Abstract
Purpose
To study the treatment and outcomes of children with retinoblastoma (RB) with extraocular tumor extension (RB-EOE) and compare them with RB without extraocular tumor extension (RB-w/o-EOE).
Design
Multicenter intercontinental collaborative prospective study from 2017 to 2020. RB-EOE cases included those with overt orbital tumor extension in treatment-naive patients. Cases with microscopic orbital extension detected postenucleation were excluded from the study.
Participants
A total of 319 children with RB-EOE and 3116 children with RB-w/o-EOE.
Intervention
Chemotherapy, enucleation, exenteration, radiotherapy.
Main Outcome Measures
Systemic metastasis and death.
Results
Of the 3435 RB patients included in this study, 309 (9%) were from low-income countries (LIC), 1448 (42%) from lower-middle income, 1012 (29%) from upper-middle income, and 666 (19%) patients from high-income countries. There was an inverse relationship between the percentage of RB-EOE and national income level, with 96 (31%) patients from LIC, 197 (6%) lower-middle income, 20 (2%) upper-middle income, and 6 (1%) patients from high-income countries (P = 0.0001). The outcomes were statistically significant for RB-EOE compared with RB-w/o-EOE: systemic metastasis (32% vs. 4% respectively; P = 0.0001) and metastasis-related death (63% vs. 6% respectively; P = 0.0001). Multimodal treatment was the most common form of treatment (n = 177; 54%) for RB-EOE, with most cases undergoing a combination of intravenous chemotherapy and enucleation (n = 97; 30%). Adjuvant external beam radiotherapy (EBRT) after surgery (enucleation/orbital exenteration) was given in only 68 (21%) cases. Kaplan–Meier analysis for systemic metastasis and metastasis-related death in RB-EOE was 28% and 57% at 1 year, 29% and 60% at 2 years, and 29% and 61% at 3 years, respectively. Cox regression analysis revealed that the risk of death from RB-EOE was greater in patients aged >4 years than <2 years (hazard ratio, 2.912; P < 0.001) and for unimodal (surgery or intravenous chemotherapy) and bimodal (surgery and intravenous chemotherapy) treatment than trimodal treatment (surgery, intravenous chemotherapy, and EBRT) (hazard ratio, 2.023; P = 0.004 and hazard ratio, 1.819; P = 0.027, respectively).
Conclusions
Retinoblastoma with extraocular tumor extension is associated with a higher risk of metastasis and death. Patients with RB-EOE are likely to benefit from trimodal treatment (intravenous chemotherapy, surgery, and EBRT) rather than treatment protocols excluding EBRT.
Financial Disclosure(s)
The authors have no proprietary or commercial interest in any materials discussed in this article.
Keywords: External beam radiotherapy, Extraocular extension, Multimodal treatment, Retinoblastoma, Tumor
Retinoblastoma (RB), the most common intraocular malignant tumor in children, is curable. However, the outcomes are not uniform across the world and differ based on various factors including national income level, Gini index, government health care financing and expenditure, education level of the population, percentage of population in rural areas, accessibility to health care, gender bias, social stigma, religious beliefs, age at presentation, and tumor stage at presentation.1, 2, 3, 4, 5, 6, 7, 8 A meta-analysis of 29 106 RB patients from 73 countries presenting during the period 1980 to 2020 revealed that the survival rates improved globally over the 4 decades, but the disparity in outcomes persisted based on the national income level.3 The survival rate of children with RB ranges from 87% to 99% in high-income countries (HIC), 71% to 92% in upper-middle income countries (UMIC), 43% to 90% in lower-middle income countries (LMIC), and 44% to 57% in low-income countries (LIC).2,3,6
When intraocular RB is left untreated, the natural progression of the disease is tumor extension beyond the globe via the optic nerve, sclera, and scleral emissary vessels, thus increasing the risk of locoregional/systemic metastasis and death. The stage of RB at presentation varies between different populations and is influenced by various factors, including availability and accessibility to health care, and socioeconomic and cultural differences.1 The incidence of RB with extraocular tumor extension (RB-EOE) is 49% in LIC, 27% in LMIC, 12% in UMIC, and 2% in HIC.1 The different treatment modalities for RB-EOE include systemic chemotherapy, enucleation, orbital exenteration, and radiotherapy.9 There is a wide variation in the treatment of RB-EOE, worldwide. Herein, we report the incidence and outcomes of RB-EOE and compare them with RB without extraocular tumor extension (RB-w/o-EOE). In this study, we also explore the differences in the treatment protocols of RB-EOE in various treatment centers across the world and provide recommendations based on our findings.
Methods
A prospective, multicenter, intercontinental, collaborative global RB study gathered data on >4000 treatment-naive RB children presenting to the various RB treatment centers across the world from January 1, 2017, to December 31, 2017.1 These patients were followed up till December 31, 2020.2 The current comparative study included a subset of prospectively followed up patients from the Global RB Outcome Study.2 All RB patients with RB-w/o-EOE and those with evidence of overt RB-EOE by clinical or radiological evaluation (i.e., RB-EOE diagnosed before initiation of treatment) without locoregional/systemic metastasis at presentation to the RB treatment center were included in this study. Those with inadequate data, those with microscopic orbital extension of RB postenucleation (these were excluded to avoid a bias toward treatment outcomes because enucleation was performed before a diagnosis of RB-EOE was made), and those with locoregional/systemic metastasis at presentation were excluded from the study. The study was approved by the Institutional Review Board at the London School of Hygiene & Tropical Medicine and at individual RB centers. A waiver of patient informed consent was obtained from the center’s ethics committee. The study adhered to the tenets of the Declaration of Helsinki.
The following data were obtained for each patient: RB treatment center, child’s country of residence, national income level, income status of the family, distance of home from the RB treatment center, age at presentation to the RB treatment center, sex, family history of RB, presenting symptoms, lag time between onset of symptoms and presentation to the RB treatment center (months), tumor laterality, and tumor classification based on the 8th edition of American Joint Committee on Cancer classification.10
All RB-EOE and RB-w/o-EOE patients who received treatment were included for analysis of outcomes. Primary and adjuvant treatment details of those with RB-EOE were further analyzed. Outcomes at the last follow-up, including systemic metastasis, the interval between initiation of treatment and systemic metastasis, death, the cause of death, and the interval between initiation of treatment and death, were noted in all patients.
