Abstract
Objective
To analyse the associations between renal function and clinical laboratory indicators and explore the renal function abnormality risk factors for gout patients in Southwest China.
Methods
Outpatient and hospitalized gout patients (n = 4384) at the First Affiliated Hospital of Chengdu Medical College between January 2017 and December 2020 were divided into normal (n = 2393) and abnormal (n = 1991) renal function groups according to their eGFR. The relationships between clinical laboratory indicators and the eGFR were analysed, and a logistic regression model was fit to identify significant risk factors.
Results
Sex, age, absolute lymphocyte count (ALC), cystatin C (CysC), homocysteine (Hcy) and thyroid stimulating hormone (TSH) were associated with renal function abnormalities (P < 0.05), whereas age [odds ratio (95% CI) = 1.06 (1.05–1.08), P < 0.001], Hcy [1.02 (1.00–1.04), P = 0.028], CysC [1.72 (1.54–1.92), P < 0.001], ALC [0.71 (0.52–0.97), P = 0.03] and TSH [1.08 (1.00–1.17), P = 0.049] were abnormal renal function risk factors for gout patients. After stratification by UA, binary logistic regression analysis identified the following risk factors: Q1 age [1.06 (1.02–1.11), P = 0.003], CysC [1.67 (1.30–2.16), P < 0.001]; Q2 age [1.09 (1.06–1.12), P < 0.001], CysC [1.55 (1.28–1.88), P < 0.001], FT3 [0.66 (0.46–0.96), P = 0.029]; Q3 age [1.06 (1.03–1.09), P < 0.001], CysC [1.75 (1.41–2.18), P < 0.001], Hcy [1.04 (1.00–1.08), P = 0.047], ALC [0.35 (0.18–0.69), P = 0.002]; Q4 age [1.05 (1.02–1.09), P = 0.004], CysC [1.79 (1.40–2.30), P < 0.001].
Conclusion
ALC and levels of TSH and serum Cys could be used for monitoring for abnormal renal function in patients with gout.
Keywords: gout, renal function, glomerular filtration rate, risk factors, cross-section
Key messages.
Age, Hcy, CysC and TSH were independent abnormal renal function risk factors for gout.
ALC is independently and inversely associated with renal dysfunction in gout patients.
ALC, TSH and CysC could be used for monitoring for abnormal renal function in patients with gout.
Introduction
Gout is the precipitation and deposition of MSU crystals in joints or other tissues due to impaired purine metabolism and/or reduced serum urate (UA) excretion and continuous elevation of blood UA levels. Gout is a metabolic and inflammatory/immune disease [1]. Many studies have shown that gout is closely related to the occurrence of heart disease, diabetes mellitus, hypertension, obstructive sleep apnoea syndrome, hyperuricaemia, obesity, renal disease (including renal insufficiency) and hyperlipidaemia [2, 3], among which gout is particularly closely related to the occurrence of kidney damage. There is a strong dose–response association between impaired renal function and the risk of developing gout [4], and the association between a lower estimated glomerular filtration rate (eGFR) and a greater risk of developing gout persists [5]. The kidney is an important organ for UA excretion and is responsible for approximately 70% of UA excretion in the human body [6]. Previous studies have demonstrated that decreased eGFR and proteinuria are closely associated with the risk of developing hyperuricaemia [7]. However, in the early stage of renal function impairment, patients often have no specific manifestations [8]. In previous studies on the relationship between gout/UA levels and renal function, chronic kidney disease (CKD; GFR <60 ml/min/1.73 m2) was used as the dependent variable, but the patients had already entered CKD stage 3a, even if the urinary albumin/creatinine ratio was normal or slightly increased, and the risk stratification of CKD was medium risk [9]. Therefore, we define eGFR <90 ml/min/1.73 m2 as abnormal renal function. Therefore, in this study, we aimed to analyse the incidence and severity of renal impairment at different UA concentrations in patients with gout and to identify relevant risk factors that may predict renal impairment in patients with gout.
Data and methods
Patient inclusion and data information
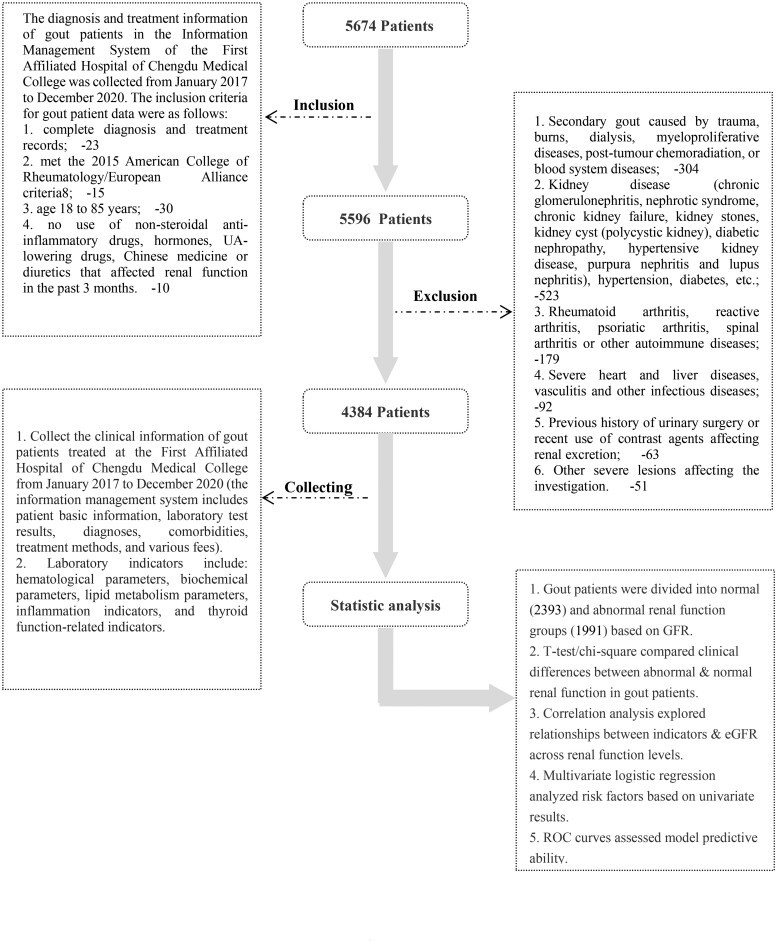
The diagnosis and treatment information of gout patients at the First Affiliated Hospital of Chengdu Medical College was collected from the information management system from January 2017 to December 2020. The information management system contains the basic information of patients, test items, test results, diagnoses, comorbidities, treatment methods and various costs and other information. The inclusion criteria for gout patients were as follows: (1) complete diagnosis and treatment records; (2) met the 2015 American College of Rheumatology/European Alliance criteria [10]; (3) aged 18–85 years; and (4) no use of nonsteroidal anti-inflammatory drugs, hormones, UA-lowering drugs, Chinese medicine or diuretics that affect renal function in the past 3 months. The above information was obtained from current or previous visit records. The exclusion criteria