Abstract
Large hemispheric infarction (LHI) is a severe form of stroke with high mortality and poor outcomes. Ultrasonic optic nerve sheath diameter (ONSD) is considered an effective indicator for intracranial hypertension. Our study aimed to validate the efficiency of ultrasonic ONSD and develop a nomogram to identify LHI patients who have 90-day mortality. We recruited 419 LHI patients (training cohort, n = 202; internal validation cohort, n = 86; and external validation cohort, n = 131) from six centers. Demographic, laboratory, computed tomography, and ultrasonic data were collected. At 90 days, 41.8% of patients died. Ultrasonic ONSD (odds ratio [OR], 7.026; 95% CI, 2.638–18.708; P < 0.001), male (OR, 8.620; 95% CI, 2.962–25.092; P < 0.001), midline shift (OR, 1.207; 95% CI, 1.085–1.342; P = 0.001), and infarction volume (OR, 1.020; 95% CI, 1.012–1.028; P < 0.001) were independent predictors. In identifying LHI patients prone to 90-day mortality, the nomogram developed using these predictors showed areas under the receiver operating characteristic curve (AUC) of 0.897, 0.824, 0.833 in the training cohort, internal and external validation cohorts, respectively. Ultrasonic ONSD complement the midline shift and infarction volume to create a reliable multimodal method for monitoring prognosis in patients with LHI.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84720-6.
Keywords: Large hemispheric infarction, Mortality, Optic nerve sheath diameter, Ultrasound
Subject terms: Neurology, Risk factors
Introduction
Large hemispheric infarction (LHI) is a severe form of ischemic stroke associated with high mortality and poor outcomes. It is usually caused by acute occlusion of the internal carotid artery (ICA) or middle cerebral artery (MCA). Over 50% of patients with LHI develop malignant cerebral edema and rapid neurological decline within 2–3 days of symptom onset1. The mortality rate can be as high as 80% in patients receiving conservative treatment2. While imaging examinations such as computed tomography (CT) and magnetic resonance imaging (MRI) aid in identifying LHI patients and monitoring disease progression, the complexity and uncertainty surrounding their condition often hinder frequent reexamination, particularly in critically ill individuals3. Therefore, there is an urgent need to establish non-invasive bedside methods for objectively assessing brain status during the early stages of LHI and identifying prognostic factors for poor outcomes.
Ultrasound is an important imaging method for patients with stroke because of its convenience and bedside operability. Recent studies have confirmed the role of ultrasound in the noninvasive assessment of intracranial pressure (ICP). Multimodal evaluation of optic nerve sheath diameter (ONSD), pulsatility index, and estimated ICP (eICP) using transcranial Doppler are correlated with invasive ICP and have acceptable accuracy in estimating intracranial hypertension4–6. Because intracranial hypertension caused by malignant brain edema is an important cause of death in patients with LHI, we hypothesized that these ultrasound features could predict the outcomes of patients with LHI. In addition, the nomogram, which has been widely used as a predictive method in recent years, can synthesize an integrated model to quantitatively predict the prognostic risks and can be used to quantify malignant risk for patients with LHI7.
Therefore, our study sought to validate the role of ultrasound indicators to predict 90-day mortality in patients with LHI. Moreover, we aimed to develop an easy bedside nomogram to evaluate the prognosis of LHI.
Materials and methods
Study design
This study was approved by the Ethics Committee (Approve number: 19K129-001) and conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the participants or their next of kin before the commencement of this study.
