Abstract
The shortwave infrared (SWIR) region is an ideal spectral window for next-generation bioimaging to harness improved penetration and reduced phototoxicity. SWIR spectral activity may also be accessed via supramolecular dye aggregation. Unfortunately, development of dye aggregation remains challenging. We propose a crystal-aided aggregate synthesis (CAASH) approach to introduce a layer of rationality for the development of J-aggregate and the successful development of a water-soluble SWIR JV-aggregate with a bisbenzannulated silicon rhodamine scaffold (ESi5). The resulting SWIR-aggregates exhibit excellent stabilities toward organic solvents, pH, sonication, photobleaching, thiols, and endogenous oxidative species. Notably, the aggregates have a high structure-dependent melting temperature of ca. 330-335 K. In fact, the heating/annealing process can be exploited to reduce aggregation disorder. The aggregates are biocompatible and have broad potential in in vivo fluorescence and photoacoustic imaging and more.
Subject terms: Optical materials, Biomedical materials, Crystal engineering
While the shortwave infrared (SWIR) region has potential for bioimaging applications, stable and biocompatible SWIR absorbers/emitters are challenging to access. Here, the authors report the approach of crystal-aided aggregate synthesis for the development of J-aggregates for in vivo bioimaging.
Introduction
Shortwave infrared (SWIR), i.e., 1000 nm and beyond, is an ideal spectral window for deep-tissue and high-contrast in vivo imaging, disease diagnosis, phototherapies, and surgery navigation1–4. SWIR-active materials of diverse nature have been reported and found applications, e.g., inorganic low-bandgap materials in infrared optoelectronics5–7 and electrochromic smart windows8, hybrid materials in optical filtering and laser shielding9, and organic dyes in infrared photography10, lasing11, and OLED12. Yet, all these existing SWIR materials have limitations to overcome to improve their potential in the aforementioned cutting-edge biomedical applications. Inorganic or hybrid SWIR materials contain heavy transition metals, the long-term toxicity of many of which are still to be systematically evaluated13. Though organic dyes typically exhibit good biocompatibility, SWIR-absorbing organic dyes have their own challenges13,14. Imparted by their high-lying HOMO and low-lying LUMO, SWIR dyes inevitably suffer from poor stabilities, which inhibit their practical and clinical applications. First, their conjugative backbones are readily destructed, either irreversibly by endogenous/photo-induced oxidative species15,16, or reversibly by ubiquitous nucleophiles17. Second, the electron-delocalization along the long conjugative backbone accounting for their SWIR spectral activity is readily disrupted by water via dipole-induced Peierls transition18. Concomitantly, their long-wavelength absorption band becomes weak, broad, and often significantly blue-shifted19. Rendering thermodynamically unstable SWIR dyes kinetically persistent in the biological milieu is the key to circumventing their instabilities. An obvious and convenient approach is supramolecular encapsulation in polymer20, serum albumin21, or porous materials22. Molecular steric shielding approaches have also been developed for squaraines23,24, cyanines25,26, and polybenzannulated xanthenoids27–29. Generally speaking, this approach becomes exponentially difficult when the absorption maximum marches deeper into the SWIR and their conjugative scaffold concomitantly becomes larger. Alternative robust strategies to develop stable and biocompatible SWIR-absorbing/emitting materials are a timely addition to the field.
The rationalization of aggregation-induced bathochromism led to the formulation of the exciton theory30, the mechanistic elucidation of the natural wonder of photosynthesis31, and the later emergence of the nowadays prosperous field of dye aggregation32–34. The highlight of this strategy for SWIR-active materials is that the resulting longer-wavelength J-aggregate can enjoy the higher stability of the shorter-wavelength monomer. Harnessing the power of J-aggregation for SWIR spectral activity is eminently promising, and yet remains an endeavor necessitating both rationality and serendipity33. An elusive but useful guideline has been empirically established in designing suitable monomeric dyes for J-aggregation. The monomeric dye ideally exhibits a large transition dipole moment to enhance dipole coupling35. Its electron push–pull headgroups should be flat to allow stacking. A molecular twist or a bulky group should exist at the center of its conjugative backbone to inhibit the undesired co-facial H-type aggregation32,36. Beyond these electronic and structural nuances, dyes should self-assemble or be organized in a topologically and orientationally precise fashion. Mother Nature has mastered both approaches and crafted astoundingly elaborate functional complexes such as photosynthetic machineries31. The nature’s prowess in protein engineering is beyond our scope of synthetic access. Matrices like DNA37, polymer38, mesoporous silica39, hydrogel40, and liposome41, have also been explored to template the aggregate synthesis. Besides, a number of environmental parameters, e.g., counterions, solvent, pH, temperature, and ionic strengths, can also be tinkered with to influence the aggregation outcome42–46. The implementation of this guideline has led to a number of elegant J-type aggregates based on, e.g., porphyrin(oid)s47,48, PDI49,50, BODIPY/aza-BODIPY51,52, squaraine53,54, and Cyanine55,56. Unfortunately, the vast majority of these J-type aggregates typically exhibit an absorption maximum well within 1000 nm55,57. Aggregates with a SWIR-absorption maximum are few and exhibit a rather broad absorption and a Stokes-shifted emission, suggesting disorder in aggregation due to the high structural flexibility of the corresponding NIR monomeric dyes43,58–60. Additionally, most SWIR aggregates are synthesized in organic solvents and lack biocompatibility. Also, some J-aggregates, e.g., double-walled nano-tube, are not thermodynamically stable and may exhibit polymorphism61–63. We recently reported a bisbenzannulated silicon-rhodamine (ESi5) dye28. The ESi5 scaffold has flat electron-donating headgroups, bulky groups at the center, a rigid conjugative scaffold, and a deep-NIR absorption maximum at 867 nm, the combination of which structural and electronic properties promote us to develop high-performance J-aggregate for in vivo bioimaging applications starting from this ESi5 scaffold.
Herein, we report a stable and biocompatible SWIR aggregate via crystal-aided aggregate synthesis (CAASH). It is a two-layer nanoribbon exhibiting a V-type intra-chain packing and a J-type inter-chain packing. The spectral signature of this unusual packing is two red-shifted peaks along with one blue-shifted sharp peak. Notably, the aggregate is stable toward H2O, pH, sonication, photobleaching, oxidative species, and sulfides. Its aggregation is temperature-dependent and fully reversible. Aggregation disorder could be reduced by repeated heating-annealing. The design and synthesis of robust molecular SWIR aggregate offer a fascinating photophysical playground to formulate/test photophysical principles and high-performance materials for numerous cutting-edge biotechnologies.
