Abstract
Uganda started implementing mass drug administration against schistosomiasis in 2003, with district used as an implementation unit. This resulted into misclassification of communities into wrong risk levels, under-or-over treatment and over request of praziquantel (PZQ) drugs. The objective of the current study was to reviewing the community data available at World Health Organization/ESPEN database to understand the status of schistosomiasis and identify pockets with infection. The decision tree assessment tool was used to analyzed schistosomiasis epidemiological data of 7501 communities. Before validation, the schistosomiasis endemicity status of 79 % of communities was not known. After validation, 58.6 %, 22.6 % and 16.3 % of communities were not endemic, had low and moderate endemicity status. Of 2362 communities classified having high endemicity using a district as implementation unit, 41.6 %, 12.7 % and 17.3 % of them were not endemic, had low and moderate endemicity, while only 22.7 % had high endemicity. Using the new treatment guidelines, 2,875,006 school aged children were adequately treated, 18,235 were under-treated and 2,250,013 were over treated. The results show a considerable change in endemicity status when communities were used as an implementation unit compared to district. Thus, the country control programme is recommended to use communities as implementation unit.
Keywords: Schistosomiasis, District, Communities, Implementation unit, Validation, Uganda
Graphical abstract
We present the above study that high lights Schistosomiasis as an endemic disease in Uganda and the country has a total of 7501 communities with different endemicity status (58.6 %, 22.6 % and 16.3 %-not endemic, low and moderate endemicity). The current analysis demonstrates that the use of a district as an implementation unit results into under treatment, overtreatment, and under-and-over request of praziquantel tablets. The analysis shows a considerable change in the endemicity status of communities when communities are used as an implementation unit. The findings call for the country to use communities as an implementation unit in order to reduce or eliminate the problem of under treatment, overtreatment, under and over request of praziquantel tablets.
Highlights
-
•
Schistosomiasis is endemic in Uganda and the country has a total of 7501 communities with different endemicity status (58.6 %, 22.6 % and 16.3 %-not endemic, low and moderate endemicity).
-
•
The current analysis demonstrates that the use of a district as an implementation unit results into under treatment, overtreatment, under-and-over request of praziquantel tablets.
-
•
The analysis shows a considerable change in the endemicity status of communities when communities are used as an implementation unit.
-
•
The findings call for the country to use communities as an implementation unit in order to reduce or eliminate the problem of under treatment, overtreatment, under and over request of praziquantel tablets.
1. Background/Introduction
Uganda is within the East African region and is among the countries endemic for schistosomiasis (Emmanuel et al., 2008; Booth et al., 2004; Kabatereine et al., 1996; Tukahebwa et al., 2004; McCullough, 1972; Brooker et al., 2009). Schistosomiasis infection was noted in the country since 1902 and both Schistosoma mansoni and Schistosoma haematobium are present in the country (Emmanuel et al., 2008). S. mansoni is widely distributed and occurs mostly in large water bodies (lakes and rivers) (Emmanuel et al., 2008; Booth et al., 2004; Tukahebwa et al., 2004; Kabatereine et al., 2004a), whereas S. haematobium is highly focal and occurs only in few areas, mostly restricted to Lake Kyoga (Adriko et al., 2018a; Adriko et al., 2018b). In Uganda, intestinal schistosomiasis is endemic in 67 % of the districts (95/146) whereas urogenital schistosomiasis is endemic in 2.9 % (4/146) of the total districts (Emmanuel et al., 2008; Kabatereine et al., 2004b). In total, 95 districts of 146 are endemic for schistosomiasis (Emmanuel et al., 2008; Kabatereine et al., 2004b). In 1995, all of the Uganda's 19.2 million people were estimated to be at risk of infection for schistosomiasis, with the national prevalence estimated at 31.9 % (Chitsulo et al., 2000). Todate, 55 % (19,000 people) of the 16.7million people are estimated to be at risk, with seven million people are estimated to be infected with schistosomiasis (Adriko et al., 2018c).
Uganda was one of the first African countries to launch a national-scale schistosomiasis and intestinal helminths control programme in 2003. The National Bilharzia and Worm control programme in Uganda was established in 2003 (Kabatereine et al., 2006a) and coordinated by the Vector Control Division (VCD) of the Uganda Ministry of Health, with support from the Schistosomiasis Control Initiative (SCI) (Kabatereine et al., 2006a). The programme is run vertically, implemented by districts using school teachers and volunteers known as community drug distributors (CDDs). The aim of the schistosomiasis control to date has been to control morbidity. The delivery strategy is through mass annual anthelmintic treatment targeted at school-aged children and high-risk groups in the endemic areas using praziquantel (PZQ) drug to treat schistosomiasis and albendazole drug to treat soil-transmitted helminths (STH) infection. The earlier intervention focused on school-and-community-based mass drug administration in one sub-county in each of the 18 most affected districts (Kabatereine et al., 2006a). The national Control Programme also included basic health education training and information on how behavioural changes can reduce transmission (Kabatereine et al., 2006a).
In 2004, a total of 1.4 million people were treated in 18 of then 38 endemic districts and in 2005, three million people were treated in 23 districts (Kabatereine et al., 2006a). In the same period, to scale-up treatment, health centre-based treatment was introduced in 11 of the 38 endemic districts? (Kabatereine et al., 2006a). In 2006, two million people were treated in 27 of the 28 endemic districts (Kabatereine et al., 2006a; Kabatereine et al., 2006b). Mass drug administration campaigns were scaled-up between 2010 and 2012, with the national coverage recorded at 33 %, 18 % and 19 % respectively in 2010, 2011 and 2012 (Fleming et al., 2009; Loewenberg, 2014; Lai et al., 2015)(WHO:PCT Databank). Following implementation of the initial control activities under the support of SCI from 2004 to 2008, the national prevalence dropped to 20.4 % in 2003 and to 15.9 % in 2010 (Kabatereine et al., 2006a; Kabatereine et al., 2006b). In 2012, the national prevalence dropped further to 9.1 % (Lai et al., 2015). The impact of MDA using praziquantel drug were also noted on schistosomiasis related morbidities in children and adult (WHO, 2011; Kabatereine et al., 2007). From 2014, efforts to control schistosomiasis (Fleming et al., 2009) were intensified by the government of Uganda in collaboration with partners (SCIF, 2020). Todate, the National Schistosomiasis and Worm control programme has implemented 16 rounds of treatment (VCD-MOH programme unpublished report). The national coverage of MDA increased from 22 % in school aged children (SAC) in 2014 to 62 % in 2019 (https://espen.afro.who.int/countries/uganda). In adult, the national coverage increased from 32 % in 2014 to 42 % in 2018 (https://espen.afro.who.int/countries/uganda). At the same period, the geographical coverage of implementation unit (IUs) requiring preventive chemotherapy increased from 44 % in 2014 to 80 % in 2019 (https://espen.afro.who.int/countries/uganda).
