Abstract
A potential non-precious metal catalyst for oxygen reduction reaction should contain metal-N4 moieties. However, most of the current strategies to regulate the distances between neighboring metal sites are not pre-designed but depend on the probability by tuning the metal loading or the support. Herein, we report a general method for the synthesis of neighboring metal-Nx moieties (metal = Fe, Cu, Co, Ni, Zn, and Mn) via an interfacial-fixing strategy. Specifically, polydopamine is used to coat nanotemplates made of metal oxides, followed by pyrolysis to form a metal-oxide skeleton coated by rich nitrogen-doped carbon shells. After chemically etching the skeleton, only interfacial metal atoms strongly bonded with the support via nitrogen atoms are retained. The high purity (>95%) of dual Fe sites was confirmed by both the direct visualization of local regions and the indirect evidence of the averaged information. When these neighboring metal-Nx moieties are applied for oxygen reduction reaction, Fe-Nx moieties exhibit the superior activity, even outperforming commercial Pt/C in the aspects of the half-wave potential, methanol tolerance, carbon monoxide tolerance, and robustness.
Subject terms: Catalyst synthesis, Synthesis and processing
Generic synthesis of metal-N4 moieties is interesting but challenging. Here the authors report an interfacial-fixing strategy to prepare neighboring metal-N4 sites, such as Fe2-N6, for oxygen reduction reaction.
Introduction
As the cathodic process of metal-air batteries and fuel cells, the oxygen reduction reaction (ORR) has garnered intensive attention, especially for searching nonprecious metals to replace platinum-based electrocatalysts. Nature routinely performs the reduction of O2 using a metalloenzyme named as cytochrome c oxidase, which only contains non-precious metals such as iron and copper1. Specifically, the active center within the cytochrome c oxidase comprises an iron heme, a distal copper in close proximity (0.5 nm) to iron heme, and surrounding organic ligands1. A promising non-precious metal catalyst for ORR should contain metal (M)-N4 moieties in close proximity rather than isolated sites. A typical approach to fabricating neighboring metal sites is based on increasing the metal loading in single-atom catalysts, following the probability that the higher the metal loading the closer the neighboring metal atoms2–4. Up to date, great efforts have been made to improve the metal-atom densities in single-atom catalysts5–13. For instance, a two-step annealing method prevents thermally induced aggregation of single atoms via stepwise ligand removal, resulting in a high metal loading of up to 23 wt%14. When graphene quantum dots, later interweaved into a carbon matrix, were used as a support, the numerous anchoring sites on the support facilitate the generation of high densities of transition-metal atoms up to 40 wt%15. Apart from varying the metal loading, other strategies for the precise fabrication of dual metal sites are mainly dependent on the coordination structures of the support. For example, the synthesis of Fe-Co dual sites was mainly based on the subtle selection of the support, Zn/Co bimetallic MOF, which encapsulated the Fe3+ moieties in the cavities via the sodalite coordination of Co2+ and Zn2+ nodes with 2-methylimidazole16. The formation of neighboring Fe atoms relied on the presence of vacancies (holes) which provided space to accommodate metal atoms17. The recent preparation of well-defined dual metal sites through in situ conversion of nanoparticles also relied on the special support comprising double carbon vacancies which could capture the evaporated metal atoms18. However, it remains a grand challenge of precisely tuning the anchoring sites on the support, leading to great difficulties in tuning the types of dual metals (limited by the host metal) and the distance between them (due to the uncontrollable adjacent defects).
Different from the previous works, we circumvented the selection of the support and turned to the metals themselves by making use of the original crystal structures for tuning the proximity of metal sites. Herein, we report a general method for fabricating neighboring M-Nx moieties (M = Fe, Cu, Co, Ni, Zn, and Mn) with specific distances via an interfacial-fixing strategy. We use Fe as the representative metal center to demonstrate our synthetic strategy. Specifically, polydopamine (PDA) was used as the confined matrix to coat Fe2O3 hollow nanotubes, followed by pyrolysis to form a Fe3O4 skeleton coated by rich nitrogen-doped carbon shells (Fig. 1a). The lattice of Fe3O4 crystals is expected to ensure the arrangement of Fe atoms at the interface. After chemically etching the skeleton, only interfacial Fe atoms strongly bonded with the support were retained. In contrast with traditional etching methods for the synthesis of single-atom catalysts, our strategy used additional urea to provide sufficient nitrogen atoms as a glue to anchor interfacial Fe atoms, even neighboring ones. Both the direct visualization of local regions and the indirect evidence of the averaged information confirm the high purity (>95%) of dual Fe sites. We successfully extended this synthetic method to fabricate various non-precious metal atom pairs including Cu, Co, Ni, Zn, and Mn. As expected, the distances between the atom pairs were consistent with those of their metal oxide counterparts. When we applied these neighboring M-Nx moieties, e.g. Fe2-N6, for ORR, Fe2-N6 exhibited the superior activity, outperforming commercial Pt/C in terms of the half-wave potential, methanol tolerance, CO tolerance, and robustness. Based on theoretical calculations, the enhanced ORR activity of Fe2-N6 derives from the parallel adsorption of O2 which induces efficient orbital overlap via both σ bonds and π bonds. Moreover, we integrated this catalyst into a Zn-air battery which exhibited the maximum power density of 190.6 mW cm-2 at 287.8 mA cm-2, outperforming the Zn-air battery with commercial Pt/C (151.7 mW cm-2, 239.8 mA cm-2).
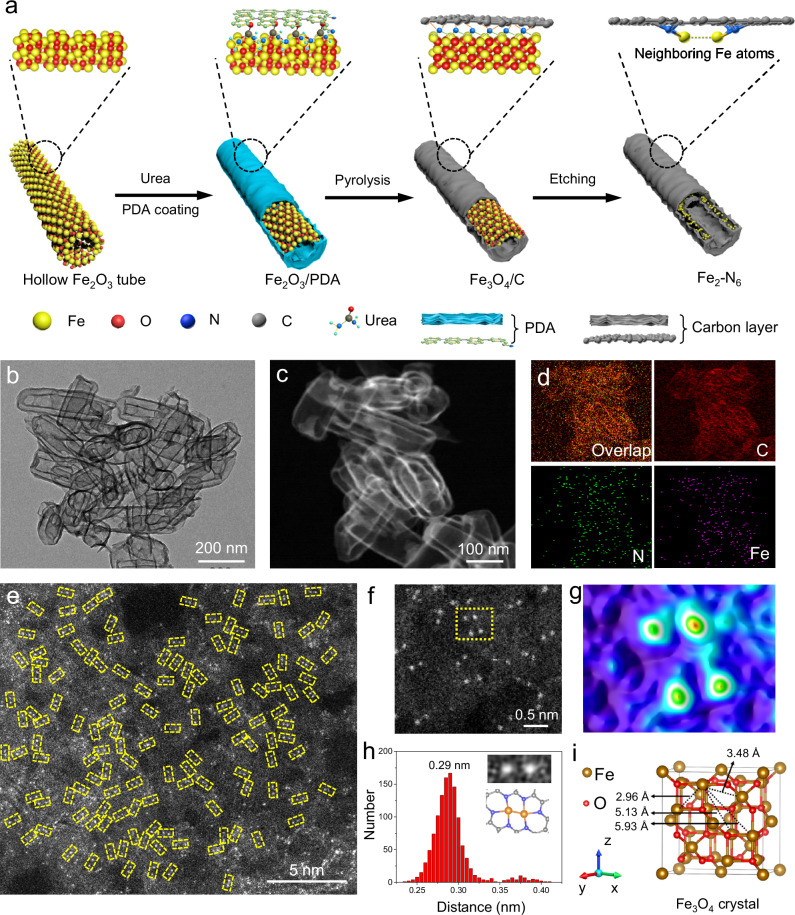
Fig. 1. Synthesis and structural characterizations.
a Schematic illustration of the synthesis of Fe2-N6. b TEM, (c) HAADF-STEM, and (d) EDS elemental mapping images of Fe2-N6. e Aberration-corrected HAADF-STEM image of Fe2-N6. f Magnified HAADF-STEM image of Fe2-N6 and (g) the corresponding 3D atom-overlapping Gaussian-function fitting maps of the region in panel f. h Distance distribution between neighboring Fe atoms. The inset images in panel h show the HAADF-STEM image and the proposed model of a typical pair of neighboring Fe atoms. i Crystal structure of Fe3O4.