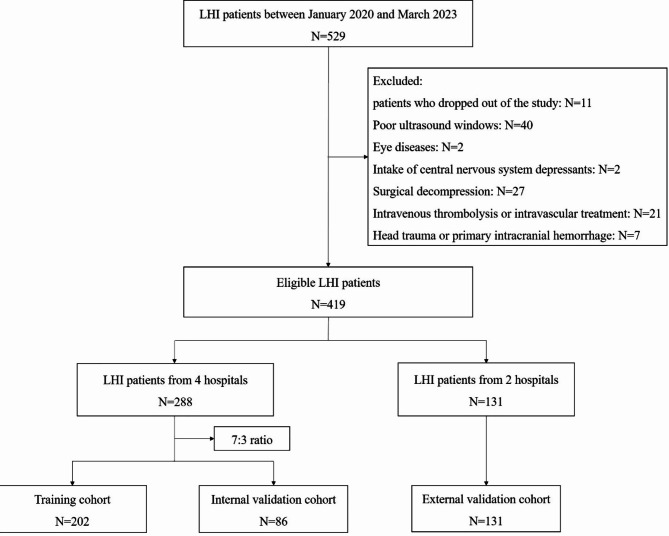
This multicenter prospective study was conducted at six large general hospitals between January 2020 and March 2023. Patients with LHI admitted to the neurological intensive care unit were eligible for enrollment. The inclusion criteria were as follows: (1) admission time ≤ 72 h after LHI onset; (2) CT and/or MRI documenting infarction in at least two-thirds of the unilateral MCA territory, with or without additional ipsilateral infarction of the anterior or posterior cerebral artery; and (3) magnetic resonance angiography or CT angiography showing ICA or MCA M1 segment vascular occlusion. The exclusion criteria were as follows: (1) patients who dropped out of the study; (2) age < 18 years; (3) poor ultrasound windows; (4) any eye diseases, such as optic neuropathy, glaucoma, eye trauma, or tumor; (5) surgical decompression; (6) intake of central nervous system depressants including sedatives, narcotics, antidepressants, antipsychotics, and antiepileptics; (7) intravenous thrombolysis or intravascular treatment; and (8) head trauma or primary intracranial hemorrhage. All patients who met the inclusion and exclusion criteria during the time period were screened. Finally, 419 patients with LHI were included in the study (Fig. 1). Among all the patients from the six hospitals, patients from four hospitals were randomly selected as the training (n = 202) and internal validation (n = 86) cohorts in a 7:3 ratio, while patients from two hospitals were selected as the external validation cohort (n = 131). All patients were treated according to the Guidelines for the Early Management of Patients with Acute Ischemic Stroke 2019 of the American Heart Association/American Stroke Association and received regular clinical care and postdischarge guidance8.
Fig. 1.
Flowchart of patients’ selection. LHI, large hemispheric infarction.
Data collection
Demographic, laboratory, CT, and ultrasound data were recorded. The demographic characteristics included age, sex, body mass index, head circumference, waistline, and vascular risk factors. Clinical assessments were evaluated on admission, including systolic blood pressure, diastolic blood pressure, mean arterial pressure (MAP), the Glasgow Coma Scale, and the National Institutes of Health Stroke Scale (NIHSS). The time from stroke onset to admission, Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification, and vascular lesions were also recorded.
Laboratory characteristics were measured within 24 h of admission, including white blood cell, neutrophil, lymphocyte, monocyte, and platelet counts, activated partial thromboplastin time, international normalized ratio, fasting blood glucose, glycosylated hemoglobin, homocysteine, serum kalium, serum sodium, serum calcium, serum albumin, uric acid, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, procalcitonin, hypersensitive C-reactive protein, N-terminal pro-B-type natriuretic peptide, and D-Dimer. The neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio were calculated.
CT characteristics were obtained within 24 h of admission, including the CT time from onset, side of infarction, midline shift, post-infarct hemorrhage, and infarction volume. CT examinations were performed using one of the four multidetector row scanners (GE, Boston, US; Philips, Amsterdam, Netherlands; Siemens, Munich, Germany; Toshiba, Tokyo, Japan). Midline shift was measured in millimeters at the level of the septum pellucidum9. Infarction volume was calculated using the ABC/2 formula10. All CT images of enrolled patients from the six centers were saved and measured by two neurologists who were blinded to the clinical data, and the final measurement of each patient was derived from the average value measured by two neurologists to minimize variability.