Results
Design guideline
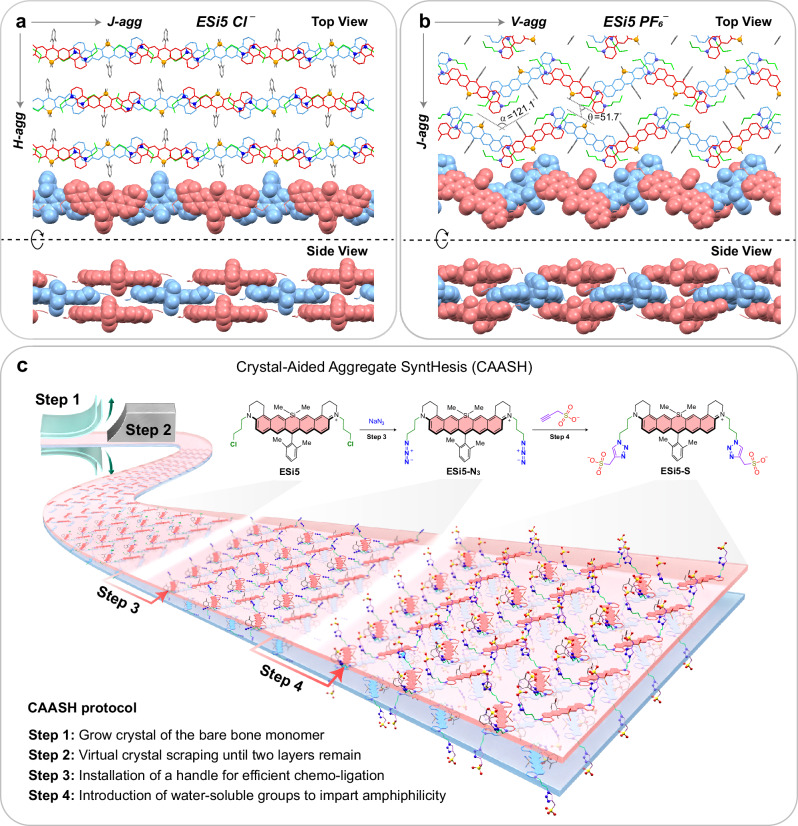
Our design of SWIR-absorbing aggregate started with the crystallization of ESi5, a bright cationic fluorophore absorbing at 867 nm. Interestingly, two distinct crystal structures of ESi5 were obtained when different counterions were used. With Cl−, ESi5 stacks into a conventional brickwork J-aggregated wall, which further aligns with other walls in an H-fashion to form the 3D crystal packing (Fig. 1a). Following the literature convention, such a packing was referred to as an HJ-aggregate64. With PF6−, ESi5 adopts a different zig-zag intra-chain packing mode with a 121.1° angle between two proximal dyes (Fig. 1b). Such an oblique-dimer was pictorially referred to as a V-dimer65,66. When two such walls are brought together in a co-facial fashion, their inter-chain packing mode is no longer H-type, but J-type with a slipping angle of 51.7°. For this reason, this aggregate is referred to as JV-aggregate. Suppose we can trim off the crystal layer by layer until the last two layers, which stack to each other via π–π interaction. To further render such two-layer stack stability and biocompatibility in aqueous medium, two flexible hydrophilic chains can be installed at the electron push–pull headgroups of the dye. Following this idea, we displaced the two chlorine atoms of the ESi5 with azido groups, and further clicked it to a terminal alkyne linked to various water-soluble groups (Fig. 1c). Such judiciously functionalized amphiphilic ESi5 is expected to spontaneously aggregate in an aqueous medium. However, the specific aggregation outcome, e.g., HJ-type, JV-type, or any other unexpected fashion, is serendipitous, but can be deduced by correlating their absorption spectral features with their packing style in the crystal structure. As for ESi5, a single red (or blue)-shifted peak would suggest a J- (or H-) aggregate, while a V-aggregate would result in a red-shifted peak along with a blue-shifted peak via energy-splitting. The relative intensities of the two peaks depend on the angle in between. Suppose the angle (α) remains at ca. 120°, the oscillation strengths of the J-band (fJ) are expected to be roughly three times that of the H-band (fH), according to fJ/fH = tg2(α/2)67.
Fig. 1. The molecular packing of ESi5 in single crystals and the design of its JV-aggregates.
The top view and side view of the packing mode of ESi5•Cl− (a) and ESi5•PF6− (b). c The crystal-aided aggregate synthesis (CAASH) with the ESi5 dye.
Photophysical properties
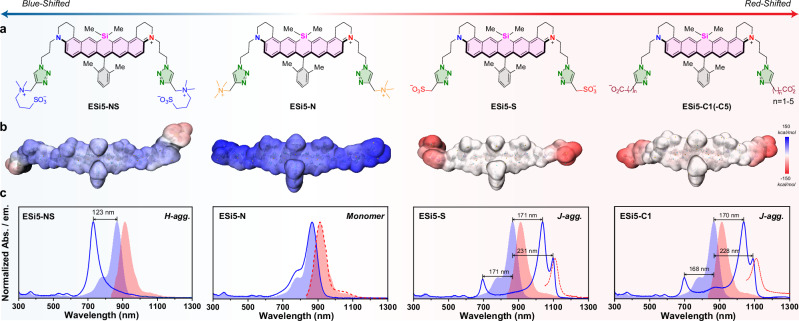
The fluorochromic core of ESi5 is cationic, and installation of anionic terminal results in amphiphilicity, imparting a driving force to aggregate in an aqueous medium. We prepared eight ESi5 derivatives with different water-soluble groups, i.e., ESi5-NS with a zwitterion, ESi5-N with a quaternary ammonium, ESi5-S with a sulfonate, ESi5-C1(-C5) with a carboxylate-caped linear alkyl chain of 1–5 carbon atoms (Fig. 2a). The surface electrostatic potential of each was mapped to qualitatively assess their potential to aggregate (Fig. 2b). An aliquot of each compound (5 mM, 2 μL) in MeOH was added to H2O (2 mL) with stirring, and their UV-Vis absorption spectra were then acquired without further sitting. No spectral feature of aggregate formation was observed for ESi5-NS and ESi5-N. The gradual addition of NaCl (200 mM) to the solution containing ESi5-NS resulted in a spectral blue shift from 867 nm of ESi5 dye to 744 nm, suggesting the formation of an undesirable H-type aggregate (Table 1). This H-aggregate of ESi5-NS was non-fluorescent. ESi5-N did not aggregate even in the presence of NaCl (200 mM), likely due to its tri-cationic feature (Fig. 2c). In sharp contrast, the ESi5-S and ESi5-C1(-C5) aggregated instantaneously upon the addition its MeOH stock into H2O (Supplementary Fig. 15). Their resulting absorption spectra exhibited similar spectral features, i.e., a lower-intensity red-shifted peak at 1098 nm, another red-shifted peak at 1038 nm of much higher intensity, a blue-shifted peak at 696 nm of comparable intensity to the peak at 1098 nm, and non-resolved vibronic bands between 696 nm and 1038 nm (Table 1).
Fig. 2. The spectral monitoring of the aggregation of ESi5 dyes with different water-soluble groups.
a The chemical structures of ESi5-NS, ESi5-N, ESi5-S, and ESi5-C1(-C5). b The electrostatic potential surfaces calculated for ESi5-NS, ESi5-N, ESi5-S and ESi5-C1 at B3LYP/6-31G level. c The UV-Vis absorption (Blue, solid line) and the fluorescence emission (Red, dash line) spectra of the resulting solution upon dilution of the MeOH stock of ESi5-NS, ESi5-N, ESi5-S, and ESi5-C1 in PBS (pH = 7.4, 10 mM). The UV-Vis absorption (Blue, space-filled) and emission spectra (Red, space-filled) of ESi5 in MeOH were plotted for comparison purposes.
Table 1.
The photophysical properties of ESi5 in MeOH and ESi5-NS, ESi5-N, ESi5-S, and ESi5-C1(-C5) in PBS (pH = 7.4, 10 mM)
| The photophysical properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Monomer | ESi5-NS | ESi5-N | ESi5-S | ESi5-C1 | ESi5-C2 | ESi5-C3 | ESi5-C4 | ESi5-C5 | |
| λabs (nm) | 867 | 744 | 866 | 1038 | 1037 | 1024 | 1022 | 1033 | 1019 |
| λabs (nm) | – | – | – | 1098 | 1095 | 1082 | 1080 | 1080 | 1075 |
| λabs (nm) | – | – | – | 696 | 699 | 700 | 708 | 701 | 709 |
| λem (nm) | 909 | – | 911 | 1106 | 1108 | 1090 | 1100 | 1097 | 1101 |
| Stokes Shift (nm) | 43 | – | 45 | 8 | 13 | 8 | 20 | 17 | 26 |
| ε (×105 cm−1 M−1) | 1.95 | – | 1.74 | 0.88 | 0.79 | 0.81 | 0.82 | 0.86 | 0.78 |
| ϕ (%) | 16 | – | 1.6 | 0.06 | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 |
| (ε × ϕ) (cm−1 M−1) | 15,054 | N/A | 2784 | 52.8 | 31.6 | 16.2 | 16.4 | 17.2 | 15.6 |
The ESi5-NS solution contains NaCl (2 M) in PBS.
The unambiguous resolution of the packing mode of an aggregate remains a grand challenge for the field. An advantage of the CAASH approach is that the crystal structure of the monomeric dye provides a reliable source of reference. Such a three-peak pattern cannot possibly be the result of an HJ-type packing in Fig. 1a, but is in agreement with the JV-type packing in Fig. 1b. The two bands at 1038 and 696 nm are presumably due to the intra-chain V-aggregation of ESi5, based on the fact that their oscillation strengths are in a ca. 3:1 ratio (vide ante) (Table 1). The remaining reddest peak at 1098 nm has to be the result of an inter-chain two-dimensional J-aggregation. The peak width is an indicator of the dipolar coupling strength and calculated via spectral deconvolution. The FWHM of all three peaks are small at 574.0 cm−1 for 696 nm, 452.6 cm−1 for 1038 nm, and 233.3 cm−1 for 1098 nm, respectively, suggesting a strong dipolar coupling between proximal ESi5 dyes. Upon photoexcitation at 696 nm, 1038 nm, or 1098 nm of a solution of ESi5-S agg or ESi5-C1(-C5) agg, the same resonant fluorescence emission band at ca. 1106 nm was observed with a very sharp FWHM of 481.9 cm−1 (Table 1). The fluorescence quantum yield was 0.06% for ESi5-S, significantly reduced compared to 1.7% of the monomer. Through a transient absorption study, a fluorescence lifetime of 58 ps was calculated (Supplementary Fig. 31). The change of the terminal anion from sulfonate to carboxylate slightly led to a reduced degree of spectral resolution between the two red-shifted peaks, and enhanced minor vibronic peaks between 1038 and 696 nm. Increasing the length of the alkyl chain from the -CH2- of ESi5-C1 to the -(CH2)5- of ESi5-C5 resulted in a slight broadening of the absorption bands (Supplementary Figs. 16–21).
