Abstract
Trachoma is targeted for elimination as a public health problem worldwide by 2030. In Nigeria, elimination activities are implemented at the local government area (LGA) level. They started in 2002 by conducting baseline population-based prevalence surveys (PBPSs), which continued in a systematic manner with engagement from the Global Trachoma Mapping Project in 2013, and subsequently Tropical Data. The results led to the development of Nigeria's first trachoma action plan and its subsequent revision with additional information. Following 449 baseline PBPSs, 122 LGAs had an active trachoma prevalence above the elimination threshold, requiring interventions, while 231 LGAs required community-based interventions for trichiasis management. By 2021, >34 million antibiotic treatments had been provided in 104 LGAs, with 89 LGAs eliminating active trachoma. Nationally, water and sanitation coverages increased by 3% and 18%, respectively, in 7 y. Systematic trichiasis case finding and management were carried out in 231 LGAs, resulting in the management of 102 527 people. Fifty-four LGAs decreased trichiasis prevalence unknown to the health system to <0.2% in persons ≥15 y of age. Where this elimination prevalence threshold was reached, trichiasis services were transitioned to routine eye/healthcare systems. Such progress relied on strong leadership and coordination from the national trachoma program and tremendous support provided by partners. Attaining elimination of trachoma as a public health problem in Nigeria by 2030 is feasible if funding support is sustained.
Keywords: elimination, neglected tropical diseases, Nigeria, trachoma, trichiasis
Introduction
In 1996, the World Health Organization (WHO) Alliance for the Global Elimination of Trachoma by 2020 was founded with the goal of eliminating trachoma as a public health problem worldwide.1 The neglected tropical diseases (NTDs) road map 2021–2030, endorsed by the World Health Assembly in 2020 through its decision 73(33), sets 2030 as the new target date for global elimination.2 The SAFE strategy (Surgery for trachomatous trichiasis (TT); Antibiotics to clear ocular Chlamydia trachomatis infection; Facial cleanliness and Environmental improvement to reduce C. trachomatis transmission) is recommended by the WHO to achieve elimination3 and is being actively implemented in endemic countries.4 SAFE is expected to drive and sustain the prevalence below the elimination thresholds (a prevalence of trachomatous inflammation–follicular (TF) of <5% in children 1–9 y of age and a prevalence of TT unknown to the health system of <0.2% in persons ≥15 y of age) set by the WHO,5 with prevalence in each case determined in evaluation units (EUs), which are administrative units for healthcare management consisting of approximately 100 000–250 000 people. By July 2023, 18 countries had reached these thresholds and been validated by the WHO as having eliminated trachoma as a public health problem.6 This article summarizes progress against trachoma in Nigeria up to the end of the year 2020.
Program overview
In Nigeria, the EU is equivalent to the local government area (LGA) level. Trachoma surveys and implementation of elimination activities are undertaken in LGAs, with local and state governments partnering with non-governmental organizations to implement the SAFE strategy. In Nigeria, the LGA is an administrative unit equivalent to a district with a population ranging between <100 000 to slightly above 250 000. Coordination is undertaken at federal level. Activities related to trachoma elimination started in 2002 with population-based prevalence surveys in Plateau and Nasarawa states,7 then in 2003 in Kebbi,8 Sokoto9,10 and Zamfara11 states, followed by Katsina,12 Yobe,13 Kano14 and Taraba15 thereafter. All LGAs suspected to be endemic (except those with security challenges) were mapped prior to or in collaboration with the Global Trachoma Mapping Project (GTMP), which started in 2012.16 Following mapping, Nigeria's national trachoma action plan17 was developed in 2013 with the aim of eliminating trachoma by 2018. The final antibiotic mass drug administration (MDA) for trachoma was planned to be completed by 2017 and the TT prevalence elimination threshold was planned to be attained in each LGA by 2018. The Federal Ministry of Health took the leadership role and collaborated with its sister ministries of education, environment and water resources to implement relevant work in the F and E aspects of the SAFE strategy. It also standardized the process for training and certification of TT surgeons, developed internal processes for requesting donated antibiotics from the International Trachoma Initiative (ITI) and produced guidance on antibiotic distribution during MDA. National guidelines for the implementation of behaviour change for personal and environmental hygiene were also developed and rolled out.
After development of the trachoma action plan in 2013, additional trachoma prevalence surveys were conducted, which influenced subsequent implementation of the SAFE strategy. In 2017, the national trachoma action plan was revised and new targets set based on the most recent data. As part of that process, the projected dates for the last antibiotic MDA and attainment of the TT elimination target in each LGA were adjusted to 2020.