were as follows: (1) secondary gout caused by trauma, burns, dialysis, myeloproliferative diseases, posttumour chemoradiation, or blood system diseases; (2) kidney disease [chronic glomerulonephritis, nephrotic syndrome, chronic kidney failure, kidney stones, kidney cyst (polycystic kidney), diabetic nephropathy, hypertensive kidney disease, purpura nephritis and lupus nephritis], hypertension, diabetes (diabetes and hypertension may have an influence on renal function. Considering that this project is a cross-sectional study, it is unable to longitudinally observe the effect of blood pressure or blood sugar on renal function. Therefore, this study excludes hypertension and diabetes); (3) rheumatoid arthritis, reactive arthritis, psoriatic arthritis, spinal arthritis or other autoimmune diseases; (4) severe heart and liver diseases, vasculitis and other infectious diseases; (5) history of urinary surgery or recent use of contrast agents affecting renal excretion and (6) other severe lesions affecting the investigation. The above information was obtained from current or previous visit records (Fig. 1). This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Chengdu Medical College (2019CYFYHEC-BA-35).
Figure 1.
Flow chart of the included patients
Data collection
Demographic characteristics (age, sex) and laboratory examination indicators were included in the analysis. The above information was obtained from the information management system of the outpatient and inpatient departments of the First Affiliated Hospital of Chengdu Medical College.
The laboratory examination indicators included white blood cell count (WBC), red blood cell count (RBC), haemoglobin (HGB), platelet count (PLT), neutrophilic granulocyte percentage (NE%), percentage of lymphocytes (LY%), percentage of monocytes (MO%), eosinophil percentage (EO%), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), monocyte count (MONO), eosinophil count (EOS), haematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin content (MCH), mean corpuscular haemoglobin concentration (MCHC), alanine transaminase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), UA, cystatin C (CysC), triglycerides (TG), cholesterol (CHOL), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), apolipoprotein A (APOA), apolipoprotein B (APOB), lipoprotein A (LPA), homocysteine (Hcy), RF, antistreptolysin ‘O’ (ASO), anti-cyclic citrullinated peptide antibody (CCP), serum-free triiodothyronine (FT3), serum-free tetraiodothyronine (FT4) and thyroid stimulating hormone (TSH). The eGFR was calculated via the CKD-EPI formula.
Grouping of UA levels
UA levels were divided into four groups according to the gout classification criteria (Supplementary Table S1, available at Rheumatology Advances in Practice online): Q1: UA ≤ 360 µmol/l; Q2: 360 µmol/l ≤ UA < 480 µmol/l; Q3: 480 µmol/l ≤ UA < 600 µmol/l; and Q4: UA ≥ 600 µmol/l.
Definition of abnormal renal function
The GFR was estimated from the serum creatinine (SCr) level, and an eGFR < 90 ml/min/1.73 m2 was defined as abnormal renal function [11]. The GFR is the best indicator for assessing human renal function [12, 13]. The primary screening for renal disease includes the GFR estimated by the SCr level and the albuminuria level assessed by the urinary albumin–creatinine ratio [11]. In this study, the CKD-EPI formula was used to estimate the eGFR to assess renal function in patients [14, 15]: a × (SCr/b)c × (0.993) age.
Female: a = 144, b = 0.7, c = −0.329 when SCr ≤ 0.7 mg/dL, c = −1.209 when SCr > 0.7 mg/dL;
Male: a = 141, b = 0.9, c = −0.411 at SCr ≤ 0.9 mg/dL, c = −1.209 at SCr > 0.9 mg/dL.
Statistical analysis
The data were statistically analysed via IBM SPSS 26.0 software (IBM Corporation, Armonk, NY, USA). Categorical variables are presented as counts or percentages, and continuous variables are presented as the mean ± standard deviation (). For the univariate analysis, we used t-tests or chi-square tests (P < 0.05). Based on the results of univariate analysis, with renal function abnormality as the dependent variable and relevant indicators as independent variables, where multicategorical variables are used in dummy variable form, they are included in the logistic regression model. Stepwise regression method was used for model fitting, and the receiver operating characteristic (ROC) curve was employed to evaluate the predictive performance of the model. Combining the number and expertise of the included factors, the threshold for retaining biomarkers in multivariate analysis is P < 0.05. For the issue of collinearity indicators, we eliminate them by calculating the variance inflation factor (VIF), and any indicator with a VIF value greater than 10 is removed. The X-axis of the ROC curve represents 1-specificity, while the Y-axis represents sensitivity. The accuracy of the model’s predictions determines the area under the ROC curve. The statistical significance was set at 0.05.
