Abstract
Background/Aim
Elderly patients with early-stage breast cancer have potentially been underrepresented in clinical trials. Thus, treatment strategies for a minority of elderly patients with hormone receptor (HR)-negative breast cancer may be inadequately informed.
Patients and Methods
We retrospectively reviewed 126 patients with HR-negative breast cancer aged ≥65 years. Patients aged ≥75 years (group A) were compared with those aged 65-74 years (group B). Of the 126 surgically treated patients, 48 were in group A and 78 were in group B.
Results
The number of patients who did not undergo axillary lymph node surgery was significantly higher in group A than that in group B (15% vs. 2%, respectively, p=0.047). The number of patients who received radiotherapy was significantly lower in group A than B (13% vs. 44%, respectively, p<0.01). The number of patients who did not receive chemotherapy was significantly higher in group A than B (79% vs. 23%, respectively, p<0.01). Breast cancer-specific survival and overall survival showed no significant difference between groups.
Conclusion
Omission of axillary surgery, radiation, or chemotherapy may not have a significant prognostic impact in patients with HR-negative breast cancer aged ≥75 years. Multiple age-related factors complicate the standardization of optimal treatment decisions for these patients.
Keywords: Geriatrics, gerontology, de-escalation, axillary surgery
In the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, an estimated 297,790 women will be new breast cancer cases in 2023; of those 45.8% women will be over 65 years old, and 19.0% over 75 years old (1). Compared to younger patients, the elderly have a greater variety of comorbidities, such as poor organ functions, cognitive function, performance status, and social background. Therefore, in many cases, decisions about management need to be made on a case-by-case basis.
The availability of prospective data to guide the treatment of elderly patients is limited, because this cohort has potentially been underrepresented in clinical trials. Consequently, the appropriate decisions required to maintain standards of care remain unclear. Elderly patients with breast cancer are often in a more advanced stage at the time of diagnosis compared to younger patients. One reason for this is that breast cancer screening among the elderly has not been adequately performed due to a lack of data demonstrating its usefulness (2). Most breast cancers in the elderly are likely to have less aggressive tumor histology, be estrogen receptor (ER)-positive, and human epidermal growth factor receptor (HER2)-negative (3-6). Therefore, breast cancer-related survival rates are not poor in elderly patients despite the less frequent use of standard adjuvant therapies (7). Nevertheless, optimal treatment strategies for a minority of elderly patients with hormone receptor (HR)-negative breast cancer remain unclear.
Therefore, we performed a retrospective study of the clinical characteristics, treatment modalities, and prognosis of elderly patients with operable HR-negative breast cancer.
Patients and Methods
Of the 2,334 breast cancer patients treated surgically at our hospital between August 2008 and April 2020, we conducted a retrospective cohort study on 126 patients ≥65 years of age with stage I, II, and III breast cancer. We excluded patients with incomplete medical records, those who had visceral metastases or other active malignancies at the time of surgery, those presenting with bilateral breast cancers, and those with a personal history of breast cancer. Histologic types with a better prognosis, such as mucinous carcinoma and adenoid cystic carcinoma, were excluded. We compared patients with HR-negative breast cancer aged 75 years and older with those aged 65-74 years. We obtained data from patients’ clinical records. Patient characteristics, comorbidities, treatment types, and mortality rates were investigated. Regarding the surgical procedure, mastectomy was indicated in cases with tumors larger than 3 cm, multicentric tumors, and cases in which postoperative radiotherapy could not be performed for some reason, and when the patient desired it, while breast-conserving surgery (BCS) was indicated in other cases. The patients who underwent BCS were recommended to receive adjuvant radiation therapy to the remaining breast tissue. The indication for sentinel lymph node biopsy was clinically negative axillary lymph node metastasis, performed by dye and/or radioisotope methods. If the frozen histological examination was positive for metastasis, axillary lymph node dissection was performed. Surgical procedures on axillary lymph nodes were omitted in patients with multiple comorbidities, reduced organ function, or frailty. Patients were followed up every six months for a minimum of five years at our hospital, with clinical breast examinations and mammograms once a year. This study was approved by our institutional review board.