Surveys
Baseline surveys
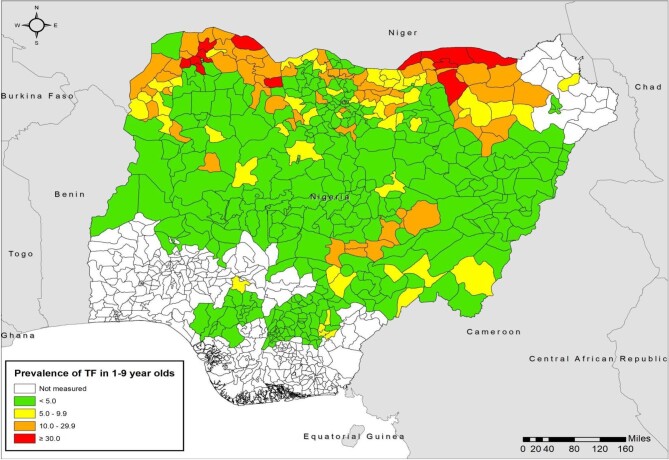
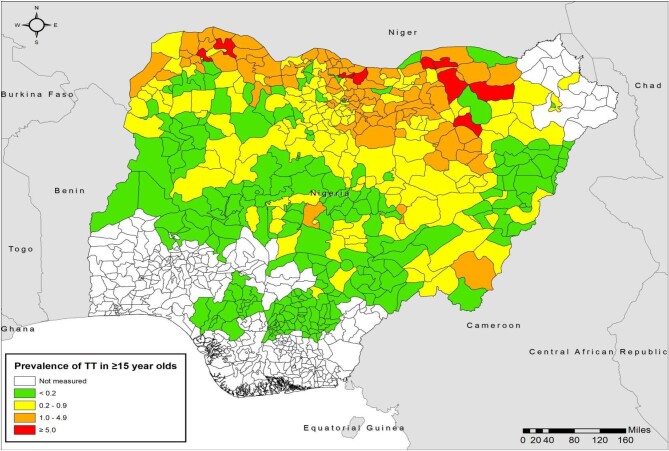
Northern Nigeria is located in Africa's ‘trachoma belt’, an arid and semi-arid area with poor water supply, poor sanitation and widespread poverty. All LGAs across the northern part of the country were therefore considered likely to be endemic for trachoma. The occurrence of cases of TT in some LGAs of more southern regions meant that surveys were also warranted there; the program mapped all LGAs in which health facilities managed or reported TT cases, plus surrounding LGAs. Geographic extension of mapping stopped when trachoma was not found to be a public health problem in the progressively more southern LGAs in Kwara18 and Kogi states.19 In total, using all available health information on trachoma in 2013, it was determined that 463 LGAs were sufficiently likely to be endemic for trachoma that population-based trachoma prevalence surveys were indicated. All surveys were undertaken at the LGA level and followed contemporary WHO guidance.20 For surveys conducted prior to the GTMP (i.e. from 2001 to 2012), sample size calculations were based on an expected TF prevalence of 20%, precision of 10%, design effect of 4 and a 10% non-response rate. The minimum sample size was determined to be 1689 participants. Study participants were selected using a two-stage cluster sampling technique. In the first stage, clusters were selected using probability proportional to cluster population size; in the second stage, households were selected using the random walk technique. In each cluster, 52 persons ≥15 y of age and 27 children ages 1–9 y were enumerated for inclusion.8,9 From 2013, all surveys followed WHO guidelines and were conducted with support from the GTMP, and from 2016 onwards, Tropical Data.16,21–24 In these surveys, the single population proportion for precision formula was used to determine the number of children 1–9 y of age to be enumerated to estimate an expected TF prevalence of 10% with an absolute precision of 3%. The sample size (n) was calculated as: n=DEFF (design effect)×p(1−p)/(2×d/(1.96×2)2)×(non-response inflation factor). We used a non-response rate inflation factor of 1.2. The design effect for cluster surveys was assumed to be 2.65, resulting in 1225 children to enumerate. First-stage sampling of clusters was based on probability proportional to cluster size, while second-stage sampling for households included random walk and compact segment techniques in various LGAs. By the end of 2020, baseline population-based prevalence trachoma surveys had been conducted in 449 LGAs (Table 1, Figures 1 and 2). Baseline surveys were still required in 14 LGAs in security-challenged Borno state.
Table 1.
Population-based trachoma prevalence surveys (baseline, impact and surveillance) conducted in Nigeria, 2002–2020
| Prevalence of TF in children 1–9 y of age | Prevalence of TT unknown to the health system in ≥15-year-oldsa | ||||||
|---|---|---|---|---|---|---|---|
| Survey type | LGAs surveyed, n | <5%: no antibiotic MDA needed | 5.0–9.9%: one round of antibiotic MDA recommended | 10.0–29.9%: three rounds of antibiotic MDA recommended | ≥30%: five rounds of antibiotic MDA recommended | <0.2%: community-based TT surgical service not needed | ≥0.2%: community-based TT surgical service needed |
| Baseline, LGAs (%) | 449 | 327 (72.8) | 52 (11.6) | 63 (14.0) | 7 (1.6) | 218 (48.6) | 231 (51.4) |
| Impact, LGAs (%) | 117 | 89 (76.1) | 14 (12.0) | 14 (12.0) | 0 | 54 (46.2) | 63 (53.8) |
| Surveillance, LGAs (%) | 31 | 29 (93.5) | 2 (6.5) | 0 | 0 | 13 (41.9) | 18 (58.1) |
aUnknown to the health system is defined as a TT patient who has not been offered any form of management by a health worker.
Figure 1.
Baseline prevalence of TF in children 1–9 y of age by LGA, Nigeria, by end of 2020.
Figure 2.
Baseline prevalence of TT in adults ≥15 y of age by LGA, Nigeria, by end of 2020.