Results
General patient data
Overall, 4384 patients were included in the statistical analysis, including their age, sex, routine blood test indicators, biochemical indicators, lipid metabolism indicators, immunologically related indicators, and thyroid function indicators (Supplementary Table S2, available at Rheumatology Advances in Practice online).
Comparison of clinical characteristics and laboratory indicators between the groups with normal and abnormal renal function among patients with gout
RBC, HGB, PLT, LY%, ALC, HCT, MCH, MCHC, ALT, TG, CHOL, LDLC, APOA, APOB and FT3 were significantly lower among the patients in the abnormal renal function group than among those in the normal renal function group (P < 0.05); female sex, age, NE%, MO%, ANC, MONO, MCV, BUN, UA, CysC, LPA, Hcy, FT4 and TSH were significantly greater among the patients in the abnormal renal function group than among those in the normal renal function group (P < 0.05; Table 1).
Table 1.
Comparison of clinical characteristics between the NRF and the ARF
| Categories | NRF (2393) | ARF (1991) | t/χ2 | P |
|---|---|---|---|---|
| Gender | 16.15 | <0.001 | ||
| Female | 8.80% | 12.60% | ||
| Male | 91.20% | 87.40% | ||
| Age | 42.54 ± 13.55 | 62.62 ± 14.74 | −46.95 | <0.001 |
| WBC | 7.98 ± 2.86 | 8.03 ± 3.29 | −0.424 | 0.671 |
| RBC | 4.95 ± 0.7 | 4.44 ± 0.84 | 17.427 | <0.001 |
| HGB | 147.05 ± 20.6 | 130.96 ± 25.74 | 21.632 | <0.001 |
| PLT | 199.43 ± 72.88 | 177.50 ± 80 | 7.545 | <0.001 |
| NE% | 65.91 ± 10.95 | 70.19 ± 17.22 | −9.248 | <0.001 |
| LY% | 24.62 ± 9.76 | 20.30 ± 9.87 | 13.877 | <0.001 |
| MO% | 7.30 ± 2.11 | 7.55 ± 2.44 | −3.404 | 0.001 |
| EO% | 1.77 ± 1.6 | 1.89 ± 2.25 | −1.948 | 0.052 |
| ANC | 5.43 ± 2.71 | 5.89 ± 3.28 | −4.809 | <0.001 |
| ALC | 1.82 ± 0.71 | 1.50 ± 0.73 | 14.293 | <0.001 |
| MONO | 0.58 ± 0.26 | 0.60 ± 0.29 | −2.434 | 0.015 |
| EOS | 0.13 ± 0.11 | 0.14 ± 0.17 | −1.804 | 0.071 |
| HCT | 44.01 ± 5.65 | 39.91 ± 7.21 | 19.852 | <0.001 |
| MCV | 90.38 ± 6.98 | 91.46 ± 7.95 | −4.549 | <0.001 |
| MCH | 30.18 ± 2.86 | 29.98 ± 3.18 | 2.102 | 0.036 |
| MCHC | 333.62 ± 14.34 | 327.24 ± 16.38 | 13.032 | <0.001 |
| ALT | 44.17 ± 41.09 | 36.36 ± 51.16 | 5.017 | <0.001 |
| AST | 31.17 ± 27.71 | 33.37 ± 45.03 | −1.745 | 0.081 |
| BUN | 5.15 ± 2.18 | 7.27 ± 3.68 | −22.472 | <0.001 |
| UA | 478.77 ± 130.08 | 505.26 ± 127.59 | −6.774 | <0.001 |
| CysC | 8.95 ± 1.66 | 13.47 ± 4.27 | −44.493 | <0.001 |
| TG | 2.52 ± 2.25 | 2.08 ± 1.77 | 5.740 | <0.001 |
| CHOL | 4.74 ± 1.09 | 4.39 ± 1.18 | 8.045 | <0.001 |
| HDLC | 1.18 ± 0.34 | 1.16 ± 0.35 | 1.698 | 0.090 |
| LDLC | 2.83 ± 0.89 | 2.55 ± 0.91 | 8.159 | <0.001 |
| APOA | 1.26 ± 0.30 | 1.23 ± 0.30 | 2.727 | 0.006 |
| APOB | 0.94 ± 0.70 | 0.85 ± 0.26 | 4.226 | <0.001 |
| LPA | 186.28 ± 229.82 | 242.23 ± 324.19 | −5.175 | <0.001 |
| Hcy | 15.90 ± 10.37 | 18.84 ± 9.47 | −7.687 | <0.001 |
| FT3 | 5.58 ± 1.06 | 5.04 ± 1.09 | 8.43 | <0.001 |
| FT4 | 11.78 ± 2.09 | 12.27 ± 3.01 | −3.30 | 0.001 |
| TSH | 2.18 ± 1.84 | 3.23 ± 5.52 | −4.58 | <0.001 |
White blood cell count (WBC), red blood cell count (RBC), haemoglobin (HGB), platelet count (PLT), neutrophil percentage (NE%), lymphocyte percentage (LY%), monocyte percentage (MO%), absolute lymphocyte value (ALC), absolute neutrophil count value (ANC), monocyte count (MONO) in the group with renal dysfunction haematocrit (HCT), mean corpuscular volume (MCV), mean erythrocyte haemoglobin content (MCH), mean erythrocyte haemoglobin concentration (MCHC), alanine aminotransferase (ALT), blood urea nitrogen (BUN), blood uric acid (UA), cystatin C (CysC), triglycerides (TG), cholesterol (CHOL), low-density lipoprotein cholestasis (LDLC), apolipoprotein A (APOA), apolipoprotein B (APOB), lipoprotein A (LPA), homocysteine (Hcy), serum triiodothyronine (FT3), serum-free tetraiodothyronine (FT4), thyrotropin (TSH) in the group with abnormal renal function were significantly different from those in the group with normal renal function (P < 0.05; indicated with bold text). NRF: normal renal function group; ARF: abnormal renal function group.
