Abstract
Background
The majority of patients diagnosed with glioblastoma are >60 years. Three randomized trials addressed the roles of radiotherapy (RT) and temozolomide (TMZ) for elderly patients. NORDIC and NOA-08 compared RT versus TMZ, while CE.6 randomized between hypofractionated RT and RT + TMZ. All showed significant benefits for the TMZ arms, especially for those patients with O6-methylguanine DNA methyltransferase (MGMT) promoter-methylated tumors. This pooled analysis aimed at identifying additional factors that could improve individualized treatment recommendations.
Methods
Analyses were performed separately in the RT and TMZ arms of the pooled NORDIC and NOA-08 data, and in the RT and TMZ/RT arms of CE.6. The prognostic value of baseline clinical factors, comorbidities, and quality of life (QoL) scores were assessed.
Results
NORDIC + NOA-08 (NN) included 715 patients and CE.6 included 562 patients. Median age for NN was 71 and 73 years for CE.6. In NN and CE.6 respectively, 66.2% versus 70.5% underwent resection and 50.9% and 75.3% were on steroids. In NN, 401 patients received RT alone and 281 in CE.6, while 314 were randomized to TMZ alone in NN and 281 to concomitant RT + TMZ in CE.6. Known clinical prognostic factors, such as extent of resection and WHO performance status were confirmed, as was MGMT promoter methylation status for TMZ-treated patients. TMZ-treated patients with 2 or 3 comorbidities; hypertension, diabetes, and/or stroke had worse survival, both in NN (P = .022) and CE.6 (P = .022). Baseline QoL had a minor association with outcome.
Conclusion
Consideration of comorbidities allows improved personalized treatment decisions for elderly glioblastoma patients.
Keywords: comorbidities, elderly glioblastoma patients, pooled analysis, prognostic factors
Key points.
The combination of hypertension, diabetes, and/or stroke leads to worse survival in TMZ-treated patients.
Extent of resection, WHO performance status, and for TMZ-treated patients MGMT promotor methylation status, are confirmed as prognostic/predictive factors.
Importance of the Study.
This is the largest pooled analysis of randomized trials (NORDIC, NOA-08, and CE.6) focusing on optimal treatment recommendations for elderly patients with glioblastoma. It confirms the importance of known clinical variables such as performance status, type of surgery, and MGMT promoter methylation status. Baseline quality of life assessment shows an association with outcome, but to a limited extent. The pooled analysis also suggests new prognostic factors, such as the combination of the comorbidities; hypertension, diabetes, and stroke. These should be included as part of a geriatric assessment during informed decision making.
Nearly half of all patients newly diagnosed with glioblastoma are older than 65 years.1,2 Elderly patients treated with standard therapy, namely radiation (RT) totaling 60Gy/30 fractions with concomitant and adjuvant temozolomide (TMZ), suffer from fatigue and cognitive deficits, often cannot complete treatment and have worse survival outcomes compared to shorter courses of treatment.2
To date, 3 randomized trials have investigated the role of RT and TMZ for elderly patients with newly diagnosed glioblastoma. The Nordic3 and NOA-084 trials compared TMZ versus RT in patients older than 60 and 65 years, respectively. The NOA-08 found noninferiority of TMZ compared to standard RT, while the Nordic study found superiority of TMZ compared to standard RT. In addition, hypofractionated RT improved overall survival (OS) when compared to standard RT in patients >70 years.3 Later, the randomized phase 3 CE.6 trial in glioblastoma patients over 65 years found significantly improved OS in favor of hypofractionated RT (40Gy/15 fractions) plus concomitant and adjuvant TMZ versus the same RT alone.5
Identifying prognostic and predictive factors for OS may improve individualized treatment recommendations. Age is known to be an important prognostic factor. All 3 trials found the tumor O6-methylguanine-DNA-methyltransferase gene (MGMT) promoter methylation status (MGMTpms) to be a predictive biomarker of benefit from TMZ for patients with a MGMT promoter-methylated tumor. The importance of using the correct cutoff for methylated versus unmethylated tumors has recently been addressed in this cohort.6
In this project the objectives were to identify prognostic factors for OS in elderly patients with newly diagnosed glioblastoma considering clinical, quality of life, and comorbidity data, using the Nordic, NOA-08 (referred to as NN), and CE.6 trial datasets.
Methods
Patients and Methods
Individual patient data from the 3 trials were used. The data from NN were pooled, so that all patients randomized to TMZ were analyzed as the TMZ cohort, despite different treatment schedules being used (Nordic TMZ 200 mg/m2 days 1–5 every 4 weeks for maximum 6 cycles; NOA-08 TMZ 100 mg/m2 7-days on-7 days off).
In a similar way, all patients that received RT were pooled into one RT cohort, regardless of fractionation, standard (1.8–2.0 Gy fractions to 60 Gy), or hypofractionated (3.4 Gy fractions to 34 Gy). The data pooled were the clinical prognostic factors age, sex, type of surgery (resection (complete and partial) versus biopsy), WHO performance status (PS), steroid use at baseline, and MGMTpms.
Data from the CE.6 trial were used to compare the findings in the pooled data from NN. RT alone patients (2.67 Gy fractions to 40 Gy) were compared to the pooled RT cohort, while findings from the TMZ arm of NN were compared to the TMZ plus RT arm, comprising of concomitant RT and TMZ followed by adjuvant TMZ (RT/TMZ > TMZ) for CE.6.
All 3 studies used the same quantitative methylation-specific PCR assay for determining MGMTpms, which was performed centrally by the same company. In the trials, IDH1 R132H status was examined, in the Nordic and CE.6 for all evaluable patients and in NOA-08 in a selected biomarker cohort. IDH1 mutation was confirmed in 1% (9/857) of patients, as expected in an elderly cohort. Other molecular markers were not generally available, therefore no other than MGMTpms was included in this analysis.
Data on comorbidities were collected in the Nordic and CE.6 trials only. The Nordic data focused on the medical history of diabetes (DM), hypertension (HT), cerebrovascular insult (CVI), thromboembolic diseases (DVT), for example, deep venous thrombosis and pulmonary embolism, epilepsy (EP) (yes/no), while the CE.6 collected more detailed information on major medical problems. For the analysis regarding comorbidities, the data from CE.6 were reviewed and registered according to the Nordic data, to allow comparison. For the analysis of the role of comorbidities for survival, baseline data were used.
Quality of life (QoL) data were collected for all patients using the EORTC Quality of Life questionnaire QLQ-C30 together with the Brain Cancer Module BN-20. For investigating the prognostic role of reported QoL for survival the baseline data were analyzed.
OS was calculated as the number of days from the date of randomization to the date of death from any cause. Patients alive or lost to follow-up were censored at the date last known to be alive.
Ethics Approval and Consent to Participate
Patients from the clinical trials cohorts provided written informed consent for their enrollment into the clinical trial and for translational research and the study was approved by the ethics committees of the participating centers.3–5 This study has been performed under institutional and international guidelines and regulations as previously reported. The study was conducted in accordance with the Declaration of Helsinki.
Statistical Analyses
See Supplement for the description of Detailed statistical analysis. For the patients’ characteristics, categorical variables were described by frequencies and percentages, whereas continuous variables were described by their median and interquartile range. In the descriptive analyses, a percentage difference of 10% or more was considered clinically relevant.
