Abstract
Background:
Clarithromycin is a widely used antibiotic, but its safety profile, particularly in different age groups, remains inadequately explored.
Objectives:
This study aims to characterize and illustrate the features of clarithromycin-related adverse events (AEs) across different age groups using the FDA Adverse Event Reporting System (FAERS) database, providing a reference for the clinical detection, prevention, and management of AEs in various age groups.
Design:
A disproportionality analysis was performed using data from the FAERS database. The study included all AE reports related to clarithromycin, stratified by age groups.
Methods:
Disproportionality analysis was conducted using reporting odds ratio, proportional reporting ratio, Bayesian confidence propagation neural network, and multiple gamma Poisson shrinkers. Statistical analyses included descriptive statistics and Chi-square tests.
Results:
A total of 7319 reports of clarithromycin AEs were retrieved from the FAERS database. Vomiting, diarrhea, drug interactions, and drug interactions were reported most frequently in the age groups 0–17, 18–44, 45–64, and ⩾65 years, respectively. Abnormal product taste, taste disorder, and medication errors related to drug interactions specified in the package insert were the strongest signals in the age groups 0–17, 18–44, 45–64, and ⩾65 years, respectively. A total of 41 Preferred Terms signals were not explicitly included in the clarithromycin package insert and were mainly associated with psychiatric disorders, skin and subcutaneous tissue disorders, and gastrointestinal disorders, among others. Specific signals for age differences were identified, with 18 signals being age-specific, including 3 in children and 15 in elderly individuals.
Conclusion:
The safety profile of clarithromycin varies across age groups. In children, it is mainly associated with vomiting, hypersensitivity, and dyspnea, while in adults, psychiatric AEs are more common. In the elderly, clarithromycin should be used cautiously, with attention to drug interactions.
Keywords: adverse event, children, clarithromycin, elderly, FAERS
Plain language summary
A study on the adverse effects of clarithromycin
Introduction:
Clarithromycin is a relatively newer macrolide antibiotic derived from erythromycin, that is included in the WHO Model List of Essential Medicines, and is one of the important drugs needed in basic healthcare systems. Currently, there are no studies mining adverse events and outcomes related to the clinical use of clarithromycin in the FDA Adverse Event Reporting System (FAERS) database. This study investigated the safety signals related to clarithromycin.
Methods:
Disproportionality analysis, including reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multiple gamma Poisson shrinker (MGPS) algorithms, were used to quantify signals of clarithromycin-related adverse events (AEs) across different age groups.
Results:
7,319 AE reports were identified, 41 PT signals were not explicitly included in the clarithromycin package insert. Specific signals for age differences were identified, with 18 signals being age specific.
Conclusion:
We discovered important safety concerns related to clarithromycin. The safety of clarithromycin is different in different age groups. Children are more closely associated with adverse events related to vomiting, drug-induced hypersensitivity, and dyspnea. In adults, it is more associated with psychiatric adverse events. In addition, the use of clarithromycin in the elderly should be strictly in accordance with the instructions and be alert to drug interactions.
Introduction
Clarithromycin is a relatively newer macrolide antibiotic derived from erythromycin, that is included in the WHO Model List of Essential Medicines and is one of the important drugs needed in basic healthcare systems.1,2 Compared to its parent compound erythromycin, clarithromycin has improved side effects, dosing regimens, and microbial activity. It is used to treat various bacterial infections, including streptococcal pharyngitis, pneumonia, skin infections, and Helicobacter pylori infection.1,3
Although clarithromycin plays an important role in treating various diseases, reports of adverse events (AEs) associated with it are gradually increasing. Currently, there are still deficiencies in the in-depth analysis of AEs related to clarithromycin. Studies have shown that adverse reactions to clarithromycin mainly include gastrointestinal reactions, allergic reactions, and liver function damage. 4 However, there has not been a comprehensive comparison and interpretation of risk factors and adverse reactions among different patient populations. The clarithromycin package clearly states that the safety of clarithromycin in pregnant and lactating women has not been confirmed, and special treatment is not recommended for children and elderly people.5,6 Currently, there are no studies mining AEs and outcomes related to the clinical use of clarithromycin in the FDA Adverse Event Reporting System (FAERS) database. To further understand clarithromycin-related AEs and better protect patients of different ages, we need to conduct comparative analyses of clarithromycin AEs.
This study aimed to characterize and illustrate the features of clarithromycin-related AEs across different age groups using FAERS data. We aimed to explore the types, frequencies, severity, and relative risks of AEs induced by clarithromycin in different age groups. Through this research, we hope to provide more comprehensive and specific information about the safety of clarithromycin, thereby enhancing patient compliance and tolerance and providing more accurate and personalized guidance for its clinical use.
Methods
Data source
We conducted a pharmacovigilance study on AEs associated with clarithromycin using the FAERS database. The FAERS is a vital public database of the FDA, that is used to collect reports of AEs and medication errors related to approved drugs.7,8 The clarithromycin reports were retrieved from the FAERS database covering the period from the first quarter of 2004 to the second quarter of 2023, using either the brand name or the generic name of clarithromycin as keywords. Data were extracted using the open-source tool OpenVigil 2.1, and the selected role code was “PS” (primary suspect). AE reports in the FAERS database are encoded according to the Preferred Terms (PTs) in the Medical Dictionary for Regulatory Activities (MedDRA), with additional classification by System Organ Class (SOC). 9
Data processing
We performed deduplication on the clarithromycin reports obtained from the FAERS database. When the CASE ID was identical, we selected the report with the most recent FDA_DT. In cases where both the CASE ID and FDA_DT were the same, the report with the larger PRIMARY_ID value was chosen. In addition, reports with identical values for fields such as gender, age, country, event date, adverse event, and indication were also identified as duplicates. Furthermore, we refined the dataset by categorizing clarithromycin reports based on the reported age, distinguishing between different age groups.
Signal mining
In the context of pharmacovigilance studies, disproportionality analysis methods are primarily used as tools to assess potential associations between specific AEs and specific drugs. The reporting of this study conforms to the READUS-PV statement. 10 Disproportionality analysis methods include frequency-based data mining approaches such as the reporting odds ratio (ROR) and proportional reporting ratio (PRR), as well as Bayesian adverse drug reaction data mining approaches like the Bayesian Confidence Propagation Neural Network (BCPNN) and the multiple gamma Poisson shrinker (MGPS).11–14
The advantage of frequency-based methods lies in their high sensitivity, simplicity of principle, ease of algorithm, and fast computation. However, they have low specificity, unstable signals, and are easily influenced by outliers. Conversely, Bayesian methods offer high specificity, stable signals, and a lower likelihood of false positives, but they typically have lower sensitivity and are computationally complex.
Considering the core principles and pros and cons of each disproportionality analysis method, this study employed four methods—ROR, PRR, BCPNN, and MGPS—for AE signal detection to reduce the likelihood of false-positive signals. A potential risk signal is identified when all four algorithms indicate a positive signal, defined as follows: the frequency of the AE occurrence is ⩾3, the lower limit of the 95% confidence interval (CI) for the ROR is >1, the PRR is ⩾2 with a chi-square value ⩾4, the lower limit of the 95% CI for the Information Component is >0, and the lower limit of the 95% CI for the Empirical Bayes Geometric Mean (EBGM) is ⩾2.
Statistical analysis
In addition to signal detection at the PT level, we also conducted a comparison across different age groups. Descriptive analysis was employed to present the characteristics of all the AE reports related to clarithromycin. The Chi-square test was used to compare the distribution differences of patients between the age groups. All statistical analyses were performed using R software (The R Foundation, version 4.4.0).