In all patients, morphological characteristics, such as histologic types and grades, were evaluated. ER, progesterone receptor (PgR), HER2 statuses were evaluated on formalin-fixed and paraffin-embedded tissue from core needle biopsy of the primary tumor before breast surgery. Cases having an immunohistochemistry score of 3+ or positive fluorescence in situ hybridization result were defined as HER2 positive. HR indicates ER and PgR, and positivity was diagnosed if at least 1% of nuclei in the tumor were stained by the immuno-histochemical method. The pathological TNM and histological classifications were registered according to the 8th edition of the Union for International Cancer Control staging system (8).
Data for continuous parameters are reported as median and interquartile ranges. Descriptive data are recorded as numbers and percentages. To ascertain the clinicopathological characteristics of older patients, we used Pearson’s chi-square test and Fisher’s Exact test, where appropriate, to determine differences between the two age groups. We constructed and compared age-group survival curves using the Kaplan–Meier method and log-rank test, respectively. All tests were two-sided, and p<0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics (version24, SPSS Inc, Chicago, IL, USA).
Results
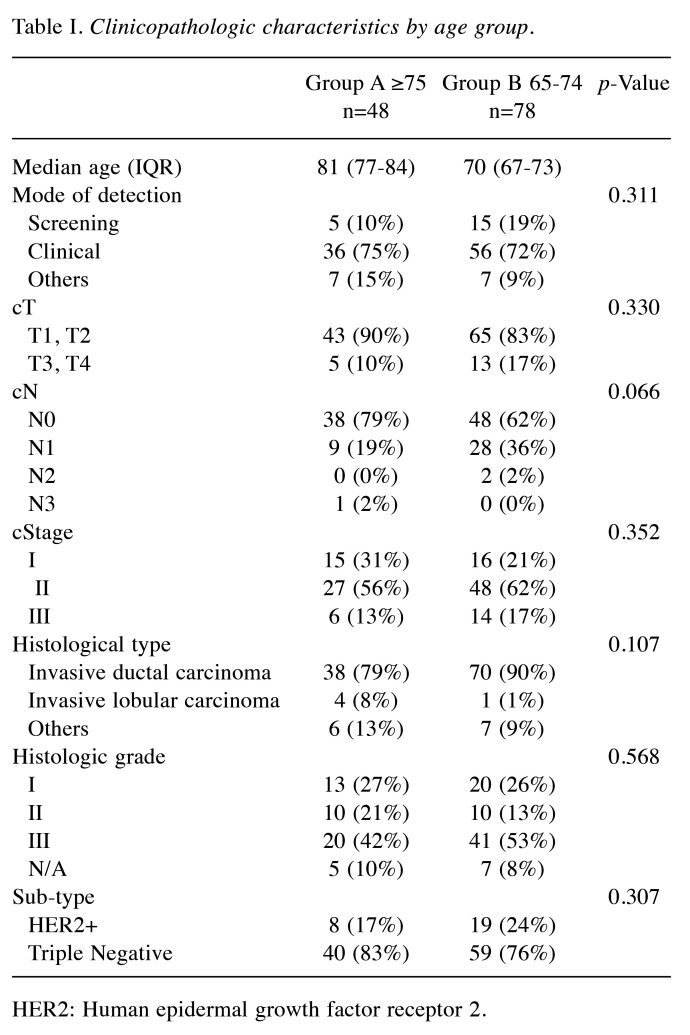
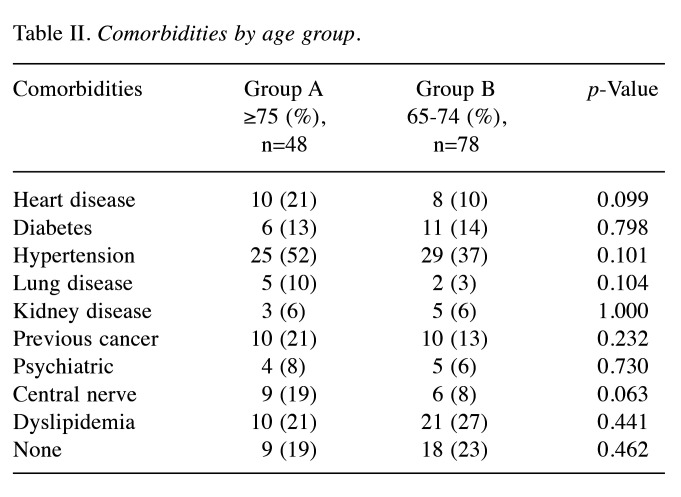
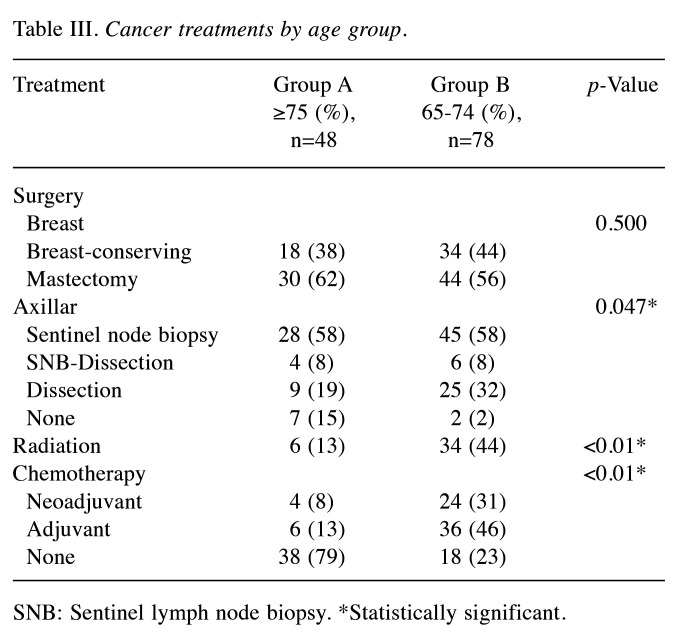
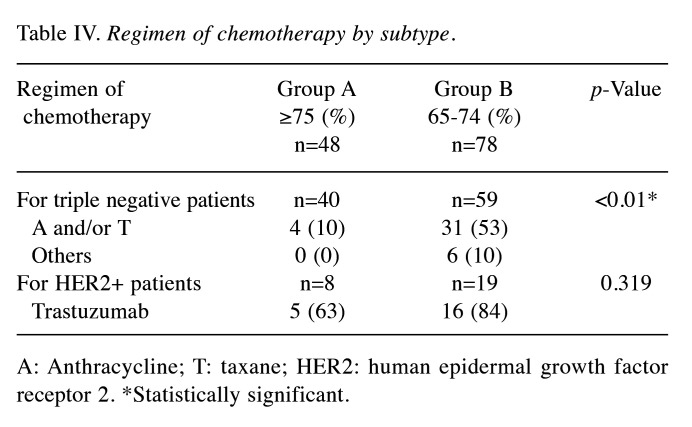
Of the 126 surgically treated patients, 48 aged 75 years and older (group A) and 78 were 65 to 74 years old (group B) (Table I). The median patient age was 81 and 70 years in group A and B, respectively. The oldest patient was 94 years of age. The characteristics of the breast cancer patients are shown in Table I. The clinical T factor was not significantly different between the two groups, with T1 and T2 accounting for >80% in both groups. There were more clinical N0 cases in group A than B (79% vs. 62%, respectively) and fewer clinical N1 cases in group A than B (19% vs. 36%, respectively), but the difference was not significant. In addition, the clinical stages were not significantly different between groups. No significant difference in histological type was observed between the two groups. There was no significant difference in the histologic grade between the two groups, with >40% in grade III. The HER2-positive subtype was lower in group A than B (17% vs. 24%, respectively), and the triple-negative subtype was higher in group A than B (83% vs. 76%, respectively), but the differences were not significant. Many patients had one or more comorbidities (Table II). The proportion of patients without comorbidities was 19% and 23% in group A and B, respectively. There was no significant difference in comorbidities between the two groups. Table III shows the results of surgery, radiotherapy, and chemotherapy. Although the surgical techniques used were similar in both groups, significantly more patients in group A than in group B did not undergo axillary lymph node surgery (15% vs. 2%, respectively) (p=0.047). The number of patients who received radiotherapy was significantly lower in group A than B (13% vs. 44%, respectively) (p<0.01). The number of patients who received chemotherapy was significantly lower in group A than B for both neoadjuvant and adjuvant chemotherapy, and the number of patients who did not receive chemotherapy was significantly higher in group A than B (79% vs. 23%, respectively) (p<0.01). The chemotherapy regimens are summarized according to subtype in Table IV. In patients with triple-negative breast cancer, the number of patients who received anthracycline or taxane was significantly lower in group A than B (10% vs. 53%, respectively) (p<0.01). Among patients with