Abstract
Pregnancy-associated changes in melanocytic nevi (MN), apart from size increase on the trunk, remain a topic of debate. We conducted the first prospective study to investigate dermoscopic changes in MN comparing pregnant with non-pregnant women on all body parts using a market-approved convolutional neural network (CNN). We included 25 pregnant and 25 non-pregnant women from Basel, Switzerland, who underwent standard skin cancer screenings and whose MN > 2 mm were digitally recorded and analysed by a CNN. Pregnant women were examined three times: in the first and third trimester and 8–12 weeks postpartum; non-pregnant women twice in an interval of 17–21 weeks. We analysed 2,799 MN. In pregnant women, diameter[p < 0.001], area[p < 0.001], number of colours [p = 0.009], shape asymmetry[p = 0.005] and border sharpness[p = 0.006] (inversely proportional value) increased while ellipseness [p < 0.001] decreased from first trimester to postpartum. Changes occurred mainly during the third trimester to postpartum. Compared to non-pregnant women (only first to third trimester) MN on the upper extremities of pregnant women increased in area[p = 0.011] and diameter[p = 0.025] and decreased in ellipseness[p = 0.037]. MN on the lower extremities increased in area[p = 0.044] and MN on the back increased in colour asymmetry[p = 0.022]. Our findings show that in all women of childbearing age MN change in their aspects with time. However, certain MN aspects might change differently in pregnant compared to non-pregnant women.
Key words: artificial intelligence, digital imaging, melanocytic nevi, melanoma, pregnancy
SIGNIFICANCE
It is currently unclear whether pregnancy can lead to changes in moles other than size increase of moles on the abdomen and chest. We therefore studied 2,799 moles in 25 pregnant and 25 non-pregnant women throughout and after pregnancy and analysed their changes with artificial intelligence. We found that in all women of childbearing age moles change over time. However, moles in pregnant women might change differently, especially regarding changes in size and symmetry – and not only on the abdomen and chest. A better understanding of changes in moles during pregnancy could help avoid unnecessary biopsies. Further, AI-assisted examinations may help doctors detect subtle changes in moles more efficiently.
Melanocytic nevi (MN) change in appearance and number throughout the lifetime. The total nevus count increases during the first 3 decades of life (1, 2). Apart from changes in nevi due to intrinsic characteristics such as skin type and family history, multiple other factors can contribute to nevus progression or regression. While ultraviolet (UV) light exposure seems to be the most impactful extrinsic influence, data on other potential factors, including pregnancy, are still very controversial (3, 4). This is also attributable to the fact that gender-specific research, particularly regarding hormonal effects on women, has not been a major subject of investigation for a long time, leading to an underrepresented research field (5, 6).
Although melanoma is the most common malignancy in pregnant women with about 30% of all cancers identified during gestation and one-third of all melanoma cases in females occur during childbearing age, there iare currently no robust data on the influence of pregnancy on melanoma (7–9).
Drastic hormonal shifts are known to occur during pregnancy. Known pregnancy-induced dermatologic changes include hyperpigmentation, appearance of melasma, striae distensae, telangiectasia, palmar erythema, and varicose veins (10). Hormonal changes have also been discussed as a potential factor for changes in MN during pregnancy (11, 12).
Only a few studies have analysed the macroscopic and dermoscopic changes of MN throughout pregnancy (13). However, so far, no other study has used artificial intelligence (AI) to assess MN changes on all body parts in pregnant women compared with non-pregnant women throughout and after pregnancy.
Using convolutional neural networks (CNNs) has several advantages. First, data analysis through deep learning-driven AI algorithms is more objective than analysis through a human eye. Second, detailed and extensive analysis of multiple images with high accuracy can be performed with higher consistency.
A better understanding of pregnancy-related changes in MN throughout and after pregnancy may avoid unnecessary biopsies and detect rare cases of melanoma in pregnant women more effectively. Here, we aimed to evaluate dermoscopic changes in MN among pregnant women prospectively. This is the first study investigating these changes in MN on all body parts with a market-approved CNN among pregnant women during and after pregnancy in comparison with non-pregnant women. By using AI, we intended to detect any MN changes with the highest accuracy in this vulnerable patient group.
MATERIALS AND METHODS
Study design and participating population
This prospective, single-centre, observational, comparative study was conducted at the Department of Dermatology at the University Hospital of Basel in Switzerland between January 2021 and February 2022. As a pilot study the sample size calculation was based on similar previous studies with 12 to 70 included patients and 21 to 703 analysed MN (13, 14).
We included 50 participants, 25 pregnant and 25 non-pregnant, and anticipated analysing approximately 50 nevi per patient. Study participants were recruited through the Department of Gynaecology and Obstetrics at the University Hospital of Basel and gynaecological practices in and around the city of Basel. Non-pregnant participants were recruited at the University of Basel and through an advertisement published on the University of Basel website. Because MN changes are age-related, recruitment aimed at balancing the 2 groups according to age. Further, both study groups were matched for skin type.
Inclusion criteria were age between 18 and 40 years, Fitzpatrick skin type I–IV, and at least 15 MN with a diameter > 2 mm. In addition, for pregnant women the first consultation had to be in the first trimester between the 12th and 16th week of pregnancy. Exclusion criteria were insufficient knowledge of project language or inability to give informed consent. Participants missed from follow-up were excluded from the analysis.
We obtained informed consent from all women for participation in the study and for possible publication of the dermoscopic images of their MN.
Study procedures
We performed baseline skin cancer screenings for both cohorts between January 2021 and October 2021. All participants completed a study-specific 18-item questionnaire concerning their UV-protective behaviours, their personal history of skin cancer, and skin cancer screening frequency at baseline. We followed up pregnant women in the first trimester (12th to 16th week of pregnancy), third trimester (29th to 33rd week of pregnancy), and postpartum (8 to 12 weeks postpartum). Non-pregnant women received 2 consultations at an interval of 17 to 21 weeks.
During every consultation study participants underwent a standard-of-care clinical skin examination by a dermatologist with a dermatoscope and an additional assessment with the CNN. Participants received total body photography (TBP) and separate dermoscopic photographs of all MN >2 mm, including lesions in the areas difficult to capture through TBP such as the scalp and genital area.