Outcome and covariates.—
The endpoint of interest was overall survival estimated using the Kaplan–Meier method. The prognostic significance of baseline clinical variables: age (≤70 or >70 years), sex (male or female), type of surgery (complete/partial resection or biopsy), PS (0, 1, or 2), steroid use at baseline (yes or no) and MGMTpms (unmethylated, methylated, or invalid/undetermined), baseline comorbidity variables (DM, HT, CVI, DVT, and EP) and baseline QoL variables C-30 and the BN-20 were utilized. For QoL, functions, symptoms, and global health status were assessed (global health status, role-, cognitive-, emotional-, physical- and social functioning, appetite loss, insomnia, fatigue, bladder control, communication deficit, drowsiness, future uncertainty, headaches, motor dysfunction, seizures, visual disorder, and weakness of legs). Seventeen QoL scales were selected based on the existing literature7 and one additional scale (role functioning) was included according to previous findings.8 The QoL scores were transformed to linear scales ranging from 0 to 100 as recommended in the EORTC guidelines.9 Baseline comorbidity data for the Nordic were analyzed in relation to survival, for each separately and also grouped regardless of the type of comorbidity. Assuming that not all comorbidities would have the same impact on survival, we hypothesized that those that could cause or were the result of cerebrovascular vessel damage might be of special interest.10 Therefore, patients with DM, HT, or CVI, were grouped as having 0–1 comorbidity or 2–3 comorbidities which were then related to survival.
Prognostic modeling.—
Separate clinical and clinical plus QoL models were fitted in the RT arms (in the pooled NN data and in CE.6 data), TMZ arm (in pooled NN data), and chemoradiation arm (RT/TMZ > TMZ in CE.6) Supplementary Table S1. As comorbidity data was not available in NOA-08, separate clinical and clinical plus comorbidities models were fitted in the RT and TMZ arms of Nordic, and in the RT and RT/TMZ > TMZ arms of CE.6. Univariate and multivariate Cox regression models were performed, and variables were selected at a 10% significance level using the Collett’s variable selection approach.11,12
Missing data.—
One patient with missing value for the type of surgery variable in the NOA-08 dataset was excluded from the analyses. For MGMTpms and the use of steroids, to limit data loss from the other variables in the model fitting, missing values were re-coded as a dummy category, labeled ‘Invalid/undetermined’ for MGMT and “Missing” for the use of steroids. For the models with the QoL variables, only patients with nonmissing data were included. Similarly, only patients with no missing value for the comorbidities were included in the comorbidity models.
Interaction tests and model assumption.—
Interaction tests for OS between the clinical variables were computed at a 5% significance level, and hazard ratios for the significant interactions were presented using forest plots. For MGMTpms and steroid use, the missing categories were excluded to allow interaction interpretation. The proportional hazard assumption for the Cox model was checked using the Kolmogorov-type supremum test with a two-sided significance level of 5%. Internal validity of the prognostic models was assessed using calibration plots and Harrell’s C-index,13 with optimism-corrected measures computed using bootstrap (1000 resamples). SAS version 9.4 (© 2002-2012 per SAS Institute Inc.) was used for the Cox models and R version 4.1.1 (rms package) was used for assessing the model validity.
Results
Patients’ Baseline characteristics
For the NN cohort, 715 patients were included (342 Nordic+ 373 NOA-08). In this cohort 401 patients belonged to the joint RT arm and 314 to the joint TMZ arm. The CE.6 trial randomized 562 patients, RT (N = 281) or RT + TMZ (N = 281). Baseline patients’ characteristics including comorbidities are presented in Table 1. Compared to patients in NN, in CE.6 a higher proportion of the patients were >70 years (70% vs 55%). Also, a higher proportion of patients in CE.6 used steroids at baseline compared to NN (75% vs 51%). The other baseline patients’ characteristics were similar in NN and CE.6. Among the comorbidities included in this analysis, HT was the most prevalent (40% in NN and 47% in CE.6), whereas CVI, EP, and DVT were less frequent (<15%). For the baseline QoL scores, there was a shift in median and interquartile range toward better scores in CE.6 for physical, role and emotional functioning, fatigue, future uncertainty, and visual disorder (see Supplementary Table S2).
Table 1.
Baseline patients’ characteristics.
| Nordic/NOA-08 | CE.6 | |||||
|---|---|---|---|---|---|---|
| RT (N = 401) |
TMZ (N = 314) |
Total (N = 715) |
RT (N = 281) |
RT/TMZ->TMZ (N = 281) |
Total (N = 562) |
|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Clinical variables | ||||||
| Sex | ||||||
| Male | 223 (55.6) | 177 (56.4) | 400 (55.9) | 172 (61.2) | 171 (60.9) | 343 (61.0) |
| Female | 178 (44.4) | 137 (43.6) | 315 (44.1) | 109 (38.8) | 110 (39.1) | 219 (39.0) |
| Age | ||||||
| Median | 70.5 | 71.0 | 71.0 | 73.0 | 73.0 | 73.0 |
| Range | 60.1–83.8 | 60.1–92.6 | 60.1–92.6 | 65.0–88.0 | 65.0–90.0 | 65.0–90.0 |
| Q1–Q3 | 67.0–74.6 | 68.0–75.0 | 67.5–74.9 | 70.0–76.0 | 70.0–76.0 | 70.0–76.0 |
| Age (categorized) | ||||||
| ≤70 | 187 (46.6) | 133 (42.4) | 320 (44.8) | 82 (29.2) | 83 (29.5) | 165 (29.4) |
| >70 | 214 (53.4) | 181 (57.6) | 395 (55.2) | 199 (70.8) | 198 (70.5) | 397 (70.6) |
| Type of surgery | ||||||
| Biopsy | 128 (31.9) | 113 (36.0) | 241 (33.7) | 82 (29.2) | 84 (29.9) | 166 (29.5) |
| Resection | 273 (68.1) | 200 (63.7) | 473 (66.2) | 199 (70.8) | 197 (70.1) | 396 (70.5) |
| Missing | 0 (0.0) | 1 (0.3) | 1 (0.1) | – | – | – |
| WHO performance status | ||||||
| 0 | 132 (32.9) | 91 (29.0) | 223 (31.2) | 57 (20.3) | 74 (26.3) | 131 (23.3) |
| 1 | 189 (47.1) | 172 (54.8) | 361 (50.5) | 160 (56.9) | 141 (50.2) | 301 (53.6) |
| 2 | 80 (20.0) | 51 (16.2) | 131 (18.3) | 64 (22.8) | 66 (23.5) | 130 (23.1) |
| MGMT promoter methylation status | ||||||
| Unmethylated | 127 (31.7) | 121 (38.5) | 248 (34.7) | 96 (34.2) | 93 (33.1) | 189 (33.6) |
| Methylated | 105 (26.2) | 59 (18.8) | 164 (22.9) | 77 (27.4) | 88 (31.3) | 165 (29.4) |
| Invalid/undetermined | 169 (42.1) | 134 (42.7) | 303 (42.4) | 108 (38.4) | 100 (35.6) | 208 (37.0) |
| Steroid use | ||||||
| No | 154 (38.4) | 141 (44.9) | 295 (41.3) | 67 (23.8) | 72 (25.6) | 139 (24.7) |
| Yes | 214 (53.4) | 150 (47.8) | 364 (50.9) | 214 (76.2) | 209 (74.4) | 423 (75.3) |
| Missing | 33 (8.2) | 23 (7.3) | 56 (7.8) | – | – | – |
| Comorbidity variables* | ||||||
|---|---|---|---|---|---|---|
| RT (N = 223) |
TMZ (N = 119) |
Total (N = 342) |
RT (N = 281) |
RT/TMZ->TMZ (N = 281) |
Total (N = 562) |
|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Diabetes (DM) | ||||||
| No | 183 (82.1) | 107 (89.9) | 290 (84.8) | 227 (80.8) | 234 (83.3) | 461 (82.0) |
| Yes | 36 (16.1) | 11 (9.2) | 47 (13.7) | 54 (19.2) | 47 (16.7) | 101 (18.0) |
| Missing | 4 (1.8) | 1 (0.8) | 5 (1.5) | – | – | – |
| Cerebrovascular insult (CVI) | ||||||
| No | 208 (93.3) | 109 (91.6) | 317 (92.7) | 272 (96.8) | 276 (98.2) | 548 (97.5) |
| Yes | 10 (4.5) | 8 (6.7) | 18 (5.3) | 9 (3.2) | 5 (1.8) | 14 (2.5) |
| Missing | 5 (2.2) | 2 (1.7) | 7 (2.0) | – | – | – |
| Hypertension (HT) | ||||||
| No | 131 (58.7) | 68 (57.1) | 199 (58.2) | 145 (51.6) | 154 (54.8) | 299 (53.2) |
| Yes | 88 (39.5) | 50 (42.0) | 138 (40.4) | 136 (48.4) | 127 (45.2) | 263 (46.8) |
| Missing | 4 (1.8) | 1 (0.8) | 5 (1.5) | -– | – | – |
| Epilepsy (EP) | ||||||
| No | 192 (86.1) | 104 (87.4) | 296 (86.5) | 277 (98.6) | 277 (98.6) | 554 (98.6) |
| Yes | 26 (11.7) | 14 (11.8) | 40 (11.7) | 4 (1.4) | 4 (1.4) | 8 (1.4) |
| Missing | 5 (2.2) | 1 (0.8) | 6 (1.8) | – | – | – |
| Deep venous thrombosis (DVT) | ||||||
| No | 207 (92.8) | 112 (94.1) | 319 (93.3) | 279 (99.3) | 273 (97.2) | 552 (98.2) |
| Yes | 11 (4.9) | 6 (5.0) | 17 (5.0) | 2 (0.7) | 8 (2.8) | 10 (1.8) |
| Missing | 5 (2.2) | 1 (0.8) | 6 (1.8) | – | – | – |
| Number of comorbidities (DT/HT/CVI) | ||||||
| 0/1 | 190 (85.2) | 109 (91.6) | 299 (87.4) | 241 (85.8) | 253 (90.0) | 494 (87.9) |
| 2/3 | 29 (13.0) | 9 (7.6) | 38 (11.1) | 40 (14.2) | 28 (10.0) | 68 (12.1) |
| Missing | 4 (1.8) | 1 (0.8) | 5 (1.5) | – | – | – |
*Comorbidity data was not available in NOA-08.
