Abstract
Fibromyalgia is a chronic pain condition contributing to significant disability worldwide. Neuroimaging studies identify abnormal effective connectivity between cortical areas responsible for descending pain modulation (pregenual anterior cingulate cortex, pgACC) and sensory components of pain experience (primary somatosensory cortex, S1). Neurofeedback, a brain-computer interface technique, can normalise dysfunctional brain activity, thereby improving pain and function. This study evaluates the safety, feasibility, and acceptability of a novel electroencephalography-based neurofeedback training, targeting effective alpha-band connectivity from the pgACC to S1 and exploring its effect on pain and function. Participants with fibromyalgia (N = 30; 15 = active, 15 = placebo) received 12 sessions of neurofeedback. Feasibility and outcome measures of pain (Brief Pain Inventory) and function (Revised Fibromyalgia Impact Questionnaire) were collected at baseline and immediately, ten-days, and one-month post-intervention. Descriptive statistics demonstrate effective connectivity neurofeedback training is feasible (recruitment rate: 6 participants per-month, mean adherence: 80.5%, dropout rate: 20%), safe (no adverse events) and highly acceptable (average 8.0/10) treatment approach for fibromyalgia. Active and placebo groups were comparable in their decrease in pain and functional impact. Future fully powered clinical trial is warranted to test the efficacy of the effective connectivity neurofeedback training in people with fibromyalgia with versus without chronic fatigue.
Keywords: Fibromyalgia, Neurofeedback, Brain computer interface, Pain
Subject terms: Health care, Signs and symptoms, Pain, Chronic pain
Introduction
Fibromyalgia is a chronic primary pain condition characterised by widespread musculoskeletal pain accompanied by secondary symptoms including fatigue, and disturbances in cognition, sleep, mood and dysautonomia1,2. Current treatments for pain associated with fibromyalgia employ a combination of non-pharmacological and pharmacological therapies3 such as anticonvulsants, antidepressants, weak opioids4,5, and lifestyle changes6. The high burden of disability in this population suggests that current treatments are inadequate7,8.
Brain alterations have been demonstrated in people with fibromyalgia, in particular, heightened activity in the salience network (amygdala, cingulate cortices, insula) and somatosensory cortex (S1)9. These changes may be associated with chronic pain or other symptoms of fibromyalgia and have shown a predictive accuracy of 93% in identifying fibromyalgia, indicating a distinctive brain signature that can be detected by artificial intelligence10. Consequently, fibromyalgia is a brain related disorder. Neuroimaging suggests a decreased descending inhibition, with decreased activity of pregenual anterior cingulate cortex (pgACC)11–15. Testing interventions targeting brain activity that drives symptoms associated with fibromyalgia is thus warranted.
Electroencephalography (EEG)-based neurofeedback utilises real time analysis of brain electrical activity, enabling individuals to learn and modify their brain patterns through visual or auditory feedback aiming to influence defective pathways towards healthier states 16,17. Traditional neurofeedback targets brain activity, aiming to either increase or decrease the power in a particular frequency band. The most common target for fibromyalgia is training the sensorimotor rhythm/theta ratio18–24. This has shown some promise, however the heterogenous nature of research conducted in this field makes it difficult to draw definitive conclusions 20.
EEG technology has advanced to use source localisation, allowing neurofeedback protocols to target specific areas of brain activity associated with pain processing. A recent study tested three different neurofeedback protocols in individuals with chronic lower back pain 25. Training that increased activity of the pgACC was found to be the most effective in improving pain outcomes. Furthermore, secondary analysis demonstrated that individuals with a higher effective connectivity (EC) from pgACC to S1 at follow up had a more significant decrease in pain intensity 26. While functional connectivity of the brain refers to synchronous activity in two given regions, EC applies a directionality to this, allowing us to infer the influence of one brain region on another 27. Therefore, we propose a novel method of neurofeedback to train EC from the pgACC to S1. We particularly selected the alpha band, as recent imaging studies have demonstrated alterations in alpha oscillations in individuals with fibromyalgia in pain processing networks 28–30.
The objectives of this study were to explore the safety, feasibility, and acceptability of a novel EEG neurofeedback training protocol, targeting EC from the pgACC to S1 in people with fibromyalgia. Additionally, we conducted exploratory analyses to determine the estimates of change in pain, function, and EEG measures following the intervention. This pilot study will inform the design of a future fully-powered randomised controlled trial.
Results
N = 30 participants were enrolled and randomised equally into two groups (Fig. 1.) Table 1 presents descriptive data for all participants at baseline, indicating both groups were comparable.
Fig. 1.
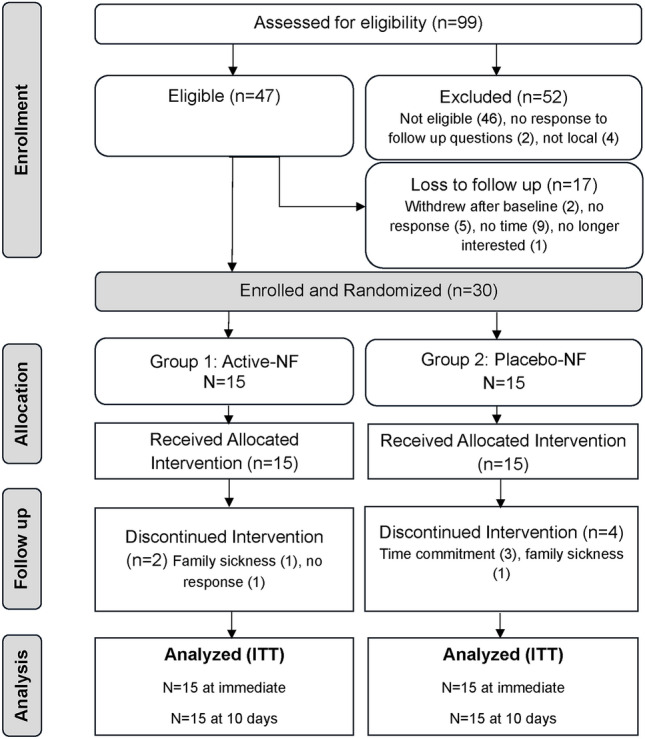
Flow of participants through the study phases. ITT = Intention to treat.
Table 1.
Demographics and clinical characteristics of participants.