The noted increases in geographical and national coverage have resulted into decline in prevalence of schistosomiasis in some districts of Uganda. The significant decline can be noted at individual communities' level rather than at the current implementation unit, the district. Alternatively, the observed changes in prevalence means that the endemicity status of communities rather than the district have changed, therefore to ensure an efficient allocation of resources and target only the population in need of treatment, it is important for the programme to change its implementation strategy from using district level data, to use data disaggregated at community levels. This process requires a review of the communities' data and risk of transmission at community levels. WHO/AFRO has provided a tool for schistosomiasis communities data optimization. This current study focuses on validating these data from Uganda using assessment tool.
2. Methodology
2.1. Study setting
Uganda is within the East African region and is divided into four regions, Central, Eastern, Northern and Western. The country has a total of 146 districts, which are divided into counties and sub-counties, which are further subdivided into parishes/wards and villages. In total, Uganda has 7501 communities (counties or sub-counties). Schistosomiasis is endemic in 67 % (95/146) districts of Uganda. Planning and implementation of schistosomiasis control activities are organized at the district level, the district is an implementation unit (IU).
2.2. Validation of the Uganda workbook
2.2.1. Data availability and analysis
Between 2018 and 2020, all countries in the African region were supported to collect all available schistosomiasis epidemiology data, in order to enrich the schistosomiasis database for better decision making. In addition, current demographic data presented at the lowest demographic level possible was obtained from the relevant government departments. During workshops organized by ESPEN in 2018, data teams entered all their available epidemiological and demographic information into the WHO/AFRO schistosomiasis community's data optimization tool (workbook) and applied the decision tree presented in the tool to determine communities' level endemicity categories. The workbook was then presented to wider stakeholders within the NTD programme in Uganda and district level managers for validation. The final validated workbook was used to apply for medicines through the WHO donation programme in 2020.
The data preparation into the WHO/AFRO communities involved filling in the input data worksheets with the available datasets for the analysis. Four worksheets were selected: - demographic data worksheet, epidemiological data worksheet, JRSM data worksheet, and the neighbouring subunits worksheet. The Joint Request for Selected Medicine (JRSM) data worksheet included data extracted from the latest Joint Application Package (JAP) submitted by the country and is used in the decision tree to inform the current implementation strategy for the communities.
For data quality check, data were categorised into three groups (i) data which were collected after 2004 using appropriate diagnostic technique, number of examined individuals and positive cases were reported and the sample size was adequate (ii) Data were collected between 2000 and 2004 using appropriate diagnostic technique, number of individual examined and positive cases were reported and sample size was adequate and (iii) Data were collected either before 2000 or after 2000 with poor quality (diagnostic methods not reported, number of individuals examined and number of positive cases not reported). Data which fulfilled criteria described in category one and two, were considered superior, and placed in quality grade one and two respectively, for further analysis. Those in category three, were only used in the absence of quality level one or two. The unit of analysis was a community (implementation unit, in the case of Uganda, the communities/county/sub-county) and from the data, various indicators were calculated as shown in the Table 1 below.
Table 1.
The implementation units from which the various the data indicators were calculated.
| Indicators | Variables calculated | Formula/narration |
|---|---|---|
| Past prevalence | Prevalence calculated by the site data of the Past quality. District level calculation: It is the average prevalence of all site prevalence of the most recent year and the Past diagnostic methods Subdistrict level: It is the highest |
Site prevalence: the site prevalence if not already calculated is calculated as below: -Number of people positives/Number of people examined *100 District Prevalence (DP): - (Total positives) / (Total examined) * 100 |
| prevalence of all site prevalence of the most recent year and the Past diagnostic methods | Prevalence of the subdistrict (SDP): Highest site prevalence among all sites (in the highest quality group) in the communities-based on parasitological technique/results | |
| Year of Past prevalence | Year of the Past prevalence of the district or communities | |
| Diagnostic methods of the Past prevalence | Diagnostic methods of the Past prevalence of the district or communities | For calculation of prevalence, the following diagnostic tests results were used: - Kato Katz technique, Urine filtration technique, Urine sedimentation technique, blood in urine (BIU), dipstick and point-of-care circulating cathodic antigen test |
| Number of sites of the Past prevalence | Number of sites used for the calculation of the Past prevalence of the district or communities | Based on the parasitological or clinical laboratory methods |
| Endemicity by the Past prevalence | Endemicity category determined by the Past prevalence of the communities or district (The endemicity category is determined as recommended in the WHO guidelines | Non-endemic, Low prevalence (<10 %), Moderate prevalence (10 %–49 %) High prevalence (50 % and above) |
| School Aged Children (SAC) needing preventive chemotherapy (PC) | Total number of school age children living in an endemic area classified low, moderate, or high risk | Low prevalence (<10 %), Moderate prevalence (10 %–49 %) High prevalence (50 % and above) |
| Adult needing PC | Number of adults needing treatment in an endemic area classified moderate, or high risk | Low risk: no adult treatment is recommended Moderate risk: 20 % of the total adult population in the sub district or district High risk: All he adult population in the sub district or district |
| Drug estimates | The number of drugs needed is calculated by multiplying the population to be treated by a factor that depends on the age. This factor is 2.5 for school age children and 3 for adults | The dose by treatment is 2.5 tablets per SAC and 3 tablets per adult. |
| Treatment adequacy | Comparison of preventive Chemotherapy (PC) regimen at district level compared to sub district level | No change in strategy: the treatment strategy does not change from district implementation to community's implementation Adequate: No change in preventive chemotherapy (PC) regimen Under treatment: PC regimen at district level is lower than PC regimen suggested by communities' analysis. Over treatment: PC regimen at district level is higher than PC regimen suggested by communities' analysis |
For the determination of the communities’ final endemicity levels, the Decision Tree was used. Despite the use of the decision tree to categorise endemicity of the communities, the National Neglected Tropical Diseases Control Programme manager and the country NTD team were involved in validating the analysis and classification of the communities.
3. Results
3.1. Demographic characteristics
Uganda has four provinces which have a total of 146 implementation units (districts) and a total of 7501 sub-implementation units (communities). The country has a total population of 42,856,905 people, of these 29.4 % are school aged children. Table 2 shows other demographic characteristics of Uganda.
Table 2.