Prognostic Models
Prognostic models involving clinical data.—
The number of patients included in each of the prognostic models is shown in Supplementary Figure S1. Data from 714 patients in NN were included: 401 patients in the RT arm model and 313 patients in the TMZ arm model (1 patient with missing data on surgery was excluded). For CE.6, data from all patients were included in the clinical model (281 patients respectively in the RT and RT + TMZ models).
Univariate models (Collett’s method, step 1)
The results of all the prognostic models with clinical data are presented in Supplementary Table S3. In the RT models; age, type of surgery, PS, and steroid use were significantly associated with OS in both NN and C.6. In the TMZ containing arms; type of surgery, PS, MGMTpms, and steroid use were significantly associated with OS in both NN and CE.6, whereas sex, and age were significant only in CE.6.
Interaction models (Collett’s method, step 2)
The interaction of PS with MGMTpms was significant in the RT arm of NN (P = .005, Supplementary Figure S2). A significant interaction was also observed between PS and steroid use in the RT arm of NN (P = .006, Supplementary Figure S3), between PS and type of surgery in the RT arm of CE.6 (P = 0.015, Supplementary Figure S4), between PS and sex in the TMZ arm of NN (P < .001, Supplementary Figure S5), between MGMTpms and sex in the TMZ arm of NN (P = .043, Supplementary Figure S6), between PS and age in the RT/TMZ > TMZ arm of CE.6 (P = .045, Supplementary Figure S8). The interaction of MGMTpms with surgery was significant in the TMZ arm of NN (P = .049, Supplementary Figure S7), as well as in the RT/TMZ > TMZ of CE.6 (P = .047, Supplementary Figure S9). Given that these interactions were either not reproducible or difficult to clinically interpret, they were not included in the final multivariate models.
Final multivariate model (main effect, Collett’s method, step 3)
In the multivariate models in the RT arm, type of surgery and PS were prognostic for OS in both NN and CE.6, whereas age and steroid use were prognostic only in NN. Resected patients had better survival compared to patients who had biopsy (NN: HR = 0.73, 90% CI = 0.61–0.88, P = .007, CE.6: HR = 0.53, 90% CI = 0.42–0.66, P = .002), and patients with good PS (PS0) had better survival compared to PS1 (HR = 1.47, 90% CI = 1.20–1.80) and PS2 (HR = 1.77, 90% CI = 1.38–2.29) (see Table 2 and Supplementary Table S3). In NN, older patients (> 70 years) had poorer survival compared to patients ≤70 years (HR = 1.22, 90% CI = 1.02–1.46, P = .061), and patients who used steroids had shorter survival compared to those who did not use steroids (HR = 1.56, 90% CI:1.29–1.89, P < .001).
Table 2.
Univariate analyses of clinical factors and factors selected in the multivariate clinical models.
| RT Arms | TMZ or RT/TMZ->TMZ arms | |||
|---|---|---|---|---|
| Nordic/NOA | CE6 | Nordic/NOA(TMZ) | CE6 (RT/TMZ->TMZ) | |
| Univariate analysis (P-value) | ||||
| Sex | 0.428 | 0.174 | 0.644 | 0.042 |
| Age | 0.007 | 0.090 | 0.207 | 0.072 |
| Type of surgery | <0.001 | <0.001 | 0.001 | 0.002 |
| WHO performance status | <0.001 | 0.005 | 0.089 | 0.078 |
| MGMT promoter methylation status | 0.883 | 0.641 | <0.001 | 0.002 |
| Steroid use | <0.001 | 0.014 | 0.026 | 0.051 |
| Multivariate analyses (P-value) | ||||
| Sex | NS | NS | NS | 0.016 |
| Age | 0.061 | NS | NS | NS |
| Type of surgery | 0.007 | 0.002 | 0.006 | 0.013 |
| WHO performance status | <0.001 | <0.001 | 0.040 | NS |
| MGMT promoter methylation status | NS | NS | <0.001 | 0.002 |
| Steroid use | <0.001 | NS | NS | NS |
NS: not significant in the Collett’s method at 10%. Significant findings in bold.
In the TMZ containing arms, in both NN and CE.6, type of surgery and MGMTpms were prognostic for OS. Resected patients had better survival compared to patients who had biopsy (NN: HR = 0.71, 90% CI = 0.57–0.87, P = .006, CE.6: HR = 0.70, 90% CI = 0.55–0.89, P = .013), and patients with MGMT promoter-methylated tumor had better survival compared to patients with MGMT promotor-unmethylated tumor (NN: HR = 0.47, 90% CI = 0.35–0.64, P < .001, CE.6: HR = 0.66, 90%=0.51–0.85, P = .002). PS was significant only in NN, with good performance status associated with better survival (PS1 (HR = 1.32, 90% CI = 1.05–1.67) and PS2 (HR = 1.59, 90%=1.15–2.20)). In CE.6, women had better survival compared to men (HR = 0.73, 90% CI = 0.59–0.91) (see Table 2 and Supplementary Table S3).
Prognostic Models Involving Clinical and Comorbidity Data (Model 1 Separated by Comorbidity)
In the first model involving the separate comorbidities, the prognostic significance of DM, CVI, HT, EP, and DVT were explored. In the univariate analyses of the RT arms, only EP (n = 4 patients) was prognostic in CE.6. In the TMZ-containing arms, CVI (n = 8) was prognostic in the Nordic, whereas EP (n = 4) was prognostic in CE.6 (Table 3 and Supplementary Table S4). In the multivariate models of the RT arms, none of the comorbidity variables was prognostic for OS after adjusting for the significant clinical variables. In the TMZ-containing arms, in the Nordic, DM (n = 11) and CVI (n = 8) were associated with OS. Patients with stroke had worse survival (HR = 2.23, 90% CI = 1.18–4.23, P = .039), whereas patients with DM had better survival (HR = 0.48, 90% CI = 0.26–0.86, P = .038). For DM we checked if the finding could be related to fewer patients being on steroids, but no significant association was found between steroid use and DM (P = .503). Stroke was also associated with poor prognosis in CE.6 (n = 5) (HR = 2.20, 90% CI = 1.02–4.73, P = 0.090) (Table 3 and Supplementary Table S4).