HER2-positive breast cancer, there was no significant difference in the administration of trastuzumab between group A and B (63% vs. 84%, respectively) (p=0.319). The median follow-up period was 49 and 50 months in group A and B, respectively (p=0.476) (Table V). The median time to recurrence was similar in group A and B (26.5 months vs. 20.5 months, respectively) (p=0.172). The mode of recurrence was similar in both groups: no recurrence was observed in 78% and 82%, only locoregional recurrence in 10% and 4%, only distant metastasis in 8% and 10%, and locoregional recurrence and distant metastasis in 4% and 4% of group A and B, respectively (p=0.525). There was no significant difference in vital status between the two groups: alive without evidence of disease was 70% and 78%; alive with disease was 12% and 10%; and died of breast cancer was 6% and 9% in group A and B, respectively (p=0.145). The number of deaths from unknown causes was 12% and 3% in group A and B, respectively but the difference was not significant (p=0.145). Breast cancer-specific survival (BCSS) and loco-regional recurrence-free survival (LRRFS) were not significantly different between the two groups (p=0.627 and 0.111, respectively) (Figure 1 and Figure 2). Although the overall survival (OS) tended to be lower in group A, the difference was not significant (p=0.222) (Figure 3).
Table I. Clinicopathologic characteristics by age group.
HER2: Human epidermal growth factor receptor 2.
Table II. Comorbidities by age group.
Table III. Cancer treatments by age group.
SNB: Sentinel lymph node biopsy. *Statistically significant.
Table IV. Regimen of chemotherapy by subtype.
A: Anthracycline; T: taxane; HER2: human epidermal growth factor receptor 2. *Statistically significant.
Table V. Outcomes by age group.
Figure 1.
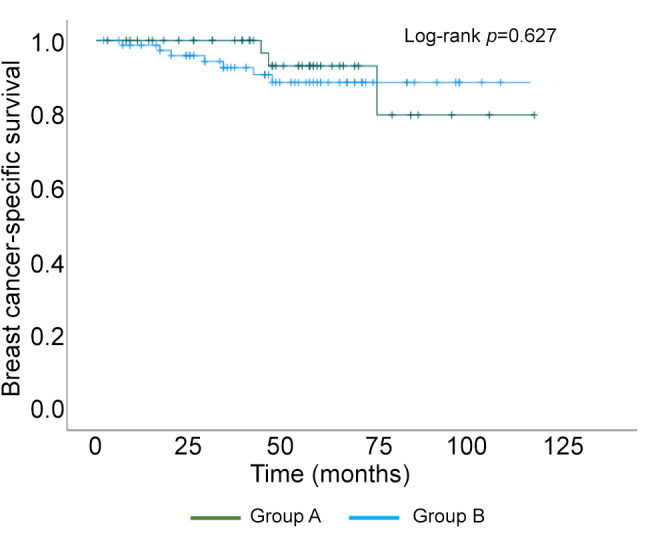
Kaplan–Meier curve of breast cancer-specific survival.
