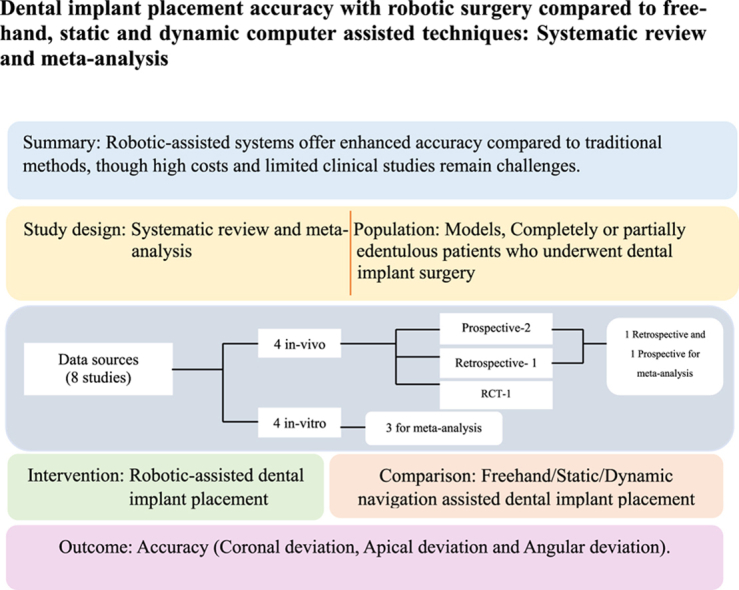
Abstract
Background
This systematic review and meta-analysis compared the accuracy of robotic-assisted dental implant placement (r-CAIS) with conventional freehand, static computer-assisted (s-CAIS), and dynamic computer-assisted (d-CAIS) techniques.
Methods
A comprehensive search was conducted in PubMed, Google Scholar, Semantic Scholar, and the Cochrane Library from January 2000 to January 2024. Studies meeting PICOST criteria, including clinical and in vitro studies, were included. Data on coronal, apical, and angular deviations were extracted for meta-analysis. The risk of bias (RoB) was assessed using the QUIN RoB and JBI RoB tools.
Results
A total of 134 models and 100 patients with edentulous and partially edentulous arches were included. Eight studies (four in vitro, four in vivo) were reviewed, demonstrating that r-CAIS offers superior accuracy compared to freehand, s-CAIS, and d-CAIS techniques. Among the studies, two in vitro and two in vivo studies had a low RoB, while others had a high RoB. The meta-analysis of five studies showed significant improvements in coronal, apical, and angular deviations with robotic systems.
Conclusion
Robotic-assisted systems showed greater accuracy than traditional non-robotic systems. However, this finding should be interpreted with caution due to the limited number of clinical studies and potential funding biases. Moreover, the high cost of robotic systems presents challenges for routine clinical implementation. Future research should focus on cost-effectiveness and seek broader clinical validation.
Keywords: Computer-assisted implant surgery, Dental implantology, Implant accuracy, Robotic-assisted implant surgery, Robotic surgery
Graphical abstract
1. Introduction
Dental implants have become a cornerstone of modern dentistry, effectively addressing partial and complete tooth loss and various maxillofacial and orthodontic challenges. The success of implant placement hinges on accurate three-dimensional positioning, angulation, and depth, all essential for achieving long-term stability, functionality, and aesthetic appeal. Traditionally, freehand implantation has relied on the clinician's expertise and radiographic guidance.1 However, technological advancements have introduced computer-assisted implant surgery (CAIS) techniques, specifically static CAIS (s-CAIS) and dynamic CAIS (d-CAIS), which significantly enhance precision.2,3
Research shows that d-CAIS, with its real-time navigation capabilities, offers superior accuracy compared to both s-CAIS and conventional freehand methods. Nevertheless, it does require specialized training, as surgeons must balance their focus between the surgical site and the navigation display. The emergence of surgical robotic systems presents an exciting development to further improve accuracy and autonomy in implant placement.4,5
The advent of robotic-assisted implant surgery (r-CAIS) introduces the potential for further enhancement in precision, operator autonomy, and reduced fatigue, promising improved postoperative outcomes.6, 7, 8 Despite these advancements, there is a lack of pooled data evidence for comparing the accuracy of r-CAIS with established techniques like freehand, s-CAIS, and d-CAIS.9
This systematic review aims to fill that gap by analyzing the positional accuracy of dental implants placed via r-CAIS relative to other non-robotic modalities. By elucidating the accuracy metrics from both model and clinical studies, the review seeks to provide valuable insights that can inform clinical practice, guide future research, and establish a foundation for standardized assessment in robotic implant surgery.
2. Material and methods
2.1. Study design
2.1.1. Procedure
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines.10 The articles were selected based on the inclusion and exclusion criteria. Two reviewers (H.S. and M.S.) independently assessed the titles and abstracts. In case of disagreement, a full-text assessment was performed. In case of any further disagreement, a third independent reviewer (S.G.) made the final decision. The study was registered in PROSPERO [CRD42023451926].
2.1.2. Selection criteria
These studies were selected based on the PICOST criteria.
-
•
Population- Models, completely or partially edentulous patients who underwent dental implant surgery.
-
•
Intervention- Robotic-assisted dental implant placement.
-
•
Comparison- Free-hand or static navigation or dynamic navigation guided implant placement.
-
•
Outcome - Accuracy (coronal, apical, and angular deviation).
-
•
Study design- Randomized controlled trial (RCT), prospective, retrospective, comparative, cohort, case-control, case series, and in-vitro studies.