Results
Synthesis of neighboring Fe atoms
A reported hydrothermal method was employed to synthesize Fe2O3 hollow nanotubes. The phase of Fe2O3 was confirmed by X-ray diffraction (XRD) pattern (Supplementary Fig. 1). Scanning electron microscopy (SEM) images showed that these nanotubes took an average length of ~260 nm and an outer diameter of ~80 nm (Supplementary Fig. 2). After being coated with a thin layer of PDA, the hollow tube-like structure was perfectly maintained without the aggregation of PDA. Additional urea was used as a nitrogen resource to increase the nitrogen content in the final products. The obtained sample was denoted as Fe2O3@PDA composites (Supplementary Fig. 3). It is worth noting that the amount of PDA plays an important role in the shape of Fe2O3@PDA composites. Afterwards, Fe2O3@PDA composites were carbonized in a tube furnace under Ar at 700 oC for 2 h. The XRD pattern of the pyrolyzed product indicated a typical phase of Fe3O4, thereby denoting the sample as Fe3O4@C (Supplementary Fig. 1). Finally, Fe3O4@C was etched by 4 M HCl solution for 72 h to remove the metal oxide bulks and 1 M H2SO4 for 12 h to remove the unstable metal species that anchored on the carbon interfaces. After chemical etching, a double-layer hollow carbon tube was obtained as evidenced by the transmission electron microscopy (TEM) and SEM images (Fig. 1b and Supplementary Fig. 4). As such, the removal of the Fe3O4 skeleton did not induce the collapse of the shape in double-layer hollow tubes. The varied contents of N species were shown in Supplementary Table 1. Pure N-C obtained from carbonized PDA contained the same amount of nitrogen as that of Fe1-N4 (5.1 wt%), which was lower than that (9.2 wt%) of Fe2-N6. As such, additional urea molecules contribute 4.1 wt% of nitrogen-containing to the Fe2-N6 sample. According to the XRD pattern of resulted product, a broad diffraction peak appeared at approximately 25o (Supplementary Fig. 5), corresponding to the {002} plane of typical amorphous carbon19–23. No other sharp peaks for the phases of iron oxides or carbides were detected, indicating the complete removal of Fe3O4 crystals. It is expected that the remaining Fe atoms after etching can retain the arrangement of initial Fe3O4 crystals in order to obtain neighboring Fe atoms. The verification of this expectation needs further characterizations at the atomic level. A schematic process was provided to illustrate the conversion of PDA/urea to N-doped carbon layers (Supplementary Fig. 6).
Characterizations of neighboring Fe-Nx moieties
Figure 1c,d shows a high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) image and the corresponding energy-dispersive X-ray spectroscopic (EDS) mapping analysis. According to these images, C, N, and Fe elements were uniformly distributed throughout the entire double-layer hollow carbon tubes. High-resolution TEM (HRTEM) images showed the absence of Fe clusters or nanoparticles on the support (Supplementary Fig. 7). TEM image observed from the top view further supported the double layer structure of the carbon matrix (Supplementary Fig. 8). In addition, the mass loading of Fe was 2.36 wt% based on inductively coupled plasma-optical emission spectrometry (ICP-OES). As revealed by bright spots and yellow dotted rectangles in Fig. 1e, highly dense pairs of Fe atoms were dispersed on the support (Supplementary Fig. 9). Further observations in the magnified HAADF-STEM image and the corresponding three-dimensional (3D) atom-overlapping Gaussian-function fitting mappings identified the exact distance between two neighboring Fe atoms (Fig. 1f,g and Supplementary Fig. 10). We further collected the distances between neighboring Fe atoms from thousands of objects, obtaining a distance distribution with the majority at 0.29 nm (Fig. 1h), indicating the successful controlling the distance between neighboring Fe atoms in a narrow distribution. A typical pair of neighboring Fe atoms with a distance of 0.29 nm was exemplified by the HAADF-STEM image and the signal intensities in the corresponding region (the inset images in Fig. 1h).
In a unit cell of the Fe3O4 crystal, there are four types of distances between Fe atoms, including 2.96, 3.48, 5.13, and 5.93 Å (Fig. 1i). In the preparation process, urea was chosen as the chemical ligand to anchor Fe atoms. The distance between two N atoms in a urea molecule is 2.73 Å. As such, urea can efficiently connect neighboring Fe atoms that are separated near this distance (2.73 Å), that is the nearest pair of Fe atoms with a distance of 2.96 Å. The main distance between neighboring Fe atoms is about 0.29 nm (Fig. 1h). Notably, the experimental distance is slightly shorter than the theoretical value (2.96 Å) of the crystal model, because the ligand with even shorter distance (2.73 Å) between nitrogen anchoring sites induced the contraction of Fe atom pairs on the surface of the Fe3O4 crystal. As for the pair of Fe atoms with a distance of 3.48 Å, they are separated too far to be fixed by one urea molecule. These Fe atoms might be coordinated with N atoms of different adjacent urea molecules. During the etching process, these Fe atoms bonded by different urea molecules are likely to be separated apart further than the theoretical distance of 3.48 Å, so that the characteristic distance (3.48 Å) cannot be well maintained. The possibility of this situation is much smaller than that linked by one urea molecule. As such, we observed a weak wide peak at around 0.35 nm in Fig. 1h. With regard to the pairs of Fe atoms with distances of 5.13 and 5.93 Å, both of them have adjacent Fe atoms with the distance of 2.96 Å, as discussed in the first situation. As such, these pairs of Fe atoms were just synthesized as designed instead of being distributed randomly. Moreover, we define the pair of Fe atoms (Fe2-N6) that can be most efficiently anchored by the urea ligand as neighboring Fe atoms. These Fe atoms are separated at a distance of about 0.29 nm. The Fe atoms with longer distances are denoted as isolated Fe atoms. We used this definition because the distance between these neighboring atoms could be facilely tuned, whereas the distances between other Fe atoms were largely dependent on the possibility. The content of neighboring Fe atoms in Fe2-N6 was as high as 98%, indicating the high purity of dual atom pairs (Supplementary Fig. 11). Intriguingly, heavy carbon layers resulting from the double usage of dopamine also achieved the formation of neighboring Fe sites (Supplementary Figs. 12 and 13).
For comparison, we prepared a reference sample with the Fe mass loading of 0.32 wt% using the same procedure except for not adding additional urea. The overall morphology also exhibited a double-layer hollow tube (Supplementary Fig. 14). The XRD pattern showed the existence of typical amorphous carbon without any iron-related phases (Supplementary Fig. 15). As shown in Supplementary Fig. 16, a and b, the distance between Fe atoms ranged from 0.45 to 0.82 nm, significantly longer than that of neighboring Fe atoms. These Fe atoms were featured as isolated ones. A typical HAADF-STEM image and its line-scanning intensity profile of isolated Fe atoms revealed a distance of 0.60 nm which was longer than 0.29 nm for neighboring ones (Supplementary Fig. 16, c and d). Therefore, the introduction of urea leads to the stabilization of neighboring Fe atoms which are presumably coordinated with N atoms. This point was supported by N 1 s X-ray photoelectron spectroscopy (XPS) analysis. For both isolated and neighboring Fe atoms, various N-containing species were identified, including oxidized N, graphitic-N, pyrrolic-N, pyridinic-N, and Fe-N (Supplementary Fig. 17a). The content of Fe-N in neighboring Fe atoms was 0.81 wt%, higher than that (0.23 wt%) in isolated ones (Supplementary Fig. 17b), verifying the more efficient stabilization of neighboring Fe atoms by N atoms. In addition, we took metallic Fe as the metal source to prepare single metal atoms, while other experimental details were the same as those for the preparation of Fe2-N6. However, no isolated metal sites were obtained (Supplementary Fig. 18). It can be ascribed to the reason that the zero-valence Fe atoms in metallic Fe are reluctant to coordinate with N atoms in urea. Thus, a basic requirement is that the metal source should strongly interact with the ligand. To investigate the role of ligand, we replaced urea with ethanediamine. It is found that the distance between Fe atoms became wider ranging from 0.27 to 0.39 nm (Supplementary Fig. 19). This is because ethylenediamine is a flexible molecule, of which distance between the two N atoms on the carbon chain can easily change, making it difficult to fix the distance between two Fe atoms. We tried another ligand, thiourea, which possesses a similar structure to that of urea. The use of thiourea ligand also led to the formation of neighboring metal atoms with the possible involvement of both N and S in the coordination configuration (Supplementary Fig. 20). Therefore, the successful preparation of dual Fe atoms lies in the perfect match of the ligand and the lattice of the template.
To investigate the coordination structures of isolated and neighboring Fe atoms, we carried out X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) measurements. The absorption threshold positions of both neighboring and isolated Fe atoms were located between Fe2O3 and Fe3O4, suggesting that the valence state of Fe atoms was between +2 and +3 (Supplementary Fig. 21a)24. Notably, the calculated XANES spectrum of the Fe2-N6 model exactly reproduced the experimental spectrum of Fe2-N6 (Supplementary Fig. 22). As shown in EXAFS and the corresponding fitting results, neighboring Fe atoms exhibited a strong peak for the Fe-N shell with a bond length (R) of 1.90 Å and a coordination number (CN) of 2.8 (Supplementary Fig. 21b and S23a, Supplementary Table 2). Another prominent peak corresponded to the Fe-Fe contribution with R of 2.62 Å and CN of 0.9 (Supplementary Fig. 21b and S23a, Supplementary Table 2). The near unit CN for Fe-Fe bonds was a typical characteristic of atom pairs25–27. As such, we denoted neighboring Fe atoms as Fe2-N6 (Supplementary Fig. 23b). For isolated Fe atoms, only the peak for Fe-N bonds was observed with R of 1.84 Å and CN of 3.8, denoting this sample as Fe1-N4 (Supplementary Fig. 21b and S24, Supplementary Table 2).