Figure 2.
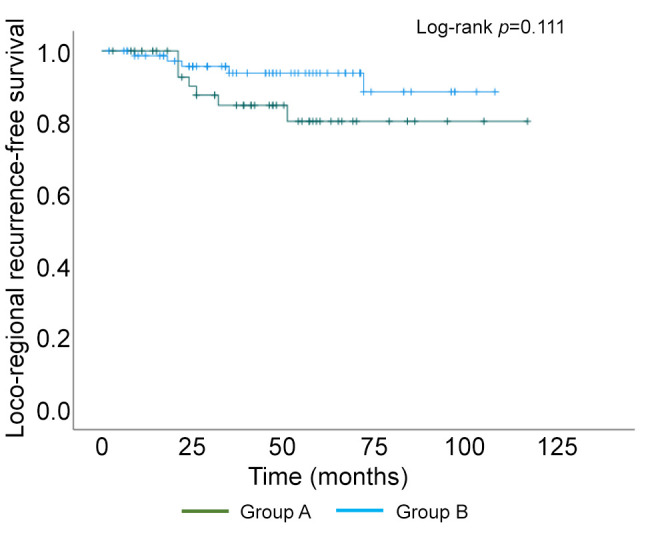
Kaplan–Meier curve of loco-regional recurrence-free survival.
Figure 3.
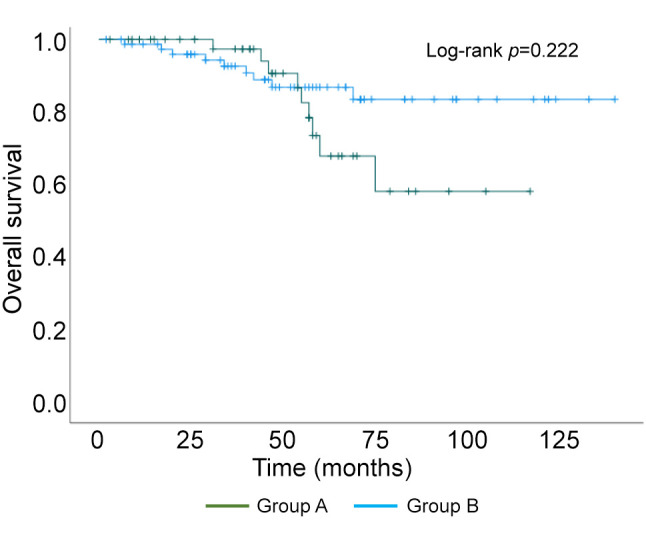
Kaplan–Meier curve of overall survival.
Discussion
Elderly breast cancer patients are generally HR-positive and their pathological characteristics are low-grade, consistent with relatively favorable tumor biology (3-6). Owing to these characteristics, the breast cancer-specific survival of elderly patients is deemed not to low, despite less frequent use of standard adjuvant therapies (5). Loss of bone mineral density is a problem with aromatase inhibitor therapy for elderly patients with HR-positive breast cancer, but has been shown to be preventable with oral bisphosphonate (9). However, the prognosis of elderly patients with HR-negative breast cancer remains unclear. This study found that LRRFS tended to be lower in those aged 75 years and older than those aged 65-74 years, although the difference was not significant. However, the BCSS was similar in both the groups. Although OS was not significantly different, it tended to be lower in the 75 years and older group than in the 65 to 74 years old group, probably due to the higher number of deaths from unknown causes in the 75 years and older group.