-
•
Time frame- January 2000 to January 2024.
Inclusion criteria were as follows: English language, study on models/in-vitro studies, study on human population (RCT, prospective, retrospective, comparative, cohort, case-control, invitro, and case series) in which there is a comparison of the accuracy of a robotic-assisted dental implant with free-hand or dynamic or static navigation guided dental implants in completely or partially edentulous patients.
The studies that have not compared the implant accuracy between robotic-guided (vs) free-hand or static or dynamic navigation-guided implant placement, implants placed in the zygomatic or pterygoid region, animal-based studies, systematic reviews, narrative reviews, scoping reviews, book chapters, and studies lacking potential data were excluded.
2.1.3. Search strategy
A broad literature search was conducted in PubMed, Google Scholar, Semantic Scholar, and Cochrane Library database between January 2000 and January 2024 using the following individual terms and combined terms: “Robotic surgery” OR “Robotics” OR “Robot-assisted” OR “Robot-assisted surgery” OR “Dental implant” OR “Dental implantology” OR “Implant-supported.” The detailed search strategy is presented in Supplementary Table 1 (S1). The resultant databases were screened for duplicates and were removed. The reference list of all the final retrieved articles was reviewed to identify relevant studies.
2.1.4. Data collection
The following data were analyzed: author name, country, year of publication, objectives, number of patients/models, number of implants, robotic system, region of implant placement, comparison, coronal deviation, apical deviation, angular deviation, and conclusion of the included studies.
2.1.5. Risk of bias assessment
Risk of bias (RoB) assessment of in-vitro studies was done using the QUIN ROB tool. Studies with a total score greater than 70 % were classified as low risk of bias, those scoring between 50 % and 70 % as medium risk of bias, and those scoring less than 50 % as high risk of bias. This classification was determined using the formula:
Final score = (Total score x100)/ (2 x number of applicable criteria).11
Risk of bias assessment of individual clinical studies was done using the JBI RoB tool for RCTs, the JBI RoB tool for cohort studies, and the JBI RoB tool for case-control studies. The RoB was categorized as low if less than three criteria were answered as ‘No’ and high RoB if more than three were responded to as ‘No.’12 Investigators (H.S and M.S) assessed the quality, following which any disagreements were settled by discussion with a third author (S.G)
2.1.6. Meta-analysis
To evaluate the positional accuracy of r-CAIS with respect to d-CAIS or s-CAIS, the weighted mean deviations were reported for both in vivo and in vitro studies. A software program (Review Manager V.5.3.5.22; Cochrane) has been used for analysis. The trials with similar treatments, participants, and results were combined, and the results were expressed as an effect measure using the highest reported mean differences (MD) in deviations and the 95 % confidence intervals (CI) that went along with them. A random effects model was used to calculate the weighted mean difference (WMD) of the treatment technique. It included studies that contained a comparison group, due to the limitation of the comparative studies, r-CAIS and d-CAIS were compared for the in-vitro studies meta-analysis whereas, r-CAIS and s-CAIS were compared for the in-vivo studies meta-analysis. A forest plot has been used to visually represent the pooled weighted size and confidence interval, showing the variability in the studies.
3. Results
3.1. Search outcome
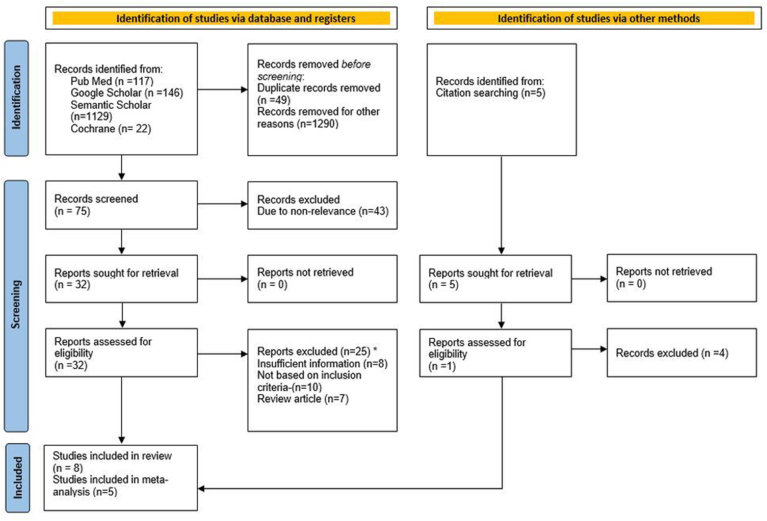
The selection process of the studies is explained in Fig. 1. After all the initial screening & cross-referencing processes, the full-text reading of 32 articles was done. Two investigators performed full-text reading and analysis of all these articles. After full-text reading, 25 articles were excluded as they did not fulfill the inclusion criteria. One article was included from cross-referencing. Final analysis and data extraction were performed for eight articles. Meta-analysis was performed for five articles.
Fig. 1.
PRISMA flowchart.
3.2. Study characteristics
3.2.1. In vitro studies
The in vitro studies included in the systematic review were published between 2022 and 2023. These studies originated from China (N = 3) and Korea (N = 1). In total, 594 dental implants were placed across 134 models. The comparisons made in these studies were between robotic-guided and static navigation-guided implants (N = 1) and between robotic-guided and dynamic navigation-guided implants (N = 3).
