade 2b/3 was significantly associated with BMI (p<0.01) and liver metastasis (p=0.02). Patients with ALBI grade 2b/3 were significantly less likely to be of normal weight (BMI ≥18.5 and <25 kg/m²) and more likely to be underweight (BMI <18.5 kg/m²). Laboratory data revealed lower serum Albumin levels in the ALBI grade 2b/3 group, with no significant difference in total bilirubin levels. Additionally, levels of GGT (p<0.01), ALP (p<0.01), and LDH (p=0.03) were increased in the ALBI grade 2b/3 group.
Table II. Association of clinical characteristics with ALBI Grade.
BMI: Body mass index; EGFR: epidermal growth factor receptor; TKIs: tyrosine kinase inhibitor; ALBI grade: albumin-bilirubin grade; Alb: serum albumin; T-Bil: total bilirubin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transpeptidase; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; Ccr: creatinine clearance; aFisher’s exact test, bPearson’s chi-square test, and cMann-Whitney U-test.
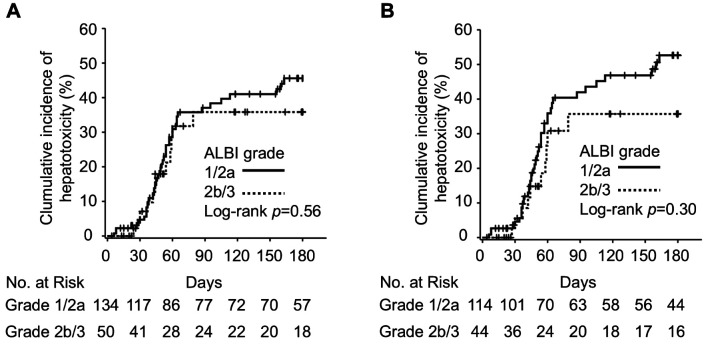
Association between ALBI grade and hepatotoxicity. We investigated hepatotoxicity by monitoring AST and ALT levels for 180 days after the initiation of TKI treatment. Hepatotoxicity occurred in 55 patients (29.9%), with grade 3/4 hepatotoxicity occurring in 33 patients (17.9%). The incidence of hepatotoxicity by the drug was as follows: gefitinib (37 patients, 36.6%), erlotinib (14 patients, 24.6%), osimertinib (four patients, 22.2%), and none with afatinib. In the ALBI grade 1/2a group, 43 patients (32.1%) developed hepatotoxicity, while 12 patients (24.0%) in the ALBI grade 2b/3 group experienced hepatotoxicity. For grade 3/4 hepatotoxicity, 26 patients (19.4%) in the ALBI grade 1/2a group and seven patients (14.0%) in the ALBI grade 2b/3 group were affected. The results of the analysis of the association between ALBI grade and the cumulative incidence of hepatotoxicity are shown in Figure 2. In the univariate Kaplan-Meier analysis, ALBI grade was not significantly related to hepatotoxicity (log-rank p=0.56).
Figure 2.
Kaplan-Meier curves of the association between occurrence of hepatotoxicity and ALBI grade. (A) Kaplan-Meier curve for all patients (n=184). (B) Kaplan-Meier curve for patients treated with gefitinib or erlotinib (n=158).
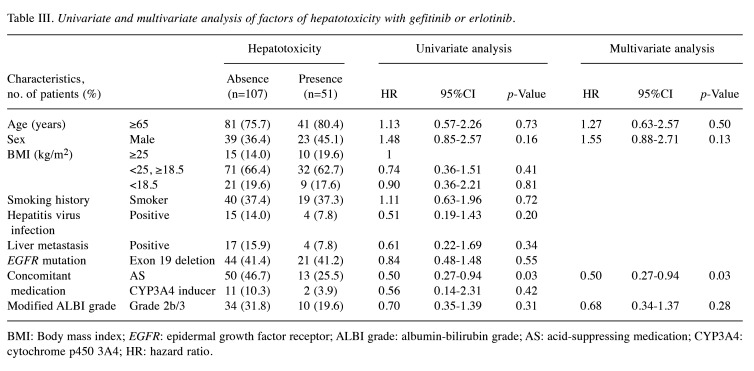
Univariate and multivariate analysis of risk factors for gefitinib or erlotinib. We investigated the factors associated with hepatotoxicity in patients treated with gefitinib or erlotinib, focusing on previously reported risk factors (Table III). In univariate analysis, ALBI grade was not significantly associated with hepatotoxicity (HR=0.70, 95%CI=0.35-1.39, p=0.31). However, concomitant use of AS medications was significantly associated with a decreased risk of hepatotoxicity (HR=0.50, 95%CI=0.27-0.94, p=0.03). This association was also significant in the multivariate analysis, which indicated that concomitant use of AS medications significantly reduced the risk of hepatotoxicity (HR=0.50, 95%CI=0.27-0.94, p=0.03).