Total number of districts, communities, and the country population number.
| Variable | Number |
|---|---|
| Number of provinces | 4 |
| Number of IUs (districts) | 146 |
| Number of sub-IUs (communities) | 7501 |
| Total Population | 42,856,905 |
| Number of school age children | 12,579,425 |
| Number of adults | 22,192,579 |
3.2. Endemicity status of district and communities in Uganda
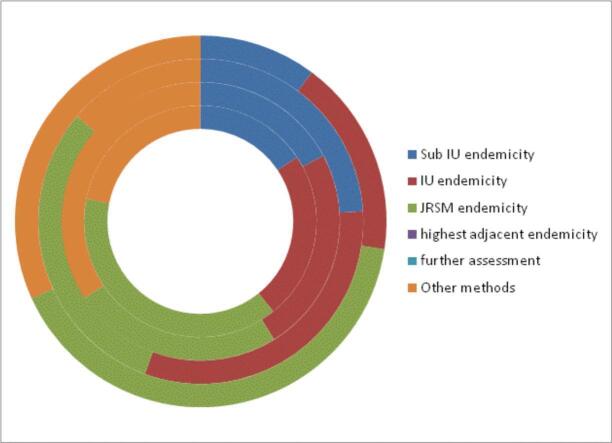
Table 3 present the results of the decision tree algorithm to assign schistosomiasis endemicity to communities', districts implementation unit, using the drug request documents and the WHO/AFRO risk assessment tool which combines information from qualitative environmental risk assessment, local knowledge by health professionals, reported clinical cases, laboratory data at local health facilities, existence of snail hosts and GIS risk maps. Of the total 7501 wards, 16.5 %, 23.7 % and 33.2 % were classified using the sub-implementation unit, district as implementation unit and JRSM documents. The remaining 26.6 %, were categorise using the WHO/AFRO risk assessment tool.
Table 3.
Categorization of wards using the decision tree algorithm for Uganda.
| Total number of wards | 1. Use sub-IU endemicity | 2. Use IU endemicity | 3. Use JRSM endemicity | 4. Use highest adjacent endemicity | 5. Need further assessment | 6. Other methods |
|---|---|---|---|---|---|---|
| 7501(100 %) | 1239 (16.5 %) | 1780(23.7 %) | 2487(33.2 %) | 0 | 0 | 1995(26.6 %) |
The implementation units were further classified based on the regions using the similar approach described above (Table 3). Using the sub-implementation unit, the Eastern (17.2 %) and the Northern (24 %) had the highest number of sub-implementation unit. However, based on JRSM, the Northern (30.6 %), Central (39 %) and Western (40.8 %) regions had the highest number of implementation units requiring drug (Table 4).
Table 4.
Categorization of implementation units by regions in Uganda using the decision tree algorithm for Uganda.
| Region | 1. Use sub-IU endemicity | 2. Use IU endemicity | 3. Use JRSM endemicity | 4. Use highest adjacent endemicity | 5. Need further assessment | 6. Other methods | Total |
|---|---|---|---|---|---|---|---|
| Central | 240 (15.7) | 360 (23.5) | 597 (39.0) | 0 | 0 | 335 (21.9) | 1532 (100) |
| Eastern | 400 (17.2) | 556 (23.9) | 572 (24.6) | 0 | 0 | 799 (34.3) | 2327 (100) |
| Northern | 396 (24.0) | 522 (31.6) | 505 (30.6) | 0 | 0 | 228 (13.8) | 1651 (100) |
| Western | 203 (10.2) | 342 (17.2) | 813 (40.8) | 0 | 0 | 633 (31.8) | 1991 (100) |
| Total | 1239 (16.5) | 1780 (23.7) | 2487 (33.2) | 0 | 0 | 1995 (26.6) | 7501 (100) |
Lastly, the sub-implementation units (communities') were classified based on the WHO endemicity criteria. Overall, 9.4 % and 7.3 % of the sub-IUs had moderate and high endemicity levels (Table 5). Majority of the sub-IUs with moderate and high endemicity levels were located in the Northern and Central regions.
Table 5.
Endemicity status of communities in Uganda.
| Regions |
Endemicity status of sub-implementation units |
||||
|---|---|---|---|---|---|
| Not endemic | Low | Moderate | High | Total | |
| Central | 979 (64.7) | 244 (16.1) | 127 (8.4) | 162 (10.7) | 1512 (100) |
| Eastern | 1151 (50.6) | 846 (37.2) | 134 (5.9) | 145 (6.4) | 2276 (100) |
| Northern | 594 (38.8) | 469 (30.6) | 335 (21.9) | 134 (8.7) | 1532 (100) |
| Western | 1671 (84.0) | 135 (6.8) | 89 (4.5) | 95 (4.8) | 1990 (100) |
| Total | 4395 (60.1) | 1694 (23.2) | 685 (9.4) | 536 (7.3) | 7310 (100) |
Key: non-endemic (0 %), low (prevalence <10 %), moderate (10 %–49 %) and high (50 % and above).
3.3. Changes in the endemicity status of sub-implementation units compared to district level implementation unit
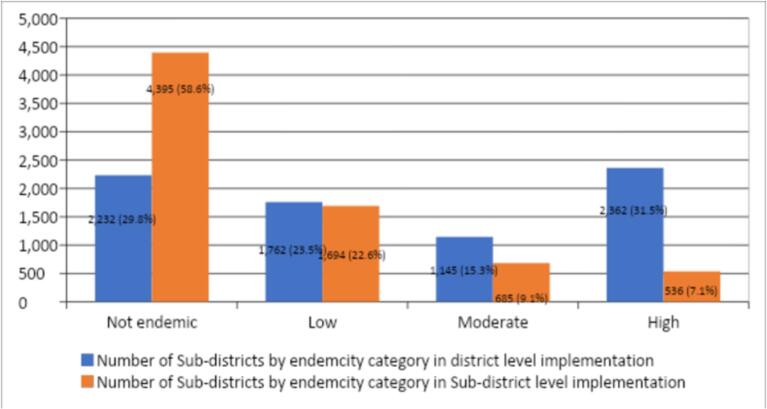
Fig. 1: shows changes in the schistosomiasis endemicity status when sub-IUs (communities') are used as the implementation unit compared to the district. Similar information is presented in Table 6. When categorization of endemicity status was done at district as IUs, 1762 sub-IUs were classified as low endemic areas, however using the sub-IUs (communities'), 46.7 % of these were classified as non-endemic (Table 6). Similarly, using the district as IUs, of 1145 district IUs categorise to have moderate endemicity, 31.7 %, 41.2 % and 23.9 % were categized as non-endemic, low and moderate endemic areas using the communities (Table 6a, Table 6b). On the other hand, using the district as an implementation unit, a total of 2362 communities were categorised to have high endemicity. However, when categorization was done using the sub-IUs (communities'), 41.6 %, 12.7 %, 16.7 % and 22.7 % were categorised into non-endemic, low, moderate and heavy endemic (Table 6a, Table 6b).