| Active (N = 15) | Placebo (N = 15) | P value | |
|---|---|---|---|
| Age (years) (Mean ± SD) | 42.4 (17) | 46.5 (13.8) | 0.52 |
| Gender | |||
| Male; n (%) | 1 (6.7) | 1 (6.7) | NA |
| Female; n (%) | 14 (93.3) | 13 (86.7) | NA |
| Non-Binary; n (%) | 0 (0) | 1 (6.7) | NA |
| Ethnicity | |||
| NZ European; n (%) | 15 (100) | 12 (80) | NA |
| Other (Hispanic (1), Nigerian (1), Scottish (1)); n (%) | 0 (0) | 3 (20) | NA |
| Employment | |||
| Employed; n (%) | 8 (53) | 7 (46.7) | NA |
| Unemployed; n (%) | 2 (13) | 4 (26.7) | NA |
| Retired; n (%) | 2 (13) | 1 (6.7) | NA |
| Looking after family; n (%) | 1 (6.7) | 2 (13) | NA |
| Self-employed; n (%) | 2 (13) | 0 (0) | NA |
| Studying; n (%) | 0 (0) | 1 (6.7) | NA |
| Highest Education | |||
| University degree; n (%) | 3 (20) | 7 (46.7) | NA |
| Trade/Apprenticeship; n (%) | 0 (0) | 0 (0) | NA |
| Certificate/Diploma; n (%) | 6 (40) | 5 (33) | NA |
| Year 12/equivalent; n (%) | 3 (20) | 1 (6.7) | NA |
| Year 10/equivalent; n (%) | 2 (13) | 1 (6.7) | NA |
| No formal qualification; n (%) | 1 (6.7) | 1 (6.7) | NA |
| Fibromyalgia | |||
| Duration of Symptoms (years) (Mean ± SD) | 14.4 (8.7) | 15.0 (10.6) | 0.64 |
| Time Since Diagnosis (years) (Mean ± SD) | 15.1 (10.6) | 11.0 (11.0) | 0.64 |
| Arthritis | |||
| Presence of Arthritis; n (%) | 3 (20) | 7 (46.7) | NA |
| Primary Clinical Outcome Measures | |||
| RFIQ (Mean ± SD) | 65.4 ± 9.6 | 59.7 ± 19.0 | 0.30 |
| BPI Pain Severity (Mean ± SD) | 5.6 ± 1.1 | 5.4 ± 2.1 | 0.76 |
| BPI Pain Interference (Mean ± SD) | 6.4 ± 1.9 | 6.1 ± 2.2 | 0.63 |
| Secondary Clinical Outcome Measures | |||
| PVAQ (Mean ± SD) | 50.3 ± 10.3 | 47.6 ± 11.8 | 0.51 |
| PCS (Mean ± SD) | 24.9 ± 14.2 | 22.9 ± 16.3 | 0.71 |
| AIMS Physical (Mean ± SD) | 2.2 ± 0.9 | 2.6 ± 1.3 | 0.68 |
| AIMS Affect (Mean ± SD) | 5.9 ± 1.4 | 4.7 ± 1.1 | 0.17 |
| AIMS Symptoms (Mean ± SD) | 5.8 ± 2.0 | 5.5 ± 1.9 | 0.80 |
| AIMS Social Interaction (Mean ± SD) | 4.6 ± 1.4 | 5.2 ± 1.3 | 0.54 |
| AIMS Role (Mean ± SD) | 4.4 ± 0.0 | 2.9 ± 0.0 | ND |
| DASS Stress (Mean ± SD) | 21.1 ± 8.6 | 19.2 ± 10.1 | 0.59 |
| DASS Anxiety (Mean ± SD) | 15.1 ± 9.1 | 17.1 ± 11.3 | 0.58 |
| DASS Depression (Mean ± SD) | 14.1 ± 10.0 | 15.7 ± 10.8 | 0.68 |
| WHO-5 Wellbeing (Mean ± SD) | 27.2 ± 16.9 | 34.4 ± 12.4 | 0.19 |
| EQ-5D Health Status (Mean ± SD) | 33.1 ± 27.0 | 40.6 ± 22.8 | 0.42 |
| EQ-5D Index Score (Mean ± SD) | 15.0 ± 27.8 | 7.0 ± 18.8 | 0.36 |
| MOS Sleep (Mean ± SD) | 56.3 ± 13.3 | 57.6 ± 14.1 | 0.79 |
| MTS (Mean ± SD) | 17.5 ± 19.6 | 17.1 ± 21.9 | 0.96 |
| PPT (Mean ± SD) | 161.8 ± 76.7 | 206.3 ± 120.5 | 0.23 |
RFIQ = Revised Fibromyalgia Impact Questionnaire, BPI = Brief Pain Inventory, PVAQ = Pain Vigilance and Awareness Questionnaire, PCS = Pain Catastrophising Scale, AIMS = Arthritis Impact Measurement Scale, DASS = Depression, Anxiety and Stress Scale, WHO-5 = World Health Organisation – 5 well-being index, EQ-5D = European Quality of life – 5 Dimensions, MOS = Medical Outcomes Scale, MTS = Mechanical Temporal Summation, PPT = Pressure Pain Threshold. NA: Not applicable; ND: Not determinable due to low sample in each group. The p values are for the between-group statistical comparisons using unpaired students’s t-tests.
Feasibility
Recruitment: The total recruitment period was five months (April to August 2023), with the last follow up completed and the trial stopped in October 2023 with attainment of the desired sample size (n = 30) and all follow ups completed. The average recruitment rate was six participants per month. The proportion of participants recruited (30) from the total number screened (99) was 30%. The main reasons for exclusion were not meeting the American College of Rheumatology (ACR) 2011 criteria and presence of one or more exclusion criteria.
Dropouts: Of the total participants enrolled and randomised, six participants discontinued treatment. Thus, the dropout rate for the placebo group was 27% (4/15) and for the active group was 13% (2/15). The overall dropout rate was 20%.
Treatment adherence and engagement: Mean session adherence excluding withdrawals was 100% in both active and placebo groups. Mean session adherence including withdrawals was 77.6% in the placebo group and 84% in the active group. Overall, mean treatment adherence was 80.5%.
Integrity of blinding: Participant blinding was deemed successful as the treatment group was not revealed to any individuals. In total, 58% incorrectly predicted the treatment group and 27% responded that they did not know. The 15% who correctly predicted their treatment group all based their decision on symptom assessment.
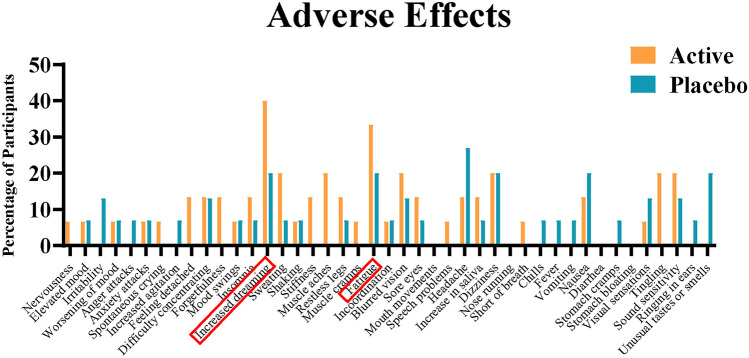
Adverse Effects: No serious adverse effects were reported. Several transient low intensity side effects were reported, rated to be related to the neurofeedback treatment, by some participants. The most common side effects in the treatment group included increase in dreaming and fatigue (Fig. 2) following sessions. No participants discontinued the study due to adverse effects.
Fig. 2.
Adverse effects reported by participants during the neurofeedback sessions.
Acceptability and satisfaction
All participants, regardless of treatment group, reported high levels of acceptability with mean ± SD of 8.4 ± 1.8 for active group and 7.6 ± 1.8 for placebo group; however, the proportion of participants that demonstrated high levels of acceptability (≥ 7) was higher in active group (90%) compared to sham group (47%). In addition, both groups also reported high levels of satisfaction with mean ± SD of 7.5 ± 2.4 active and 7.1 ± 3.2 placebo; however, the proportion of participants that demonstrated high levels of satisfaction (≥ 7) was higher in active group (60%) compared to sham group (40%).
Outcome measures
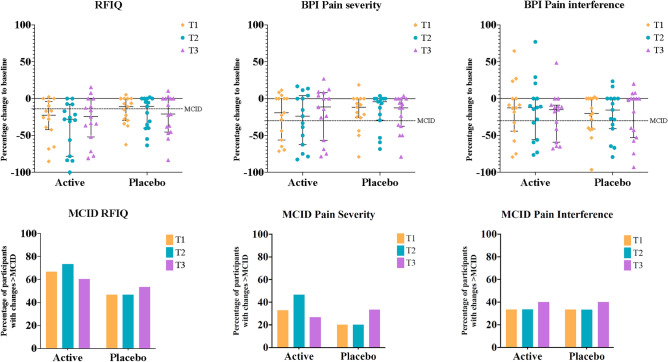
Clinical Outcomes: Table 2 presents data for primary and secondary pain and functional measures. Primary clinical outcomes are presented graphically in Fig. 3. Figure 3 suggests that in general, participants from both active treatment and placebo groups showed a decrease in pain at all time points. This is seen across both components of the Brief pain inventory-short form (BPI), pain severity and interference. The measures are comparable between active treatment and placebo groups, which suggest improvement in function at all time points. Figure 3 also presents the percentage of participants who demonstrated changes greater than the Minimal Clinical Important Difference (MCID) in primary outcomes between active treatment and placebo groups. Percentage of participants who demonstrated clinically important changes for function and pain were comparable between treatment groups.