Table III. Univariate and multivariate analysis of factors of hepatotoxicity with gefitinib or erlotinib.
BMI: Body mass index; EGFR: epidermal growth factor receptor; ALBI grade: albumin-bilirubin grade; AS: acid-suppressing medication; CYP3A4: cytochrome p450 3A4; HR: hazard ratio.
Association between ALBI grade and TTF. The median TTF was 8.4 months (95%CI=6.4-10.4). We analyzed the relationship between TTF and ALBI grade (Figure 3). In the univariate Kaplan-Meier analysis, the median TTF was 10.6 months for the ALBI grade 1/2a group and 5.8 months for the ALBI grade 2b/3 group (HR=1.66, 95%CI=1.19-2.33, p=0.003). We also examined the relationship between TTF and patient characteristics (Table IV). In univariate analysis, factors associated with a shortened TTF included being male (HR=1.57, 95%CI=1.14-2.15, p<0.01), having a history of smoking (HR=1.49, 95%CI=1.09-2.03, p=0.01), and concomitant use of CYP3A4 inducers (HR=2.12, 95%CI=1.17-3.85, p=0.01). Conversely, treatment with osimertinib was associated with a prolonged TTF (HR=0.51, 95%CI=0.29-0.93, p=0.03) compared to treatment with other TKIs. Additionally, a multivariate analysis was conducted to assess the relationship between TTF and patient characteristics using the Cox proportional hazards model. Our results showed that ALBI grade 2b/3 was associated with a shortened TTF (HR=1.64, 95%CI=1.16-2.30, p<0.01), while treatment with osimertinib was associated with a prolonged TTF (HR=0.53, 95%CI=0.29-0.97, p=0.04) compared to treatment with other TKIs.
Figure 3.
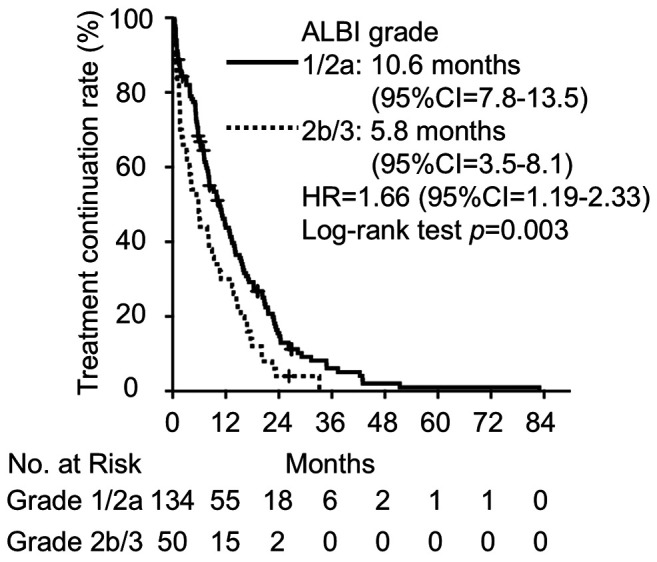
Kaplan-Meier curve for the comparison of time to treatment failure between ALBI grades 1/2a and 2b/3.
Table IV. Univariate and multivariate analysis for time to treatment failure.
BMI: Body mass index; EGFR: epidermal growth factor receptor; TKI: tyrosine kinase inhibitor; ALBI grade: albumin-bilirubin grade; AS: acid-suppressing medication; CYP3A4: cytochrome p450 3A4; HR: hazard ratio.
Discussion
In this study, we retrospectively investigated the association between ALBI grade, hepatotoxicity, and TTF in patients with NSCLC treated with EGFR-TKIs. We found that ALBI grade is a prognostic factor for TTF but not for hepatotoxicity.
