Abstract
Selenium is an essential trace element for human health. To date, a hotspot of functional foods is strengthening the content of organic Se in food using biological Se enrichment. Herein, Se-enriched rice pancakes were produced by directly adding different sodium selenite concentrations into the fermentation process. The effects of sodium selenite addition on the texture properties, structure, and Se species of rice pancakes were investigated. Meanwhile, the bioaccessibility of Se and the changes of Se species in Se-enriched rice pancakes were determined by digestion experiments in vitro. The results showed significant differences in hardness, adhesiveness, chewiness, porosity, and flavor substances of Se-enriched rice pancakes after adding sodium selenite (p < 0.05). In Se-enriched rice pancakes, selenocystine (SeCys2) and methylselenocysteine (MeSeCys) are the main Se species. When sodium selenite was added at 3.3 μg/mL, the maximum values of SeCys2 and MeSeCys were 328.35 ± 33.43 and 311.11 ± 49.48 μg/kg, respectively. Se bioaccessibility was negatively correlated with sodium selenite content. The electronic nose results of Se-enriched rice pancakes showed that the sulfur compounds, nitrogen substances, alcohol substances, alkane substances, alcohols, aldehydes, and ketones in rice pancakes significantly increased following sodium selenite addition. The results can provide a significant basis for developing high efficiency Se-enriched fermented food and the processing of Se-enriched rice pancakes.
Keywords: Selenium-enriched rice pancake, Fermentation, Sodium selenite, Seleno-amino acid, Electronic nose
Graphical abstract
Highlights
-
•
TPA and X-ray CT were used to study the structure of Se-enriched rice pancakes.
-
•
SeCys2 and SeMeCys were the main Se species in Se-enriched rice pancakes.
-
•
High bioavailable Se content in Se-enriched rice pancake after in vitro digestion.
-
•
Se supplementation improved the content of flavor substances in rice pancakes.
1. Introduction
Selenium is an indispensable trace element for humans, animals, and plants. As the active center of various selenoproteins and selenoenzymes, Se plays a significant role in maintaining oxidation–reduction homeostasis (Zhang et al., 2020), cardiovascular and cerebrovascular disease prevention (Grosicka-Maciąg et al., 2012), and thyroid hormone metabolism regulation (Feng et al., 2021). Previous research has confirmed that Se intake can reduce a range of cancers due to the antioxidant role of Se in the very early preneoplastic onset (Tian et al., 2020). Human Se intake is derived from the daily diet, which is mainly provided by the soil through the food chain (Emmanuelle et al., 2012). However, among the residents in China, 39 %–61 % have a daily Se intake lower than the level recommended by the World Health Organization (WHO) (55 μg/day), and the upper limit that humans can tolerate is 400 μg/day (Dong et al., 2021). Therefore, seeking suitable sources of dietary Se supplementation and developing various Se-enriched functional foods on this basis to meet the daily Se intake needs of Chinese residents are significant.
The nutritional value of Se largely depends on the speciation and concentration of Se present or supplemented in food (Kieliszek & Błażejak, 2013). Se mainly exists in two forms, including inorganic and organic Se, among which inorganic Se has high toxicity and low bioavailability (Souza et al., 2022). The organic Se can be combined with functional macromolecules, which can produce a synergistic effect in antioxidant, antitumor, and other bioactivities, and has a high bioavailability (Nie et al., 2023). Converting inorganic Se into organic Se is performed using the following three methods: plant transformation, animal transformation, and microbial transformation (D'Amato et al., 2020). Compared with plant and animal conversion methods, microbial transformation is unaffected by seasonal and climatic factors, and is characterized by fast reproduction, short production cycle, high adaptability, and metabolic capacity (Eswayah et al., 2017). Alzate et al. (Alzate et al., 2008) investigated the preparation of Se-enriched food products through a fermentation process, showing that yeasts and lactic acid bacteria can accumulate inorganic Se and convert it into organic Se compounds. In Hubei Province, China, rice pancakes are a kind of food with a long history and strong local characteristics. They are made from rice flour and yeast by fermenting and steaming roast. The fermentation process of yeast in rice pancakes is an effective way for converting exogenous inorganic Se into bioavailable and easily absorbed organic Se.
Currently, there is limited research on the preparation of Se-enriched rice products through the direct addition of inorganic selenium during the fermentation process. Mainly focus on flour products. Nunzio et al. (di Nunzio et al., 2018) and Sanchez-Martinez et al. (Sánchez-Martínez et al., 2015) reported that selenium amino acids in yeast fermented bread after adding high concentrations of sodium selenite accounted for more than 80 % of the total selenium content. Nunzio et al. (di Nunzio et al., 2018) made Italian flatbread (piadina) by adding 5.66 mg of sodium selenite per kilogram of dough. The total content of bioaccessible selenium in organic form in piadina was increased, and led to protective effects counteracting oxidative damage in cultured cells. Sanchez-Martinez et al. (Sánchez-Martínez et al., 2015) added selenite directly to dough to prepare white and whole wheat bread with 100 % and 40 % selenium bioaccessibility, respectively. SeMet is the main selenium species found in Se-enriched bread. Lazo-Vélez et al. (Lazo-Vélez et al., 2013) reported that there were not statistical differences (p < 0.05) in yeast CO2 production and dough pH during the fermentation of sponge bread treated with different concentrations of sodium selenite (4.33, 7.83 and 23.8 mg Se/g),but the density of bread increased and the height is significantly reduced. Du et al. (Du et al., 2023) added 0–80 mg Se/kg sodium selenite to flour to prepare steamed breads, As the Se content increased, the steamed bread showed a more homogeneous crumb structure with an improved number and area of stomata cells. Although yeast fermentation can effectively transform inorganic selenium into organic selenium, the effect of exogenous selenium on the quality of fermented rice products is still inconclusive. Wang et al. (Wang et al., 2021) directly added sodium selenite in the fermentation process of Pleurotus eryngii, and found that the contents of alcohols, aldehydes and ketones flavor compounds in Se-enriched Pleurotus eryngii decreased, while the contents of esters and furans increased significantly. The results indicated that the addition of sodium selenite would have a certain effect on the flavor characteristics of fermented food.
This study designed three sodium selenite addition amounts (1.1, 2.2, and 3.3 μg/mL) based on the daily safe range of selenium. Se-enriched rice pancakes were obtained by adding sodium selenite to the rice slurry before fermentation. To investigate the effects of different concentrations of sodium selenite treatment on the structural characteristics and volatile flavor components of rice pancakes, the texture characteristics, microstructure, and electronic nose of Se-enriched rice pancakes were analyzed. Meanwhile, exploring the effects of sodium selenite treatment on the total Se content, Se species, and bioaccessibility in Se-enriched rice pancakes through in vitro gastrointestinal simulation tests. This study comprehensively evaluated the effect of sodium selenite on the quality of Se-enriched rice pancake, and provided a basis for developing Se-enriched fermented rice products.