3.2.2. In vivo studies
The clinical studies included in the review were all published in 2023 and were conducted in China (N = 4). The study designs comprised prospective (N = 2), retrospective (N = 1), and RCT (N = 1). These studies involved 100 patients and placed 145 dental implants. The patient cohort included one completely edentulous and three partially edentulous individuals. Comparisons in these studies were made between robotic-guided and free-hand implants (N = 1), robotic-guided and static navigation-guided implants (N = 3), and robotic-guided and dynamic navigation-guided implants (N = 1).
Demographic characteristics and details on accuracy are summarized in Table 1, Table 2.
Table 1.
Demographic, characteristics and accuracy details (Invitro studies).
| S.NO. | Author, country, year | Study design | Objective | Number of models | Number of implants | Robotic system used | Area of implant placement | Comparison | Coronal deviation | Apical deviation | Angular deviation | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Jin X, South korea, (2022)18 | Invitro | To evaluate the tracking accuracy of a robot-guided implant surgery system and compare the spatial accuracy of robot-assisted implant surgery with that of static stent guided implant surgery for implant placement | 4 | 14 | Autonomous (da Vinci) Robotic system | 3-D printed mandible model | Robotic vs static | Robotic (0.61 ± 0.29) vs static (0.49 ± 0.39) | Robotic (0.50 ± 0.14) vs static (0.72 ± 0.34) | Robotic (2.38 ± 0.62) vs static (3.16 ± 2.36) | Accuracy in implant placement using robot-assisted implant surgery was comparable to that of static-guided surgery |
| 2. | Tao B, China, (2022)19 | Invitro | To compare the accuracy of dental implant placement using a dynamic navigation and a robotic system. | 80 | 480 | Hybrid robotic system | 3-D printed mandible model | Robotic vs dynamic | Robotic (0.83 ± 0.55) vs Dynamic (0.96 ± 0.57) | Robotic (0.96 ± 0.57) vs Dynamic (1.06 ± 0.59) | Robotic (1 ± 0.48) vs Dynamic (2.41 ± 1.42) | Implant positioning accuracy of the robotic system was superior to that of the dynamic navigation system |
| 3. | Chen, China, (2023)20 | Invitro | To compare the accuracy of dental implant placement using a novel dental implant robotic system (THETA) and a dynamic navigation system (Yizhimei) by a vitro model experiment | 10 | 20 | THETA robotic system | 3-D printed mandible model | Robotic vs dynamic | Robotic (0.46 ± 0.29) vs Dynamic (0.70 ± 0.21) | Robotic (0.56 ± 0.30) vs Dynamic (0.85 ± 0.25) | Robotic (1.36 ± 0.54) vs Dynamic (3.44 ± 1.38) | Implant positioning accuracy of the robotic system, especially the angular deviation was superior to that of the dynamic navigation system |
| 4. | Chen, Zhuong, China, (2023)8 | Invitro | To compare the accuracy of dental implant placement in a single tooth gap, including the postextraction site and healed site, using a task-autonomous robotic system and a dynamic navigation system. | 40 | 80 | Remebot robotic system | 3-D printed maxilla model | Robotic vs dynamic | Robotic (0.58 ± 0.31) vs dynamic (0.73 ± 0.20) | Robotic (0.69 ± 0.29) v/s Dynamic (0.86 ± 0.33) | Robotic (1.08 ± 0.66) vs Dynamic (2.32 ± 0.71) | The position in both immediate and conventional implant placement was more precise with the task-autonomous robotic system than with the dynamic navigation system |
Table 2.
Demographic, characteristics and accuracy details (invivo studies).
| S.NO. | Author, country, year | Study design | Objective | Number of patients | Number of implants | Robotic system used | Area of implant placement | Comparison | Coronal deviation | Apical deviation | Angular deviation | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Shasha Jia, China, 202321 | R | To compare the robotic with static in terms of the accuracy of dental implantation in dentition defects | 39 | 39 | Yake robot limited tech | Maxilla & mandible | Robotic v/s static navigation | Robotic (0.43 ± 0.18) v/s static (1.31 ± 0.62) | Robotic (0.56 ± 0.18) v/s Static (1.47 ± 0.65) | Robotic (1.48 ± 0.59°) v/s static (2.42 ± 1.55°) | The accuracy of the implant position using the ADIR robotic system was significantly higher than with static CAIS |
| 2. | Wenxue Wang, China, 202322 | P | To compare the accuracy of the Yake bot dental implant robotic system with that of fully guided static computer assisted implant surgery (CAIS) template in edentulous implantation. | 13 | 84 | Yake robot limited tech | Maxilla & mandible | Robotic v/s static navigation | Robotic (0.65 ± 0.25) v/s static (1.37 ± 0.72) | Robotic (0.65 ± 0.22) v/s static (1.28 ± 0.68) | Robotic (1.43 ± 1.18°) v/s static (3.47 ± 2.02°) | The accuracy of robotic system in edentulous implant placement was superior to that of the static CAIS |
| 3. | Wei Chen, China, 202323 | P | To assess the accuracy of implant positions using a robotic system in partially edentulous patients. | 381 | 726 | Den core, Lancet Robotics Co., LTD | Maxilla & mandible | Robotic v/s static navigation vs dynamic navigation | Robotic (0.53 ± 0.23) v/s static (0.91 ± 0.12) v/s dynamic (1.28) | Robotic (0.53 ± 0.24) v/s static (1.26 ± 0.12) v/s dynamic (1.68°) | Robotic (2.81 ± 1.13°) v/s static (3.25° ± 0.41) v/s dynamic (3.79°) | robotic system appears to achieve higher accuracy in implant positions than static & dynamic CAIS in partially edentulous patients |
| 4. | Jun-Yu Shi, China, 20236 | RCT | To Compare the implant accuracy, safety and morbidity between robot-assisted and freehand dental implant placement. | 20 | 20 | Theta implant system | Maxilla & mandible | Robotic v/s free hand | Robotic (1.23) vs freehand (1.9) | Robotic (1.40) vs freehand (2.1) | Robotic (3.0) vs freehand (6.7) | Robot-assisted implant placement enabled greater positional accuracy of the implant compared to freehand placement in this pilot trial. |
3.2.3. Risk of bias
Among the included in-vitro studies (N = 4), as per the QUIN tool, two had a low RoB, and two had a medium RoB (Supplementary Table S2). For invivo studies (N = 4), as per the JBI tool, two had a low RoB, and two had a high RoB (Supplementary Table S3.1, S3.2, S3.3).