Medical device and convolutional neural network
The FotoFinder ATBM master® device with its Moleanalyzer pro® system (FotoFinder Systems GmbH, Bad Birnbach, Germany, Version 3.3.1.0) is approved as a medical device for the European market (Conformité Européenne mark). The system is based on a modified architecture of GoogleNet’s Inception_v4. Details regarding the CNN architecture and training have been described previously in studies validating the system (15–19). For our study the CNN supplied data on various MN metrics. The metrics included in the study’s analysis were diameter, area, number of colours per nevi, number of dots and globules per nevi, colour asymmetry, shape asymmetry, ellipseness, and border sharpness (inverse proportional value). Ellipseness is a score between 0 and 1 calculated by the CNN. The more regular the mole’s borders and the more elliptic the mole, the closer the score is to 1. Further explanations of the terms and their calculations can be found in Appendix S1.
Statistical analysis
We presume that MN measured within the same patient are not statistically independent, and consequently analysing changes at the level of the nevus entails a large risk of statistical bias. Therefore, we evaluated MN changes at the level of the patient. This approach has an additional benefit, which is that, according to the central-limit theorem, the expected mean of a random variable follows a normal distribution regardless of the distribution of the averaged variable, thus allowing us to use linear mixed-effect models with normal distribution of the residuals.
Because change in nevus aspect in pregnancy may be more or less pronounced depending on the localization of the nevus, we ran separate models for each of the nevus localizations. Appendix S2 displays the metrics and localizations used in the analysis.
All endpoints were analysed using linear mixed-effect models. Mean aspect metrics were used as the dependent variable, pregnancy status, screening time, and the interaction between pregnancy status and screening time as the explanatory fixed factors, age and BMI as covariates, and the patient ID as a random factor to account for the non-independence of the repeated measures over time within a given patient.
To allow comparisons of the strength of the association between pregnancy and change in nevi aspect across all endpoints describing a nevus’s aspect, we computed p-values and standardized effect sizes in the form of Cohen’s d along with 95% confidence intervals.
All analyses were performed using R version 4.2.1 (2022-06-23; R Foundation for Statistical Computing, Vienna, Austria). Further details regarding the statistical analysis are mentioned in Appendix S2.
RESULTS
Of 26 pregnant and 30 non-pregnant women screened, 54 were included in the study. Due to dropouts, ultimately 50 women (25 in each cohort) were included in the statistical analysis. A total of 2,799 MN in 50 women (mean age 29.5 ± 4.9 years) were included in the analysis (Tables I and II, and Table SI). Patient characteristics were balanced between the two groups regarding age, BMI, skin type, sun exposure through working outdoors, and tanning habits (Table I), as well as risk factors such as previous melanoma, nicotine and alcohol consumption, and educational level (Table SI). Table SII shows the mean and total number of MN on different localizations.
Table I.
Patient characteristics of non-pregnant and pregnant women
| Characteristic | Overall, n = 50a | Non-pregnant, n = 25a | Pregnant, n = 25a |
|---|---|---|---|
| Age, mean (SD) (min; max) | 29.5 (4.9) (19.09; 40.09) | 26.7 (3.9) (19.09; 36.0) | 32.3 (4.1) (25.0; 40.0) |
| Severe sunburn (n, %) | |||
| No | 24 (48) | 9 (36) | 15 (60) |
| Yes | 26 (52) | 16 (64) | 10 (40) |
| Fitzpatrick skin type (n, %) | |||
| II | 27 (54) | 15 (60) | 12 (48) |
| III | 21 (42) | 9 (36) | 12 (48) |
| IV | 2 (4.0) | 1 (4.0) | 1 (4.0) |
| History of skin cancer (n, %) | |||
| No | 50 (100) | 25 (100) | 25 (100) |
| Yes | 0 (0) | 0 (0) | 0 (0) |
| Family history of melanoma (n, %) | |||
| No | 50 (100) | 25 (100) | 25 (100) |
| Yes | 0 (0) | 0 (0) | 0 (0) |
| Work outdoors > 4 h/day (n, %) | |||
| No | 48 (96) | 24 (96) | 24 (96) |
| Yes | 2 (4.0) | 1 (4.0) | 1 (4.0) |
| Tanning (n, %)a | |||
| Never/Seldom | 14 (28) | 10 (40) | 4 (16) |
| Sometimes | 24 (48) | 10 (40) | 14 (56) |
| Often/Always | 12 (24) | 5 (20) | 7 (28) |
| BMI, mean (SD) (min; max) | 22.7 (3.7) (17.2; 35.8) | 21.8 (2.8) (17.2; 28.3) | 23.7 (4.2) (18.6; 35.8) |
Intentional sun exposure during summer/spring months.
Table II.
Nevi characteristics in pregnant and non-pregnant women
| Characteristic | Overall, n = 50 Mean (SD) (min; max) Total MN: 2,799 | Non-pregnant, n = 25 Mean (SD) (min; max) Total MN: 1,422 | Pregnant, n = 25 Mean (SD) (min; max) Total MN: 1,377 |
|---|---|---|---|
| Number of nevi | 56.0 (33.1) (4.0; 151.0) | 56.9 (34.3) (20.0; 151.0) | 55.1 (32.5) (4.0; 122.0) |
| Diameter (mm) | 3.3 (0.5) (2.3; 4.4) | 3.5 (0.4) (2.8; 4.2) | 3.1 (0.4) (2.3; 4.4) |
| Area (mm2) | 5.3 (1.3) (3.1; 8.8) | 5.6 (1.4) (3.3; 8.8) | 4.9 (1.2) (3.1; 7.7) |
| Number of colours | 2.7 (0.2) (2.2; 3.1) | 2.7 (0.3) (2.2; 3.9) | 2.7 (0.2) (2.3; 3.0) |
| Number of dots and globules | 5.7 (2.2) (2.4; 11.8) | 5.9 (2.1) (3.2; 9.5) | 5.5 (2.4) (2.4; 11.8) |
| Colour asymmetry | 0.1 (0.02) (0.1; 0.2) | 0.1(0.02) (0.1; 0.2) | 0.1 (0.02) (0.1; 0.2) |
| Shape asymmetry | 0.3 (0.1) (0.2; 0.5) | 0.3 (0.03) (0.2; 0.4) | 0.3 (0.1) (0.2; 0.5) |
| Ellipseness | 0.7 (0.1) (0.4; 0.9) | 0.7 (0.1) (0.5; 0.9) | 0.7 (0.1) (0.4; 0.9) |
Dermoscopic variation of nevi between first trimester and postpartum only in pregnant women
We observed significant changes in how mean nevus aspect metrics changed from baseline to third trimester and postpartum in pregnant women averaged across all localizations in mean diameter (p = < 0.001), area (p = < 0.001), number of colours (p = 0.009), shape asymmetry (p = 0.005), border sharpness (p = 0.006), and ellipseness (p = < 0.001) (Table SIII).