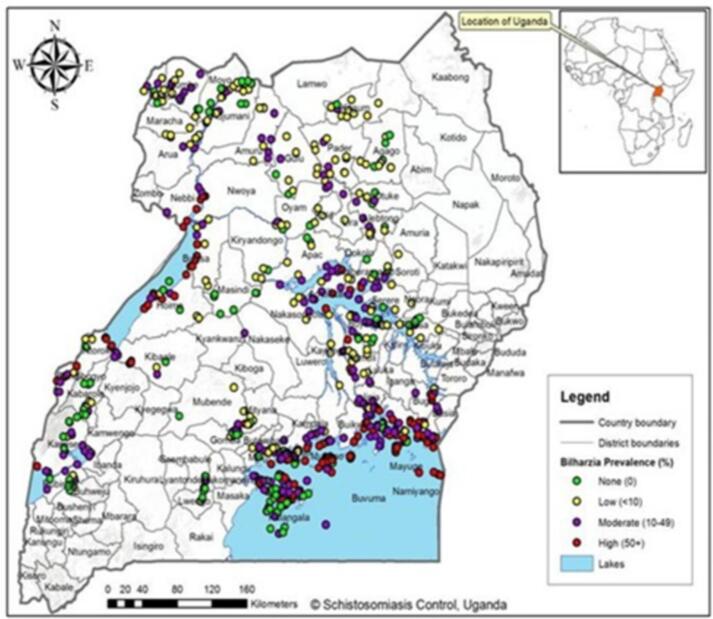
Fig. 1.
Geographical Current distribution Schistosomiasis in Uganda.
Table 6a.
Changes in endemicity category of communities compared to district level implementation.
| Endemicity category by district level implementation | Endemicity category by community's level implementation | ||||
|---|---|---|---|---|---|
| Categories | Popn | Not endemic | Low | Moderate | High |
| Not endemic | 2232 | 2226 | 3 | 3 | 0 |
| Low | 1762 | 823 | 919 | 0 | 0 |
| Moderate | 1145 | 363 | 472 | 274 | 0 |
| High | 2362 | 983 | 300 | 408 | 536 |
| Total | 7501 | 4395 | 1694 | 685 | 536 |
Table 6b.
Projected changes in endemicity category within district level when the classification is by district the implementation unit in Uganda.
| Endemicity category by community's level implementation | Number | Percentage (%) |
|---|---|---|
| Classified as Not endemic at District level implementation (N = 2232) | ||
| Not endemic | 2226 | 99.7 |
| Low | 3 | 0.1 |
| Moderate | 3 | 0.1 |
| High | 0 | 0.0 |
| Classified as Low at District level implementation (N = 1762) | ||
| Not endemic | 823 | 46.7 |
| Low | 919 | 52.2 |
| Moderate | 0 | 0.0 |
| High | 0 | 0.0 |
| Classified as Moderate at District level implementation (N = 1145) | ||
| Not endemic | 363 | 31.7 |
| Low | 472 | 41.2 |
| Moderate | 274 | 23.9 |
| High | 0 | 0.0 |
| Classified as High at District level implementation (N = 2362) | ||
| Not endemic | 983 | 41.6 |
| Low | 300 | 12.7 |
| Moderate | 408 | 17.3 |
| High | 536 | 22.7 |
3.4. Projection of school-aged children and adult living in communities' and their level of risk
Overall, a total of 5,143,254 school aged children were estimated to be living in areas with different endemicity level of schistosomiasis (Table 7). Of these, 27 % and 20 % live in communities with moderate and high endemicity for schistosomiasis. Majority of the adult population live in areas with high endemicity for schistosomiasis (Fig. 2).
Table 7.
Number of sub-IUs by endemicity categories and related populations in Uganda.
| Endemicity categories of communities' | Number of communities' | Number of school age children | Number of adults |
|---|---|---|---|
| Not endemic | 4395 (58.6) | 0 | 0 |
| Low | 1694 (22.6) | 2,718,794 (52.9) | 0 |
| Moderate | 685 (9.1) | 1,388,635 (27.0) | 457,911 (20.8) |
| High | 536 (7.1) | 1,035,825 (20.1) | 1,742,190 (79.2) |
| Total | 7501 (100) | 5,143,254 (100) | 2,200,101 (100) |
Fig. 2.
Comparison of number of communities' and their endemicity categorised using district as an implementation unit and communities has an implementation unit.
3.5. Comparison of the population requiring medicine by district as an implementation unit versus communities as an implementation unit
In general, the results indicate a significant variation in the communities' requiring treatment is the district is used as an implementation unit (Table 8). There was a discrepancy of 44.7 % of the number of communities' requiring treatment when the district is used as an implementation unit. Similarly, there was a discrepancy of 35.1 % of the total number of school children requiring treatment when the district is used as an implementation unit. Importantly, there was a variation of 35.1 % with a total of 2,315,950 extra praziquantel tablets requested when the district was used as an implementation unit.
Table 8.
Comparison of target population and medicines by district implementation versus community's implementation.
| Variable | District level implementation | Communities level implementation | Variation |
|---|---|---|---|
| Number of Communities | 5269 | 2915 | −2354 (−44.7 %) |
| School age children requiring treatment | 3,562,789 | 2,636,409 | −926,380 (−35.1 %) |
| PZQ Estimates | 8,906,972 | 6,591,022 | −2,315,950 (−35.1 %) |
3.6. Communities with under treatment and over treatment when using the district is used as an implementation unit
Table 9 present the findings on the number of communities' which had either under treatment or over treatment when the district was used as an implementation unit. All the 823 communities' which were not required to received treatment received treatment rounds. These communities were classified as low endemic areas using the district as an implementation unit. However, using the communities as an implementation unit, all these communities were categorised as non-endemic. Similarly, of the 1691 areas categorised to have moderate endemicity using the district as an implementation unit, received treatment. When using communities has an implementation unit, 58.1 % (983 sub-IUs) and 17.7 % (300 sub-IUs) were categorised as non-endemic and with low endemicity (Table 9). Only 12.2 % (408) of these communities qualified for treatment (Table 9). Again, 50 % of the communities were under treated when the district was used as an implementation unit.
Table 9.