Morphology of ESi5 aggregates
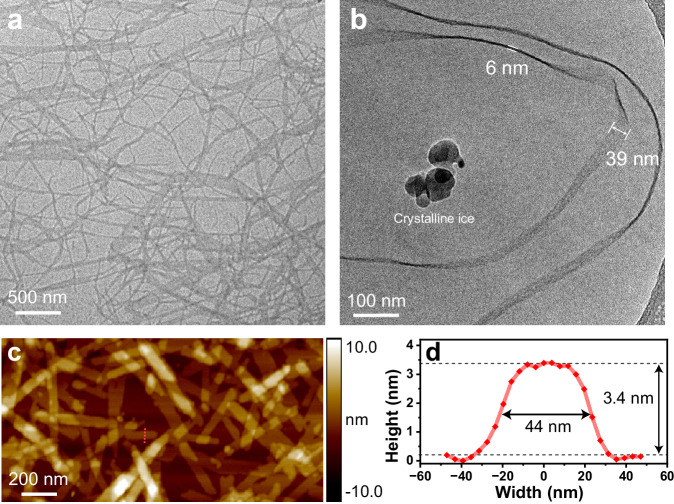
The morphology of the ESi5-S agg was different with respect to the sitting time of the solution. Without sitting time, the aggregate adopted the form of small particulate with a diameter of 7.7 ± 0.6 nm (Supplementary Fig. 29A). In 6 h, the particulate could still be seen while the aggregate predominantly existed in form of short nanoribbon with an average width of 39.6 ± 1.6 nm, and an average length of 0.44 μm (Supplementary Fig. 29B). In 24 h, the short nanoribbon further grew into a network. Some ribbons exhibited an increased width of up to 116.2 ± 2.2 nm (Fig. 3a, Supplementary Fig. 29C). Some ribbons folded back and forth suggesting a soft and flexible nature. The nanoribbon morphology of ESi5-S agg in solution was clearly captured by the cryo-electron microscopy (cryo-EM) image (Fig. 3b). The thickness of the ribbon was determined to be ca. 3.4 nm by atomic force microscopy (AFM), in agreement with the hypothesized double-layer aggregate model (Fig. 3c, d). The transmission electron microscopy (TEM) images of ESi5-C1/C2/C3 agg revealed that their morphologies were similar to that of ESi5-S agg (Supplementary Figs. 24–28). As for ESi5-C4/C5 agg, the ribbon was significantly shorter and wider. The existence of particulate in the TEM images of ESi5-C4/C5 agg suggested that their aggregation displayed inhomogeneity, likely due to the enhanced structural flexibility of the monomer dye (Supplementary Figs. 27 and 28). The thickness of aggregates increased with respect to the chain length from 3.4 nm of ESi5-C1 agg to 5.8 nm of ESi5-C5 agg (Supplementary Figs. 24–28). Despite the morphological change, it was interesting to note that the absorption spectra of the bulk aggregate solution remained unchanged. This indicated that the molecular packing of the monomer in the particulate at 0 h, short nanoribbon at 6 h, and long nanoribbon network at 24 h remained unchanged.
Fig. 3. Morphology of ESi5-S agg.
The TEM image (a) and the Cryo-EM image (b) of ESi5-S agg (200 μM) in H2O. c The AFM image of ESi5-S agg with cross-section analyses (d) along red dashed lines. The dark spots are crystalline ice. Each experiment was repeated independently with similar results (n = 3).
Governing parameters on aggregation
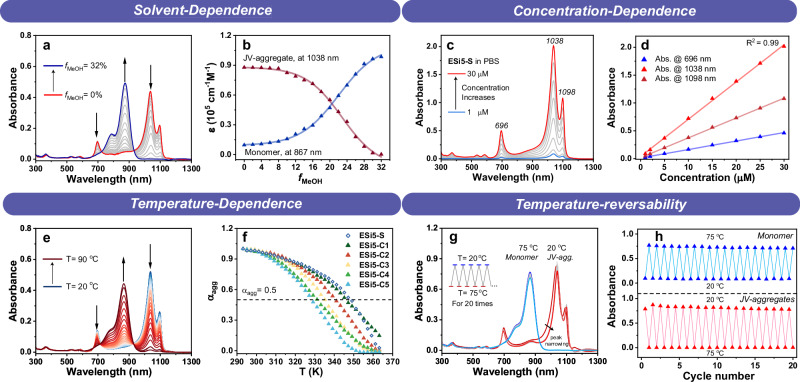
With ESi5-S agg as an example, the stabilities of the JV-aggregates of ESi5 dyes toward organic solvent, dye concentration, sonication, pH, temperature, light irradiation, and oxidants were thoroughly evaluated. The UV-Vis absorption spectra of ESi5-S agg (5 μM) in a mixture of neutral PBS buffer and varying percentages of MeOH (0%–32%, v/v) were acquired (Fig. 4a, b). The absorption spectrum remained essentially unchanged when the MeOH percentage was below 6%. When the MeOH percentage went above 6%, the peaks of the JV-aggregate started to decrease, and the peak of the monomer at 867 nm started to increase accordingly. When the MeOH percentage was 22%, a 50% aggregation was observed. Raising the MeOH beyond 32% eventually totally inhibited the formation of JV-aggregate. ESi5-S exhibited a high tendency to aggregate. In the literature, a high dye concentration of 10−4–10−3 M−1 was routinely used to promote aggregation. Yet, ESi5-S at even 1 μM is completely aggregated. Further increasing the dye concentration gave a linear increase in the absorbance at 696, 1038, and 1098 nm with respect to the dye concentration, tested up to 30 μM (Fig. 4c, d). This experiment could help to determine the ideal concentration for bioimaging.
Fig. 4. The stabilities of JV-aggregate toward different stimuli.
a Solvent-dependent changes of the absorption spectra of ESi5-S (5 μM) in PBS (pH = 7.4, 10 mM) with different MeOH fractions (fMeOH) from 0% to 32% at room temperature. b The plot of molar absorptivities of ESi5-S monomer (867 nm) and JV-aggregate (1038 nm) with respect to the fMeOH. c The concentration-dependent absorption spectra of ESi5-S in PBS (pH = 7.4, 10 mM, containing <2% MeOH) at room temperature from 1 to 30 μM. d The plot of absorbance at 696, 1038, and 1098 nm, respectively, as a function of the concentration of ESi5-S. e The temperature-dependent absorption spectra of ESi5-S agg (5 μM) in PBS (PH = 7.4, 10 mM) with 5% MeOH from 20 to 90 °C. f The plot of aggregation percentage (αagg) with respect to the temperature. g The absorption spectra of ESi5-S (10 μM) in PBS (pH = 7.4, 10 mM) with 10% MeOH upon repeated heating/annealing between 20 and 75 °C. h The reversible absorbance changes of the monomer at 867 nm and the ESi5-S agg at 1038 nm with respect to heating and annealing cycles.
The temperature dependence of the aggregation was studied with a solution of ESi5-S (5 μM) in PBS with 5% MeOH. When the temperature gradually raised from room temperature to 90 °C, the aggregation degree (αagg) decreased from near unity at 25 °C, to ca. 2.8% at 90 °C (Fig. 4e). Its melting temperature (Tm, when αagg = 50%), was 74 °C (Fig. 4f, Table 2). When the solution temperature was further lowered back to 25 °C from 90 °C, the absorption of the aggregate was essentially restored. Interestingly, the absorption band at 1038 nm and the 1098 nm became narrower, and their separation became slightly wider. Likely, the heating/annealing process eliminated some of the aggregation defects and further enhanced dipolar coupling. We then repeated the heating/annealing processes over 20 times, and the system showed excellent reversibility (Fig. 4g, h). This experiment not only highlighted the stability of the aggregate, but also was a manifestation of the high stability of the monomeric ESi5 dyes at demanding conditions, i.e., 75 °C in PBS for hours. The aggregate equilibrium constant (Ke) and Gibbs free energy (ΔG) of ESi5-S aggregate were calculated to be 8.2 105 M−1, and −36.6 kJ•M−1, respectively. Changing the sulfonate of ESi5-S into the carboxylate of ESi5-C1 led to a slight decrease of Tm to 69 °C, Ke to 7.1 105 M−1, and ΔG to −36.2 kJ•M−1 (Table 2). Further elongation of the alkyl chain led to a notable further decrease of the aggregate stability (Table 2, Supplementary Fig. 36). The heating-annealing results with ESi5-S agg promoted us to wonder if the same method could be applied to the ESi5-C1(-C5) since their aggregation disorder seemed to be more pronounced than ESi5-S. The solutions of ESi5-C1(-C5) aggregates in PBS with 5% MeOH were heated to 90 °C and then cooled to room temperature (Supplementary Fig. 15). The effect was very obvious. Three major bands at ca. 1098 nm, ca. 1038 nm, and ca. 696 nm became sharper in shape. The two spectrally unresolved red bands were now totally resolved. The two red bands slightly red-shifted by ca. 8 nm, and the blue band slightly blue-shifted by ca. 12 nm. These were indications of the reduction of aggregate disorder. ESi5-S agg was also stable toward sonication and pH change (Supplementary Figs. 34 and 35).