We also found significant changes in mean nevus aspect metrics from baseline to third trimester and postpartum when analysing changes according to different localizations.
On all localizations an increase in size and a decrease in ellipseness was observed from first trimester to postpartum. On the back as well as upper and lower extremities (UE/LE), shape asymmetry and the border sharpness value increased throughout time. Lastly, the number of colours on the back and LE decreased from third trimester to postpartum and the number of dots from MN on the back increased during pregnancy and decreased to baseline postpartum (Table III).
Table III.
Changes of nevi aspects on different localizations in relation from first to third trimester and postpartum in pregnant women and according to age and BMI
| Factor | Predictors | Third trimestera | Postpartuma |
|---|---|---|---|
| Diameter (abdomen) | Estimate | 0.21 | 0.26 |
| 95% CI | 0.02–0.39 | 0.08–0.45 | |
| p | 0.034 | 0.007 | |
| Area (abdomen) | Estimate | 0.11 | 0.03 |
| 95% CI | 0.01–0.21 | –0.07–0.13 | |
| p | 0.032 | 0.497 | |
| Ellipseness index (abdomen) | Estimate | –0.07 | –0.09 |
| 95% CI | –0.13 to –0.01 | –0.15 to –0.02 | |
| p | 0.031 | 0.007 | |
| Diameter (back) | Estimate | 0.06 | 0.41 |
| 95% CI | –0.09–0.21 | 0.27–0.56 | |
| p | 0.406 | < 0.001 | |
| Number of colours (back) | Estimate | –0.13 | –0.27 |
| 95% CI | –0.34–0.08 | –0.48 to –0.06 | |
| p | 0.222 | 0.014 | |
| Number of dots and globules (back) | Estimate | –0.18 | –0.14 |
| 95% CI | –0.33 to –0.03 | –0.29–0.01 | |
| p | 0.018 | 0.072 | |
| Shape asymmetry (back) | Estimate | 0.03 | 0.06 |
| 95% CI | –0.00–0.07 | 0.02–0.10 | |
| p | 0.081 | 0.001 | |
| Ellipseness index (back) | Estimate | –0.07 | –0.17 |
| 95% CI | –0.15–0.00 | –0.25 to –0.09 | |
| p | 0.056 | < 0.001 | |
| Border sharpness (back) | Estimate | 0.47 | 1.23 |
| 95% CI | –0.38–1.32 | 0.37–2.09 | |
| p | 0.273 | 0.006 | |
| Area (chest) | Estimate | 0.06 | 0.09 |
| 95% CI | –0.02–0.14 | 0.01–0.17 | |
| p | 0.123 | 0.035 | |
| Ellipseness index (chest) | Estimate | –0.01 | –0.05 |
| 95% CI | –0.05–0.03 | –0.09 to –0.01 | |
| p | 0.574 | 0.020 | |
| Diameter (head) | Estimate | 0.11 | 0.32 |
| 95% CI | –0.10–0.32 | 0.10–0.53 | |
| p | 0.312 | 0.005 | |
| Ellipseness index (head) | Estimate | –0.05 | –0.11 |
| 95% CI | –0.13–0.03 | –0.19 to –0.04 | |
| p | 0.191 | 0.005 | |
| Diameter (LE) | Estimate | 0.11 | 0.33 |
| 95% CI | –0.00–0.23 | 0.21–0.45 | |
| p | 0.057 | < 0.001 | |
| Area (LE) | Estimate | 0.10 | 0.14 |
| 95% CI | 0.04–0.16 | 0.08–0.20 | |
| p | 0.001 | < 0.001 | |
| Shape asymmetry (LE) | Estimate | 0.02 | 0.02 |
| 95% CI | –0.00–0.04 | 0.00–0.05 | |
| p | 0.061 | 0.034 | |
| Ellipseness index (LE) | Estimate | –0.06 | –0.11 |
| 95% CI | –0.12 to –0.01 | –0.16 to –0.06 | |
| p | 0.016 | < 0.001 | |
| Diameter (UE) | Estimate | 0.20 | 0.56 |
| 95% CI | 0.03–0.37 | 0.39–0.72 | |
| p | 0.019 | < 0.001 | |
| Area (UE) | Estimate | 0.06 | 0.04 |
| 95% CI | 0.02–0.10 | –0.00–0.07 | |
| p | 0.005 | 0.079 | |
| Number of colours (UE) | Estimate | –0.09 | –0.48 |
| 95% CI | –0.25–0.06 | –0.64 to –0.32 | |
| p | 0.221 | < 0.001 | |
| Shape asymmetry (UE) | Estimate | 0.02 | 0.08 |
| 95% CI | –0.02–0.07 | 0.03–0.12 | |
| p | 0.317 | 0.002 | |
| Ellipseness index (UE) | Estimate | –0.07 | –0.20 |
| 95% CI | –0.14 to –0.00 | –0.27 to –0.13 | |
| p | 0.037 | < 0.001 | |
| Border sharpness (UE) | Estimate | 0.74 | 1.72 |
| 95% CI | –0.07–1.56 | 0.89–2.54 | |
| p | 0.074 | < 0.001 |
Significant p-values are marked in bold.
Estimates for third trimester and postpartum are in reference to the first trimester.
LE: Lower extremity; UE: upper extremity
Dermoscopic changes in nevi in pregnant compared to non-pregnant women over the period of 17–21 weeks
We found that in both study groups MN changed in their aspects with time. However, certain MN aspects on certain localizations changed significantly more in pregnant compared to non-pregnant women from initial screening to 17 to 21 weeks later (Table IV) (see Figs 2 and 3).
Table IV.