Results
Baseline characteristics
The demographic characteristics of the patients included in the clarithromycin AE reports are shown in Table 1. From the first quarter of 2004 to the second quarter of 2023, a total of 7319 clarithromycin AE reports were retrieved from the FAERS database. Among them, 679 were in the age group of 0–17 years, 2056 were in the age group of 18–44 years, 2192 were in the age group of 45–64 years, and 2392 were in the age group of 65 years and above. The most common age group was ⩾65 years (32.7%). The proportion of female reports (59.9%) was higher than that of male reports (38.5%). A total of 42.5% of the reports originated from the United Kingdom. A total of 16.3% of the AE reports did not specify the route of administration. Among the known routes of administration, oral administration accounted for 69.0%, while injection accounted for 1.4%. Except for unknown indications, the reported AEs associated with clarithromycin are primarily related to lower respiratory tract infection and pneumonia. The most reported outcome was Other (52.3%), followed by Hospitalization—Initial or Prolonged (31.1%) and Life-Threatening (6.3%).
Table 1.
Demographic characteristics of patients who received clarithromycin and AE reports and percentages (n (%)).
| Variables | 0–17 (N = 679) | 18–44 (N = 2056) | 45–64 (N = 2192) | ⩾65 (N = 2392) | p-Value |
|---|---|---|---|---|---|
| Gender | <0.001 | ||||
| Male | 312 (45.9) | 652 (31.7) | 881 (40.2) | 975 (40.8) | |
| Female | 345 (50.8) | 1384 (67.3) | 1272 (58.0) | 1382 (57.8) | |
| Unknown | 22 (3.2) | 20 (1.0) | 39 (1.8) | 35 (1.5) | |
| Reporter country | <0.001 | ||||
| United Kingdom | 180 (26.5) | 990 (48.2) | 932 (42.5) | 1008 (42.1) | |
| United States | 74 (10.9) | 184 (8.9) | 252 (11.5) | 201 (8.4) | |
| Italy | 97 (14.3) | 182 (8.9) | 182 (8.3) | 223 (9.3) | |
| Japan | 31 (4.6) | 85 (4.1) | 141 (6.4) | 209 (8.7) | |
| Other | 297 (43.7) | 615 (29.9) | 685 (31.3) | 751 (31.4) | |
| Route | <0.001 | ||||
| Oral | 373 (54.9) | 1209 (58.8) | 1235 (56.3) | 1407 (58.8) | |
| Injection | 8 (1.2) | 21 (1.0) | 19 (0.9) | 35 (1.5) | |
| Transplacental/transmammary | 6 (0.9) | 5 (0.2) | 0 (0.0) | 0 (0.0) | |
| Other | 180 (26.5) | 492 (23.9) | 565 (25.8) | 570 (23.8) | |
| Indication | <0.001 | ||||
| Lower respiratory tract infection | 94 (13.8) | 114 (5.5) | 316 (14.4) | 313 (13.1) | |
| Pneumonia | 85 (12.5) | 77 (3.7) | 194 (8.9) | 309 (12.9) | |
| Bronchitis | 53 (7.8) | 67 (3.3) | 73 (3.3) | 124 (5.2) | |
| Tonsillitis | 30 (4.4) | 145 (7.1) | 37 (1.7) | 7 (0.3) | |
| Helicobacter infection | 20 (2.9) | 154 (7.5) | 304 (13.9) | 272 (11.4) | |
| Other | 202 (29.7) | 884 (43.0) | 615 (28.1) | 621 (26.0) | |
| Unknown | 195 (28.7) | 615 (29.9) | 653 (29.8) | 746 (31.2) | |
| Outcome | <0.001 | ||||
| Death | 16 (2.1) | 42 (1.8) | 107 (4.2) | 193 (6.4) | |
| Life-threatening | 32 (4.2) | 99 (4.3) | 160 (6.2) | 256 (8.5) | |
| Disability | 35 (4.6) | 130 (5.6) | 141 (5.5) | 146 (4.9) | |
| Congenital anomaly | 3 (0.4) | 3 (0.1) | 0 (0.0) | 1 (0.0) | |
| Hospitalization—initial or prolonged | 269 (35.1) | 542 (23.4) | 817 (31.8) | 1066 (35.6) | |
| Required intervention to prevent permanent impairment/damage | 3 (0.4) | 19 (0.8) | 23 (0.9) | 23 (0.8) | |
| Other | 408 (53.3) | 1484 (64.0) | 1321 (514) | 1311 (43.8) |
AE, adverse event.
Reporting year
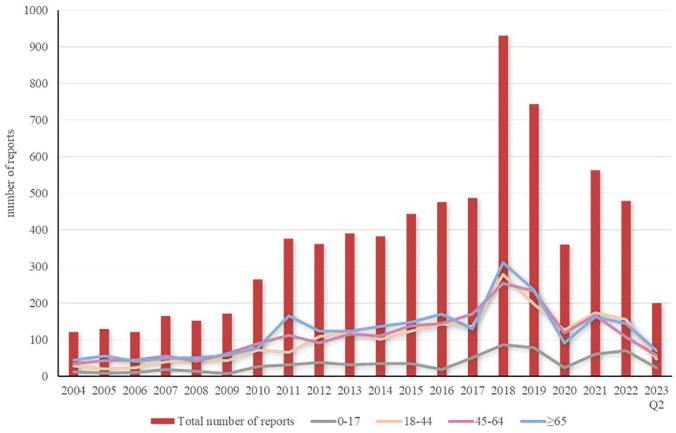
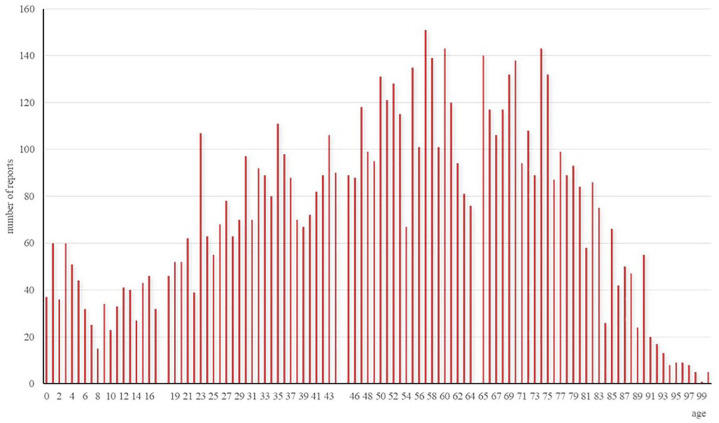
The trend of clarithromycin AE reports over time is illustrated in Figure 1. The number of reports was relatively low and stable from 2004 to 2009. There was an increase in the number of reports in 2010, followed by a slow increase from 2011 to 2017. The number of reports surged in 2018, reaching a peak of 931 cases. The number of reports decreased from 2019 to 2020. There was an increase in the number of reports in 2021. The distribution of clarithromycin AE reports across different age groups is shown in Figure 2.
Figure 1.
Annual AE reports of patients receiving clarithromycin.
AE, adverse event.
Figure 2.
AE reports of clarithromycin across different age groups.
AE, adverse event.