Number of communities' with under treated and over treated in district level implementation by endemicity category.
| Preventive Chemotherapy strategy adequacy | District level implementation | Communities level implementation |
||||
|---|---|---|---|---|---|---|
| Communities endemicity category | ||||||
| Not endemic | Low | Moderate | High | |||
| Adequate Treatment | Not endemic | 2226 (100) | 2226 (100) | 0 | 0 | 0 |
| Low | 919 (100) | 0 | 919 (100) | 0 | 0 | |
| Moderate | 274 (100) | 0 | 0 | 274(100) | 0 | |
| High | 536 (100) | 0 | 0 | 0 | 536(100) | |
| Total | 3955(100) | 2226(56.3) | 919 (23.2) | 274(6.9) | 536(13.6) | |
| Under Treatment | Not endemic | 6 (100) | 0 | 3 (50.0) | 3 (50.0) | 0 |
| Low | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | |
| High | 0 | 0 | 0 | 0 | 0 | |
| Total | 6(100) | 0 | 3 (50.0) | 3 (50.0) | 0 | |
| Over Treatment | Not endemic | 0 | 0 | 0 | 0 | 0 |
| Low | 823(100) | 823(100) | 0 | 0 | 0 | |
| Moderate | 835(100) | 363(43.5) | 472(56.5) | 0 | 0 | |
| High | 1691(100) | 983(58.1) | 300(17.7) | 408 (24.1) | 0 | |
| Total | 3349(100) | 2169(64.8) | 772(23.1) | 408 (12.2) | 0 | |
| Grand total | 7310(100) | 4395(60.1) | 1694(23.2) | 685(9.4) | 536 (7.3) | |
3.7. Treatment adequacy for targeted population
Overtreatment was noted in a total of 867,000 individuals who were classified living in moderate endemicity using the district as an implementation unit (Table 10). Using the communities as IUs, all these individuals were classified in low endemicity requiring no treatment. Similarly, of 1,383,013 individuals targeted for treatment using the district as an IUs, 42.8 % (591,928) of them were categorised into low endemicity, when the communities were used as IUs and did not require treatment. On the other hand, of 18,235 individuals classified to be living in non-endemic areas using the district as IUs, 72 % of them were undertreated/missed treatment when classification was done using communities as IUs (Table 10). These individuals were classified to be living in moderate endemicity areas.
Table 10.
Treatment adequacy for target populations by endemicity categories.
| PC strategy adequacy |
District level implementation |
Sub-IU level implementation |
||||
|---|---|---|---|---|---|---|
| Sub-IU Endemicity Category | ||||||
| Not endemic | Low | Moderate | High | |||
| Adequate Treatment | Not endemic | 0 | 0 | 0 | 0 | 0 |
| Low | 1,254,803 (100) | 0 | 1,254,803 (100) | 0 | 0 | |
| Moderate | 584,378 (100) | 0 | 0 | 584,378 (100) | 0 | |
| High | 1,035,825 (100) | 0 | 0 | 0 | 1,035,825 (100) | |
| Total | 2,875,006 (100) | 0 | 1,254,803 (43.6) | 584,378 (20.3) | 1,035,825 (36.0) | |
| Under Treatment | Not endemic | 18,235 (100) | 0 | 5063 (27.8) | 13,172 (72.2) | 0 |
| Low | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | |
| High | 0 | 0 | 0 | 0 | 0 | |
| Total | 18,235 (100) | 0 | 5063 (27.8) | 13,172 (72.2) | 0 | |
| Over Treatment | Not endemic | 0 | 0 | 0 | 0 | 0 |
| Low | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 867,000 (100) | 0 | 867,000 (100) | 0 | 0 | |
| High | 1,383,013 (100) | 0 | 591,928 (42.8) | 791,085 (57.2) | 0 | |
| Total | 2,250,013 (100) | 0 | 1,458,928 (64.8) | 791,085 (35.2) | 0 | |
| Grand total | 5,143,254 (100) | 0 | 2,718,794 (52.9) | 1,388,635 (27.0) | 1,035,825(20.1) | |
Table 11 summarizes the number of school aged children adequately treated and missed treatment in all the sub-IUs in Uganda when the district was used as an IUs compared to the use of communities as IUs. Overall, 18,235 and 2,250,013 school aged children missed treatment and were unnecessary treated. There was also an under-estimation of treatment and over estimation of targeted population as shown in the Table 11. These data are categorised by regions of Uganda in Table 12. The Eastern (38.5 %) and Northern (32.6 %) region had the highest number of the SAC who were adequately treated where the Central region had the highest number of SAC who were undertreated (Table 12). The highest number of over treatments of SAC were observed in Eastern (38.2 %) and Northern (30.9 %) regions. These regions had also the highest percentage of excess PZQ tablets requested at 27.6 % and 34.5 % (Table 12).
Table 11.
Treatment adequacy of Sub-IUs in previous IU level implementation Vs Sub-IU level.
| Number of sub-IUs | SAC adequately treated | SAC missing treatment | SAC unnecessary treated | Under estimations gaps | Over estimations excess |
|---|---|---|---|---|---|
| 7501 | 2,875,006 | 18,235 | 2,250,013 | 20,642 | 2,348,805 |
Table 12.
Treatment adequacy of Sub-IUs in previous IU level implementation Vs Sub-IU level by province.
| Region | Number of Ward | SAC adequately treated | SAC under treated | SAC over treated | Under treatment gaps in PZQ | Over treatment excess in PZQ |
|---|---|---|---|---|---|---|
| Central | 1532 | 518,739 | 18,235 | 431,778 | 20,642 | 545,731 |
| Eastern | 2327 | 1,108,186 | 0 | 861,053 | 0 | 647,786 |
| Northern | 1651 | 938,014 | 0 | 694,752 | 0 | 810,987 |
| Western | 1991 | 310,067 | 0 | 262,430 | 0 | 344,301 |
| Total | 7501 | 2,875,006 | 18,235 | 2,250,013 | 20,642 | 2,348,805 |
3.8. Before and after validation of communities' and population requiring treatment
Table 13 summarizes the total number of communities of Uganda and their schistosomiasis endemicity status before validation. Of the total 7501 communities, 404 (5.4 %), 729 (9.7 %) and 444(5.9 %) were categorised as not endemic, low, and moderate endemicity. The endemicity status of 5924 communities was not known. Before validation, a total of 2,537,230 (SAC = 944,184 and adult = 1,593,046) required treatment. After validation, 4395 (58.6 %), 1694 (22.6 %), 1221 (16.3 %) were not endemic, low, and moderate endemicity (Table 14). Overall, a total of 2,424,460 and 4,031,741 SAC and adult required treatment.
Table 13.