Estimated difference between non-pregnant and pregnant women at 17–21 weeks post-screening
| Location | Aspect | Estimate | Estimate 95% CI | p-value | Cohen’s d | Cohen’s d 95% CI |
|---|---|---|---|---|---|---|
| Abdomen | Diametera | 0.088 | (–0.085, 0.259) | 0.323 | 0.322 | (–0.314, 0.953) |
| Areaa | 0.101 | (–0.018, 0.219) | 0.105 | 0.532 | (–0.110, 1.167) | |
| Number of colours | 0.255 | (–0.141, 0.646) | 0.212 | 0.406 | (–0.230, 1.037) | |
| Number of dots and globulesa | 0.080 | (–0.294, 0.456) | 0.677 | 0.134 | (–0.492, 0.758) | |
| Colour asymmetrya | 0.139 | (–0.227, 0.505) | 0.471 | 0.165 | (–0.283, 0.612) | |
| Shape asymmetry | –0.001 | (–0.045, 0.043) | 0.968 | –0.013 | (–0.634, 0.609) | |
| Ellipseness | 0.022 | (–0.063, 0.106) | 0.619 | 0.160 | (–0.466, 0.783) | |
| Border sharpnessa | –0.271 | (–1.217, 0.666) | 0.576 | –0.18 | (–0.805, 0.448) | |
| Back | Diametera | –0.031 | (–0.122, 0.059) | 0.501 | –0.196 | (–0.762, 0.373) |
| Areaa | –0.041 | (–0.089, 0.007) | 0.104 | –0.479 | (–1.050, 0.097) | |
| Number of colours | –0.115 | (–0.470, 0.241) | 0.531 | –0.182 | (–0.748, 0.386) | |
| Number of dots and globulesa | –0.105 | (–0.314, 0.104) | 0.331 | –0.283 | (–0.851, 0.287) | |
| Colour asymmetrya | 0.198 | (0.034, 0.361) | 0.022 | 0.683 | (0.098, 1.262) | |
| Shape asymmetry | 0.002 | (–0.028, 0.032) | 0.904 | 0.035 | (–0.531, 0.601) | |
| Ellipseness | –0.032 | (–0.132, 0.068) | 0.536 | –0.180 | (–0.746, 0.388) | |
| Border sharpnessa | –0.010 | (–0.999, 0.979) | 0.984 | –0.006 | (–0.571, 0.560) | |
| Chest | Diametera | –0.022 | (–0.239, 0.195) | 0.843 | –0.065 | (–0.700, 0.572) |
| Areaa | 0.061 | (–0.028, 0.151) | 0.188 | 0.435 | (–0.211, 1.075) | |
| Number of colours | –0.010 | (–0.515, 0.496) | 0.971 | –0.009 | (–0.464, 0.447) | |
| Number of dots and globulesa | 0.027 | (–0.199, 0.253) | 0.814 | 0.077 | (–0.560, 0.712) | |
| Colour asymmetrya | 0.050 | (–0.289, 0.389) | 0.773 | 0.094 | (–0.543, 0.730) | |
| Shape asymmetry | –0.017 | (–0.070, 0.036) | 0.542 | –0.2 | (–0.836, 0.439) | |
| Ellipseness | 0.041 | (–0.075, 0.157) | 0.490 | 0.226 | (–0.413, 0.863) | |
| Border sharpnessa | –0.165 | (–1.108, 0.778) | 0.734 | –0.111 | (–0.747, 0.526) | |
| Head | Diametera | 0.024 | (–0.178, 0.227) | 0.816 | 0.07 | (–0.515, 0.654) |
| Areaa | 0.021 | (–0.127, 0.169) | 0.784 | 0.082 | (–0.503, 0.666) | |
| Number of colours | –0.148 | (–0.520, 0.224) | 0.44 | –0.232 | (–0.817, 0.355) | |
| Number of dots and globulesa | –0.084 | (–0.351, 0.184) | 0.543 | –0.183 | (–0.767, 0.404) | |
| Colour asymmetrya | 0.048 | (–0.172, 0.268) | 0.67 | 0.128 | (–0.458, 0.712) | |
| Shape asymmetry | 0.003 | (–0.068, 0.074) | 0.941 | 0.022 | (–0.562, 0.606) | |
| Ellipseness | –0.045 | (–0.134, 0.043) | 0.319 | –0.300 | (–0.886, 0.289) | |
| Border sharpnessa | 0.225 | (–0.906, 1.356) | 0.703 | 0.114 | (–0.471, 0.698) | |
| Lower extremity | Diametera | 0.026 | (–0.079, 0.132) | 0.627 | 0.143 | (–0.431, 0.714) |
| Areaa | 0.081 | (0.005, 0.158) | 0.044 | 0.605 | (0.018, 1.187) | |
| Number of colours | –0.045 | (–0.298, 0.208) | 0.728 | –0.102 | (–0.674, 0.471) | |
| Number of dots and globulesa | –0.082 | (–0.272, 0.108) | 0.404 | –0.246 | (–0.819, 0.329) | |
| Colour asymmetrya | 0.097 | (–0.093, 0.286) | 0.324 | 0.291 | (–0.285, 0.864) | |
| Shape asymmetry | 0.005 | (–0.026, 0.036) | 0.756 | 0.091 | (–0.481, 0.663) | |
| Ellipseness | –0.007 | (–0.058, 0.043) | 0.781 | –0.082 | (–0.653, 0.491) | |
| Border sharpnessa | –0.198 | (–1.070, 0.673) | 0.658 | –0.13 | (–0.702, 0.443) | |
| Upper extremity | Diametera | 0.185 | (0.029, 0.342) | 0.025 | 0.668 | (0.084, 1.246) |
| Areaa | 0.064 | (0.016, 0.112) | 0.011 | 0.76 | (0.171, 1.342) | |
| Number of colours | –0.072 | (–0.299, 0.155) | 0.538 | –0.179 | (–0.745, 0.389) | |
| Number of dots and globulesa | 0.081 | (–0.098, 0.260) | 0.379 | 0.256 | (–0.313, 0.823) | |
| Colour asymmetrya | 0.038 | (–0.091, 0.167) | 0.57 | 0.165 | (–0.403, 0.731) | |
| Shape asymmetry | 0.017 | (–0.018, 0.051) | 0.342 | 0.277 | (–0.293, 0.844) | |
| Ellipseness | –0.074 | (–0.139, –0.008) | 0.032 | –0.638 | (–1.215, –0.055) | |
| Border sharpnessa | 1.018 | (–0.097, 2.133) | 0.08 | 0.516 | (–0.062, 1.089) |
Significant p-values are marked in bold.
Log-transformed.