Table 2.
Mean and percentage change in clinical outcomes.
| Clinical measure | Time point | Active (Mean ± SD) | Placebo (Mean ± SD) |
|---|---|---|---|
| RFIQ | T0 | 65.4 ± 9.6 | 59.7 ± 19.0 |
| T1 | 46.9 ± 19.6 | 50.1 ± 21.5 | |
| T2 | 40.7 ± 24.0 | 48.0 ± 23.0 | |
| T3 | 46.2 ± 19.9 | 46.5 ± 25.0 | |
| T1-T0 (% Change) | -28.1 ± 27.1 | -16.6 ± 18.9 | |
| T2-T0 (% Change) | -38.1 ± 33.7 | -20.3 ± 22.2 | |
| T3-T0 (% Change) | -28.5 ± 30.8 | -23.0 ± 27.1 | |
| BPI Pain Severity | T0 | 5.6 ± 1.1 | 5.4 ± 2.1 |
| T1 | 4.5 ± 2.2 | 4.5 ± 2.4 | |
| T2 | 4.2 ± 2.4 | 4.4 ± 2.2 | |
| T3 | 4.6 ± 2.3 | 4.3 ± 2.2 | |
| T1-T0 (% Change) | -23.5 ± 30.4 | -18.1 ± 24.5 | |
| T2-T0 (% Change) | -27.6 ± 35.5 | -17.0 ± 24.3 | |
| T3-T0 (% Change) | -19.6 ± 34.4 | -20.7 ± 24.6 | |
| BPI Pain Interference | T0 | 6.4 ± 1.9 | 6.1 ± 2.2 |
| T1 | 5.4 ± 2.4 | 4.7 ± 2.7 | |
| T2 | 5.3 ± 2.5 | 4.8 ± 2.7 | |
| T3 | 5.0 ± 2.4 | 4.8 ± 3.0 | |
| T1-T0 (% Change) | -15.7 ± 38.6 | -23.7 ± 27.7 | |
| T2-T0 (% Change) | -16.5 ± 40.9 | -21.7 ± 31.0 | |
| T3-T0 (% Change) | -23.8 ± 31.8 | -23.1 ± 34.5 | |
| PVAQ | T0 | 50.3 ± 10.3 | 47.6 ± 11.8 |
| T1 | 42.1 ± 12.0 | 43.9 ± 13.0 | |
| T2 | 41.5 ± 10.0 | 37.7 ± 14.5 | |
| T3 | 39.6 ± 14.4 | 37.2 ± 15.4 | |
| T1-T0 (% Change) | -15.3 ± 21.7 | -8.2 ± 12.1 | |
| T2-T0 (% Change) | -16.3 ± 17.4 | -21.8 ± 18.9 | |
| T3-T0 (% Change) | -20.7 ± 24.1 | -23.7 ± 20.2 | |
| PCS | T0 | 24.9 ± 14.2 | 22.9 ± 16.3 |
| T1 | 18.7 ± 12.7 | 14.8 ± 12.3 | |
| T2 | 17.9 ± 13.3 | 15.2 ± 15.7 | |
| T3 | 17.2 ± 14.3 | 13.8 ± 14.0 | |
| T1-T0 (% Change) | -23.6 ± 24.5 | -30.2 ± 28.0 | |
| T2-T0 (% Change) | -30.5 ± 30.4 | -37.3 ± 33.0 | |
| T3-T0 (% Change) | -34.8 ± 36.4 | -39.9 ± 32.9 | |
| DASS Stress | T0 | 21.1 ± 8.6 | 19.2 ± 10.1 |
| T1 | 16.3 ± 11.0 | 17.2 ± 9.2 | |
| T2 | 17.5 ± 10.0 | 16.9 ± 9.3 | |
| T3 | 17.3 ± 9.3 | 16.3 ± 9.7 | |
| T1-T0 (% Change) | -27.7 ± 38.1 | -4.2 ± 33.7 | |
| T2-T0 (% Change) | -18.9 ± 34.1 | -5.6 ± 32.1 | |
| T3-T0 (% Change) | -16.9 ± 33.2 | -4.7 ± 50.9 | |
| DASS Anxiety | T0 | 15.1 ± 9.1 | 17.1 ± 11.3 |
| T1 | 8.9 ± 7.6 | 13.1 ± 11.4 | |
| T2 | 9.7 ± 8.0 | 12.3 ± 11.5 | |
| T3 | 9.6 ± 6.9 | 13.3 ± 11.8 | |
| T1-T0 (% Change) | -39.6 ± 35.8 | -5.6 ± 91.0 | |
| T2-T0 (% Change) | -28.8 ± 51.0 | -22.1 ± 39.4 | |
| T3-T0 (% Change) | -30.2 ± 45.4 | -6.3 ± 94.1 | |
| DASS Depression | T0 | 14.1 ± 10.0 | 15.7 ± 10.8 |
| T1 | 13.1 ± 10.4 | 11.9 ± 9.6 | |
| T2 | 13.6 ± 10.3 | 12.3 ± 10.6 | |
| T3 | 12.9 ± 9.7 | 12.7 ± 11.8 | |
| T1-T0 (% Change) | -16.9 ± 44.9 | -21.2 ± 31.0 | |
| T2-T0 (% Change) | -10.9 ± 48.2 | -18.1 ± 43.7 | |
| T3-T0 (% Change) | -12.7 ± 57.4 | -20.8 ± 53.9 | |
| WHO-5 Wellbeing | T0 | 27.2 ± 16.9 | 34.4 ± 12.4 |
| T1 | 39.5 ± 18.3 | 39.2 ± 11.4 | |
| T2 | 37.1 ± 19.3 | 38.9 ± 13.0 | |
| T3 | 35.7 ± 24.6 | 40.3 ± 17.4 | |
| T1-T0 (% Change) | 41.0 ± 50.6 | 22.8 ± 46.2 | |
| T2-T0 (% Change) | 24.3 ± 35.9 | 19.4 ± 45.7 | |
| T3-T0 (% Change) | 18.7 ± 71.0 | 16.7 ± 35.0 | |
| EQ5D Health Status | T0 | 33.1 ± 27.0 | 40.6 ± 22.8 |
| T1 | 42.8 ± 29.8 | 41.8 ± 23.0 | |
| T2 | 40.5 ± 29.8 | 43.3 ± 23.7 | |
| T3 | 37.2 ± 28.6 | 44.2 ± 25.3 | |
| T1-T0 (% Change) | 70.4 ± 118.8 | 7.3 ± 20.2 | |
| T2-T0 (% Change) | 55.9 ± 109.1 | 12.5 ± 28.5 | |
| T3-T0 (% Change) | 47.0 ± 110.7 | 12.8 ± 30.9 | |
| EQ5D Index | T0 | 15.0 ± 27.8 | 7.0 ± 18.8 |
| T1 | 16.1 ± 29.1 | 9.0 ± 22.2 | |
| T2 | 15.8 ± 28.2 | 11.9 ± 29.9 | |
| T3 | 15.9 ± 29.1 | 9.2 ± 23.0 | |
| T1-T0 (% Change) | 10.5 ± 28.5 | 7.3 ± 20.2 | |
| T2-T0 (% Change) | 3.4 ± 33.5 | 12.5 ± 28.5 | |
| T3-T0 (% Change) | 12.4 ± 32.0 | 12.8 ± 30.9 | |
| MOS Sleep Scale | T0 | 56.3 ± 13.3 | 57.6 ± 14.1 |
| T1 | 52.7 ± 12.4 | 54.1 ± 18.6 | |
| T2 | 52.5 ± 12.7 | 50.1 ± 16.5 | |
| T3 | 51.2 ± 16.3 | 50.9 ± 19.2 | |
| T1-T0 (% Change) | -5.3 ± 14.3 | -6.9 ± 17.4 | |
| T2-T0 (% Change) | -5.9 ± 13.3 | -12.6 ± 19.6 | |
| T3-T0 (% Change) | -9.1 ± 20.1 | -12.0 ± 22.0 | |
| MTS | T0 | 17.5 ± 19.6 | 17.1 ± 21.9 |
| T1 | 17.3 ± 14.3 | 17.8 ± 14.4 | |
| T2 | 17.9 ± 15.2 | 15.8 ± 17.1 | |
| T3 | 19.5 ± 18.9 | 14.3 ± 14.8 | |
| T1-T0 (% Change) | -37.0 ± 206.3 | 56.8 ± 223.3 | |
| T2-T0 (% Change) | -17.3 ± 155.9 | -27.0 ± 62.5 | |
| T3-T0 (% Change) | -20.4 ± 284.2 | -37.7 ± 64.0 | |
| PPT | T0 | 161.8 ± 76.7 | 206.3 ± 120.5 |
| T1 | 178.3 ± 82.9 | 188.7 ± 103.7 | |
| T2 | 183.5 ± 85.8 | 198.9 ± 110.1 | |
| T3 | 174.6 ± 85.3 | 210.1 ± 109.3 | |
| T1-T0 (% Change) | 17.9 ± 56.7 | -4.9 ± 30.2 | |
| T2-T0 (% Change) | 19.9 ± 49.6 | 4.5 ± 44.7 | |
| T3-T0 (% Change) | 14.4 ± 49.3 | 10.1 ± 45.1 |
Mean is calculated at each time point of baseline (T0) and follow ups (T1, T2, T3) and presented with the standard deviation (SD). Percentage change is calculated for each participant between each follow up and their baseline scores, and the group mean with SD is presented. RFIQ = Revised Fibromyalgia Impact Questionnaire, BPI = Brief Pain Inventory, PVAQ = Pain Vigilance and Awareness Questionnaire, PCS = Pain Catastrophising Scale, AIMS = Arthritis Impact Measurement Scale, DASS = Depression, Anxiety, and Stress Scale, WHO-5 = World Health Organisation – 5 well-being index, EQ-5D = European Quality of life – 5 Dimensions, MOS = Medical Outcomes Scale, MTS = Mechanical Temporal Summation, PPT = Pressure Pain Threshold.