Risk factor analysis of patients with gout
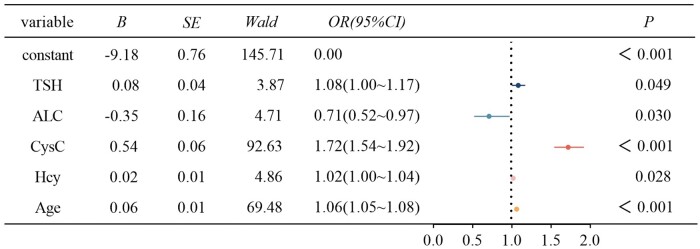
Following the univariate analysis and exclusion of collinearity indicators, renal function was used as the dependent variable, with sex, age, HGB, PLT, NE%, LY%, MO%, ANC, ALC, ALC, MCH, MCHC, ALT, BUN, UA, CysC, TG, CHOL, LDLC, APOA, APOB, LPA, Hcy, FT3, FT4 and TSH as the independent variables, and the qualitative factors were included in the multivariate logistic regression model and model fitting by stepwise regression. Age, ALC, CysC, Hcy and TSH remained risk factors for abnormal renal function in gout patients; age, CysC, Hcy and TSH were risk factors for abnormal renal function in gout patients and ALC was a protective factor (Fig. 2).
Figure 2.
Logistic regression analysis of risk factors related to abnormal renal function in patients with gout. Age, cystatin C (CysC), homocysteine (Hcy), and thyroid stimulating hormone (TSH) were risk factors for gout with abnormal renal function; and absolute lymphocyte count (ALC) was a protective factor. The included variables were gender, age, haemoglobin (HGB), platelet count (PLT), neutrophil percentage (NE%), percentage lymphocytes (LY%), percentage monocytes (MO%), absolute neutrophil count (ANC), ALC, mean red cell haemoglobin content (MCH), mean cell haemoglobin concentration (MCHC), alanine aminotransferase (ALT), blood urea nitrogen (BUN), serum urate (UA), CysC, triglycerides (TG), cholesterol (CHOL), low-density lipoprotein cholesterol (LDLC), apolipoprotein A (APOA), apolipoprotein B (APOB), lipoprotein A (LPA), Hcy, serum-free triiodothyronine (FT3), serum-free tetraiodothyronine (FT4) and TSH
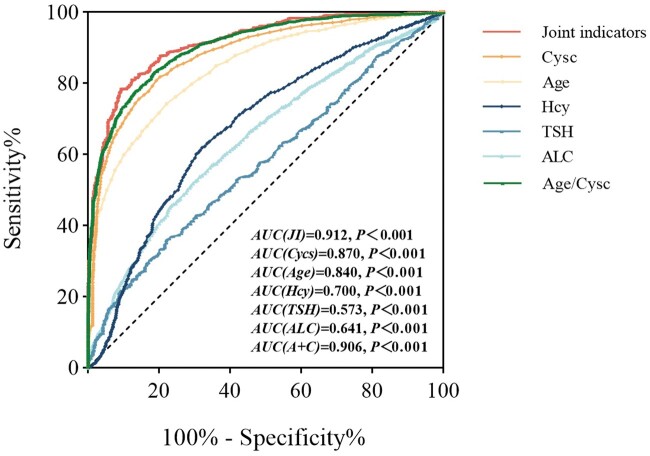
After applying age, ALC, CysC, Hcy and TSH individually or in combination for the identification of renal dysfunction in gout patients, the combined prediction had greater value. The area under the ROC curve (AUC) was 0.912, with a sensitivity of 77.5% and a specificity of 90.9% (Fig. 3).
Figure 3.
Receiver operating characteristic (ROC) curves predicted by age, absolute lymphocyte count (ALC), cystatin C (CysC), homocysteine (Hcy) and thyroid stimulating hormone (TSH) alone and jointly. The combined prediction of age, ALC, CysC, Hcy and TSH yielded an area under the ROC curve (AUC) of 0.912, with a sensitivity of 77.5% and specificity of 90.9%. The AUC for CysC was 0.87, for age was 0.84, for Hcy was 0.7, for TSH was 0.573 and for ALC was 0.641
Risk factor analysis of gout patients with renal function abnormalities in subgroups with different UA levels
The gout patients were divided into four groups according to the quartiles of their UA levels, and the average eGFRs in the Q1–Q4 groups were 95.07 ml/min/1.73 m2, 90.06 ml/min/1.73 m2, 88.79 ml/min/1.73 m2, and 85.21 ml/min/1.73 m2, respectively. The analysis results revealed that the eGFR of the Q1 group was significantly greater than that of the other three groups and that the eGFR of the Q4 group was significantly lower than that of the other three groups (P < 0.001). There was no significant difference in the eGFR between the Q2 and Q3 groups (P > 0.05). The proportions of patients with abnormal renal function in the Q1–Q4 groups were 35.2%, 45.2%, 47% and 51.1%, respectively. The χ2 results revealed that the proportion of patients with abnormal renal function in the Q1 group was significantly lower than that in the Q2, Q3 and Q4 groups (P < 0.001). There were no significant differences among the Q2, Q3 and Q4 groups (P > 0.05). The proportion of patients with abnormal renal function generally increased with increasing UA levels (Fig. 4).