Risk signals associated with clarithromycin
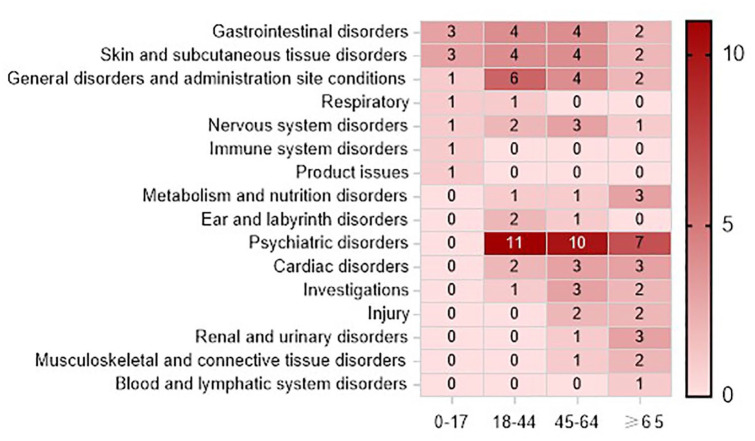
The detection of AE signals at the SOC and PT levels using five methods—ROR, PRR, comprehensive standard method, BCPNN, and MGPS—is illustrated in Figure 3 for clarithromycin-related AE signals across different age groups. The 45- to 64-year-old age group (n = 37) had the greatest number of signals, followed by the 18- to 44-year-old age group (n = 34), and the 65-year-old age group (n = 30), while the 0- to 17-year-old age group (n = 11) had the least signals. At the SOC level, AE signals in the 0–17 age group involved 7 SOCs, primarily concentrated in gastrointestinal disorders (n = 3) and skin and subcutaneous tissue disorders (n = 3). AE signals in the 18–44 age group involved 10 SOCs, mainly concentrated in psychiatric disorders (n = 11) and general disorders and administration site conditions (n = 6). AE signals in the 45–64 age group involved 12 SOCs, mainly concentrated in psychiatric disorders (n = 10), gastrointestinal disorders (n = 4), skin and subcutaneous tissue disorders (n = 4), and general disorders and administration site conditions (n = 4). AE signals in the age group of 65 years and above involved 12 SOCs, mainly concentrated in psychiatric disorders (n = 7), with the remaining signals distributed relatively evenly.
Figure 3.
Clarithromycin AE signals detected using four algorithms.
AE, adverse event.
The AE signals detected at the PT level are shown in Table 2.
Table 2.
AE signals detected and strength of clarithromycin.
| No. | PT | SOC | N | ROR025 | PRR | χ2 | IC − 2 SD | EB05 | Whether included in the package insert |
|---|---|---|---|---|---|---|---|---|---|
| 0–17 Years | |||||||||
| 1 | Product taste abnormal | Product issues | 14 | 12.91 | 21.74 | 245.81 | 0.07 | 12.14 | No |
| 2 | Dysgeusia | Nervous system disorders | 17 | 12.69 | 20.25 | 280.74 | 0.41 | 11.92 | Yes |
| 3 | Lip swelling | Gastrointestinal disorders | 15 | 5.38 | 8.85 | 95.18 | 0.16 | 5.2 | No |
| 4 | Drug interaction | General disorders and administration site conditions | 38 | 4.27 | 5.66 | 140.95 | 1.58 | 4.04 | Yes |
| 5 | Stevens-Johnson syndrome | Skin and subcutaneous tissue disorders | 15 | 3.33 | 5.48 | 50.13 | 0.08 | 3.24 | Yes |
| 6 | Urticaria | Skin and subcutaneous tissue disorders | 35 | 3.09 | 4.18 | 81.88 | 1.08 | 2.95 | Yes |
| 7 | Vomiting | Gastrointestinal disorders | 89 | 2.79 | 3.16 | 134.21 | 1.31 | 2.52 | Yes |
| 8 | Drug hypersensitivity | Immune system disorders | 15 | 2.4 | 3.94 | 29.93 | 0.05 | 2.34 | Yes |
| 9 | Dyspnea | Respiratory, thoracic and mediastinal disorders | 36 | 2.32 | 3.14 | 50.81 | 0.88 | 2.23 | No |
| 10 | Erythema | Skin and subcutaneous tissue disorders | 20 | 2.08 | 3.19 | 27.98 | 0.23 | 2.03 | No |
| 11 | Abdominal pain upper | Gastrointestinal disorders | 23 | 2.07 | 3.08 | 30.33 | 0.37 | 2.02 | Yes |
| 18–44 Years | |||||||||
| 1 | Taste disorder | Nervous system disorders | 23 | 21.8 | 32.87 | 645.85 | 0.02 | 20.53 | Yes |
| 2 | Dysgeusia | Nervous system disorders | 150 | 18.9 | 20.85 | 2734.49 | 3.34 | 17.08 | Yes |
| 3 | Nightmare | Psychiatric disorders | 51 | 7.31 | 9.46 | 373.06 | 0.86 | 7.06 | No |
| 4 | Lip swelling | Gastrointestinal disorders | 44 | 7.29 | 9.66 | 328.75 | 0.57 | 7.05 | No |
| 5 | Hallucination | Psychiatric disorders | 62 | 6.37 | 8.01 | 369.87 | 1.07 | 6.14 | Yes |
| 6 | Tinnitus | Ear and labyrinth disorders | 49 | 6.2 | 8.07 | 293.76 | 0.72 | 6.00 | Yes |
| 7 | Psychotic disorder | Psychiatric disorders | 58 | 5.76 | 7.31 | 307.21 | 0.92 | 5.57 | Yes |
| 8 | Panic attack | Psychiatric disorders | 63 | 5.54 | 6.94 | 312.6 | 1.06 | 5.35 | No |
| 9 | Rash maculopapular | Skin and subcutaneous tissue disorders | 24 | 5.36 | 7.96 | 137.61 | 0.05 | 5.25 | No |
| 10 | Disorientation | Psychiatric disorders | 37 | 5.2 | 7.11 | 186.46 | 0.36 | 5.08 | Yes |
| 11 | Angioedema | Skin and subcutaneous tissue disorders | 42 | 5.12 | 6.84 | 201.96 | 0.48 | 4.98 | Yes |
| 12 | Adverse drug reaction | General disorders and administration site conditions | 38 | 4.8 | 6.53 | 171.13 | 0.39 | 4.69 | Yes |
| 13 | Palpitations | Cardiac disorders | 102 | 4.86 | 5.69 | 389.57 | 1.69 | 4.63 | Yes |
| 14 | Mania | Psychiatric disorders | 27 | 4.36 | 6.31 | 114.55 | 0.1 | 4.27 | Yes |
| 15 | Insomnia | Psychiatric disorders | 166 | 4.42 | 4.85 | 507.56 | 1.94 | 4.11 | Yes |
| 16 | Vertigo | Ear and labyrinth disorders | 45 | 4.09 | 5.41 | 156.62 | 0.51 | 3.99 | Yes |
| 17 | Paranoia | Psychiatric disorders | 26 | 3.81 | 5.55 | 91.99 | 0.07 | 3.74 | No |
| 18 | Drug interaction | General disorders and administration site conditions | 90 | 3.83 | 4.58 | 248.8 | 1.4 | 3.68 | Yes |
| 19 | Confusional state | Psychiatric disorders | 76 | 3.79 | 4.63 | 212.98 | 1.13 | 3.66 | Yes |
| 20 | Swelling face | General disorders and administration site conditions | 38 | 3.35 | 4.56 | 101.77 | 0.3 | 3.29 | No |
| 21 | Diarrhea | Gastrointestinal disorders | 179 | 3.39 | 3.7 | 355.83 | 1.62 | 3.16 | Yes |
| 22 | Abdominal pain upper | Gastrointestinal disorders | 88 | 3.05 | 3.67 | 168.91 | 1.13 | 2.95 | Yes |
| 23 | Peripheral swelling | General disorders and administration site conditions | 32 | 2.88 | 4.04 | 70.03 | 0.14 | 2.83 | No |
| 24 | Erythema | Skin and subcutaneous tissue disorders | 63 | 2.7 | 3.4 | 104.64 | 0.72 | 2.63 | No |
| 25 | Heart rate increased | Investigations | 48 | 2.68 | 3.52 | 84.1 | 0.44 | 2.63 | No |
| 26 | Nervousness | Psychiatric disorders | 25 | 2.66 | 3.91 | 51.2 | 0.01 | 2.62 | Yes |