Fig. 3.
Median percentage change to baseline for the primary clinical measures at all time points, displayed with percentage of participants who showed improvement greater than the MCID. Clinical outcomes are presented as median with 95% CI, MCID are presented as the percentage of participants in each group who reached 45% decrease in RFIQ and 30% decrease in BPI at each time point. n (active) = 15, n (placebo) = 15. RFIQ = Revised Fibromyalgia Impact Questionnaire, BPI = Brief Pain Inventory, MCID = Minimal Clinical Important Difference.
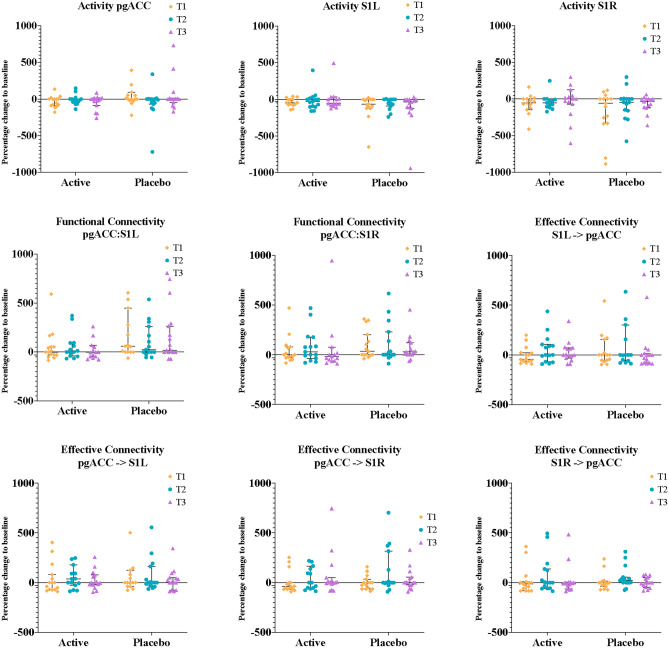
EEG Outcomes: Changes in all three regions of interest (ROI) analysed showed no distinct trends. These data are presented in Fig. 4. Percentage change in EEG measures between the active treatment and placebo groups are comparable.
Fig. 4.
Median percentage change to baseline of activity, functional connectivity and EC in the three ROI targeted: S1R, S1L and pgACC. Results are log transformed and are presented as median with 95% CI, n (active) = 15, n (placebo) = 15. S1 = Primary somatosensory cortex, pgACC = pre-genual Anterior Cingulate Cortex.
Discussion
This study focused on exploring the feasibility and safety of a novel intervention: EC EEG neurofeedback, a form of brain-computer interface training based on strengthening directional functional connectivity (= EC). Our findings show that conducting a fully powered trial is feasible. Significant interest and willingness to participate in the intervention was observed, with high retention and adherence rates. However, the time intensive nature of the intervention, requiring thrice-weekly visits, posed a barrier that led to self-exclusion and withdrawal due to conflicting work or family commitments and fatigue. Regarding safety, no serious adverse events were reported among participants. Some participants reported symptoms during the study period, although these were either linked to pre-existing secondary symptoms of fibromyalgia or were mild, transient, and self-resolving. Increased fatigue and dreaming were notable side effects in the active treatment group.
Interestingly, the active group reported higher occurrences of increased dreaming and fatigue compared to the placebo group, suggesting a potential correlation with brain learning in neurofeedback training. Enhanced dreaming, linked to nondeclarative learning following neurofeedback training, could potentially serve as an early indicator of brain response to a protocol 25,31. Fatigue experienced during the study seemed linked to the training period and may be an initial response to the cognitive demands required to engage with neurofeedback. Interestingly, studies which investigated fatigue pre- and post-treatment have seen improvement in fatigue 18,19,32,33. One hypothesis is that fatigue may be caused when sympathetic overdrive is reduced with neurofeedback, before the reference state is reset 34. Further investigation correlating autonomic nervous system measures to fatigue during neurofeedback training in a larger study could validate fatigue as an early indicator of efficacy.
Considering that neurofeedback EC training did not significantly affect clinical measures or alpha band EEG measures above the level of placebo effect, we propose three distinct mechanisms why this might be occurring. 1) a high placebo response. High placebo response rates in fibromyalgia trials 35, driven by psychological factors and patient motivation, complicate treatment efficacy assessments 36,37, highlighting the need for robust placebo controls in neurofeedback studies. 2) comorbid fatigue in fibromyalgia. Fatigue is a well-established secondary symptom of fibromyalgia, and comorbidity with chronic fatigue syndrome (CFS) is common 38. Mental fatigue reduces cognitive function, resulting from atrophy of the caudate 39. As neurofeedback learning critically involves the caudate 16, this may explain why participants with an exhausted brain may not be capable of neurofeedback mediated learning. Presence of CFS was not part of our exclusion criteria therefore 10% of participants had diagnosis of CFS and many more regularly reported high levels of fatigue. 3) methodological challenges. A proportion of individuals responded well to the EC training, while others did not. Previous findings have shown a proportion of individuals are unable to learn to modulate their brain by neurofeedback, and therefore show no benefits of treatment 16,40. Our sample size is not large enough to draw conclusions on responder analysis, however it is an important concept to consider. Likewise, it is important to consider neurological variability between individuals with fibromyalgia, especially given the high comorbidity with other conditions 41,42. If the cortical connectivity of each individual participant in our study was different to that of the group level characteristics we attempted to train, this may be a contributing factor as to why many participants did not respond to the EC training used in this study.