Figure 4.
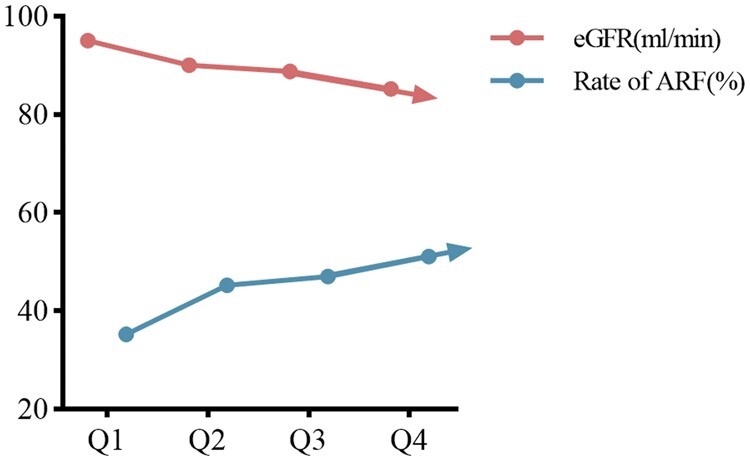
Proportion of abnormal renal function (ARF) at different serum urate levels and trend plot of mean eGFR level. The proportion of ARF increases with serum urate levels. Rate of ARF: proportion of abnormal renal function; eGFR: estimated glomerular filtration rate
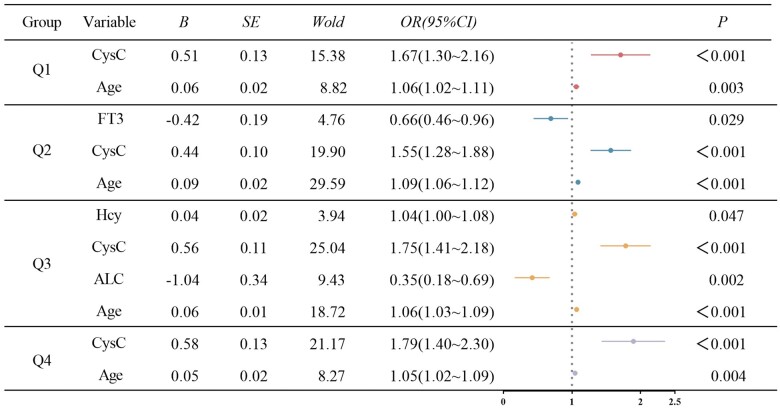
According to the univariate results and excluding collinearity indicators, multivariate binary logistic regression analysis was conducted for the Q1–Q4 groups. The results revealed that age and CysC were risk factors for gout combined with abnormal renal function in the Q1 group. In the Q2 group, age and CysC were risk factors for gout combined with abnormal renal function, and FT3 was a protective factor. In the Q3 group, age, CysC and Hcy were risk factors for gout associated with abnormal renal function, and ALC was a protective factor. In the Q4 group, age and CysC were risk factors for gout combined with abnormal renal function (Fig. 5).
Figure 5.
Logistic regression analysis of risk factors related to renal function abnormalities in patients with gout with different serum urate levels. Age and cystatin C (CysC) were risk factors for gout combined with abnormal renal function in the Q1 group. In the Q2 group, age and CysC were risk factors for gout combined with abnormal renal function, and serum-free triiodothyronine (FT3) was a protective factor. In the Q3 group, age, CysC and homocysteine (Hcy) were risk factors for gout associated with abnormal renal function, and absolute lymphocyte count (ALC) was a protective factor. In the Q4 group, age and CysC were risk factors for gout combined with abnormal renal function. Variables include: gender, age, haemoglobin (HGB), platelet count (PLT), neutrophil percentage (NE%), percentage lymphocytes (LY%), percentage monocytes (MO%), absolute neutrophil count (ANC), ALC, mean corpuscular haemoglobin content (MCH), mean corpuscular haemoglobin concentration (MCHC), alanine transaminase (ALT), blood urea nitrogen (BUN), serum urate (UA), cystatin C (CysC), triglycerides (TG), cholesterol (CHOL), low-density lipoprotein cholesterol (LDLC), apolipoprotein A (APOA), apolipoprotein B (APOB), lipoprotein A (LPA), Hcy, FT3, serum-free tetraiodothyronine (FT4) and thyroid stimulating hormone (TSH)
Discussion
In recent years, the prevalence and incidence of gout have been increasing annually, and the age trend indicates that the average age of patients with gout is decreasing, which places a heavy economic burden on patients [3, 16]. Gout affects the function of joints, and abnormal UA metabolism and persistent hyperuricaemia are associated with the occurrence of multiple types of systemic damage [3]. In the pathogenesis of gout, renal damage is highly prevalent, and some studies have shown that at autopsy, almost all patients with gout exhibit some degree of renal damage, including glomerular sclerosis, arteriolar sclerosis, and interstitial fibrosis [17]. However, in the early stage of renal function impairment, patients often have no specific manifestations [8]. In this study, an eGFR <90 ml/min/1.73 m2 was defined as abnormal renal function, which is highly important for the early prevention of CKD.
Studies have confirmed that the relationship between gout and the risk of developing CKD is bidirectional, and although reduced renal function can precede the development of gout, gout can also have adverse effects on renal function [18, 19]. Our results revealed that the proportion of patients with renal function abnormalities reached 45.4%, and the proportion of patients with renal function abnormalities gradually increased with age.
