Abstract
Retinopathy of prematurity (ROP) is a major cause of preventable blindness in preterm infants. The association between red blood cell (RBC) parameters and the development of ROP remains unclear. The objectives of the present study were to evaluate the association between RBC parameters and ROP treatment. This single-center, retrospective cohort study included preterm infants born at < 30 weeks of gestation. Data pertaining to RBC parameters and ROP treatment were obtained from the medical records. A receiver operating characteristic (ROC) analysis was performed to determine the cut-off values of the RBC parameters according to the need for ROP treatment. Multiple logistic regression analyses assessed the association between ROP treatment and RBC parameters at birth and on day of life (DOL) 28. We included 202 infants, and 44.1% were treated for ROP. After adjusting for confounders, we observed associations between ROP treatment and mean corpuscular volume (MCV) values > 117.3 fL at birth (adjusted odds ratio [aOR]:2.3; 95% confidence intervals CI 1.0–5.3). Additionally, on DOL 28, hemoglobin (Hb) values < 9.9 g/dL (aOR:3.0; 95% CI 1.4–6.7), hematocrit (Hct) values < 31.0% (aOR:2.7; 95% CI 1.3–5.6), and red cell distribution width (RDW) values > 18.5% (aOR:2.6; 95% CI 1.1–6.2) were associated with ROP treatment. In conclusion, our study indicated that infants born at < 30 weeks of gestation with an MCV > 117.3 fL at birth, along with Hb < 9.9 g/dL, Hct < 31.0%, and RDW > 18.5% on DOL 28, had an increased risk of requiring ROP, warranting treatment.
Subject terms: Biomarkers, Diseases, Medical research, Risk factors
Introduction
Retinopathy of prematurity (ROP) is a major cause of preventable blindness in preterm infants1. It is characterized by abnormal retinal vascularization that can lead to retinal detachment, severe visual impairment, or blindness. In 2010, approximately 184,700 preterm infants worldwide developed ROP of any stage; 20,000 developed ROP-induced blindness or severe visual impairment, and 12,300 developed mild or moderate visual impairment2. In the USA, the incidence of severe ROP in preterm infants born at a gestational age of ≤ 30 weeks increased from 3.4% in 2009 to 5.3% in 20183. In Japan, the mortality rate of extremely preterm infants is low, whereas the incidence of severe ROP in such infants is approximately 15%, much higher than that reported in other countries4,5. ROP is a multifactorial disease with risk factors including maternal, prenatal, and perinatal factors; demographics; medical interventions; comorbidities of prematurity; nutrition; and genetics6. Moreover, low birth weight, low gestational age, and high or fluctuating oxygen levels at birth and during the neonatal period are well-known risk factors for ROP7–12.
Most stage 1, 2, and early stage-3 ROP spontaneously regress without any serious residual eye disease; blindness or serious visual impairment results from progression to retinal detachment or severe posterior retinal distortion13. Therefore, the eyes should be screened for ROP to ensure timely treatment and prevention of ROP-induced blindness. However, ROP screening can be stressful for preterm infants and can cause apnea, bradycardia, and serious gastrointestinal complications. Therefore, efforts should be made to enable safe and efficient retinal examination on infants scheduled for multiple examinations. Predictive biomarkers of ROP may be useful in determining ROP screening schedules. Additionally, there have been several reports on the association between anemia and the development of ROP14–19. However, the results of these studies are controversial. Furthermore, reports on the association between red blood cell (RBC) parameters and ROP treatments are limited20–22. Thus, we aimed to evaluate the association between RBC parameters and ROP treatment.