The primary limitations are the small sample size and use of descriptive comparisons to infer trends in clinical and EEG outcomes. Future research with a fully powered sample size would allow calculation of statistical significance to compare clinical and EEG outcomes between treatment and placebo groups. Future studies might look at the trend of EC over the course of the training sessions. This may allow determination of the change in connectivity required to see a clinical effect, and to quantify when an individual has gained the maximum benefit from the protocol.
Neurofeedback training is not a one-size-fits-all solution. It is impractical to anticipate that a single protocol could effectively address the diverse needs of everyone with fibromyalgia, as different clinical subgroups 43 likely have different brain networks involved in generating symptomatology. This study has ascertained that the protocol is both feasible and safe for participants. Individuals with fibromyalgia in Dunedin showed substantial interest in participating in the study, participants rated the protocol highly acceptable and there were no significant adverse effects reported.
Methodology
Trial registration and ethical approval
Prospectively registered in Australian and New Zealand Clinical Trials Registry under registration number: ACTRN12623000244606p (date: 07/03/2023). Ethical approval was obtained from Health and Disability Ethics Committee (ref 2023EXP15164). All experiments were performed in accordance with the declaration of Helsinki.
Study design
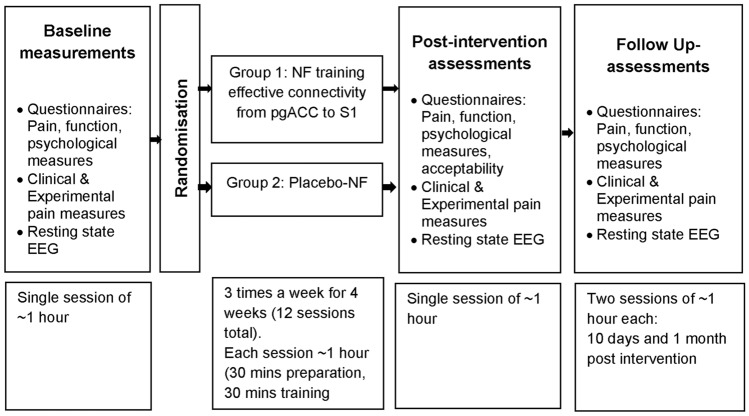
A double blinded (participant/outcome assessor), randomised, placebo-controlled pilot trial with two intervention arms (Fig. 5) was conducted at a tertiary institution. Randomisation was completed by a research administrator not involved in treatment or assessment procedures, using an open-access randomisation software, splitting eligible volunteers on a 1:1 basis to either Group 1: Targeted EEG neurofeedback training EC from pgACC to S1 region, or Group 2: Placebo neurofeedback. Blinding of participants and outcome assessors involved concealment of group allocation. After completion of the intervention and follow-up, participants were asked to identify the type of treatment they believed they received, rate their confidence on a scale from 0 to 10, provide reasons for the judgement and confirm whether the intervention was revealed to them.
Fig. 5.
Study design and time-duration for each study phase. EEG: Electroencephalography, NF = neurofeedback, pgACC = pregenual anterior cingulate cortex, S1 = primary somatosensory cortex.
Participants and eligibility criteria
Thirty participants (15 per group; as this was feasibility pilot trial sample size calculation was not performed) were recruited from Dunedin, NZ, through flyers, social media, and fibromyalgia support groups. They underwent eligibility screening via an online questionnaire and, if eligible and willing to adhere to the protocol, received study information and enrolment into the study. Consent was obtained before baseline testing. Inclusion and exclusion criteria: Modified American College of Rheumatology (ACR) 2011 Fibromyalgia Diagnostic Criteria 44 (sensitivity: 86%, specificity: 90% 45) was used as an eligibility screening tool consisting of: the presence of generalised pain, defined as pain in at least four of five regions (four quadrants and axial), symptoms present at a similar level for > 3 months and a score of ≥ 7 on Widespread Pain Index (WPI) and ≥ 5 on Symptom Severity Scale (SS) or a score of 4–6 on WPI and of ≥ 9 on SS 44. Participants were also required to be capable of understanding and signing an informed consent form, aged between 18 to 75 years on the day of consent, have a score of ≥ 4 on the 11-point numeric pain rating scale (NPRS, 0 = No pain to 10 = Worst pain) in the past 7 days and have a disability score of ≥ 50 on Revised Fibromyalgia Impact Questionnaire (RFIQ) 46–48. Written informed consent was obtained from all participants before entering the trial.
Participants with following were excluded: neurological conditions, cognitive and psychiatric disorders, epilepsy, substance abuse, pregnancy or within six-months post-partum, or those with unstable medical conditions. Participants were permitted to continue their medications for the duration of the trial, with the type and dosage of medication being recorded throughout the duration of the trial.
Intervention
Source localised EEG neurofeedback was administered three times a week (30 min/session) for a total of four weeks (i.e., 12sessions) by a researcher experienced in delivering neurofeedback techniques. A 21channel DC coupled amplifier and BrainAvatar™ Standardised low-resolution brain electromagnetic tomography (sLORETA) software version 4.7.5 for Discovery manufactured by BrainMaster Technologies (Inc., Bedford, OH, USA) was used. The sLORETA software utilizes the MNI152 standard template- a well-validated method for estimating the cortical sources of EEG activity, which allows us to localize brain activity in three dimensions. This approach has been extensively validated against modalities such as fMRI and PET, and it is widely accepted in EEG research. The dosage of the intervention was chosen based on our previous studies 25,49. During each session, participants were asked to sit on a back-supported chair and relax for 10 min, allowing the trainer to prepare the participant for neurofeedback training. Comby EEG lead cap with sensors (Ag/AgCl) were fitted to the scalp, with reference electrodes placed at mastoids. The impedance of active electrodes was monitored through amplifier and kept below 5 kilo-ohms. The participants were emphasised to minimise their movements to minimise motion artifact in EEG.
The method of neurofeedback utilized for the study was sLORETA neurofeedback, constructed to measure Isolated Effective Coherence (Icoh) as proposed by Pascual-Marqui50. This method identifies causal information flow with a multivariate autoregressive model that identifies dependencies within time series data. Specifically, Icoh provides a frequency representation of directional information flow which is in general agreement with Granger Causality. Icoh correctly identifies a time series transmitted from region #1 to region #2, that can be characterized as region #1 directing region #2. This relationship may be strengthened in sLORETA neurofeedback by coupling the increase in Icoh with a reward sound. Thus, through operant conditioning of the strength of the signal, it was hypothesized that pgACC would direct S1. The pain inhibition region would direct the pain sensing area in cortex leading to better outcomes in people with fibromyalgia.
Active neurofeedback: Participants were instructed to relax with closed eyes and listen to sounds played. The neurofeedback program reinforces and trains participants using the real-time EEG EC from pgACC to the S1 bilaterally in the alpha band by providing sound feedback when EC reaches the desired threshold between pgACC and S1 regions. Reward threshold was adjusted in real-time between 60–80%, meaning that for 60–80% of the time, sound feedback was delivered by the system when participant’s EC at targeted regions met the desired alpha magnitude.
Placebo neurofeedback: Conditions and preparation for placebo-neurofeedback group were same as active-neurofeedback, except participants did not receive a sound feedback based on their real-time EC but received a pre-recorded neurofeedback session. This was sourced from a database of recorded audio files (using audacity software) of healthy participants who underwent neurofeedback training targeting the ratio between pgACC and dACC + S1. This process has shown to effectively blind participants from the treatment group 25,49.