Implementation of A, F and E
Antibiotic treatments for trachoma elimination started in 2004 in Sokoto, Kebbi and Zamfara states, before the national trachoma program was formally established. By the end of 2016, when most baseline surveys had been concluded, there were an estimated 21 837 512 people living in districts with TF requiring antibiotic MDA.25 Overall, baseline surveys showed that seven LGAs (which had been mapped prior to GTMP and national integration of trachoma activities) required five MDA rounds, 63 LGAs required three MDA rounds and 52 LGAs required one MDA round, plus activities to enhance facial cleanliness and improve the environment. Nigeria adopted community-based MDA with the use of house-to-house and central site distribution strategies and prioritized LGAs requiring more rounds of MDA. Health education on social and behavioural changes was integrated within MDA campaigns. MDA treatment data are recorded in community registers in triplicate according to the households treated. Data from these registers are manually collated at the health facility level and transmitted to the LGA level. Community and school-based education on personal and environmental hygiene was undertaken during MDA campaigns, community meetings and at marketplaces. Essential messages conveyed related to trachoma blindness and its relationship to poor personal and environmental hygiene, the need for personal hygiene and the importance of keeping the environment clean through the use of latrines and proper disposal of waste. While accurate records of treatment prior to 2010 are no longer available, from 2010 to 2020, >34 million antibiotic treatments were provided in 104 LGAs (Table 2). In 2019, of 43 LGAs that received antibiotic treatments, 40 (93%) LGAs achieved the minimum 80% coverage, while in 2020, 23 (92%) achieved the minimum coverage. District implementation of F and E activities resulted in an increase in open defecation free (ODF) communities. In 2013, there were no ODF communities, but this increased to 27 communities by 2020. Marginal increases in water (64% to 67%) and sanitation (24% to 48%) coverage were also recorded in Nigeria between 2013 and 2020.26,27
Table 2.
Antibiotic MDA carried out in Nigeria for trachoma elimination purposes, 2010–2020
| State | LGAs, n | LGAs requiring MDA, n | LGAs offered one round of MDA, n | LGAs offered three rounds of MDA, n | Total antibiotic treatments given, n |
|---|---|---|---|---|---|
| Adamawa | 21 | 0 | 0 | 0 | 0 |
| Bauchi | 20 | 2 | 2 | 0 | 536 133 |
| Borno | 27 | 3 | 3 | 0 | 279 532 |
| Benue | 23 | 3 | 3 | 0 | 635 163 |
| Ebonyi | 13 | 4 | 4 | 0 | 710 081 |
| Edo | 18 | 1 | 1 | 0 | 320 189 |
| Enugu | 17 | 0 | 0 | 0 | 0 |
| FCT | 6 | 0 | 0 | 0 | 0 |
| Gombe | 11 | 0 | 0 | 0 | 0 |
| Jigawa | 27 | 23 | 12 | 11 | 6 598 892 |
| Kaduna | 23 | 1 | 1 | 0 | 558 761 |
| Kano | 44 | 10 | 6 | 4 | 780 659 |
| Katsina | 34 | 12 | 6 | 6 | 1 224 831 |
| Kebbi | 21 | 8 | 4 | 4 | 2 461 584 |
| Kogi | 21 | 0 | 0 | 0 | 0 |
| Kwara | 16 | 0 | 0 | 0 | 0 |
| Nasarawa | 13 | 4 | 0 | 4 | 1 033 616 |
| Niger | 25 | 2 | 1 | 1 | 168 621 |
| Plateau | 17 | 3 | 0 | 3 | 1 004 200 |
| Sokoto | 23 | 7 | 6 | 1 | 8 089 536 |
| Taraba | 16 | 3 | 3 | 0 | 101 240 |
| Yobe | 17 | 16 | 4 | 12 | 6 692 058 |
| Zamfara | 14 | 2 | 2 | 0 | 3 483 302 |
| Total | 467 | 104 | 58 | 46 | 34 678 398 |
Impact and surveillance surveys
After the implementation of the required elimination activities based on the outcome of the baseline surveys, impact surveys were conducted with GTMP and Tropical Data support at least 6 months after the last scheduled round of MDA. These surveys provide information on whether the elimination thresholds had been reached or not.28 Surveillance surveys (conducted 2 y after an impact survey shows TF prevalence in 1- to 9-year-olds to be <5%) provide information on whether districts had sustained a subthreshold TF prevalence in the absence of antibiotic pressure.29 A total of 117 impact (conducted in some of the 122 LGAs that had received MDA) and 31 surveillance surveys were conducted by the end of 2020 (Table 1) and results are shown in Figure 3. The sample size for these surveys was calculated to estimate an expected TF prevalence of 4%, with an absolute precision of 2% and a design effect of 2.63. After inflating by a factor of 1.2 to account for non-response, the expected number of children to enumerate was 1164.
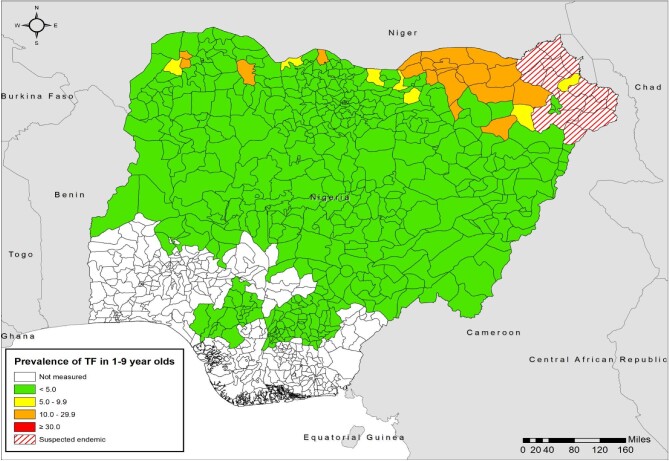
Figure 3.