Statistical Analysis
Descriptive continuous data were expressed as mean, median, and range, and categorical data were expressed as proportions. The demographics, clinical features, and outcomes of children with RB-EOE and RB-w/o-EOE were compared. The statistical analysis was performed using Statistical Package for Social Sciences, version 29.0.2.0 (20). Continuous data were compared using the student t test and Mann–Whitney U test based on the normality of distribution. Categorical data were compared using the chi-square test. A P value of ≤0.05 was considered statistically significant. Kaplan–Meier estimates were used to predict the rates of systemic metastasis and death in children with RB-EOE. Cox proportional hazard regression was used to estimate the impact of age and treatment modalities on the outcomes in children with RB-EOE. To assess the benefit of external beam radiotherapy (EBRT), patients who received trimodal treatment (intravenous chemotherapy, surgery [enucleation or orbital exenteration], and EBRT) were compared against bimodal therapy (intravenous chemotherapy and surgery [enucleation or orbital exenteration]).
Results
Of the total 4064 patients from the Global RB Outcome Study,2 a total of 3435 patients were included in this study. Of these, 319 (9%) patients had RB-EOE, and 3116 (91%) patients had RB-w/o-EOE in 1 or both eyes with no evidence of metastasis at presentation. Based on national income level, 309 (9%) patients were from LIC, 1448 (42%) from LMIC, 1012 (29%) from UMIC, and 666 (19%) patients from HIC. There was an inverse relationship between the percentage of patients with RB-EOE and national income level with 96 (31%) patients from LIC, 197 (6%) from LMIC, 20 (2%) from UMIC, and 6 (1%) patients from HIC (P = 0.0001) (Table 1).
Table 1.
RB with and without Extraocular Tumor Extension: Demographics and Clinical Presentation
| Feature | RB with Extraocular Tumor Extension n = 319 n (%) |
RB without extraocular tumor extension n = 3116 n (%) |
P Value |
|---|---|---|---|
| National income level | |||
| Low | 96 (30) | 213 (7) | 0.0001 |
| Lower-middle | 197 (62) | 1251 (40) | 0.0001 |
| Upper-middle | 20 (6) | 992 (32) | 0.0001 |
| High | 6 (2) | 660 (21) | 0.0001 |
| National income level | |||
| Low (n = 309) | 96 (31) | – | – |
| Lower-middle (n = 1448) | 197 (14) | – | – |
| Upper-middle (n = 1012) | 20 (2) | – | – |
| High (n = 666) | 6 (1) | – | – |
| P value | 0.0001 | ||
| Family income | |||
| Low | 96 (30) | 227 (7) | 0.0001 |
| Lower-middle | 196 (61) | 1267 (41) | 0.0001 |
| Upper-middle | 23 (7) | 1003 (32) | 0.0001 |
| High (n = 626) | 4 (1) | 619 (20) | 0.0001 |
| Distance to RB care (miles), mean (median, range) | 216 (160, <1–2620) | 268 (119, <1–7470) | 0.113 |
| Lag time from onset of symptoms to presentation at RB center (mos), mean (median, range) | 7 (2, <1–54) | 4 (1, <1–117) | 0.0001 |
| Age at presentation (mos), mean (median, range) | 37 (36, 2–137) | 24 (19, <1–281) | 0.0001 |
| <2 yrs | 74 (24) | 1791 (58) | 0.0001 |
| 2–4 | 148 (47) | 600 (20) | 0.0001 |
| ≥4 yrs | 91 (29) | 682 (22) | 0.009 |
| Sex | |||
| Male | 185 (58) | 1709 (55) | 0.29 |
| Female | 134 (42) | 1407 (45) | |
| Family history of RB | 4 (1) | 177 (6) | 0.0002 |
| Tumor laterality | |||
| Unilateral | 244 (76) | 2078 (67) | 0.0003 |
| Bilateral | 75 (24) | 1038 (33) | |
| Bilateral orbital extension | 8 (3) | NA | |
| 8th edition AJCC classification (n = 4328 eyes) | n = 327 eyes | n = 4001 eyes | |
| cT1 | 0 (0) | 815 (21) | |
| cT2 | 0 (0) | 1484 (37) | |
| cT3 | 0 (0) | 1702 (42) | |
| cT4 | 327 (100) | 0 (0) | 0.0001 |
AJCC = American Joint Committee on Cancer; NA = not applicable; RB = retinoblastoma.
The following features and outcomes were statistically significant for RB-EOE compared with those with RB-w/o-EOE: mean lag time from onset of symptoms to the presentation to RB treatment center (7 months vs. 4 months; P = 0.0001), mean age at presentation (37 months vs. 24 months; P = 0.0001), family history of RB (1% vs. 6%; P = 0.0001), and unilaterality of RB (76% vs. 67%; P = 0.0003). Based on the 8th edition American Joint Committee on Cancer classification, all (100%) RB-EOE belonged to cT4, and a majority (42%) of RB-w/o-EOE belonged to cT3.
Of 327 eyes with RB-EOE, 83% were treated, 4% were advised palliative care, and 13% refused treatment, and of 4001 eyes with RB-w/o-EOE, 98% were treated. Table 2 lists the treatment details for children with RB-EOE. Multimodal treatment was the most common form of treatment (n = 177, 54%) for RB-EOE, with most cases undergoing bimodal treatment, including intravenous chemotherapy and enucleation (n = 97; 30%). Adjuvant EBRT after surgery (enucleation/orbital exenteration) was given in only 68 (21%) cases.
Table 2.
Retinoblastoma with Extraocular Extension: Management
| Treatment | n = 327, Eyes of 319 Patients n (%) |
|---|---|
| Primary enucleation | 13 (4) |
| Intravenous chemotherapy | 64 (20) |
| External beam radiotherapy | 1 (<1) |
| Primary orbital exenteration | 16 (5) |
| Combination therapy | 177 (54) |
| IVC + orbital exenteration | 11 (3) |
| IVC + enucleation | 97 (30) |
| IVC + enucleation + EBRT | 66 (20) |
| IVC + orbital exenteration + EBRT | 2 (1) |
| Enucleation + EBRT | 1 (<1) |
| Palliative care | 12 (4) |
| Refusal to treatment | 44 (13) |
EBRT = external beam radiotherapy; IVC = intravenous chemotherapy.