2. Methods
2.1. Materials and reagents
Rice flour (Erawan brand, Cho Heng Rice Vermicelli Factory Co., Ltd.) and Saf-instant dry yeast (Lesaffre) were purchased from local supermarkets in Hubei Province, China; sodium selenite, nitric acid, hydrochloric acid, artificial saliva, artificial gastric juice, artificial intestinal juice, alpha-amylase, pepsin, pancreatic enzyme, protease E, and protease K were all purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China); and standards used in the analysis of Se species, including Se (IV), Se (VI), SeMet, SeCys2, and SeMeCys, were obtained from the National Institute of Metrology (Beijing, China).
2.2. Preparation of Se-enriched rice pancakes
First, prepare 1.1, 2.2, and 3.3 μg/mL sodium selenite solutions and subsequently mix with rice flour at a material–liquid ratio of 1:1 to prepare rice slurry. After adding 1 % yeast and 5 % sugar, fermentation was performed in a constant temperature incubator at 30 °C for 6 h. After fermentation, 10 % white sugar and 0.2 % baking soda were added to the rice slurry, which was poured into the film and baked in a pan for 7 min to produce Se-enriched rice pancakes. FX-1, FX-2, and FX-3 represent Se-enriched rice pancakes with the addition of sodium selenite at 1.1, 2.2, and 3.3 μg/mL concentrations, respectively. Meanwhile, rice pancakes without sodium selenite were prepared as a blank control group.
2.3. Texture analysis of Se-enriched rice pancakes
The method of (Dou et al., 2023) was followed, with some modifications. The TPA of rice pancake samples was performed using a texture analyzer (Rapid TA; Shanghai Tengba Instrument Technology Co., Ltd., Shanghai, China), and the samples (Φ 7 mm)were evaluated using a cylindrical probe (P/36R). During each test, the samples were directly placed under the probe and were successively examined by two 50 % deformation compression tests. The following were the detecting parameters: Pretest speed, 2.0 mm/s; test speed, 1.0 mm/s; posttest speed, 1.0 mm/s; compression height, 20 mm; trigger force, 5.0 g; and interval between two compressions, 5 s.
2.4. X-ray computed tomography
The method of (Ghodki et al., 2019) was followed, with some modifications. The microstructure of rice pancake samples was analyzed using an X-ray CT system (NAOMI-CT 002 L, RF Co., Nagano, Japan). The following were the scanning parameters: imaging area, Φ146 × 153–185 mm; voxel number, 900 × 900 × 1100; gray scale range, approximately −32,768 to 32,767 (16 bits); and scanning time, 120 s.
2.5. Determination of the total colony count
Collect samples at 0, 3, and 6-h intervals and prepare diluted sample suspensions of 10−2, 10−3, and 10−4. Transfer 1 mL of the sample suspension to sterile petri dishes and simultaneously transfer 1 mL of blank diluted solution to two other sterile petri dishes as a blank control. Immediately pour 15–20 mL of plate counting agar culture medium into the dishes and rotate them to ensure even mixing. Allow the agar to solidify horizontally by leveling the dishes before inverting them for incubation at 36 °C for a period of 48 h.
2.6. Electronic nose analysis
cNose-18 electronic nose (Shanghai Baosheng Industrial Development Co., Ltd.) was used to analyze the overall odor characteristics of the rice pancake samples (Jeong et al., 2022). The 3.0 g Se-enriched rice pancake sample was put into a headspace bottle and heated in a metal bath at 50 °C for 10 min. The electronic nose probe was inserted into the headspace bottle, and volatile substances were analyzed and determined by suction of the top gas. The following were the parameters used: cleaning duration, 120 s; injection time, 150 s; and injection speed, 0.4 L/min.
2.7. Total Se content determination
The determination of total Se content followed the method of (Huang et al., 2022), with slight modifications. Here, 0.1 g of the lyophilized sample and 7 mL of HNO3 were added to the digestion tank, which was subsequently put into the microwave digestion instrument (W-8000, Shanghai Yiyao Instrument Technology Development Co., Ltd., Shanghai, China). After digestion, the digestion tank was transferred to the 180 °C acid drive instrument to drive the solution to 1 mL. After the digestion tank was cooled to room temperature, to reduce Se (VI) to Se (IV), 5 ml 50 % HCL was added and gently shaken until the hydrochloric acid and digestion solution were fully mixed. Subsequently, it was transferred to the acid drive instrument and heated for approximately 50 min. After the reduction was completed and cooled to room temperature, the dissolved sample solution was diluted to 10 mL with 10 % HCL. The total Se content was determined using hydride atomic fluorescence spectrometry. The working conditions of atomic fluorescence spectrum were set as follows: negative high voltage, 300 V; atomizer height, 8 mm; lamp current, 80 mA; carrier gas, high purity argon; reading time, 16 s; and delay time, 5 s.
2.8. Determination of Se species
The determination of Se species content followed the method of (Dong et al., 2021), with slight modifications. The 0.5 g lyophilized sample was weighed and added with 5 mL Tris-HCl buffer (75 mmol/L, pH 7.5, containing 10-mg protease E and 10 mg protease K), and oscillated at 37 °C at 200 rpm for 24 h. Subsequently, the mixture was centrifuged at 5000 r/min at 4 °C for 10 min. The supernatant was collected and filtered through a 0.22 μm hydrophilic filter membrane. The filtered samples were injected into high performance liquid chromatography–inductively coupled plasma mass spectrometry (HPLC–ICP–MS), and 20 mmol of citric acid (pH 4, pH 6, and pH adjusted by ammonia) was used as the mobile phase for gradient elution at a flow rate of 1 mL/min. The sample injection volume was 10 μL. A Hamilton PRP-X100 (250 mm × 4.1 mm, 10 μm) anion exchange column was used for separation using HPLC, and the Se retention time was monitored using ICP–MS.
2.9. In vitro-simulated gastrointestinal digestion test
The method described by (Yang et al., 2023) was followed, with some modifications. At a material–liquid ratio of 1:1 (m/v), 10 mL of oral digestive juice was added to 10 g of lyophilized sample, and 0.3 g of α-amylase (>5 U/mg) was added after mixing and stirred in a 37 °C water bath for 2 min away from light. After simulated oral digestion, 20 mL of gastric digestive juice and 0.025 g of pepsin (>500 U/mg) were added to the sample, and the samples were stirred in a 37 °C water bath for 2 h in the dark, and the pH was maintained at 3.0 with 1 mol/L HCL. Following simulated stomach digestion, 40 mL of intestinal digestive juice, 0.3 g of pancreatic enzyme, and 0.32 g of bile salt were added to the sample. The samples were stirred in a 37 °C water bath for 2 h in the dark, and the pH was maintained at 7.0 with 1 mol/L NaOH. After sampling, the digestion was centrifuged at 6000 rpm at 4 °C for 10 min. The supernatant was used for the determination of total Se and Se species.