Table 2.
The thermodynamic parameters of ESi5-S, and ESi5-C1(-C5) aggregation
| The thermodynamic parameters | ||||||
|---|---|---|---|---|---|---|
| Tm (K) | Ke (105 M−1) | DPN | ΔH (kJ mol−1) | ΔS (kJ mol−1) | ΔG (kJ mol−1) | |
| ESi5-S | 349 | 8.2 | 2.59 | −74.8 | 118.3 | −36.6 |
| ESi5-C1 | 345 | 7.1 | 2.45 | −79.9 | 135.3 | −36.2 |
| ESi5-C2 | 341 | 6.3 | 2.31 | −86.7 | 157.6 | −35.8 |
| ESi5-C3 | 335 | 4.1 | 2.02 | −96.3 | 190.6 | −34.7 |
| ESi5-C4 | 331 | 2.9 | 1.80 | −100.3 | 206.0 | −33.8 |
| ESi5-C5 | 328 | 2.2 | 1.66 | −111.9 | 243.9 | −33.1 |
SWIR-absorbing dyes are usually very susceptible to nucleophilic attack of ONOO−. Yet, the stability of the JV-aggregate of ESi5-S and ESi5-C1(-C5) was all very high. The addition of up to ONOO− (50 μM, ca. 100-fold of the maximum physiological concentration) into its solution resulted in only 10.5% decrease of the aggregate absorbance. Addition of H2O2 (5 mM), GSH (60 mM), cysteine (5 mM), and HS− (tested to 1 mM) did not induce absorbance decrease (Supplementary Fig. 33). Also, the aggregate and monomer exhibited high photostability and irradiation of the solution at 808 nm or 1064 nm for 30 min resulted in an absorbance decrease of only ca. 4.8% and 11.8%, respectively, while the absorbances of ICG at 808 nm irradiation and IR1064 at 1064 nm irradiation decreased by 98.7% and 85.9%, respectively (Supplementary Fig. 32).
In vivo fluorescence imaging
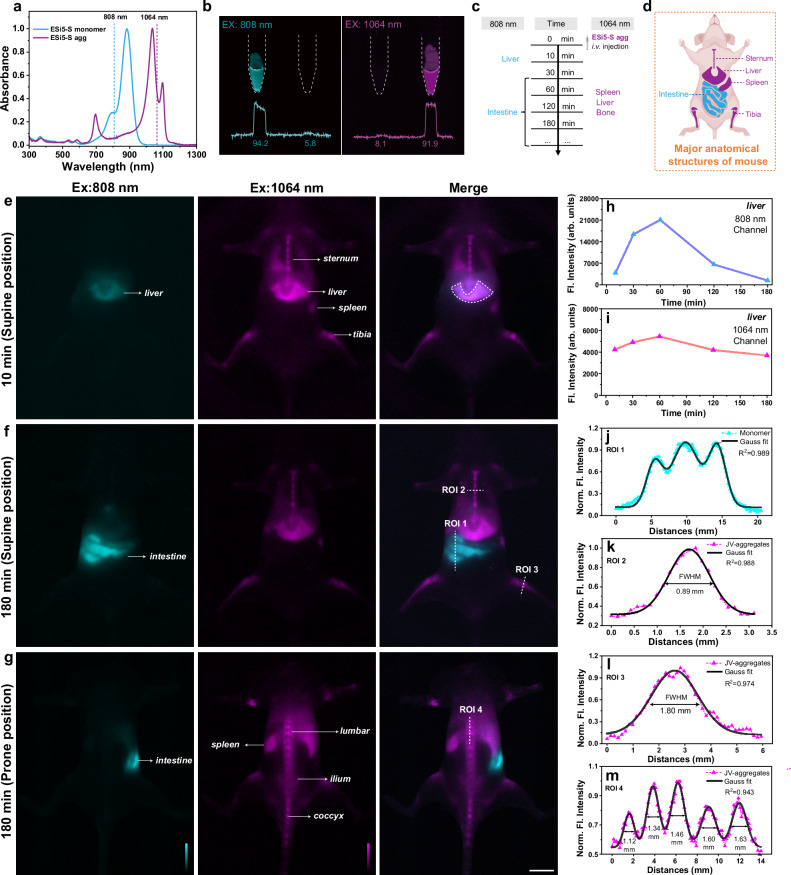
ESi5-S agg exhibited good biocompatibility. ESi5-S agg was essentially non-cytotoxic when its concentration was smaller than 30 μM, since the cells viability was higher than 85% after a 24 h incubation with HeLa cells (Supplementary Fig. 38). Incubating ESi5-S agg (100 μM) with red blood cells for 24 h did not induce noticeable hemolysis (<5%) (Supplementary Fig. 39). An acute-toxicity study was carried out to verify that up to 2.5 mg/kg did not exhibit toxicity based on monitoring of the weight and post-mortal H&E histological analysis (Supplementary Figs. 45 and 46). We further tested the feasibility of ESi5-S agg for in vivo fluorescence imaging harnessing its intense and narrowband SWIR absorption and high stability in an aqueous medium. Upon i.v. injection, an aggregate is diluted and re-establishes the aggregation-disaggregation equilibrium in blood vessels. Therefore, a dual-channel imaging experiment may be carried out to monitor the fluorescence of the aggregate (λex = 1064 nm) and that of the dissociated monomer (λex = 808 nm). Since the aggregate and the monomer are drastically different in terms of their size, charge, and morphology, they are expected to exhibit distinct metabolic profiles and in vivo distribution. We further verified that the aggregate can be selectively excited by a 1064 nm laser and the monomer by an 808 nm laser, respectively, with minimal cross-talk (Fig. 5a, b). An aliquot of ESi5-S agg solution (500 μM in PBS, 100 μL) was injected into a BALB/c mouse through the tail vein to render a final vasculature concentration of 2.5 mg/kg, equivalent to a biocompatible blood concentration of ca. 30 μM. The first set of images was acquired 10 min post injection (Fig. 5e). The fluorescence signal of the aggregate was found from such organs as the liver, spleen, and bones, whereas the signal of the monomer localized in the liver (Fig. 5e–g). The blood vessels were not observed in either channel. This suggests that the aggregate exhibited a notable stability against dissociation even in blood and was distributed through circulation. Its affinity toward bone was likely due to its polyanionic nature. This bone affinity was also in agreement with many other polyanionic materials, due to its affinity to hydroxyapatite68 (Supplementary Fig. 47). The ratio of the fluorescence intensities between the vertebrae and soft tissue reached a peak of 3.9 in 180 min (Supplementary Fig. 41). Besides, the analysis crossing the vertebrae and shin also showed good differentiation between bone and others, with FWHM of 0.89 mm and 1.80 mm (Fig. 5k, l).
Fig. 5. The in vivo dual-channel fluorescence (Fl.) imaging of BALB/c mouse using ESi5-S agg.
a The normalized absorption spectra of ESi5-S monomer and ESi5-S agg and the compatible common laser lines. b The epi-fluorescence images of the ESi5-S Monomer (Left, 500 μM in water with 1% tween 80) and ESi5-S agg (500 μM in PBS) solutions in EP tubes excited by 808 (60 mW•cm−2) and 1064 nm (80 mW•cm−2), respectively. c The timeline of the in vivo fluorescence mouse imaging experiment. 808 nm channel: λex = 808 nm (60 mW•cm−2), long pass filter: 1200 nm, exposure time: 100 ms. 1064 nm channel: λex = 1064 nm (80 mW•cm−2), long pass filter: 1200 + 1350 nm, exposure time: 500 ms. d A schematic diagram of major anatomical structures of mouse related to this experiment. Created in BioRender. Yang, Y. (2024) https://BioRender.com/q64i385. The two-channel fluorescence images of BALB/c mice in the supine position (e) 10 min and (f) 180 min post i.v. injection, and in the prone position (g) at 180 min post i.v. injection. The changes of the liver region fluorescence intensity (h) in the 808 nm channel and (i) in the 1064 nm channel. The line intensities of (j) the ROI 1 for the intestine in the 808 nm channel, (k) the ROI 2 for the sternum in the 1064 nm channel, (l) the ROI 3 for the tibia in the 1064 nm channel, and (m) the ROI 4 for the lumbar vertebra in the 1064 nm channel. Scale bar: 10 mm.