Moreover, we conducted N and O K-edge soft XANES measurements of Fe2-N6, Fe1-N4, and carbon matrix. As shown in N K-edge XANES spectra (Supplementary Fig. 25a), obvious absorption at 400.5 eV appeared for Fe2-N6 and Fe1-N4, corresponding to the Fe-N bonding based on the previous papers28. This absorption was absent for the carbon matrix. As such, there existed Fe-N bonds in both Fe2-N6 and Fe1-N4. The O K-edge XANES spectra of these three samples were similar, showing a prominent peak at 532.8 eV for the π* state of C = O bonds (Supplementary Fig. 25b)29. This result implies that the existing O species were bonded with the carbon matrix rather than coordinated with Fe atoms. As a result, the Fe atoms were only coordinated by N atoms. This result also corresponded to the synthetic procedure. Specifically, urea was used as a coating layer which was inserted between the Fe2O3 template and the PDA layer. Fe atoms on the surface of Fe2O3 and Fe3O4 were preferentially coordinated with N atoms in urea molecules. Meanwhile, the chemical bonds between O and Fe atoms in Fe3O4 crystal were broken down when they underwent strong chemical etching (by 4 M HCl solution for 72 h and 1 M H2SO4 for 12 h). As such, it is reasonable that O atoms did not take part in the coordination with Fe atoms in Fe2-N6 but existed on the carbon matrix.
Actually, the evidence of HAADF-STEM images is not convincing enough to quantify the purity of neighboring Fe atoms, since it only reflects the local information. To this end, we conducted temperature-programmed desorption (TPD) measurements with mass spectroscopy (MS) using acetylene (C2H2) as a probe molecule to quantify the averaged purity of neighboring Fe atoms. Since the adsorption strength of acetylene molecules on isolated Fe sites differs from that on dual Fe sites, the temperatures for the desorption of acetylene molecules from isolated and dual Fe sites should be different. We analyzed the data in Supplementary Fig. 26a, b. The C2H2-TPD-MS profile of Fe1-N4 shows a symmetric peak at 103.2 oC which we assigned to the adsorption on isolated Fe sites. As for the C2H2-TPD-MS profile of Fe2-N6, the peak was shifted to a higher temperature. This phenomenon is rational since the adsorption on dual Fe sites is stronger than that on isolated sites. Specifically, the acetylene molecule is physically adsorbed on Fe1-N4 with an adsorption energy (Eads) of -0.542 eV (Supplementary Fig. 26c). As for Fe2-N6, the acetylene molecule is adsorbed via a flat-lying bidentate configuration, with an Eads of -1.620 eV, where the C-C bond was stretched to 1.306 Å (Supplementary Fig. 26d). Moreover, the shape of this peak was asymmetric, indicating the existence of more than one peak. In this work, we attributed the other peak to that for isolated Fe sites. As such, we adopted the temperature of 103.2 oC for isolated Fe sites and the peak temperature of 123.6 oC to fit the profile of Fe2-N6. The latter we assigned to the adsorption on dual Fe sites. As shown in the fitting profile of Fe2-N6, the peak area ratio of dual Fe sites to isolated Fe sites was 10.6:1 (Supplementary Fig. 26b). When we assume that one Fe site adsorbs one acetylene molecule, the purity of dual sites in the Fe2-N6 sample was 91.4%. Actually, it is more rational that one isolated Fe site adsorbs one acetylene molecule via an end-on mode, while a pair of dual Fe sites adsorb one acetylene molecule via a bidentate mode. In other words, for dual sites, one acetylene molecule corresponds to two Fe atoms. Thus, the purity of dual sites in the Fe2-N6 sample should be 95.5%, close to the value (98%) from the statistics of HAADF-STEM images. Overall, both the direct visualization of local regions and the indirect evidence of the averaged information confirm the high purity (>95%) of dual Fe sites in Fe2-N6.
In addition, we adjusted the amount of urea added during the synthesis process and measured the Fe loading and the distance between Fe atoms. As the amount of urea increased from 10 to 100 mg, the Fe loading increased from 0.55 to 2.36 wt%, while the distance between iron atoms decreased from 0.54 to 0.29 nm, with the distribution of distances between Fe atoms becoming more concentrated (Supplementary Fig. 27). When the amount of urea exceeded 100 mg (e.g., 120 mg), the Fe loading and the distance between iron atoms remained largely unchanged at 2.37 wt% and 0.29 nm, respectively, due to saturation (Supplementary Fig. 28). Also, we tuned the pyrolysis temperature to minimize the decomposition loss of urea molecules while ensuring the occurrence of the pyrolysis reaction. we lowered the pyrolysis temperature to 500 °C and reduced the urea content to 50 mg (see Supplementary Table 3 for synthesis condition comparison). The Fe content in the synthesized sample was reduced to 1.62%, while maintaining high Fe diatomic purity (Supplementary Fig. 29). Notably, the Fe content of 1.62% at a pyrolysis temperature of 500 °C was higher than that (1.32%) at 700 oC, implying that lowering the pyrolysis temperature indeed decreased the loss of urea molecules and thereby increased the amount of anchored Fe atoms.
Synthesis of other neighboring M-Nx moieties
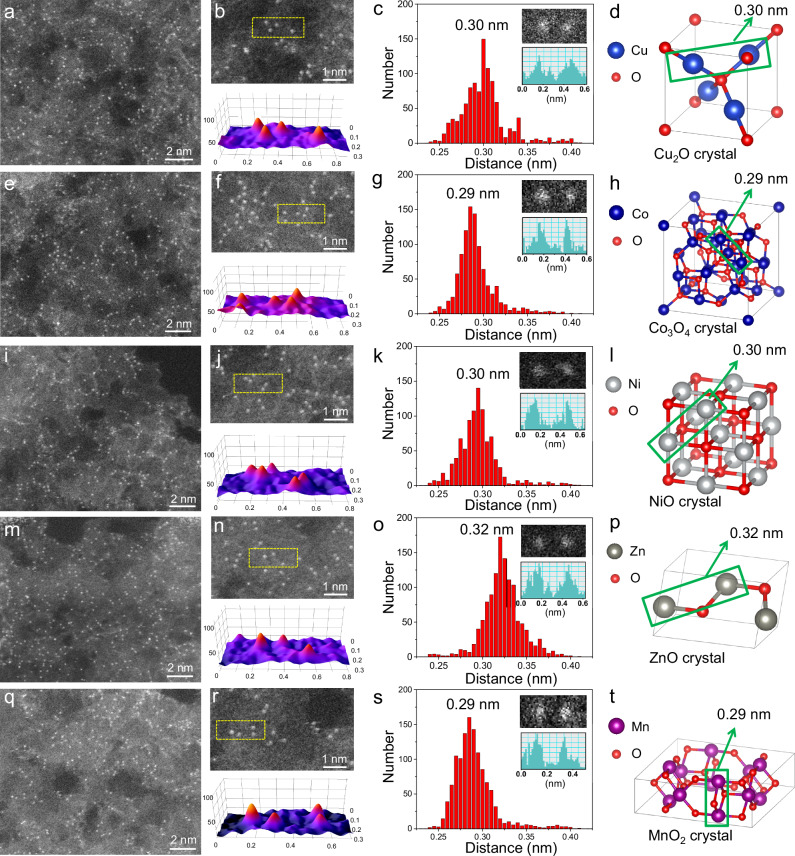
To explore whether our interfacial-fixing method can be extended to prepare other neighboring M-Nx moieties, we used Cu2O as the substrate to synthesize neighboring Cu-Nx moieties. Multi-scale characterization evidence ranging from subnanometers to millimeters was integrated to exclude the existence of clusters or nanoparticles (Supplementary Fig. 30). To quantify the distance between neighboring Cu atoms, we conducted aberration-corrected HAADF-STEM images (Fig. 2a). Magnified HAADF-STEM image and the corresponding 3D atom-overlapping Gaussian-function fitting mappings identified the exact distance between two neighboring Cu atoms (Fig. 2b). The main distance between adjacent Cu atoms was determined to be 0.30 nm (Fig. 2c), which is consistent with the distance between two Cu atoms in the Cu2O crystal (Fig. 2d). These results demonstrate the successful synthesis of neighboring Cu-Nx moieties with presupposed distances.
Fig. 2. Structural characterizations of different neighboring M-Nx moieties.
Aberration-corrected HAADF-STEM images of neighboring (a) Cu-Nx, (e) Co-Nx, (i) Ni-Nx, (m) Zn-Nx, and (q) Mn-Nx moieties. Enlarged HAADF-STEM images and corresponding 3D atom-overlapping Gaussian-function fitting maps of neighboring (b) Cu-Nx, (f) Co-Nx, (j) Ni-Nx, (n) Zn-Nx, and (r) Mn-Nx moieties. Distance distribution between neighboring (c) Cu-Nx, (g) Co-Nx, (k) Ni-Nx, (o) Zn-Nx, and (s) Mn-Nx moieties. Crystal structures of (d) Cu2O, (h) Co3O4, (l) NiO, (p) ZnO, and (t) MnO2.
Other non-precious metal moieties, including Co, Ni, Zn, and Mn were also fabricated via the same synthetic method. The absence of clusters or nanoparticles was confirmed by a series of characterizations (Supplementary Fig. 31-35). As shown in Fig. 2e-t, the distances between neighboring metal atoms were all consistent with those of their metal-oxide counterparts. These results confirm the generality of our synthetic strategy for neighboring M-Nx moieties.