The Se bioaccessibility in Se-enriched rice pancakes was expressed as BA%, and the following was the calculation formula used:
| (1) |
where Se in GI is the Se contents (μg/kg) in Se-enriched rice pancakes in vitro gastrointestinal digestive juice.
2.10. Statistical analysis
Microsoft Excel 2016 was used for data processing, and the experimental results were expressed as “average ± standard deviation” (n = 3). IBM SPSS Statistics Version 19 (SPSS Inc., Chicago, IL, USA) was used for the significance test (one-way analysis of variance), and differences among mean values of each treatment were compared using Duncan's test. Statistical significance was determined at a p value of less than 0.05. Origin 2021 (OriginLab Corporation, MA, USA) was used for diagram plotting.
3. Results and discussion
3.1. Texture characteristics of Se-enriched rice pancakes
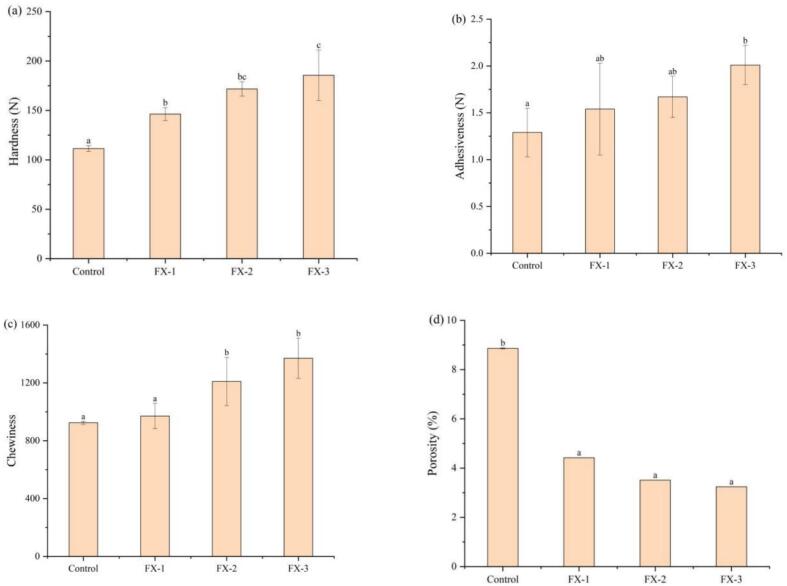
TPA can obtain more intuitive and quantifiable parameters than human sensory evaluation (Nishinari & Fang, 2018). Hardness refers to the force necessary for the sample to reach a certain deformation; in sensory sense, it refers to the force used to break the sample using the teeth. Springiness indicates the ratio of a deformed sample to its height or volume before deformation after recovering the deformation force. Chewiness indicates the energy required to chew the sample into a swallowing state and is numerically equal to hardness × chewiness × springiness (Paula & Conti-Silva, 2014). Based on the texture characteristics of rice pancakes (Table 1 and Figs. 1a, b, and c), FX-1, FX-2, and FX-3 had lower springiness and cohesiveness than the control group; however, no significant difference was noted among the three samples (p > 0.05). Compared with the control, the hardness, adhesiveness and chewiness of rice pancake significantly increased (p < 0.05) after adding sodium selenite. Moreover, the hardness, adhesiveness and chewiness increased with the increase in sodium selenite concentration. These results shown that adding sodium selenite has a certain effect on the texture properties of rice pancakes. However, no significant difference in the specific volume of rice pancakes after adding sodium selenite was noted (Table 1). Therefore, further analysis will be conducted on the basis of the microstructure parameters.
Table 1.
Texture and microstructure analysis of Se-enriched rice pancakes.
| Sample | Specific volume(ml/g) | Texture Microstructure parameters |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hardness (N) | Adhesiveness (N) | Cohesiveness \ | Springiness \ | Chewiness \ | Porosity (%) | Mean cell volume(mm3) | Mean cell area(mm2) | Cell number | ||
| Control | 1.37 ± 0.12ab | 111.44 ± 2.93a | 1.29 ± 0.26a | 0.83 ± 0.03c | 10 ± 0.00a | 924.34 ± 10.48a | 8.86 % ± 0.02b | 0.47 ± 0.13c | 2.20 ± 0.39b | 10,017.33 ± 2938.1a |
| FX-1 | 1.45 ± 0.02b | 146.30 ± 6.49b | 1.54 ± 0.49ab | 0.7 ± 0.00a | 9.63 ± 0.57a | 971.08 ± 87.52a | 4.42 % ± 0.003a | 0.35 ± 0.05bc | 1.84 ± 0.14b | 9892.67 ± 1242.21a |
| FX-2 | 1.39 ± 0.01ab | 171.73 ± 7.18bc | 1.67 ± 0.22ab | 0.77 ± 0.01b | 9.17 ± 1.41a | 1209.87 ± 166.90b | 3.51 % ± 0.003a | 0.19 ± 0.02a | 1.21 ± 0.07a | 9451 ± 830.14a |
| FX-3 | 1.30 ± 0.01a | 185.62 ± 25.45c | 2.01 ± 0.21b | 0.75 ± 0.01b | 9.82 ± 1.41a | 1370.07 ± 139.22b | 3.24 % ± 0.003a | 0.23 ± 0.02ab | 1.31 ± 0.10a | 7437.33 ± 93.55a |
Note: Results are presented as means ± standard deviation (n = 3). Values with different letters within each column are significantly different at a significance level of p < 0.05.
Fig. 1.
Structural characteristics of Se-enriched rice pancakes: (a) hardness; (b) adhesiveness; (c) chewiness; (d) porosity. Different lowercase letters indicate significant differences between samples treated with different concentrations of sodium selenite (p < 0.05).
3.2. Microstructure analysis of Se-enriched rice pancakes
To analyze the microstructure of rice pancake samples, X-ray CT was employed. The decrease in springiness and increase in hardness of baked products (e.g., muffins and bread) were correlated with the decrease in pore number (Baixauli et al., 2008), which was also observed in this study. The greater the hardness of Se-enriched rice pancakes, the smaller the number of pores (Table 1). As shown in Fig. 1d, the porosity of rice pancakes precipitously decreases after adding sodium selenite. The hardness of rice pancakes in the control group was the lowest, whereas the porosity was the largest, reaching 8.86 %. The difference in porosity between FX-1, FX-2, and FX-3 was not significant. Compared with the control, adding sodium selenite reduced the porosity by 50.11 %, 60.38 %, and 63.43 %, respectively. Additionally, the average pore volume of FX-1, FX-2, and FX-3 was significantly decreased (p < 0.05) by 25.53 %, 59.57 %, and 51.066 %, and the average pore surface area was decreased by 16.36 %, 45 %, and 40.45 %, respectively (Table 1).