During a course of 180 min, the fluorescence images of these two channels were acquired intermittently. The distribution pattern was essentially unchanged except for a gradual decrease in signal intensity. The fluorescence images of the liver region of the two channels provided a good estimate of the metabolic kinetics of the monomer and the aggregate to be on the order of a few hours (Fig. 5h, i). This suggested a gradual dissociation and metabolism of the aggregate. In 180 min, the monomer was metabolized from the liver to the intestine (Fig. 5f). Line intensity profile could be used to calculate the size of various anatomical structures, e.g., sternum to be ca. 0.89 mm (Fig. 5k) and tibia to be ca. 1.80 mm (Fig. 5l). Likewise, imaging of the prone state (Fig. 5g) also displayed a high sensitivity and SBR in the normal bones, including the lumbar vertebra, ilium, caudal vertebra, and femur. Specifically, line-intensity analysis (red line) along the lumbar vertebra was extracted and afforded the dimensions of all the vertebrae in the range of 1.12–1.63 mm (Fig. 5m). The lack of the monomer signal in other organs except the liver suggested that the dissociation of aggregate occurred predominantly in the liver. Overall, ESi5-S agg is a specifically bone-binding nano-assemblies for visualizing the whole skeletal system in vivo, and its enterohepatic metabolism processes are revealed by dual-channel imaging.
In vivo photoacoustic imaging
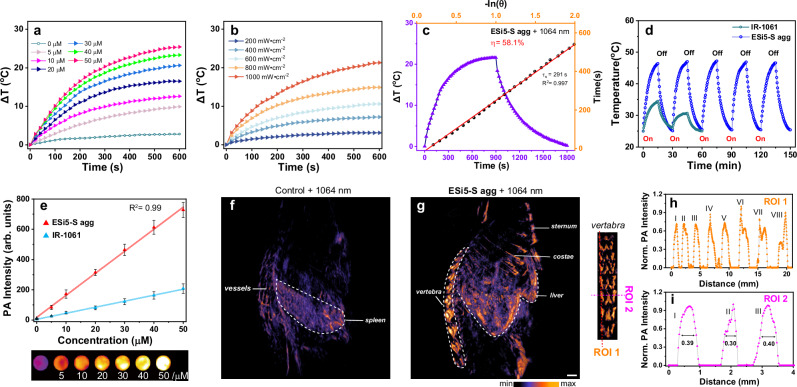
An additional proof-of-concept photoacoustic imaging application was carried out to further showcase the potential of this series of aggregates with ESi5-S agg as an example for different modalities of bioimaging. PA signal is mainly related to the photothermal conversion and subsequent thermal-to-acoustic transformation of a light-absorbing material. The temperature increase profiles of the ESi5-S agg solution in PBS correlated with both the aggregate concentrations and laser power density (Fig. 6a, b). Fitting of the photothermal conversion profile yielded a photothermal conversion efficiency of 58.1% (Fig. 6c). Further, the thermal stability of the ESi5-S agg was evaluated by repeated heating/cooling cycles in the range of 25–46.6 °C (Fig. 6d). After five cycles, no sign of fatigue was observed, while IR-1061 did not survive two around of heating/cooling cycles under the same conditions. The photoacoustic effect of ESi5-S agg was further tested at various concentrations (0–50 μM) (Fig. 6e). A dose-dependence was observed between the photoacoustic intensity and the ESi5-S agg concentration. Notably, the photoacoustic signal intensity was 3.5 times higher than that of IR-1061 (50 μM in PBS).
Fig. 6. The in vivo photoacoustic (PA.) imaging of BALB/c mouse using ESi5-S agg.
The concentration (a) and laser intensity (b) dependent photothermal property of ESi5-S agg in PBS. c The calculation of the photothermal conversion efficiency of ESi5-S agg (30 μM) under continuous 1064 nm laser (1000 mW•cm−2) irradiation. d Photothermal stability of ESi5-S agg (30 μM) in PBS and IR-1061 (30 μM) in PBS under 1064 nm laser (1000 mW•cm−2) irradiation. e The photoacoustic intensity of the ESi5-S agg and IR-1061 in PBS excited by 1064 nm laser (n = 3, Data are presented as mean values ± SD). Photoacoustic imaging of BALB/c mice acquired (f) without ESi5-S agg and (g) 180 min post injection of ESi5-S agg (2.5 mg/kg in PBS). h, i Line intensities of the highlighted ROIs of the images of vertebra. Scale bar: 1 mm.
The feasibility of ESi5-S agg for in vivo photoacoustic imaging was carried out with BALB/c mouse. First, we acquired the background photoacoustic imaging with mouse without injecting ESi5-S agg. The presence of weak signals from the vasculature-rich area/organs was due to a weak absorption of hemoglobin at this SWIR region (Fig. 6f). Then, a solution of ESi5-S agg (2.5 mg/kg) in PBS was injected into shaved BALB/c mouse through tail vein. Due to its intense absorption at 1064 nm, a good contrast could be obtained over the signal from the hemoglobin. In 180 min, the photoacoustic experiment was carried out with a 1064 nm excitation and a three-dimensional photoacoustic tomography was reconstructed (Fig. 6g). The photoacoustic signal was concentrated in the liver and the bone (including sternum, costae, and lumbar), in agreement with the fluorescence imaging results. For lumbar, specifically, vertical and horizontal analysis (ROI 1 and ROI 2) along the lumbar vertebra showed eight and three peaks of photoacoustic intensity respectively, and the FWHMs were high enough to discriminate different vertebrae including posterior articular protrusion (Fig. 6h, i). From the above experimental results, it can be concluded that the ESi5-S exhibits an excellent PA imaging performance.
Discussion
In conclusion, we proposed an approach, i.e., CAASH, to make J-aggregate development more of a rational and less of a serendipitous endeavor. We chose ESi5 as the monomeric dye to develop SWIR aggregate because it fulfills the basic set of criteria: (1) deep-NIR absorption maximum at 867 nm due to a large transition dipole moment; (2) flat electron push–pull headgroups; (3) bulky groups at the center of the conjugative backbone. Implementation of the CAASH approach to the scaffold of ESi5 was a two-step process. First, the tendency of the ESi5 to form aggregate was evaluated by growing the crystals of the bare bone ESi5 fluorophore. Interestingly, we successfully obtained two distinct crystals of ESi5 when different counteranions were used. With the success of the first step, we then installed two hydrophilic chains to the electron push–pull headgroups of ESi5 scaffold to impart additional driving force to aggregate in an aqueous medium. Upon addition of the amphiphilic ESi5 dyes, e.g., ESi5-S, or ESi5-C1(-C5), into H2O, spectral features suggesting the formation of an aggregate were immediately observed. The intense absorption at 867 nm of ESi5 monomer disappeared and three new peaks at 696, 1038, and 1098 nm appeared. Taking into account both the basic exciton theory and the crystal packing styles of ESi5 crystal, it was deduced that the 696 and 1038 nm bands were presumably from intra-chain V-aggregation, and the 1098 nm band from inter-chain J-aggregation. Upon photoexcitation, ESi5 aggregates exhibited by a sharp resonant fluorescence emission band and a greatly reduced fluorescence lifetime (τ = 58 ps). The JV-aggregate from ESi5 dyes exhibited unexpectedly high stability toward organic solvent, temperature, photobleaching, and reactive chemical species. Notably, we found that heating/annealing not only did not lead to destruction of the JV-aggregate, but also helped to reduce aggregation disorder and promote aggregation coupling strength. ESi5-S agg exhibited high biocompatibility, e.g., high aqueous stability, aqueous solubility, low toxicity, and low hemolysis capability. Together, these properties rendered the ESi5 aggregates broad potential for cutting-edge biotechnologies exploiting the SWIR region. In this work, we intravenously injected an aliquot of ESi5-S agg to BALB/c mouse. The ESi5-S agg exhibited good stability in the bloodstream and can be used for dual-channel fluorescence bioimaging. The 1064 nm laser line can be used to excite ESi5-S agg, which exhibits high affinities toward bones, along with some uptake in the liver and spleen. The 808 nm laser line can be used to excite the monomeric ESi5-S dye, which was found only to exist in the liver and metabolized to the intestine in the course of a few hours. ESi5-S agg also enabled photoacoustic tomography of mouse bones, including sternum, costae, and vertebra. Multiplexed imaging with minimal cross-talks between different channels is a challenge for the near-infrared region due to the broad absorption bands of the NIR dyes. Sletten et al. noted that the aggregates with sharp absorption bands are viable candidates for multiplexing in this spectral region39. Therefore, developing aggregates that can pair with ESi5-S agg for multiplexed imaging is a viable direction for future research.