3.3. Meta-analysis
3.3.1. In vitro studies
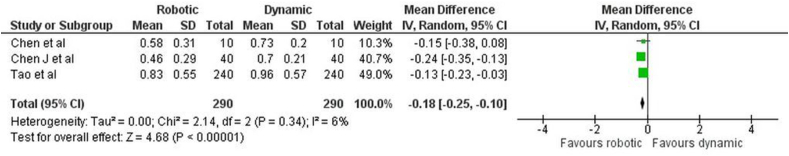
Coronal deviation: The forest plot (Fig. 2) for the coronal deviation between r-CAIS and d-CAIS showed a mean difference of −0.18 (−0.25, −0.10), demonstrating a statistically significant overall effect (z = 4.68, p < 0.001). There was notable heterogeneity, with a Tau2 of 0.00, a Chi2 of 2.14 with 2 degrees of freedom (df) (p = 0.34), and an I2 of 6 %, indicating low variability in effect sizes.
Fig. 2.
Forest plot representing the coronal deviation of the Invitro study.
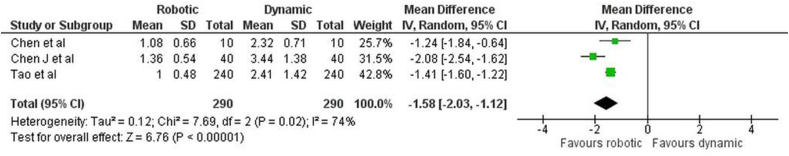
Angular deviation: The forest plot (Fig. 3) for the angular deviation between r-CAIS and d-CAIS showed a mean difference of −1.58 (−2.03, −1.12), reflecting a statistically significant overall effect (z = 6.76, p < 0.001). This analysis demonstrated significant heterogeneity, with a Tau2 of 0.12, a Chi2 of 7.69 with 2 df (p = 0.02), and an I2 of 74 %, suggesting substantial variability in effect sizes.
Fig. 3.
Forest plot representing the angular deviation of the Invitro study.
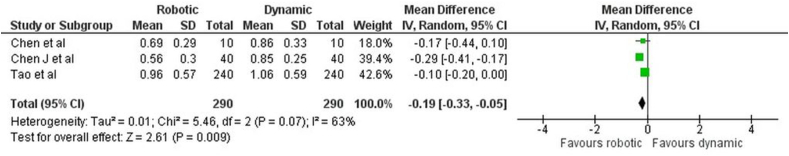
Apical deviation: The forest plot (Fig. 4) comparing apical deviation between r-CAIS and d-CAIS revealed a mean difference of −0.19 (−0.33, −0.05), indicating a statistically significant overall effect (z = 2.61, p < 0.01). There was considerable heterogeneity, with a Tau2 of 0.01, a Chi2 of 5.46 with 2 df (p = 0.07), and an I2 of 63 %.
Fig. 4.
Forest plot representing the apical deviation of the Invitro study.
3.3.2. In vivo studies
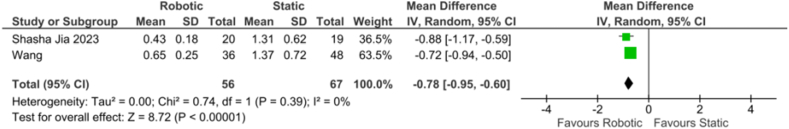
Coronal deviation: The forest plot (Fig. 5) for the comparison of coronal deviation between r-CAIS and s-CAIS showed a mean difference of −0.78 (−0.95, −0.60), indicating a statistically significant overall effect (z = 8.72, p < 0.001). There was notable heterogeneity among the studies, with a Tau2 of 0.00, a Chi2 of 0.74 with 1 df (p = 0.39), and an I2 of 0 %, suggesting minimal variability in effect sizes across studies.
Fig. 5.
Forest plot representing the coronal deviation of the Invivo study.
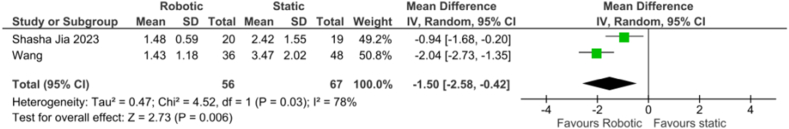
Angular deviation: The forest plot (Fig. 6) for the angular deviation between r-CAIS and s-CAIS revealed a mean difference of −1.50 (−2.58, −0.42), with a statistically significant overall effect (z = 2.73, p < 0.01). This analysis showed considerable heterogeneity, with a Tau2 of 0.47, a Chi2 of 4.52 with 1 df (p = 0.03), and an I2 of 78 %, indicating substantial variability in effect sizes.
Fig. 6.
Forest plot representing the angular deviation of the Invivo study.