Results
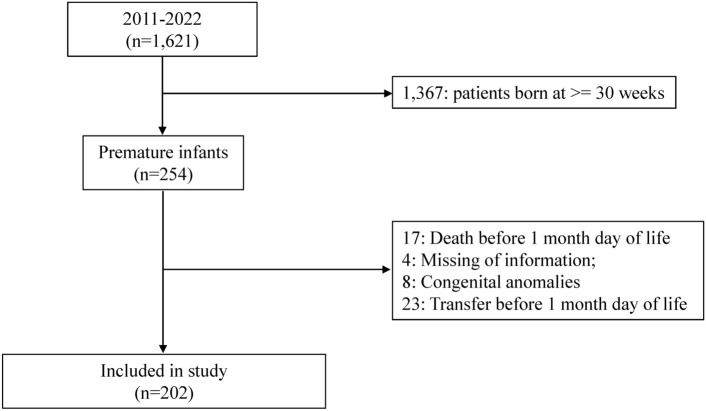
Information on 1,621 infants was obtained from medical records, of which 202 were eligible for participation in the study (Fig. 1). Of the 202 infants, 89 (44.1%) underwent ROP treatment (Table 1). A higher incidence of ROP treatment was associated with lower gestational age, lower birth weight, antenatal steroid use, history of premature rupture of membrane (PROM), lower Apgar scores at 1 and 5 min, inhaled nitric oxide (iNO) use, oxygen supplementation on day of life (DOL) 28, mechanical ventilation on DOL 28, and RBC transfusion before DOL 28 (Table 2). The mean values of hemoglobin (Hb), hematocrit (Hct), and mean corpuscular hemoglobin concentration (MCH) at birth and on DOL 28, mean corpuscular volume (MCV) at birth, and red blood cell distribution width (RDW) on DOL 28 were significantly different (Table 2). No differences were detected in mean MCV on DOL 28 or RDW at birth (Table 2).
Fig. 1.
Flow diagram of sample selection for analysis.
Table 1.
Characteristics of the study participants.
| Characteristics | Value |
|---|---|
| GA, weeks, mean ± SD* | 26.0 ± 2.0 |
| Birth weight, g, mean ± SD* | 793 ± 251 |
| SGA, n (%) | 32 (15.8%) |
| Male, n (%) | 104 (51.5%) |
| Antenatal steroid use, n (%) | 161 (79.7%) |
| CAM, n (%) | 96 (47.8%) |
| PROM, n (%) | 53 (26.2%) |
| HDP, n (%) | 22 (10.9%) |
| Apgar score at 1 min, mean ± SD* | 4.0 ± 2.0 |
| Apgar score at 5 min, mean ± SD* | 6.2 ± 2.1 |
| RDS, n (%) | 163 (80.7%) |
| PDA, n (%) | 108 (53.5%) |
| iNO, n (%) | 22 (10.9%) |
| Oxygen supplementation on DOL 28, n (%) | 95 (47.0%) |
| Mechanical ventilation on DOL 28, n (%) | 160 (79.2%) |
| RBC transfusion before DOL 28, n (%) | 112 (55.5%) |
| Hb at birth, g/dL, mean ± SD* | 15.0 ± 2.6 |
| Hb on DOL 28, g/dL, mean ± SD* | 10.4 ± 1.7 |
| Hct at birth, %, mean ± SD* | 44.4 ± 6.9 |
| Hct on DOL 28, %, mean ± SD* | 31.1 ± 5.0 |
| MCV at birth, fL, mean ± SD* | 117.7 ± 8.1 |
| MCV on DOL 28, fL, mean ± SD* | 98.3 ± 7.3 |
| MCH at birth, pg, mean ± SD* | 39.6 ± 2.4 |
| MCH on DOL 28, pg, mean ± SD* | 32.7 ± 2.2 |
| RDW at birth, %, mean ± SD* | 16.1 ± 1.5 |
| RDW on DOL 28, %, mean ± SD* | 20.0 ± 3.3 |
| ROP, n (%) | 89 (44.1%) |
*Normalized data.
CAM, chorioamnionitis; DOL, day of life; GA, gestational age; Hb, hemoglobin; Hct, hematocrit; HDP, hypertensive disorders of pregnancy; iNO, inhaled nitric oxide; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; PDA, patent ductus arteriosus; PROM, premature rupture of membrane; RBC, red blood cells; RDS, respiratory distress syndrome; RDW, red cell distribution width; ROP, retinopathy of prematurity; SGA, Small for gestational age.
Table 2.