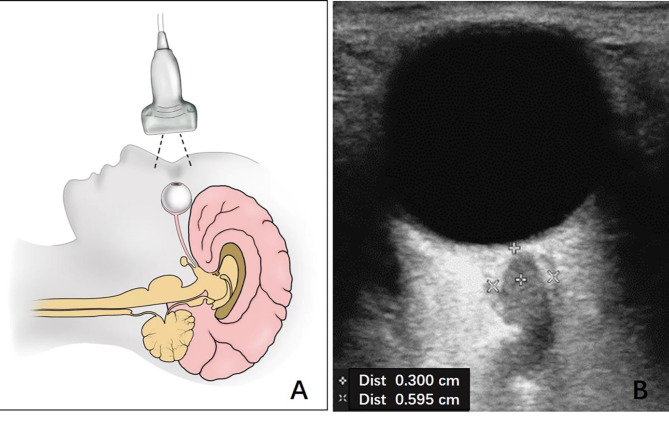
Ultrasound characteristics were obtained within 24 h of admission, including ultrasound time from onset, peak systolic velocity, end diastolic velocity (EDV), mean flow velocity (MFV), pulsatility index of the affected and unaffected hemispheres, ONSD of the affected and unaffected eyes, and eICP. Ultrasound examinations were performed on a Philips iU22 ultrasound system (Andover, Massachusetts, USA). The velocity measurements were performed bilaterally on MCA using a 5 − 1 MHz phased array transducer. The ONSD measurements were performed on the axial and sagittal planes 3 mm behind the retina in both eyes using a high-resolution 9-MHz linear array transducer, as previously published (Fig. 2)4,6. They were measured on the inside of the dura mater11. Following the “as low as reasonably achievable” principles, TI and MI were both set to 0.1 and the inspection time was minimized as much as possible. Sufficient ultrasound gel was used to avoid globe pressure12. eICP was calculated using a validated formula, eICP = MAP×(1-EDV/MFV)-14, which includes simultaneous velocity measurements using transcranial color Doppler and blood pressure readings to standardize measurements4,13. All ultrasound images of enrolled patients from the six centers were saved and measured by two sonographers who were blinded to the clinical data, and final measurement of each patient was derived from the average value measured by two sonographers to minimize variability.
Fig. 2.
Schematic (A) and ultrasonic (B) images of measurement of optic nerve sheath diameter. Software Adobe Illustrator (version 26.0, http://www.adobe.com/cn/products/illustrator.html) was used to create 2 A.
Patients were followed up for 90 days after stroke onset via telephone interviews. The 90-day mortality rate of the patients was considered as the outcome.
Statistical analyses
The normality of distribution was assessed using the Kolmogorov-Smirnov test. Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range, IQR), and categorical variables are expressed as frequencies and percentages. Continuous variables were analyzed using the independent samples t-test or Mann–Whitney U-test, and categorical variables were analyzed using the chi-squared test. In the training cohort, potential prognostic factors were screened using the least absolute shrinkage and selection operator (LASSO) regression. Factors selected in the LASSO regression were further analyzed using multivariable logistic regression analysis to identify prognostic factors associated with death in patients with LHI. Factors that were statistically significant in the multivariable analysis were used to construct a nomogram. Nomogram performance was evaluated with respect to discrimination and calibration. The nomogram was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC) for discrimination. For calibration, the nomogram was assessed using calibration curves. Internal and external validations were performed to validate the nomogram, and 1000 bootstrapping resamples were performed in each group for internal and external validation. Decision curve analysis was used to evaluate the clinical benefits and utility of the nomogram. The 95% CIs and two-tailed P values were calculated. A P value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 26.0, IBM Corporation, Chicago, IL, USA) and R version 4.2.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
In total, 419 patients with LHI were included in this study. The mean age was 66.7 years, and most were male (69.7%). The 90-day mortality was 41.8%. Supplemental Table 1 summarizes the patient characteristics in the training and validation cohorts.