ORR performance
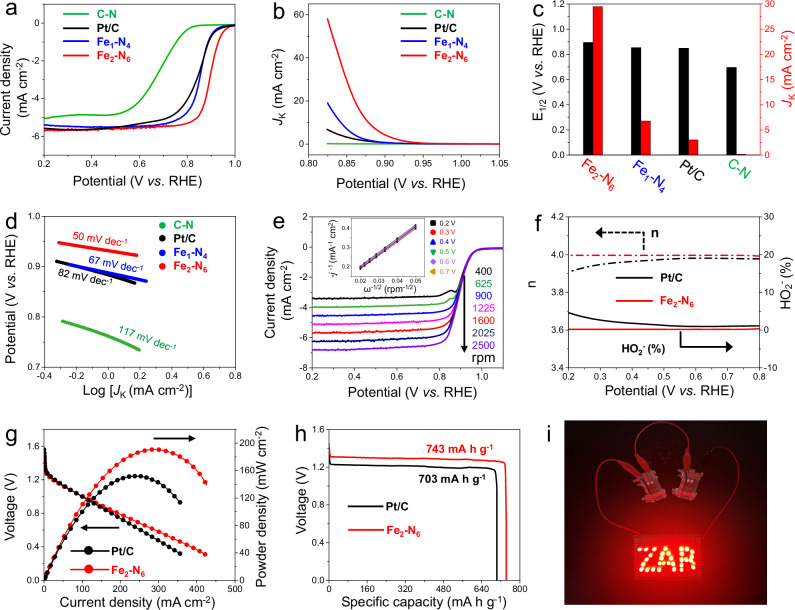
In order to evaluate the enhancement of catalytic capability by the achieved neighboring single atoms, Fe2-N6 and Fe1-N4 were explored toward ORR in a three-electrode system. Similar specific surface area and porosity of Fe1-N4 and Fe2-N6 can exclude the differences in catalytic performance that resulted from the pore structure (Supplementary Fig. 36 and Supplementary Table 4). Moreover, the porous structure can ensure the fully exposure of Fe atoms, which can be utilized during the ORR process. ORR activities were investigated by cyclic voltammetry (CV) measurements in O2 and N2 saturated KOH solution (0.1 M). The peak potential of Fe2-N6 was 0.92 V vs. reversible hydrogen electrode (RHE), more positive than that (0.87 V) of Fe1-N4 (Supplementary Fig. 37). The ORR activities were also evaluated by rotating disk electrode (RDE) measurements with a rotating rate of 1600 rpm. The linear sweep voltammetry (LSV) results indicated that Fe2-N6 achieved the good ORR catalytic activities with an onset potential (0.95 V) and the most positive half-wave potential (0.90 V), outperforming those of Fe1-N4 (0.91 V and 0.86 V) and commercial Pt/C catalysts (0.92 V and 0.85 V) (Fig. 3a). In order to quantitatively assess the ORR activity, the kinetic current density (Jk) was calculated based on the LSV curves in Fig. 3a. Figure 3b exhibited the whole kinetics control and mixed kinetics-diffusion control regions of the catalysts. Jk of Fe2-N6 was apparently higher than those of the other three samples at 0.92 - 0.82 V. Specifically, at 0.85 V, the Jk value of Fe2-N6 reached 29.4 mA cm-2, being 4.2 and 9.9 times higher than that of Fe1-N4 (7.02 mA cm−2) and that of commercial Pt/C (2.98 mA cm−2), respectively (Fig. 3c). Strikingly, the Fe2-N6 catalyst exhibited the lowest Tafel slope (50 mV·dec−1) (Fig. 3d) compared with Fe1-N4 (67 mV dec−1) and Pt/C (82 mV dec−1). According to electrochemical impedance spectroscopy (EIS), Fe2-N6 and commercial Pt/C showed the smallest charge transfer resistance among the samples (Supplementary Fig. 38), resulting in the fast reaction kinetics of Fe2-N6 catalyst. Considering the difference in Fe contents between Fe2-N6 and Fe1-N4, we mixed Fe2-N6 with the C-N matrix in an electrode to ensure the same content of Fe atoms in the prepared electrode as that in Fe1-N4 (see details in the experimental section). In spite of the same content of Fe, Fe2-N6/C-N still exhibited a higher E1/2 (0.88 V vs. RHE) value than that (0.86 V vs. RHE) of Fe1-N4 (Supplementary Fig. 39). As such, the dual Fe pairs were intrinsically more active than isolated Fe sites during ORR. The ORR performance of Fe2-N6 surpassed most of the reported Fe-based catalysts (Supplementary Fig. 40 and Supplementary Table 5). Moreover, Fe2-N6 also outperformed other neighboring M-Nx (M = Cu, Co, Ni, Zn, and Mn) moieties (Supplementary Fig. 41). Therefore, Fe2-N6 possessed a superior ORR activity compared with commercial Pt/C in an alkaline medium.
Fig. 3. ORR properties.
a Linear sweep voltammetry curves, b kinetic current density curves, (c) E1/2 and Jk, and (d) corresponding Tafel plots of Fe2-N6, Fe1-N4, commercial Pt/C, and C-N matrix. e LSV curves of Fe2-N6 in O2-saturated 0.1 M KOH with various rotating rates. f Electron transfer number (n) and peroxide species yield of Fe2-N6 and commercial Pt/C. g Discharge polarization curves and corresponding power density curves of the aqueous Zn-air batteries using Fe2-N6 and commercial Pt/C as air electrode. h Galvanostatic discharge curves of Zn-air batteries with Fe2-N6 and commercial Pt/C as an air electrode at a current density of 2 mA cm-2. i Photograph of lit red LED display powered by Zn-air batteries with Fe2-N6 air electrode.
The reaction kinetics were further explored. Figure 3e shows the LSV curves of Fe2-N6 at different rotating rates. The current densities of catalysts were obviously enhanced with the increase in rotational speeds. The Koutechy-Levich (K-L) plots derived from the LSV curves of Fe2-N6 showed high linearity in the potential range of 0.2 to 0.7 V, implying first-order reaction kinetics for ORR (the inset of Fig. 3e). Based on the K-L plots, the electron transfer numbers (n) for Fe2-N6 at different potentials approached to the theoretical value of 4, indicating that the ORR proceeded mainly in a four-electron reduction process. Additionally, K-L plots of Fe1-N4 exhibited electron transfer numbers of 3.85-3.96 (Supplementary Fig. 42), while the carbon matrix achieved electron transfer numbers of 3.82-3.87 (Supplementary Fig. 43). To support the above results, we further measured the yields of peroxide species. In the rotating ring disk electrode (RRDE) tests, the yield of peroxide species for Fe2-N6 was fairly low (<1.0%) (Fig. 3f). The electron transfer number (n) was calculated at around 4 (Fig. 3f), consistent with the results from the K-L plots. In comparison, the yields of peroxide species for Fe1-N4 and carbon matrix were higher than that for Fe2-N6 at the applied potentials, exhibiting the n values of 3.79-3.87 and 3.69-3.81, respectively (Supplementary Fig. 44).
We further evaluated the ORR stability of Fe2-N6 in comparison with commercial Pt/C in O2-saturated 0.1 M KOH solution. As shown in Supplementary Fig. 45, the Fe2-N6 still maintained 97% of its initial current after 10,000 s, whereas commercial Pt/C maintained only 85% of the initial current. These results revealed a high stability of Fe2-N6. In methanol fuel cells, the tolerance to methanol crossover is of great importance for practical applications, as the efficiency of commercial Pt/C is generally degraded by the presence of methanol in the electrolyte. The methanol tolerance of Fe2-N6 was evaluated by injecting a methanol solution during the chronoamperometric measurement. After the addition of methanol, Fe2-N6 still maintained a stable current density with negligible fluctuation, whereas the current of commercial Pt/C catalyst decreased to less than 70% (Supplementary Fig. 46). These results demonstrate that Fe2-N6 possessed high tolerance to methanol crossover. CO tolerance performance of Fe2-N6 and Pt/C was further evaluated. Compared with Pt/C, Fe2-N6 was much more tolerant against CO poisoning (Supplementary Fig. 47), while the advantage of Fe2-N6 became more prominent with the extension of reaction time (see detailed analysis in the Note of Supplementary Table 6). Moreover, the long-term durability of Fe2-N6 in the practical environment was conducted according to an accelerated durability test (ADT). As shown in Supplementary Fig. 48, a small E1/2 decay (10 mV) of Fe2-N6 was observed even after 50,000 ADT cycles, smaller than those of Fe1-N4 (36 mV) and commercial Pt/C (55 mV), revealing outstanding long-term durability of Fe2-N6 for ORR.
To demonstrate the potential application of Fe2-N6, we constructed Zn-air batteries using Fe2-N6 as the air cathode and Zn foil as the anode. 6 M KOH aqueous solution with 0.2 M zinc acetate was used as the electrolyte. The Zn-air batteries based on Fe2-N6, Fe1-N4 and commercial Pt/C exhibited open circuit voltage of 1.425, 1.380 and 1.410 V, respectively (Supplementary Fig. 49). The maximum power density reached 190.6 mW cm−2 at a current density of 287.8 mA cm−2 (Fig. 3g), which was much higher than that of the Pt/C-based Zn-air battery (151.7 mW cm−2, 239.8 mA cm−2). The calculated specific capacities of Fe2-N6-based Zn-air battery achieved a high value of 743 mA h g−1 at 2 mA cm−2 (Fig. 3h), which was larger than that of commercial Pt/C (703 mA h g−1). Moreover, a mini fan and a red light-emitting diode (LED) can be powered by two Fe2-N6-based Zn-air batteries (Fig. 3i, Supplementary Movies 1 and 2). These results demonstrate the superior catalytic activities of Fe2-N6 for ORR. Rechargeable Zn-air batteries with Fe2-N6, Fe1-N4 and commercial Pt/C acting as cathodes were also assembled, where Fe2-N6 were stably cycled up to 70 h, much more stable than Fe1-N4 and commercial Pt/C (Supplementary Fig. 50).