With regard to surgery for elderly patients with breast cancer, standard surgical approaches are recommended in most patients with minimal comorbidities and are expected to have a relatively long-life expectancy. Surgery for elderly patients with breast cancer may be considered as safe as that for younger patients (10,11). Although elderly patients with breast cancer tend to undergo mastectomy compared to breast-conserving surgery (BCS) and omit axillary lymph node surgery, the BCSS rate was similar among age categories (7,12,13). In particular, omitting axillary surgery has little effect on OS, particularly in women aged≥75 years, and studies indicate a local progression of less than 10%, which, in most cases, can be controlled with either further surgery or radiotherapy (14,15). Many of these reports include mainly results of HR-positive breast cancers, and it is not clear what the consequences of omitting surgery of the axillary lymph nodes would be in HR-negative breast cancers. One retrospective study showed that not performing radiotherapy and axillary lymph node dissection had no effect on 5-year disease-free survival, OS, or BCSS in elderly patients with breast cancer, irrespective of breast cancer subtypes (16). In this study, BCS was similar in approximately 40% of patients in both groups, but omission of axillary surgery was more common in those over 75 years than those who were 65-74 years old. Although no significant differences were found, LRRFS tended to be lower in those over 75 than those in the 65 to 74 years old group, possibly due to the omission of axillary lymph node dissection. However, the BCSS was similar in both groups.
Omission of radiotherapy is acceptable in elderly patients with stage I and HR-positive breast cancer when endocrine therapy is used. In the Cancer and Leukemia group-B-initiated CALGB 9343, a randomized trial comparing the efficacy of radiation therapy in older women with HR-positive, clinical stage Ⅰ breast cancer, there were no significant differences in BCSS or OS between the tamoxifen and radiation therapy and tamoxifen alone groups (17). In the PRIME II trial, a randomized phase III trial of patients with HR-positive, low-risk, and 65 years or older breast cancer with or without radiotherapy after breast-conserving surgery, regional recurrences, and OS at five years were identical between the two treatment groups (18). However, the effects of omitting radiotherapy in elderly patients with HR-negative tumors are not well understood. Although the number of patients was small, local recurrence occurred in six of 65 non-irradiated patients with HR-negative tumors compared to no recurrence in the 55 irradiated patients in the PRIME II trial (18). In one study using SEER data to evaluate the impact of radiotherapy among elderly (≥80 years) patients with HR-negative breast cancer, the difference in the need for future mastectomy was 3.4% vs. 6.9% (p=0.05) with or without radiotherapy, respectively, with a smaller magnitude of effect from radiotherapy (19). In our study, there was significantly more omission of radiotherapy in those over 75 years than those who were 65 to 74 years old. The LRRFS and BCSS were similar in both groups, although LRRFS tended to be lower in those over 75 years than those who were 65-74 years old, suggesting that omission of radiotherapy may not have a significant impact on the prognosis of elderly patients with breast cancer aged ≥75 years.
Chemotherapy demonstrated a survival benefit for women aged 67-79 years with ER-negative lymph node-positive disease (20,21). It has been widely reported that age alone is not a contraindication for chemotherapy because chemotherapy results in similar prolonged survival and reduced recurrence in older women and younger patients. By contrast, although primary systemic chemotherapy was omitted more frequently in elderly patients, the BCSS was similar between age categories (7). In the present work, there was significantly more omission of chemotherapy in those aged 75 years and older than 65 to 74 years old, but the BCSS was comparable. Individualized therapies that consider the patient’s general status, comorbidities, and life expectancy remain the key to optimal outcomes, both from the perspective of cancer outcome and preserving the quality of life. Recently, molecular-targeted agents have been increasingly applied to clinical practice: CDK4/6 inhibitors have been shown to be effective in patients with HR-positive HER2-negative breast cancer, and their efficacy and toxicity have been reported to be similar in patients over 75 years of age as in those under 75 years of age (22). Novel targeted cancer treatments with a better safety profile than standard chemotherapy would provide further opportunities for improving outcomes, especially for subtypes with limited options currently, such as HR-negative disease. In particular, in patients with HER2-positive breast cancer, adjuvant trastuzumab monotherapy can be considered an option for selected elderly patients (23). In our study, the use of trastuzumab was similar in both the 75 years and older group and the 65-74 years old group of patients with HER2-positive.