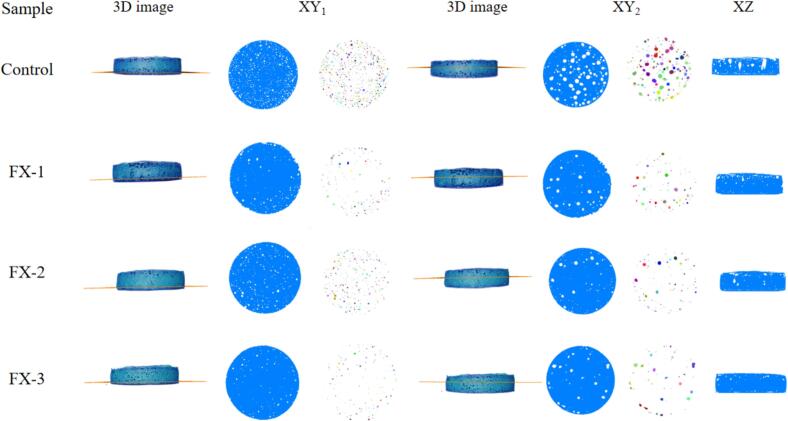
The pore structure inside the rice pancakes can be more intuitively observed from the X-ray CT scanned image (Fig. 2). Based on the three-dimensional images, the appearance of the rice pancakes was not very different. Therefore, a top view (XY1 and XY2) in the XY axis direction was taken at the positions with the number of slice layers of 20 and 60, respectively, and the center front view in the direction of XZ axis to observe the pore structure of the sample. The colorful images in XY1 and XY2 were two-dimensional slice images of separated pores. The pores were evenly distributed in the bottom layer and large pores in the top layer. With increased sodium selenite concentration, the colorful area gradually decreased, indicating the gradual decrease in the number of pores in rice pancakes. Moreover, it can be more clearly observed from the section diagram of XY2 and XZ that the large pores were evenly distributed in the upper layer of the rice pancake in a cylindrical form. In the control, upper layers exhibited the highest number of large pores, predominantly consisting of open pores that were fractured. This can be attributed to the thermal expansion of gas in the rice pancake during steaming roast, causing some large pores in the upper layer to rupture. Consequently, despite an increased pore count in the control group, there would be no significant elevation in rice pancake height. This observed phenomenon can indicate that although there was no statistically significant difference (p > 0.05) in specific volume between the control and experimental groups, there was a noticeable downward trend in porosity and average pore volume within the experimental group compared to the control group. A positive correlation was observed between the number of pores and the gas production capacity of yeast (Sun et al., 2023). Du et al. (Du et al., 2024) reported that the yeast gas production rate changed, the time to reach the maximum gas production rate was delayed, and the yeast gas production capacity was inhibited owing to the increase in Se concentrations. Therefore, the number of pores will gradually decrease with the increase in sodium selenite concentrations.
Fig. 2.
Comparison of 3D and 2D (XY-top view and XZ-Center front view) images of Se-enriched rice pancakes treated with different concentrations of sodium selenite. XY1 represents the top view taken at 20 slice layers;XY2 represents the top view taken at 60 slice layers.
3.3. Effect of sodium selenite on the total colony count during fermentation
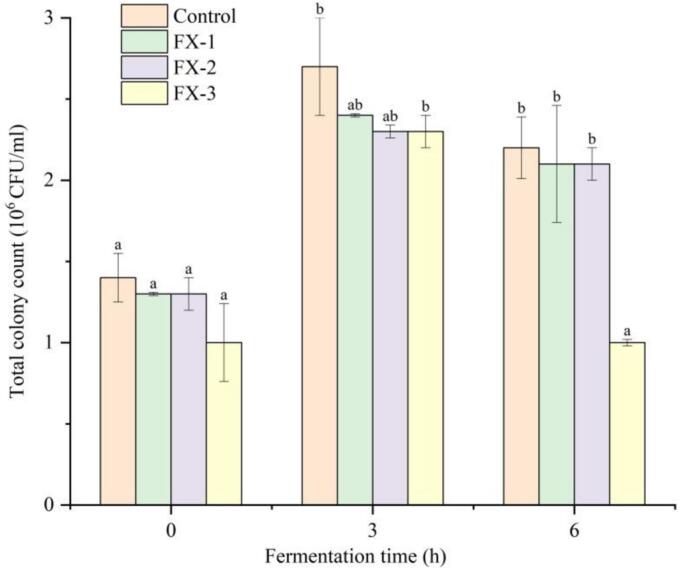
The fermentation of rice slurry was achieved through the addition of active dry yeast in this study. The change in total colony count serves as an indicator of microbial growth and reproduction during rice slurry fermentation. As depicted in Fig. 3, there was a rapid increase in total colony count at 3 h compared to 0 h for both the control group and all experimental groups, indicating vigorous microbial growth and favorable fermentation progress during the early stage. Towards the end of fermentation (6 h), the accumulation of metabolites, particularly ethanol, led to inhibition of microbial growth and a significant reduction in total colony count (Tofalo et al., 2020). There were no significant differences in total colonies among all groups at 0-h fermentation (p > 0.05). At 3-h fermentation, the control exhibited the highest total colonies at 2.7 × 106 CFU/mL. The FX-1, FX-2, and FX-3 had significantly lower total colonies than the control at this time point, showing a slight decreasing trend with increasing sodium selenite concentration. When the fermentation period was 6 h, there was a decrease in the total colonies. The control still exhibited a higher total colonies compared to all experimental groups, with no significant difference between FX-1 and FX-2 when compared to the control (p > 0.05). However, the total colonies in FX-3 significantly decreased (p < 0.05) to only 1.0 × 106 CFU/mL.
Fig. 3.
Effect of sodium selenite addition on the total colony count during rice slurry fermentation. Different lowercase letters indicate significant differences between samples treated with different concentrations of sodium selenite (p < 0.05).
These results indicate that the addition of sodium selenite can inhibit the growth of microorganisms, which is consistent with findings reported by Kieliszek et al. (Kieliszek et al., 2019), suggesting that an increase in selenium levels can have a certain effect on yeast growth and metabolic activity. Du et al. (Du et al., 2024) found that when the Se level was increased, the yeast gas production rate changed. Furthermore, there was a delay in reaching the maximum gas production rate. Se-enriched yeast could reduce the elasticity and viscosity modulus of dough. It could also prolong the time for the dough to reach its maximum height. It was mainly attributed to the fact that Se altered the yeast metabolic activity. In this study, the total number of colonies in rice slurry was inhibited by sodium selenite, indicating that sodium selenite inhibited the growth and gas production capacity of yeast in rice slurry. This result can further prove the phenomenon described in section 3.2 that with the increase of selenium level, the gas production capacity of yeast is inhibited, thus reducing the porosity and pore number of Se-enriched rice pancakes. Meanwhile, the texture characteristics of rice pancake were further affected.