In this study, we used the modified ALBI Grade classification. Matsukane et al. demonstrated that pretreatment ALBI grade can predict the efficacy of ICIs in NSCLC (24). In their study, patients were classified by modified ALBI grade, with 37.1% having grade 1, 25.7% having grade 2a, 32.2% having grade 2b, and 5.0% having grade 3. These proportions were largely consistent with our data. Therefore, we divided the patients into ALBI grade 1/2a and ALBI grade 2b/3 groups using the same criteria to evaluate the efficacy and hepatotoxicity of EGFR-TKIs. Lower serum Alb levels were observed in the ALBI grade 2b/3 group; however, there was no difference in T-Bil levels. This suggests ALBI grade classifications in patients with NSCLC may exhibit similar patterns. Decreased serum Alb levels in patients with cancer can result from reduced Alb synthesis due to impaired liver function, poor nutritional status, and increased Alb consumption related to tumor hypermetabolism. While T-Bil levels did not differ significantly, GGT, ALP, and LDH levels were significantly higher in the ALBI grade 2b/3 group. Additionally, there were significantly more patients with liver metastases in the ALBI grade 2b/3 group. This suggests that patients with impaired liver function can be classified as having an ALBI grade 2b/3. Furthermore, there were significantly fewer patients with normal weight (BMI ≥18.5 and <25 kg/m²) and more underweight patients (BMI <18.5 kg/m²) in the ALBI grade 2b/3 group, indicating patients with poor nutritional status could be classified as having ALBI grade 2b/3. While ALBI grade has been proposed primarily as an assessment tool for hepatic function in patients with HCC, our results suggest that it could also be used to evaluate liver function and nutritional status in patients with NSCLC.
The ALBI grade was not associated with hepatotoxicity due to EGFR-TKIs in the overall patient population or those treated with gefitinib or erlotinib. The ALBI Score may influence metabolic enzymes, such as CYP3A4, and a deterioration in the ALBI score can reduce the clearance of medications metabolized by CYP3A4 (28). It has been reported that gefitinib and erlotinib are metabolized in hepatocytes by CYP3A4, and their reactive metabolites can induce hepatotoxicity (29). Although hepatocytes may be more vulnerable in patients with deteriorated ALBI scores, decreased metabolism in these cells might reduce TKI toxicity. Our results suggest that AS medications reduce the risk of hepatotoxicity induced by gefitinib or erlotinib. Gefitinib and erlotinib have their solubility altered by the stomach pH, which affects their bioavailability. Concomitant use of proton pump inhibitors or H2 receptor antagonists can reduce the area under the curve (AUC) by approximately 50% in patients receiving gefitinib or erlotinib (30,31). Therefore, reduced exposure to TKIs due to concomitant use of AS medications can decrease the risk of hepatotoxicity. However, some reports suggest that AS may also be a risk factor for hepatotoxicity induced by TKIs (10-12). AS and TKIs may interact with the ATP-binding cassette subfamily G member 2 (ABCG2), a membrane transporter expressed in the liver. ABCG2 negatively regulates uptake into hepatocytes (32). Gefitinib may accumulate in hepatocytes due to the inhibition of ABCG2 by AS but AS plasma concentrations 50-200 times higher than the therapeutic level would have a significant effect (33). Furthermore, a meta-analysis showed no significant association between ABCG2 polymorphism and hepatotoxicity induced by EGFR-TKIs (34). This suggests that the impact of this interaction via ABCG2 may have been negligible in this study.
In this study, the multivariate analysis of TTF for TKIs identified ALBI grade 2b/3 and osimertinib treatment as independent predictors. Treatment with osimertinib was associated with a longer TTF compared to treatment with other TKIs, confirming its benefits over other TKIs (5,7). However, our results also indicated that ALBI grade 2b/3 was associated with a shorter TTF. This suggests that the ALBI grade can predict TTF with its ability to assess liver function and nutritional status in patients with NSCLC. An association between nutritional status and prognosis in NSCLC has been reported (35). Malnutrition affects the immune system, inflammation, and cachexia and is a risk factor for mortality, treatment resistance, and increased chemotherapy toxicity (36). We hypothesize that deterioration to ALBI grade 2b/3 impacts the pharmacokinetics, efficacy, and adverse effects of TKIs. TKIs exhibit a strong serum protein-binding capacity, and low serum Alb levels can lead to higher plasma concentrations of TKIs (37,38). Elevated plasma concentrations may negatively impact patient prognosis due to off-target effects (39,40). Off-target effects occur when a molecularly targeted drug inhibits or activates a molecule other than its intended target (41). Consistent with this, low serum Alb levels have been identified as a poor prognostic factor for TKI treatment (39,42-44). Additionally, hepatic impairment may elevate TKI exposure by reducing clearance due to decreased CYP3A4 activity (28,45). Therefore, deterioration in ALBI grade may lead to off-target effects due to increased plasma concentrations of TKIs. Additionally, increased exposure to TKIs not only diminishes efficacy but also heightens the risk of adverse events (46,47). Therefore, deterioration in ALBI grade results in a shorter TTF for TKI treatment due to its effects on both efficacy and safety.