Characteristics of the infants in the ROP treatment and non-ROP treatment groups.
| Characteristics | ROP treatment (n = 89) | Non-ROP treatment (n = 113) | p-value | |
|---|---|---|---|---|
| GA, weeks, mean ± SD | 24.8 ± 1.3 | 27.0 ± 1.9 | < 0.001 | |
| Birth weight, g, mean ± SD | 648.2 ± 151.7 | 907.1 ± 256.0 | < 0.001 | |
| SGA, n (%) | 15 (16.9%) | 17 (15.0%) | 0.727 | |
| Male, n (%) | 51 (57.3%) | 53 (46.9%) | 0.142 | |
| Antenatal steroids use, n (%) | 81 (91.0%) | 80 (70.8%) | < 0.001 | |
| CAM, n (%) | 46 (51.7%) | 50 (44.2%) | 0.321 | |
| PROM, n (%) | 31 (34.8%) | 22 (19.5%) | < 0.05 | |
| HDP, n (%) | 8 (9.0%) | 14 (12.4%) | 0.441 | |
| Apgar score at 1 min, mean ± SD | 3.3 ± 1.9 | 4.5 ± 2.0 | < 0.001 | |
| Apgar score at 5 min, mean ± SD | 5.7 ± 2.4 | 6.6 ± 1.9 | < 0.01 | |
| RDS, n (%) | 75 (84.3%) | 88 (77.9%) | 0.253 | |
| PDA, n (%) | 54 (60.7%) | 54 (47.8%) | 0.068 | |
| iNO, n (%) | 15 (16.9%) | 7 (6.2%) | < 0.05 | |
| Oxygen supplementation on DOL 28, n (%) | 63 (70.8%) | 32 (28.3%) | < 0.001 | |
| Mechanical ventilation on DOL 28, n (%) | 88 (98.9%) | 72 (63.7%) | < 0.001 | |
| RBC transfusion before DOL 28, n (%) | 66 (74.2%) | 46 (40.7%) | < 0.001 | |
| Hb at birth, g/dL, mean ± SD | 14.1 ± 2.4 | 15.7 ± 2.6 | < 0.001 | |
| Hb on DOL 28, g/dL, mean ± SD | 9.8 ± 1.8 | 10.9 ± 1.5 | < 0.001 | |
| Hct at birth, %, mean ± SD | 42.3 ± 6.6 | 46.1 ± 6.7 | < 0.001 | |
| Hct on DOL 28, %, mean ± SD | 29.6 ± 5.2 | 32.3 ± 4.5 | < 0.001 | |
| MCV at birth, fL, mean ± SD | 121.2 ± 8.0 | 114.8 ± 6.9 | < 0.001 | |
| MCV on DOL 28, fL, mean ± SD | 98.1 ± 7.7 | 98.5 ± 6.9 | 0.7017 | |
| MCH at birth, pg, mean ± SD | 40.4 ± 2.5 | 39.1 ± 2.2 | < 0.001 | |
| MCH on DOL 28, pg, mean ± SD | 32.3 ± 2.4 | 33.0 ± 2.0 | < 0.05 | |
| RDW at birth, %, mean ± SD | 16.1 ± 1.6 | 16.0 ± 1.4 | 0.6514 | |
| RDW on DOL 28, %, mean ± SD | 21.5 ± 3.3 | 18.8 ± 2.7 | < 0.001 | |
CAM, chorioamnionitis; DOL, days of life, GA, gestational age; Hb, hemoglobin; Hct, hematocrit; HDP, hypertensive disorders of pregnancy; iNO, inhaled nitric oxide; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; PDA, patent ductus arteriosus; PROM, premature rupture of membrane; RBC, red blood cells; RDS, respiratory distress syndrome; RDW, red cell distribution width; ROP, retinopathy of prematurity; SGA, small for gestational age.
The cut-off values for Hb, Hct, MCV, MCH, and RDW at birth and on DOL 28 were 16.7 g/dL (AUC:0.659) and 9.9 g/dL (0.674), 49.5% (0.639) and 31.0% (0.668), 117.3 fL (0.725) and 92.3 fL (0.528), 39.4 pg (0.642) and 31.8 pg (0.600), and 19.2% (0.513) and 18.5% (0.744), respectively (Table 3).
Table 3.