Number of sub-units by PC regimen and targeted populations before validation.
| Region | Total No. of communities | Total population | Not endemic |
<10 % (No PC) |
≥ 10 % (1 round/year) |
≥ 10 % (2 rounds/year) |
Unknown |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of communities' | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | |||
| Central | 1532(100) | 457,912(100) | 93 (6.1) | 0 | 0 | 16,410.7) | 0 | 0 | 68 (4.4) | 147,346 (32.2) | 310,566 (67.8) | 0 | 0 | 0 | 1207(78.8) | 0 | 0 |
| Eastern | 2327(100) | 1,061,412(100) | 75 (3.2) | 0 | 0 | 265(11.4) | 0 | 0 | 170 (7.3) | 413,932 (39.0) | 647,480 (61.0) | 0 | 0 | 0 | 1817(78.1) | 0 | 0 |
| Northern | 1651(100) | 779,194(100) | 96 (5.8) | 0 | 0 | 217(13.1) | 0 | 0 | 155(9.4) | 300,741 (38.6) | 478,453 (61.4) | 0 | 0 | 0 | 1183(71.7) | 0 | 0 |
| Western | 1991(100) | 238,712(100) | 140 (7.0) | 0 | 0 | 83(4.2) | 0 | 0 | 51(2.6) | 82,165 (34.4) | 156,547 (65.6) | 0 | 0 | 0 | 1717(86.2) | 0 | 0 |
| Total | 7501(100) | 2,537,230(100) | 404 (5.4) | 0 | 0 | 729(9.7) | 0 | 0 | 444(5.9) | 944,184 (37.2) | 1,593,046 (62.8) | 0 | 0 | 0 | 5924 (79.0) | 0 | 0 |
Table 14.
Number of communities by PC regimen and targeted populations after validation.
| Region | Total No. of communities | Total population | Not endemic |
<10 % (No PC) |
≥ 10 % (1 round/year) |
≥ 10 % (2 rounds/year) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of communities | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | No. of communities | No. of SAC | No. of adults | |||
| Central | 1532 (100) | 1,581,209 (100) | 979 (63.9) | 0 | 0 | 244 (15.9) | 0 | 0 | 289 (18.9) | 534,346 (33.8) | 1,046,863 (66.2) | 0 | 0 | 0 |
| Eastern | 2327 (100) | 1694,186 (100) | 1151 (49.5) | 0 | 0 | 846 (36.4) | 0 | 0 | 279 (12.0) | 667,989 (39.4) | 1,026,197 (60.6) | 0 | 0 | 0 |
| Northern | 1651 (100) | 2,248,158 (100) | 594 (36.0) | 0 | 0 | 469 (28.4) | 0 | 0 | 469 (28.4) | 888,196 (39.5) | 1,359,962 (60.5) | 0 | 0 | 0 |
| Western | 1991 (100) | 932,648 (100) | 1671 (83.9) | 0 | 0 | 135 (6.8) | 0 | 0 | 184 (9.2) | 333,929 (35.8) | 598,719 (64.2) | 0 | 0 | 0 |
| Total | 7501 (100) | 6,456,201 (100) | 4395 (58.6) | 0 | 0 | 1694 (22.6) | 0 | 0 | 1221 (16.3) | 2,424,460 (37.6) | 4,031,741 (62.4) | 0 | 0 | 0 |
4. Discussion
Review and validation of the Uganda workbook and other documents available at WHO/ESPEN has given an opportunity to understand the changes which have occurred in the country after over 15 years of MDA and the importance of using communities versus districts has an implementation unit for planning and executing MDA. The findings indicate that the use of a district as an UIs resulted into a misclassification of the infection risk levels of communities/wards which translated into underestimation or overestimation of the number of people requiring preventive chemotherapy, the number of PZQ tablets requested for the MDA campaigns and in-country budgeting for the MDA exercise. For instance, before validation, Uganda had a total of 7501 communities but schistosomiasis endemicity status was known for only 21 % (5.4 %-not endemic, 9.7 %-low endemicity and 5.9 %-moderate endemicity) of the communities. After validation, the endemicity status of 97.5 % of the communities was known, with 58.6 % (4395) were noted to not endemic, 22.6 % (1694) had low endemicity and 16.3 % (1221) had moderate endemicity. Similar picture was noted in the population required preventive chemotherapy, before validation, a total of 2,537,230 people (944,184 school aged children and 1,593,046 adult) were indicated to require preventive chemotherapy. After validation, 6,456,201 people (2,424,460 school aged children and 4,031,741 adults) required preventive chemotherapy. The gaps observed in the risk level of communities and the number of people before and after validation of the Uganda workbook shows (i) the importance of periodic review of the in-country data after repeated rounds of MDA (ii) the importance of using communities as an IUs rather the district as an IUs which has a number of limitations and (iii) the importance of having a living workbook for schistosomiasis endemic countries.
Uganda is one of the first African countries to launch a national-scale schistosomiasis control in 2003 (Fenwick et al., 2009). Mass drug administration has been a key intervention against schistosomiasis in Uganda and repeated rounds of MDA have resulted in changes in the prevalence and intensities of schistosomiasis, which translate to changes in the schistosomiasis endemicity status of the district as an IUs. It is clear that the changes in endemicity status can vary within a single district or community. Thus, using the district as IUs to define the endemicity status of communities can lead to misclassification of wards/counts. A noted change in the endemicity status of communities was observed when the communities were used as IUs. Over 50 % of the communities was noted to be not endemic for schistosomiasis and the country had no any communities which had high endemicity. In addition, considering the recent release of treatment guidelines by WHO (WHO, 2022), the country will have an additional of 22.6 % (1694 with prevalence <10 %) communities which will not require treatment rounds and only 16.3 % (1221) requiring only one round of treatment. These results will have a significant impact on the country’s request for PZQ tablets to reduce drug wastage, reduce the in-country budget for MDA implementation and monitoring and finally, will allow the national control programme to implement a highly focused MDA targeting only communities remaining with pockets of infection (Tchuenté et al., 2017).
Among the disadvantages of using the district as an IUs is undertreatment and overtreatment which is simply translated into wastage of medicine. The current analysis and the consideration of the new treatment guidelines (WHO, 2022) will help Uganda overcome the problem of undertreatment and overtreatment. This will allow the country to plan for the delivery of a highly focused treatment plan by delivering drugs to the most needed areas. In addition, the country will have space to deliver drug to the most at-risk population of adult and pre-school aged children. The analysis has further shown that, a total of 6,546,201 people (2,424,460 school aged children and 4,031,741 adult) require preventive chemotherapy and are living in areas categorised by the new treatment guidelines requiring at least one round of treatment. It is worthwhile to note that the discrepancy observed between the two-implementation unit, the district, and the sub-district, arises from comparing the number of those at risk when the prevalence threshold was aggregated at a higher level, the district IU versus the population estimated to be at risk when the prevalence was disaggregated to smaller sub-units, the sub-district IU. The analysis identified the gaps which were previously not addressed. However, the difference in the number of people requiring the treatment from the two IU, does not imply an increased need for resources because the target districts remain the same, but it does indeed indicate a higher need for donation of PZQ drug. Furthermore, in this analysis is important to note that the population requiring treatment increased at sub-district analysis due to the more granular analysis, again this does not imply a higher logistical demand.