| 27 | Urticaria | Skin and subcutaneous tissue disorders | 78 | 2.69 | 3.28 | 122.17 | 0.93 | 2.61 | Yes |
| 28 | Anxiety | Psychiatric disorders | 158 | 2.75 | 3.06 | 221.87 | 1.3 | 2.59 | Yes |
| 29 | Dyspnea | Respiratory, thoracic, and mediastinal disorders | 150 | 2.66 | 2.99 | 200.18 | 1.22 | 2.52 | No |
| 30 | Chest discomfort | General disorders and administration site conditions | 44 | 2.49 | 3.3 | 68.5 | 0.33 | 2.44 | Yes |
| 31 | Decreased appetite | Metabolism and nutrition disorders | 49 | 2.48 | 3.24 | 73.77 | 0.45 | 2.43 | Yes |
| 32 | Dyspepsia | Gastrointestinal disorders | 32 | 2.29 | 3.21 | 46.51 | 0.1 | 2.25 | Yes |
| 33 | Tachycardia | Cardiac disorders | 50 | 2.12 | 2.77 | 54.93 | 0.39 | 2.08 | Yes |
| 34 | Chest pain | General disorders and administration site conditions | 65 | 2.1 | 2.64 | 64.92 | 0.57 | 2.05 | Yes |
| 45–64 Years | |||||||||
| 1 | Labeled drug–drug interaction medication error | Injury, poisoning, and procedural complications | 55 | 39.06 | 50.16 | 2474.46 | 1.22 | 36.29 | Yes |
| 2 | Erythema multiforme | Skin and subcutaneous tissue disorders | 37 | 20.77 | 28.43 | 925.23 | 0.5 | 19.88 | No |
| 3 | Mania | Psychiatric disorders | 42 | 15.32 | 20.49 | 744.36 | 0.71 | 14.76 | Yes |
| 4 | Dysgeusia | Nervous system disorders | 159 | 15.72 | 17.27 | 2388.76 | 3.57 | 14.44 | Yes |
| 5 | Drug interaction | General disorders and administration site conditions | 265 | 13.97 | 14.15 | 3206.48 | 3.55 | 12.27 | Yes |
| 6 | Drug level increased | Investigations | 35 | 12.11 | 16.71 | 493.24 | 0.42 | 11.74 | No |
| 7 | Electrocardiogram QT prolonged | Investigations | 53 | 9.47 | 12.19 | 527.59 | 1.03 | 9.16 | Yes |
| 8 | Paranoia | Psychiatric disorders | 27 | 8.67 | 12.57 | 272.73 | 0.13 | 8.48 | No |
| 9 | Face edema | General disorders and administration site conditions | 27 | 8.07 | 11.7 | 250.76 | 0.13 | 7.9 | No |
| 10 | Psychotic disorder | Psychiatric disorders | 32 | 7.31 | 10.25 | 255.59 | 0.29 | 7.15 | Yes |
| 11 | Drug eruption | Skin and subcutaneous tissue disorders | 23 | 7.21 | 10.8 | 192.96 | 0.03 | 7.08 | Yes |
| 12 | Hallucination | Psychiatric disorders | 53 | 7.21 | 9.29 | 381 | 0.95 | 7 | Yes |
| 13 | Nightmare | Psychiatric disorders | 40 | 6.82 | 9.19 | 281.61 | 0.54 | 6.65 | No |
| 14 | Ageusia | Nervous system disorders | 23 | 6.73 | 10.07 | 177.31 | 0.03 | 6.6 | Yes |
| 15 | Swollen tongue | Gastrointestinal disorders | 39 | 6.58 | 8.9 | 263.77 | 0.51 | 6.42 | No |
| 16 | Lip swelling | Gastrointestinal disorders | 33 | 5.71 | 7.96 | 192.79 | 0.3 | 5.6 | No |
| 17 | Rhabdomyolysis | Musculoskeletal and connective tissue disorders | 47 | 5.54 | 7.27 | 246.83 | 0.73 | 5.41 | Yes |
| 18 | Tinnitus | Ear and labyrinth disorders | 41 | 5.54 | 7.43 | 220.56 | 0.54 | 5.41 | Yes |
| 19 | Disorientation | Psychiatric disorders | 35 | 5.17 | 7.13 | 177.38 | 0.35 | 5.07 | Yes |
| 20 | Confusional state | Psychiatric disorders | 102 | 5.04 | 5.92 | 412.47 | 1.91 | 4.82 | Yes |
| 21 | Dysarthria | Nervous system disorders | 34 | 4.91 | 6.81 | 162.02 | 0.3 | 4.82 | No |
| 22 | Hypoglycemia | Metabolism and nutrition disorders | 28 | 3.52 | 5.06 | 87.05 | 0.09 | 3.47 | Yes |
| 23 | Adverse drug reaction | General disorders and administration site conditions | 29 | 3.47 | 4.96 | 87.45 | 0.13 | 3.42 | Yes |
| 24 | Agitation | Psychiatric disorders | 38 | 3.33 | 4.53 | 100.95 | 0.35 | 3.27 | No |
| 25 | Arrhythmia | Cardiac disorders | 26 | 3.21 | 4.69 | 71.75 | 0.04 | 3.17 | Yes |
| 26 | Erythema | Skin and subcutaneous tissue disorders | 75 | 3.26 | 4 | 166.29 | 1.19 | 3.16 | No |
| 27 | Acute kidney injury | Renal and urinary disorders | 66 | 3.18 | 3.98 | 144.65 | 0.96 | 3.1 | No |
| 28 | Palpitations | Cardiac disorders | 60 | 2.99 | 3.79 | 121.09 | 0.8 | 2.93 | Yes |
| 29 | Anxiety | Psychiatric disorders | 99 | 2.57 | 3.05 | 136.25 | 1.13 | 2.49 | Yes |
| 30 | Abdominal pain upper | Gastrointestinal disorders | 71 | 2.43 | 3.01 | 94.21 | 0.84 | 2.37 | Yes |
| 31 | Swelling face | General disorders and administration site conditions | 28 | 2.36 | 3.39 | 44.94 | 0.03 | 2.33 | No |
| 32 | Toxicity to various agents | Injury, poisoning, and procedural complications | 67 | 2.34 | 2.92 | 83.46 | 0.75 | 2.29 | No |
| 33 | Urticaria | Skin and subcutaneous tissue disorders | 56 | 2.31 | 2.96 | 71.1 | 0.57 | 2.26 | Yes |
| 34 | Insomnia | Psychiatric disorders | 91 | 2.26 | 2.71 | 98.05 | 0.92 | 2.2 | Yes |
| 35 | Tachycardia | Cardiac disorders | 31 | 2.19 | 3.09 | 41.92 | 0.08 | 2.16 | Yes |
| 36 | Heart rate increased | Investigations | 35 | 2.17 | 3 | 44.69 | 0.15 | 2.14 | No |
| 37 | Dry mouth | Gastrointestinal disorders | 27 | 2.12 | 3.07 | 35.72 | 0 | 2.1 | Yes |
| ⩾65 Years | |||||||||
| 1 | Labeled drug–drug interaction medication error | Injury, poisoning, and procedural complications | 62 | 17.67 | 22.26 | 1207.73 | 1.66 | 16.83 | Yes |
| 2 | Drug interaction | General disorders and administration site conditions | 487 | 20.16 | 18.08 | 7764.35 | 3.95 | 16.04 | Yes |
| 3 | Torsade de pointes | Cardiac disorders | 32 | 14.29 | 20.09 | 548.97 | 0.32 | 13.81 | Yes |
| 4 | Erythema multiforme | Skin and subcutaneous tissue disorders | 30 | 13.72 | 19.51 | 497.45 | 0.24 | 13.27 | No |
| 5 | Dysgeusia | Nervous system disorders | 97 | 10.26 | 12.14 | 969.6 | 2.55 | 9.77 | Yes |
| 6 | Electrocardiogram QT prolonged | Investigations | 58 | 8.71 | 11.07 | 515.77 | 1.29 | 8.42 | Yes |
| 7 | Drug resistance | General disorders and administration site conditions | 26 | 8.04 | 11.74 | 241.78 | 0.11 | 7.86 | Yes |