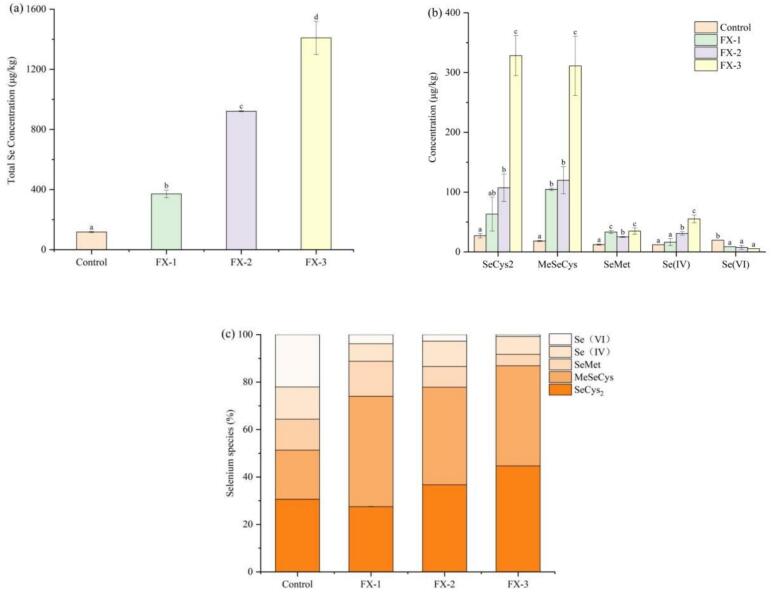
3.4. Total se content and se species of Se-enriched rice pancakes
The total Se contents of FX-1, FX-2, and FX-3 were 371.08, 921.94, and 1409.05 μg/kg, respectively (Fig. 4a). It increased by 2.16 fold, 6.85 fold, and 11.00 fold compared with the control group, respectively. SeCys2, MeSeCys, SeMet, Se(IV) and Se(VI) in Se-enriched rice pancakes were qualitatively and quantitatively analyzed using ICP-MS, and the difference of Se species in rice pancakes at different sodium selenite concentrations was analyzed. The Se species in Se-enriched rice pancakes is shown in Fig. 4b, wherein SeCys2 and MeSeCys show an increasing trend with increased sodium selenite concentrations. The maximum values of SeCys2 and MeSeCys in FX-3 were 328.35 μg/kg and 311.11 μg/kg, which were 11.12 fold and 16.00 fold of the control group, respectively. The SeMet content was small, with a maximum of 34.76 μg/kg (FX-3). Additionally, the inorganic Se content in the Se-enriched rice pancakes was very low. The Se(VI) content is significantly decreased with the increase in sodium selenite addition. Se-enriched food can be divided into natural and artificially Se-enriched food (Chen et al., 2023). Se in artificially enriched food is frequently present in the form of organic Se compounds, including selenocysteine (SeCys2), selenomethionine (SeMet), and methylselenocysteine (MeSeCys) (Han & Liu, 2022). However, Selenomethionine (SeMet) is the main form of Se in natural Se-enriched agricultural products (e.g., Se-enriched tea and Se-enriched rice) (Tangjaidee et al., 2022).
Fig. 4.
Concentrations of total selenium (a) and main selenium species (b) in rice pancakes with different concentration of sodium selenite. (c) The proportion of seleno-amino acids in Se-enriched rice pancakes treated with different concentrations of sodium selenite. Different lowercase letters indicate significant differences between samples treated with different concentrations of sodium selenite (p < 0.05).
The relative proportion of the five Se forms in Se-enriched rice pancakes treated with different sodium selenite concentrations is shown in Fig. 4c. After adding sodium selenite, the total relative proportion of the three seleno-amino acids in the sample was significantly increased. Compared with the control group, the SeCys2 relative proportion of FX-1 was not significantly different; however, the MeSeCys relative proportion was significantly increased. The relative proportion of seleno-amino acids of FX-3 was the largest, reaching 91.67 %. The proportions of Se(IV) and Se(VI) in FX-1, FX-2, and FX-3 were lower than those in the control, and the relative proportions of Se(IV) in FX-1, FX-2, and FX-3 gradually decreased to 3.85 %, 2.74 %, and 0.74 %, respectively. The results showed that exogenous inorganic Se could be fully transformed into available organic Se with high safety during rice pancake fermentation.
3.5. Bioaccessibility and species of Se following in vitro digestion
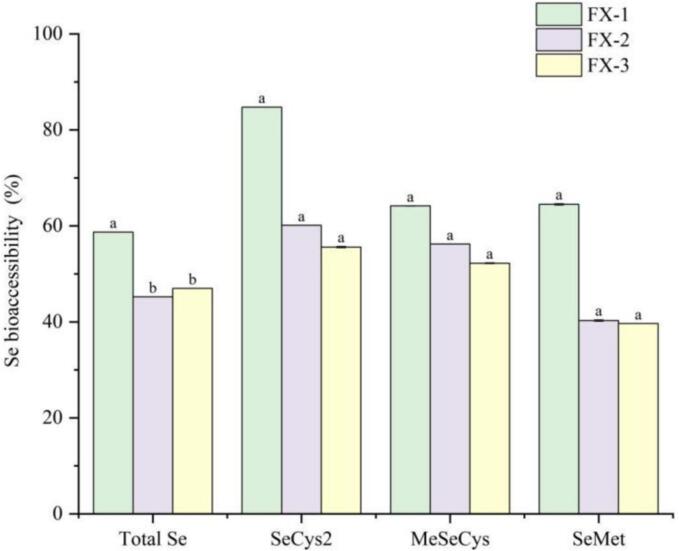
According to formula (1), the bioaccessibility of total Se and three seleno-amino acids in Se-enriched rice pancakes was calculated. As shown in Fig. 5, the bioaccessibility of total Se in FX-1, FX-2, and FX-3 was 58.72 %, 45.23 %, and 46.98 %, respectively. The bioaccessibility of three seleno-amino acids in FX-1 was the highest, particularly SeCyS2 (84.73 %), MeSeCys (64.16 %), and SeMet (64.49 %), respectively. The bioaccessibility of total Se and three seleno-amino acids decreased with the increase in sodium selenite addition. This is similar to the findings of Wang et al. (Wang et al., 2022), where the bioaccessibility of Se in wheat flour treated with high sodium selenite concentrations decreased compared with that treated with low sodium selenite concentrations. In vivo human tests show that Se levels in plasma or whole blood increased significantly after supplementation with Se-fortified wheat (Kirby et al., 2008). This indicates that Se-enriched foods can be absorbed by the human body after digestion.
Fig. 5.
Selenium bioaccessibility (%) of total selenium and main selenium species. Different lowercase letters indicate significant differences between samples treated with different concentrations of sodium selenite (p < 0.05).