Feature selection
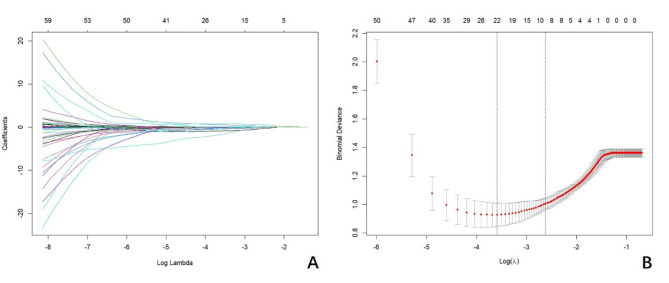
We reduced the initial 68 characteristics of 202 patients in the training cohort to 10 potential predictors of 90-day mortality of patients with LHI: sex (coefficient, − 4.047), hyperlipidemia (coefficient, − 1.529), serum Ca (coefficient, − 5.083), triglyceride (coefficient, − 1.052), NIHSS on admission (coefficient, 0.016), midline shift (coefficient, 0.051), infarction volume (coefficient, 0.008), ultrasound time from onset (coefficient, 0.001), ONSD on the affected side (coefficient, 0.257), and mean ONSD (coefficient, 0.588). The LASSO regression model was used to select the characteristic variables (Fig. 3).
Fig. 3.
LASSO regression model was used to select characteristic impact factors. (A) LASSO coefficients of 68 features; (B) Selection of tuning parameter (λ) for the LASSO model.
Nomogram construction and validation
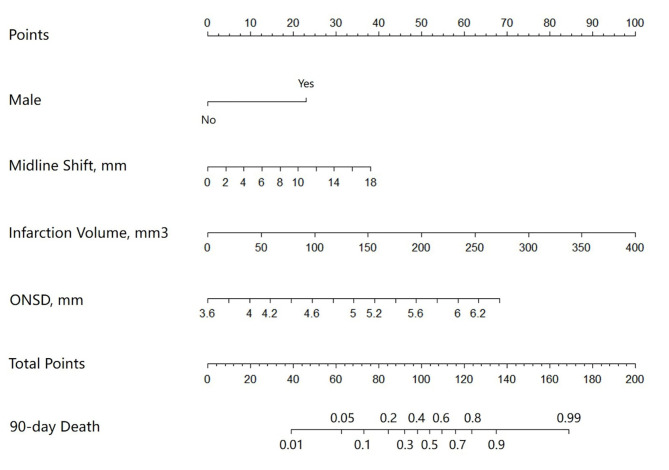
The ten factors selected in the LASSO regression were further analyzed using multivariable logistic regression analysis (Table 1). In the multivariable logistic regression model, male sex, midline shift, infarction volume, and mean ONSD were independently associated with increased 90-day mortality in patients with LHI. The final logistic model incorporating the four independent predictors was developed into a simple-to-use nomogram (Fig. 4). In the tool, the total score was obtained by summing the calculated points of each variable; thus, the 90-day mortality of patients with LHI was estimated. For example, a male with LHI, a midline shift of 10 mm, an infarction volume of 100 mm3, and an ONSD of 6 mm had an approximately 85% probability of 90-day death based on this nomogram.
Table 1.
Multivariable regression model based on LASSO regression results.
| Variables | Multivariable logistics model | ||
|---|---|---|---|
| Coefficients | OR (95% CI) | P value | |
| Male | 8.620 | 2.962–25.092 | < 0.001 |
| Hyperlipidemia | 2.076 | 0.704–6.117 | 0.185 |
| Serum Ca, mmol/L | 0.025 | 0.001–1.156 | 0.059 |
| Triglyceride, mmol/L | 0.513 | 0.276–1.052 | 0.051 |
| NIHSS on admission | 1.044 | 0.973–1.120 | 0.231 |
| Midline shift, mm | 1.207 | 1.085–1.342 | 0.001 |
| Infarction volume, cm3 | 1.020 | 1.012–1.028 | < 0.001 |
| US time from onset, h | 1.027 | 0.999–1.057 | 0.062 |
| ONSD, affected side, mm | 2.186 | 0.542–8.817 | 0.272 |
| ONSD, mean, mm | 7.026 | 2.638–18.708 | < 0.001 |
OR, odds ratio; NIHSS, National Institutes of Health Stroke Scale; US, ultrasound; ONSD, optic nerve sheath diameter.
Fig. 4.
The nomogram to predict 90-day mortality of patients with large hemispheric infarction. ONSD, optic nerve sheath diameter.