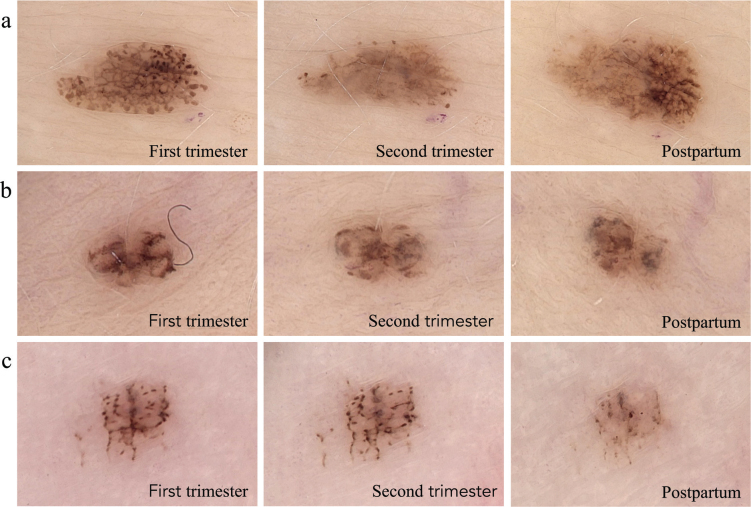
Fig. 2.
Evolution of melanocytic nevi in 3 pregnant women. (A) MN on the abdomen; (B) MN on the lower back; and (C) MN on the right palm.
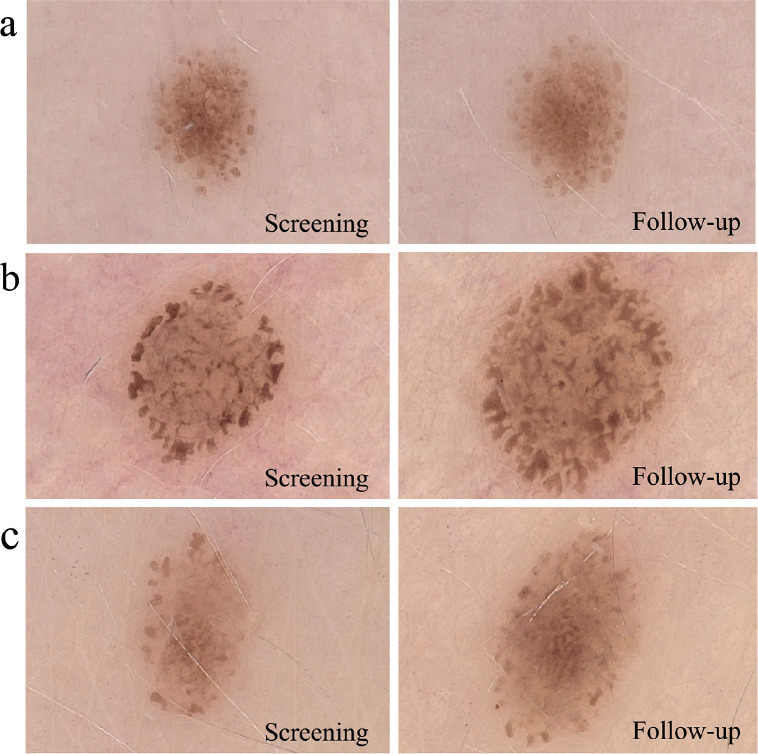
Fig. 3.
Evolution of melanocytic nevi in 3 non-pregnant women. (A) MN on the right upper arm; (B) MN on the upper chest; and (C) MN on the lower back.
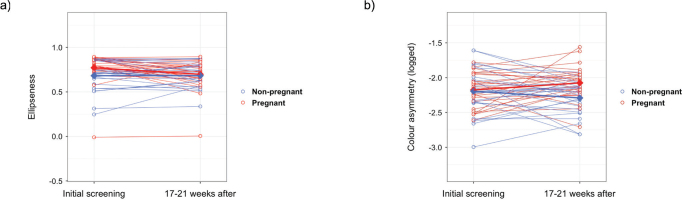
We saw that, on the UE, diameter and area increased more in pregnant women (p = 0.025; p = 0.011) (Fig. S2.1.). Further, on the UE, ellipseness decreased in pregnant women while it increased in non-pregnant women (p = 0.037) (Fig. 1A). On the LE, area increased slightly more in pregnant women (p = 0.044) (Fig. S2.2.). On the back, colour asymmetry increased in pregnant women (p = 0.022) but decreased in non-pregnant women (Fig. 1B). On other body parts nevi did not significantly change differently in pregnant women compared with non-pregnant women.
Fig. 1.
Scatter plots of mean nevus aspects in pregnant and non-pregnant women. (A) Ellipseness on the upper extremities. (B) Colour asymmetry on the back. Open circles represent individual patient data, diamonds represent averages at a given examination session. Thin and solid lines connect data points across examination sessions. Initial screening was between the 12th and 16th week of pregnancy in pregnant women.
DISCUSSION
Our findings emphasize that pregnancy might contribute to dermoscopic and macroscopic changes in MN. A unique aspect of our study is that to the best of our knowledge this is the first and largest investigation to compare pregnancy-related changes of MN on all body parts using a market-approved CNN.
Previous comparable studies have analysed MN changes at the level of the nevus. We argue that MN measured within the same patient are not statistically independent, and analysing changes at the level of the nevus entails a large risk of statistical bias. Therefore, we evaluated MN changes at the level of the patient.
Nevi changes in area and diameter
We found that in MN of pregnant women diameter not only increased on the abdomen but also on the back, head, LE, and UE.
Compared with non-pregnant women, MN in pregnant women showed a larger increase in area and diameter on the UE and LE. A larger increase in size was not seen on the abdomen and chest, body parts where skin distension and therefore size increase of MN would be expected during pregnancy. This might partly be explained by the smaller number of MN on the abdomen compared with the extremities. Second, our data showed a more pronounced increase in size between the third trimester and postpartum in pregnant women. As non-pregnant women were only followed-up once, no comparison could be made for this period.