Cut-off values of the RBC parameters for the treatment of ROP and its AUC, sensitivity, and specificity.
| Parameter | Cut-off | AUC | Sensitivity | Specificity |
|---|---|---|---|---|
| Hb at birth | 16.7 g/dL | 0.6592 | 37.2 | 89.9 |
| Hb on DOL 28 | 9.9 g/dL | 0.6741 | 76.1 | 51.7 |
| Hct at birth | 49.5% | 0.639 | 30.1 | 92.1 |
| Hct on DOL 28 | 31.0% | 0.6678 | 65.5 | 62.9 |
| MCV at birth | 117.3 fL | 0.7248 | 68.5 | 69.9 |
| MCV on DOL 28 | 92.3 fL | 0.5281 | 84.1 | 23.6 |
| MCH at birth | 39.4 pg | 0.642 | 62.9 | 58.4 |
| MCH on DOL 28 | 31.8 pg | 0.6002 | 74.3 | 42.7 |
| RDW at birth | 19.2% | 0.513 | 3.5 | 89.9 |
| RDW on DOL 28 | 18.5% | 0.7439 | 83.2 | 50.4 |
AUC, area under the curve; DOL, days of life; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW, red cell distribution width; ROP, retinopathy of prematurity.
In the univariate analysis, the need for ROP treatment was significantly associated with Hb < 16.7 g/dL, Hct < 49.5%, MCV > 117.3 fL, and MCH > 39.4 pg at birth, and Hb < 9.9 g/dL, Hct < 31.0%, MCH < 31.8 pg, and RDW > 18.5% at DOL 28 (Table 5). Gestational age, birth weight, antenatal steroid use, PROM, iNO, oxygen supplementation on DOL 28, mechanical ventilation on DOL 28, and red blood cell transfusions were selected for confounders from the significant results of the univariate analysis. After adjusting for these confounders, the need for ROP treatment was significantly associated with MCV values > 117.3 fL at birth (adjusted odds ratio [aOR] = 2.3; 95% CI 1.0–5.3); and Hb values < 9.9 g/dL (aOR = 3.0; 95% CI 1.4–6.7], Hct values < 31.0% (aOR = 2.7; 95% CI 1.3–5.6], and RDW values > 18.5% (aOR = 2.6; 95% CI 1.1–6.2) at DOL 28 (Tables 4, 5).
Table 5.
Association between the risk of developing ROP warranting treatment and RBC parameters at birth and on DOL 28.
| Parameter | cOR (95% CI) | aOR (95% CI) |
|---|---|---|
| Hb at birth < 16.7 g/dL | 5.3 (2.4–11.6) | 1.9 (0.6–5.8) |
| Hb on DOL 28 < 9.9 g/dL | 3.4 (1.9–6.2) | 3.4 (1.5–8.0) |
| Hct at birth < 49.5% | 5.0 (2.1–12.0) | 2.2 (0.7–7.3) |
| Hct on DOL 28 < 31.0% | 3.2 (1.8–5.8) | 2.5 (1.2–5.5) |
| MCV at birth > 117.3 fL | 5.3 (2.9–9.7) | 2.3 (1.0–5.3) |
| MCV on DOL 28 < 92.3 fL | 1.6 (0.8–3.3) | 1.6 (0.6–4.4) |
| MCH at birth > 39.4 pg | 2.5 (1.4–4.5) | 0.7 (0.3–1.5) |
| MCH on DOL 28 < 31.8 pg | 2.2 (1.2–3.9) | 2.2 (0.9–5.6) |
| RDW at birth > 19.2% | 2.3 (0.7–8.2) | 1.2 (0.3–5.7) |
| RDW on DOL 28 > 18.5% | 5.8 (3.0–11.3) | 2.6 (1.1–6.2) |
aOR, adjusted odds ratio; CI, confidence interval; cOR, crude odds ratio; DOL, day of life; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW, red cell distribution width; ROP, retinopathy of prematurity.
aOR: The model was adjusted for potential confounders, including gestational age, birth weight, antenatal steroid use, premature rupture of membrane, inhaled nitric oxide, oxygen supplementation on DOL 28, mechanical ventilation on DOL 28, and red blood cell transfusions.
Table 4.