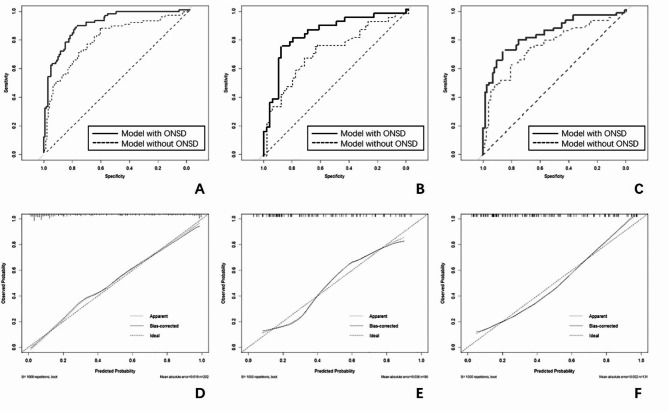
ROC analysis was used to evaluate the predictive efficiency of the nomogram model (Fig. 5). When only using the three predictors of male sex, midline shift, and infarction volume, the AUC values of the model in the training, internal validation, and external validation cohorts were 0.794 (95% CI, 0.729–0.860), 0.712 (95% CI, 0.597–0.827), and 0.754 (95% CI, 0.668–0.841), respectively. When adding the ONSD to the model, the AUC values in the training, internal validation, and external validation cohorts were 0.897 (95% CI, 0.854–0.941), 0.824 (95% CI, 0.732–0.917), and 0.833 (95% CI, 0.761–0.905), respectively. The AUC values of the model with ONSD were significantly higher than those of the model without ONSD in the training (P < 0.001), internal validation (P < 0.01), and external validation cohorts (P = 0.011). The calibration of the model was assessed using calibration curves. The calibration curve demonstrated good agreement between the prediction and observation in all cohorts (Fig. 5). The clinical applicability of the nomogram was assessed using decision curve analysis. The results showed that the nomogram had a wide threshold probability range and good clinical applicability for predicting 90-day mortality in patients with LHI (Supplemental Fig. 1).
Fig. 5.
ROC curves and Calibration plots for the nomogram. ROC curves for the nomogram in (A) the training cohort, (B) the internal validation cohort, and (C) the external validation cohort; Calibration plots for the nomogram in (D) the training cohort, (E) the internal validation cohort, and (F) the external validation cohort. ROC, the receiver operating characteristic curve; ONSD, optic nerve sheath diameter.
Risk stratification based on the nomogram scores
The optimal cutoff value for the total nomogram score was 115. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio were 88.9%, 77.7%, 72.7%, 91.3%, 3.99, and 0.14, respectively, in the training cohort, 72.2%, 86.0%, 78.8%, 81.1%, 5.16, 0.32, respectively, in the internal validation cohort, and 72.4%, 65.8%, 80.8%, 75.0%, 2.12, 0.42, respectively, in the external validation cohort.
Discussion
Our multicenter study analyzed the clinical, laboratory, CT and ultrasound characteristics of patients with LHI and found that ultrasonic ONSD, male, midline shift, and infarction volume were independently associated with increased 90-day mortality. This study further developed and validated an easy bedside nomogram to predict 90-day mortality for the early identification of patients with LHI who are likely to have a poor prognosis.
It is crucial to identify patients with LHI who are likely to have a poor prognosis. Previous studies have found some radiographic features associated with the development of malignant edema in patients with LHI, such as early hypodensity involving greater than half of the MCA territory, infarct volume > 220 mL, and midline shift > 3.7 to 5 mm on computed tomography (CT), as well as apparent diffusion coefficient values < 80% compared with the contralateral hemisphere with lesion volume ≥ 80 mL within the first 6 h, and diffusion-weighted imaging infarct volume > 145 mL on magnetic resonance imaging (MRI)3. However, patients with LHI are often in the intensive care unit due to their severe condition. They are not always suitable for MRI and cannot be moved frequently for CT. Therefore, serial imaging may be difficult to perform in patients with LHI. Moreover, most of these factors can only be qualitatively evaluated and cannot accurately quantify the risk of patients with LHI. Therefore, novel, quantitative, convenient, and bedside biomarker for LHI is required.