Prevalence of TF in children 1–9 y of age by LGA, Nigeria, by end of 2020. For each LGA, the most recent prevalence estimate generated to that date is represented.
Impact surveys showed that 89 (76%) LGAs did not require further antibiotic MDA, while 28 (24%) LGAs required one (n=14) or three (n=14) more rounds of MDA. Of the 31 LGAs that had surveillance surveys, 29 (94%) had sustained a subthreshold TF prevalence, while 2 (6%) LGAs again had a TF prevalence above the 5% elimination threshold. This phenomenon of TF prevalence returning to ≥5% at the surveillance survey has since been defined as recrudescent active trachoma.30 LGAs affected by recrudescence have been scheduled for more frequent than annual (MFTA) antibiotic treatment and the use of serology and polymerase chain reaction (PCR) testing during impact surveys to investigate Chlamydia trachomatis transmission dynamics.
In all, between the commencement of implementation of the SAFE strategy in 2002 and the end of 2020, 89 LGAs attained the elimination threshold for TF, with only 28 LGAs still qualifying for MDA. Between 2016 and the end of 2020, the estimated number of people living in districts with TF prevalence indicating a need for the A, F and E components of SAFE had dropped by 71% to 6 310 385.31
Implementation of S
TT surgery was provided by certified TT surgeons across all endemic states. In Nigeria, eye nurses working in eye care were trained and certified as TT surgeons to provide community-based TT surgery. The national trachoma program trained/retrained and certified 186 TT surgeons across all endemic states starting in 2013 (Table 3). Each trained and certified surgeon was provided with three TT surgical kits. Along with the surgeons, the program trained at least two community health workers or general nurses per surgeon as surgeon assistants. Following the 449 baseline surveys, the backlog of TT cases was estimated at 235 734. The TT backlog was calculated by multiplying the point prevalence of TT in each LGA by the population ≥15 y of age. In 231 (51%) LGAs (Table 1) in which the prevalence of TT was above the elimination threshold, community-based TT management was provided. TT surgeons trained community volunteers as TT case finders and provided them with a pen torch32 and pictures of various types of TT (major TT, minor TT and epilated cases). A total of 49 743 trained case finders undertook house-to-house case finding within 2 weeks of training and referred all suspected cases to an agreed upon surgical outreach site for confirmation and management on a prespecified date. Case finders were also asked to escort suspected cases to the outreach site on the day of the outreach. Case finders visited the homes of persons suspected of having TT who failed to show up at outreach sites and encouraged those patients to present. In some instances, TT surgeons went to the homes of such patients and offered them management after confirmation of TT. The proportion of TT cases confirmed from the list of suspected cases was between 30% and 60%. A total of 102 527 cases were managed by the end of 2020, not including work done prior to setting up the national program.
Table 3.
TT management, Nigeria, 2003–2020
| State | Trichiasis surgeons trained (including uncertified trainees), n | Estimated TT backlog from baseline survey, n | LGAs in the state, n | LGAs with TT prevalence above threshold for elimination at baseline, n | Estimated TT backlog from the most recent survey, n | TT cases managed by health facilities (in transitioned LGAs)a, n | TT cases managed during outreach, n | Total TT cases managed in the state, n |
|---|---|---|---|---|---|---|---|---|
| Adamawa23 | 2 | 3205 | 21 | 3 | 177 | 0 | 0 | 0 |
| Bauchi34 | 21 | 28 706 | 20 | 19 | 23 318 | 0 | 9544 | 9544 |
| Benue35 | 3 | 1596 | 23 | 3 | 358 | 0 | 37 | 37 |
| Borno24 | 0 | 6222 | 27 | 10 | 6222 | 0 | 0 | 0 |
| Ebonyi | 0 | 575 | 13 | 0 | 0 | 0 | 0 | 0 |
| Edo | 0 | 1169 | 18 | 3 | 273 | 0 | 0 | 0 |
| Enugu | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 |
| FCT36 | 3 | 1055 | 6 | 2 | 101 | 0 | 59 | 59 |
| Gombe37 | 10 | 19 548 | 11 | 11 | 16 899 | 0 | 720 | 720 |
| Jigawa38 | 23 | 37 540 | 27 | 26 | 25 252 | 0 | 20 054 | 20 054 |
| Kaduna39 | 10 | 7685 | 23 | 10 | 2316 | 0 | 707 | 707 |
| Kano40 | 23 | 47 823 | 44 | 42 | 27 215 | 0 | 21 991 | 21 991 |
| Katsina41 | 32 | 27 225 | 34 | 32 | 14 238 | 35 | 9061 | 9096 |
| Kebbi9,42 | 11 | 8447 | 21 | 14 | 4797 | 0 | 12 915 | 12 915 |
| Nasarawa7 | 1 | 1348 | 13 | 4 | 120 | 0 | 131 | 131 |
| Niger43 | 2 | 2743 | 25 | 5 | 327 | 0 | 0 | 0 |
| Plateau7 | 1 | 1272 | 17 | 2 | 179 | 0 | 313 | 313 |
| Sokoto9,10 | 13 | 10 692 | 23 | 15 | 347 | 79 | 8423 | 8502 |
| Taraba44 | 6 | 5049 | 16 | 7 | 1760 | 0 | 904 | 904 |
| Yobe13 | 20 | 16 871 | 17 | 17 | 13 605 | 0 | 16 461 | 16 461 |
| Zamfara11 | 5 | 6963 | 14 | 6 | 4467 | 0 | 1093 | 1093 |
| Total | 186 | 235 734 | 430 | 231 | 141 971 | 114 | 102 413 | 102 527 |
aTT cases managed after LGAs had fully transitioned local TT management from campaign-style intervention to routine health system action.