Characteristics of the infants in the ROP treatment and non-ROP treatment groups for confounders and RBC parameters.
| Characteristic | ROP treatment (n = 89) n (%) | Non-ROP treatment (n = 113) n (%) | p-value |
|---|---|---|---|
| GA < 28 weeks | 87 (97.8%) | 70 (61.9%) | < 0.001 |
| Birth weight < 1,000 g | 88 (98.9%) | 71 (62.8%) | < 0.001 |
| Antenatal steroids use, n (%) | 81 (91.0%) | 80 (70.8%) | < 0.001 |
| PROM, n (%) | 31 (34.8%) | 22 (19.5%) | < 0.05 |
| iNO, n (%) | 15 (16.9%) | 7 (6.2%) | < 0.05 |
| Oxygen supplementation on DOL 28 | 63 (70.8%) | 32 (28.3%) | < 0.001 |
| Mechanical ventilation on DOL 28 | 88 (98.9%) | 72 (63.7%) | < 0.001 |
| RBC transfusion before DOL 28 | 66 (74.2%) | 46 (40.7%) | < 0.001 |
| Hb at birth < 16.7 g/dL | 80 (89.9%) | 71 (62.8%) | < 0.001 |
| Hb on DOL 28 < 9.9 g/dL | 52 (58.4%) | 27 (23.9%) | < 0.001 |
| Hct at birth < 49.5% | 82 (92.1%) | 79 (69.9%) | < 0.001 |
| Hct on DOL 28 < 31.0% | 58 (65.2%) | 45 (39.8%) | < 0.001 |
| MCV at birth > 117.3 fL | 61 (68.5%) | 33 (29.2%) | < 0.001 |
| MCV on DOL 28 < 92.3 fL | 33 (37.1%) | 34 (30.1%) | 0.295 |
| MCH on birth > 39.4 pg | 55 (61.8%) | 44 (38.9%) | < 0.01 |
| MCH on DOL 28 < 31.8 pg | 12 (13.5%) | 8 (7.1%) | 0.13 |
| RDW at birth > 19.2% | 7 (7.9%) | 4 (3.5%) | 0.179 |
| RDW on DOL 28 > 18.5% | 69 (77.5%) | 47 (41.6%) | < 0.001 |
DOL: day of life; GA, gestational age; Hb, hemoglobin; Hct, hematocrit; iNO, inhaled nitric oxide; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; PROM, premature rupture of membrane; RBC, red blood cells; RDW, red cell distribution width; ROP, retinopathy of prematurity; SGA, small for gestational age.
Discussion
Our analyses demonstrated that MCV values of 117.3 fL at birth, and Hb values < 9.9 g/dL, Hct values < 31.0%, and RDW values > 18.5% on DOL 28 were independent risk factors for ROP treatment. These associations remained significant even after adjusting for gestational age, birth weight, antenatal steroid use, PROM, iNO, oxygen supplementation on DOL 28, mechanical ventilation on DOL 28, and RBC transfusion before DOL 28. ROP is a multifactorial disease characterized by perinatal and postnatal risk factors. Moreover, ROP treatment is always performed after DOL 28. Therefore, predictive biomarkers of ROP on DOL28 are more useful for ROP screening and treatment. To the best of our knowledge, this is the first study to evaluate the association between a wide range of RBC parameters, including RDW, and the need for ROP treatment, and to determine the cut-off values of the RBC parameters.
Several reports have examined the association between anemia, Hb levels, and ROP development. However, reports on RBC parameters, such as RDW and ROP, are limited. Tandon et al. determined that anemia is a significant risk factor for ROP development and identified the statistically significant mean Hb levels for each ROP stage: stage 1, 10.41 g/dL; stage 2, 10.56 g/dL; stage 3, 9.47 g/dL; stage 4, 9.3 g/dL; and matured retina, 12.15 g/dL22. Logistic regression performed by Akyüz Ünsal et al. revealed that the risk of ROP development was negatively correlated with Hb levels four weeks postnatally and positively correlated with RDW at four weeks postnatally. Furthermore, MCH (cutoff: 34.43 pg) was the most prominent risk factor according to the Classification and Regression Tree20. In a multicenter, prospective, observational cohort study, Fevereiro-Martins et al. determined that MCV was significantly and independently associated with the development of ROP in Portuguese infants born at a gestational age of 29.6 weeks or a birth weight of 1175.7 g21. In our study, MCH were not independent risk factors for ROP after adjusting for multiple confounders. These differences may be due to variable sample sizes, gestational ages, birth weights, follow-up times, case definitions, and adjustments for confounding factors. Furthermore, our study included infants with a lower gestational age (26 weeks) and birth weight (793 g) after adjusting for multiple confounders.