Many studies have confirmed that ultrasonic indicators can assess intracranial hypertension noninvasively and accurately, including ONSD, PI, eICP4,14. In our study, we collected all these ultrasonic indicators and found that ultrasonic ONSD could also predict the prognosis of patients with LHI. Similarly, previous studies found that ONSD on CT or MRI could accurately distinguish between benign and malignant LHI15–17. A recent study enrolled 22 patients with LHI and found that ONSD on the first MRI can predict the risk of large middle cerebral artery infarcts requiring decompressive hemicraniectomy16. However, in all these studies, ONSD was repeatedly measured using CT or MRI, which are not always applicable to patients with LHI. Ultrasound has proven to be an accurate, convenient, bedside, and repeatable method for measuring ONSD. Our study enrolled 419 patients with LHI and confirmed that the ONSD measured using ultrasound was an independent predictor of 90-day mortality. So ultrasonic ONSD may be a good biomarker for the prognosis of LHI.
Furthermore, we developed a nomogram based on ultrasonic ONSD to predict the 90-day mortality of patients with LHI. We collected 68 characteristics from the clinical, laboratory, CT, and ultrasound data of 419 patients with LHI. We found that ultrasonic ONSD, male, midline shift, and infarction volume were predictors of 90-day mortality in patients with LHI. Male sex, midline shift, and infarction volume were known biomarkers for predicting the prognosis of acute ischemic stroke18–20. Our results found that the addition of ultrasonic ONSD could accurately evaluate the prognosis of patients with LHI.
In addition to serving as a prognostic tool for patients with LHI, we believe that the nomogram also has potential future utility. For patients who may have poor prognosis, recent studies showed that early rather than late decompressive hemicraniectomy can significantly improve clinical outcomes2. Decompressive hemicraniectomy can decrease intracranial pressure and improves perfusion and blood flow, not only in ipsilateral penumbral tissue but in the contralateral hemisphere as well3. Therefore, the nomogram can help early identify the patients with LHI who are likely to have poor prognosis and candidates for decompressive hemicraniectomy. In addition, the nomogram can also be used in communication with family members of the patient or in allocation of resources. It can help to identify futile cases in which further intensive care maybe just a waste of resources and a prolonging of suffering for the patients and their family.
Our study has some limitations. First, a recent study showed a large and significant sex difference in diagnostic accuracy of ONSD as a screening tool for elevated ICP21. We will explore the potential role of sex in ONSD and other predictors in our multivariable models for patients with LHI in future studies. Second, although we enrolled 419 patients with LHI in six centers over three years, the sample size was still relatively small. In the future, additional data will be used for further validation.
In conclusion, we found that ultrasonic ONSD complement the male sex, midline shift and infarction volume to create a reliable multimodal method for monitoring prognosis in patients with LHI. We further developed and validated an easy bedside nomogram to predict 90-day mortality for the early identification of patients with LHI who are likely to have poor prognoses.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge all patients who participated in this study.
Author contributions
J.Z.: Conceptualization, Methodology, Investigation, Writing- Original draft preparation; S.Z., Y.Z., L.A., D.L., Z.L.: Methodology, Data curation, Writing- Original draft preparation, Writing-Reviewing and Editing; L.W.: Conceptualization, Resources, Supervision, Writing-Reviewing and Editing.