To ensure the quality of surgery, TT surgeons were supervised during outreach by their trainers and the program required that each active surgeon was audited within 6 months of starting surgeries once the required number of eyes for an audit had been attained. Surgeon supervisors were trained to provide supportive supervision33 to surgeons during outreach and to conduct surgeon audits. By the end of 2020, of 117 LGAs that had had impact surveys, 54 LGAs had attained a TT prevalence unknown to the health system of <0.2% in people ≥15 y of age; of the 31 LGAs that had surveillance surveys, 13 had reached this prevalence threshold. The LGAs that achieved the TT elimination threshold underwent transitioning and TT surgery was devolved to routine eye care services, with 114 TT cases managed over this period in static facilities across 16 LGAs (Figure 4 and Table 3). In the 166 LGAs with TT prevalence above the elimination threshold, no surveys were scheduled as the TF elimination threshold had been attained. These LGAs have undergone or are undergoing documented full geographic coverage through case finding and management to attain TT elimination.
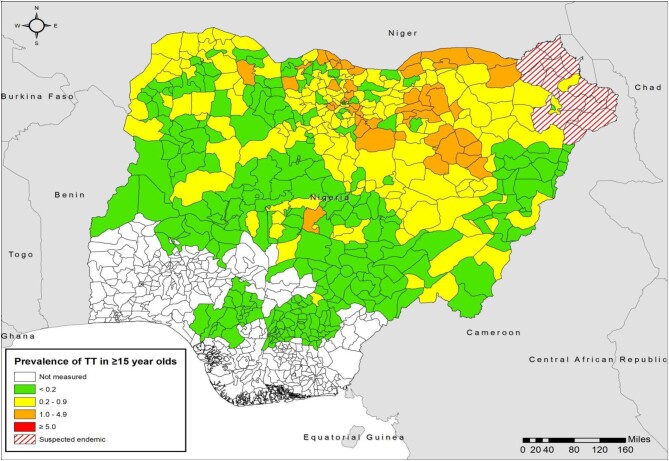
Figure 4.
Prevalence of TT in adults ≥15 y of age by LGA, Nigeria, by end of 2020. For each LGA, the most recent prevalence estimate generated to that date is represented.
Health information system
Data collection
TT surgical data were initially collected on paper. In 2016, the TT tracker, an electronic phone-based data collection tool,45 was introduced. The application was piloted in Kano state and then, from 2019, rolled out to five other states: Bauchi, Jigawa, Katsina, Yobe and Taraba. The tool supports real-time surgical data entry and visualization across these states and is likely to have improved outcomes by increasing the proportion of patients seen at the 3- to 6-month postoperative follow-up visit. Surgeons are given prompts about postoperative patients due for follow-up. The tool also enables easy calculation of surgeon-level surgical outcomes to prioritize individual surgeons for routine audits.
The Federal Ministry of Health is developing an NTD-focused District Health Information System (DHIS2) platform with the long-term intention of integrating it into the existing national DHIS2/Health Management Information System (HMIS). When fully developed, the number of trichiasis cases managed will be reported as part of the HMIS, facilitating alert creation to serve as a tool for surveillance in the unusual circumstance of a sudden increase in managed cases.
Transition from community-based outreach to routine health service delivery for TT management
Discussions on transitioning to static services were started as LGAs approached the elimination threshold for TT. Health and eye care facilities were made aware of the need to assume management of TT cases that presented when outreach services were no longer required. Advocacy was undertaken at the LGA governance level to support TT surgical services being provided in the same way as management is offered for other eye conditions. Where user fees are the norm, it was advocated that patients pay a minimum fee to receive TT management. Where available, health insurance covered the cost of services. Trained ophthalmic nurses at district hospitals continued to provide TT services to presenting patients as part of their routine eye care work. All transitioned LGAs currently have active surgeons, but guidelines exist for situations where there is no trained TT surgeon in an LGA. TT surgeons from neighbouring LGAs will visit to provide surgery on agreed upon dates. Provision of surgical kits and surgical consumables at the end of the active case finding and outreach in an LGA facilitated the handover to static facilities. Surgeon assistants were encouraged to be advocates for TT surgery in the communities in which they live. Mobilization of community groups, including women's groups, also facilitated provision of TT management through static services in some LGAs. The trachoma program leveraged existing eye care programs, such as cataract camps, to strengthen the provision of TT management at static sites.
Discussion
Progress made so far in Nigeria can be attributed to several factors. First is the critical leadership provided by the national and state trachoma programs. The national program, guided by the National Trachoma Task Force (NTTF), coordinated activities in the various states. The national program ensured antibiotics for MDA were requested on time and ensured timely distribution to the states once received, while states ensured that antibiotics reached the LGAs and then the communities requiring treatment. Coordination at the national level ensured antibiotics could be moved between states as needed. The national trachoma program also coordinated the training and certification of TT surgeons with the National Eye Centre based on the needs in each state.