Table 3.
Univariate analyses of comorbidity factors and factors selected in the multivariate models.
| RT Arms | TMZ or RT/TMZ->TMZ arms | |||
|---|---|---|---|---|
| Nordic | CE6 | Nordic (TMZ) | CE6 (RT/TMZ->TMZ) | |
| Univariate analysis (P-value) | ||||
| Diabetes | 0.604 | 0.286 | 0.163 | 0.647 |
| Cerebrovascular insult | 0.980 | 0.536 | 0.033 | 0.212 |
| Hypertension | 0.454 | 0.143 | 0.238 | 0.664 |
| Epilepsy | 0.743 | 0.049 | 0.676 | 0.077 |
| Deep venous thrombosis | 0.983 | 0.234 | 0.903 | 0.817 |
| Multivariate analyses (P-value) | ||||
| Sex | NS | NS | NS | 0.013 |
| Age | NS | NS | NS | NS |
| Type of surgery | NS | 0.002 | 0.074 | 0.020 |
| WHO performance status | 0.022 | <0.001 | <0.001 | NS |
| MGMT promoter methylation status | NS | NS | 0.015 | 0.001 |
| Steroid use | <0.001 | NS | NS | NS |
| Diabetes | NS | NS | 0.038 | NS |
| Cerebrovascular insult | NS | NS | 0.039 | 0.090 |
| Hypertension | NS | NS | NS | NS |
| Epilepsy | NS | NS | NS | NS |
| Deep venous thrombosis | NS | NS | NS | NS |
NS: not significant in the Collett’s method at 10%. Significant findings for comorbidities in bold.
Prognostic models involving clinical and comorbidity data (model 2 grouped comorbidities).—
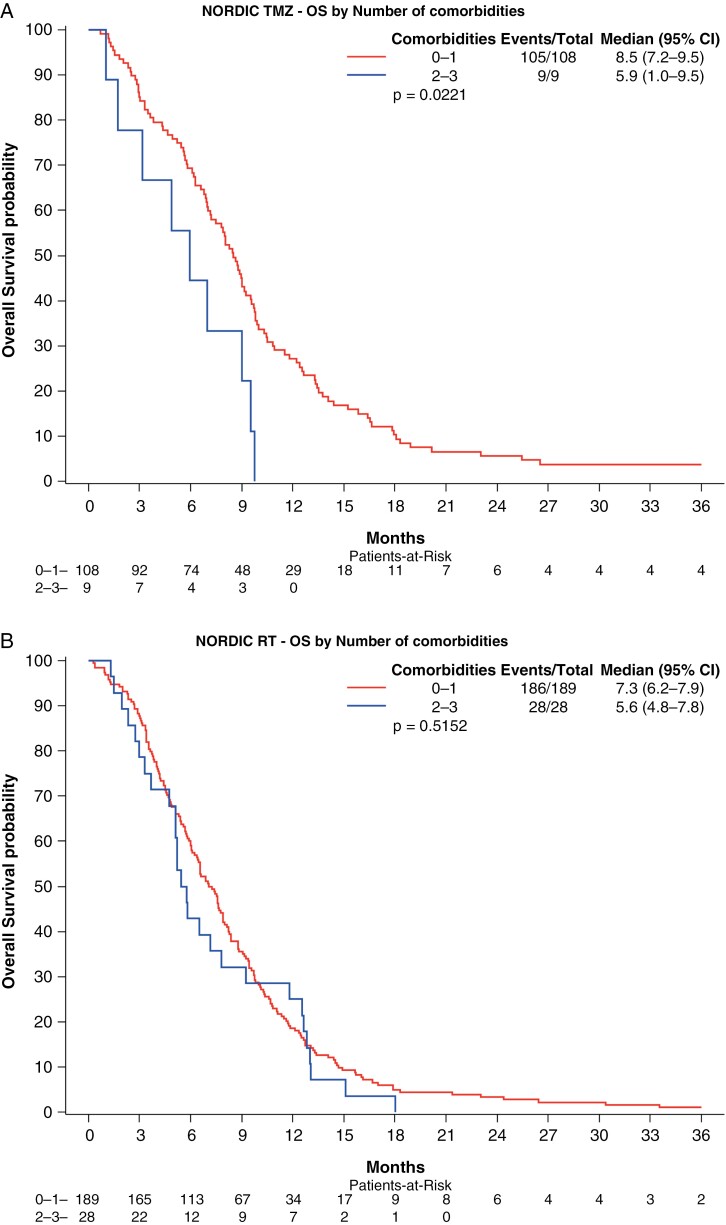
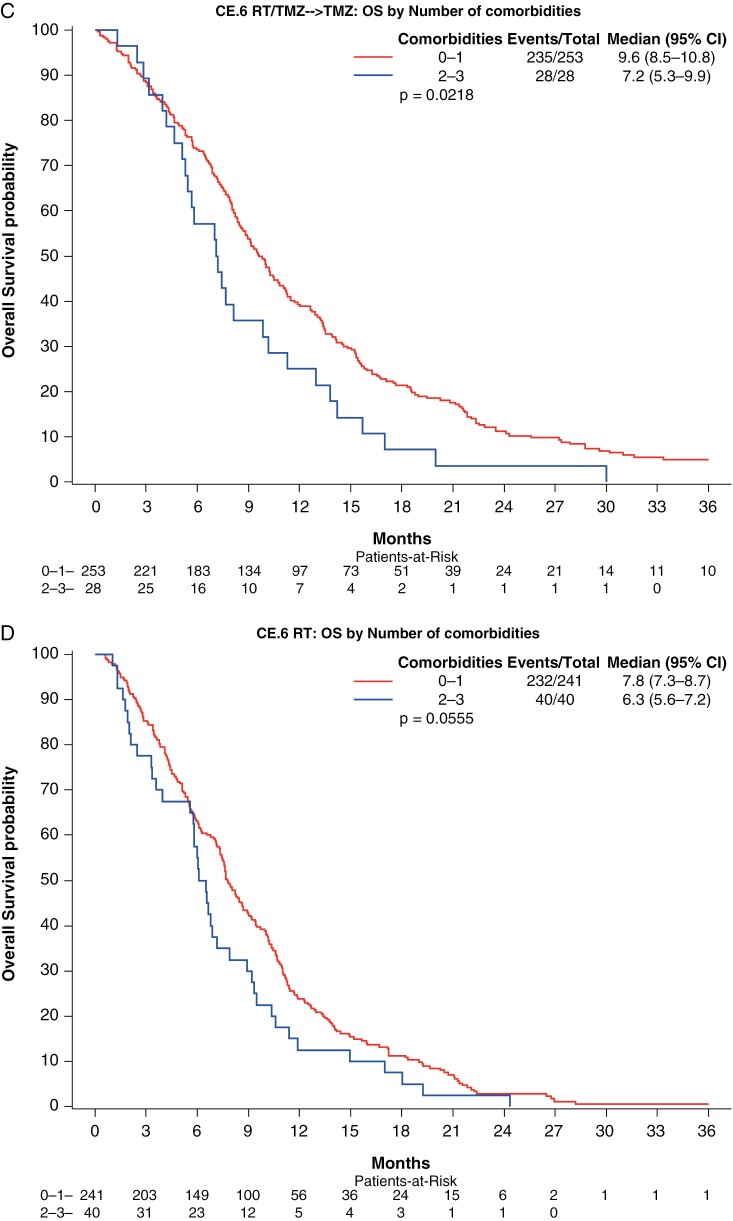
We first analyzed whether the number of comorbidities among all 5, affected survival, comparing 0–1 comorbidity versus 2 or more comorbidities, but as assumed did not find a correlation (data not shown). We then analyzed the number of comorbidities among DM, HT, and CVI (0 or 1 vs 2 or 3 comorbidities). In the univariate analyses, there was a significant association with OS in the RT arm of CE.6, and in the TMZ-containing arms of both the Nordic and CE.6 (Figure 1, Table 4, and Supplementary Table S5). In multivariate analyses, the number of comorbidities was not correlated to survival in the RT arms of the Nordic or CE.6. However, in the TMZ-containing arms, patients with 2 or 3 comorbidities (DM, HT, and CV) had significantly worse OS compared to those with 0 or 1 (Nordic: HR = 2.00, 90% CI = 1.09–3.66, P = .059. CE.6: HR = 1.56, 90% CI = 1.12–2.18, P = .029) (Table 4 and Supplementary Table S5).