The causes of abnormal renal function in gout patients in the present study may be related to the following factors: (1) increased blood UA levels, UA excretion aggravating the kidney burden, or the deposition of urate crystals in the kidney, causing kidney stones, interstitial nephritis and acute and chronic renal failure; (2) kidney injury may be mediated through the activation of the renin–angiotensin–aldosterone system, which can mediate oxidative stress, cause mitochondrial dysfunction, promote vascular smooth muscle proliferation, damage endothelial cells, affect renal cell phenotypic transformation, stimulate inflammation and the immune response, and promote the occurrence and development of CKD [20]; (3) continuous hyperuricaemia promotes the development of hypertension, and hypertension further promotes kidney injury [21]. Current studies on the relationship between UA levels and renal function are not uniform, and some studies have shown a strong positive association between elevated UA levels and the risk of developing CKD [22, 23], which is a moderate independent risk factor for developing nephropathy in the general population in the USA [19]. Other studies have concluded that the opposite is true. Studies published in the New England Journal of Medicine in 2020 indicated that using allopurinol to lower UA levels does not have clinically significant benefits for kidney outcomes, including the progression of CKD [24–26]. Our study showed through univariate analysis that increased UA was associated with abnormal renal function in patients with gout, but after multivariate analysis, the UA level was no longer a risk factor for abnormal renal function in patients with gout. We further stratified the analysis of UA and showed that the proportion of patients with abnormal renal function in the Q4 group was significantly greater, whereas the proportion of patients with abnormal renal function in the Q1 group was significantly lower, suggesting that controlling UA at normal or slightly low (UA ≤ 360 µmol/l) levels may be more beneficial for protecting renal function.
Numerous studies have confirmed that age is a risk factor [27, 28]. Our study revealed that age was a risk factor for gout combined with abnormal renal function, which coincided with the findings of existing studies. Hcy is known to be a nonessential amino acid, an important intermediate formed by methionine metabolism, and is metabolized by remethylation and transsulfuration [29]. Clinical research indicates that increased Hcy is causally linked to renal impairment and further CKD [30–35]. Multivariate logistic regression analysis revealed that Hcy was a risk factor for abnormal renal function in gout patients. These findings suggest that damage can be indirectly predicted by analysing changes in Hcy levels during the diagnosis and treatment of gout.
CysC, a cysteine protease inhibitor in humans, is synthesized at a relatively constant rate within all nucleated cells and is freely filtered by the glomerulus, where it is reabsorbed and catabolic in the proximal tubule [36]. CysC is a sensitive indicator for the early diagnosis of renal damage [37–42]. In this study, the serum CysC level was a risk factor for gout combined with abnormal renal function, suggesting that dynamic observation of the serum CysC level could aid in the early diagnosis of abnormal renal function.
TSH, which is secreted by the adenohypophysis and is a key glycoprotein hormone regulated by the hypothalamic–pituitary–thyroid axis, is a sensitive indicator of thyroid function [43, 44]. It has been shown that increased TSH levels are associated with a decreased GFR and the prevalence of CKD [45–47]. Our results revealed that TSH is a risk factor for abnormal renal function in patients with gout, which is consistent with the above findings and suggests that TSH can be used as an important predictor of abnormal renal function in the clinical diagnosis and treatment of gout.
Previous studies have demonstrated a correlation between lymphocyte counts and the risk of developing CKD [48–50]. According to Kim and Kim, the ALC may be related to the progression of CKD and that a lower relative lymphocyte count is independently associated with the rapid progression of CKD [50]. Our study revealed that the ALC is independently and inversely associated with renal dysfunction in gout patients, suggesting that renal function can be indirectly predicted in gout patients by evaluating the ALC.
In our current study, we further analysed the relationships between renal function and relevant clinical laboratory indicators under different UA levels. The results revealed that the proportion of patients with abnormal renal function in the Q1 group was significantly lower than that in the Q2, Q3 and Q4 groups. Multivariate binary logistic regression analysis revealed that age and CysC were risk factors for gout combined with abnormal renal function in the Q1 group. In the Q2 group, age and CysC were risk factors for gout combined with abnormal renal function, and FT3 was a protective factor. In the Q3 group, age, CysC and Hcy were risk factors for gout associated with abnormal renal function, and the ALC was a protective factor. In the Q4 group, age and CysC were risk factors for gout combined with abnormal renal function. The results of this study further suggested that the proportion of patients with abnormal renal function increased significantly with increasing UA levels and that age and CysC were always independent risk factors associated with gout at different UA levels. These results suggest that in the clinical diagnosis and treatment of gout, we should pay close attention to changes in CysC levels in gout patients, especially in elderly individuals and those with a long course of gout. Active UA-lowering and treatment standards should be used to minimize renal damage due to gout.
In conclusion, our study revealed that sex, age, HGB, PLT, NE%, ALC, MONO, EOS, MCH, MCHC, ALT, BUN, UA, CysC, TG, CHOL, LDLC, LPA, Hcy, FT3, FT4 and TSH were associated with abnormal renal function in patients with gout; age, Hcy, CysC and TSH were found to be independent risk factors for renal abnormalities in patients with gout; and the ALC was found to be an independent protective factor against renal abnormalities in patients with gout. Moreover, this study revealed that the indicators associated with abnormal renal function varied at different UA levels. This finding indicates that the UA level of gout patients fluctuates between 360 mmol/l and 600 mmol/l, which may require clinicians to pay more attention to the multisystemic laboratory examination of patients to comprehensively judge renal function, identify renal function abnormalities in a timely manner and reasonably protect renal function. However, this study has several limitations. First, this was a cross-sectional study in which all the indicators were measured only once; therefore, causality could not be verified, and the results were inevitably disturbed by experimental instruments, human factors and some confounding bias. Second, this was a single-centre study subject to selection bias. Third, the study included only age, sex and important laboratory indicators, whereas BMI, smoking status, diet structure and alcohol intake were not included. Fourth, in the stratified multivariate binary logistic regression analysis, some sample sizes were too small because of the absence of some indicators. Therefore, based on this study’s findings, a more advanced prospective study can be carried out in the future, which is expected to further clarify the risk factors for abnormal renal function in patients with gout.