Study limitations. First, it was a retrospective study. Liver function can be influenced by various factors; however, data on alcohol intake and underlying diseases are lacking. Additionally, information on PS and the presence of brain metastases, both of which are reported prognostic factors for EGFR-TKI efficacy in NSCLC, is missing (48). The results may be confounded by unaccounted background factors, as we did not evaluate all potential variables that could impact the outcomes. A randomized prospective study is required to validate our findings. Second, there was sample size bias among the TKIs used in this study. Gefitinib was administered to more than half of the patients, whereas osimertinib and afatinib were used in smaller sample sizes. Consequently, these results should be interpreted with caution. Third, we were unable to assess the pharmacokinetic effects. To clarify our results further, it is necessary to evaluate the association between blood levels and the safety and efficacy of the ALBI grade classification. Fourth, our results were based on ALBI grade classification before treatment initiation. As liver function and nutritional status can change over time, an analysis that reflects changes in ALBI grade throughout the course of treatment is also needed.
In conclusion, our study indicates that pretreatment ALBI grade classification, which assesses liver function and nutritional status, may predict the efficacy of EGFR-TKI treatment in patients with NSCLC.
Funding
None.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
Conception and design: H. Arihara, H. Nagamatsu, Y. Hayakawa, H. Mase; Data acquisition: H. Arihara, H. Nagamatsu, Y. Hayakawa; Analysis and interpretation of data: H. Arihara, H. Nagamatsu, Y. Hayakawa, H. Mase; Writing and revision of the manuscript: H. Arihara; review of the manuscript: H. Nagamatsu, Y. Hayakawa, H. Mase, T. Araya, T. Kita; Study supervision: T. Araya and T. Kita.
Acknowledgements
The Authors thank Professor Yukio Suga and a member of the Department of Clinical Pharmacy and Healthcare Science for their useful discussions and advice.
References
- 1.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5(9):2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 3.Akamatsu H, Ninomiya K, Kenmotsu H, Morise M, Daga H, Goto Y, Kozuki T, Miura S, Sasaki T, Tamiya A, Teraoka S, Tsubata Y, Yoshioka H, Hattori Y, Imamura CK, Katsuya Y, Matsui R, Minegishi Y, Mizugaki H, Nosaki K, Okuma Y, Sakamoto S, Sone T, Tanaka K, Umemura S, Yamanaka T, Amano S, Hasegawa K, Morita S, Nakajima K, Maemondo M, Seto T, Yamamoto N. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24(7):731–770. doi: 10.1007/s10147-019-01431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A, Miyazawa H, Tanaka T, Ikebuchi K, Nukiwa T, Morita S, Hagiwara K, North East Japan Gefitinib Study Group First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27(9):1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC, FLAURA Investigators Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 6.Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kölbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 7.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, FLAURA Investigators Osimertinib in untreated EGFR -mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 8.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74–79. doi: 10.1016/j.lungcan.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Nishio M, Kato T, Niho S, Yamamoto N, Takahashi T, Nogami N, Kaneda H, Fujita Y, Wilner K, Yoshida M, Isozaki M, Wada S, Tsuji F, Nakagawa K. Safety and efficacy of first-line dacomitinib in Japanese patients with advanced non-small cell lung cancer. Cancer Sci. 2020;111(5):1724–1738. doi: 10.1111/cas.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho S, Yee J, Kim JY, Jeong Rhie S, Gwak HS. Effects of concomitant medication use on gefitinib-induced hepatotoxicity. J Clin Pharmacol. 2018;58(2):263–268. doi: 10.1002/jcph.1010. [DOI] [PubMed] [Google Scholar]