Figure 1.
Association of number of the comorbidities DT, HT, and/or CVI with OS by treatment arm for each trial. Kaplan–Meier survival curve of overall survival in patients with 0–1 comorbidity versus 2 or more comorbidities for each trial and treatment combination. (A) Nordic TMZ arm, (B) Nordic RT arm, (C) CE.6 RT/TMZ arm, and (D) CE.6 RT arm. Significant OS differences were observed in the RT arm of CE.6, and in the TMZ arms of both the Nordic and CE.6.
Table 4.
Univariate and multivariate analyses of number of comorbidities DM/HT/CVI, 0/1 versus 2/3.
| RT Arms | TMZ or RT/TMZ->TMZ arms | |||
|---|---|---|---|---|
| Nordic | CE6 | Nordic (TMZ) | CE6 (RT/TMZ->TMZ) | |
| Univariate analysis (P-value) | ||||
| Number of comorbidities | 0.515 | 0.056 | 0.022 | 0.022 |
| Multivariate analyses (P-value) | ||||
| Sex | NS | NS | NS | 0.024 |
| Age | NS | NS | NS | NS |
| Type of surgery | NS | 0.002 | 0.036 | 0.022 |
| WHO performance status | 0.022 | <0.001 | 0.008 | NS |
| MGMT promoter methylation status | NS | NS | 0.012 | 0.001 |
| Steroid use | <0.001 | NS | NS | NS |
| Number of comorbidities (DT/HT/CVI) | NS | NS | 0.059 | 0.029 |
NS: not significant in the Collett’s method at 10%. Significant findings for the comorbidities in bold.
Prognostic models involving clinical and quality of life data.—
In both the univariate and multivariate analyses of only the QoL variables, in the RT and TMZ arms of NN and CE.6 several different symptoms and functions were significantly associated with OS (Table 5 and Supplementary Table S6), but only physical functioning for the RT arms and cognitive functioning in the TMZ arms were confirmed in both cohorts.
Table 5.
Univariate analyses of QoL factors and factors selected in the multivariate models.
| RT Arms | TMZ or RT/TMZ->TMZ arms | |||
|---|---|---|---|---|
| Nordic/ NOA | CE6 | Nordic/NOA(TMZ) | CE6 (RT/TMZ->TMZ) | |
| Univariate analysis of QoL prognostic factors for overall survival (P-value) | ||||
| QLQ-C30 | ||||
| Physical Functioning | 0.028 | <0.001 | 0.582 | 0.104 |
| Role Functioning | 0.077 | <0.001 | 0.932 | 0.401 |
| Emotional Functioning | 0.290 | 0.040 | 0.829 | 0.136 |
| Cognitive Functioning | 0.346 | 0.001 | 0.057 | 0.001 |
| Social Functioning | 0.945 | <0.001 | 0.654 | 0.753 |
| Global health status/QoL | 0.804 | 0.026 | 0.131 | 0.135 |
| Fatigue | 0.129 | 0.015 | 0.541 | 0.340 |
| Insomnia | 0.117 | 0.444 | 0.832 | 0.238 |
| Appetite loss | 0.033 | 0.570 | 0.338 | 0.632 |
| QLQ-BN20 | ||||
| Future uncertainty | 0.212 | 0.165 | 0.281 | 0.887 |
| Visual disorder | 0.973 | 0.004 | 0.149 | 0.043 |
| Motor dysfunction | 0.027 | <0.001 | 0.597 | 0.033 |
| Communication deficit | 0.377 | 0.065 | 0.322 | 0.046 |
| Headaches | 0.696 | 0.135 | 0.887 | 0.514 |
| Seizures | 0.799 | 0.484 | 0.135 | 0.817 |
| Drowsiness | 0.924 | <0.001 | 0.507 | 0.287 |
| Weakness legs | 0.186 | 0.071 | 0.590 | 0.019 |
| Bladder control | 0.941 | 0.116 | 0.239 | 0.249 |
| Multivariate analysis of quality-of-life prognostic factors for overall survival (P-value) | ||||
| QLQ-C30 | ||||
| Physical Functioning | 0.034 | 0.005 | NS | NS |
| Cognitive Functioning | NS | NS | 0.008 | <0.001 |
| Social Functioning | NS | 0.070 | NS | NS |
| Global health status/QoL | NS | NS | 0.012 | NS |
| Insomnia | NS | NS | NS | 0.024 |
| Appetite loss | 0.042 | NS | NS | NS |
| QLQ-BN20 | ||||
| Future uncertainty | 0.046 | NS | NS | NS |
| Visual disorder | NS | 0.024 | NS | NS |
| Seizures | NS | NS | 0.062 | NS |
| Multivariate analysis of quality-of-life and clinical prognostic factors for overall survival (P-value) | ||||
| Sex | NS | NS | NS | 0.061 |
| Age | 0.059 | NS | NS | NS |
| Type of surgery | 0.001 | <0.001 | 0.003 | 0.030 |
| WHO performance status | NS | NS | 0.082 | NS |
| MGMT promoter methylation status | NS | NS | 0.001 | 0.002 |
| Steroid use | <0.001 | NS | NS | NS |
| Physical Functioning | NS | NS | NS | NS |
| Cognitive Functioning | NS | NS | 0.001 | 0.004 |
| Social Functioning | NS | 0.072 | NS | NS |
| Global health status/QoL | NS | NS | 0.001 | NS |
| Insomnia | NS | NS | NS | 0.015 |
| Appetite loss | 0.003 | NS | NS | NS |
| Future uncertainty | 0.008 | NS | NS | NS |
| Visual disorder | NS | 0.024 | NS | NS |
| Seizures | NS | NS | NS | NS |
NS: not significant at 10%. Significant finding for QoL in bold. Significant QoL scales in any of the arms are shaded in red. In these multivariate analyses, significant QoL variables were first selected using the Collett’s variable selection approach, and the significant clinical variables identified in the clinical models were added.
After adjusting the significant variables from the multivariate QoL models for the prognostic clinical variables, only cognitive functioning in the TMZ arms of both NN (HR = 0.92, 90% CI = 0.89–0.96, P = .001) and CE.6 (HR = 0.93, 90% CI = 0.89–0.97, P = .004) were significantly associated to OS (Table 5 and Supplementary Table S6).
Summary of Findings
Among the clinical parameters, type of surgery and PS, age, and steroid use were prognostic for OS. Adjusted for these clinical factors, the number of comorbidities of HT, DM, and CVI (2–3 vs 0–1) was prognostic for OS, but only in the TMZ-containing arms. Among QoL factors, cognitive functioning, social functioning, global health status (QoL), insomnia, appetite loss and future uncertainty, visual disorder, and seizures were also prognostic for OS after adjustment although, these factors were not consistently prognostic for all treatment arms, apart from cognitive functioning in the TMZ-containing arms.