In our study, ROP treatment was significantly associated with Hb levels of < 16.7 g/dL at birth; however, after adjusting for confounders, there was no statistically significant difference. Lundgren et al. found that Hb levels during the first week of life were significantly lower in infants requiring ROP treatment than in those who did not require treatment (12.5 g/dL vs. 13.8 g/dL, p < 0.001). Furthermore, they determined that the number of days with anemia during the first week of life is an independent risk factor for ROP, warranting treatment even after adjusting for gestational age14. In another prospective study of 78 infants born below a gestational age of 28 weeks in Sweden, Lundgren et al. found that infants who required ROP treatment developed anemia more frequently than those who did not require treatment during the first (42.9% vs. 8.0%, p = 0.003) and second (40.9% vs. 6.3%, p = 0.002) postnatal week15. In our study, the Hb cut-off values at birth were routinely measured prior to ROP treatment; however, anemia at birth or the number of early postnatal days with anemia might be important factors affecting ROP treatment. At our institution, blood samples are routinely collected at birth and on DOL 28; however, they are not routinely collected during the early postnatal period. Therefore, we could not evaluate the relationship between anemia during early postnatal weeks and ROP development.
A two-phase hypothesis regarding the development of ROP has been proposed. In phase 1, immediately following birth, there is delayed physiological retinal vascularization, vaso-attenuation, and obliteration, which are thought to be related to premature neonatal physiological stressors, extrauterine hyperoxia, low levels of insulin-like growth factor 1, and delayed expression of VEGF receptor 2. In phase 2 (approximately 4–8 weeks after birth), there is abnormal proliferation of retinal vascular cells and neovascularization of the retina and vitreous. This is stimulated by the increased VEGF levels in the peripheral avascular retina in response to local hypoxia induced by metabolic cellular demands23,24. Therefore, preventing hyperoxia during resuscitation and up to 30–32 weeks of postmenstrual age decreases the risk of ROP. In contrast, preventing hypoxia beyond 32 weeks of postmenstrual age decreases the risk of ROP. This is consistent with our findings that Hb values < 9.9 g/dL and Hct values < 31.0% on DOL 28 are independent risk factors for ROP treatment, which can lead to hypoxia beyond DOL 28.
The main strength of our study is the use of logistic regression to control for multiple confounders. Additionally, cut-off values were calculated using a wide range of RBC parameters. The limitations of our study include its single-center retrospective nature, the small number of treated infants, and lack of comparison between RBC parameters other than those at birth and on DOL 28. The rate of ROP treatment was very high (44.1%), which may be related to the high oxygen supplementation on DOL 28 (70.8%). However, the mortality rate of very preterm infants is the lowest in Japan. Therefore, the incidence of severe ROP in Japan is thought to be higher than that in other countries.
In conclusion, our study demonstrated that infants born at < 30 weeks of gestation with MCV values > 117.3 fL at birth as well as Hb values < 9.9 g/dL, Hct values < 31.0%, and RDW values > 18.5% on DOL 28 had an increased risk of developing ROP, warranting treatment. Large prospective studies are required to validate the association between RBC parameters and ROP treatment.
Methods
Study design
This single-center, retrospective cohort study was conducted at the neonatal intensive care unit (NICU) of Fukushima Medical University Hospital in Fukushima, Japan, between January 1, 2011, and July 31, 2022. Preterm infants born at < 30 weeks’ gestation were included in this study. Infants with congenital anomalies, missing data, and those who died or were transferred to another hospital within one month of life were excluded. The Ethics Committee of Fukushima Medical University, guided by local policy, national law, and the World Medical Association Declaration of Helsinki, approved this study without requiring informed consent from guardians, but consent could be rescinded in the form of opt-out (REC2022-005).