- 11.Kim MK, Yee J, Cho YS, Jang HW, Han JM, Gwak HS. Risk factors for erlotinib-induced hepatotoxicity: a retrospective follow-up study. BMC Cancer. 2018;18(1):988. doi: 10.1186/s12885-018-4891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han JM, Han HW, Yee J, Kim MK, Moon JY, Cho S, Jung D, Cho YS, Seo I, Kim JY, Gwak HS. Factors affecting high-grade hepatotoxicity of tyrosine kinase inhibitors in cancer patients: a multi-center observational study. Eur J Clin Pharmacol. 2020;76(8):1183–1191. doi: 10.1007/s00228-020-02897-x. [DOI] [PubMed] [Google Scholar]
- 13.Qian J, Zhang X, Zhang B, Yan B, Wang L, Gu P, Wang W, Wang H, Han B. Tyrosine kinase inhibitor-related hepatotoxicity in patients with advanced lung adenocarcinoma: a real-world retrospective study. Cancer Manag Res. 2020;12:3293–3299. doi: 10.2147/CMAR.S237968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai H, Shimada T, Takahashi Y, Nishikawa M, Tozuka H, Yamamoto Y, Niwa O, Takahara Y, Fujita A, Nagase K, Kasahara K, Yano S, Sai Y. Evaluation of factors affecting epidermal growth factor receptor tyrosine kinase inhibitor-induced hepatotoxicity in Japanese patients with non-small cell lung cancer: a two-center retrospective study. J Pharm Health Care Sci. 2022;8(1):28. doi: 10.1186/s40780-022-00258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M, Takayama T, Kokudo T, Kashiwabara K, Kudo M. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325–336. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YS, Tseng SY, Chen WW, Chang TT, Peng CY, Lo GH, Hsu CW, Hu CT, Huang YH. Clinical characteristics and outcomes of drug-induced liver injury in Taiwan: With emphasis on the impact of chronic hepatitis B infection. J Chin Med Assoc. 2022;85(3):286–294. doi: 10.1097/JCMA.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 18.Asai Y, Yamamoto T, Sato Y. Risk assessment of micafungin-induced liver injury using spontaneous reporting system data and electronic medical records. J Infect Chemother. 2022;28(5):690–695. doi: 10.1016/j.jiac.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe D, Fujii H, Yamada Y, Matsuhashi N, Makiyama A, Iihara H, Takahashi T, Kiyama S, Kobayashi R, Yoshida K, Suzuki A. Association of albumin-bilirubin score in patients with colorectal cancer receiving later-line chemotherapy with regorafenib. Int J Clin Oncol. 2021;26(7):1257–1263. doi: 10.1007/s10147-021-01910-2. [DOI] [PubMed] [Google Scholar]
- 20.Iizuka A, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Proposal of a scoring scale to estimate risk of the discontinuation of S-1 adjuvant monotherapy in patients with stage II to III gastric cancer: a multi-institutional dataset analysis. World J Surg. 2019;43(8):2016–2024. doi: 10.1007/s00268-019-04942-y. [DOI] [PubMed] [Google Scholar]
- 21.Ju M, Aoyama T, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, Morita J, Onodera A, Endo K, Hashimoto I, Kano K, Hara K, Cho H, Nakazono M, Segami K, Ishiguro T, Onuma S, Oshima T, Yukawa N, Rino Y. The albumin-bilirubin score is a prognostic factor for gastric cancer patients who receive curative treatment. Anticancer Res. 2022;42(8):3929–3935. doi: 10.21873/anticanres.15887. [DOI] [PubMed] [Google Scholar]
- 22.Aoyama T, Ju M, Machida D, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, Kano K, Hara K, Segami K, Hashimoto I, Nagasawa S, Nakazono M, Oshima T, Yukawa N, Rino Y. Clinical impact of preoperative albumin-bilirubin status in esophageal cancer patients who receive curative treatment. In Vivo. 2022;36(3):1424–1431. doi: 10.21873/invivo.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gon H, Komatsu S, Omiya S, Kido M, Fukushima K, Urade T, Yoshida T, Arai K, Ishida J, Nanno Y, Tsugawa D, Yanagimoto H, Toyama H, Fukumoto T. The albumin-bilirubin grade as prognostic indicator for recurrent hepatocellular carcinoma needing repeat liver resection. Anticancer Res. 2024;44(5):2031–2038. doi: 10.21873/anticanres.17006. [DOI] [PubMed] [Google Scholar]