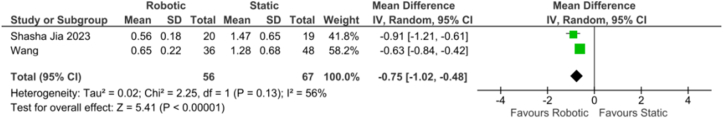
Apical deviation: The forest plot (Fig. 7) comparing apical deviation between r-CAIS and s-CAIS presented a mean difference of −0.75 (−1.02, −0.48), with a statistically significant overall effect (z = 5.41, p < 0.01). The analysis indicated considerable heterogeneity: a Tau2 of 0.02, a Chi2 of 2.25 with 1 df (p = 0.13), and an I2 of 56 %.
Fig. 7.
Forest plot representing the apical deviation of the Invivo study.
4. Discussion
Conventional dental implant surgery has progressively evolved in the modern era. Integrating modern techniques, such as static and dynamic navigation systems, has greatly facilitated treatment planning and surgery execution. Numerous systematic reviews and meta-analyses have demonstrated the enhanced precision achieved through these methods compared to the conventional freehand approach in dental implant placement.13, 14, 15
Evidence synthesis indicates that static and dynamic navigation systems exhibit comparable accuracy in dental implant placement. Notably, dynamic navigation presents as an accessible option even for novice practitioners. However, despite their advantages, these systems are not devoid of limitations, including computer data-based errors, potential distraction from split-screen interfaces, and the inherent risk of human error during implant placement. Although robotic systems with robotic arms have been posited to mitigate manual errors associated with other techniques, the existing literature lacks pooled evidence of clinical studies supporting their efficacy.16,17
Thus, this systematic review compares robotic-assisted implant surgery with conventional freehand, static, and dynamic navigation-guided implant surgeries.
4.1. Invitro studies
Jin et al. (2022)18 conducted an in vitro study using four 3D-printed mandibular phantom models, placing 14 implants in the mandibular canine and premolar regions. They compared robotic-guided to static-guided implant placement, reporting coronal deviations of 0.61 ± 0.29 mm for robotic and 0.49 ± 0.39 mm for static guidance. Angular deviations were 2.38 ± 0.62° for robotic and 3.16 ± 2.36° for static guidance. Apical deviations were 0.50 ± 0.14 mm for robotic and 0.72 ± 0.34 mm for static guidance. The study concluded that robotic-guided implant placement is either more accurate or comparable to static guidance.
Tao et al. (2022)19 performed an in vitro study comparing implant placement accuracy using robotic and dynamic navigation systems across 80 phantom models. They found coronal deviations of 0.83 ± 0.55 mm for robotic and 0.96 ± 0.57 mm for dynamic navigation. Angular deviations were 1.00 ± 0.48° for robotic and 2.41 ± 1.42° for dynamic navigation. Apical deviations were 0.96 ± 0.57 mm for robotic and 1.06 ± 0.59 mm for dynamic navigation. The results indicated that the robotic system demonstrated statistically significant superior accuracy compared to the dynamic navigation system.
Chen et al. (2023)20 conducted an in vitro study with 40 partially edentulous models, placing 80 implants using robotic and dynamic navigation systems. They reported coronal deviations of 0.58 ± 0.31 mm for robotic and 0.73 ± 0.20 mm for dynamic navigation, angular deviations of 1.08 ± 0.66° for robotic and 2.32 ± 0.71° for dynamic navigation, and apical deviations of 0.69 ± 0.29 mm for robotic and 0.86 ± 0.33 mm for dynamic navigation. The study concluded that the robotic system provided more precise implant placement than the navigation system.
Chen et al. (2023)8 similarly compared implant placement accuracy on 10 3D-printed mandibular models using robotic and dynamic navigation systems. They found coronal deviations of 0.46 ± 0.29 mm for robotic and 0.70 ± 0.21 mm for dynamic navigation, angular deviations of 1.08 ± 0.66° for robotic and 2.32 ± 0.71° for dynamic navigation, and apical deviations of 0.69 ± 0.29 mm for robotic and 0.86 ± 0.33 mm for dynamic navigation. The study concluded that the robotic system achieved higher accuracy, particularly in angular deviation, than the navigation system.
4.2. In vivo studies
Shasha et al. (2023)21 conducted a retrospective study involving partially edentulous patients, comparing robotic and static computer-assisted surgery. The reported outcomes for robotic-assisted surgery were as follows: coronal deviation of 0.43 ± 0.18 mm, apical deviation of 0.56 ± 0.18 mm, and angular deviation of 1.48 ± 0.59°. In contrast, static computer-assisted surgery showed deviations of 1.31 ± 0.62 mm, 1.47 ± 0.65 mm, and 2.42 ± 1.55°, respectively.
Wenxue Wang et al. (2023)22 performed a prospective study on edentulous cases, reporting outcomes for robotic-assisted surgery as 0.65 ± 0.25 mm for coronal deviation, 0.65 ± 0.22 mm for apical deviation, and 1.43 ± 1.18° for angular deviation. The corresponding values for static-assisted surgery were 1.37 ± 0.72 mm, 1.28 ± 0.68 mm, and 3.47 ± 2.02°, respectively.
Wei Chen et al. (2023)23 conducted a prospective study on partially edentulous patients, integrating previous meta-analyses and employing Objective Performance Goals (OPG) scores for comparison. The study concluded that robotic-assisted implant placement demonstrated significantly higher accuracy than static and dynamic-assisted surgeries. The results for robotic-assisted surgery were 0.53 ± 0.23 mm for coronal deviation, 0.53 ± 0.24 mm for apical deviation, and 2.81 ± 1.13° for angular deviation. In contrast, static-assisted and dynamic-assisted surgeries showed coronal deviations of 0.91 mm vs. 1.28 mm, apical deviations of 1.26 mm vs. 1.68 mm, and angular deviations of 3.79° vs. 3.25°, respectively.