The bioaccessibility of Se pertains to the portion released from the food matrix within the gastrointestinal tract (Jian et al., 2023), Bioavailable Se refers to the portion that reaches the blood further through effective absorption of the mucosa of the small intestine (Bodnar et al., 2016). In research conducted by Thiry et al. (Thiry, Ruttens, et al., 2013), the relationship between the bioaccessibility of Se in three Se-enriched food supplements after in vitro digestion and the bioavailability of Se following translocation through a monolayer of Caco-2 cells was examined. The findings indicated that the bioavailable fraction of Se constitutes a subpart of the bioaccessible Se, and the degree of Se absorption through the Caco-2 monolayer was strongly species-dependent, with seleno-amino acids demonstrating higher bioavailability. In rats, Se concentrations in blood and liver were reported to be up to four times higher following SeMet supplementation compared with selenite (Se(IV)) supplementation (Thiry, Schneider, et al., 2013). It shows that seleno-amino acids are more easily absorbed. These studies imply that the bioaccessibility of Se can be employed to initially characterize the digestion and absorption of Se in Se-enriched foods.
Although the bioaccessibility of Se decreased with the increase in sodium selenite concentrations, the content of bioavailable Se in the digestive supernatant of FX-1, FX-2, and FX-3 was significantly higher than that in the control group (p < 0.05). The contents of total Se and different Se species following simulated in vitro digestion are presented in Table 2. Compared with the control group, the total Se content following digestion in FX-1, FX-2, and FX-3 gradually increased, and the total Se content in the in vitro digestive supernatant of FX-3 reached 661.99 μg/kg. The bioavailable SeCys2 and MeSeCys in FX-3 reached 182.5 and 162.42 μg/kg, respectively. Furthermore, no seleno-amino acids were detected in the digestive supernatant in vitro owing to the low Se content in the control group. In all three experimental groups, the SeMet content following in vitro digestion was relatively low, and no significant difference was noted (10.1–21.45 μg/kg).
Table 2.
Concentrations of total selenium and main selenium species in Se-enriched rice pancakes and digestive supernatant.
| Sample | Se-enriched rice pancakes |
Supernatant after digestion in vitro |
||||||
|---|---|---|---|---|---|---|---|---|
| Total Se (μg/kg) | SeCyS2 (μg/kg) | MeSeCys (μg/kg) | SeMet (μg/kg) | Total Se (μg/kg) | SeCyS2 (μg/kg) | MeSeCys (μg/kg) | SeMet (μg/kg) | |
| Control | 117.43 ± 3.66a | 27.100 ± 0.00a | 18.300 ± 0.00a | 12.20 ± 0.99a | 71.20 ± 0.61a | ND | ND | ND |
| FX-1 | 371.08 ± 25.96b | 63.29 ± 28.17ab | 104.68 ± 1.92b | 33.26 ± 2.42c | 217.91 ± 14.46b | 53.62 ± 3.64a | 67.17 ± 7.56a | 21.45 ± 6.59a |
| FX-2 | 921.94 ± 3.76c | 107.29 ± 22.98b | 119.82 ± 22.72b | 25.08 ± 0.73b | 416.97 ± 7.82c | 64.51 ± 3.27a | 67.35 ± 3.96a | 10.1 ± 4.67a |
| FX-3 | 1409.05 ± 118.28d | 328.35 ± 33.43c | 311.11 ± 49.48c | 34.76 ± 5.11c | 661.99 ± 16.53d | 182.5 ± 60.97b | 162.42 ± 41.52b | 13.78 ± 1.53a |
Note: Results are presented as means ± standard deviation (n = 3). Values with different letters within each column are significantly different at a significance level of p < 0.05.
3.6. Analysis of electronic nose results of Se-enriched rice pancakes
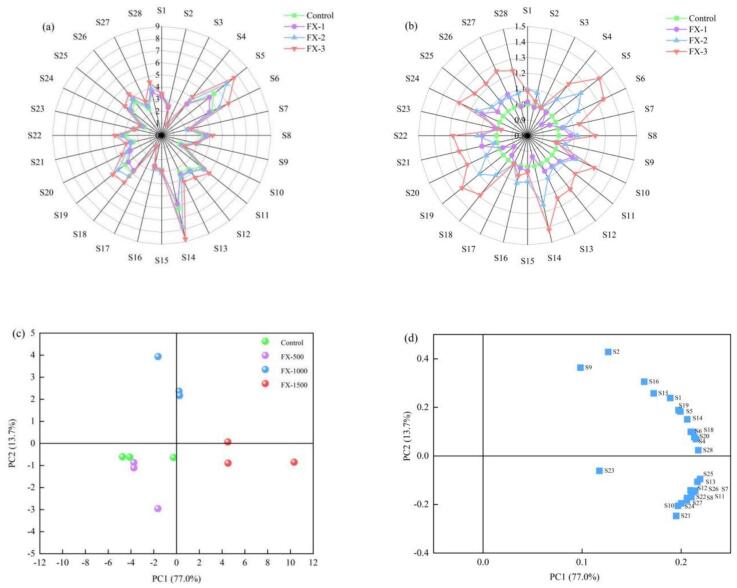
The whole odor analysis of Se-enriched rice pancakes was based on the electronic nose sensor array technology, with each sensor having different sensitivity to the measured gas. The response values of 28 electronic nose sensors to the volatile flavor substances of Se-enriched rice pancakes treated with different sodium selenite concentrations were presented in the form of a radar map. The greater the response value obtained by the sensor, the more flavor components were produced. As shown in Fig. 6a, samples corresponding to different sodium selenite concentrations show differences in flavor substances; however, no significant difference is noted in the overall radar profile, and the difference characterization of flavor substances among samples is not obvious. Therefore, all response values are normalized to obtain Fig. 6b, which demonstrates that with the increase in sodium selenite addition, 20 sensors (S4–S8, S10–S14, S18–S22, and S24–S28) show significant differences in the response values of volatile flavor substances of Se-enriched rice pancakes added with different sodium selenite concentrations. According to the electronic nose sensor performance in Table S1, the types of volatile flavor substances changing in Se-enriched rice pancakes are mainly concentrated in sulfides, nitrogen substances, alcohol substances, alkane substances, alcohols, aldehydes, and ketones, and they increase with increased sodium selenite concentrations.
Fig. 6.
Electronic nose radar map(a) and normalization (b) of Se-enriched rice pancakes with different concentrations of sodium selenite; PCA score diagram (c) and loading diagram (d) of flavor substances in Se-enriched rice pancakes treated with different concentrations of sodium selenite.