| 8 | Gout | Metabolism and nutrition disorders | 32 | 7.85 | 11.02 | 278.36 | 0.32 | 7.67 | No |
| 9 | Nightmare | Psychiatric disorders | 32 | 7.29 | 10.23 | 254.61 | 0.3 | 7.13 | No |
| 10 | Chromaturia | Renal and urinary disorders | 29 | 7.02 | 10.04 | 224.61 | 0.18 | 6.87 | No |
| 11 | Rhabdomyolysis | Musculoskeletal and connective tissue disorders | 68 | 7.03 | 8.74 | 455.17 | 1.55 | 6.8 | Yes |
| 12 | International normalized ratio increased | Investigations | 62 | 5.44 | 6.86 | 303.18 | 1.31 | 5.29 | Yes |
| 13 | Hallucination | Psychiatric disorders | 26 | 5.2 | 7.59 | 141.14 | 0.08 | 5.11 | Yes |
| 14 | Hypoglycemia | Metabolism and nutrition disorders | 60 | 5.17 | 6.54 | 275.25 | 1.16 | 5.03 | Yes |
| 15 | Oral pain | Gastrointestinal disorders | 23 | 4.99 | 7.48 | 121.72 | 0.01 | 4.91 | Yes |
| 16 | Lip swelling | Gastrointestinal disorders | 25 | 4.11 | 6.05 | 99.77 | 0.04 | 4.05 | No |
| 17 | Delirium | Psychiatric disorders | 39 | 3.85 | 5.22 | 128.4 | 0.41 | 3.78 | No |
| 18 | Renal failure acute | Renal and urinary disorders | 47 | 3.74 | 4.92 | 142.52 | 0.61 | 3.66 | No |
| 19 | Pancytopenia | Blood and lymphatic system disorders | 47 | 3.71 | 4.88 | 140.69 | 0.63 | 3.63 | No |
| 20 | Hallucination | Psychiatric disorders | 49 | 3.48 | 4.55 | 131.9 | 0.7 | 3.41 | Yes |
| 21 | Toxicity to various agents | Injury, poisoning, and procedural complications | 62 | 3.02 | 3.82 | 126.32 | 0.89 | 2.95 | No |
| 22 | Hyperkalemia | Metabolism and nutrition disorders | 36 | 2.95 | 4.06 | 79.85 | 0.27 | 2.91 | No |
| 23 | Agitation | Psychiatric disorders | 27 | 2.65 | 3.84 | 53.79 | 0.04 | 2.62 | No |
| 24 | Bradycardia | Cardiac disorders | 41 | 2.55 | 3.43 | 68.32 | 0.37 | 2.51 | No |
| 25 | Acute kidney injury | Renal and urinary disorders | 85 | 2.52 | 3.05 | 116.02 | 1.03 | 2.45 | No |
| 26 | Palpitations | Cardiac disorders | 39 | 2.46 | 3.33 | 61.47 | 0.27 | 2.42 | Yes |
| 27 | Confusional state | Psychiatric disorders | 80 | 2.28 | 2.79 | 91.03 | 0.89 | 2.23 | Yes |
| 28 | Insomnia | Psychiatric disorders | 70 | 2.22 | 2.77 | 77.66 | 0.8 | 2.17 | Yes |
| 29 | Muscular weakness | Musculoskeletal and connective tissue disorders | 41 | 2.07 | 2.79 | 45.48 | 0.27 | 2.04 | No |
| 30 | Hyperhidrosis | Skin and subcutaneous tissue disorders | 35 | 2.04 | 2.83 | 39.68 | 0.14 | 2.02 | Yes |
AE, adverse event; IC, Information Component; PT, preferred term; PRR, proportional reporting ratio; ROR, reporting odds ratio; SOC, system organ class.
Vomiting (n = 89), diarrhea (n = 179), drug interactions (n = 265), and drug interactions (n = 487) were the most commonly reported in the age groups of 0–17, 18–44, 45–64, and 65 years and above, respectively.
In the 0–17 years age group, strong signals detected included abnormal product taste (EBGM 95% CI = 12.14) and dysgeusia (EBGM 95% CI = 11.92). In the 18–44 years age group, strong signals detected included taste disorders (EBGM 95% CI = 20.53) and dysgeusia (EBGM 95% CI = 17.08). In the 45- to 64-year-old age group, strong signals detected included labeled drug-drug interaction medication errors (EBGM 95% CI = 36.29) and erythema multiforme (EBGM 95% CI = 19.88). In the 65 years and older age group, strong signals detected included labeled drug–drug interaction medication errors (EBGM 95% CI = 16.83) and drug interactions (EBGM 95% CI = 16.04).
A total of 41 PT signals were not explicitly listed in the clarithromycin package insert, and were primarily concentrated in psychiatric disorders (n = 9), skin and subcutaneous tissue disorders (n = 6), gastrointestinal disorders (n = 5), etc. Among these signals, 4 signals were not explicitly listed in the package insert for the age group of 0–17 years, 9 signals for the age group of 18–44 years, 14 signals for the age group of 45–64 years, and 14 signals for the age group of 65 years and above.
Signals related to age differences
Using the ROR method, the above warning signals are separately calculated for age-related signals. When the ratio of the signal value for children to the signal value for other age groups is >1.5, or when children produce signals while other age groups do not, these signals are considered specific to children; similarly, when the ratio of the signal value for elderly people to the signal value for other age groups is >1.5, or when elderly people produce signals while other age groups do not, these signals are considered specific to the elderly.15–17 Through computation, it was found that there were 18 age-specific signals, including 3 for children and 15 for elderly people. Table 3 shows the results.
Table 3.
Signals related to age differences.
| Child-specific signals | Elderly specific signals | ||||
|---|---|---|---|---|---|
| Adverse event | Frequency | Signal risk ratio | Adverse event | Frequency | Signal risk ratio |
| Vomiting a | 89 | 2.79 | Gout b | 32 | 7.85 |
| Drug hypersensitivity a | 15 | 2.4 | Pancytopenia b | 47 | 3.71 |
| Dyspnea | 36 | 2.32 | Hyperkalemia b | 36 | 2.95 |
| Chromaturia | 29 | 2.86 | |||
| Bradycardia b | 41 | 2.55 | |||
| Drug resistance | 26 | 2.43 | |||
| Drug interaction | 487 | 2.38 | |||
| Torsade de pointes | 32 | 2.36 | |||
| Rhabdomyolysis | 68 | 2.25 | |||
| Electrocardiogram QT prolonged | 58 | 2.22 | |||
| Muscular weakness b | 41 | 2.07 | |||
| Hyperhidrosis b | 35 | 2.04 | |||
| Hypoglycemia | 60 | 1.97 | |||
| Oral pain | 23 | 1.88 | |||
| Renal failure acute | 85 | 1.76 | |||
Signals generated by children rather than signals not generated by children.