Supplementary Material
Acknowledgements
This study was made possible by the collaborative efforts of doctors, nurses and administrators at the recruiting hospital. We thank everyone who contributed their time and expertise.
Contributor Information
Ting Zhang, Department of Rheumatology and Immunology, Clinical Medical College, The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, PR China.
Ziqian Zeng, Department of Epidemiology and Health Statistics, School of Public Health, Chengdu Medical College, Chengdu, Sichuan, PR China.
Dan Xu, Clinical Medical College, The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, PR China.
Wantai Dang, Department of Rheumatology and Immunology, Clinical Medical College, The First Affiliated Hospital of Chengdu Medical College, Chengdu, Sichuan, PR China.
Supplementary material
Supplementary material is available at Rheumatology Advances in Practice online.
Data availability
Data are available upon reasonable request to the corresponding author. All data relevant to this study are included in the article.
Funding
This work was supported by the Basic Application Research Project of Sichuan Science and Technology Department (No. 2022NSFSC0692).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Wu M, Tian Y, Wang Q, Guo C.. Gout: a disease involved with complicated immunoinflammatory responses: a narrative review. Clin Rheumatol 2020;39:2849–59. doi: 10.1007/s10067-020-05090-8. [DOI] [PubMed] [Google Scholar]
- 2. Blagojevic-Bucknall M, Mallen C, Muller S. et al. The risk of gout among patients with sleep apnea: a matched cohort study. Arthritis Rheumatol 2019;71:154–60. doi: 10.1002/art.40662. [DOI] [PubMed] [Google Scholar]
- 3. Dehlin M, Jacobsson L, Roddy E.. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol 2020;16:380–90. doi: 10.1038/s41584-020-0441-1. [DOI] [PubMed] [Google Scholar]
- 4. Juraschek SP, Kovell LC, Miller ER 3rd, Gelber AC.. Association of kidney disease with prevalent gout in the United States in 1988-1994 and 2007-2010. Semin Arthritis Rheum 2013;42:551–61. doi: 10.1016/j.semarthrit.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan VS, Garg AX, McArthur E. et al. The 3-year incidence of gout in elderly patients with CKD. Clin J Am Soc Nephrol 2017;12:577–84. doi: 10.2215/CJN.06790616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep 2012;14:179–88. doi: 10.1007/s11926-012-0240-z. [DOI] [PubMed] [Google Scholar]
- 7. Russo E, Viazzi F, Pontremoli R, Working Group on UricAcid and Cardiovascular Risk of the Italian Society of Hypertension et al. Association of uric acid with kidney function and albuminuria: the Uric Acid Right for heArt Health (URRAH) Project. J Nephrol 2022;35:211–21. doi: 10.1007/s40620-021-00985-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forbes A, Gallagher H.. Chronic kidney disease in adults: assessment and management. Clin Med (Lond) 2020;20:128–32. doi: 10.7861/clinmed.cg.20.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99:S1–87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 10. Neogi T, Jansen TLTA, Dalbeth N. et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol 2015;67:2557–68. doi: 10.1002/art.39254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. August P. Chronic kidney disease–another step forward. N Engl J Med 2023;388:179–80. doi: 10.1056/NEJMe2215286. [DOI] [PubMed] [Google Scholar]
- 12. Webster A, Nagler E, Morton R, Masson P.. Chronic kidney disease. Lancet 2017;389:1238–52. doi: 10.1016/s0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Ingelfinger JR, Grams ME, Inker LA.. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med 2022;386:2120–8. doi: 10.1056/NEJMra2201153. [DOI] [PubMed] [Google Scholar]
- 14. Das SK, Roy DK, Chowdhury AA. et al. Correlation of eGFR by MDRD and CKD-EPI formula with creatinine clearance estimation in CKD patients and healthy subjects. Mymensingh Med J 2021;30:35–42. [PubMed] [Google Scholar]
- 15. Michels WM, Grootendorst DC, Verduijn M. et al. Performance of the Cockcroft-Gault, MDRD, and New CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5:1003–9. doi: 10.2215/cjn.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rai SK, Burns LC, De Vera MA. et al. The economic burden of gout: a systematic review. Semin Arthritis Rheum 2015;45:75–80. doi: 10.1016/j.semarthrit.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 17. Johnson RJ, Nakagawa T, Jalal D. et al. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant 2013;28:2221–8. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jing J, Kielstein JT, Schultheiss UT, GCKD Study Investigators et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant 2015;30:613–21. doi: 10.1093/ndt/gfu352. [DOI] [PubMed] [Google Scholar]
- 19. Kielstein JT, Pontremoli R, Burnier M.. Management of hyperuricemia in patients with chronic kidney disease: a focus on renal protection. Curr Hypertens Rep 2020;22:102. doi: 10.1007/s11906-020-01116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balakumar P, Alqahtani A, Khan NA, Mahadevan N, Dhanaraj SA.. Mechanistic insights into hyperuricemia-associated renal abnormalities with special emphasis on epithelial-to-mesenchymal transition: pathologic implications and putative pharmacologic targets. Pharmacol Res 2020;161:105209. doi: 10.1016/j.phrs.2020.105209. [DOI] [PubMed] [Google Scholar]
- 21. Sato Y, Feig DI, Stack AG. et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 2019;15:767–75. doi: 10.1038/s41581-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 22. Kumagai T, Ota T, Tamura Y. et al. Time to target uric acid to retard CKD progression. Clin Exp Nephrol 2017;21:182–92. doi: 10.1007/s10157-016-1288-2. [DOI] [PubMed] [Google Scholar]