The results of the electronic nose were further analyzed using principal component analysis (PCA). The PCA score graph of flavor substances in Se-enriched rice pancakes treated with different sodium selenite concentrations is depicted in Fig. 6c, showing that the cumulative contribution rate of the two principal components, PC1 and PC2, reaches 90.7 %, indicating that the established PCA model can more completely reflect the flavor information of the sample. The control group and FX-1 are in the same quadrant, and some overlap is observed, indicating that the volatile flavor substances in these two groups of samples are similar, and the difference in volatile flavors is small. FX-2 and FX-3 are in different quadrants and far away from the other groups, indicating that FX-2 and FX-3 are significantly different from the other groups in terms of volatile flavor components. The contribution of volatile flavor substances to the flavor in Se-enriched rice pancakes can be effectively measured using the loading values shown in the PCA loading diagram (Fig. 6d). As shown in the figure, the flavor substances represented by S4, S13, S20, S25, and S28 have the largest contribution, including sulfides, alkanes, alcohols, ketones, and aldehydes, which are consistent with the results of electronic nose normalization.
4. Conclusion
Se-enriched rice pancakes had three main species of organic Se, among which SeCys2 and MeSeCys contents were positively correlated with sodium selenite addition. In FX-3, the contents of SeCys2 and MeSeCys reached 328.35 and 311.11 μg/kg, respectively. Rice pancake fermentation effectively transformed inorganic Se into organic Se, which was available to the body. The bioavailability of Se in Se-enriched rice pancakes treated with different sodium selenite concentrations was high; however, it tended to decrease with the increase in sodium selenite concentrations. Additionally, after adding sodium selenite, the structural characteristics and flavor substances of rice pancakes showed significant differences. The porosity and total colony count of rice pancakes gradually decreased with the increase in sodium selenite concentrations, and the hardness, adhesiveness, chewiness increased. Moreover, the flavor substances such as nitrogen, alcohols, aldehydes and ketones significantly enriched with the increase in sodium selenite concentrations. In summary, Se-enriched rice pancakes are a good source of dietary Se, which is expected to provide a new way to supplement Se for Se-deficient individuals.
CRediT authorship contribution statement
Feiran An: Writing – original draft, Validation, Methodology, Investigation, Formal analysis. Kun Zhuang: Writing – review & editing, Software, Methodology, Formal analysis. Lingling Shangguan: Supervision, Investigation. Lan Yao: Writing – review & editing. Jun Dai: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the National Natural Science Foundations of China [Grant Nos.31871789 and 41876114], the key project of Hubei Provincial Department of Education [No.T2022011], the Natural Science Foundation of Hubei Province [No. 2024AFB803], the Natural Science Foundation of Zhejiang Province [No. LY23D060005], and the Science Foundation of Donghai Laboratory [No. DH-2022KF0218].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.102064.
Appendix A. Supplementary data
Supplementary material
Data availability
The authors do not have permission to share data.
References
- Alzate A., Fernández-Fernández A., Pérez-Conde M.C., Gutiérrez A.M., Cámara C. Comparison of biotransformation of inorganic selenium by lactobacillus and saccharomyces in lactic fermentation process of yogurt and kefir. Journal of Agricultural and Food Chemistry. 2008;56:8728–8736. doi: 10.1021/jf8013519. [DOI] [PubMed] [Google Scholar]
- Baixauli R., Salvador A., Martínez-Cervera S., Fiszman S.M. Distinctive sensory features introduced by resistant starch in baked products. LWT - Food Science and Technology. 2008;41:1927–1933. doi: 10.1016/j.lwt.2008.01.012. [DOI] [Google Scholar]
- Bodnar M., Szczyglowska M., Konieczka P., Namiesnik J. Methods of selenium supplementation: Bioavailability and determination of selenium compounds. Critical Reviews in Food Science and Nutrition. 2016;56:36–55. doi: 10.1080/10408398.2012.709550. [DOI] [PubMed] [Google Scholar]
- Chen Z., Lu Y., Dun X., Wang X., Wang H. Research Progress of Selenium-Enriched Foods. Nutrients. 2023;15(19) doi: 10.3390/nu15194189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato R., Regni L., Falcinelli B., Mattioli S., Benincasa P., Dal Bosco A., Pacheco P., Proietti P., Troni E., Santi C., Businelli D. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. Journal of Agricultural and Food Chemistry. 2020;68:4075–4097. doi: 10.1021/acs.jafc.0c00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Liu Y., Dong G., Wu H. Effect of boiling and frying on the selenium content, speciation, and in vitro bioaccessibility of selenium-biofortified potato (Solanum tuberosum L.) Food Chemistry. 2021;348 doi: 10.1016/j.foodchem.2021.129150. [DOI] [PubMed] [Google Scholar]
- Dou X., Lv M., Ren X., He Y., Liu L., Zhang G.…Yang C.H. Test conditions of texture profile analysis for frozen dough. Italian Journal of Food Science. 2023;35:58–68. doi: 10.15586/ijfs.v35i4.2401. [DOI] [Google Scholar]
- Du C., Zhu S., Li Y., Yang T., Huang D. Selenium-enriched yeast, a selenium supplement, improves the rheological properties and processability of dough: From the view of yeast metabolism and gluten alteration. Food Chemistry. 2024;458 doi: 10.1016/j.foodchem.2024.140256. [DOI] [PubMed] [Google Scholar]
- Du C., Zhu S., Li Y., Yu R., Yang T., Huang D. Alterations in the multilevel structure and depolymerization behavior of gluten induced by selenium in fermented dough. Food Bioscience. 2023;56 doi: 10.1016/j.fbio.2023.103389. [DOI] [Google Scholar]
- Emmanuelle B., Virginie M., Fabienne S., Isabelle I., Martine P.-G., Bernard L., Sylvie R. Selenium exposure in subjects living in areas with high selenium concentrated drinking water: Results of a French integrated exposure assessment survey. Environment International. 2012;40:155–161. doi: 10.1016/j.envint.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Eswayah A.S., Smith T.J., Scheinost A.C., Hondow N., Gardiner P.H.E. Microbial transformations of selenite by methane-oxidizing bacteria. Applied Microbiology and Biotechnology. 2017;101:6713–6724. doi: 10.1007/s00253-017-8380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M., Wang X., Xiong H., Qiu T., Zhang H., Guo F., Jiang L., Sun Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264.7 macrophages via NF-κB/MAPKs signaling pathways. Journal of Functional Foods. 2021;76 doi: 10.1016/j.jff.2020.104320. [DOI] [Google Scholar]
- Ghodki B.M., Dadlani G., Ghodki D.M., Chakraborty S. Functional whole wheat breads: Compelling internal architecture. LWT. 2019;108:301–309. doi: 10.1016/j.lwt.2019.03.066. [DOI] [Google Scholar]