Jun-Yu Shi et al. (2023)6 conducted a randomized controlled trial comparing robotic-assisted and freehand techniques in partially edentulous participants. They reported coronal deviations of 1.23 mm (range 0.9–1.4 mm) for robotic and 1.9 mm (range 1.2–2.3 mm) for freehand, apical deviations of 1.40 mm (range 1.1–1.6 mm) for robotic and 2.1 mm (range 1.7–3.9 mm) for freehand, and angular deviations of 3.0° (range 0.9–6.0°) for robotic and 6.7° (range 2.2–13.9°) for freehand. Statistically significant differences were found favouring robotic-assisted surgery for coronal and apical deviations (p < 0.05 and p < 0.01, respectively), although angular deviation did not reach statistical significance (p > 0.05).
Robotic-assisted techniques consistently demonstrate superior accuracy to conventional freehand methods and static and dynamic navigation systems. This finding is consistent with trends observed in orthopaedic procedures, including knee, hip prostheses, and spine screw positioning.24, 25, 26
Systematic reviews of in vitro studies have shown that robotic systems achieve higher implant accuracy.27 This advantage is primarily due to the reduction of manual errors from hand tremors, as robotic systems operate independently of direct human manipulation. The real-time feedback and efficiency of robotic arms further enhance accuracy, while human interactions such as controlling speed, positioning the robotic arm within the surgical field, and withdrawing the arm in response to unexpected patient movements add predictability and safety to the procedure.28, 29, 30
The observed variations in robotic system accuracy across different studies can be attributed to several factors, including the operator's experience with robotic technology, the complexity and precision of preoperative procedures such as system registration and calibration, and the quality of Cone Beam Computed Tomography images used for measuring deviations.8,15,31 These variables contribute to outcome variability. Despite these challenges, robotic systems consistently show high accuracy and reliability, significantly reducing human error compared to traditional methods. This underscores the potential of robotic systems to enhance surgical precision on models and improve patient outcomes across various medical procedures.32 However, this finding should be interpreted with caution due to the limited number of clinical studies and associated study heterogeneity.
When evaluating the efficacy of robotic systems in clinical settings, it's essential to balance precision with practical considerations. Patient satisfaction plays a significant role in influencing compliance and overall outcomes. Additionally, recovery durations are crucial; faster recovery can enhance patient experience and reduce hospital stays.23,25 Overall implant success rates also provide a tangible measure of the robotic system's performance. By integrating these factors, healthcare providers can better assess the true value of robotic systems beyond just technical precision, ensuring that they contribute positively to patient care and outcomes.3,24
To our knowledge, this systematic review and meta-analysis is the first to compare the accuracy of robotic-assisted dental implant placement with conventional methods—including freehand placement, static navigation, and dynamic navigation—within a clinical context. However, several limitations must be acknowledged.
Firstly, there is a paucity of clinical studies directly comparing robotic systems with other dental implant techniques, and the included studies often have limited sample sizes. Additionally, there is significant heterogeneity among the studies with respect to design, comparison methodologies, patient characteristics (e.g., partial vs. complete edentulism), and the specific robotic systems utilized. Furthermore, the review is constrained by the absence of randomized clinical trials directly comparing robotic-assisted dental implant surgery with static or dynamic navigation-assisted procedures.
Several clinical studies have highlighted various limitations of robotic implant placement systems.18,31,32 One key issue is that the robotic arm does not have the same flexibility as the human hand, which makes it challenging to reach the posterior regions of the jaws, particularly in areas with limited interocclusal space. Increased angular deviations in these posterior zones further exacerbate this difficulty. Apical deviation is seen to be affected by implant length, most likely as a result of the guide's crown side being secured while the apical side remained movable. Moreover, the drills were usually directed toward less resistant areas of bone during osteotomy preparation, like freehand surgery and guided implant placement, robotic-assisted surgery poses a risk of drill drifting.33 Additionally, each robotic surgery system's methodology and learning curve vary. The capabilities of the robotic surgery devices currently on the market are restricted to implant osteotomy and placement. Moreover, sufficient regulatory, ethical, and legal frameworks may not be accessible for each progress in robotic CAIS technology.25,34
Further, major drawbacks of robotic implant systems, such as extended preparation time before surgery and detailed cost considerations, were not thoroughly addressed in the available studies, and most of the in vitro and in vivo studies were noted to be funded. The cost of dental robotic systems ranges from approximately $600,000 to $2.5 million, encompassing semi-active, active, and passive systems.35,36 This substantial investment challenges incorporating robotic systems into routine dental implantology practice, mostly in developing countries.
A thorough evaluation of the learning curve for robotic technology in surgery reveals that structured training, effective mentorship, and ongoing assessment are crucial for developing proficiency. Understanding this curve benefits surgeons and enhances patient safety and surgical outcomes, making it vital to integrate robotic systems into clinical practice.14,32
To address these limitations, future research should include non-manufacturer-sponsored studies that provide detailed cost analyses and examine time constraints associated with robotic systems. Additional studies should focus on broader clinical contexts and larger sample sizes to offer more comprehensive insights and facilitate clearer conclusions regarding integrating robotic systems into regular dental implant practices.