In order to meet the increasing demand for wearable electronics, we further tested whether Fe2-N6 could be used as a cathode for fabricating flexible Zn-air batteries with Zn foil as an anode and alkaline polyvinyl alcohol gel solid-state as an electrolyte (Supplementary Fig. 51a). This flexible Zn-air battery exhibited a stable open-circuit voltage of ~1.47 V (Supplementary Fig. 51b). A red LED was lit up by both flat and bend Zn-air batteries (Supplementary Fig. 51c). The charge and discharge platforms were well maintained by alternative changing the Fe2-N6-based battery between flat and bend states (Supplementary Fig. 51d). These results confirm the remarkable electrochemical performance of the Fe2-N6 catalyst as a flexible air electrode. HAADF-STEM image of prepared Fe2-N6 catalyst after long-term cycling showed the preservation of Fe atom pairs, suggesting the robustness of this catalyst (Supplementary Fig. 52).
DFT studies
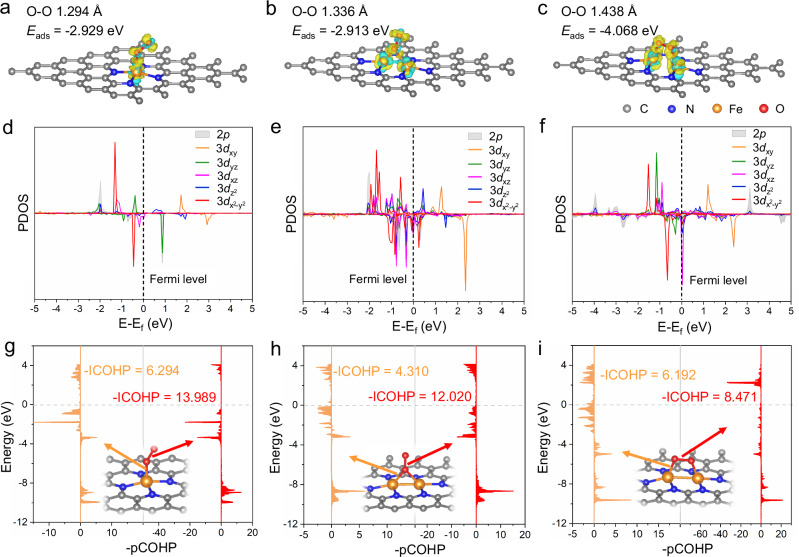
To further scrutinize the ORR mechanism, we conducted density functional theory (DFT) calculations. Various reported literature that contained theoretical calculations of ORR over Fe-based catalysts demonstrated that the activation of O = O double bonds was the rate determining step30,31. Taking these papers as reference, we used the cleavage of O = O bonds as the descriptor to evaluate the catalytic properties of the developed catalysts. The adsorption configurations of oxygen molecules on Fe1-N4 and Fe2-N6 were built. Specifically, Fe1-N4 adopts the end-on adsorption of O2 (Fe1-N4-O2) with an adsorption energy (Eads) of -2.929 eV and an O-O bond length of 1.294 Å (Fig. 4a, Supplementary Data 1). As for Fe2-N6, the O2 molecule can be adsorbed on the bridge sites of neighboring Fe atoms in a vertical mode (Fe2-N6-O2-ver, Fig. 4b, Supplementary Data 2). This adsorption configuration exhibits an Eads of -2.913 eV and an O-O bond length of 1.336 Å. When the adsorbed O2 takes the flat-lying bidentate configuration on neighboring Fe atoms in a parallel mode (Fe2-N6-O2-par), the length of the O-O bond is stretched to 1.438 Å, while the adsorption is strengthened to -4.068 eV (Fig. 4c, Supplementary Data 3). As such, the most efficient activation of O2 occurs on Fe2-N6 with the parallel mode considering the most negative Eads and the longest of O-O bonds among these configurations.
Fig. 4. DFT studies.
The charge density difference of (a) Fe1-N4-O2, (b) Fe2-N6-O2-ver, and (c) Fe2-N6-O2-par, respectively. The isosurface value of the color region is 0.01 Bohr-3. Yellow and cyan regions refer to the increased and decreased charge distribution, respectively. PDOS for Fe 3 d orbitals and O 2p orbitals of (d) Fe1-N4-O2, (e) Fe2-N6-O2-ver, and (f) Fe2-N6-O2-par. pCOHP of (g) Fe1-N4-O2, (h) Fe2-N6-O2-ver, and (i) Fe2-N6-O2-par at different positions. The insets in panels g-i show the corresponding structures.
To reveal the underlying mechanism from the perspective of electronic structures, we carried out spin-polarized project density of states (PDOS) analysis. As shown in Supplementary Fig. 53, the d-band center of Fe2-N6 (-1.48 eV) is much closer to the Fermi level than that of Fe1-N4 (-3.02 eV), signifying stronger chemisorption of Fe2-N632. Furthermore, we analyzed Fe 3 d and O 2p orbitals of Fe1-N4 and Fe2-N6 after oxygen adsorption, which is responsible for O2 activation. The adsorbed O2 on Fe1-N4 induces the formation of σ bonds which derives from the interaction of O 2p orbitals with the Fe orbital (Fig. 4d). The O 2p and Fe 3 d orbitals of Fe2-N6-O2-ver exhibit a similar overlap to that of Fe1-N4-O2 (Fig. 4e). As for Fe2-N6-O2-par, the bonding interaction between O 2p and Fe orbitals move away from Fermi level, indicating a weak σ bond (Fig. 4f). Moreover, the additional π bonds arise from the O 2p-Fe hybridization on Fe2-N6 (Fig. 4f), accounting for the elevated charge density of oxygen atoms, leading to a strong interaction between O atoms and Fe atoms33. Therefore, neighboring Fe atoms favor parallel adsorption of O2 which induces both σ bonds and π bonds.
The bonding of O2 with Fe atoms on Fe1-N4 and Fe2-N6 was further analyzed on the basis of crystal orbital Hamilton populations (COHP). The projected COHP profiles (pCOHP) for Fe-O bonds and O-O bond show the bonding (-pCOHP > 0) and anti-bonding (-pCOHP <0) interaction of O2 adsorbed on Fe1-N4 and Fe2-N6 (Fig. 4g-i, Supplementary Fig. 54). Integrating the pCOHP to the Fermi level (E = 0 eV) gives the ICOHP which is a measure of bond strength. The -ICOHP value (6.192) of Fe-O bonds in Fe2-N6-O2-par is between that (4.310) in Fe2-N6-O2-ver and that (6.294) in Fe1-N4-O2 (Fig. 4g-i), indicating a moderate interaction between O2 and Fe atoms in Fe2-N6-O2-par. Notably, Fe2-N6-O2-par exhibits a much lower -ICOHP value (8.471) of O-O bond than that (12.020) in Fe2-N6-O2-ver and that (13.989) in Fe1-N4-O2 (Fig. 4g-i). As such, the O-O bond is significantly weakened when O2 is parallelly adsorbed on neighboring Fe atoms, accounting for the facile oxygen activation.
Discussion
In summary, we demonstrated a general method for synthesizing neighboring M-Nx moieties with a specific pre-designed distance via an interfacial-fixing strategy. The precise preparation lies in the interfacial-fixing strategy where the distance between neighboring metal atoms relies on the crystal structure of the skeleton and the type of ligand. This work offers a top-down strategy to tune the distance between neighboring atoms, which is pre-designed instead of depending on the probability of metal loading. Precisely tuning the distance between neighboring metal atoms advances the fundamental understanding of single-atom catalysis.
Methods
Chemicals
All chemical reagents were used as received without further purification. Iron (III) chlorideanhydrous (FeCl3; ≥ 99.9%), ammonium dihydrogen phosphate (NH4H2PO4; ≥ 99.8%), tris (hydroxymethyl) aminomethane (≥ 99.9%), urea (≥ 99%), dopamine (≥ 98%), Cu(NO3)2⋅3H2O (99.0~102.0%), Co(NO3)2⋅6H2O (99%), Ni(NO3)2⋅6H2O ( ≥ 98.5%), Zn(NO3)2⋅6H2O (98%), NaBH4 (98%), KOH ( ≥ 85%), Zn(OAc)2 ( ≥ 99%), acrylamide (≥ 98%), N-N methylene bisacrylamide (≥ 99%), potassium persulfate (≥ 99.5%), dimethyl sulfoxide (≥ 99.5%), montmorillonite and KMnO4 (≥ 99%) were obtained from Sinopharm Chemical Reagent Co. Ltd. Commercial Pt/C (20 wt%) catalyst was purchased from Sigma-Aldrich. Concentrated sulfuric acid (95.0 ~ 98.0%) and hydrochloric acid (36.0 ~ 38.0%) were purchased from Sinopharm Chemical Reagent (Shanghai, China). Deionized (DI) water was used throughout the experiments.