- 24.Matsukane R, Watanabe H, Hata K, Suetsugu K, Tsuji T, Egashira N, Nakanishi Y, Okamoto I, Ieiri I. Prognostic significance of pre-treatment ALBI grade in advanced non-small cell lung cancer receiving immune checkpoint therapy. Sci Rep. 2021;11(1):15057. doi: 10.1038/s41598-021-94336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita F, Yamashita T, Oku Y, Kosai K, Ono Y, Wakasu S, Haratake N, Toyokawa G, Takenaka T, Tagawa T, Shimokawa M, Nakashima N, Mori M. Prognostic impact of albumin-bilirubin (ALBI) grade on non-small lung cell carcinoma: a propensity-score matched analysis. Anticancer Res. 2021;41(3):1621–1628. doi: 10.21873/anticanres.14924. [DOI] [PubMed] [Google Scholar]
- 26.WHO Expert Consultation. Appropriate body-mass index for asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 27.Imai H, Kuwako T, Kaira K, Masuda T, Miura Y, Seki K, Sakurai R, Utsugi M, Shimizu K, Sunaga N, Tomizawa Y, Ishihara S, Ishizuka T, Mogi A, Hisada T, Minato K, Takise A, Saito R, Yamada M. Evaluation of gefitinib efficacy according to body mass index, body surface area, and body weight in patients with EGFR-mutated advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;79(3):497–505. doi: 10.1007/s00280-016-3232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokubun H, Takigawa C, Chihara S, Hara S, Uezono Y. Population pharmacokinetics of methadone after oral administration in Japanese patients with cancer-related pain. J Pain Palliat Care Pharmacother. 2020;34(4):203–210. doi: 10.1080/15360288.2020.1785070. [DOI] [PubMed] [Google Scholar]
- 29.Li XH, Kamenecka TM, Cameron MD. Bioactivation of the epidermal growth factor receptor inhibitor gefitinib: implications for pulmonary and hepatic toxicities. Chem Res Toxicol. 2009;22(10):1736–1742. doi: 10.1021/tx900256y. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Tomkinson H, Masson E. Effect of sustained elevated gastric pH levels on gefitinib exposure. Clin Pharmacol Drug Dev. 2017;6(5):517–523. doi: 10.1002/cpdd.337. [DOI] [PubMed] [Google Scholar]
- 31.Kletzl H, Giraudon M, Ducray PS, Abt M, Hamilton M, Lum BL. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals. Anticancer Drugs. 2015;26(5):565–572. doi: 10.1097/CAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 32.Galetti M, Petronini PG, Fumarola C, Cretella D, La Monica S, Bonelli M, Cavazzoni A, Saccani F, Caffarra C, Andreoli R, Mutti A, Tiseo M, Ardizzoni A, Alfieri RR. Effect of ABCG2/BCRP expression on efflux and uptake of gefitinib in NSCLC cell lines. PLoS One. 2015;10(11):e0141795. doi: 10.1371/journal.pone.0141795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, Sawada Y, Kohda Y. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67(1):44–49. doi: 10.1111/j.1365-2125.2008.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L, Zhang C, He H, Pan Z, Fan D, He Y, You H, Li Y. Associations between ABCG2 gene polymorphisms and gefitinib toxicity in non-small cell lung cancer: a meta-analysis. Onco Targets Ther. 2018;11:665–675. doi: 10.2147/OTT.S154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Li C, Yang R, Jin J, Liu D, Li W. Prognostic value of the Geriatric Nutritional Risk Index in non-small cell lung cancer patients: a systematic review and meta-analysis. Front Oncol. 2022;11:794862. doi: 10.3389/fonc.2021.794862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Edwards BJ. Malnutrition in older adults with cancer. Curr Oncol Rep. 2019;21(9):80. doi: 10.1007/s11912-019-0829-8. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Brahmer J, Messersmith W, Hidalgo M, Baker SD. Binding of gefitinib, an inhibitor of epidermal growth factor receptor-tyrosine kinase, to plasma proteins and blood cells: in vitro and in cancer patients. Invest New Drugs. 2006;24(4):291–297. doi: 10.1007/s10637-006-5269-2. [DOI] [PubMed] [Google Scholar]