The outcomes differed between those with RB-EOE and RB-w/o-EOE, including systemic metastasis (32% vs. 4%; P = 0.0001) and metastasis-related death (63% vs. 6%; P = 0.0001) (Table 3). The analysis revealed an odds ratio of 13.5 for metastasis and 33 for metastasis-related death in patients with RB-EOE compared with those with RB-w/o-EOE. The overall cumulative incidence by Kaplan–Meier analysis at 1 year, 2 years, 3 years, and 4 years for RB-EOE was 28%, 29%, 29%, and 29%, respectively, for systemic metastasis and 57%, 60%, 61%, and 61%, respectively, for death (Table 4). Cox regression analysis based on age revealed hazard ratios of 3.163 (95% confidence interval, 1.149–8.708, P = 0.026) for systemic metastasis and 2.912 (95% confidence interval, 1.603–5.290, P < 0.001) for death, for children aged >4 years when compared with children <2 years of age. Lag time of >2 months showed no effect on outcomes (metastasis and death) of patients with RB-EOE (Table 5).
Table 3.
RB with and without Extraocular Tumor Extension: Outcomes
| Feature | RB with Extraocular Tumor Extension n = 319 n (%) |
RB without Extraocular Tumor Extension n = 3116 n (%) |
Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Duration of follow-up (mos), mean (median, range) | 16 (11, <1–48) | 30 (36, <1–51) | NA | 0.0001 |
| Systemic metastasis | 67 (32)∗ | 105 (4)† | 13.488 (9.645–18.862) | 0.0001 |
| Time interval between presentation and systemic metastasis (mos), mean (median, range) | 5 (4, <1–22) | 16 (12, <1–41) | NA | 0.0001 |
| Death due to RB | 140 (63)‡ | 164 (6)§ | 33.004 (23.731–45.889) | 0.0001 |
| Time interval between presentation and death (mos), mean (median, range) | 11 (9, <1–35) | 17 (15, <1–43) | NA | 0.0001 |
CI = confidence interval; NA = not applicable; RB = retinoblastoma.
Outcomes were unknown in 107 patients.
Outcomes were unknown in 593 patients.
Outcomes were unknown in 97 patients.
Outcomes were unknown in 451 patients.
Table 4.
Retinoblastoma with Extraocular Extension: Kaplan–Meier Survival Analysis
| Months | Systemic Metastasis |
Death |
||
|---|---|---|---|---|
| No. of Events | Cumulative Incidence | No. of Events | Cumulative Incidence | |
| <12 | 32 | 20% | 72 | 42% |
| 12 | 9 | 28% | 23 | 57% |
| 24 | 1 | 29% | 4 | 60% |
| 36 | 0 | 29% | 1 | 61% |
| 48 | 0 | 29% | 0 | 61% |
Table 5.
Retinoblastoma with Extraocular Extension: Cox Regression Analysis Based on Age, Lag Time, and Treatment Modality
| Feature | Systemic Metastasis |
Death |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age∗ | ||||
| 2–4 yrs | 2.459 (0.931–6.497) | 0.069 | 1.773 (0.986–3.187) | 0.056 |
| >4 yrs | 3.163 (1.149–8.708) | 0.026 | 2.912 (1.603–5.290) | <0.001 |
| Lag time | ||||
| >2 mos vs. ≤2 mos | 0.885 (0.459–1.706) | 0.715 | 1.024 (0.575–1.826) | 0.936 |
| Treatment modality† | ||||
| Trimodal‡ vs. unimodal§ + bimodal‖ | 2.253 (1.309–3.880) | 0.003 | 1.179 (0.701–1.984) | 0.534 |
| Trimodal‡ vs. unimodal (surgery) | 2.712 (1.011–7.277) | 0.048 | 0.644 (0.234–1.774) | 0.395 |
| Trimodal‡ vs. unimodal (chemotherapy) | 4.496 (2.081–9.714) | <0.001 | 2.023 (1.244–3.240) | 0.004 |
| Trimodal‡ vs. bimodal‖ | 2.187 (0.830–5.763) | 0.113 | 1.819 (1.063–3.177) | 0.027 |
| Primary treatment modality in combination therapy¶ | ||||
| Primary surgery | 6.500 (1.310–32.246) | 0.022 | 1.117 (0.363–2.704) | 0.844 |
CI = confidence interval.
Reference: Age <2 years.
Reference: Trimodal therapy.
Trimodal: Surgery, intravenous chemotherapy, and external beam radiotherapy.
Unimodal: Surgery or intravenous chemotherapy.
Bimodal: Surgery and intravenous chemotherapy.
Reference: Primary chemotherapy.