Synthesis of Fe2O3 nanotubes
Fe2O3 nanotubes were synthesized via a hydrothermal treatment, as described in34. Specifically, 14.0 mL of a 0.5 M FeCl₃ aqueous solution and 12.6 mL of a 0.02 M NH₄H₂PO₄ aqueous solution were mixed under vigorous stirring. Deionized (DI) water was then added to the mixture until the total volume reached 350 mL, followed by continued stirring for 10 minutes. The resulting mixture was transferred to a 500-mL Teflon-lined stainless-steel autoclave, and the hydrothermal reaction was conducted at 220 °C for 60 hours. After the reaction, the autoclave was allowed to cool naturally to room temperature. The red precipitates were then separated by centrifugation, washed three times with DI water and ethanol, and dried at 80 °C under vacuum.
Synthesis of metallic Fe template with zero valence state
Metallic Fe with a zero-valence state was synthesized following the procedure outlined in ref. 35. First, 50 mg of FeCl3 was dispersed in 50 mL of deionized water using ultrasonication for 30 minutes. Then, a freshly prepared aqueous NaBH4 solution (50 mg of NaBH4 in 50 mL of DI water) was added dropwise to the mixture under stirring, resulting in the formation of a black dispersion. The mixture was stirred continuously for 1 hour to ensure the complete reduction of the metal salt. Finally, the dispersion was filtered, washed five times with water, and dried in a vacuum oven at 60 °C overnight to yield the metallic Fe sample.
Synthesis of CuO, Co3O4, NiO, ZnO and MnO2 templates
Carbonaceous microspheres (CMS) templates were synthesized via a hydrothermal method36,37. Metal precursors, including Cu(NO3)2·3H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Zn(NO3)2·6H2O, and KMnO4, were used to synthesize CuO, Co3O4, NiO, ZnO, and MnO2 templates, respectively. In a typical procedure, 0.6 g of freshly prepared CMSs was dispersed in 50 mL of a 0.1 M precursor aqueous solution by ultrasonication for 30 minutes. The resulting suspension was allowed to stand for 6 hours, followed by vacuum filtration. The precipitate was washed three times with deionized water and then dried at 100 °C overnight. The resulting CMS-M (M = Cu, Co, Ni, Zn, Mn) composites were heated to 500 °C in air at a rate of 2 °C min⁻¹, maintained at 500 °C for 1 hour, and then cooled naturally. The hollow metal oxides, including CuO, Co3O4, NiO, ZnO, and MnO2, were collected.
Synthesis of Fe2-N6
300.0 mg of Fe2O3 nanotubes were dispersed in 200.0 mL of freshly prepared tris-buffer solution (10 mM, pH 8.5) by ultrasonication for 1 h to form a suspension. Then 10.0 mL of urea (100.0 mg) and 20.0 mL of dopamine hydrochloride (310.0 mg) were added to the buffer solution. The mixed solution was stirred for 18 h at room temperature to finish the polymerization of dopamine, resulting in the formation of Fe2O3@polydopamine (Fe2O3@PDA). The resulting products were washed with DI water and ethanol three times and collected by centrifugation. After being dried at 80 °C, the Fe2O3@PDA was carbonized at 700 °C for 2 h under Ar atmosphere with a heating rate of 3 °C min-1 in a tube furnace. Then, the pyrolyzed products were etched with 4 M HCl solution for 72 h and further etched by 1 M H2SO4 for 12 h. Finally, 42 mg of double-layer hollow nanotubes containing Fe2-N6 was obtained after being filtered, washed with deionized water, and dried under vacuum. Following the same procedure, we synthesized the Fe2-N6-containing nanotubes with different thicknesses by adjusting the content of dopamine hydrochloride. Pure PDA nanoparticles were synthesized according to the same procedure without the addition of Fe2O3 seeds. After high-temperature treatment, nitrogen-doped carbon was achieved.
The sample yields were calculated as follows. In the synthesis process, 310 mg of dopamine hydrochloride was used as a polymer precursor for the synthesis of PDA layer that coated on the surface of hollow Fe2O3 tubes. After polymerization, pyrolysis and etching treatments, 42 mg of Fe2-N6 catalyst was obtained (2.36 wt% of Fe atoms anchored on double-layered carbon nanotubes), realizing a yield efficiency of ~13.2% (considered from the polymer precursor). Considering the etching process, we also calculated the yield of Fe during the synthesis. 300 mg of Fe2O3 nanotubes were used as a seed precursor for the preparation of Fe2-N6. Finally, 0.99 mg of Fe were fixed as single metal atoms that existed in Fe2-N6. As such, the yield of Fe atoms was only 0.33%. Although the majority of Fe atoms were etched by HCl, the obtained FeCl2 and FeCl3 could be re-used and oxidized to FeCl3 aqueous solution. This solution was just the chemical reagent (Fe precursor) for the preparation of Fe2O3 hollow nanotubes, realizing the recycling of the Fe element.
Synthesis of Fe2-N6 with other ligands or different pyrolysis temperature
Fe2-N6 catalysts were also synthesized according the same procedure except for substituting urea with other ligands in terms of the same amount, such as thiourea and ethanediamine. Also, the Fe2-N6 catalysts were also synthesized by adjusting the amount of urea, as seen in supplementary Table S3. The usages of urea are 0, 10, 30, 50, 100 and 120 mg, respectively. Furthermore, Fe2-N6 catalyst with 50 mg of urea has also been produced at low temperature of 500 °C.
Synthesis of Fe1-N4
For comparison, double layer hollow nanotubes containing Fe1-N4 were prepared by the same procedure of Fe2-N6 except for adding urea.
Synthesis of Cu-Nx, Co-Nx, Ni-Nx, Zn-Nx, Mn-Nx moieties
For the preparation of Cu-Nx, Co-Nx, Ni-Nx, Zn-Nx, and Mn-Nx moieties, metal oxide nanomaterials (CuO, Co3O4, NiO, ZnO and MnO2) were used as supporting templates, while PDA was coated on their surface with urea as an additional N resource. Specially, Cu2O templates were synthesized according to a wet chemistry approach38. Co3O4, NiO, ZnO, and MnO2 templates were synthesized according to the previously reported literature39,40. The following preparation processes including coating with PDA and urea, pyrolysis, as well as chemical etching are similar to that of Fe2-N6.
Synthesis of Fe moiety with ligand of ethanediamine and thiourea
Different ligands, including ethanediamine and thiourea, were also used to produce Fe moieties. The synthesis procedures were similar to that of Fe2-N6 except for using ethanediamine and thiourea to replace urea, respectively.
Acetylene TPD experiments
Temperature-programmed desorption (TPD) studies of acetylene were conducted utilizing a Micromeritics AutoChem II analyzer for chemisorption, connected to a Pfeiffer OmniStar mass spectrometer41,42. In a typical experiment, 50 mg of the sample was pre-treated with a 10% H2/He mixture at 350 °C for a duration of 2 hours, then subjected to He purging at 300 °C for 1 hour. The sample was allowed to cool down to ambient temperature in a He atmosphere. After cooling, acetylene was admitted to the system to achieve saturation levels on the sample surface. A He purge at 25 °C was applied for 1 hour to remove any unreacted acetylene. The temperature was then elevated from 25 °C to 300 °C at a controlled rate of 10 °C per minute in a He flow, while concurrent acquisition of TPD spectra.
Characterizations
The crystallographic structure of prepared samples were determined by an XRD diffractometer (Bruker. D8 Advanced), scanning over an 2Theta range of 5 - 80o with Cu Kα radiation at a wavelength of 0.154056 nm. SEM images were carried out on a Hitachi SU8010 with a field emission gun, operating at an acceleration voltage of 5 kV. TEM images were characterized by a FEI Tecnai G2 F20. EDS detections were captured with a super-X detector with X-ray fluorescence emission system. XPS experiments were conducting using PHI QUANTUM2000 spectrometer. The iron concentration was quantified by ICP-OES on a Shimadzu ICPS-8100 instrument. HAADF-STEM was employed for the Fe2-N6 and Fe1-N4 catalysts, utilizing a JEOL JEM ARM300F microscope with a resolution capability of 0.063 nm. Nitrogen adsorption-desorption isotherms were measured with a Micrometrics ASAP 2020 system. The specific surface areas and pore size distribution were calculated applying the BET equation and the nonlinear DFT model. The nitrogen content in the final Fe2-N6 sample was quantified using an Element Analyzer from UNICUBE in Germany.
Electrochemical measurements for ORR
RDE measurements
Electrochemical measurements were conducted utilizing a standard three-electrode system with a CHI760E electrochemical workstation (CH Instruments), complemented by a rotation apparatus from Pine Research Instrumentation. A glassy carbon disk electrode, procured from Pine Research Instrumentation in USA, functioned as the working electrode, and an Ag/AgCl (KCl, 3 M) electrode served as the reference. A platinum wire fulfilled the role of the counter electrode. Reported potentials are referenced to the standard reversible hydrogen electrode (RHE).