Signals generated by elderly individuals rather than signals not generated by elderly individuals.
Discussion
Although adverse reactions related to clarithromycin have been reported and studied in clinical trials, there is a lack of comprehensive research on these AEs and insufficient studies focusing on specific populations. This study is the first pharmacovigilance investigation of clarithromycin-related AEs based on real-world data from the FAERS database. By utilizing a specific time frame of FAERS data for comparison, we conducted a disproportionality analysis to identify AEs significantly associated with clarithromycin therapy, revealing differences in AE risks across various age groups. Our research represents the largest post-marketing study of clarithromycin AEs conducted in a real-world setting to date. Through this large-scale, widely encompassing database, we can provide a more comprehensive depiction of the potential AEs encountered by different age groups during clarithromycin use, particularly in elderly and pediatric populations, where specific risks are highlighted.
Analysis of signals in children
In the 0–17 age group, the highest number of reports occurred in the 1–4 age group. This may be related to the relatively lower safety profile of clarithromycin in this age group, possibly due to factors such as increased susceptibility to adverse effects. Studies by Bourgeois et al. and Kimland et al. have shown that adverse reactions are more common in younger children, with 43%–61% of events originating from children aged 0 to 4.18,19 The most frequently reported adverse reactions to clarithromycin in the 0–17 age group are gastrointestinal disorders, mainly vomiting and upper abdominal pain, which is consistent with the clarithromycin label. 5 Among them, vomiting is also a high-risk signal for clarithromycin in children, with a high frequency of occurrence. Vomiting is a common symptom in children and is usually benign. However, clinical physicians must be able to identify this complication promptly and avoid serious complications. 20 The adverse reaction with the strongest signal intensity was abnormal product taste, which was not mentioned in the clarithromycin label. Clarithromycin has a strong bitter taste, which affects patient compliance and treatment efficacy. Therefore, improvements in design can optimize taste masking. 21
In addition to vomiting, two specific signals were detected in children receiving clarithromycin (drug hypersensitivity, dyspnea). Research by Marrs et al. indicated that children under 4 years old most commonly present to drug allergy clinics, suggesting that young children may be more susceptible to antibiotic allergies. 22 Guvenir et al. evaluated hypersensitivity reactions in children to non-β-lactam antibiotics, among which clarithromycin (63.6%) was the most commonly reported cause of hypersensitivity reactions. 23 Conducting oral provocation tests to diagnose clarithromycin-induced hypersensitivity reactions is crucial because they can manifest in various clinical presentations, ranging from mild skin reactions to life-threatening severe skin reactions. 24 Skin reactions are closely associated with hypersensitivity reactions, with rash being the most common hypersensitivity reaction. 25 In addition, there are severe adverse skin reactions, including Stevens-Johnson syndrome. 26 Suleyman et al. reported that confirmed β-lactam allergy is a risk factor for clarithromycin hypersensitivity reactions, especially in patients who develop rash following amoxicillin–clavulanic acid treatment before clarithromycin therapy. 27 For individuals allergic to this drug, its use should be prohibited. Dyspnea is a specific signal in children and is not included in the label. Research by Gangemi et al. during drug provocation tests revealed that after administering a 1/4 therapeutic dose of clarithromycin, patients exhibited dyspnea, coughing, and bronchospasm in all lung fields. 28 Clinical physicians should note that dyspnea is a pediatric-specific adverse reaction not listed on the label.
Signal analysis in adults
The signals detected in the 18- to 44-year-old and 45- to 64-year-old age groups for clarithromycin were similar, with AE signals predominantly related to psychiatric disorders in these populations. The signals mainly included nightmare, hallucination, psychotic disorder, panic attack, disorientation, mania, insomnia, paranoia, confusional state, nervousness, anxiety, and agitation, among which four signals—nightmare, panic attack, paranoia, and agitation—were not labeled in the instructions. Wallace et al. first reported clarithromycin-induced neurotoxicity in 1993, describing central nervous system side effects in seven patients receiving high-dose antibiotics (1200 mg/day) with mild renal impairment. The observed adverse reactions included altered consciousness, dizziness, and insomnia, but these symptoms improved when the dose was reduced to 1000 mg/day. 29 Rare neurological and psychiatric sequelae of clarithromycin have been reported. 30 Bandettini di Poggio et al. reviewed the literature on adult neurotoxicity induced by clarithromycin and reported adverse reactions in the central nervous system, including central nervous system depression (altered consciousness and lethargy) or excitation (agitation, insomnia, delirium, and psychosis). 3 Studies have shown that both high and low doses of clarithromycin may cause neurological side effects, and drug interactions are important underlying causes of neurotoxicity. When patients simultaneously use clarithromycin and other drugs metabolized by the cytochrome P450 enzyme of the CYP3A family, the risk of neurotoxicity is greater.3, 31 –35
Due to the widespread use of clarithromycin, clinicians should be aware of its neurotoxicity and be vigilant for potential neurological and psychiatric symptoms. Early detection of clarithromycin (CLA)-induced neurotoxicity and prompt discontinuation of the drug are crucial. Bandettini di Poggio et al. recommend performing electroencephalography (EEG) in the diagnostic evaluation of CLA-induced neurotoxicity. 3
Signal analysis in elderly
In elderly individuals (⩾65 years), strong signals for clarithromycin infection mainly include labeled drug–drug interaction medication errors, drug interactions, and cardiac-related AEs such as prolonged torsade de pointes and electrocardiogram QT, all of which are specific signals for clarithromycin in the elderly population.