- 23. Weiner DE, Tighiouart H, Elsayed EF. et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol 2008;19:1204–11. doi: 10.1681/asn.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jordan DM, Choi HK, Verbanck M. et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 2019;16:e1002725. doi: 10.1371/journal.pmed.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badve SV, Pascoe EM, Tiku A, CKD-FIX Study Investigators et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 2020;382:2504–13. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 26. Doria A, Galecki AT, Spino C, PERL Study Group et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020;382:2493–503. doi: 10.1056/NEJMoa1916624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Xu X, Zhang M. et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med 2023;183:298–310. doi: 10.1001/jamainternmed.2022.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalbeth N, Gosling AL, Gaffo A, Abhishek A.. Gout. Lancet 2021;397:1843–55. doi: 10.1016/s0140-6736(21)00569-9. [DOI] [PubMed] [Google Scholar]
- 29. Esse R, Barroso M, Tavares de Almeida I, Castro R.. The contribution of homocysteine metabolism disruption to endothelial dysfunction: state-of-the-art. Int J Mol Sci 2019;20:867. doi: 10.3390/ijms20040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen E, Margalit I, Shochat T, Goldberg E, Krause I.. The relationship between the concentration of plasma homocysteine and chronic kidney disease: a cross sectional study of a large cohort. J Nephrol 2019;32:783–9. doi: 10.1007/s40620-019-00618-x. [DOI] [PubMed] [Google Scholar]
- 31. Xiong Y, Zhang Y, Zhang F. et al. Genetic evidence supporting the causal role of homocysteine in chronic kidney disease: a Mendelian randomization study. Front Nutr 2022;9:843534. doi: 10.3389/fnut.2022.843534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chao M-C, Hu S-L, Hsu H-S. et al. Serum homocysteine level is positively associated with chronic kidney disease in a Taiwan Chinese population. J Nephrol 2014;27:299–305. doi: 10.1007/s40620-013-0037-9. [DOI] [PubMed] [Google Scholar]
- 33. Zhang S, Zhang Y, Zhang X. et al. Nitrative stress-related autophagic insufficiency participates in hyperhomocysteinemia-induced renal aging. Oxid Med Cell Longev 2020;2020:4252047–11. doi: 10.1155/2020/4252047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi ST, Kim JS, Song J-S.. Elevated serum homocysteine levels were not correlated with serum uric acid levels, but with decreased renal function in gouty patients. J Korean Med Sci 2014;29:788–92. doi: 10.3346/jkms.2014.29.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slot O. Homocysteine, a marker of cardiovascular disease risk, is markedly elevated in patients with gout. Ann Rheum Dis 2013;72:457. doi: 10.1136/annrheumdis-2012-202023. [DOI] [PubMed] [Google Scholar]
- 36. Siklos M, BenAissa M, Thatcher GR.. Cysteine proteases as therapeutic targets: does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharm Sin B 2015;5:506–19. doi: 10.1016/j.apsb.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang H, Zhao Y, Tan C, Liu Y.. Significance of serum markers and urinary microalbumin in the diagnosis of early renal damage in patients with gout. Clin Lab 2021;67. doi: 10.7754/Clin.Lab.2020.200722. [DOI] [PubMed] [Google Scholar]
- 38. Spencer S, Desborough R, Bhandari S.. Should cystatin C eGFR become routine clinical practice? Biomolecules 2023;13:1075. doi: 10.3390/biom13071075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shlipak MG, Katz R, Sarnak MJ. et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 2006;145:237–46. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 40. Gharaibeh KA, Hamadah AM, El-Zoghby ZM. et al. Cystatin C predicts renal recovery earlier than creatinine among patients with acute kidney injury. Kidney Int Rep 2018;3:337–42. doi: 10.1016/j.ekir.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shlipak MG, Sarnak MJ, Katz R. et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 42. Ix JH, Shlipak MG, Chertow GM, Whooley MA.. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation 2007;115:173–9. doi: 10.1161/circulationaha.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yazdaan HE, Jaya F, Sanjna F. et al. Advances in thyroid function tests: precision diagnostics and clinical implications. Cureus 2023;15:e48961. doi: 10.7759/cureus.48961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duan J, Xu P, Luan X. et al. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature 2022;609:854–9. doi: 10.1038/s41586-022-05173-3. [DOI] [PubMed] [Google Scholar]
- 45. Gopinath B, Harris DC, Wall JR, Kifley A, Mitchell P.. Relationship between thyroid dysfunction and chronic kidney disease in community-dwelling older adults. Maturitas 2013;75:159–64. doi: 10.1016/j.maturitas.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 46. Toda A, Hara S, Kato M, Tsuji H, Arase Y.. Association of thyrotropin concentration with chronic kidney disease in a Japanese general population cohort. Nephron 2019;142:91–7. doi: 10.1159/000497326. [DOI] [PubMed] [Google Scholar]
- 47. Kim HJ, Park SJ, Park HK. et al. Subclinical thyroid dysfunction and chronic kidney disease: a nationwide population-based study. BMC Nephrol 2023;24:64. doi: 10.1186/s12882-023-03111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K.. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr 2009;90:407–14. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agarwal R, Light RP.. Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol 2011;6:1393–9. doi: 10.2215/cjn.10521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim SM, Kim HW.. Relative lymphocyte count as a marker of progression of chronic kidney disease. Int Urol Nephrol 2014;46:1395–401. doi: 10.1007/s11255-014-0687-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to the corresponding author. All data relevant to this study are included in the article.