For the preparation of the working electrode, a mixture of 2 mg of the sample and 5 μL of a 5 wt% Nafion solution in 400 μL of ethanol was sonicated for 1 hour to achieve a homogeneous ink. Then, 10 μL of this ink was pipetted onto a glassy carbon electrode with a diameter of 5 mm, resulting in a catalyst mass loading of 0.255 mg cm⁻². As a compared sample, 2 mg of Pt/C was dispersed in 400 μL of ethanol along with 5 μL of the 5 wt% Nafion solution, and applying 10 μL to a glassy carbon electrode. The inks were allowed to dry at room temperature to ensure an uniform distribution across the electrode surface.
Electrochemical experiments were executed in an O₂-saturated 0.1 M KOH aqueous electrolyte. Cyclic voltammetry (CV) measurements were performed at a scan rate of 50 mV s⁻1. Linear sweep voltammetry (LSV) curves were recorded by using a rotating disk electrode (RDE) with a rotation speed of 1600 rpm and a scan rate of 10 mV s⁻1. LSV polarization curves were derived at various rotation speeds ranging from 400 to 2500 rpm, with background currents being corrected using data from Ar-saturated electrolyte. To assess the stability of the oxygen reduction reaction (ORR), an accelerated durability test (ADT) was conducted at potential of 0.6 V and 1.2 V, with a scan rate of 100 mV s⁻1 over 50,000 cycles. Following this, the ORR steady-state polarization curves were obtained in the O₂-saturated 0.1 M KOH solution. The methanol tolerance of samples was assessed by introducing 2 mL of methanol into the electrolyte at 500 seconds mark of the chronoamperometric measurements.
The applied potential relative to Ag/AgCl (KCl, 3 M) is converted to the reversible hydrogen electrode (RHE) potential using the following equation:
| 1 |
where EAg/AgCl was the experimentally measured potential using Ag/AgCl as the reference electrode and E°Ag / AgCl was 0.199 V.
The number of electrons transferred (n) was calculated according to the K-L equation:
| 2 |
| 3 |
| 4 |
J, JK and JL represent the current density, the kinetic and diffusion-limiting current densities, respectively. The angular velocity of the rotating electrode is defined as ω = 2πN, where N is the linear rotation speed. F refers to the Faraday constant, which is 96485 C mol−1. The concentration of O2 in 0.1 M KOH is given as C0 = 1.2×10−6 mol cm−3, and the diffusion coefficient of O2 in this solution is D0 = 1.9×10−5 cm2 s−1. Additionally, ν denotes the kinematic viscosity of the electrolyte, with a value of 0.01 cm2 s−1, and k represents the electron transfer rate constant. Electrochemical impedance spectroscopy (EIS) tests were performed over a frequency range from 100 kHz to 100 mHz, using an amplitude of 5 mV to facilitate IR correction.
Catalytic activity comparison between Fe1-N4 and Fe2-N6
In order to make a comparison between the catalytic activities of Fe1-N4 and Fe2-N6 samples, we mixed Fe2-N6 with the C-N matrix in an electrode to ensure the same content of Fe atoms in the prepared electrode as that in Fe1-N4. For Fe1-N4, 2 mg of the sample (0.32 wt% Fe) and 5 μL of Nafion solution (5 wt%) were dispersed in 400 μL of ethanol solution by sonication for 1 h to form a homogeneous ink. Next, 10 μL of the catalyst dispersion was pipetted onto a glassy carbon electrode with a diameter of 5 mm. The Fe content in this electrode was 1.6 × 10-4 mg. For Fe2-N6, 0.678 mg of the sample (2.36 wt%) and 4.32 mg of C-N sample were mixed with 12 μL of Nafion solution in 1000 μL of ethanol solution by sonication for 1 h to form homogeneous ink. Next, 10 μL of the catalyst dispersion was pipetted onto a glassy carbon electrode with a diameter of 5 mm. The mixed catalyst was denoted as Fe2-N6/C-N. As such, the Fe content in this electrode was also 1.6 × 10-4 mg, equal to that of the electrode containing Fe1-N4. And the electrochemical analysis was the same as RDE measurements mentioned above.
RRDE measurements
Catalyst inks and electrodes were prepared as aforementioned in RDE measurements The disk electrode underwent cathodic polarization at a linear sweep rate of 5 mV s⁻¹, with the ring electrode held at a fixed potential of 0.5 V versus Ag/AgCl. The data gleaned from the rotating ring-disk electrode (RRDE) studies facilitated the calculation of HO2⁻ yield and the electron transfer number (n), employing the following equations.
| 5 |
| 6 |
where id and ir denote the disk current and the ring current, respectively. And N represents the current collection efficiency of the Pt ring with a value of 0.37, which was provided by the manufacturer.
CO-tolerance experiments
The tolerance of Fe2-N6 to CO compared with those of Fe1-N4 and Pt/C (20%) electrocatalyst was carried out by the chronoamperometric response at 0.1 V for the three electrodes. CO (v/v = 1:9, CO/O2) was introduced into the O2-saturated 0.1-M KOH electrochemical cell at 1550 s. Afterwards, the response of current density was recorded for another 8450 s.
XAFS measurements
Data pertaining to X-ray absorption fine structure (XAFS) at the Fe K-edge were acquired at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF), which functions at an energy of 3.5 GeV with a constant current of 220 mA, operating in “top-up” mode. The XAFS measurements were performed in fluorescence mode, using a 32-element Ge solid-state detector array. The energy calibration was referenced against the absorption edge of pure Fe powder. The Athena and Artemis software packages were used for data extraction and profile fitting.
For X-ray absorption near-edge structure (XANES) analysis, the experimental absorption coefficients, represented as a function of energy, μ(E), underwent background removal and normalization to yield “normalized absorption” spectra. For the extended X-ray absorption fine structure (EXAFS) analysis, the data were subjected to Fourier-transformed (FT) to obtain the radial distribution in R space. This analysis was conducted using either a first-shell approximation or a metallic Fe model to account for Fe-O and Fe-Fe scattering paths. The passive electron factor (S₀²) was ascertained by fitting the Fe foil data, with the coordination number (CN) of the Fe-Fe shell fixed to enable further analysis of the measured samples. The fitting process allowed for the variation of parameters describing the electronic properties (such as the correction to the photoelectron energy origin, E₀), as well as the local structural environment, including CN, bond distance (R), and Debye-Waller factor (σ²) around the absorbing atoms.
Assembly of Zn-air batteries
All the electrochemical performance of Zn-air batteries was evaluated in home-built electrochemical devices. Fe2-N6 catalyst ink was loaded on a PTFE-treated porous carbon paper (1 × 1 cm2) and further used as the air cathode. A polished zinc foil was utilized as the anode. 6 M KOH aqueous solution with 0.2 M zinc acetate was used as the electrolyte. Polarization curves and galvanostatic discharge tests were carried out on an electrochemical workstation (CHI 760E) and LAND testing systems. Amount of catalysts used in cathode was 0.25 mg cm-2 for Fe2-N6, Fe1-N4 and commercial Pt/C.
Assembly of flexible solid-state Zn-air batteries
A polished zinc plate was used as anode, 6 M KOH + 0.2 M Zn(OAc)2 filled PAM/MMT organic hydrogel was used as solid electrolyte and Arc-Co SAC or Pt/C+Ir/C catalyst on carbon cloth was used as the air cathodes. To form the solid polymer electrolyte, 5 g acrylamide (AM), 5 mg N-N methylene bisacrylamide (MBA), and 12.5 mg potassium persulfate (KPS) were added into 20 mL mixed solvent of deionized water and dimethyl sulfoxide (DMSO) (1:1, water: DMOS). The mixed solution was stirred for 30 min before 15 mg montmorillonite (MMT) was added and the obtained mixture was kept stirring for 30 min. The obtained gel was moved to the mold and the polymerization was occurred at 60 °C for 16 h. After cooling process, the obtained PAM/MMT organic hydrogel was immersed in 6 M KOH + 0.2 M Zn(OAc)2 solution for 72 h before used. To package the flexible solid Zn-Air battery, carbon cloth that coated with catalyst and the polished zinc plate were placed on opposite sides of the polymer electrolyte film. All tests for the flexible solid Zn-Air battery were performed on the LAND-CT2001A test system (Land). A cycling test was performed using recurrent galvanostatic pulses for 10 min of discharge, followed by 10 min of charge. And for the large current density, the discharge and charge time were changed to 20 min.