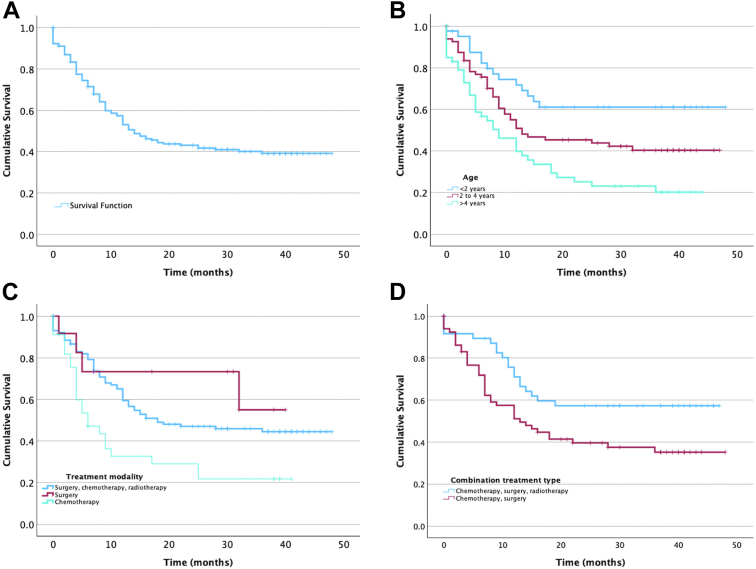
In patients with RB-EOE receiving combination treatment, Cox regression analysis of outcomes was performed based on the various treatment modalities (Table 5). When compared with a reference of combination therapy with surgery (enucleation or orbital exenteration), intravenous chemotherapy, and radiotherapy (trimodal treatment), patients who received unimodal (surgery or intravenous chemotherapy) or bimodal (surgery and intravenous chemotherapy) treatments were at 2.253 times greater risk of systemic metastasis (P < 0.003). Similarly, patients who underwent only surgical treatment were at 2.712 times greater risk of developing systemic metastasis (P = 0.048) and patients treated with chemotherapy alone were at 4.496 times greater risk of systemic metastasis (P < 0.001) and 2.0 times greater risk of death (P = 0.004). A comparison of patients who received trimodal treatment vs. those who received bimodal therapy revealed that the patients who did not receive adjuvant EBRT had a hazard ratio of 1.819 for death compared with the former (P = 0.027). In patients receiving trimodal treatment, patients who received primary surgical intervention had a worse prognosis for systemic metastasis (hazard ratio, 6.500; 95% confidence interval, 1.310–32.246, P = 0.022) than those who received neoadjuvant intravenous chemotherapy (Fig 1).
Figure 1.
Retinoblastoma with extraocular extension: Kaplan–Meier curves depicting overall survival (A), survival based on age (B), treatment modality (C), and type of combination treatment (D).
Discussion
Overall, the incidence of RB-EOE is less common compared with RB-w/o-EOE. However, the incidence of RB-EOE is greatly influenced by the national income level. In the Global RB-outcomes study that included 4064 patients from 149 countries, the incidence of RB-EOE was 1% in HIC, 5% in UMIC, 20% in LMIC, and 43% in LIC.2 A similar trend of inverse relationship between national income level and incidence of RB-EOE was seen in the current study at 1% in HIC, 2% in UMIC, 6% in LMIC, and 31% in LIC. Though this study cohort is derived from the Global RB-outcomes study, the relatively lower percentage of RB-EOE is due to the exclusion of metastatic cases and microscopic RB-EOE from the current study. Data from single centers in LMIC or LIC have shown a much higher incidence of RB-EOE, accounting for 52% to 85% of cases. However, over the years, a decreasing trend in the occurrence of RB-EOE has been observed in many countries.3
The occurrence of advanced RB at presentation may be related to a lack of awareness about RB, resulting in delayed diagnosis and delayed access to appropriate care.11 In this study, the lag time between the onset of symptoms and presentation to the RB treatment center, as well as the mean age at presentation to the RB treatment center, was much higher for RB-EOE compared with RB-w/o-EOE. However, within the RB-EOE cohort, the lag time was not predictive of the outcomes including systemic metastasis or death. Family history of RB was also less common in the children with RB-EOE compared with those with RB-w/o-EOE. The occurrence of RB in a family member may increase awareness about RB, resulting in earlier health-seeking behavior.
Retinoblastoma with extraocular tumor extension is a poor prognostic factor with a higher risk of metastasis and death. A multivariate analysis of 15 variables in 361 cases by Kopelman et al12 revealed that RB-EOE has an odds ratio of 21.6 for RB metastasis. Retinoblastoma metastasis is associated with high rates of mortality. Rootman et al13 noted that only 9.4% of patients with RB-EOE lived for >2 years after diagnosis, and death in these cases is likely due to systemic metastasis. Based on our study, patients with RB-EOE have a 13.5 times higher risk of metastasis and 33 times higher risk of metastasis-related death compared with RB-w/o-EOE. Most deaths occurred within 1 year of diagnosis of RB-EOE in our study. This underscores the severity and more aggressive nature of RB-EOE compared with when the cancer is contained within the eye.
Protocol-based treatment plays a crucial role in improving outcomes in cancer care. Although well-documented protocols exist for the management of RB-w/o-EOE14 and are followed in the majority of oncology centers across the globe, there is no uniform protocol for RB-EOE. There seems to be a wide variation in the management of RB-EOE, as observed in the present global-scale study. Although multimodal treatment was used in 54% of cases, bimodal treatment (surgery and intravenous chemotherapy, 33%) was more common than trimodal treatment (surgery, intravenous chemotherapy, and EBRT, 21%). Based on our study, isolated treatment with primary enucleation or primary orbital exenteration, or intravenous chemotherapy is unlikely to increase the likelihood of life salvage in RB-EOE. Various studies have shown that an aggressive therapeutic approach, including combination therapy with chemotherapy, surgery, and EBRT, is beneficial in the treatment of RB-EOE.9,14, 15, 16, 17, 18, 19, 20, 21, 22, 23 In a long-term study of 20 patients with RB-EOE, a survival rate of 71% was achieved at a median follow-up of 77 months, with the use of multimodal treatment comprising 3 to 9 cycles of neoadjuvant chemotherapy followed by secondary enucleation/orbital exenteration, 3 to 9 cycles of adjuvant chemotherapy (a total of 12 cycles of chemotherapy including both neoadjuvant and adjuvant chemotherapy), and EBRT.9 In a prospective international Children’s Oncology Group trial (ARET-0321), including patients with microscopic RB-EOE and overt RB-EOE, treated with a multimodal treatment comprising chemotherapy, surgery, and EBRT, the 3-year event-free survival was 88%.23 In the current study, the patients who underwent only chemotherapy and surgery had 1.8 times higher chances of death compared with those receiving multimodal treatment comprising chemotherapy, surgery, and EBRT. The results did not show a statistically significant increase in metastasis risk among patients who did not receive trimodal treatment, which may be attributed to missing metastasis data. Though our study did not include microscopic RB-EOE, the results were similar to the ARET-0321 study.23 This indicates that the inclusion of EBRT in the treatment regimen significantly enhances survival rates. Based on these findings, we recommend multimodal treatment, including chemotherapy, surgery, and EBRT for all patients with RB-EOE. Although the availability of EBRT in LICs and some LMICs remains a challenge, efforts should continue to ensure that EBRT is accessible at all RB treatment centers. This would help improve treatment outcomes and survival rates for RB-EOE patients globally.