5. Conclusion
This systematic review and meta-analysis provide the first comprehensive evaluation of robotic-assisted dental implant placement compared to conventional freehand, static, and dynamic navigation techniques. Our findings indicate that robotic-assisted systems consistently offer superior accuracy in coronal, apical, and angular deviations, highlighting their potential to enhance implant placement precision and surgical outcomes. The automation of robotic systems reduces manual errors and provides real-time feedback, contributing to improved predictability and safety. However, the review reveals limitations, including a lack of direct comparative clinical studies, significant variability in study designs, and high robotic system costs, ranging from $600,000 to $2.5 million. Future research should address these gaps by including more extensive, non-manufacturer-sponsored studies with detailed cost analyses and focusing on broader clinical contexts to better understand the practical implications of robotic systems in routine dental implantology.
Ethics statement
The following review on dental implant placement accuracy with robotic surgery compared to free-hand, static and dynamic computer assisted techniques did not require ethics approval.
Patients consent
This is a review article on comparing the accuracy in the dental implant placement between robotic system and non-robotic systems. Thus, no patient consent was needed.
Funding sources
Nil.
Declaration of Competing Interest
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work has been done at the All India Institute of Medical Sciences (AIIMS), Bathinda.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2024.12.005.
Contributor Information
Hariram Sankar, Email: meethariram205@gmail.com.
M. Shalini, Email: ms418598@gmail.com.
Anjana Rajagopalan, Email: anj26795@gmail.com.
Satish Gupta, Email: guptasatish_27@yahoo.com.
Amit Kumar, Email: thakur.amit100@gmail.com.
Rukhsar Shouket, Email: rukhsarshowkat2611@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Yeshwante B., Baig N., Tambake S.S., Tambake R., Patil V., Rathod R. Mastering dental implant placement: a review. J Appl Dent Med Sci. 2017;3(2):220–227. [Google Scholar]
- 2.Rawal S. vol. 127. 2022. (Guided Innovations: Robot-Assisted Dental Implant Surgery). [DOI] [PubMed] [Google Scholar]
- 3.Fortin T., Champleboux G., Bianchi S., Buatois H., Coudert J.L. Precision of transfer of preoperative planning for oral implants based on cone-beam CT-scan images through a robotic drilling machine. Clin Oral Implants Res. 2002;13(6):651–656. doi: 10.1034/j.1600-0501.2002.130612.x. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z., Xiao Y., Zhou L., et al. Accuracy and efficiency of robotic dental implant surgery with different human-robot interactions: an in vitro study. J Dent. 2023;137 doi: 10.1016/j.jdent.2023.104642. [DOI] [PubMed] [Google Scholar]
- 5.Li P., Chen J., Li A., Luo K., Xu S., Yang S. Accuracy of autonomous robotic surgery for dental implant placement in fully edentulous patients: a retrospective case series study. Clin Oral Implants Res. 2023;34(12):1428–1437. doi: 10.1111/clr.14188. [DOI] [PubMed] [Google Scholar]
- 6.Shi J., Liu B., Wu X., et al. Improved positional accuracy of dental implant placement using a haptic and machine‐vision‐controlled collaborative surgery robot: a pilot randomized controlled trial. J Clin Periodontol. 2023;51(1):24–32. doi: 10.1111/jcpe.13893. [DOI] [PubMed] [Google Scholar]
- 7.Ivashchenko A.V., Yablokov A.E., Komlev S.S., Stepanov G.V., Tsimbalistov A.V. [Robot-assisted and robotic systems used in dentistry] Stomatologiia (Sofiia) 2020;99(1):95–99. doi: 10.17116/stomat20209901195. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Bai X., Ding Y., et al. Comparison the accuracy of a novel implant robot surgery and dynamic navigation system in dental implant surgery: an in vitro pilot study. BMC Oral Health. 2023;23(1):179. doi: 10.1186/s12903-023-02873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J., Li H. Accuracy assessment of robot-assisted implant surgery in dentistry: a systematic review and meta-analysis. J Prosthet Dent. 2024;132(4):747.e1–747.e15. doi: 10.1016/j.prosdent.2023.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth V.H., Shah N.P., Jain R., Bhanushali N., Bhatnagar V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: the QUIN. J Prosthet Dent. 2022;131(6):1038–1042. doi: 10.1016/j.prosdent.2022.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Barker T.H., Stone J.C., Sears K., et al. Revising the JBI quantitative critical appraisal tools to improve their applicability: an overview of methods and the development process. JBI Evid Synth. 2023;21(3):478–493. doi: 10.11124/JBIES-22-00125. [DOI] [PubMed] [Google Scholar]
- 13.van Riet T.C., Sem K.T.C.J., Ho J.P.T., Spijker R., Kober J., de Lange J. Robot technology in dentistry, part two of a systematic review: an overview of initiatives. Dent Mater. 2021;37(8):1227–1236. doi: 10.1016/j.dental.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang S., Chen J., Li A., Deng K., Li P., Xu S. Accuracy of autonomous robotic surgery for single-tooth implant placement: a case series. J Dent. 2023;132 doi: 10.1016/j.jdent.2023.104451. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs R., Salmon B., Codari M., Hassan B., Bornstein M.M. Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health. 2018;18:1–16. doi: 10.1186/s12903-018-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y., Zheng Y., Chen R., et al. Accuracy of a novel semi-autonomous robotic-assisted surgery system for single implant placement: a case series. J Dent. 2023 doi: 10.1016/j.jdent.2023.104766. Published online. [DOI] [PubMed] [Google Scholar]