Outcome measures
Feasibility and safety measures were collected throughout, while clinical measures and EEG were collected at baseline (T0), immediately post intervention (T1), and 10 days (T2) and 1 month (T3) post intervention (Fig. 1). Feasibility outcomes included recruitment rate (number of participants recruited per month), proportion of participants recruited from total number screened, with reasons for exclusion (expressed as a percentage), adherence to intervention (number of treatment sessions attended by each participant expressed as a percentage of the total number of sessions), drop-out rates (number of participants who dropped-out in each group, expressed as a percentage of the total number of participants enrolled in the study.) Safety Measures: Adverse effects and related dropouts were recorded. The Discontinuation-Emergent Sign and Symptom (DESS) scale was used to record deterioration in any side effects compared to status prior to previous session and record any new symptoms 51,52. Intervention acceptability and satisfaction: Participant satisfaction levels regarding treatment and acceptability of neurofeedback was recorded on a numeric rating scale (0-Not at all satisfied/acceptable to 10-Very satisfied/acceptable) 53.
Clinical measures
Pain intensity and interference in daily activities: was captured using the BPI 54,55; MCID: average pain: 2.1 (32.3%); pain severity: 2.2 (34.2%) 56.
Function: was assessed using Revised Fibromyalgia Impact Questionnaire (RFIQ) 48 (MCID 14% improvement). As fibromyalgia co-exists with arthritis in many cases, the Arthritis Impact Measurement Scale (AIMS) was used to measure the impact of arthritis on physical, social, and emotional well-being 57.
Psychological measures: Depression, Anxiety, and Stress Scale (DASS) was used to measure three psychological constructs: depression, anxiety, and stress 58; (MCID: 5points 59). The normal scores are 0–4 for depression, 0–3 for anxiety, 0–7 for stress and 0–14 for total scores 60. Pain Catastrophising Scale (PCS) was used to measure the extent of catastrophic thoughts and feelings about their pain 61; (MCID: 3points to distinguish between responder and non-responder 62). The threshold for healthy controls is 11 for men, 15.7 for women and 13.9 in general 61. Pain Vigilance and Awareness Questionnaire (PVAQ) was used to measure the frequency of habitual ‘attention to pain’ 63.
Quality of life and Well-being: was assessed using European Quality of Life–5 Dimensions (EQ-5D) scale 64 and World Health Organisation-Five Well-Being Index (WHO-5) 65 respectively.
Sleep: quality and quantity were assessed using Medical Outcomes Study-Sleep Scale 54,66.
Measures of sensitisation: Pressure Pain Threshold, defined as the minimum force applied which induces pain, was measured using a digital handheld algometer 67 at the non-dominant wrist region. Temporal Summation (TS), an increased pain perception to repetitive mechanical stimuli, was assessed using a nylon monofilament (Touchtest Sensory Evaluator 300 g). Participants reported the perceived pain intensity immediately after first contact with monofilament and then following ten contacts over the non-dominant wrist region. The TS index is defined as the ratio the of "follow-up" pain rating divided by “baseline” pain rating 68,69.
Resting-state Electroencephalography
EEG was obtained in a quiet room while the participant was sitting upright in a comfortable chair with their eyes closed for 10 min using the SynAmps RT Amplifier (Compudemics Neuroscan). A total of 64electrodes were placed in the standard 10–20 International placement and impedances were checked to remain below 5kΩ. Participants were instructed not to drink alcohol for 24 h prior to EEG recording or caffeinated beverages one hour before recording to avoid alcohol or caffeine-induced changes in the EEG stream. The alertness of participants was checked by monitoring both slowing of the alpha rhythm and appearance of spindles in the EEG stream to prevent possible enhancement of the theta power due to drowsiness during recording. The EEG data was resampled (128 Hz), band-pass filtered (fast Fourier transform filter) to 0.01–44 Hz and re-referenced to the average reference using the EEGLAB function in MATLAB. The data was plotted in ICoN for a careful inspection of artifacts and all episodic artifacts suggestive of eye blinks, eye movements, jaw tension, teeth clenching, or body movement were manually removed from the EEG stream.
Data analysis
SPSS version 29.0 was used for all statistical analyses. As this is a feasibility study, tests for significance to compare measures between study groups were not performed, instead descriptive statistics were used. Feasibility and safety measures are presented in the form of proportions of participants. For primary clinical measures (pain severity, pain interference and function) and EEG measures, we calculated percentage change from baseline for each time point as below (e.g., for T3):
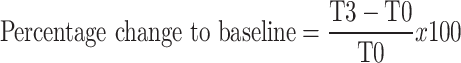 |
For primary clinical measures, we also calculated the proportion of participants that demonstrated changes greater than the MCID70–72 at each time point when compared to baseline; which was used to descriptively compare the two treatment groups. The MCID represents the smallest improvement considered worthwhile by a participant and is used in clinical research to determine treatment effectiveness and depicting patient satisfaction in reference to that treatment. The MCID for RFIQ and BPI was defined as a 45% decrease and a 30% decrease respectively from baseline.
All clinical outcomes were analysed based on intention-to-treat (ITT) principle and as per the originally assigned groups. Last observation carried forward methodology was used to impute missing data. Mean ± SDs, and mean differences were calculated from baseline to each interim and primary endpoint (T3) for all clinical and experimental pain measures, and descriptively compared between groups.
EEG analysis
Standardised LORETA was used to estimate the intracerebral electrical sources that generates the scalp-recorded activity in the alpha (8–12 Hz) band. The following analyses were conducted:
Region of interest analyses: sLORETA was used to calculate and compare the log transformed current density changes at the targeted brain regions (pgACC and S1). The ROI maker one function in sLORETA was used to define the region of interest. A seed point was provided for each ROI and all voxels within a radius of 20 mm were averaged to calculate the current density.
Lagged phase connectivity (Functional connectivity): Coherence and phase synchronisation between time series corresponding to different spatial locations are interpreted as indicators of “functional connectivity”. As this is highly contaminated with an instantaneous, non-physiological contribution due to volume conduction, Pascual-Marqui introduced a new measure of coherence by considering only non-instantaneous (lagged) connectivity 73. Hence, effectively removing the confounding factor of volume conduction. Measures of linear dependence (coherence) between the multivariate time series are defined. The measures are non-negative, taking the value zero only when there is independence, and were defined in the frequency domain of alpha (8-12 Hz). Time-series of current density was extracted for different region of interests using sLORETA. Power in all 6,239 voxels was normalised to a power of one and log-transformed at each time point. ROI values thus reflect the log-transformed fraction of total power across all voxels separately for specific frequencies. ROI included the targeted brain regions (pgACC, and S1) for the alpha band. Percentage changes in the functional connectivity were calculated between the pre- and post-treatment measurements for the targeted ROIs (i.e., pgACC to S1 in alpha band), and compared between groups.
Effective connectivity: Granger causality reflects the strength of EC (i.e., causal interactions) from one region to another by quantifying how much the signal in the seed region can predict the signal in the target region74. Hence, it can be considered directed functional connectivity. The isolated effective coherence (iCoh) function in LORETA-key software was used to calculate the Granger causality between the targeted ROIs (pgACC and left and right S1). Granger causality is based on formulating a multivariate autoregressive model and calculating the corresponding partial coherences after setting all irrelevant connections to zero. In general, the autoregressive coefficients correspond to Granger causality. Granger causality is defined and calculated as the log-ratio between the error variance of a reduced model, which predicts one time series based only on its own past values, and that of the full model, which in addition includes the past values of another time series. It is important to note that Granger causality does not imply anatomical connectivity between regions but directional functional connectivity between two sources. Granger causality analysis was calculated between the targeted ROIs (i.e., pgACC to S1 in alpha band). Percentage changes were calculated between the pre- and post-treatment measurements and compared between groups.
Acknowledgements
This work was funded by the Otago Medical School Trust Funding, and the Brain Health Research Centre (through a Philanthropist).