Funding
The article was supported by the Natural Science Foundation of Jilin Province (No.20230508100RC).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Hospital of Jilin University (approve number: 19K129-001) and conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the participants or their next of kin before the commencement of this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liebeskind, D. S. et al. Cerebral edema associated with large hemispheric infarction. Stroke50, 2619–2625. 10.1161/STROKEAHA.118.024766 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hofmeijer, J. et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy after Middle cerebral artery infarction with life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol.8, 326–333. 10.1016/S1474-4422(09)70047-X (2009). [DOI] [PubMed] [Google Scholar]
- 3.Lin, J. & Frontera, J. A. Decompressive Hemicraniectomy for large Hemispheric strokes. Stroke52, 1500. 10.1161/STROKEAHA.120.032359 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Robba, C. et al. Multimodal non-invasive assessment of intracranial hypertension: an observational study. Crit Care24, 379. 10.1186/s13054-020-03105-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernando, S. M. et al. Diagnosis of elevated intracranial pressure in critically ill adults: systematic review and meta-analysis. BMJ366, l4225. 10.1136/bmj.l4225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, L. J. et al. Ultrasonography Assessments of Optic Nerve Sheath Diameter as a noninvasive and dynamic method of detecting changes in intracranial pressure. JAMA Ophthalmol.136, 250–256. 10.1001/jamaophthalmol.2017.6560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park, S. Y. Nomogram: An analogue tool to deliver digital knowledge. J. Thorac. Cardiovasc. Surg.155, 1793. 10.1016/j.jtcvs.2017.12.107 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Powers, W. J. et al. Guidelines for the early management of patients with Acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of Acute ischemic stroke: a Guideline for Healthcare professionals from the American Heart Association/American Stroke Association. Stroke50, e344–e418. 10.1161/STR.0000000000000211 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Ng, F. C. et al. Cerebral edema in patients with large Hemispheric Infarct undergoing reperfusion treatment: a HERMES Meta-Analysis. Stroke52, 3450–3458. 10.1161/STROKEAHA.120.033246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedraza, M. I. et al. Brain atrophy and the risk of futile endovascular reperfusion in Acute ischemic stroke. Stroke51, 1514–1521. 10.1161/STROKEAHA.119.028511 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Pansell, J. et al. Optic nerve sheath diameter measurement by ultrasound: evaluation of a standardized protocol. J. Neuroimaging. 32, 104. 10.1111/jon.12936 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Hirzallah, M. I. et al. Optic nerve sheath diameter point-of-care Ultrasonography Quality Criteria Checklist: An International Consensus Statement on Optic nerve sheath diameter imaging and measurement. Crit. Care Med.52, 1543–1556. 10.1097/CCM.0000000000006345 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Czosnyka, M. et al. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J. Neurosurg.88, 802–808. 10.3171/jns.1998.88.5.0802 (1998). [DOI] [PubMed] [Google Scholar]
- 14.de Moraes, F. M. et al. Multimodal monitoring intracranial pressure by invasive and noninvasive means. Sci Rep13, 18404. 10.1038/s41598-023-45834-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lochner, P. et al. Sonography of optic nerve sheath diameter identifies patients with middle cerebral artery infarction at risk of a malignant course: a pilot prospective observational study. J. Neurol.267, 2713–2720. 10.1007/s00415-020-09906-0 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Legros, V. et al. Optic nerve and perioptic sheath diameter (ONSD), Eyeball transverse diameter (ETD) and ONSD/ETD ratio on MRI in large middle cerebral artery infarcts: a case-control study. J. Stroke Cerebrovasc. Dis.30, 105500. 10.1016/j.jstrokecerebrovasdis.2020.105500 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Guzeldag, S. et al. Measuring the Optic nerve sheath diameter with Ultrasound in Acute Middle cerebral artery stroke patients. J. Stroke Cerebrovasc. Dis.30, 105523. 10.1016/j.jstrokecerebrovasdis.2020.105523 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Carcel, C. et al. Sex differences in treatment and outcome after stroke: pooled analysis including 19,000 participants. Neurology93, e2170–e2180. 10.1212/WNL.0000000000008615 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, K. et al. Risk factors for poor outcomes of spontaneous supratentorial cerebral hemorrhage after surgery. J. Neurol.269, 3015–3025. 10.1007/s00415-021-10888-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takagi, S. & Shinohara, Y. Internal carotid occlusion: volume of cerebral infarction, clinical findings, and prognosis. Stroke12, 835–839. 10.1161/01.str.12.6.835 (1981). [DOI] [PubMed] [Google Scholar]
- 21.Pansell, J. et al. Sex differences in the diagnostic value of optic nerve sheath diameter for assessing intracranial pressure. Sci. Rep.14, 9553. 10.1038/s41598-024-60489-6 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.