Discussion
In this pooled analysis of data from elderly patients with glioblastoma included in 3 clinical trials, we found that the risk of death was doubled when TMZ-treated patients had more than one of the comorbidities DM, HT, or CVI. The high impact on survival of exclusively these, among the analyzed comorbid conditions, constitutes a new and important clinical prognostic factor. Although numbers were small, the findings were confirmed in both the NN and CE.6 studies. This information should be integrated into clinical evaluation including cardiovascular and metabolic status in addition to imaging and pathological data when choosing the most appropriate cancer therapy. For patients with MGMT methylated tumor affected by 2 or 3 of these comorbidities, RT is not suggested as an alternative to TMZ as survival for these patients was similar to those receiving RT only, and data from the Nordic trial3 suggest poorer QoL for RT than for TMZ.
Known prognostic factors for adult patients with glioblastoma, namely PS, use of baseline steroids,14MGMTpms,15,16 and extent of resection,17 were confirmed in our elderly patient dataset. Quality of life variables also showed a significant association with prognosis, although with less impact (HR varying from 0.9 to 1.12). The cohorts in the pooled studies were generally comparable regarding clinical prognostic factors, comorbidities, and QoL. As CE.6 included patients 65 years or older, while the Nordic patients were ≥60, more patients in the NN-cohort, 45%, were ≤70 years compared to only 29% for CE.6. Despite this, the patients in CE.6 reported better function for several QoL scales. Interestingly, steroid use at baseline was considerably higher in the CE.6-cohort, 75%, compared to 51% in NN.
Diabetes Mellitus
Unexpectedly, DM was associated with longer survival in the Nordic patients receiving TMZ. This was not related to any difference in the prescription of steroids at baseline and could be due to small numbers (n = 11). Of note, persistent high glucose serum levels are associated with cardiovascular pathology, but also with initiation and progression of cancer.18 Much research has been performed regarding in vitro antitumor activity of the antidiabetic drug metformin. However, the EORTC brain tumor group analyzed data from the AVAglio, CENTRIC, and CORE trials, pooling data of 124 diabetic patients using metformin, and found no positive effect on survival.19
Hypertension
Hypertension affects an estimated 1.3 billion people aged 30–79 years worldwide and is the leading preventable risk factor for cardiovascular disease. The prevalence reaches nearly 45% in adults in the US and increases with age.20 The trial participants in our study showed a similar prevalence of hypertension, around 45% in CE.6 and 40% in Nordic. Of course, it would not be unexpected if impaired perfusion, hypoxia, and hypertensive complications would have negative effects on patients with glioma. However, as single comorbidity, there was no effect noted in our cohorts of elderly glioblastoma patients, while combined with DM and/or CVI, HT had a detrimental effect.
Stroke (CVI)
We found that patients with documented CVI at baseline had poorer survival, although the numbers were small (n = 32). The cause of the stroke was not documented, therefore could have had different etiology in different patients. The 2022 World Stroke fact sheet reports that there are over 12.2 million strokes every year; 70% of them being associated with metabolic risk factors, for example, increased blood pressure, hyperglycemia, high body mass index, increased cholesterol levels, and decreased renal function.21 Therefore, stroke cannot be considered an independent prognostic factor, but as it causes severe damage to the brain it appears justifiable to examine its impact on elderly glioblastoma patients. In addition, strokes occur as peri-operative complications after neurosurgical procedures and are an important cause of morbidity in affected patients.22 Acute ischemic strokes can be seen on postoperative diffusion-weighted imaging, mostly after resection of tumors in the insula or in the temporal lobe. In a retrospective study on 239 patients with glioblastoma resection, an acute ischemic stroke was seen in 30 patients (12.5%), and 13 patients (5%) developed new neurological deficits.23 The damage induced by stroke deeply influences the functionality of the brain, as well on the damaged as on the contralateral side, making clear that additional damage caused by an invasive tumor might exceed the compensatory mechanisms of the affected patients. New onset postoperative neurological motor and language dysfunction have also been shown to negatively correlate to survival in glioblastoma patients.24 For some patients also stroke is the presenting symptom of their glioblastoma, caused by bleeding into the tumor.25
Although the proportion of patients with a history of stroke was low in this study with 5% (n = 18) in the Nordic and 2.5% (n = 14) in the CE.6 study, stroke was significantly associated with mortality in the TMZ arms (HR 2.2, respectively).
Effects of Comorbidity Accumulation
In the Nordic trial data were collected for the comorbidities: HT, DM, CVI, DVT, and EP, the same comorbidities were then also analyzed for the CE.6 trial. We found that not all of these comorbidities had the same impact on survival when comparing patients with none or one comorbidity versus patients with more than one comorbidity. This indicates that some comorbidities might be more important for prognosis than others. The combination of 2 or all 3 of DM, HT, and CVI was of clinical importance, with the majority of patients having the combination of DM and HT. DM and HT are known to aggravate cerebrovascular vessel damage which in turn causes CVI. The negative effect was confirmed even when other clinical prognostic factors were included in the analyses. For TMZ-treated patients, having several of these comorbidities led to worse survival, similar to the poorer survival of the patients in the RT arms. This was observed in both the Nordic and CE.6 studies. Unlike chemotherapy, these comorbidities had no impact on outcome following radiotherapy.
Other Prognostic Factors
In our study, extent of surgery (in all arms), PS (in all arms except in CE.6 TMZ arm), MGMTpms (in TMZ arms only), age, and steroid use (in NN RT arm), were prognostic for OS. There was no consistent survival difference between sexes (only in CE.6 TMZ arm). This might be due to the overall short survival of elderly patients with glioblastoma, but is also in line with previous findings from a national cohort.26 Tumor resection, methylated MGMT promoter, and good PS are well-known prognostic factors in younger patients.14
In multivariate analyses, steroid use was identified as a negative prognostic factor for OS. A recent retrospective single-center analysis of 360 glioblastoma patients confirmed the negative impact of steroids in the early postoperative period, leading to prolonged hyperglycemia, increased rate of infections, and prolonged lymphopenia. Increasing total doses of dexamethasone was found to be associated with an increase in mortality.27
As expected, MGMTpms was of importance for patients treated with TMZ in both cohorts, but not for patients who were only irradiated.
Quality of Life
Elderly trial patients reported similar baseline quality of life on symptom scales compared to younger patients, for example, in phase III trials testing the addition of bevacizumab to standard of care.28,29 Conversely, elderly patients´ scores for functional scales were lower. The largest differences between the elderly and those in the bevacizumab trials seem to be in the domains of cognitive function and global health status. A significant negative effect of baseline cognitive impairment was found in both NN and CE.6 in the TMZ arms and is supported in the literature.30 Apart from this, many of the QoL domains were found to be significantly associated with OS. Mainly, when the scales indicated adequate functioning, it was found to be a positive prognostic factor and when symptom scales reported neurological deficits it was a negative prognostic factor. But this was then only found in 1 of the 4 subcohorts. The impact of all QoL factors was moderate with hazard ratios varying from 0.9 to 1.15, but was conserved in multivariate analysis. The contribution of QoL at baseline to predict survival on top of clinical variables was only 2-4%.