Data collection
We extracted the following data from medical records: gestational age; birth weight; small for gestational age (SGA); sex; antenatal steroid use; a history of chorioamnionitis, PROM or hypertensive disorders of pregnancy; Apgar score at 1 min and 5 min; the presence of respiratory distress syndrome or patent ductus arteriosus; a history of iNO use, oxygen supplementation on DOL 28, mechanical ventilation on DOL 28, or RBC transfusion before DOL 28; laboratory values; and a history of ROP. Blood samples (approximately 250 µL) were collected in EDTA tubes from the peripheral veins of premature infants at birth and on DOL 28. Complete blood counts, including Hb, Hct, MCV, MCH, and RDW, were measured using a coagulation analyzer (Sysmex XE-5000; Sysmex, Kobe, Japan). SGA was defined as both birth weight and length below the 10th percentile for gestational age or birth weight or length of ≤ -2.0 standard deviation scores (SDS), which was calculated according to sex-specific standards for birth weight and length or height during infancy in a Japanese population, for gestational age25. In this study, we included premature infants treated with iNO in the first 28 days of life for hypoxic respiratory failure (defined as the need for mechanical ventilation with an oxygenation index score of ≥ 10) or pulmonary hypertension identified on echocardiography. Indications for RBC transfusion in the NICU were Hb values < 7 g/dL, Hb values < 11 g/dL for infants requiring oxygen supplementation, and Hb values < 12 g/dL within 24 h of birth.
ROP screening
All infants were screened and diagnosed with ROP by three ophthalmologists with sufficient knowledge and experience to accurately locate and identify sequential changes in ROP. Infants were examined during the study period using the International Classification of Retinopathy of Prematurity Revised (ICROP)26. The initial screening was performed at 31–33 weeks of gestation for infants born before 28 weeks and 4–6 weeks after birth for those born after 28 weeks. The ophthalmologists decided on follow-up examinations and treatments with laser photocoagulation or anti-vascular endothelial growth factor (VEGF) injections based on the ICROP findings.
Outcomes and confounding factors
The study outcome was the association between ROP treatment and RBC parameters at birth and on DOL 28. Infants were assigned to ROP treatment or non-ROP treatment groups based on the need for ROP treatment. Possible confounders were identified based on the significant results of the univariate analysis.
Statistical analyses
The characteristics of the mothers and their children were summarized according to the ROP treatment. Receiver operating characteristic (ROC) curve analysis was performed to determine the RBC parameter cut-off values according to ROP treatment. Cut-off values were determined using Youden’s index analysis. The sensitivity and specificity of the cut-off values and the area under the curve (AUC) were also calculated. Chi-square test and one-way analysis of variance were used to compare categorical and continuous variables. Multiple logistic regression analysis was performed to determine the association between the need for ROP treatment and RBC parameters by calculating the odds ratio (OR), which was adjusted for the confounders and 95% confidence intervals (CIs). All the statistical analyses were performed using Stata (version 15.0; Stata StataCorp LLC, College Station, TX, USA). p-values of < 0.05 were considered statistically significant.
Author contributions
H.M. had primary responsibility for protocol development, patient screening, enrollment, outcome assessment, preliminary data analysis and writing the manuscript. H.G. and H.I. participated in the development of the protocol and analytical framework for the study and contributed to the writing of the manuscript. S.H., H.I., Y.S., and K.O. contributed in the same ways as HG and HI and was responsible for patient screening. N.M., T.S., and M.H. supervised the design and execution of the study, performed the final data analyses and contributed to the writing of the manuscript.