Various combinations of chemotherapeutic agents, including vincristine, etoposide, carboplatin, cyclophosphamide, doxorubicin, idarubicin, and cisplatin in standard or high-dose have been attempted for RB-EOE.9,15, 16, 17, 18, 19, 20, 21, 22, 23, 24 In a recent prospective comparative study of 54 patients with RB-EOE receiving multimodal treatment protocol including chemotherapy, surgery, and EBRT, it was noted that patients treated with a 3-drug combination of high-dose vincristine, etoposide, and carboplatin had better 4-year survival rates at 63%, compared with 25% in patients treated with the 5-drug combination of carboplatin and etoposide alternating with cyclophosphamide, idarubicin, and vincristine.15 This finding highlights that the 3-drug regimen is more effective in improving long-term survival for patients with RB-EOE than the more complex 5-drug regimen. In our study, data on the specific chemotherapeutic agents and their doses were lacking. Therefore, it is difficult to comment upon ideal chemotherapy agents and their doses for the treatment of RB-EOE, based on the current study. In our study, patients with RB-EOE who received protocol-based treatment were more likely to have undergone a 3-drug chemotherapy regimen.
In this study, patients who underwent primary surgery (enucleation or orbital exenteration) for RB-EOE had a 6.5 times higher risk of systemic metastasis compared with those who received neoadjuvant chemotherapy before surgery. Based on these findings, we recommend avoidance of the primary surgical approach for patients with RB-EOE. Neoadjuvant chemotherapy may play a role in reducing the risk of systemic metastasis in patients with RB-EOE, potentially improving overall outcomes and highlighting the importance of considering multimodal treatment approaches in managing RB-EOE. Neoadjuvant chemotherapy causes a dramatic response in RB-EOE, resulting in phthisis bulbi in most cases, and rendering the eyes amenable to secondary enucleation.9,15,18, 19, 20, 21, 22 With neoadjuvant chemotherapy, secondary orbital exenteration can be avoided in most cases of RB-EOE.9,15,18, 19, 20, 21, 22
Combining the strengths of a good representation of RB-EOE cases that presented to treatment centers across the world in 2017 and unbiased prospective data collection, this study likely provides robust and reliable insights into RB-EOE treatment outcomes. Because this study was not based on a uniform treatment protocol, indeed, the variation in treatment protocols among different treatment centers can offer a unique opportunity to identify the most beneficial protocol for managing RB-EOE. The limitations of the study include: (1) the capture rate of RB cases may not be 100%, especially in LICs where many patients may not even reach the treatment center; (2) details of histopathology data of the enucleated eyes were limited and hence did not allow us to perform an in-depth analysis of the data based on the specific high-risk histopathological features such as extrascleral invasion and optic nerve invasion; (3) the details of chemotherapy, including drugs, dose, and regimen, the dose of EBRT, and complications of treatment, were not available for a detailed analysis; (4) this study enrolled patients from 2017 to 2020, but the availability of treatment resources in the participating centers and practice patterns may have changed over the next few years; (5) disease presentation was heterogeneous across various nations; and (6) the outcomes were not recorded for nearly one-third of the patients with RB-EOE, as they did not return for follow-up.
In conclusion, according to this global-scale analysis, the risk of systemic metastasis and metastasis-related death was 13.5 and 33 times higher in patients with RB-EOE compared with RB-w/o-EOE at presentation. Patients who underwent only chemotherapy and surgery had 1.8 times higher chances of death compared with those receiving multimodal treatment comprising chemotherapy, surgery, and EBRT. Patients who underwent primary surgery for RB-EOE had a 6.5 times higher risk of systemic metastasis compared with those who received neoadjuvant chemotherapy before surgery. Overall, these findings bring about the differences in the treatment patterns for RB-EOE across the world and provide valuable insights into the prognosis and management of RB-EOE, thus informing clinical decision-making and emphasizing the importance of multimodal treatment strategies in improving patient outcomes. Furthermore, the study emphasizes the need to work toward a collaborative, standardized, evidence-based approach to the management of RB-EOE.
Manuscript no. XOPS-D-24-00198R2.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosures:
All authors have completed and submitted the ICMJE disclosures form.
The authors have no proprietary or commercial interest in any materials discussed in this article.
Support provided by The Operation Eyesight Universal Institute for Eye Cancer (S.K.), Hyderabad Eye Research Foundation (S.K.), and Prateek Menezes Memorial Foundation (S.K.). The funders had no role in preparing, reviewing, or approving the manuscript.
HUMAN SUBJECTS: Human subjects were included in this study. The study was approved by the Institutional Review Board at the London School of Hygiene and Tropical Medicine and at individual retinoblastoma centers. A waiver of patient informed consent was obtained from the center’s ethics committee. The study adhered to the tenets of the Declaration of Helsinki.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Kaliki
Data collection: Kaliki, Fabian
Analysis and interpretation: Kaliki, Vempuluru
Obtained funding: N/A
Overall responsibility: Kaliki
Contributor Information
Swathi Kaliki, Email: kalikiswathi@yahoo.com.