Our results suggest that while diameter and area might be affected by pregnancy-related weight gain and expansion of skin during pregnancy, especially around the abdomen, size might also be influenced by other factors concomitant with pregnancy, leading to an increase of size in areas not considerably affected by skin expansion. Previous studies investigating MN in anatomical locations unaffected by skin expansion have not found significant changes in size in these regions (20, 21). However, these studies analysed only an average of 1.8 to 5.8 MN per patient and, apart from Rubegni et al. (20), all used old imaging systems with manual diameter measurement or no dermoscopic imaging at all. A more recent study including about 39 MN per patient and using total body photography and dermoscopic imaging also found MN on the limbs, face, neck, and back to enlarge during pregnancy (22). Currently, most speculated on growth of MN, dysplasia, and appearance of melanoma is the influence of pregnancy-related hormones, more specifically oestrogen receptor beta(ERβ) (23–26). However, as of today, the clinical significance of hormonal receptors in the evolution of MN throughout life in both sexes remains unclear. Our results on a more pronounced increase in MN size on the limbs in pregnant women compared with non-pregnant women support hypotheses such as the oestrogen theory. Future laboratory and clinical investigations on sex- and hormone-specific MN evolution are needed to gain a better translational understanding of hormone-related changes.
Nevi changes in dots and globules
In our study, population the number of dots and globules on the back increased during pregnancy and decreased postpartum.
Compared with non-pregnant women, however, the changes in dots and globules did not differ between first and third trimester. Previous studies also found an increase in the number of dots in lesions with globular patterns during pregnancy (20, 22, 27, 28) and a reduction in the number and size of dots and globules from the third trimester to postpartum (21), suggesting that pregnancy itself – apart from mechanical distension of the skin – might have an influence on this increase. However, apart from Rubegni et al. (20) none of these studies included a control population. Our results underline the importance of including a control group in further studies in this field to identify which changes can be attributed to pregnancy and which not.
Nevi changes in colour
In pregnant women the number of colours in MN decreased significantly from third trimester to postpartum on the back and UE. Further, pregnant women compared with non-pregnant women showed a higher increase in colour asymmetry on the back from first to third trimester.
Our findings on colour-associated changes in pregnant women correspond with another AI-assisted comparative study (20) detecting the variable “entropy” to increase more in pregnant women throughout and after pregnancy (reticular pattern less organized, colour-cluster size less homogeneous and distribution more chaotic).
Other studies did not analyse changes in number of colours or colour asymmetry but focused on changes towards hyper- or hypopigmentation. Whereas previous studies have given rise to the popular belief that MN undergo hyperpigmentation during pregnancy (29–31), more recent studies show contradictory results regarding pregnancy-inducing hyperpigmentation in MN (22, 32, 33).
Due to the potential bias of digital photography and external factors resulting in darkening and lightening of MN, we did not analyse hypo- and hyperpigmentation of MN in this study. To date, no study has investigated the pathophysiology of (asymmetrical) loss of number of colours towards the end of pregnancy. Further studies are needed to clarify the significance of these findings, as knowledge on such pregnancy-related colour changes could prevent unnecessary biopsies.
Nevi changes in ellipseness, shape asymmetry, and border sharpness
In our pregnant study population, the ellipseness score increased on all body parts throughout and after pregnancy, meaning the MN borders became more irregular over time. Contrarily, in non-pregnant women borders became more regular. Further, shape asymmetry and border sharpness values increased from late pregnancy to postpartum, meaning MN became more asymmetrical and the transition between mole and surrounding skin became less distinct, making it more difficult to determine where exactly the mole begins or ends. We hypothesize that shape asymmetry and border sharpness increased more in pregnant compared with non-pregnant women, assuming that enlarging MN in young to middle-aged adults generally grow in a symmetrical manner (34, 35). Our prospective study is the first to detect pregnancy-related changes towards shape and border asymmetry, and irregularity. Our observations are strengthened by previous histological findings of pregnant and non-pregnant women with increased mitotic figures and rates and a trend towards a higher Ki-67 proliferation index (36). The authors suggest the sustained increase in oestrogen levels as the most likely physiologic mechanism for these findings. Whether or not pregnancy increases the risk for MN irregularity, dysplasia, or even melanoma remains a controversial topic of debate. Although no dysplastic nevus or melanoma was detected in our real-world setting, more registry data are needed to find out whether pregnancy itself is a driver of pregnancy-associated melanoma, one of the most common cancers in pregnancy worldwide (7, 8).
Strengths and limitations
This study’s primary strength is it being the first to investigate dermoscopic changes in MN in pregnant vs non-pregnant women on all body parts using AI. Further, no previous study on this topic has ever analysed more MN (n = 2,799, in pregnant women n = 1,377). A review on studies using dermoscopy to observe changes in MN in pregnant women (13) showed that the highest number of MN previously analysed was 703 (in 18 women) by Martins-Costa & Bakos (22) and the highest number of analysed patients was by Rubegni et al. (20) with 70 participants (35 pregnant, 35 non-pregnant) totalling only 204 MN.
By using an AI-supported medical device to capture, characterize, and analyse MN and their changes, our study results are more objective than those in previous studies (20–22). Lastly, we tried to balance out the amount of time each woman was exposed to the sun by including patients at different time points throughout the year. Despite this effort, confounding effects of seasonal change may still have occurred because baseline screening took place only between January and October 2021.
Limitations to our study include the relatively small number of participants as well as the missing second follow-up for non-pregnant women. A second follow-up was refrained from to avoid a high dropout rate in the young, healthy control population. However, it must be mentioned that most previous studies did not have a control population or a postpartum follow-up at all.
For practical reasons, pregnant women were included in the analysis only when already pregnant. This might have influenced the changes we were able to detect. Further, the third trimester follow-up was positioned as early as 29 to 33 weeks to avoid high dropout rates due to high stress levels when moving closer to the date of birth as well as possible premature birth.
Due to our inclusion criteria our study may not provide comparable results in women with Fitzpatrick skin types IV–VI. As our study was a single-centre study, it is important to be cautious when generalizing the results, especially with regard to demographics.
Conclusions
MN in women of childbearing age change in their aspects over time. Our findings suggest that certain MN aspects show a trend towards changing differently in pregnant women compared with non-pregnant women of the same age. MN in pregnant women, especially during the third trimester, increased more as regards diameter, area, colour, and shape asymmetry; borders became less sharp and ellipseness decreased.
Therefore, changes that occur in an MN of a pregnant patient might be physiological and a too soon exaggerated melanoma suspicion could be avoided in daily clinical practice.