Author contributions
D.D.R., P.G., R.M. and D.B.A. conceived the experiment, M.S. programmed the Neurofeedback protocol, L.A. and C.V.S. conducted the experiment, L.A. analyzed the results, and prepared the original manuscript. All authors reviewed the manuscript.
Data availability
The study protocol and the datasets generated during and/or analysed during current study are available from corresponding author on reasonable request.
Declarations
Competing interests
Mark Smith is the owner of Neurofeedback Therapy Services of New York. Mark helped with programming the neurofeedback protocols tested in the current study. The cortical areas (pgACC, S1) to be targeted, its co-ordinates and the training protocol (effective connectivity) were provided by the principal investigators (D.B.A and D.D.R). All other authors (L.A., P.G., R.M., C.S., D.D.R., and D.B.A) declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bair, M. J. & Krebs, E. E. Fibromyalgia. Ann. Intern. Med.172, Itc33-itc48. 10.7326/aitc202003030 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Kashikar-Zuck, S. & Ting, T. V. Juvenile fibromyalgia: Current status of research and future developments. Nat. Rev. Rheumatol.10, 89–96. 10.1038/nrrheum.2013.177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrot, S. & Russell, I. J. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur. J. Pain18, 1067–1080. 10.1002/ejp.564 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Welsch, P., Bernardy, K., Derry, S., Moore, R. A. & Häuser, W. Mirtazapine for fibromyalgia in adults. Cochrane Database Syst. Rev.10.1002/14651858.CD012708.pub2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsch, P., Üçeyler, N., Klose, P., Walitt, B. & Häuser, W. Serotonin and noradrenaline reuptake inhibitors for fibromyalgia. Cochrane Database Syst. Rev.2, Cd010292. 10.1002/14651858.CD010292.pub2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarmiento-Hernández, I. et al. effectiveness of invasive techniques in patients with fibromyalgia: Systematic review and meta-analysis. Pain Med.21, 3499–3511. 10.1093/pm/pnaa321 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Sarzi-Puttini, P., Giorgi, V., Marotto, D. & Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol.16, 645–660. 10.1038/s41584-020-00506-w (2020). [DOI] [PubMed] [Google Scholar]
- 8.Häuser, W., Thieme, K. & Turk, D. C. Guidelines on the management of fibromyalgia syndrome – A systematic review. Eur. J. Pain14, 5–10. 10.1016/j.ejpain.2009.01.006 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Dehghan, M. et al. Coordinate-based (ALE) meta-analysis of brain activation in patients with fibromyalgia. Hum. Brain Mapp.37, 1749–1758. 10.1002/hbm.23132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Solà, M. et al. Towards a neurophysiological signature for fibromyalgia. Pain158, 34–47. 10.1097/j.pain.0000000000000707 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamping, S., Bomba, I. C., Kanske, P., Diesch, E. & Flor, H. Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain154, 1846–1855. 10.1016/j.pain.2013.06.003 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Robinson, M. E., Craggs, J. G., Price, D. D., Perlstein, W. M. & Staud, R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J. Pain12, 436–443. 10.1016/j.jpain.2010.10.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valet, M. et al. Patients with pain disorder show gray-matter loss in pain-processing structures: A voxel-based morphometric study. Psychosom. Med.71, 49–56. 10.1097/PSY.0b013e31818d1e02 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Jensen, K. B. et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthr. Rheum.65, 3293–3303. 10.1002/art.38170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ridder, D. & Vanneste, S. occipital nerve field transcranial direct current stimulation normalizes imbalance between pain detecting and pain inhibitory pathways in fibromyalgia. Neurotherapeutics14, 484–501. 10.1007/s13311-016-0493-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitaram, R. et al. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci.18, 86–100. 10.1038/nrn.2016.164 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Jensen, M. P. et al. Steps toward developing an EEG biofeedback treatment for chronic pain. Appl. Psychophysiol. Biofeedback38, 101–108 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Caro, X. J. & Winter, E. F. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl. Psychophysiol. Biofeedback36, 193–200. 10.1007/s10484-011-9159-9 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Kayıran, S., Dursun, E., Dursun, N., Ermutlu, N. & Karamürsel, S. neurofeedback intervention in fibromyalgia syndrome; A Randomized, controlled, rater blind clinical trial. Appl. Psychophysiol. Biofeedback35, 293–302. 10.1007/s10484-010-9135-9 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Torres, C. B., Barona, E. J. G., Molina, M. G., Sánchez, M.E.G.-B. & Manso, J. M. M. A. systematic review of EEG neurofeedback in fibromyalgia to treat psychological variables, chronic pain and general health. Eur. Arch. Psych. Clin. Neurosci.10.1007/s00406-023-01612-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farnia, S.K.-C. et al. Comparison of alpha–theta neurofeedback versus sensorimotor rhythm neurofeedback in the treatment of patients with fibromyalgia: A randomized, double-blind, controlled clinical trial. Chro. Dis. J.8, 105–111 (2020). [Google Scholar]
- 22.Wu, Y.-L., Fang, S.-C., Chen, S.-C., Tai, C.-J. & Tsai, P.-S. Effects of neurofeedback on fibromyalgia: A randomized controlled trial Pain. Manag. Nurs.22(755), 763. 10.1016/j.pmn.2021.01.004 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Barbosa-Torres, C., & Cubo-Delgado, S. Clinical Findings in SMR neurofeedback protocol training in women with fibromyalgia syndrome. Brain Sci.11, (2021). 10.3390/brainsci11081069 [DOI] [PMC free article] [PubMed]
- 24.Barbosa-Torres, C., & Cubo-Delgado, S. Efficacy of the SMR protocol in women with fibromyalgia for the improvement of chronic pain, sleep, and quality of life. Behavioral Psychology/Psicología Conductual29, 549-560 (2021). 10.51668/bp.8321302n
- 25.Adhia, D. B. et al. Exploring electroencephalographic infraslow neurofeedback treatment for chronic low back pain: a double-blinded safety and feasibility randomized placebo-controlled trial. Sci. Rep.13, 1177. 10.1038/s41598-023-28344-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhia, D. B., Mani, R., Turner, P. R., Vanneste, S. & De Ridder, D. Infraslow neurofeedback training alters effective connectivity in individuals with chronic low back pain: A secondary analysis of a pilot randomized placebo-controlled study. Brain Sci.12, 1514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston, K. J. Functional and effective connectivity: A review. Brain Connect.1, 13–36. 10.1089/brain.2011.0008 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Vanneste, S. & De Ridder, D. Chronic pain as a brain imbalance between pain input and pain suppression. Brain Commun.3, fcab014. 10.1093/braincomms/fcab014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Ridder, D., Vanneste, S., Smith, M. & Adhia, D. Pain and the triple network model. Front. Neurol.13, 757241. 10.3389/fneur.2022.757241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ridder, D., Adhia, D. & Vanneste, S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci. Biobehav. Rev.130, 125–146. 10.1016/j.neubiorev.2021.08.013 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Yonah, R. Postsession dreaming in neurofeedback as an indication of nondeclarative learning. NeuroRegulation7, 2–2 (2020). [Google Scholar]
- 32.Mueller, H. H., Donaldson, C. C., Nelson, D. V. & Layman, M. Treatment of fibromyalgia incorporating EEG-driven stimulation: A clinical outcomes study. J. Clin. Psychol.57, 933–952. 10.1002/jclp.1060 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Nelson, D. V. et al. Neurotherapy of fibromyalgia?. Pain Med.11, 912–919. 10.1111/j.1526-4637.2010.00862.x (2010). [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Lavin, M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthr. Res Ther.9, 216. 10.1186/ar2146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammond, D. C. Placebos and neurofeedback: A case for facilitating and maximizing placebo response in neurofeedback treatments. J. Neurother.15, 94–114. 10.1080/10874208.2011.570694 (2011). [Google Scholar]