Methods
Animals
The animal experiment protocol was approved by the Laboratory Animal Welfare and Ethics Committee of Shanghai University of Traditional Chinese Medicine (PZSHUTCM220613018, June 13, 2022). The mice which purchased from Shanghai JieSiJie Laboratory. Six to eight-week-old female BALB/c mice with a body weight of ~20 g were chosen for research. The mice were housed in an environment with a temperature of ~25 °C and a 12 h light/dark cycle, suitable humidity (typically 40%–60%). Sex was not noted in the mouse imaging studies because the results are applicable to both male and female mice.
General materials
Chemicals were purchased from major venders based in China and used without further purification. Analytical grade solvents, including petroleum ether, ethyl acetate, dichloromethane, methanol, dimethyl sulfoxide (DMSO), and N,N-dimethylformamide (DMF), were obtained from Titan Scientific. IR-1061 (CAS: 155614-01-0) and ICG (CAS: 3599-32-4) was purchased from Sigma Aldrich. Anhydrous solvents for reactions (e.g. tetrahydrofuran and DMF) were procured from Adamas with activated 4A molecular sieves. Silica gel (200–300 or 300–400 mesh) for flash chromatography was acquired from Haiyang Chem, China. The HeLa cells were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Sheep red blood cells were purchased from SenBeiJia Biological Technology Co., Ltd.
Instruments
The 1H-NMR and 13C-NMR spectra were acquired over Bruker Avance 400 or Avance 600 spectrometers. The ESI-HRMS spectra were obtained using Micromass GCT spectrometer at the Analysis and Testing Center of East China University of Science and Technology.
The UV-Vis absorption spectra were acquired using a SHIMADZU UV-2600 UV-VIS spectrophotometer and the fluorescence spectra were acquired with a PTI-QM4 steady-state fluorimeter with 1 cm cuvette and an InGaAs photodetector. The femtosecond transient absorption spectra were acquired with Helios fire, ultrafast systems. The single-crystal X-ray diffraction data were acquired with a Rigaku Synergy-R X-ray single-crystal diffractometer. The in vivo fluorescence imaging was carried out with MARS (Artemis Intelligent Imaging) equipped with an InGaAs camera (NIRvana, Teledyne Princeton Instruments). The photoacoustic imaging of solutions and in vivo photoacoustic imaging of mice were performed with the LOIS 3D imaging system (TomoWave Laboratories, USA).
The 1H-NMR and 13C-NMR spectra were processed using MestReNova. The spectral data were processed using Origin 2024b. The microscopy images were analyzed with Image J 1.48. The X-ray crystal diffraction data (cif file) were analyzed using Mercury 3.8, CCDC.
Electrostatic potential (ESP) calculations
All theoretical calculations were accomplished by Gaussian 16. The structural optimization and ESP distribution were derived from density-functional theory calculations at the B3LYP/6-31G (d) level. And the surface density minima or maxima analysis was performed using Multiwfn (Comput. Chem. 2012, 33, 580–592).
Preparation of ESi5 aggregates
Take ESi5-S for example, a stock solution of ESi5-S (5.0 mM) was prepared in organic solvents, e.g., MeOH, and an aliquot was subsequently added to a PBS (pH = 7.4, 10 mM) solution with stirring. The aggregation was completed essentially instantaneously.
Fluorescence quantum yield (ϕ) determination
The fluorescence quantum yield of ESi5 dyes in organic solvent (i.e. DMSO, MeOH, MeCN, DMF) and ESi5-N in PBS were referenced to ECXb (ϕ = 13.3% in CH2Cl2), with excitation at 880 nm. The fluorescence quantum yield of ESi5 JV-aggregates in PBS (λem > 1000 nm) were referenced to IR26 (ϕ = 0.5% in 1,2-dichloroethane), with excitation at 980 nm. Specifically, the fluorescence quantum yield of the sample was calculated according to literature methods: Due to the fact that there still lacks a stable and bright dye absorbing in SWIR region with robustly calibrated fluorescence quantum yield, this quantum yield should be considered as an estimate.
Spectral deconvolution
Spectra deconvolution was conducted following literature methods (J. Am. Chem. Soc. 2022, 144, 14351–14362).
Electron microscopy characterization
The stock solution containing ESi5-S or ESi5-C1(-C5) (5.0 mM, 40 μL) was prepared in methanol (MeOH) and then added to double-distilled water (960 μL) to induce aggregation. The resulting aggregate solution (0.2 mM) was incubated in the dark for 24 h, and the percentage of MeOH in the solution was diluted to 4%.
TEM samples were prepared by depositing 5 μL of aggregate solution on a 400-mesh formvar-supported copper grids coated with ultra-thin carbon film. After a 10-min deposition period, excess solution was removed using filter paper. The aggregate samples were imaged using JEM-1400 and JEM-2100 at an acceleration voltage of 100 kV.
Three microliters of solution containing aggregate was incubated on a mica sheet for 5 min and then was blotted with the filter paper until only a thin solution film remained. The AFM images were captured using a Bruker Dimension Fast Scan, using ScanAsyst mode under the ambient conditions.
For cryo-EM, the solution of aggregate (5 μL) was applied to glow-discharged holey carbon copper grids before plunge-frozen in liquid ethane after blotting with filter paper using Vitrobot Mark IV(FEI). Cryo-EM micrographs were acquired on a Tecnai T12 TEM (FEI), operated at an acceleration voltage of 120 kV.
Chemo-stability toward thiols and reactive oxygen species
An aliquot of GSH (0–10 mM) in H2O, Cys (0–100 mM) in H2O, NaSH (0–1 mM) in H2O, H2O2 (0–10 mM) in H2O, NaClO (0–50 μM) in H2O (pH = 10), ONOO− (0–50 μM) in H2O (pH = 10) were added into a solution of ESi5-S agg. The solution was stirred for 5 min before the absorption spectra were acquired.
Photostability tests
The ESi5-S monomer and ICG in DMSO with same absorbance (A = 0.2) in 808 nm were irradiated by an 808 nm laser (500 mW•cm−2) with vigorous stirring for 30 min. The ESi5-S agg in PBS and IR-1061 in DMSO with same absorbance (A = 0.2) in 1064 nm were irradiated by a 1064 nm laser (520 mW•cm−2) with vigorous stirring for 30 min. The absorption of solutions was measured every 1 or 2 min.
The thermodynamic parameters for aggregation
The degree of aggregation and thermodynamic parameters for aggregation were calculated following literature methods (Angew. Chem. Int. Ed. 2012, 51, 5615–5619).
In vitro hemolysis assay
Sheep red blood cells (2%, 0.5 mL) were incubated with Triton X-100 (2%, +), PBS buffer (pH = 7.4, 10 mM, −), and incremental concentration gradient of ESi5-S agg (20, 40, 60, 80, 100 μM) for 4 h on an oscillator at 37 °C, respectively. The suspension supernatant was concentrated by centrifugation (10,000 rpm, 5 min) and placed in a 96-well plate. The absorbance at 540 nm of each well was measured by enzyme marker, and the hemolytic percentage value was calculated according to Equation:
Where ODsample, ODsaline, and ODTriton stand for the absorbance of sheep red blood cells incubated with different ESi5-S agg, PBS, and triton X-100, respectively.
In vivo dual-color fluorescence imaging by MARS system
Six to eight-week-old female BALB/c mice with a body weight of ~20 g were chosen for in vivo dual-color color fluorescence imaging. An aliquot of ESi5-S agg solution (500 μM in PBS, containing 4% DMSO, 100 μL) was injected into a BALB/c mouse through the tail vein to render a final vasculature concentration of 2.5 mg/kg, equivalent to a biocompatible blood concentration of ca. 30 μM. The whole-body images were acquired 10, 30, 60, 120, 180 min post injection. The images with excitation at 808 and 1064 nm were acquired sequentially. When the excitation of 808 nm (exposure time: 100 ms, 60 mW•cm−2) was used, a long pass filter with a cutoff of 1200 nm was used for emission collection. When the excitation of 1064 nm (exposure time: 500 ms, 80 mW•cm−2) was used, two long pass filters at 1350 and 1200 nm were used for emission collection. All mice were shaved before fluorescence imaging.