Geriatric Assessment
Considering all data derived from this analysis, it becomes clear that choosing an optimal therapeutic regimen for an elderly patient with glioblastoma requires consideration of neurological functioning, performance status, comorbidities, and molecular characteristics of the tumor. In addition, assessment of medical capacity for decision making, the availability of family caregivers, and ability to comply with the logistical demands of the treatment are essential for mitigation of treatment complications such as early treatment stops and even premature death.31 During the last decades, geriatric oncology has developed and validated the geriatric assessment (GA) for this purpose. Geriatric assessment is increasingly recommended in oncology and has been part of some treatment guidelines since 2018.32 Reconsideration of incorporating these assessments in neuro-oncology is important as neither GA nor short screening tests are widely used.33 These assessments can take less than one hour and can be done by nonmedical personnel and may inform which elderly patients will develop serious therapy-related side effects, show severe functional decline, or die prematurely.28,34,35
The large impact of comorbidities and frailty in elderly patients was recently shown in a retrospective single-center analysis of 110 glioblastoma patients, including comorbidity reporting and a frailty score.36 Lombardi et al. reported a retrospective single-center study administering GA to 113 elderly patients with newly diagnosed glioblastoma, which demonstrated the prognostic accuracy of GA.37
Limitations and Strengths
This pooled analysis has some limitations. The treatment arms in the trials were only partly the same, therefore not allowing for a formal meta-analysis. The TMZ arms differed between the single-arm treatments for the Nordic and NOA-08, and for CE.6 which included RT as well. Despite this, several findings with regard to comorbidities were congruent. Radiotherapy included both standard and hypofractionated schedules, although this has not been shown to have a major impact on elderly and/or frail glioblastoma patients in some trials.38,39 This is in contrast to the findings in the Nordic trial, where patients >70 years had significantly worse survival when treated with 60Gy RT compared to 34Gy over 2 weeks.3
Only baseline data were available in this analysis and we cannot know, for example, if steroid doses were tapered for patients with DM, which may explain their better outcome in one trial. Without specific data on concurrent medications, any correlation of specific drugs with outcomes cannot be determined. The absolute number of patients with the comorbidities HT, DM and/or stroke, and their combinations was low. The analyses, while strongly suggesting a survival impact of these comorbidities, ultimately require validation in future studies. These should collect more comprehensive and detailed data on comorbidities for correlation with outcome.
The strength of this analysis is that it includes data from close to 1300 patients and is the largest pooled analyses of data from randomized trials focusing on treatment options for the elderly glioblastoma population. Apart from confirming the importance of known clinical prognostic factors, we found a significant impact on survival from a combination of HT, DM, and stroke in the elderly.
Conclusion
The findings from this pooled analysis strongly suggest the performance of a geriatric assessment, including comorbidities, to facilitate the assessment of expected tolerability and benefit from oncological treatment, to be considered together with patient wishes and therapy goals, as part of individualized treatment recommendations.
Supplementary material
Supplementary material is available online at Neuro-Oncology Advances (https://academic.oup.com/noa).
Acknowledgments
The authors wish to express their appreciation for the contributions of Roger Stupp, Didier Frappaz, Henrik Schultz, Ufuk Abacioglu, Björn Tavelin, Benoit Lhermitte, Monika E Hegi, Johan Rosell for the Nordic Clinical Brain Tumour Study Group (NCBTSG), Michael Platten, Christoph Meisner, Jörg Felsberg, Ghazaleh Tabatabai, Matthias Simon, Guido Nikkhah, Kirsten Papsdorf, Joachim P Steinbach, Michael Sabel, Stephanie E Combs, Jan Vesper, Christian Braun, Jürgen Meixensberger, Ralf Ketter, Regine Mayer-Steinacker for the NOA-08 Study Group of the Neuro-oncology Working Group (NOA) of the German Cancer Society and Alba A. Brandes, Johan Menten, Claire Phillips, Michael Fay, Ryo Nishikawa, J. Gregory Cairncross, Wilson Roa, David Osoba, John P. Rossiter, Arjun Sahgal, Hal Hirte, Florence Laigle-Donadey, Enrico Franceschi, Olivier Chinot, Vassilis Golfinopoulos, Laura Fariselli, Antje Wick, Loic Feuvret, Michael Back, Michael Tills, Chad Winch, Brigitta G. Baumert, Keyue Ding for the Trial Investigators CCTG-EORTC (CE.6) as well as all our colleagues who contributed to these studies.
Preliminary data from this analysis were reported at WFNOS 2022.
Contributor Information
Annika Malmström, Department of Advanced Home Care in Linköping and Division of Cell and Neurobiology, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden.
Felix B Oppong, EORTC Headquarters, Brussels, Belgium.
Christopher J O`Callaghan, Canadian Cancer Trials Group, Kingston, Canada.
Wolfgang Wick, Neurology Clinic and National Center for Tumor Diseases, University Hospital Heidelberg, Heidelberg University and Clinical Cooperation Unit Neurooncology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Normand Laperriere, Department of Radiation Oncology, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada.
Thierry Gorlia, EORTC Headquarters, Brussels, Belgium.
Michael Weller, Department of Neurology, University Hospital and University of Zurich, Zurich, Switzerland.
Roger Henriksson, Department of Radiation Sciences, Oncology, Umeå University, Umeå, Sweden.
Warren Mason, Department of Medicine, Princess Margaret Cancer Centre and University of Toronto, Toronto, Canada.
Michael Platten, Department of Neurology, Medical Faculty Mannheim, MCTN, Heidelberg University, Mannheim and Clinical Cooperation Unit Neuroimmunology and Brain Tumor Immunology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Eva Cantagallo, Statistics Department, European Organisation for Research and Treatment of Cancer (EORTC), Brussels, Belgium.
Bjørn H Grønberg, Department of Clinical and Molecular Medicine, NTNU, Norwegian University of Science and Technology and Department of Oncology, St Olav´s Hospital, Trondheim University Hospital, Trondheim, Norway.
Guido Reifenberger, Institute of Neuropathology, Medical Faculty, Heinrich Heine University and University Hospital Düsseldorf, Düsseldorf, Germany.
Christine Marosi, Clinical Division of Palliative Care, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria.
James R Perry, Odette Cancer Centre, Department of Medicine, University of Toronto, Toronto, Canada.
NCBTSG, NOA, CCTG and EORTC Brain Tumor Group:
Roger Stupp, Didier Frappaz, Henrik Schultz, Ufuk Abacioglu, Björn Tavelin, Benoit Lhermitte, Monika E Hegi, Johan Rosell, Michael Platten, Christoph Meisner, Jörg Felsberg, Ghazaleh Tabatabai, Matthias Simon, Guido Nikkhah, Kirsten Papsdorf, Joachim P Steinbach, Michael Sabel, Stephanie E Combs, Jan Vesper, Christian Braun, Jürgen Meixensberger, Ralf Ketter, Regine Mayer-Steinacker, Alba A Brandes, Johan Menten, Claire Phillips, Michael Fay, Ryo Nishikawa, J Gregory Cairncross, Wilson Roa, David Osoba, John P Rossiter, Arjun Sahgal, Hal Hirte, Florence Laigle-Donadey, Enrico Franceschi, Olivier Chinot, Vassilis Golfinopoulos, Laura Fariselli, Antje Wick, Loic Feuvret, Michael Back, Michael Tills, Chad Winch, and Brigitta G Baumert
Funding
A.M. Career grants, Region Östergötland, Sweden. R.H. Cancer Foundation North and the Swedish Cancer Foundation. F.B.O. and E.C. work as Fellows at EORTC Headquarters was supported by a grant from the EORTC Cancer Research Fund from Belgium. The Nordic was supported by an unrestricted grant from Merck Sharp & Dohme (formerly Schering Plough) to R.H. The NOA-08 trial was supported by a grant from Merck Sharp & Dohme (formerly Schering Plough) to M.W. and W.W. The CE.6 trial was supported by grants (015469 and 021039) from the Canadian Cancer Society Research Institute, by an unrestricted grant from Schering-Plough (now Merck), and by the EORTC Cancer Research Fund from Belgium. F.O., C.O.C., N.L., T.G., W.M., M.P., E.C., B.H.G., G.R., C.M., and J.P. no funding.