As this was a retrospective observational study with a small number of patients, there might be selection bias and residual confounding by factors for which we did not have data. In addition, it is possible that only patients in good general condition who could undergo surgery were selected by the attending physician, especially in those patients aged 75 years and older. In addition, comprehensive geriatric assessments such as the Charlson Comorbidity Index or other methods, were not performed in this study. Further research is required to determine the overall tolerability and outcomes of various treatment regimens in elderly patients.
Conclusion
In this study of patients with HR-negative breast cancer who underwent surgery, omission of axillary surgery, radiotherapy, or chemotherapy was significantly more common in patients aged ≥75 years than in those aged 65-74 years. Nevertheless, there were no significant differences in BCSS, LRRFS, and OS between the two groups, suggesting that it may be possible to omit these treatments by selecting patients appropriately. Even in patients with HR-negative breast cancer, it is important to select the optimal treatment for elderly breast cancer patients over 75 years of age, considering the patient's general status, comorbidities, life expectancy, wishes, and treatment-related adverse events.
Conflicts of Interest
The Authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors’ Contributions
T.I. conceived the presented idea. T.I., M.T., M.T., and D.O. acquired data, conducted data analysis, and drafted the manuscript. S.A. and D.O. interpreted the data and revised the manuscript. All Authors reviewed the final manuscript and approved it for final submission.
Acknowledgements
The Authors thank all of the people who contributed to this report.
References
- 1.An interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute. Available at: https://seer.cancer.gov/statfacts/html/breast.html. [Last accessed on May 12, 2024]
- 2.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, U.S. Preventive Services Task Force. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727. doi: 10.7326/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angarita FA, Chesney T, Elser C, Mulligan AM, McCready DR, Escallon J. Treatment patterns of elderly breast cancer patients at two Canadian cancer centres. Eur J Surg Oncol. 2015;41(5):625–634. doi: 10.1016/j.ejso.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Cyr A, Gillanders WE, Aft RL, Eberlein TJ, Margenthaler JA. Breast cancer in elderly women (≥ 80 years): variation in standard of care. J Surg Oncol. 2011;103(3):201–206. doi: 10.1002/jso.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamtani A, Gonzalez JJ, Neo D, Slanetz PJ, Houlihan MJ, Herold CI, Recht A, Hacker MR, Sharma R. Early-stage breast cancer in the octogenarian: tumor characteristics, treatment choices, and clinical outcomes. Ann Surg Oncol. 2016;23(10):3371–3378. doi: 10.1245/s10434-016-5368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Water W, Seynaeve C, Bastiaannet E, Markopoulos C, Jones SE, Rea D, Hasenburg A, Putter H, Hille ET, Paridaens R, de Craen AJ, Westendorp RG, van de Velde CJ, Liefers GJ. Elderly postmenopausal patients with breast cancer are at increased risk for distant recurrence: a tamoxifen exemestane adjuvant multinational study analysis. Oncologist. 2013;18(1):8–13. doi: 10.1634/theoncologist.2012-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawaki M, Yamada A, Kumamaru H, Miyata H, Nakayama K, Shimizu C, Miyashita M, Honma N, Taira N, Saji S. Clinicopathological characteristics, practical treatments, prognosis, and clinical issues of older breast cancer patients in Japan. Breast Cancer. 2021;28(1):1–8. doi: 10.1007/s12282-020-01188-8. [DOI] [PubMed] [Google Scholar]
- 8.Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. New Jersey, USA, 8th Edition, Wiley-Blackwell. 2016 [Google Scholar]