Medication interactions are a common reason for hospital admission in elderly individuals. 36 Clarithromycin interacts with a variety of drugs, being a stronger CYP450 inhibitor than erythromycin, and appears to have more frequent and severe drug interactions. 37 Clarithromycin interacts with statin drugs, particularly those metabolized by CYP3A4, increasing the risk of skeletal muscle toxicity. 38 Kunakorntham et al. and Pasqualetti et al. reported associations between clarithromycin and rhabdomyolysis.39,40 Rhabdomyolysis is a clinical syndrome of skeletal muscle injury characterized by muscle pain, weakness, dark-colored urine, and acute kidney injury, with the most common presenting symptoms being muscle pain, weakness, and tea-colored urine. 41 Rhabdomyolysis, pigmenturia, muscle weakness, and acute kidney injury are specific signals in elderly individuals, possibly resulting from interactions between clarithromycin drugs. Coadministration of clarithromycin with calcium channel blockers can also cause acute kidney injury. A large retrospective cohort study revealed that patients prescribed both calcium channel blockers and clarithromycin had an increased risk of hospitalization and acute kidney injury. 42 The Girardeau algorithm confirmed previously identified signals of acute kidney injury associated with clarithromycin and calcium channel blockers. 43 Interactions between clarithromycin and colchicine can also lead to renal impairment. 44 Villa Zapata et al.’s retrospective study revealed that concurrent use of colchicine and clarithromycin can result in leukopenia, thrombocytopenia, rhabdomyolysis, and renal failure. 45 Clinicians should monitor white blood cell count, creatine kinase (CK), renal function, and liver function, with symptom monitoring including identification of the aforementioned symptoms. Concurrent use of clarithromycin and sulfonylureas can lead to hypoglycemia, 46 which is also a specific signal in elderly individuals using clarithromycin. Case reports have shown severe hypoglycemia related to the interaction between clarithromycin and repaglinide. 47 Kennedy et al. found a significant association between clarithromycin and hypoglycemia through analysis of adverse reactions of hypoglycemia in the FAERS. 48 Adverse reactions involving cardiac organs, such as torsades de pointes and prolonged QT intervals on electrocardiograms, have been reported, suggesting an increased risk of adverse cardiac reactions with clarithromycin.49,50 Currently, the Summary of Product Characteristics for clarithromycin advises caution in patients with coronary artery disease and recommends against use in patients with a history of ventricular arrhythmias. 51
Drug interactions are preventable causes of morbidity and mortality. Before prescribing clarithromycin, clinicians should strictly adhere to the label instructions and review the patient’s medication list. Patients with heart disease should not use clarithromycin. Electrolyte levels should be monitored during oral clarithromycin therapy. Patients with diabetes using sulfonylureas and patients with hyperlipidemia undergoing lipid-lowering therapy should use clarithromycin cautiously, with timely monitoring of blood glucose and biochemical markers related to rhabdomyolysis, including renal function, CK, blood potassium, and myoglobinuria. Patients using calcium channel blockers should closely monitor related drug concentrations.52–54
Limitations
This study has several limitations. First, the FAERS database is a global spontaneous reporting system that has inherent issues such as underreporting, overreporting, misreporting, incomplete information, and non-standardized data. 55 The number of patients receiving clarithromycin treatment who did not report AEs is unknown, and there is a lack of denominator data. As a result, we cannot establish a causal relationship between clarithromycin and AEs, nor can we calculate the true incidence of clarithromycin-related AEs. Furthermore, each report lacks specific treatment duration data, which hinders our ability to conduct further risk analysis on clarithromycin-related AEs. Third, there is a lack of certain key information, such as the patient’s medical history, concomitant medications, or treatment regimen, which may influence the occurrence of AEs. It is challenging to fully obtain this information. This study also did not completely differentiate between the drug indications, as reports of unknown indications were relatively common, and missing values were frequent. In addition, spontaneous reporting data are typically less reliable than data collected in clinical trials and cohort studies, and comparisons between different age groups are limited by potential imbalances in patient characteristics. Despite the limitations of FAERS, our findings provide insights into the basic aspects of clarithromycin-related AEs across different age groups and may serve as a foundation for subsequent rigorous prospective studies.
Conclusion
In summary, this study provides an objective reference for pharmacovigilance by exploring the safety signals of clarithromycin use across different age groups. The safety of clarithromycin is different in different age groups. Children are more closely associated with AEs related to vomiting, drug-induced hypersensitivity, and dyspnea. In adults, it is more associated with psychiatric AEs. In addition, the use of clarithromycin in the elderly should be strictly in accordance with the instructions and be alert to drug interactions.
Acknowledgments
None.
Footnotes
ORCID iDs: Zhenpo Zhang  https://orcid.org/0009-0006-8175-4972
https://orcid.org/0009-0006-8175-4972
Jingping Zheng  https://orcid.org/0000-0002-9770-4308
https://orcid.org/0000-0002-9770-4308
Contributor Information
Haiyan Mai, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China.
Zhenpo Zhang, College of Pharmacy, Jinan University, Guangzhou, Guangdong, China.
Yankun Liang, College of Pharmacy, Jinan University, Guangzhou, Guangdong, China.
Jingping Zheng, College of Pharmacy, Jinan University, Guangzhou, Guangdong, China.
Ling Su, College of Pharmacy, Jinan University, Guangzhou, Guangdong 511436, China.
Declarations
Ethics approval and consent to participate: Not applicable. FDA Adverse Event Reporting System is a spontaneous reporting system, the publicly available data are anonymized, and therefore, obtaining consent to participate is not applicable. The present pharmacovigilance study was conducted using a public database of spontaneous reports. Given the use of deidentified data, ethical approval was not considered necessary.
Consent for publication: Not applicable.
Author contributions: Haiyan Mai: Data curation; Resources; Writing – original draft; Writing – review & editing.
Zhenpo Zhang: Data curation; Resources; Writing – original draft; Writing – review & editing.
Yankun Liang: Investigation; Methodology; Software.
Jingping Zheng: Software; Supervision.
Ling Su: Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets generated and/or analyzed during the current study are available in the US FAERS repository (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers).
Code availability: The R script developed for the analyses is available from the corresponding author upon reasonable request.
References
- 1. Vancocin. The American Society of Health-System Pharmacists, https://www.drugs.com/monograph/vancomycin.html (2015, accessed 1 March 2024).
- 2. World Health Organization. WHO model list of essential medicines, http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1 (2013, accessed 1 March 2024).
- 3. Bandettini di, Poggio M, Anfosso S, Audenino D, et al. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3): 313–318. [DOI] [PubMed] [Google Scholar]
- 4. Wikipedia Contributors. Clarithromycin. Wikipedia. The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Clarithromycin&oldid=1210187822 (accessed 1 March 2024).
- 5. Biaxin Package Insert. Drugs.com, https://www.drugs.com/pro/biaxin.html (2023, accessed 1 March 2024).