- 38.Yokota H, Sato K, Sakamoto S, Okuda Y, Fukuda N, Asano M, Takeda M, Nakayama K, Miura M. Effects of CYP3A4/5 and ABC transporter polymorphisms on osimertinib plasma concentrations in Japanese patients with non-small cell lung cancer. Invest New Drugs. 2022;40(6):1254–1262. doi: 10.1007/s10637-022-01304-9. [DOI] [PubMed] [Google Scholar]
- 39.Hashino Y, Hatsuyama T, Iwayama K, Hoshi T, Wakamoto A, Ohtaki K, Toda T, Sato H. The relationship between efficacy and safety of osimertinib blood concentration in patients with EGFR mutation-positive lung cancer: a prospective observational study. In Vivo. 2023;37(6):2669–2677. doi: 10.21873/invivo.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodier T, Puszkiel A, Cardoso E, Balakirouchenane D, Narjoz C, Arrondeau J, Fallet V, Khoudour N, Guidi M, Vidal M, Declèves X, Csajka C, Alexandre J, Cadranel J, Fabre E, Wislez M, Goldwasser F, Blanchet B. Exposure-response analysis of osimertinib in patients with advanced non-small-cell lung cancer. Pharmaceutics. 2022;14(9):1844. doi: 10.3390/pharmaceutics14091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenfeld AJ, Yu HA. The evolving landscape of resistance to osimertinib. J Thorac Oncol. 2020;15(1):18–21. doi: 10.1016/j.jtho.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Park MJ, Lee J, Hong JY, Choi MK, Yi JH, Lee SJ, Oh SJ, Ahn JS, Park K, Ahn MJ. Prognostic model to predict outcomes in nonsmall cell lung cancer patients treated with gefitinib as a salvage treatment. Cancer. 2009;115(7):1518–1530. doi: 10.1002/cncr.24151. [DOI] [PubMed] [Google Scholar]
- 43.Fiala O, Pesek M, Finek J, Racek J, Minarik M, Benesova L, Bortlicek Z, Sorejs O, Kucera R, Topolcan O. Serum albumin is a strong predictor of survival in patients with advanced-stage non-small cell lung cancer treated with erlotinib. Neoplasma. 2016;63(03):471–476. doi: 10.4149/318_151001N512. [DOI] [PubMed] [Google Scholar]
- 44.Kwok WC, Ho JCM, Tam TCC, Ip MSM, Lam DCL. Serum protein level as a predictor of therapeutic response and adverse effects associated with afatinib use. J Thorac Dis. 2022;14(6):1880–1889. doi: 10.21037/jtd-21-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horak J, White J, Harris AL, Verrill M, Carmichael J, Holt A, Cantarini M, Macpherson M, Swaisland A, Swaisland H, Twelves C. The effect of different etiologies of hepatic impairment on the pharmacokinetics of gefitinib. Cancer Chemother Pharmacol. 2011;68(6):1485–1495. doi: 10.1007/s00280-011-1611-2. [DOI] [PubMed] [Google Scholar]
- 46.Wada Y, Koyama S, Kuraishi H, Miyahara T, Yoshiike F, Agatsuma T, Yamamoto R, Ono Y, Suzuki T, Hachiya T, Gomi D, Tateishi K, Hanaoka M, Koizumi T. Clinical analysis of patients treated with afatinib for advanced non-small cell lung cancer: A Nagano Lung Cancer Research Group observational study. Respir Investig. 2016;54(6):462–467. doi: 10.1016/j.resinv.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa E, Yokoyama Y, Chishima H, Kasai H, Kuniyoshi O, Kimura M, Hakamata J, Nakada H, Suehiro N, Nakaya N, Nakajima H, Ikemura S, Kawada I, Yasuda H, Terai H, Jibiki A, Kawazoe H, Soejima K, Muramatsu H, Suzuki S, Nakamura T. Population pharmacokinetics, pharmacogenomics, and adverse events of osimertinib and its two active metabolites, AZ5104 and AZ7550, in Japanese patients with advanced non-small cell lung cancer: a prospective observational study. Invest New Drugs. 2023;41(1):122–133. doi: 10.1007/s10637-023-01328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibson AJW, D’Silva A, Elegbede AA, Tudor RA, Dean ML, Bebb DG, Hao D. Impact of Asian ethnicity on outcome in metastatic EGFR-mutant non-small cell lung cancer. Asia Pac J Clin Oncol. 2019;15(6):343–352. doi: 10.1111/ajco.13234. [DOI] [PubMed] [Google Scholar]