Since changes of symmetry, size, and colour in MN during pregnancy may not always be noticed by the patient or even the treating dermatologist, AI-assisted dermoscopy might be a fast and beneficial tool to perceive pregnancy-related changes and early changes towards malignancy more efficiently. CNNs can detect subtle changes and improve diagnostic accuracy not only in MN of pregnant women but in MN of all patient groups. However, their clinical utility hinges on their ability to distinguish between changes that are statistically significant but not clinically concerning. Minor dynamic changes of MN detected by CNNs may potentially increase the risk of overtreatment of benign lesions. Therefore, it is important that the results generated by CNNs are interpreted by clinicians who understand the context and clinical background of the patient. Integrating CNNs into clinical workflows requires careful consideration to enhance rather than complicate complex clinical decision-making, ensuring that the changes identified lead to improved patient outcomes without increasing the burden of unnecessary interventions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all participants who made this study possible.
Funding Statement
Founding sources This research project was funded by the Department of Dermatology of the University Hospital of Basel, as well as in minor part by the Voluntary Academic Society Grant, Basel, Switzerland. FotoFinder ATBM® Systems GmbH did not have the opportunity to comment on or influence the results or manuscript of the study.
Footnotes
The other authors have no conflicts of interest to declare.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
All authors confirm that they accept and agree with the UN’s Declaration of Human Rights. This study complies with all the relevant national regulations, institutional policies, and is in accordance with the tenets of the Helsinki Declaration. The study was approved by the local Ethics Committee (2020-02494) and was registered with ClinicalTrials.gov (NCT05148455). The participants in the study have given written informed consent to participation in the study and publication of their case details including dermoscopic and histologic images of their melanocytic nevi.
Conflict of interest disclosures
With no relation to the present manuscript, ERR reported being a shareholder of Maximon and its holding ventures and receives grants from the Goldschmidt Jacobson Foundation and Swiss National Foundation. With no relation to the present manuscript, AAN received funding for study personnel from Canfield, and declares being a consultant and adviser and/or receiving speaking fees and/or grants and/or served as an investigator in clinical trials for AbbVie, Almirall, Amgen, Biomed, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK, LEO Pharma, Janssen-Cilag, MSD, Novartis, Pfizer, Pierre Fabre Pharma, Regeneron, Sandoz, Sanofi, and UCB. For the present study, LVM received a grant from the Voluntary Academic Society in Basel. With no relation to the present manuscript, LVM received grants from the Research Fund of the University of Basel, the Voluntary Academic Society, as well as the ProPatient Foundation of the University Hospital Basel. Further, with no relation to the present manuscript, LVM has served as adviser and/or received speaking fees and/or participated in clinical trials sponsored by Almirall, Amgen, Eli Lilly, Incyte, MSD, Novartis, Pierre Fabre, Roche, and Sanofi.
REFERENCES
- 1.Di Brizzi EV, Pampena R, Licata G, Calabrese G, Longo C, Argenziano G. Are we born and do we die without nevi? A cross-sectional study. Int J Dermatol 2021; 60: 1405–1410. 10.1111/ijd.15668 [DOI] [PubMed] [Google Scholar]
- 2.Mackie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. Br J Dermatol 1985; 113: 167–174. 10.1111/j.1365-2133.1985.tb02060.x [DOI] [PubMed] [Google Scholar]
- 3.De Giorgi V, Gori A, Greco A, Savarese I, Alfaioli B, Grazzini M, et al. Sun-protection behavior, pubertal development and menarche: factors influencing the melanocytic nevi development – the results of an observational study of 1,512 children. J Invest Dermatol 2018; 138: 2144–2151. 10.1016/j.jid.2018.02.046 [DOI] [PubMed] [Google Scholar]
- 4.Lanna C, Tartaglia C, Caposiena Caro RD, Mazzilli S, Ventura A, Bianchi L, et al. Melanocytic lesion in children and adolescents: an Italian observational study. Sci Rep 2020; 10: 8594. 10.1038/s41598-020-65690-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woitowich NC, Beery A, Woodruff T. A 10-year follow-up study of sex inclusion in the biological sciences. eLife 2020; 9: e56344. 10.7554/eLife.56344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong BY, Haugh IM, Schlosser BJ, Getsios S, Paller AS. Mind the gap: sex bias in basic skin research. J Invest Dermatol 2016; 136: 12–14. 10.1038/JID.2015.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmartello M, Negri E, La Vecchia C, Scarfone G, Buonomo B, Peccatori FA, et al. Frequency of pregnancy-associated cancer: a systematic review of population-based studies. Cancers 2020; 12: 1356. 10.3390/cancers12061356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg FE, Stensheim H, Ullenhag GJ, Sahlgren HM, Lindemann K, Fredriksson I, et al. Risk factors for the increasing incidence of pregnancy-associated cancer in Sweden: a population-based study. Acta Obstet Gynecol Scand 2024; 103: 669–683. 10.1111/aogs.14677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson TM, Hieken TJ, Glasgow AE, Habermann EB, Yan Y. Pregnancy-associated melanoma: characteristics and outcomes from 2002 to 2020. Melanoma Res 2024; 34: 175–181. 10.1097/CMR.0000000000000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnawi AM, Barnawi GM, Alamri AM. Women’s health: most common physiologic and pathologic cutaneous manifestations during pregnancy. Cureus 2021; 13: e16539. 10.7759/cureus.16539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieber AK, Martires KJ, Stein JA, Grant-Kels JM, Driscoll MS, Pomeranz MK. Pigmentation and pregnancy: knowing what is normal. Obstet Gynecol 2017; 129: 168–173. 10.1097/AOG.0000000000001806 [DOI] [PubMed] [Google Scholar]