The Nigeria trachoma program also involved other ministries and departments in the implementation of trachoma elimination activities. The ministries of education, water resources and environment are represented in the NTTF and use information on water and sanitation access generated during trachoma surveys for advocacy about provision of water and sanitation in trachoma-endemic communities.46
All program staff have demonstrated enduring commitment to the various activities implemented towards elimination of trachoma. Community drug distributors ensured drug coverage was high and provided education on water and sanitation, while TT case finders went house-to-house to find TT cases and surgeons were willing to visit remote communities to provide the required management.
Partnerships between governments and non-governmental development organizations (NGDOs) were well coordinated. This ensured that each endemic state was paired with an NGDO partner, which provided funding support for implementation of the SAFE strategy as required. NGDO partners leveraged funding from external sources to support trachoma elimination activities.
There have been challenges on the road to achieving trachoma elimination in Nigeria. In many areas, mapping was delayed due to insecurity; at the time of submission of this manuscript, this remained a problem for 14 LGAs in Borno state. A lack of data on the prevalence of trachoma in these LGAs means that the requirements to reach elimination remain incompletely known. Insecurity has also impacted on the implementation of elimination activities in some LGAs, where TT case finding, TT case management and MDA coverage may have been suboptimal. As in other countries,47 attempts have been made to develop innovative ways of using local community volunteers to screen TT patients and transport those thought to need management to secure areas for further review. Importation of antibiotics for MDA experienced occasional delays due to shipping issues and challenges with clearing medicines from the ports. MDA rounds were either delayed or missed in some instances. The program has developed ways to prevent import delays as well as expedited clearance of drugs at the ports of entry by using dedicated clearing agents.
Ensuring that individuals suspected of having TT by case finders present for management remains a challenge. Many suspected cases do not present at outreach sites for confirmation and management. Outreach teams have had to send case finders back to encourage such individuals to be reviewed and, in some cases, TT surgeons have travelled to examine people at home. These efforts may be critical: comparison of the changing prevalence maps for TF (Figures 1 and 3) and TT (Figures 2 and 4) show a profound retreat in the geographic extent of active trachoma, while the reduction of the area in which TT is a public health problem has been less marked. While important to achieve elimination of TT as a public health problem, individual follow-up requires considerable additional time, effort and cost. Therefore, continued commitment from the national trachoma program and its supporting partners is essential to achieve and maintain the elimination targets.
Conclusions
Substantial progress has been made towards elimination of trachoma in Nigeria, although the elimination target date of 2020 was not achieved. Despite many challenges, there has been a >70% reduction in the number of persons living in districts requiring implementation of the A, F and E components of the SAFE strategy. Systematic TT case finding and case management have saved thousands from the risk of trachomatous blindness. With sustained funding support and the momentum already generated, providing security does not continue to prevent access to endemic populations, elimination of trachoma as a public health problem in Nigeria by 2030 is feasible.
Acknowledgements
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. The boundaries and names shown and the designations used in the maps in this article do not imply the expression of any opinion whatsoever on the part of the authors, or the institutions with which they are affiliated, concerning the legal status of any country, territory, city or area or of its authorities or concerning the delimitation of its frontiers or boundaries.
Contributor Information
Caleb D Mpyet, Sightsavers, Nigeria Country Office, Kaduna, Nigeria; Department of Ophthalmology, University of Jos, Jos, Nigeria.
Nicholas Olobio, Department of Public Health, Federal Ministry of Health, Abuja, Nigeria.
Sunday Isiyaku, Sightsavers, Nigeria Country Office, Kaduna, Nigeria.
Teyil Wamyil-Mshelia, Sightsavers, Nigeria Country Office, Kaduna, Nigeria.
Grace Ajege, Sightsavers, Nigeria Country Office, Kaduna, Nigeria.
Christopher Ogoshi, Health and Development Support Programme, Jos, Nigeria.
Francisca Olamiju, Mission to Save the Helpless, Jos, Nigeria.
Ijeoma Achu, Mission to Save the Helpless, Jos, Nigeria.
Mohammed Dantani Adamu, Ophthalmology Department, Usmanu Danfodiyo University, Sokoto, Nigeria.
Nasiru Muhammad, Ophthalmology Department, Usmanu Danfodiyo University, Sokoto, Nigeria.
Aliyu Mohammed Jabo, Helen Keller International, Abuja, Nigeria.
Philomena Orji, Helen Keller International, Abuja, Nigeria.
Adamani William, CBM International, Abuja Nigeria.
Alice Venyir Ramyil, Department of Ophthalmology, University of Jos, Jos, Nigeria.
Ana Bakhtiari, Task Force for Global Health, Decatur, GA, USA.
Sarah Boyd, Task Force for Global Health, Decatur, GA, USA.
Michaela Kelly, Sightsavers, Haywards Heath, UK.
Cristina Jimenez, Sightsavers, Haywards Heath, UK.
Amir Bedri Kello, World Health Organization Regional Office for Africa, Brazzaville, Congo.
Anthony W Solomon, Global Neglected Tropical Diseases Programme, World Health Organization, Geneva, Switzerland.
Emma M Harding-Esch, Clinical Research Department, London School of Hygiene and Tropical Medicine, London, UK.
Paul Courtright, Kilimanjaro Centre for Community Ophthalmology, Division of Ophthalmology, University of Cape Town, Cape Town, South Africa.
Authors’ contributions
This manuscript was conceived by CDM and PC, all authors collected relevant data and participated in writing, editing and approval of this manuscript.