- 9.Ogata N, Toh U, Sudou T, Ogata S, Takao Y, Sugihara R, Watanabe H, Matushima S, Akagi Y. Efficacy and safety of monthly minodronate therapy in postmenopausal breast cancer patients receiving aromatase inhibitors. Anticancer Res. 2022;42(8):4139–4143. doi: 10.21873/anticanres.15912. [DOI] [PubMed] [Google Scholar]
- 10.Angarita FA, Acuna SA, Cordeiro E, Elnahas A, Sutradhar S, Jackson T, Cil TD. Thirty-day postoperative morbidity and mortality in elderly women with breast cancer: an analysis of the NSQIP database. Breast Cancer Res Treat. 2018;170(2):373–379. doi: 10.1007/s10549-018-4747-5. [DOI] [PubMed] [Google Scholar]
- 11.Buonomo OC, Pellicciaro M, Materazzo M, Berardi S, Gigliotti PE, Caspi J, Meucci R, Perretta T, Portarena I, Dauri M, Pistolese CA, Vanni G. Surgical treatments for ductal carcinoma in situ (DCIS) in elderly patients. Anticancer Res. 2023;43(4):1555–1562. doi: 10.21873/anticanres.16305. [DOI] [PubMed] [Google Scholar]
- 12.Yamada A, Narui K, Sugae S, Shimizu D, Takabe K, Ichikawa Y, Ishikawa T, Endo I. Operation with less adjuvant therapy for elderly breast cancer. J Surg Res. 2016;204(2):410–417. doi: 10.1016/j.jss.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertolo A, Rosso C, Voutsadakis IA. Breast cancer in patients 80 years-old and older. Eur J Breast Health. 2020;16(3):208–212. doi: 10.5152/ejbh.2020.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truong PT, Bernstein V, Wai E, Chua B, Speers C, Olivotto IA. Age-related variations in the use of axillary dissection: a survival analysis of 8038 women with T1–ST2 breast cancer. Int J Radiat Oncol Biol Phys. 2002;54(3):794–803. doi: 10.1016/s0360-3016(02)02973-5. [DOI] [PubMed] [Google Scholar]
- 15.International Breast Cancer Study Group, Rudenstam CM, Zahrieh D, Forbes JF, Crivellari D, Holmberg SB, Rey P, Dent D, Campbell I, Bernhard J, Price KN, Castiglione-Gertsch M, Goldhirsch A, Gelber RD, Coates AS. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10-93. J Clin Oncol. 2006;24(3):337–344. doi: 10.1200/JCO.2005.01.5784. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Y, Xu Y, Zhou Y, Mao F, Lin Y, Guan J, Shen S, Pan B, Wang C, Peng L, Huang X, Li Y, Wang X, Sun Q. Breast-conserving surgery without axillary lymph node surgery or radiotherapy is safe for HER2-positive and triple negative breast cancer patients over 70years of age. Breast Cancer Res Treat. 2020;182(1):117–126. doi: 10.1007/s10549-020-05686-3. [DOI] [PubMed] [Google Scholar]
- 17.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, Muss HB, Smith BL, Hudis CA, Winer EP, Wood WC. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 19.Eaton BR, Jiang R, Torres MA, Kahn ST, Godette K, Lash TL, Ward KC. Benefit of adjuvant radiotherapy after breast-conserving therapy among elderly women with T1-T2N0 estrogen receptor-negative breast cancer. Cancer. 2016;122(19):3059–3068. doi: 10.1002/cncr.30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS. Adjuvant chemotherapy and survival in older women with hormone receptor–negative breast cancer: assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24(18):2757–2764. doi: 10.1200/JCO.2005.03.6053. [DOI] [PubMed] [Google Scholar]
- 21.Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 22.Pacilio C, Rosati G, Crispo A, Bimonte S, DI Rella F, Nuzzo F, DE Laurentiis M. An overview of the roles of CDK4/6 inhibitors in metastatic breast cancer elderly patients. In Vivo. 2023;37(4):1445–1449. doi: 10.21873/invivo.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawaki M, Taira N, Uemura Y, Saito T, Baba S, Kobayashi K, Kawashima H, Tsuneizumi M, Sagawa N, Bando H, Takahashi M, Yamaguchi M, Takashima T, Nakayama T, Kashiwaba M, Mizuno T, Yamamoto Y, Iwata H, Kawahara T, Ohashi Y, Mukai H, for the RESPECT study group. Randomized controlled trial of trastuzumab with or without chemotherapy for HER2-positive early breast cancer in older patients. J Clin Oncol. 2020;38(32):3743–3752. doi: 10.1200/JCO.20.00184. [DOI] [PubMed] [Google Scholar]