- 12.Koh SS, Roehmholdt BF, Cassarino DS. Immunohistochemistry of p16 in nevi of pregnancy and nevoid melanomas. J Cutan Pathol 2018; 45: 891–896. 10.1111/cup.13350 [DOI] [PubMed] [Google Scholar]
- 13.Cosgarea I, Trevisan-Herraz M, Ungureanu L, Zalaudek I. Dermatoscopic features of naevi during pregnancy: a mini review. Front Med 2021; 8: 727319. 10.3389/fmed.2021.727319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bieber AK, Martires KJ, Driscoll MS, Grant-Kels JM, Pomeranz MK, Stein JA. Nevi and pregnancy. J Am Acad Dermatol 2016; 75: 661–666. 10.1016/j.jaad.2016.01.060 [DOI] [PubMed] [Google Scholar]
- 15.Winkler JK, Sies K, Fink C, Toberer F, Enk A, Deinlein T, et al. Melanoma recognition by a deep learning convolutional neural network: performance in different melanoma subtypes and localisations. Eur J Cancer 2020; 127: 21–29. 10.1016/j.ejca.2019.11.020 [DOI] [PubMed] [Google Scholar]
- 16.Fink C, Blum A, Buhl T, Mitteldorf C, Hofmann-Wellenhof R, Deinlein T, et al. Diagnostic performance of a deep learning convolutional neural network in the differentiation of combined naevi and melanomas. J Eur Acad Dermatol Venereol 2020; 34: 1355–1361. 10.1111/jdv.16165 [DOI] [PubMed] [Google Scholar]
- 17.Haenssle HA, Fink C, Toberer F, Winkler J, Stolz W, Deinlein T, et al. Man against machine reloaded: performance of a market-approved convolutional neural network in classifying a broad spectrum of skin lesions in comparison with 96 dermatologists working under less artificial conditions. Ann Oncol 2020; 31: 137–143. 10.1016/j.annonc.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 18.Goessinger EV, Cerminara SE, Mueller AM, Gottfrois P, Huber S, Amaral M, et al. Consistency of convolutional neural networks in dermoscopic melanoma recognition: a prospective real-world study about the pitfalls of augmented intelligence. J Eur Acad Dermatol Venereol 2024; 38: 945–953. 10.1111/jdv.19777 [DOI] [PubMed] [Google Scholar]
- 19.Cerminara SE, Cheng P, Kostner L, Huber S, Kunz M, Maul JT, et al. Diagnostic performance of augmented intelligence with 2D and 3D total body photography and convolutional neural networks in a high-risk population for melanoma under real-world conditions: a new era of skin cancer screening? Eur J Cancer 2023; 190: 112954. 10.1016/j.ejca.2023.112954 [DOI] [PubMed] [Google Scholar]
- 20.Rubegni P, Sbano P, Burroni M, Cevenini G, Bocchi C, Severi FM, et al. Melanocytic skin lesions and pregnancy: digital dermoscopy analysis. Skin Res Technol 2007; 13: 143–147. 10.1111/j.1600-0846.2007.00180.x [DOI] [PubMed] [Google Scholar]
- 21.Zampino MR, Corazza M, Costantino D, Mollica G, Virgili A. Are melanocytic nevi influenced by pregnancy? A dermoscopic evaluation. Dermatol Surg 2006; 32: 1497–1504. 10.1111/j.1524-4725.2006.32362.x [DOI] [PubMed] [Google Scholar]
- 22.Martins-Costa GM, Bakos R. Total body photography and sequential digital dermoscopy in pregnant women. Dermatol Pract Concept 2019; 9: 126–131. 10.5826/dpc.0902a08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosci I, Grande G, Di Nisio A, Rocca MS, Del Fiore P, Benna C, et al. Cutaneous melanoma and hormones: focus on sex differences and the testis. Int J Mol Sci 2022; 24: 599. 10.3390/ijms24010599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt AN, Nanney LB, Boyd AS, King LE, Ellis DL. Oestrogen receptor-β expression in melanocytic lesions. Exp Dermatol 2006; 15: 971–980. 10.1111/j.1600-0625.2006.00502.x [DOI] [PubMed] [Google Scholar]
- 25.Spałkowska M, Dyduch G, Broniatowska E, Damiani G, Wojas-Pelc A. Molecular proof of a clinical concept: expression of estrogen alpha-, beta-receptors and g protein-coupled estrogen receptor 1 (GPER) in histologically assessed common nevi, dysplastic nevi and melanomas. Medicina (Mex) 2021; 57: 1228. 10.3390/medicina57111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Giorgi V, Mavilia C, Massi D, Gozzini A, Aragona P, Tanini A, et al. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol 2009; 145: 30–36. 10.1001/archdermatol.2008.537 [DOI] [PubMed] [Google Scholar]
- 27.Aktürk A, Bilen N, Bayrämgürler D, Demirsoy E, Erdogan S, Kiran R. Dermoscopy is a suitable method for the observation of the pregnancy–related changes in melanocytic nevi. J Eur Acad Dermatol Venereol 2007; 21: 1086–1090. 10.1111/j.1468-3083.2007.02204.x [DOI] [PubMed] [Google Scholar]
- 28.Gunduz K, Koltan S, Sahin MT, Filiz E. Analysis of melanocytic naevi by dermoscopy during pregnancy. J Eur Acad Dermatol Venereol 2003; 17: 349–351. 10.1046/j.1468-3083.2003.00792_2.x [DOI] [PubMed] [Google Scholar]
- 29.Wong RC, Ellis CN. Physiologic skin changes in pregnancy. J Am Acad Dermatol 1984; 10: 929–940. 10.1016/S0190-9622(84)80305-9 [DOI] [PubMed] [Google Scholar]
- 30.Parmley T, O’Brien TJ. Skin changes during pregnancy. Clin Obstet Gynecol 1990; 33: 713–717. 10.1097/00003081-199012000-00004 [DOI] [PubMed] [Google Scholar]
- 31.Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol 1982; 6: 977–998. 10.1016/S0190-9622(82)70083-0 [DOI] [PubMed] [Google Scholar]
- 32.Strumia R. Digital Epiluminescence microscopy in nevi during pregnancy. Dermatology 2002; 205: 186–187. 10.1159/000063901 [DOI] [PubMed] [Google Scholar]
- 33.Wyon Y, Synnerstad I, Fredrikson M, Rosdahl I. Spectrophotometric analysis of melanocytic naevi during pregnancy. Acta Derm Venereol 2007; 87: 231–237. 10.2340/00015555-0227 [DOI] [PubMed] [Google Scholar]
- 34.Bajaj S, Dusza SW, Marchetti MA, Wu X, Fonseca M, Kose K, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol 2015; 151: 1338. 10.1001/jamadermatol.2015.2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cengiz FP, Yılmaz Y, Emiroglu N, Onsun N. Dermoscopic evolution of pediatric nevi. Ann Dermatol 2019; 31: 518. 10.5021/ad.2019.31.5.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan MP, Chan MM, Tahan SR. Melanocytic nevi in pregnancy: histologic features and Ki–67 proliferation index. J Cutan Pathol 2010; 37: 843–851. 10.1111/j.1600-0560.2009.01491.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.