Funding
ABK and AWS are staff of WHO. The GTMP was funded by the UK government's Department for International Development, subsequently known as UK AID, with additional funding from the US Agency for International Development (USAID). Core Tropical Data funding was provided by the International Trachoma Initiative (ITI), Sightsavers and RTI International through the USAID Act to End NTDs | East program. The SAFE strategy in Nigeria has been funded by UK AID, the Queen Elizabeth Diamond Jubilee Trust, the Commonwealth Fund, USAID and multiple private donors, while implementation and funding was through a network of non-governmental organizations including Sightsavers, Christoffel Blinden Mission, Helen Keller International, Health and Development Support Programme, Mission To Save the Helpless, RTI, Amen Health Care and Empowerment Foundation and the Carter Center.
Competing interests
AB and SB are employed by ITI, which receives an operating budget and research funding from Pfizer, the manufacturers of Zithromax (azithromycin). EMHE receives salary support from ITI.
Ethical approval
Not required.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author
Dedication
This article is dedicated to Mr Christopher Sunday Ogoshi for his exemplary leadership in the fight against trachoma, unfortunately he died before Nigeria could attain elimination.
References
- 1. World Health Organization . Future approaches to trachoma control: report of a global scientific meeting, Geneva, 17–20 June 1996. WHO/PBL/96.56. Geneva: World Health Organization; 1997. [Google Scholar]
- 2. World Health Organization . Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/338565 [accessed 28 January 2021]. [Google Scholar]
- 3. Francis V, Turner V. Achieving community support for trachoma control. WHO/PBL/93.36. Geneva: World Health Organization; 1993. [Google Scholar]
- 4. World Health Organization . WHO Alliance for the Global Elimination of Trachoma: progress report on elimination of trachoma, 2021. Wkly Epidemiol Rec. 2023;98(28):297–314. [Google Scholar]
- 5. World Health Organization . Validation of elimination of trachoma as a public health problem. WHO/HTM/NTD/2016.8. Geneva: World Health Organization; 2016. [Google Scholar]
- 6. World Health Organization . Iraq eliminates trachoma as a public health problem. Geneva: World Health Organization; 2023. Available from: https://www.who.int/news/item/31-07-2023-iraq-eliminates-trachoma-as-a-public-health-problem [accessed 21 September 2023]. [Google Scholar]
- 7. King JD, JIP N, Jugu YS et al. Mapping trachoma in Nasarawa and Plateau States, central Nigeria. Br J Ophthalmol. 2010;94(1):14–19. [DOI] [PubMed] [Google Scholar]
- 8. Muhammad N, Rabiu MM. Prevalence of trachoma in 6 local government areas of Kebbi State. Kaduna: National Eye Centre; 2006. [Google Scholar]
- 9. Muhammad N, Mohammed A, Isiyaku S et al. Mapping trachoma in 25 local government areas of Sokoto and Kebbi states, northwestern Nigeria. Br J Ophthalmol. 2014;98(4):432–7. [DOI] [PubMed] [Google Scholar]
- 10. Mansur R, Muhammad N, Liman IRN. Prevalence and magnitude of trachoma in a local government area of Sokoto state, north western Nigeria. Niger J Med. 2007;16(4):348–53. [DOI] [PubMed] [Google Scholar]
- 11. Rabiu MM, Muhammad N. Report of trachoma population based survey in 6 local government areas of Zamfara State. Kaduna: Sightsavers International; 2005. [Google Scholar]
- 12. Jip N, King JD, Diallo MO et al. Blinding trachoma in Katsina state, Nigeria: population-based prevalence survey in ten local government areas. Ophthalmic Epidemiol. 2008;15:294–302. [DOI] [PubMed] [Google Scholar]
- 13. Mpyet C, Ogoshi C, Goyol M. Prevalence of trachoma in Yobe state, northeastern Nigeria. Ophthalmic Epidemiol. 2008;15(5):303–7. [DOI] [PubMed] [Google Scholar]
- 14. Mpyet C, Lass BD, Yahaya HB et al. Prevalence of and risk factors for trachoma in Kano State, Nigeria. PLoS One. 2012;7(7):e40421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mission To Save The Helpless . Report of trachoma population-based survey in 3 local government areas of Taraba State. Jos, Nigeria: Mission To Save The Helpless; 2009. [Google Scholar]
- 16. Solomon AW, Pavluck A, Courtright P et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22 (3):214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National plan for the elimination of blinding trachoma from Nigeria. Abuja, Nigeria: Federal Ministry of Health; 2013. [Google Scholar]
- 18. Alada JJ, Mpyet C, Florea VV et al. Prevalence of and risk factors for trachoma in Kwara state, Nigeria: results of eight population-based surveys from the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2018;25(Suppl 1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alada JJ, Mpyet C, Florea VV et al. Prevalence of trachoma in Kogi State, Nigeria: results of four local government area-level surveys from the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2018;25(Suppl 1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases . Design parameters for population-based trachoma prevalence surveys. WHO/HTM/NTD/PCT/2018.07. Geneva: World Health Organization; 2018. [Google Scholar]
- 21. Solomon AW, Zondervan M, Kuper H, et al. Trachoma control: a guide for programme managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 22. Courtright P, MacArthur C, Macleod C et al. Tropical Data: training system for trachoma prevalence surveys (version 2). London: International Coalition for Trachoma Control; 2016. [Google Scholar]
- 23. Adamu MD, Mohammed Jabo A, Orji P et al. Baseline prevalence of trachoma in 21 local government areas of Adamawa State, North East Nigeria. Ophthalmic Epidemiol. 2023:30(6):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adamu MD, Jabo AM, Orji P et al. Baseline prevalence of trachoma in 13 local government areas of Borno State, Nigeria. Ophthalmic Epidemiol. 2023;30(6):628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . WHO Alliance for the Global Elimination of blinding Trachoma by the year 2020. Progress report on elimination of trachoma, 2016. Wkly Epidemiol Rec. 2017;92(26):359–68.28664685 [Google Scholar]
- 26. World Health Organization . Investing in water and sanitation: increasing access, reducing inequalities. Geneva: World Health Organization; 2014. [Google Scholar]
- 27. UNICEF . Water, sanitation and hygiene national outcome routine mapping report 2021. Available from: https://www.unicef.org/nigeria/reports/water-sanitation-and-hygiene-national-outcome-routine-mapping-report-2021 [accessed].