- 6. Clarithromycin. Tuberculosis (Edinb) 2008; 88(2): 92–95. [DOI] [PubMed] [Google Scholar]
- 7. Edwards BJ, Bunta AD, Lane J, et al. Bisphosphonates and nonhealing femoral fractures: analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: a systematic review from the Research on Adverse Drug Events and Reports (RADAR) project. J Bone Joint Surg Am 2013; 95(4): 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alatawi YM, Hansen RA. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf 2017; 16(7): 761–767. [DOI] [PubMed] [Google Scholar]
- 9. Brown EG. Using MedDRA: implications for risk management. Drug Saf 2004; 27(8): 591–602. [DOI] [PubMed] [Google Scholar]
- 10. Fusaroli M, Salvo F, Begaud B, et al. The reporting of a disproportionality analysis for drug safety signal detection using individual case safety reports in pharmacovigilance (READUS-PV): development and statement. Drug Saf 2024; 47(6): 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 2004; 13(8): 519–523. [DOI] [PubMed] [Google Scholar]
- 12. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001; 10(6): 483–486. [DOI] [PubMed] [Google Scholar]
- 13. Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 1998; 54(4): 315–321. [DOI] [PubMed] [Google Scholar]
- 14. Dumouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat 1999; 53(3): 177–190. [Google Scholar]
- 15. Tang XW, Jia YT, Tian XJ, et al. Detection and analysis of adverse reaction signals for sex differences of anti-MRSAdugs. Chin J Hosp Phram 2018; 38(3): 262–265, 274. [Google Scholar]
- 16. Si FG, Cui J. Literature analysis of drug-induced autoimmune hepatitis induced by infliximab. Chin J New Drugs 2020; 29(24): 2874–2877. [Google Scholar]
- 17. Tian XJ, Tang XW, Ji HH, et al. Data mining and analysis based on gender differences in statin-associated muscular adverse events: data mining of the pharmacovigilance databases of the United States. Chin J Hosp Pharm 2019; 39(14): 1480–1484. [Google Scholar]
- 18. Bourgeois FT, Shannon MW, Valim C, et al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf 2010; 19: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kimland E, Rane A, Ufer M, et al. Paediatric adverse drug reactions reported in Sweden from 1987 to 2001. Pharmacoepidemiol Drug Saf 2005; 14: 493–499. [DOI] [PubMed] [Google Scholar]
- 20. Shields TM, Lightdale JR. Vomiting in children. Pediatr Rev 2018; 39(7): 342–358. [DOI] [PubMed] [Google Scholar]
- 21. Ntemi PV, Walker RB, Khamanga SMM. Design, evaluation and optimization of taste masked clarithromycin powder. Pharmazie 2019; 74(12): 721–727. [DOI] [PubMed] [Google Scholar]
- 22. Marrs T, Fox AT, Lack G, et al. The diagnosis and management of antibiotic allergy in children: systematic review to inform a contemporary approach. Arch Dis Child 2015; 100(6): 583–588. [DOI] [PubMed] [Google Scholar]
- 23. Guvenir H, Dibek Misirlioglu E, Capanoglu M, et al. Proven non-β-lactam antibiotic allergy in children. Int Arch Allergy Immunol 2016; 169(1): 45–50. [DOI] [PubMed] [Google Scholar]
- 24. Shaeer KM, Chahine EB, Varghese Gupta S, et al. Macrolide allergic reactions. Pharmacy 2019; 7(3): 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu LJ, Liu AY, Wong PH, et al. Road less traveled: drug hypersensitivity to fluoroquinolones, vancomycin, tetracyclines, and macrolides. Clin Rev Allergy Immunol 2022; 62(3): 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pejčić AV. Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of macrolide antibiotics: a review of published cases. Int J Dermatol 2021; 60(1): 12–24. [DOI] [PubMed] [Google Scholar]
- 27. Suleyman A, Yucel E, Sipahi Cimen S, et al. Clarithromycin hypersensitivity in children: is there a link with β-lactam hypersensitivity? Pediatr Allergy Immunol 2021; 32(8): 1781–1787. [DOI] [PubMed] [Google Scholar]
- 28. Gangemi S, Ricciardi L, Fedele R, et al. Immediate reaction to clarithromycin. Allergol Immunopathol (Madr) 2001; 29(1): 31–32. [DOI] [PubMed] [Google Scholar]
- 29. Jiménez P, Navarro-Ruiz A, Sendra P, et al. Hallucinations with therapeutic doses of clarithromycin. Int J Clin Pharmacol Ther 2002; 40(1): 20–22. [DOI] [PubMed] [Google Scholar]
- 30. Young MJ, Caplan RA, Connolly I, et al. Closed-eye visual hallucinations associated with clarithromycin. J Neuropsychiatry Clin Neurosci 2021; 33(3): 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yasui N, Otani K, Kaneko S, et al. Carbamazepine toxicity induced by clarithromycin coadministration in psychiatric patients. Int Clin Psychopharmacol 1997; 12(4): 225–229. [DOI] [PubMed] [Google Scholar]
- 32. Gélisse P, Hillaire-Buys D, Halaili E, et al. Carbamazépine et clarithromycine: une interaction médicamenteuse cliniquement significative [Carbamazepine and clarithromycin: a clinically relevant drug interaction]. Rev Neurol (Paris) 2007; 163(11): 1096–1099. [DOI] [PubMed] [Google Scholar]
- 33. Pollak PT, Sketris IS, MacKenzie SL, et al. Delirium probably induced by clarithromycin in a patient receiving fluoxetine. Ann Pharmacother 1995; 29(5): 486–488. [DOI] [PubMed] [Google Scholar]
- 34. Jaber BL, Lobon LF, Madias NE. The serotonin syndrome complicating co-prescription of paroxetine and clarithromycin. Am J Med 2006; 119(4): e3. [DOI] [PubMed] [Google Scholar]
- 35. Prime K, French P. Neuropsychiatric reaction induced by clarithromycin in a patient on highly active antiretroviral therapy (HAART). Sex Transm Infect 2001; 77(4): 297–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cunningham G, Dodd TR, Grant DJ, et al. Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing 1997; 26(5): 375–382. [DOI] [PubMed] [Google Scholar]
- 37. Eljaaly K, Botaish A, Bahobail F, et al. Systematic review and meta-analysis of the safety of erythromycin compared to clarithromycin in adults and adolescents with pneumonia. J Chemother 2020; 32(1): 1–6. [DOI] [PubMed] [Google Scholar]
- 38. Hougaard Christensen MM, Bruun Haastrup M, Øhlenschlaeger T, et al. Interaction potential between clarithromycin and individual statins—a systematic review. Basic Clin Pharmacol Toxicol 2020; 126(4): 307–317. [DOI] [PubMed] [Google Scholar]
- 39. Kunakorntham P, Pattanaprateep O, Dejthevaporn C, et al. Detection of statin-induced rhabdomyolysis and muscular related adverse events through data mining technique. BMC Med Inform Decis Mak 2022; 22(1): 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pasqualetti G, Bini G, Tognini S, et al. Clarithromycin-induced rhabdomyolysis: a case report. Int J Gen Med 2012; 5: 283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest 2013; 144(3): 1058–1065. [DOI] [PubMed] [Google Scholar]
- 42. Yan R. Clarithromycin combined with calcium channel blockers increases the risk of hospitalisation for acute kidney injury in elderly patients. Chin J Evid Based Cardiovasc Med 2013; 5(6): 663. [Google Scholar]
- 43. Girardeau Y, Trivin C, Durieux P, et al. Detection of drug-drug interactions inducing acute kidney injury by electronic health records mining. Drug Saf 2015; 38(9): 799–809. [DOI] [PubMed] [Google Scholar]
- 44. Hung IFN, Wu AKL, Cheng VCC, et al. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: a retrospective study. Clin Infect Dis 2005; 41: 291–300. [DOI] [PubMed] [Google Scholar]
- 45. Villa Zapata L, Hansten PD, Horn JR, et al. Evidence of clinically meaningful drug-drug interaction with concomitant use of colchicine and clarithromycin. Drug Saf 2020; 43(7): 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014; 10: 711–722. [DOI] [PubMed] [Google Scholar]
- 47. Khamaisi M, Leitersdorf E. Severe hypoglycemia from clarithromycin-repaglinide drug interaction. Pharmacotherapy 2008; 28: 682–684. [DOI] [PubMed] [Google Scholar]
- 48. Kennedy KE, Teng C, Patek TM, et al. Hypoglycemia associated with antibiotics alone and in combination with sulfonylureas and meglitinides: an epidemiologic surveillance study of the FDA Adverse Event Reporting System (FAERS). Drug Saf 2020; 43(4): 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chang NL, Shah P, Bikkina M, et al. Clarithromycin-induced torsades de pointes. Am J Ther 2016; 23(3): e955–e956. [DOI] [PubMed] [Google Scholar]
- 50. Soraci L, Cherubini A, Paoletti L, et al. Safety and tolerability of antimicrobial agents in the older patient. Drugs Aging 2023; 40(6): 499–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Root AA, Wong AY, Ghebremichael-Weldeselassie Y, et al. Evaluation of the risk of cardiovascular events with clarithromycin using both propensity score and self-controlled study designs. Br J Clin Pharmacol 2016; 82(2): 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Corsonello A, Abbatecola AM, Fusco S, et al. The impact of drug interactions and polypharmacy on antimicrobial therapy in the elderly. Clin Microbiol Infect. 2015; 21: 20–26. [DOI] [PubMed] [Google Scholar]
- 53. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging 2018; 13: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 2003; 289(13): 1652–1658. [DOI] [PubMed] [Google Scholar]
- 55. Sakaeda T, Tamon A, Kadoyama K, et al. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci 2013; 10(7): 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]