In vivo toxicity study
Six-week BALB/c female mouse (n = 6) was used as an animal model and randomly divided into three groups. An aliquot of PBS (10 mM, pH = 7.4, 0.1 mL) and ESi5-S agg (2.5 mg/kg, 5.0 mg/kg) solution was injected through the tail vein, respectively. The mice were raised for a period of 14 days, during which period the body weight and vitality of the mouse were recorded.
For H&E histology, the mice were sacrificed 24 h post i.v. injection of PBS (10 mM, pH = 7.4, 0.1 mL) and ESi5-S agg (2.47 mg/kg in PBS) and the major organs are harvested for H&E histology analysis for acute-toxicity features.
Photothermal conversion performance
The increase in temperature of ESi5-S agg solution (2 mL, in PBS) with different concentrations (0, 5, 10, 20, 30, 40, and 50 μM) under 1064 nm laser (1000 mW•cm−2) and ESi5-S agg solution (30 μM in 2 mL PBS) under different power densities (200, 400, 600, 800, and 1000 mW•cm−2) were measured using a temperature detector. The photothermal conversion efficiency (η) was calculated according to Equation:
Where is the heat transfer coefficient and stands for the surface area of the quartz sample cell. and are the maximum steady-state temperature under laser and the surrounding environment temperature, respectively. is the heat dissipation from the light absorbed by the solvent and the quartz sample cell. was the incident laser power, and A1064 represent the absorbance intensity of the solution at 1064 nm.
In vivo photoacoustic imaging
The photoacoustic signals of ESi5-S agg and IR-1061 solutions with different concentrations (0, 5, 10, 20, 30, 40, and 50 μM) were carried out with LOIS 3D (TomoWave Laboratories, USA) under 1064 nm laser.
For in vivo photoacoustic imaging, 6–8-week-old female BALB/c mice with a body weight of ~20 g (n = 3) were injected with ESi5-S agg (2.5 mg/kg, in PBS containing 4% DMSO). The PA imaging was performed by LOSI 3D imaging system before injected and post-injected of 120 min under 1064 nm laser. All mice were shaved before photoacoustic imaging.
Calcium-binding experiments
Hydroxyapatite (HA), calcium phosphate (CP), calcium pyrophosphate salts (CPP), calcium carbonate (CC), calcium oxalate (CO), and manganese dioxide (MnO2) (10 mg/mL) were incubated separately with ESi5-S agg (10 μM) in PBS (pH = 7.4, 10 mM). The mixtures were vortexed for 60 min and then washed three times with PBS before centrifugated to remove unbound JV-aggregate. The precipitate was diluted in 200 μL PBS and the fluorescence emission intensity was tested.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the National Key Research and Development Program of China (no. 2022YFD1700800), the National Natural Science Foundation of China (nos. 22078098 and 22278138), the Fundamental Research Funds for the Central Universities, the Shanghai Academic/Technology Research Leader (22XD1421000), the Open Funding Project of the State Key Laboratory of Bioreactor Engineering, and the State Key Laboratory of Fine Chemicals (KF2202). The authors thank Qi Zhang, Weiping Zhu, and Haibo Yang for insightful discussions.
Author contributions
C.Y. was responsible for synthesis, spectral studies, draft preparation, and editing. R.W., X.Z., and R.C. contributed to the synthesis. J.Z. and J.C. carried out transient absorption studies. Y.S. and J.R. contributed to TEM. W.X. and C.L. contributed to cryo-EM studies. D.Q. contributed to the vary-temperature experiment. Y.W. contributed to AFM studies. X.L. Y.D., and G.G. contributed to in vivo imaging. X.L. and X.Q. were responsible for advising and editing. Y.Y was responsible for advising, editing, and conceptualization.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
Supplementary information is available. Requests for materials should be addressed to the corresponding author. The source data generated in this study have been deposited in the FigShare database under the accession code 10.6084/m9.figshare.27959742.v1. Source data are provided alongside the manuscript. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2120399 (ESi5•Cl−), and 2364282 (ESi5•PF6−). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Data are available from the authors on request. Source data are provided with this paper.
Competing interests
A Chinese patent application (2024115961715) with Y.Y., X.Q., X.L., and C.Y. listed as inventors is filed for the structures, preparation, and potential applications of the aggregates described in this manuscript. Y.Y., X.Q., X.L., and C.Y. declare no other competing interests. Other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55445-x.
References
- 1.Oliinyk, O. S. et al. Deep-tissue SWIR imaging using rationally designed small red-shifted near-infrared fluorescent protein. Nat. Methods20, 70–74 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang, F., Zhong, Y., Bruns, O., Liang, Y. & Dai, H. In vivo NIR-II fluorescence imaging for biology and medicine. Nat. Photonics18, 535–547 (2024). [Google Scholar]
- 3.Ibrahim, N. E.-S. Shortwave-infrared imaging of cancer vaccine uncovers immune response. Nat. Rev. Bioeng.1, 694–694 (2023). [Google Scholar]
- 4.Kantamneni, H. et al. Surveillance nanotechnology for multi-organ cancer metastases. Nat. Biomed. Eng.1, 993–1003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang, X., Ackerman, M. M., Chen, M. & Guyot-Sionnest, P. Dual-band infrared imaging using stacked colloidal quantum dot photodiodes. Nat. Photonics13, 277–282 (2019). [Google Scholar]
- 6.Saran, R. & Curry, R. J. Lead sulphide nanocrystal photodetector technologies. Nat. Photonics10, 81–92 (2016). [Google Scholar]
- 7.Na, N. et al. Room temperature operation of germanium–silicon single-photon avalanche diode. Nature627, 295–300 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Baetens, R., Jelle, B. P. & Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: a state-of-the-art review. Sol. Energy Mater. Sol. Cells94, 87–105 (2010). [Google Scholar]
- 9.Wang, Z. Y. Near-Infrared Organic Materials and Emerging Applications. (CRC Press, 2013). 10.1201/b14775.
- 10.Tani, T., Suzumoto, T., Kemnitz, K. & Yoshihara, K. Picosecond kinetics of light-induced electron transfer from J-aggregated cyanine dyes to silver bromide microcrystals: effect of aggregate size. J. Phys. Chem.96, 2778–2783 (1992). [Google Scholar]
- 11.Stuke, M. Dye Lasers: 25 Years Vol. 70 (Springer Berlin Heidelberg, 1992).
- 12.Xie, Y. et al. Bright short-wavelength infrared organic light-emitting devices. Nat. Photonics16, 752–761 (2022). [Google Scholar]
- 13.Liu, Y. et al. Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: from strategic design toward molecular imaging and theranostics. Chem. Rev.122, 209–268 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Meador, W. E. et al. Silicon-RosIndolizine fluorophores with shortwave infrared absorption and emission profiles enable in vivo fluorescence imaging. Nat. Chem.16, 970–978 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukushima, H. et al. Cyanine phototruncation enables spatiotemporal cell labeling. J. Am. Chem. Soc.144, 11075–11080 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nani, R. R., Kelley, J. A., Ivanic, J. & Schnermann, M. J. Reactive species involved in the regioselective photooxidation of heptamethine cyanines. Chem. Sci.6, 6556–6563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, A. & Rivera-Fuentes, P. A general strategy to develop fluorogenic polymethine dyes for bioimaging. Nat. Chem.16, 28–35 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolbert, L. M. & Ogle, M. E. How far can a carbanion delocalize? Carbon-13 NMR studies on soliton model compounds. J. Am. Chem. Soc.112, 9519–9527 (1990). [Google Scholar]
- 19.Brooker, L. G. S., Sprague, R. H., Smyth, C. P. & Lewis, G. L. Color and constitution. I. Halochromism of anhydronium bases related to the Cyanine Dyes1. J. Am. Chem. Soc.62, 1116–1125 (1940). [Google Scholar]
- 20.Tao, Z. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem.125, 13240–13244 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Antaris, A. L. et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun.8, 15269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei, Y. et al. Supra-fluorophores: ultrabright fluorescent supramolecular assemblies derived from conventional fluorophores in water. Adv. Mater.36, 2401346 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumes, J. M. et al. Storable, thermally activated, near-infrared chemiluminescent dyes and dye-stained microparticles for optical imaging. Nat. Chem.2, 1025–1030 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, W., Johnson, A. & Smith, B. D. Guest back-folding: a molecular design strategy that produces a deep-red fluorescent host/guest pair with picomolar affinity in water. J. Am. Chem. Soc.140, 3361–3370 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Li, D.-H., Schreiber, C. L. & Smith, B. D. Sterically shielded heptamethine cyanine dyes for bioconjugation and high performance near-infrared fluorescence imaging. Angew. Chem. Int. Ed.59, 12154–12161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, D.-H. et al. Doubly strapped zwitterionic NIR-I and NIR-II heptamethine cyanine dyes for bioconjugation and fluorescence imaging. Angew. Chem. Int. Ed.62, e202305062 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei, Z. et al. Bright, stable, and biocompatible organic fluorophores absorbing/emitting in the deep near-infrared spectral region. Angew. Chem. Int. Ed.56, 2979–2983 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Li, J. et al. Stable, bright, and long-fluorescence-lifetime dyes for deep-near-infrared bioimaging. J. Am. Chem. Soc.144, 14351–14362 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Wei, R. et al. Rigid and photostable shortwave infrared dye absorbing/emitting beyond 1200 nm for high-contrast multiplexed imaging. J. Am. Chem. Soc.145, 12013–12022 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Kasha, M. Energy transfer mechanisms and the molecular exciton model for molecular aggregates. Radiat. Res.20, 55–70 (1963). [PubMed] [Google Scholar]
- 31.Nelson, N. & Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol.5, 971–982 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Hu, X. et al. J-aggregation strategy toward potentiated NIR-II fluorescence bioimaging of molecular fluorophores. Adv. Mater.36, 2304848 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Würthner, F., Kaiser, T. E. & Saha-Möller, C. R. J-aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed.50, 3376–3410 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev.115, 11718–11940 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Tian, Y., Yin, D. & Yan, L. J-aggregation strategy of organic dyes for near-infrared bioimaging and fluorescent image-guided phototherapy. WIREs Nanomed. Nanobiotechnol.15, e1831 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi, T. J-Aggregates Vol. 2. (World Scientific, 2012).