Global Retinoblastoma Study Group:
Ido Didi Fabian, Elhassan Abdallah, Shehu U. Abdullahi, Rula A. Abdulqader, Aminatu A. Abdulrahaman, Sherif Abouelnaga, Dupe S. Ademola-Popoola, Adedayo Adio, Mahmoud A. Afifi, Armin R. Afshar, Priyanka Aggarwal, Ada E. Aghaji, Alia Ahmad, Marliyanti N.R. Akib, Adeseye M. Akinsete, Lamis Al Harby, Saleh A. Al Mesfer, Mouroge H. Al Ani, Silvia Alarcón Portabella, Safaa A.F. Al-Badri, Ana Patricia A. Alcasabas, Saad A. Al-Dahmash, Amanda Alejos, Ernesto Alemany-Rubio, Amadou I. Alfa Bio, Yvania Alfonso Carreras, Christiane E. Al-Haddad, Hamoud H.Y. Al-Hussaini, Amany M. Ali, Donjeta B. Alia, Mazin F. Al-Jadiry, Usama Al-Jumaly, Hind M. Alkatan, Charlotta All-Eriksson, Ali A.R.M. Al-Mafrachi, Argentino A. Almeida, Khalifa M. Alsawidi, Athar A.S.M. Al-Shaheen, Entissar H. Al-Shammary, Doreen Amankwaa-Frempong, Primawita O. Amiruddin, Inggar Armytasari, Nicholas J. Astbury, Hatice T. Atalay, Eda Ataseven, La-ongsri Atchaneeyasakul, Rose Atsiaya, Rudolf Autrata, Julia Balaguer, Ruhengiz Balayeva, Honorio Barranco, Paulina Bartoszek, Katarina Bartuma, Covadonga Bascaran, Nikolaos E. Bechrakis, Maja Beck Popovic, Ainura S. Begimkulova, Sarra Benmiloud, Rokia C. Berete, Jesse L. Berry, Anirban Bhaduri, Sunil Bhat, Arpita Bhattacharyya, Eva M. Biewald, Elaine Binkley, Sharon Blum, Nadia Bobrova, H. Culver Boldt, Maria Teresa B.C. Bonanomi, Gabrielle C. Bouda, Hédi Bouguila, Rachel C. Brennan, Bénédicte G. Brichard, Jassada Buaboonnam, Aléine Budiongo, Matthew Burton, Patricia Calderón-Sotelo, Doris A. Calle Jara, Jayne E. Camuglia, Miriam R. Cano, Michael Capra, Shani Caspi, Nathalie Cassoux, Guilherme Castela, Luis Castillo, Jaume Català-Mora, Isabel Caviedes, Arthika Chandramohan, Guillermo L. Chantada, Shabana Chaudhry, Bhavna Chawla, Wensi Chen, Faraja S. Chiwanga, Tsengelmaa Chuluunbat, Krzysztof Cieslik, Antony Clark, Ruellyn L. Cockcroft, Codruta Comsa, Maria G. Correa Llano, Timothy W. Corson, Line Couitchere, Kristin E. Cowan-Lyn, Monika Csóka, Wantanee Dangboon, Anirban Das, Pranab Das, Sima Das, Jacquelyn M. Davanzo, Alan Davidson, Sonia De Francesco, Patrick De Potter, Karina Q. Delgado, Hakan Demirci, Laurence Desjardins, Rosdali Y. Diaz Coronado, Helen Dimaras, Andrew J. Dodgshun, Carla R. Donato Macedo, Monica D. Dragomir, Yi Du, Magritha Du Bruyn, Johannes P. Du Plessis, Gagan Dudeja, Katrin Eerme, I Wayan Eka Sutyawan, Asmaa El Kettani, Amal M. Elbahi, James E. Elder, Alaa M. Elhaddad, Moawia M.A. Elhassan, Mahmoud M. Elzembely, Connor Ericksen, Vera A. Essuman, Ted Grimbert A. Evina, Ifeoma R. Ezegwui, Zehra Fadoo, Adriana C. Fandiño, Mohammad Faranoush, Oluyemi Fasina, Delia D.P.G. Fernández, Ana Fernández-Teijeiro, Allen Foster, Shahar Frenkel, Ligia D. Fu, Soad L. Fuentes-Alabi, Juan L. Garcia, David García Aldana, Henry N. Garcia Pacheco, Jennifer A. Geel, Fariba Ghassemi, Ana V. Girón, Marco A. Goenz, Aaron S. Gold, Hila Golberg, Glen A. Gole, Nir Gomel, Efren Gonzalez, Graciela Gonzalez Perez, Liudmira González-Rodríguez, Malka Gorfine, Jaime Graells, Pernille A. Gregersen, Nathalia D.A.K. Grigorovski, Koffi M. Guedenon, D Sanjeeva Gunasekera, Ahmet K. Gündüz, Himika Gupta, Sanjiv Gupta, Vineeta Gupta, Theodora Hadjistilianou, Patrick Hamel, Syed A. Hamid, Norhafizah Hamzah, Eric D. Hansen, J William Harbour, M. Elizabeth Hartnett, Murat Hasanreisoglu, Sadiq Hassan, Shadab Hassan, Wojciech Hautz, Huda A. Haydar, Stanislava Hederova, Laila Hessissen, Hoby Lalaina, Suradej Hongeng, Diriba F. Hordofa, G. Baker Hubbard, Marlies Hummlen, Kristina Husakova, Allawi N. Hussein Al-Janabi, Affiong A. Ibanga, Russo Ida, Vesna R. Ilic, Ziyavuddin Islamov, Vivekaraj Jairaj, Teyyeb A. Janjua, Irfan Jeeva, Xunda Ji, Dong Hyun Jo, Michael M. Jones, Theophile B. Amani Kabesha, Rolande L. Kabore, Swathi Kaliki, Abubakar Kalinaki, Pius Kamsang, Mehmet Kantar, Noa Kapelushnik, Tamar Kardava, Rejin Kebudi, Jonny Keomisy, Tomas Kepak, Petra Ketteler, Zohora J. Khan, Hussain A. Khaqan, Vikas Khetan, Alireza Khodabande, Zaza Khotenashvili, Jonathan W. Kim, Jeong Hun Kim, Hayyam Kiratli, Tero T. Kivelä, Artur Klett, Irem Koç, Jess Elio Kosh Komba Palet, Dalia Krivaitiene, Mariana Kruger, Kittisak Kulvichit, Mayasari W. Kuntorini, Alice Kyara, Geoffrey C. Lam, Scott A. Larson, Slobodanka Latinović, Kelly D. Laurenti, Yotam Lavi, Alenka Lavric Groznik, Amy A. Leverant, Cairui Li, Kaijun Li, Ben Limbu, Chun-Hsiu Liu, Quah Boon Long, Juan P. López, Robert M. Lukamba, Sandra Luna-Fineman, Delfitri Lutfi, Lesia Lysytsia, Shiran Madgar, George N. Magrath, Amita Mahajan, Puja Maitra, Erika Maka, Emil K. Makimbetov, Azza M.Y. Maktabi, Carlos Maldonado, Ashwin Mallipatna, Rebecca Manudhane, Lyazat Manzhuova, Nieves Martín Begue, Sidra