Data availability
The datasets analyzed during the current study are not publicly available, because the consent obtained from the participants specified that the data can be used only for research purposes at our institution. The datasets can only be available from the corresponding author upon reasonable request and after the approval of the ethics committee of Fukushima Medical University.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sabri, K., Ells, A. L., Lee, E. Y., Dutta, S. & Vinekar, A. Retinopathy of prematurity: A global perspective and recent developments. Pediatrics10.1542/peds.2021-053924 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Blencowe, H., Lawn, J. E., Vazquez, T., Fielder, A. & Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res.74(Suppl 1), 35–49. 10.1038/pr.2013.205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cudjoe, G. A. et al. National trends in the incidence and management of retinopathy of prematurity in the United States, 2009–2018. J. Neonatal. Perinatal. Med.15, 553–557. 10.3233/npm-210826 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Isayama, T. The clinical management and outcomes of extremely preterm infants in Japan: Past, present, and future. Transl. Pediatr.8, 199–211. 10.21037/tp.2019.07.10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isayama, T. et al. Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics130, e957-965. 10.1542/peds.2012-0336 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Kim, S. J. et al. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol.63, 618–637. 10.1016/j.survophthal.2018.04.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali, A. A., Gomaa, N. A. S., Awadein, A. R., Al-Hayouti, H. H. & Hegazy, A. I. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr.106, 1919–1927. 10.1111/apa.14019 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Chen, M. L., Guo, L., Smith, L. E., Dammann, C. E. & Dammann, O. High or low oxygen saturation and severe retinopathy of prematurity: A meta-analysis. Pediatrics125, e1483-1492. 10.1542/peds.2009-2218 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Fiore, J. M. et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr.157, 69–73. 10.1016/j.jpeds.2010.01.046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins, R. D. Oxygen saturation and retinopathy of prematurity. Clin. Perinatol.46, 593–599. 10.1016/j.clp.2019.05.008 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Lundgren, P. et al. Low birth weight is a risk factor for severe retinopathy of prematurity depending on gestational age. PLoS ONE9, e109460. 10.1371/journal.pone.0109460 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yucel, O. E., Eraydin, B., Niyaz, L. & Terzi, O. Incidence and risk factors for retinopathy of prematurity in premature, extremely low birth weight and extremely low gestational age infants. BMC Ophthalmol.22, 367. 10.1186/s12886-022-02591-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju, R. H. et al. Spontaneous regression of retinopathy of prematurity: Incidence and predictive factors. Int. J. Ophthalmol.6, 475–480. 10.3980/j.issn.2222-3959.2013.04.13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundgren, P. et al. Duration of anaemia during the first week of life is an independent risk factor for retinopathy of prematurity. Acta Paediatr.107, 759–766. 10.1111/apa.14187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundgren, P. et al. Erythropoietin serum levels, versus anaemia as risk factors for severe retinopathy of prematurity. Pediatr. Res.86, 276–282. 10.1038/s41390-018-0186-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen, T. T. B., Bui, V. T., Pham, V. P. T. & Pham, T. N. Retinopathy of prematurity: A study of incidence and risk factors in a tertiary hospital in Vietnam. Clin. Ophthalmol.16, 3361–3367. 10.2147/opth.S386808 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rekha, S. & Battu, R. R. Retinopathy of prematurity: Incidence and risk factors. Indian Pediatr.33, 999–1003 (1996). [PubMed] [Google Scholar]
- 18.Ugurbas, S. C. et al. Comparison of UK and US screening criteria for detection of retinopathy of prematurity in a developing nation. J. Aapos14, 506–510. 10.1016/j.jaapos.2010.07.012 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Yau, G. S. et al. Incidence and risk factors of retinopathy of prematurity from 2 neonatal intensive care units in a Hong Kong Chinese population. Asia Pac. J. Ophthalmol. (Phila)5, 185–191. 10.1097/apo.0000000000000167 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Akyüz Ünsal, A. et al. Can complete blood count parameters predict retinopathy of prematurity?. Turk. J. Ophthalmol.50, 87–93. 10.4274/tjo.galenos.2019.45313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fevereiro-Martins, M., Santos, A. C., Marques-Neves, C., Guimarães, H. & Bicho, M. Complete blood count parameters as biomarkers of retinopathy of prematurity: A Portuguese multicenter study. Graefes Arch. Clin. Exp. Ophthalmol.10.1007/s00417-023-06072-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandon, M., Ranjan, R., Muralidharan, U. & Kannan, A. Influence of anaemia on multifactorial disease retinopathy of prematurity: A prospective observational study. Cureus14, e27877. 10.7759/cureus.27877 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dammann, O., Hartnett, M. E. & Stahl, A. Retinopathy of prematurity. Dev. Med. Child Neurol.65, 625–631. 10.1111/dmcn.15468 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Hartnett, M. E. & Penn, J. S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med.367, 2515–2526. 10.1056/NEJMra1208129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita, K. et al. Prevalence of small for gestational age (SGA) and short stature in children born SGA who qualify for growth hormone treatment at 3 years of age: Population-based study. Pediatr. Int.58, 372–376. 10.1111/ped.12859 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Fierson, W. M. Screening examination of premature infants for retinopathy of prematurity. Pediatrics10.1542/peds.2018-3061 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available, because the consent obtained from the participants specified that the data can be used only for research purposes at our institution. The datasets can only be available from the corresponding author upon reasonable request and after the approval of the ethics committee of Fukushima Medical University.