- 37.Hannah, K. C. & Armitage, B. A. DNA-templated assembly of helical cyanine dye aggregates: a supramolecular chain polymerization. Acc. Chem. Res.37, 845–853 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Li, K. et al. J-aggregates of meso-[2.2]paracyclophanyl-BODIPY dye for NIR-II imaging. Nat. Commun.12, 2376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen, W. et al. Shortwave infrared imaging with j-aggregates stabilized in hollow mesoporous silica nanoparticles. J. Am. Chem. Soc.141, 12475–12480 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grande, V., Soberats, B., Herbst, S., Stepanenko, V. & Würthner, F. Hydrogen-bonded perylene bisimide J-aggregate aqua material. Chem. Sci.9, 6904–6911 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huynh, E. et al. In situ conversion of porphyrin microbubbles to nanoparticles for multimodality imaging. Nat. Nanotechnol.10, 325–332 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Sun, C. et al. J-aggregates of cyanine dye for NIR-II in vivo dynamic vascular imaging beyond 1500 nm. J. Am. Chem. Soc.141, 19221–19225 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Chen, Z. et al. Near-IR absorbing J-aggregate of an amphiphilic BF2-azadipyrromethene dye by kinetic cooperative self-assembly. Angew. Chem. Int. Ed.56, 5729–5733 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Draper, E. R. et al. pH-directed aggregation to control photoconductivity in self-assembled perylene bisimides. Chem2, 716–731 (2017). [Google Scholar]
- 45.Kim, J. H. et al. An efficient narrowband near-infrared at 1040 nm organic photodetector realized by intermolecular charge transfer mediated coupling based on a squaraine dye. Adv. Mater.33, 2100582 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma, X. et al. J-aggregates formed by NaCl treatment of aza-coating heptamethine cyanines and their application to monitoring salt stress of plants and promoting photothermal therapy of tumors. Angew. Chem. Int. Ed.62, e202216109 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Lovell, J. F. et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater.10, 324–332 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Wei, K. et al. A light-triggered J-aggregation-regulated therapy conversion: from photodynamic/photothermal therapy to long-lasting chemodynamic therapy for effective tumor ablation. Angew. Chem. Int. Ed.63, e202404395 (2024). [DOI] [PubMed] [Google Scholar]
- 49.Cai, K. et al. Concurrent cooperative J-aggregates and anticooperative H-aggregates. J. Am. Chem. Soc.140, 5764–5773 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Herbst, S. et al. Self-assembly of multi-stranded perylene dye J-aggregates in columnar liquid-crystalline phases. Nat. Commun.9, 2646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Q. et al. Bright and stable NIR-II J-aggregated AIE dibodipy-based fluorescent probe for dynamic in vivo bioimaging. Angew. Chem. Int. Ed.60, 3967–3973 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Guo, X. et al. Tuning shortwave-infrared J-aggregates of aromatic ring-fused aza-BODIPYs by peripheral substituents for combined photothermal and photodynamic therapies at ultralow laser power. Angew. Chem.136, e202319875 (2024). [DOI] [PubMed] [Google Scholar]
- 53.Freytag, E. et al. Chiroptical properties of planar benzobisthiazole-bridged squaraine dimers. J. Org. Chem.88, 10777–10788 (2023). [DOI] [PubMed] [Google Scholar]
- 54.Shen, C.-A., Stolte, M., Kim, J. H., Rausch, A. & Würthner, F. Double J-coupling strategy for near infrared emitters. J. Am. Chem. Soc.143, 11946–11950 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Eisele, D. M. et al. Utilizing redox-chemistry to elucidate the nature of exciton transitions in supramolecular dye nanotubes. Nat. Chem.4, 655–662 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Hou, T.-C., Wu, Y.-Y., Chiang, P.-Y. & Tan, K.-T. Near-infrared fluorescence activation probes based on disassembly-induced emission cyanine dye. Chem. Sci.6, 4643–4649 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bricks, J. L., Slominskii, Y. L., Panas, I. D. & Demchenko, A. P. Fluorescent J-aggregates of cyanine dyes: basic research and applications review. Methods Appl. Fluoresc.6, 012001 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Feng, L. et al. A facile structural isomerization-induced 3D spatial D-A interlocked network for achieving NIR-II phototheranostic agents. Angew. Chem. Int. Ed.61, e202212673 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Lee, K.-W. et al. Anti-quenching NIR-II J-aggregates of benzo[c]thiophene fluorophore for highly efficient bioimaging and phototheranostics. Adv. Mater.35, 2211632 (2023). [DOI] [PubMed] [Google Scholar]
- 60.Kaiser, T. E., Wang, H., Stepanenko, V. & Würthner, F. Supramolecular construction of fluorescent J-aggregates based on hydrogen-bonded perylene dyes. Angew. Chem. Int. Ed.46, 5541–5544 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Deshmukh, A. P. et al. Near-atomic-resolution structure of J-aggregated helical light-harvesting nanotubes. Nat. Chem.16, 800–808 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey, A. D. et al. Exploring the design of superradiant J-aggregates from amphiphilic monomer units. Nanoscale15, 3841–3849 (2023). [DOI] [PubMed] [Google Scholar]
- 63.Eisele, D. M., Knoester, J., Kirstein, S., Rabe, J. P. & Vanden Bout, D. A. Uniform exciton fluorescence from individual molecular nanotubes immobilized on solid substrates. Nat. Nanotechnol.4, 658–663 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Hestand, N. J. & Spano, F. C. Expanded theory of H- and J-molecular aggregates: the effects of vibronic coupling and intermolecular charge transfer. Chem. Rev.118, 7069–7163 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Shi, Y. et al. Small energetic disorder enables ultralow energy losses in non-fullerene organic solar cells. Adv. Energy Mater.13, 2300458 (2023). [Google Scholar]
- 66.Han, G. & Yi, Y. Molecular insight into efficient charge generation in low-driving-force nonfullerene organic solar cells. Acc. Chem. Res.55, 869–877 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Tkacheva, T. N., Yefimova, S. L., Klochkov, V. K., Borovoy, I. A. & Malyukin, Y. V. Spectroscopic study of ordered hybrid complexes formation between dye aggregates and ReVO4:Eu3 + (Re = Y, Gd, La) nanoparticles. J. Mol. Liq.199, 244–250 (2014). [Google Scholar]
- 68.Wang, H. et al. P800SO3-PEG: a renal clearable bone-targeted fluorophore for theranostic imaging. Biomater. Res.26, 51 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Supplementary information is available. Requests for materials should be addressed to the corresponding author. The source data generated in this study have been deposited in the FigShare database under the accession code 10.6084/m9.figshare.27959742.v1. Source data are provided alongside the manuscript. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2120399 (ESi5•Cl−), and 2364282 (ESi5•PF6−). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Data are available from the authors on request. Source data are provided with this paper.