For the 4,031,741 adults requiring treatment, the analysis was based on the WHO guidelines that recommend treating all at risk persons in areas where the prevalence of schistosomiasis exceeds 10 %. While no communities were classified as having “high endemicity,” in Uganda, the large number of adults requiring preventive chemotherapy reflected the inclusion of moderately endemic areas where adults still need treatment, as per WHO's threshold for mass drug administration. The data showing over 50 % of communities as non-endemic is accurate after district-level disaggregation, but the large adult population needing treatment comes from those moderate-endemicity areas. On the other hand, the increased the number of people requiring preventive chemotherapy, especially adults and the reduced number of communities requiring treatment, the country can be in track to achieve the required minimum treatment coverage of 75 % as recommended by the WHO (WHO, 2002). The impact of repeated MDA has been demonstrated in Africa (Kokaliaris et al., 2022), with a reduction of prevalence of 60 %, gives a clear way forward that the continent is on the right track to achieve the 2030 vision (WHO, 2020). These results call for the inclusion of other supplementary measures such as provision of clean water, improved sanitation, and hygiene.
5. Limitation
Even though the assessment tool has provided useful information about the need for changing of implementation units, from district to communities and the milestone the country has reached in fighting against schistosomiasis, there are limitations which are worthwhile to mention. Parasitological data used in the analysis tool were based on a single stool and urine samples, which can lead to under estimation of the true prevalence and intensity of infection (Berhe et al., 2007). The use of the prevalence data of adjacent geographical area to estimate the prevalence of the nearby geographical area may have led to either underestimation or overestimation of the true prevalence. Thus, verification of each of the endemic communities is highly recommended.
6. Conclusion
The assessment tools have clearly demonstrated that, Uganda has reduced the number of communities requiring preventive chemotherapy and the tools have further demonstrated the gaps when using the district as implementation unit versus using the community as implementation unit. There are more advantages of using the communities in planning and implementation of mass drug administration which will reduce wastage of drugs, financial resources and allow the control programme to implement a focused mass drug administration targeting the most in need communities in order to achieve the elimination goals.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of generative AI and Ai-Assisted Technologies
The author declare that there was no use of generative AI and AI-assisted technologies during the writing process and submission of the manuscript.
Ethical standards
Data used in the current study were collected by the National Bilharzia and Worm control programme of Uganda and shared with the World Health Organization. The Ministry of Health of Uganda signed consent with WHO/AFRO to allow for sharing of PC-NTD programme data on the ESPEN portal https://espen.who.int.
CRediT authorship contribution statement
Moses Adriko: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Benjamin Tinkitina: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Moses Arinaitwe: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Edridah M. Tukahebwa: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Alfred Mubangizi: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jorge Cano Ortega: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Honorat Zoure: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Pauline N. Mwinzi: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Boniface Kinvi: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Amadou Garba Djirmay: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sammy Njenga: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Humphrey D. Mazigo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that there are no conflicts of interest in the authorship of this publication.
Acknowledgements
The authors are thankful to the Uganda's Vector Borne and NTD Control Division and WHO-ESPEN for the provision of data for this manuscript. We also acknowledge the contribution of all authors during the review of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parepi.2024.e00394.
Contributor Information
Moses Adriko, Email: adrikomoses@gmail.com.
Humphrey D. Mazigo, Email: humphreymazigo@gmail.com.
Appendix A. Supplementary data
Supplementary material
References
- Adriko M., et al. The epidemiology of schistosomiasis in Lango region Uganda 60 years after Schwetz 1951: can schistosomiasis be eliminated through mass drug administration without other supportive control measures? Acta Trop. 2018;185:412–418. doi: 10.1016/j.actatropica.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Adriko M., et al. Data on the pre-MDA and post MDA interventions for Schistosoma mansoni and Schistosoma haematobium in a co-endemic focus in Uganda: 1951–2011. Data Brief. 2018;20:991–998. doi: 10.1016/j.dib.2018.08.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriko M., et al. Low praziquantel treatment coverage for Schistosoma mansoni in Mayuge District, Uganda, due to the absence of treatment opportunities, rather than systematic non-compliance. Trop. Med. Infect. Dis. 2018;3(4):111. doi: 10.3390/tropicalmed3040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe N., Myrvang B., Gundersen S.G. Intensity of Schistosoma mansoni, hepatitis B, age, and sex predict levels of hepatic periportal thickening/fibrosis (PPT/F): a large-scale community-based study in Ethiopia. Am. J. Trop. Med. Hyg. 2007;77(6):1079–1086. [PubMed] [Google Scholar]
- Booth M., et al. Hepatosplenic morbidity in two neighbouring communities in Uganda with high levels of Schistosoma mansoni infection but very different durations of residence. Trans. R. Soc. Trop. Med. Hyg. 2004;98(2):125–136. doi: 10.1016/s0035-9203(03)00018-x. [DOI] [PubMed] [Google Scholar]
- Brooker S., et al. An updated atlas of human helminth infections: the example of East Africa. Int. J. Health Geogr. 2009;8(1):1–11. doi: 10.1186/1476-072X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsulo L., et al. The global status of schistosomiasis and its control. Acta Trop. 2000;77(1):41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel I., Aginya O., Doehring E. Epidemiology, of bilharzias (schistosomiasis) in Uganda from 1902 until 2005. Afr. Health Sci. 2008;8(4):239–243. [PMC free article] [PubMed] [Google Scholar]
- Fenwick A., et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136(13):1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- Fleming F., et al. Process evaluation of schistosomiasis control in Uganda, 2003 to 2006: perceptions, attitudes and constraints of a national programme. Parasitology. 2009;136(13):1759–1769. doi: 10.1017/S0031182009990709. [DOI] [PubMed] [Google Scholar]
- Kabatereine N., Odongo-Aginya E., Lakwo T. Schistosoma mansoni along Lake Albert, Kibale District, Western Uganda. East African medical journal. 1996;73(8):502–504. [PubMed] [Google Scholar]
- Kabatereine N.B., et al. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Trop. Med. Int. Health. 2004;9(3):372–380. doi: 10.1046/j.1365-3156.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Kabatereine N., et al. Epidemiology and morbidity of Schistosoma mansoni infection in a fishing community along Lake Albert in Uganda. Trans. R. Soc. Trop. Med. Hyg. 2004;98(12):711–718. doi: 10.1016/j.trstmh.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kabatereine N.B., et al. The control of schistosomiasis and soil-transmitted helminths in East Africa. Trends Parasitol. 2006;22(7):332–339. doi: 10.1016/j.pt.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kabatereine N.B., et al. Progress towards countrywide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Trans. R. Soc. Trop. Med. Hyg. 2006;100(3):208–215. doi: 10.1016/j.trstmh.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kabatereine N.B., et al. Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bull. World Health Organ. 2007;85(2):91–99. doi: 10.2471/BLT.06.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaliaris C., et al. Effect of preventive chemotherapy with praziquantel on schistosomiasis among school-aged children in sub-Saharan Africa: a spatiotemporal modelling study. Lancet Infect. Dis. 2022;22(1):136–149. doi: 10.1016/S1473-3099(21)00090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.-S., et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect. Dis. 2015;15(8):927–940. doi: 10.1016/S1473-3099(15)00066-3. [DOI] [PubMed] [Google Scholar]