Masud, Ibrahim O. Matende, Clarissa C.D.S. Mattosinho, Marchelo Matua, Ismail Mayet, Freddy B. Mbumba, John D. McKenzie, Azim Mehrvar, Aemero A. Mengesha, Vikas Menon, Gary John V.D.D. Mercado, Marilyn B. Mets, Edoardo Midena, Audra Miller, Divyansh K.C. Mishra, Furahini G. Mndeme, Ahmed A. Mohamedani, Mona T. Mohammad, Annette C. Moll, Margarita M. Montero, Claude Moreira, Prithvi Mruthyunjaya, Mchikirwa S. Msina, Gerald Msukwa, Sangeeta S. Mudaliar, Hassan Muhammad, Kangwa I. Muma, Francis L. Munier, Timothy G. Murray, Kareem O. Musa, Asma Mushtaq, Anne A. Musika, Hamzah Mustak, Tajudeen Mustapha, Okwen M. Muyen, Khumo H. Myezo, Gita Naidu, Natasha Naidu, Akshay Gopinathan Nair, Sundaram Natarajan, Larisa Naumenko, Paule Aïda Ndoye Roth, Yetty M. Nency, Vladimir Neroev, Yvonne Ng, Marina Nikitovic, Elizabeth D. Nkanga, Henry E. Nkumbe, Marcel N. Numbi, Kalle Nummi, Murtuza Nuruddin, Mutale Nyaywa, Chinsisi Nyirenda, Ghislaine Obono-Obiang, Scott C.N. Oliver, Joaquin Ooporto, Miriam Ortega-Hernández, Alexander Oscar, Diego Ossandon, Halimah Pagarra, Vivian Paintsil, Luisa Paiva, Mahesh Shanmugam Palanivelu, Ruzanna Papyan, Raffaele Parrozzani, Claudia R. Pascual Morales, Katherine E. Paton, Jacob Pe'er, Jesús Peralta Calvo, Sanja Perić, Chau T.M. Pham, Remezo Philbert, David A. Plager, Pavel Pochop, Rodrigo A. Polania, Vladimir Polyakov, Jimena Ponce, Ali O. Qadir, Seema Qayyum, Jiang Qian, Ardizal Rahman, Purnima Rajkarnikar, Rajesh Ramanjulu, Aparna Ramasubramanian, Marco A. Ramirez-Ortiz, Jasmeen K. Randhawa, Léa Raobela, Riffat Rashid, M. Ashwin Reddy, Lorna A. Renner, David Reynders, Dahiru Ribadu, Petra Ritter-Sovinz, Anna Rogowska, Duangnate Rojanaporn, Livia Romero, Soma R. Roy, Raya H. Saab, Svetlana Saakyan, Ahmed H. Sabhan, Mandeep S. Sagoo, Azza M.A. Said, Rohit Saiju, Beatriz Salas, Sonsoles San Román Pacheco, Gissela L. Sánchez, Alma Janeth Sanchez Orozco, Phayvanh Sayalith, Trish A. Scanlan, Christoph Schwab, Ahad Sedaghat, Rachna Seth, Mariana Sgroi, Ankoor S. Shah, Shawkat A. Shakoor, Manoj K. Sharma, Sadik T. Sherief, Carol L. Shields, David Sia, Sorath Noorani Siddiqui, Sidi Sidi cheikh, Sónia Silva, Arun D. Singh, Usha Singh, Penny Singha, Rita S. Sitorus, Alison H. Skalet, Hendrian D. Soebagjo, Tetyana Sorochynska, Grace Ssali, Andrew W. Stacey, Sandra E. Staffieri, Erin D. Stahl, David M. Steinberg, David K. Stones, Caron Strahlendorf, Maria Estela Coleoni Suarez, Sadia Sultana, Xiantao Sun, Rosanne Superstein, Eddy Supriyadi, Supawan Surukrattanaskul, Shigenobu Suzuki, Karel Svojgr, Fatoumata Sylla, Gevorg Tamamyan, Deborah Tan, Alketa Tandili, Jing Tang, Fanny F. Tarrillo Leiva, Maryam Tashvighi, Bekim Tateshi, Kok Hoi Teh, Edi S. Tehuteru, Luiz F. Teixeira, Manca Tekavcic Pompe, Abdullah Dahan M. Thawaba, Tuyisabe Theophile, Helen Toledano, Doan L. Trang, Fousseyni Traoré, Devjyoti Tripathy, Samuray Tuncer, Harba Tyau-Tyau, Ali B. Umar, Emel Unal, Ogul E. Uner, Steen F. Urbak, Tatiana L. Ushakova, Rustam H. Usmanov, Sandra Valeina, Paola Valente, Milo van Hoefen Wijsard, Jacqueline Karina Vasquez Anchaya, Leon O. Vaughan, Nevyana V. Veleva-Krasteva, Nishant Verma, Andi A. Victor, Maris Viksnins, Edwin G. Villacís Chafla, Victor M. Villegas, Victoria Vishnevskia-Dai, Keith Waddell, Amina H. Wali, Yi-Zhuo Wang, Nutsuchar Wangtiraumnuay, Julie A. Wetter, Widiarti P. Riono, Matthew W. Wilson, Amelia D.C. Wime, Atchareeya Wiwatwongwana, Damrong Wiwatwongwana, Charlotte Wolley Dod, Emily S. Wong, Phanthipha Wongwai, Si-qi Wu, Daoman Xiang, Yishuang Xiao, Bing Xu, Kang Xue, Antonio Yaghy, Jason C. Yam, Huasheng Yang, Jenny M. Yanga, Muhammad A. Yaqub, Vera A. Yarovaya, Andrey A. Yarovoy, Huijing Ye, Roberto I. Yee, Yacoub A. Yousef, Putu Yuliawati, Arturo M. López, Ekhtelbenina Zein, Yi Zhang, Katsiaryna Zhilyaeva, Nida Zia, Othman A.O. Ziko, Marcia Zondervan, Sabrina Schlüter, and Richard Bowman
Supplementary Data
References
- 1.Global Retinoblastoma Study Group, Fabian I.D., Abdallah E., et al. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020;6:685–695. doi: 10.1001/jamaoncol.2019.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Retinoblastoma Study Group The Global Retinoblastoma Outcome Study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob Health. 2022;10:e1128–e1140. doi: 10.1016/S2214-109X(22)00250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong E.S., Choy R.W., Zhang Y., et al. Global retinoblastoma survival and globe preservation: a systematic review and meta-analysis of associations with socioeconomic and health-care factors. Lancet Glob Health. 2022;10:e380–e389. doi: 10.1016/S2214-109X(21)00555-6. [DOI] [PubMed] [Google Scholar]