- 17.Linn T.Y., Salamanca E., Aung L.M., Huang T.K., Wu Y.F., Chang W.J. Accuracy of implant site preparation in robotic navigated dental implant surgery. Clin Implant Dent Relat Res. 2023;25(5):881–891. doi: 10.1111/cid.13224. [DOI] [PubMed] [Google Scholar]
- 18.Jin X., Kim R.J.Y., Park J.M., et al. Accuracy of surgical robot system compared to surgical guide for dental implant placement: a pilot study. J Implantol Appl Sci. 2022;26(1):27–38. [Google Scholar]
- 19.Tao B., Feng Y., Fan X., et al. Accuracy of dental implant surgery using dynamic navigation and robotic systems: an in vitro study. J Dent. 2022;123 doi: 10.1016/j.jdent.2022.104170. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Zhuang M., Tao B., Wu Y., Ye L., Wang F. Accuracy of immediate dental implant placement with task‐autonomous robotic system and navigation system: an in vitro study. Clin Oral Implants Res. 2023;35(8):973–983. doi: 10.1111/clr.14104. [DOI] [PubMed] [Google Scholar]
- 21.Jia S., Wang G., Zhao Y., Wang X. Accuracy of an autonomous dental implant robotic system versus static guide-assisted implant surgery: a retrospective clinical study. J Prosthet Dent. 2023 doi: 10.1016/j.prosdent.2023.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Wang W., Xu H., Mei D., et al. Accuracy of the Yakebot dental implant robotic system versus fully guided static computer‐assisted implant surgery template in edentulous jaw implantation: a preliminary clinical study. Clin Implant Dent Relat Res. 2024;26(2):309–316. doi: 10.1111/cid.13278. [DOI] [PubMed] [Google Scholar]
- 23.Chen W., Al‐Taezi K.A., Chu C.H., et al. Accuracy of dental implant placement with a robotic system in partially edentulous patients: a prospective, single‐arm clinical trial. Clin Oral Implants Res. 2023;34(7):707–718. doi: 10.1111/clr.14083. [DOI] [PubMed] [Google Scholar]
- 24.Thilak J., Babu B.C., Thadi M., et al. Accuracy in the execution of pre-operative plan for limb alignment and implant positioning in robotic-arm assisted total knee arthroplasty and manual total knee arthroplasty: a prospective observational study. Indian J Orthop. 2021;55:953–960. doi: 10.1007/s43465-020-00324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunze K.N., Huddleston H.P., Romero J., Chiu Y.F., Jerabek S.A., McLawhorn A.S. Accuracy and precision of acetabular component position does not differ between the anterior and posterior approaches to total hip arthroplasty with robotic assistance: a matched-pair analysis. Arthroplasty Today. 2022;18:68–75. doi: 10.1016/j.artd.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogalo C., Meena A., Abermann E., Fink C. Complications and downsides of the robotic total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2023;31(3):736–750. doi: 10.1007/s00167-022-07031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain S., Sayed M.E., Ibraheem W.I., et al. Accuracy comparison between robot-assisted dental implant placement and static/dynamic computer-assisted implant surgery: a systematic review and meta-analysis of in vitro studies. Medicina (Mex) 2023;60(1):11. doi: 10.3390/medicina60010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kan T., Cheng K., Liu Y., et al. Evaluation of a custom‐designed human–robot collaboration control system for dental implant robot. Int J Med Robot. 2022;18(1) doi: 10.1002/rcs.2346. [DOI] [PubMed] [Google Scholar]
- 29.Cheng K jie, shu Kan T., feng Liu Y., et al. Accuracy of dental implant surgery with robotic position feedback and registration algorithm: an in-vitro study. Comput Biol Med. 2021;129 doi: 10.1016/j.compbiomed.2020.104153. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z., Zhou L., Han B., et al. Accuracy of dental implant placement using different dynamic navigation and robotic systems: an in vitro study. NPJ Digit Med. 2023;7(1):182. doi: 10.1038/s41746-024-01178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolding S.L., Reebye U.N. Accuracy of haptic robotic guidance of dental implant surgery for completely edentulous arches. J Prosthet Dent. 2022;128(4):639–647. doi: 10.1016/j.prosdent.2020.12.048. [DOI] [PubMed] [Google Scholar]
- 32.Qiao S., Wu X., Shi J., Tonetti M.S., Lai H. Accuracy and safety of a haptic operated and machine vision controlled collaborative robot for dental implant placement: a translational study. Clin Oral Implants Res. 2023;34(8):839–849. doi: 10.1111/clr.14112. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y., Liao Y., Wu X., Zhang Y., Shi B., Yan Q. Effect of the number and distribution of fiducial markers on the accuracy of robot-guided implant surgery in edentulous mandibular arches: an in vitro study. J Dent. 2023;134 doi: 10.1016/j.jdent.2023.104529. [DOI] [PubMed] [Google Scholar]
- 34.Xu Z., Xiao Y., Zhou L., et al. Accuracy and efficiency of robotic dental implant surgery with different human-robot interactions: an in vitro study. J Dent. 2023;137 doi: 10.1016/j.jdent.2023.104642. [DOI] [PubMed] [Google Scholar]
- 35.Kayani B., Konan S., Ayuob A., Onochie E., Al-Jabri T., Haddad F.S. Robotic technology in total knee arthroplasty: a systematic review. EFORT Open Rev. 2019;4(10):611–617. doi: 10.1302/2058-5241.4.190022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dam P., Hauspy J., Verkinderen L., et al. Are costs of robot‐assisted surgery warranted for gynecological procedures? Obstet Gynecol Int. 2011;2011(1) doi: 10.1155/2011/973830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.