- 28. Solomon AW, Hooper PJ, Bangert M et al. The importance of failure: how doing impact surveys that fail saves trachoma programs money. Am J Trop Med Hyg. 2020;103(6):2481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases . Technical consultation on trachoma surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA. WHO/HTM/NTD/2015.02. Geneva: World Health Organization; 2015. [Google Scholar]
- 30. Informal consultation on end-game challenges for trachoma elimination, December 7–9, 2021, Task Force for Global Health, Decatur, USA. Geneva: World Health Organization; 2022. Available from: https://apps.who.int/iris/bitstream/handle/10665/363591/9789240048089-eng.pdf [accessed 18 October 2022]. [Google Scholar]
- 31. World Health Organization . WHO Alliance for the Global Elimination of blinding trachoma by the year 2020. Progress report on elimination of trachoma by 2020. Wkly Epidemiol Rec. 2021:96:353–64. [Google Scholar]
- 32. Mpyet C, Ramyil A, Dami N et al. Use of an inexpensive magnifier with light source in the diagnosis of trichiasis among community-based case finders in Nigeria. Ophthalmic Epidemiol. 2018;25(Suppl 1):138–42. [DOI] [PubMed] [Google Scholar]
- 33. International Coalition for Trachoma Control . Supportive supervision for trachomatous trichiasis programmes. London: International Coalition for Trachoma Control; 2017. Available from: http://www.trachomacoalition.org/sites/default/files/content/resources/files/ICTC%20Trichiasis%20Supervision%20Manual.pdf [accessed 12 June 2018]. [Google Scholar]
- 34. Mpyet C, Muhammad N, Adamu MD et al. Prevalence of trachoma in Bauchi State, Nigeria: results of 20 local government area-level surveys. Ophthalmic Epidemiol. 2016;23(Suppl 1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mpyet C, Tagoh S, Boisson S et al. Prevalence of trachoma and access to water and sanitation in Benue State, Nigeria: results of 23 population-based prevalence surveys. Ophthalmic Epidemiol. 2018;25(Suppl 1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muhammad N, Mpyet C, Adamu MD et al. Prevalence of trachoma in the area councils of the Federal Capital Territory, Nigeria: results of six population-based surveys. Ophthalmic Epidemiol. 2018;25(Suppl 1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mpyet C, Muhammad N, Adamu MD et al. Trachoma mapping in Gombe State, Nigeria: results of 11 local government area surveys. Ophthalmic Epidemiol. 2016;23(6):406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mpyet C, Muhammad N, Adamu MD et al. Prevalence of trachoma in four local government areas of Jigawa State, Nigeria. Ophthalmic Epidemiol. 2018;25(Suppl 1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muhammad N, Mpyet C, Adamu MD et al. Mapping trachoma in Kaduna State, Nigeria: results of 23 local government area-level, population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mpyet C, Muhammad N, Adamu MD et al. Prevalence of trachoma in Kano state, Nigeria: results of 44 local government area-level surveys. Ophthalmic Epidemiol. 2017;24(3):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mpyet C, Muhammad N, Adamu MD et al. Prevalence of trachoma in Katsina state, Nigeria: results of 34 district-level surveys. Ophthalmic Epidemiol. 2016;23(Suppl 1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mpyet C, Muhammad N, Adamu MD et al. Impact survey results after SAFE strategy implementation in fifteen local government areas of Kebbi, Sokoto and Zamfara States, Nigeria. Ophthalmic Epidemiol. 2018;25(Suppl 1):103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adamu MD, Mpyet C, Muhammad N et al. Prevalence of trachoma in Niger State, North Central Nigeria: results of 25 population-based prevalence surveys carried out with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Umar MM, Mpyet C, Muhammad N et al. Prevalence of trachoma in 13 local government areas of Taraba State, Nigeria. Ophthalmic Epidemiol. 2018;25(Suppl 1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Informal consultation on a tracking system for patients with trachomatous trichiasis. Rollins School of Public Health, Emory University, Atlanta, USA, Wednesday 30 September 2015. WHO/HTM/NTD/2016.1. Geneva: World Health Organization; 2016. [Google Scholar]
- 46. Boisson S, Wohlgemuth L, Yajima A et al. Building on a decade of progress in water, sanitation and hygiene to control, eliminate and eradicate neglected tropical diseases. Trans R Soc Trop Med Hyg. 2021;115(2):185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yaya G. Lessons learned in the implementation of programmes to eliminate trachoma within conflict zones. Trans R Soc Trop Med Hyg. 2022;116(11):979–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author