- 36.Louiza, K., Laura Specker, S. & Anna, W. Neurofeedback as placebo: a case of unintentional deception?. J. Med. Ethics.48, 1037. 10.1136/medethics-2021-107435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thibault, R. T. & Raz, A. The psychology of neurofeedback: Clinical intervention even if applied placebo. American Psychol.72, 679–688. 10.1037/amp0000118 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Aaron, L. A. et al. Comorbid clinical conditions in chronic fatigue: A co-twin control study. J. Gen. Intern. Med.16, 24–31. 10.1111/j.1525-1497.2001.03419.x (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavelin, H. M. et al. Mental fatigue in stress-related exhaustion disorder: Structural brain correlates, clinical characteristics and relations with cognitive functioning. Neuroimage Clin.27, 102337. 10.1016/j.nicl.2020.102337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alkoby, O., Abu-Rmileh, A., Shriki, O. & Todder, D. Can we predict who will respond to neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG neurofeedback learning. Neuroscience378, 155–164. 10.1016/j.neuroscience.2016.12.050 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Giorgi, V. et al. Fibromyalgia: One year in review 2022. Clin. Exp. Rheumatol.40, 1065–1072. 10.55563/clinexprheumatol/if9gk2 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Mayr, A. et al. Individually unique dynamics of cortical connectivity reflect the ongoing intensity of chronic pain. PAIN163, 1987–1998. 10.1097/j.pain.0000000000002594 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Plazier, M., Ost, J., Stassijns, G., De Ridder, D. & Vanneste, S. Pain characteristics in fibromyalgia: understanding the multiple dimensions of pain. Clin. Rheumatol.34, 775–783. 10.1007/s10067-014-2736-6 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Wolfe, F. & Häuser, W. Fibromyalgia diagnosis and diagnostic criteria. Ann. Med.43, 495–502. 10.3109/07853890.2011.595734 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Wolfe, F. et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthr. Rheum.46, 319–329. 10.1016/j.semarthrit.2016.08.012 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Childs, J. D., Piva, S. R. & Fritz, J. M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976)30, 1331–1334. 10.1097/01.brs.0000164099.92112.29 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Jensen, M. P. & McFarland, C. A. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain55, 195–203. 10.1016/0304-3959(93)90148-i (1993). [DOI] [PubMed] [Google Scholar]
- 48.Bennett, R. M. et al. The revised fibromyalgia impact questionnaire (FIQR): Validation and psychometric properties. Arthr. Res. Ther.11, R120. 10.1186/ar2783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathew, J., Adhia, D. B., Smith, M. L., De Ridder, D. & Mani, R. Source localized infraslow neurofeedback training in people with chronic painful knee osteoarthritis: A randomized, double-blind, sham-controlled feasibility clinical trial. Front. Neurosci.16, 899772. 10.3389/fnins.2022.899772 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pascual Marqui, R. D. et al. Isolated effective coherence (iCoh): causal information flow excluding indirect paths. arXiv6 (2014). [DOI] [PMC free article] [PubMed]
- 51.Rogel, A. et al. Transient adverse side effects during neurofeedback training: A randomized, sham-controlled. Doubl. Blind Stud. Appl. Psychophysiol. Biofeedback40, 209–218. 10.1007/s10484-015-9289-6 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum, J. F., Fava, M., Hoog, S. L., Ascroft, R. C. & Krebs, W. B. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol. Psychiat.44, 77–87. 10.1016/s0006-3223(98)00126-7 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Farrar, J. T., Young, J. P. Jr., LaMoreaux, L., Werth, J. L. & Poole, M. R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain94, 149–158. 10.1016/s0304-3959(01)00349-9 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Williams, D. A. & Arnold, L. M. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ). Arthr. Care Res. (Hoboken)63(Suppl 11), S86-97. 10.1002/acr.20531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song, C. Y. et al. Validation of the brief pain inventory in patients with low back pain. Spine (Phila Pa 1976)41, E937-e942. 10.1097/brs.0000000000001478 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Mease, P. J. et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthr. Care Res. (Hoboken)63, 821–826. 10.1002/acr.20449 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Meenan, R. F., Mason, J. H., Anderson, J. J., Guccione, A. A. & Kazis, L. E. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthr. Rheum.35, 1–10 (1992). [DOI] [PubMed] [Google Scholar]
- 58.Parkitny, L. et al. Rasch analysis supports the use of the depression, anxiety, and stress scales to measure mood in groups but not in individuals with chronic low back pain. J. Clin. Epidemiol.65, 189–198. 10.1016/j.jclinepi.2011.05.010 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Yohannes, A. M., Casaburi, R., Dryden, S. & Hanania, N. A. Minimum clinically important difference in the Depression Anxiety Stress Scale-21 using an anchor-based methodology in response to pulmonary rehabilitation. CHEST162, A2288–A2289. 10.1016/j.chest.2022.08.1896 (2022). [Google Scholar]
- 60.Lovibond, P. F. & Lovibond, S. H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav. Res. Therapy33, 335–343. 10.1016/0005-7967(94)00075-U (1995). [DOI] [PubMed] [Google Scholar]
- 61.Osman, A. et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J. Behav. Med.23, 351–365. 10.1023/a:1005548801037 (2000). [DOI] [PubMed] [Google Scholar]
- 62.Sabourin, S., Tram, J., Sheldon, B. L. & Pilitsis, J. G. Defining minimal clinically important differences in pain and disability outcomes of patients with chronic pain treated with spinal cord stimulation. J. Neurosurg. Spine10.3171/2020.11.Spine201431 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Roelofs, J., Peters, M. L., McCracken, L. & Vlaeyen, J. W. S. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain101, 299–306. 10.1016/s0304-3959(02)00338-x (2003). [DOI] [PubMed] [Google Scholar]
- 64.Sullivan, T., Turner, R. M., Derrett, S. & Hansen, P. New Zealand population norms for the EQ-5D-5L constructed from the personal value sets of participants in a national survey. Value Health24, 1308–1318. 10.1016/j.jval.2021.04.1280 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Topp, C. W., Østergaard, S. D., Søndergaard, S. & Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom.84, 167–176. 10.1159/000376585 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Stewart, A. L., & Ware, J. E. (Eds.). (1992). Measuring functioning and well-being: the medical outcomes study approach. Duke university Press.
- 67.Uddin, Z. & MacDermid, J. C. Quantitative sensory testing in chronic musculoskeletal pain. Pain Med.17, 1694–1703. 10.1093/pm/pnv105 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Goodin, B. R. et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom. Med.76, 302–310. 10.1097/psy.0000000000000058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hübscher, M. et al. Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. Pain154, 1497–1504. 10.1016/j.pain.2013.05.031 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Lee, E. C., Whitehead, A. L., Jacques, R. M. & Julious, S. A. The statistical interpretation of pilot trials: should significance thresholds be reconsidered?. BMC Med. Res. Methodol.14, 41. 10.1186/1471-2288-14-41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tickle-Degnen, L. Nuts and bolts of conducting feasibility studies. Am. J. Occup. Ther.67, 171–176. 10.5014/ajot.2013.006270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kannan, S. & Gowri, S. Pilot studies: Are they appropriately reported?. Perspect. Clin. Res.6, 207–210. 10.4103/2229-3485.167097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pascual-Marqui, R. D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp. Clin. Pharmacol.24(Suppl D), 5–12 (2002). [PubMed] [Google Scholar]
- 74.Wang, W. & Sun, W. Using EEG effective connectivity based on granger causality and directed transfer function for emotion recognition. Int. J. Adv. Comput. Sci. Appl.10.14569/IJACSA.2023.0140990 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol and the datasets generated during and/or analysed during current study are available from corresponding author on reasonable request.