- 4.Vempuluru V.S., Maniar A., Kaliki S. Global retinoblastoma studies: a review. Clin Exp Ophthalmol. 2024;52:334–354. doi: 10.1111/ceo.14357. [DOI] [PubMed] [Google Scholar]
- 5.Finger P.T., Tomar A.S. Retinoblastoma outcomes: a global perspective. Lancet Glob Health. 2022;10:e307–e308. doi: 10.1016/S2214-109X(21)00598-2. [DOI] [PubMed] [Google Scholar]
- 6.Tomar A.S., Finger P.T., Gallie B., et al. Global retinoblastoma treatment outcomes: association with national income level. Ophthalmology. 2021;128:740–753. doi: 10.1016/j.ophtha.2020.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Tomar A.S., Finger P.T., Gallie B., et al. A multicenter, international collaborative study for American Joint Committee on Cancer staging of retinoblastoma: part I: metastasis-associated mortality. Ophthalmology. 2020;127:1719–1732. doi: 10.1016/j.ophtha.2020.05.050. [DOI] [PubMed] [Google Scholar]
- 8.Tomar A.S., Finger P.T., Gallie B., et al. A multicenter, international collaborative study for American Joint Committee on Cancer staging of retinoblastoma: part II: treatment success and globe salvage. Ophthalmology. 2020;127:1733–1746. doi: 10.1016/j.ophtha.2020.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Kaliki S., Patel A., Iram S., Palkonda V.A.R. Clinical presentation and outcomes of stage III or stage IV retinoblastoma in 80 Asian Indian patients. J Pediatr Ophthalmol Strabismus. 2017;54:177–184. doi: 10.3928/01913913-20161019-01. [DOI] [PubMed] [Google Scholar]
- 10.Mallipatna A., Gallie B.L., Chévez-Barrios P., et al. In: AJCC Cancer Staging Manual. 8th ed. Amin M.B., Edge S.B., Greene F.L., editors. Springer; New York, NY: 2017. Retinoblastoma; pp. 819–831. [Google Scholar]
- 11.Kaliki S., Ji X., Zou Y., et al. Lag time between onset of first symptom and treatment of retinoblastoma: an international collaborative study of 692 patients from 10 countries. Cancers (Basel) 2021;13:1956. doi: 10.3390/cancers13081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopelman J.E., McLean I.W., Rosenberg S.H. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology. 1987;94:371–377. doi: 10.1016/s0161-6420(87)33436-0. [DOI] [PubMed] [Google Scholar]
- 13.Rootman J., Ellsworth R.M., Hofbauer J., Kitchen D. Orbital extension of retinoblastoma: a clinicopathological study. Can J Ophthalmol. 1978;13:72–80. [PubMed] [Google Scholar]
- 14.Ancona-Lezama D., Dalvin L.A., Shields C.L. Modern treatment of retinoblastoma: a 2020 review. Indian J Ophthalmol. 2020;68:2356–2365. doi: 10.4103/ijo.IJO_721_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla B., Hasan F., Seth R., et al. Multimodal therapy for stage III retinoblastoma (international retinoblastoma staging system): a prospective comparative study. Ophthalmology. 2016;123:1933–1939. doi: 10.1016/j.ophtha.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Goble R.R., McKenzie J., Kingston J.E., et al. Orbital recurrence of retinoblastoma successfully treated by combined therapy. Br J Ophthalmol. 1990;74:97–98. doi: 10.1136/bjo.74.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chantada G., Fandiño A., Casak S., et al. Treatment of overt extraocular retinoblastoma. Med Pediatr Oncol. 2003;40:158–161. doi: 10.1002/mpo.10249. [DOI] [PubMed] [Google Scholar]
- 18.Honavar S.G., Singh A.D. Management of advanced retinoblastoma. Ophthalmol Clin North Am. 2005;18:65–73. doi: 10.1016/j.ohc.2004.09.001. viii. [DOI] [PubMed] [Google Scholar]
- 19.Ali M.J., Reddy V.A.P., Honavar S.G., Naik M. Orbital retinoblastoma: where do we go from here? J Cancer Res Ther. 2011;7:11–14. doi: 10.4103/0973-1482.80429. [DOI] [PubMed] [Google Scholar]
- 20.Ali M.J., Honavar S.G., Reddy V.A.P. Orbital retinoblastoma: present status and future challenges – a review. Saudi J Ophthalmol. 2011;25:159–167. doi: 10.1016/j.sjopt.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honavar S.G., Manjandavida F.P., Reddy V.A.P. Orbital retinoblastoma: an update. Indian J Ophthalmol. 2017;65:435–442. doi: 10.4103/ijo.IJO_352_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaliki S., Palkonda V.A.R. Management of retinoblastoma with extraocular tumour extension. Community Eye Health. 2018;31:18–19. [PMC free article] [PubMed] [Google Scholar]
- 23.Dunkel I.J., Piao J., Chantada G.L., et al. Intensive multimodality therapy for extraocular retinoblastoma: a Children’s Oncology Group trial (ARET0321) J Clin Oncol. 2022;40:3839–3847. doi: 10.1200/JCO.21.02337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doz F., Khelfaoui F., Mosseri V., et al. The role of chemotherapy in orbital involvement of retinoblastoma. The experience of a single institution with 33 patients. Cancer. 1994;74:722–732. doi: 10.1002/1097-0142(19940715)74:2<722::aid-cncr2820740228>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.