Conflict of interest statement
A.M., F.B.O., C.O.C., N.L., T.G., E.C., G.R., C.M., and J.P. none. M.W. has received research grants from Quercis and Versameb, and honoraria for lectures or advisory board participation or consulting from Bayer, Curevac, Medac, Novartis, Novocure, Orbus, Philogen, Roche, and Servier. R.H. has since the start of the Nordic study received honoraria for lectures or advisory board participation or consulting from Novocure, MSD, Roche, Astra Zeneca, and BrainCool. W.W. Honoraria for consultation or non-financial clinical trial support from Apogenix, Bayer, Merck Sharp & Dome, AstraZeneca, Merck Serono, Novartis, Roche and Mundipharma, with compensation paid to the Medical Faculty at Heidelberg University. B.H.G. research funding from AstraZeneca and Roche, and honoraria from MSD, AstraZeneca, Pfizer, Janssen, Sanofi, Takeda, Eli Lilly, BMS, Gilead and Debiopharm. W.M. consultant for Merck, Servier, Novocure, Boehringer Ingelheim, and chairs a data monitoring committee for Ono Therapeutics. M.P. has received research grants and support from Bayer, Merck, Pfizer and Roche, and advisory board participation compensation from Cellula Therapeutics.
Authorship statement
Conception and design: A.M., F.B.O., T.G., M.W., W. W., C.M. Acquisition of data (enrolled and managed patients): J.R. P., W.W., R. H., N.J. L., A. M., M. W., B.H. G., C. M. Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): A.M., F.B.O., T. G. Writing, review, and/or revision of the manuscript: A.M., F. B. O., C.J.O., W.W., N.L., T.G., M. W., R. H., W.M., M. P., E. C., B. H. G., G. R., C. M., J. R. P. Study supervision: A.M., T. G., M. W.
Data availability
Data can be requested at Data And Sample Sharing—EORTC (https://www.eortc.org/data-sharing/).
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wohrer A, Waldhor T, Heinzl H, et al. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95(3):401–411. [DOI] [PubMed] [Google Scholar]
- 3. Malmstrom A, Gronberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 4. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 5. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 6. Hegi ME, Oppong FB, Perry JR, et al. No benefit from TMZ treatment in GB with truly unmethylated MGMT promoter: reanalysis of the CE.6 and the pooled Nordic/NOA-08 trials in elderly GB patients. Neuro Oncol. 2024;26(10):1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mauer ME, Taphoorn MJ, Bottomley A, et al. ; EORTC Brain Cancer Group. Prognostic value of health-related quality-of-life data in predicting survival in patients with anaplastic oligodendrogliomas, from a phase III EORTC brain cancer group study. J Clin Oncol. 2007;25(36):5731–5737. [DOI] [PubMed] [Google Scholar]
- 8. Malmstrom A, Akesson L, Milos P, et al. “Do I want to know it all?” A qualitative study of glioma patients’ perspectives on receiving information about their diagnosis and prognosis. Support Care Cancer. 2021;29(6):3339–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fayers PM, Aaronson N, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 10. Guzik A, Bushnell C.. Stroke epidemiology and risk factor management. . Continuum (Minneap Minn). 2017;23(1, Cerebrovascular Disease):15–39. [DOI] [PubMed] [Google Scholar]
- 11. Collet D. Modelling Survival Data in Medical Research. London: Chapman & Hall; 1994. [Google Scholar]
- 12. Fox J, Monette G.. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–183. [Google Scholar]
- 13. Harrell FE, Jr, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 14. Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–1184. [DOI] [PubMed] [Google Scholar]
- 15. Hegi ME, Genbrugge E, Gorlia T, et al. MGMT promoter methylation cutoff with safety margin for selecting glioblastoma patients into trials omitting temozolomide: a pooled analysis of four clinical trials. Clin Cancer Res. 2019;25(6):1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 17. Stummer W, Meinel T, Ewelt C, et al. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol. 2012;108(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon JM, Thomas F, Czernichow S, et al. Hyperglycaemia is associated with cancer-related but not non-cancer-related deaths: evidence from the IPC cohort. Diabetologia. 2018;61(5):1089–1097. [DOI] [PubMed] [Google Scholar]
- 19. Seliger C, Genbrugge E, Gorlia T, et al. ; EORTC Brain Tumor Group. Use of metformin and outcome of patients with newly diagnosed glioblastoma: pooled analysis. Int J Cancer. 2020;146(3):803–809. [DOI] [PubMed] [Google Scholar]
- 20. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT.. Hypertension prevalence among adults aged 18 and over: United States, 2017–2018. NCHS Data Brief. 2020;Apr(364):1–8. [PubMed] [Google Scholar]
- 21. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022, 2022. https://doi.org/ 10.1177/17474930211065917 [DOI] [PubMed] [Google Scholar]
- 22. Ulmer S, Braga TA, BarkerFG, 2nd, et al. Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology. 2006;67(9):1668–1670. [DOI] [PubMed] [Google Scholar]
- 23. Berger A, Tzarfati GG, Serafimova M, et al. Risk factors and prognostic implications of surgery-related strokes following resection of high-grade glioma. Sci Rep. 2022;12(1):22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGirt MJ, Mukherjee D, Chaichana KL, et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463–9; discussion 469. [DOI] [PubMed] [Google Scholar]
- 25. Joseph DM, O’Neill AH, Chandra RV, Lai LT.. Glioblastoma presenting as spontaneous intracranial haemorrhage: case report and review of the literature. J Clin Neurosci. 2017;40:1–5. [DOI] [PubMed] [Google Scholar]
- 26. Tavelin B, Malmstrom A.. Sex differences in glioblastoma-findings from the Swedish National Quality Registry for primary brain tumors between 1999-2018. J Clin Med. 2022;11(3):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mistry AM, Jonathan SV, Monsour MA, et al. Impact of postoperative dexamethasone on survival, steroid dependency, and infections in newly diagnosed glioblastoma patients. Neurooncol Pract. 2021;8(5):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wefel JS, Armstrong TS, Pugh SL, et al. Neurocognitive, symptom, and health-related quality of life outcomes of a randomized trial of bevacizumab for newly diagnosed glioblastoma (NRG/RTOG 0825). Neuro Oncol. 2021;23(7):1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;33(19):2166–2175. [DOI] [PubMed] [Google Scholar]
- 30. Bruhn H, Blystad I, Milos P, et al. Initial cognitive impairment predicts shorter survival of patients with glioblastoma. Acta Neurol Scand. 2022;145(1):94–101. [DOI] [PubMed] [Google Scholar]
- 31. Hamaker ME, Rostoft S.. Geriatric assessment in older patients with cancer: a new standard of care. Lancet. 2021;398(10314):1853–1855. [DOI] [PubMed] [Google Scholar]
- 32. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chakiba C, Bellera C, Etchepare F, et al. The prognostic value of G8 for functional decline. J Geriatr Oncol. 2019;10(6):921–925. [DOI] [PubMed] [Google Scholar]
- 34. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. [DOI] [PubMed] [Google Scholar]
- 35. Lorimer CF, Walsh G, MacKinnon M, et al. Geriatric assessment of glioblastoma patients is feasible and may provide useful prognostic information. Neurooncol Pract. 2020;7(2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider M, Potthoff AL, Scharnbock E, et al. Newly diagnosed glioblastoma in geriatric (65 +) patients: impact of patients frailty, comorbidity burden and obesity on overall survival. J Neurooncol. 2020;149(3):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lombardi G, Bergo E, Caccese M, et al. Validation of the comprehensive geriatric assessment as a predictor of mortality in elderly glioblastoma patients. Cancers (Basel). 2019;11(10):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 39. Roa W, Kepka L, Kumar N, et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be requested at Data And Sample Sharing—EORTC (https://www.eortc.org/data-sharing/).