- Loewenberg S. Uganda’s struggle with schistosomiasis. Lancet. 2014;383(9930):1707–1708. doi: 10.1016/s0140-6736(14)60817-5. [DOI] [PubMed] [Google Scholar]
- McCullough F. The distribution of Schistosoma mansoni and S. haematobium in East Africa. Trop. Geogr. Med. 1972;24(3):199–207. [PubMed] [Google Scholar]
- SCIF Uganda Resumes Mass Treatment Against Parasitic Worms. 2020. https://schistosomiasiscontrolinitiativeorg/news/2020/7/22/uganda-resumes-mass-treatment-against-parasitic-worms
- Tchuenté L.-A.T., et al. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect. Dis. Poverty. 2017;6(01):12–25. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukahebwa E.M., et al. 2004. Epidemiology and Geography of Schistosoma mansoni in Uganda: Implications for Planning Control. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization Technical Report Series. Vol. 912. 2002. World Health Organization Expert Committee Report on Prevention and control of schistosomiasis and soil-transmitted helminthiasis; p. i. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2011. Report of an INFORMAL CONSULTATION on Schistosomiasis Control World Health Organization,Geneva,30 March-1 April, 2011. [Google Scholar]
- WHO . 2020. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. [Google Scholar]
- WHO . World Health Organization; 2022. WHO guideline on control and elimination of human schistosomiasis. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Between 2018 and 2020, all countries in the African region were supported to collect all available schistosomiasis epidemiology data, in order to enrich the schistosomiasis database for better decision making. In addition, current demographic data presented at the lowest demographic level possible was obtained from the relevant government departments. During workshops organized by ESPEN in 2018, data teams entered all their available epidemiological and demographic information into the WHO/AFRO schistosomiasis community's data optimization tool (workbook) and applied the decision tree presented in the tool to determine communities' level endemicity categories. The workbook was then presented to wider stakeholders within the NTD programme in Uganda and district level managers for validation. The final validated workbook was used to apply for medicines through the WHO donation programme in 2020.
The data preparation into the WHO/AFRO communities involved filling in the input data worksheets with the available datasets for the analysis. Four worksheets were selected: - demographic data worksheet, epidemiological data worksheet, JRSM data worksheet, and the neighbouring subunits worksheet. The Joint Request for Selected Medicine (JRSM) data worksheet included data extracted from the latest Joint Application Package (JAP) submitted by the country and is used in the decision tree to inform the current implementation strategy for the communities.
For data quality check, data were categorised into three groups (i) data which were collected after 2004 using appropriate diagnostic technique, number of examined individuals and positive cases were reported and the sample size was adequate (ii) Data were collected between 2000 and 2004 using appropriate diagnostic technique, number of individual examined and positive cases were reported and sample size was adequate and (iii) Data were collected either before 2000 or after 2000 with poor quality (diagnostic methods not reported, number of individuals examined and number of positive cases not reported). Data which fulfilled criteria described in category one and two, were considered superior, and placed in quality grade one and two respectively, for further analysis. Those in category three, were only used in the absence of quality level one or two. The unit of analysis was a community (implementation unit, in the case of Uganda, the communities/county/sub-county) and from the data, various indicators were calculated as shown in the Table 1 below.
Table 1.
The implementation units from which the various the data indicators were calculated.
| Indicators | Variables calculated | Formula/narration |
|---|---|---|
| Past prevalence | Prevalence calculated by the site data of the Past quality. District level calculation: It is the average prevalence of all site prevalence of the most recent year and the Past diagnostic methods Subdistrict level: It is the highest |
Site prevalence: the site prevalence if not already calculated is calculated as below: -Number of people positives/Number of people examined *100 District Prevalence (DP): - (Total positives) / (Total examined) * 100 |
| prevalence of all site prevalence of the most recent year and the Past diagnostic methods | Prevalence of the subdistrict (SDP): Highest site prevalence among all sites (in the highest quality group) in the communities-based on parasitological technique/results | |
| Year of Past prevalence | Year of the Past prevalence of the district or communities | |
| Diagnostic methods of the Past prevalence | Diagnostic methods of the Past prevalence of the district or communities | For calculation of prevalence, the following diagnostic tests results were used: - Kato Katz technique, Urine filtration technique, Urine sedimentation technique, blood in urine (BIU), dipstick and point-of-care circulating cathodic antigen test |
| Number of sites of the Past prevalence | Number of sites used for the calculation of the Past prevalence of the district or communities | Based on the parasitological or clinical laboratory methods |
| Endemicity by the Past prevalence | Endemicity category determined by the Past prevalence of the communities or district (The endemicity category is determined as recommended in the WHO guidelines | Non-endemic, Low prevalence (<10 %), Moderate prevalence (10 %–49 %) High prevalence (50 % and above) |
| School Aged Children (SAC) needing preventive chemotherapy (PC) | Total number of school age children living in an endemic area classified low, moderate, or high risk | Low prevalence (<10 %), Moderate prevalence (10 %–49 %) High prevalence (50 % and above) |
| Adult needing PC | Number of adults needing treatment in an endemic area classified moderate, or high risk | Low risk: no adult treatment is recommended Moderate risk: 20 % of the total adult population in the sub district or district High risk: All he adult population in the sub district or district |
| Drug estimates | The number of drugs needed is calculated by multiplying the population to be treated by a factor that depends on the age. This factor is 2.5 for school age children and 3 for adults | The dose by treatment is 2.5 tablets per SAC and 3 tablets per adult. |
| Treatment adequacy | Comparison of preventive Chemotherapy (PC) regimen at district level compared to sub district level | No change in strategy: the treatment strategy does not change from district implementation to community's implementation Adequate: No change in preventive chemotherapy (PC) regimen Under treatment: PC regimen at district level is lower than PC regimen suggested by communities' analysis. Over treatment: PC regimen at district level is higher than PC regimen suggested by communities' analysis |
For the determination of the communities’ final endemicity levels, the Decision Tree was used. Despite the use of the decision tree to categorise endemicity of the communities, the National Neglected Tropical Diseases Control Programme manager and the country NTD team were involved in validating the analysis and classification of the communities.