- Grosicka-Maciąg E., Grosicka-Maciąg E., Szumiło M., Kurpios-Piec D., Rahden-Staroń I. Biomedical effects of selenium in a human organism. Journal of Elementology. 2012;22:1269–1284. doi: 10.5601/jelem.2017.22.1.1357. [DOI] [Google Scholar]
- Han M., Liu K. Selenium and selenoproteins: Their function and development of selenium-rich foods. International Journal of Food Science & Technology. 2022;57:7026–7037. doi: 10.1111/ijfs.16096. [DOI] [Google Scholar]
- Huang Y., Fan B., Lei N., Xiong Y., Liu Y., Tong L., Wang F., Maesen P., Blecker C. Selenium biofortification of soybean sprouts: Effects of selenium enrichment on proteins, protein structure, and functional properties. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.849928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C.-H., Lee S.-H., Kim H.-Y. Microbiological composition and sensory characterization analysis of fermented sausage using strains isolated from Korean fermented foods. Food Science of Animal Resources. 2022;42:928–941. doi: 10.5851/kosfa.2022.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian F., Zhang Z., Li D., Luo F., Wu Q., Lu F., Gu Q. Evaluation of the digestibility and antioxidant activity of protein and lipid after mixing nuts based on in vitro and in vivo models. Food Chem. 2023;414:135706. doi: 10.1016/j.foodchem.2023.135706. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Błażejak S. Selenium: Significance, and outlook for supplementation. Nutrition. 2013;29:713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Błażejak S., Bzducha-Wróbel A., Kot A.M. Effect of selenium on growth and antioxidative system of yeast cells. Molecular Biology Reports. 2019;46:1797–1808. doi: 10.1007/s11033-019-04630-z. [DOI] [PubMed] [Google Scholar]
- Kirby J.K., Lyons G.H., Karkkainen M.P. Selenium speciation and bioavailability in biofortified products using species-unspecific isotope dilution and reverse phase ion pairing−inductively coupled plasma−mass spectrometry. Journal of Agricultural and Food Chemistry. 2008;56(5):1772–1779. doi: 10.1021/jf073030v. [DOI] [PubMed] [Google Scholar]
- Lazo-Vélez M.A., Gutiérrez-Díaz V.A., Ramírez-Medrano A., Serna-Saldívar S.O. Effect of sodium selenite addition and sponge dough fermentation on selenomethionine generation during production of yeast-leavened breads. Journal of Cereal Science. 2013;58(1):164–169. doi: 10.1016/j.jcs.2013.03.019. [DOI] [Google Scholar]
- Nie X., Yang X., He J., Liu P., Shi H., Wang T., Zhang D. Bioconversion of inorganic selenium to less toxic selenium forms by microbes: A review. Frontiers in Bioengineering and Biotechnology. 2023;11:1167123. doi: 10.3389/fbioe.2023.1167123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinari K., Fang Y. Perception and measurement of food texture: Solid foods. Journal of Texture Studies. 2018;49:160–201. doi: 10.1111/jtxs.12327. [DOI] [PubMed] [Google Scholar]
- di Nunzio M., Bordoni A., Aureli F., Cubadda F., Gianotti A. Sourdough fermentation favorably influences selenium biotransformation and the biological effects of flatbread. Nutrients. 2018;10(12) doi: 10.3390/nu10121898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula A.M., Conti-Silva A.C. Texture profile and correlation between sensory and instrumental analyses on extruded snacks. Journal of Food Engineering. 2014;121:9–14. doi: 10.1016/j.jfoodeng.2013.08.007. [DOI] [Google Scholar]
- Sánchez-Martínez M., Pérez-Corona T., Cámara C., Madrid Y. Preparation and characterization of a laboratory scale Selenomethionine-enriched bread. Selenium bioaccessibility. Journal of Agricultural and Food Chemistry. 2015;63(1):120–127. doi: 10.1021/jf505069d. [DOI] [PubMed] [Google Scholar]
- Souza S.O., Ávila D.V.L., Cerdà V., Araujo R.G.O. Selenium inorganic speciation in beers using MSFIA-HG-AFS system after multivariate optimization. Food Chemistry. 2022;367 doi: 10.1016/j.foodchem.2021.130673. [DOI] [PubMed] [Google Scholar]
- Sun X., Wu S., Li W., Koksel F., Du Y., Sun L., Fang Y., Hu Q., Pei F. The effects of cooperative fermentation by yeast and lactic acid bacteria on the dough rheology, retention and stabilization of gas cells in a whole wheat flour dough system – a review. Food Hydrocolloids. 2023;135 doi: 10.1016/j.foodhyd.2022.108212. [DOI] [Google Scholar]
- Tangjaidee P., Swedlund P., Xiang J., Yin H., Quek S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.962312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry C., Ruttens A., Pussemier L., Schneider Y.J. An in vitro investigation of species-dependent intestinal transport of selenium and the impact of this process on selenium bioavailability. The British Journal of Nutrition. 2013;109(12):2126–2134. doi: 10.1017/S0007114512004412. [DOI] [PubMed] [Google Scholar]
- Thiry C., Schneider Y.J., Pussemier L., de Temmerman L., Ruttens A. Selenium bioaccessibility and bioavailability in se-enriched food supplements. Biological Trace Element Research. 2013;152(1):152–160. doi: 10.1007/s12011-013-9604-0. [DOI] [PubMed] [Google Scholar]
- Tian J., Wei X., Zhang W., Xu A. Effects of selenium nanoparticles combined with radiotherapy on lung cancer cells. Frontiers in Bioengineering and Biotechnology. 2020;8 doi: 10.3389/fbioe.2020.598997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofalo R., Fusco V., Bohnlein C., Kabisch J., Logrieco A.F., Habermann D., Franz C. The life and times of yeasts in traditional food fermentations: A review. Critical Reviews in Food Science and Nutrition. 2020;60(18):3103–3132. doi: 10.1080/10408398.2019.1677553. [DOI] [PubMed] [Google Scholar]
- Wang B., Zhao N., Li J., Xu R., Wang T., Guo L., Wei X. Selenium-enriched Lactobacillus plantarum improves the antioxidant activity and flavor properties of fermented pleurotus eryngii. Food Chemistry. 2021;345 doi: 10.1016/j.foodchem.2020.128770. doi:j.foodchem.2020.128770. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhou F., Cheng N., Chen P., Ma Y., Zhai H.…Liang D. Soil and foliar selenium application: Impact on accumulation, speciation, and bioaccessibility of selenium in wheat (Triticum aestivum L.) Frontiers in Plant Science. 2022;13 doi: 10.3389/fpls.2022.988627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Hu Y., Yue P., Li H., Wu Y., Hao X., Peng F. Structure, stability, antioxidant activity, and controlled-release of selenium nanoparticles decorated with lichenan from Usnea longissima. Carbohydrate Polymers. 2023;299 doi: 10.1016/j.carbpol.2022.120219. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Roh Y.J., Han S.-J., Park I., Lee H.M., Ok Y.S.…Lee S.-R. Role of selenoproteins in redox regulation of signaling and the antioxidant system: A review. Antioxidants. 2020;9:383. doi: 10.3390/antiox9050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The authors do not have permission to share data.