DFT calculations
All density functional theory (DFT) calculations were implemented using the Vienna Ab initio Simulation Package (VASP)43. The generalized gradient approximation (GGA) with Perdew-Burke-Ernzerhof (PBE) functional and the projector augmented wave (PAW) potential were used to describe the exchange-correlation energy and the electron-ion interaction, respectively44,45. The DFT-D3 empirical correction method was employed to accurately describe the van der Waals interaction46. The Fe1-N4 model contains a Fe atom, four nitrogen atoms and forty-four carbon atoms in a supercell, and Fe2-N6 model contains two Fe atoms, six nitrogen atoms and forty carbon atoms. The convergence of energy and forces were 1×10-5 eV and 0.005 eV·Å−1, respectively. The cut-off energy for the plane wave basis set is 500 eV. Γ-centered k-grids of 5×5×1 and 17×17×1 with the Monkhorst-Pack scheme are used in geometry optimizations and electronic-structure calculations, respectively47. The adsorption energy (Ea) was calculated according to the following equation:
| 7 |
where Etotal, Esubstrate and EO2 are the energies of O2 absorbed on matrix, Fe single atom matrix or adjacent Fe single atom matrix, and O2, respectively. The charge difference for substrate-adsorbate hybrids can be determined using the following equation:
| 8 |
where ρtotal, ρsubstrate, and ρO2 correspond to the charge density of substrate after O2 adsorption, charge density of pure substrate and change density of O2, respectively.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors acknowledge the fund support of the National Key Research and Development Program of China (2021YFA1500500, 2023YFA1508003), CAS Project for Young Scientists in Basic Research (YSBR-051), National Science Fund for Distinguished Young Scholars (21925204), NSFC (22475003, 22479001, 22221003, 22250007, 22308346, 22209063), the DNL Cooperation Fund, CAS (DNL202003), Anhui National Science Fund for Distinguished Young Scholars (2308085J16, 2408085J008), the Key Research and Development Program of Xuzhou (KC22497), the Natural Science Foundation of Xuzhou City (KC21283), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Postgraduate Research and Practical Innovation Program of Jiangsu Province (KYCX20_2230), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (YLU-DNL Fund 2022012), Joint Funds from the Hefei National Synchrotron Radiation Laboratory (KY9990000202), Natural Science Foundation of Anhui Province (2208085QB42), the State Key Laboratory of Catalysis (2024SKL-A-01), and USTC Research Funds of the Double First-Class Initiative (YD9990002014). J.Z. acknowledges support from the New Cornerstone Science Foundation through the XPLORER PRIZE. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. This work was also partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication.
Author contributions
Y.Y., M.L., H.L., and J.Z. designed the studies and wrote the paper. Y.Y., R.Y., and M.L. synthesized catalysts. M.L., Z.Q., J.Y., and H.L. performed catalytic tests. M.L., S.H., and H.L. conducted characterizations. Y.Y. contributed to DFT calculations. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Jin-Song Hu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mingkai Liu, Email: liumingkai@ahut.edu.cn.
Hongliang Li, Email: lihl@ustc.edu.cn.
Jie Zeng, Email: zengj@ustc.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55630-y.
References
- 1.Collman, J. P. et al. A Cytochrome c Oxidase Model Catalyzes Oxygen to Water Reduction Under Rate-Limiting Electron Flux. Science315, 1565–1568 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao, X. et al. Atomic Bridging Structure of Nickel–Nitrogen–Carbon for Highly Efficient Electrocatalytic Reduction of CO2. Angew. Chem. Int. Ed.61, e202113918 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Fu, J. et al. Synergistic Effects for Enhanced Catalysis in a Dual Single-Atom Catalyst. ACS Catal.11, 1952–1961 (2021). [Google Scholar]
- 4.Wang, Y. et al. Flexible carbon nanofiber film with diatomic Fe-Co sites for efficient oxygen reduction and evolution reactions in wearable zinc-air batteries. Nano Energy87, 106147 (2021). [Google Scholar]
- 5.Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotech.13, 411–417 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science352, 797–800 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Cao, S. et al. High-loading single Pt atom sites [Pt-O(OH)x] catalyze the CO PROX reaction with high activity and selectivity at mild conditions. Sci. Adv. 6, eaba3809. [DOI] [PMC free article] [PubMed]
- 8.Zhou, Y. et al. Dual-Metal Interbonding as the Chemical Facilitator for Single-Atom Dispersions. Adv. Mater.32, 2003484 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Bai, L., Hsu, C.-S., Alexander, D. T. L., Chen, H. M. & Hu, X. A Cobalt–Iron Double-Atom Catalyst for the Oxygen Evolution Reaction. J. Am. Chem. Soc.141, 14190–14199 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Ding, T. et al. Atomically Precise Dinuclear Site Active toward Electrocatalytic CO2 Reduction. J. Am. Chem. Soc.143, 11317–11324 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Zhang, X. et al. Platinum–copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun.10, 5812 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han, A. et al. An Adjacent Atomic Platinum Site Enables Single-Atom Iron with High Oxygen Reduction Reaction Performance. Angew. Chem. Int. Ed.60, 19262–19271 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Chukwu, E. & Yang, M. Uncertainties in the reactivity of atomically dispersed catalytic metal: Can any single-atom catalyst work like a charm? Chem Catal. 3 (2023).
- 14.Hai, X. et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat. Nanotech.17, 174–181 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Xia, C. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem.13, 887–894 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Wang, J. et al. Design of N-Coordinated Dual-Metal Sites: A Stable and Active Pt-Free Catalyst for Acidic Oxygen Reduction Reaction. J. Am. Chem. Soc.139, 17281–17284 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Li, J. et al. Modulating the Electronic Coordination Configuration and d-Band Center in Homo-Diatomic Fe2N6 Catalysts for Enhanced Peroxymonosulfate Activation. ACS Appl. Mater. Interfaces14, 37865–37877 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Hao, Q. et al. Nickel dual-atom sites for electrochemical carbon dioxide reduction. Nat. Synth.1, 719–728 (2022). [Google Scholar]
- 19.Zhu, C. et al. Hierarchically Porous M–N–C (M = Co and Fe) Single-Atom Electrocatalysts with Robust MN Active Moieties Enable Enhanced ORR Performance. Adv. Energy Mater.8, 1801956 (2018). [Google Scholar]
- 20.Han, L. et al. Modulating Single-Atom Palladium Sites with Copper for Enhanced Ambient Ammonia Electrosynthesis. Angew. Chem. Int. Ed.60, 345–350 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Wei, Y.-S. et al. Fabricating Dual-Atom Iron Catalysts for Efficient Oxygen Evolution Reaction: A Heteroatom Modulator Approach. Angew. Chem. Int. Ed.59, 16013–16022 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Jiao, L. et al. Non-Bonding Interaction of Neighboring Fe and Ni Single-Atom Pairs on MOF-Derived N-Doped Carbon for Enhanced CO2 Electroreduction. J. Am. Chem. Soc.143, 19417–19424 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Zhang, M. et al. Metal (Hydr)oxides@Polymer Core–Shell Strategy to Metal Single-Atom Materials. J. Am. Chem. Soc.139, 10976–10979 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Liu, M. et al. A “Pre-Constrained Metal Twins” Strategy to Prepare Efficient Dual-Metal-Atom Catalysts for Cooperative Oxygen Electrocatalysis. Adv. Mater.34, 2107421 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Jin, Z. et al. Understanding the inter-site distance effect in single-atom catalysts for oxygen electroreduction. Nat. Catal.4, 615–622 (2021). [Google Scholar]
- 26.Gu, H. et al. Adjacent single-atom irons boosting molecular oxygen activation on MnO2. Nat. Commun.12, 5422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao, J. et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem.11, 222–228 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Wan, J. et al. In Situ Phosphatizing of Triphenylphosphine Encapsulated within Metal–Organic Frameworks to Design Atomic Co-P1N3 Interfacial Structure for Promoting Catalytic Performance. J. Am. Chem. Soc.142, 8431–8439 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Pacilé, D. et al. Electronic properties and atomic structure of graphene oxide membranes. Carbon49, 966–972 (2011). [Google Scholar]
- 30.Wang, M. et al. Non-planar Nest-like [Fe2S2] Cluster Sites for Efficient Oxygen Reduction Catalysis. Angew. Chem. Int. Ed.62, e202300826 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Zhang, S., Qin, Y., Ding, S. & Su, Y. A DFT Study on the Activity Origin of Fe−N−C Sites for Oxygen Reduction Reaction. ChemPhysChem23, e202200165 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Xin, H., Vojvodic, A., Voss, J., Nørskov, J. K. & Abild-Pedersen, F. Effects of $d$-band shape on the surface reactivity of transition-metal alloys. Phys. Rev. B89, 115114 (2014). [Google Scholar]
- 33.Shang, H. et al. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun.11, 3049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan, H. M. et al. Shape-Controlled Synthesis of Single-Crystalline Fe2O3 Hollow Nanocrystals and Their Tunable Optical Properties. J. Phys. Chem. C.113, 9928–9935 (2009). [Google Scholar]
- 35.Guo, J., Wang, R., Tjiu, W. W., Pan, J. & Liu, T. Synthesis of Fe nanoparticles@graphene composites for environmental applications. J. Hazard. Mater.225−226, 63–73 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Lai, X. et al. General Synthesis and Gas-Sensing Properties of Multiple-Shell Metal Oxide Hollow Microspheres. Angew. Chem. Int. Ed.50, 2738–2741 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Wang, J. et al. Multi-shelled metal oxides prepared via an anion-adsorption mechanism for lithium-ion batteries. Nat. Energy1, 16050 (2016). [Google Scholar]
- 38.Fang, Y., Luan, D., Chen, Y., Gao, S. & Lou, X. W. D. Rationally Designed Three-Layered Cu2S@Carbon@MoS2 Hierarchical Nanoboxes for Efficient Sodium Storage. Angew. Chem. Int. Ed.59, 7178–7183 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Wang, J. et al. Accurate Control of Multishelled Co3O4 Hollow Microspheres as High-Performance Anode Materials in Lithium-Ion Batteries. Angew. Chem. Int. Ed.52, 6417–6420 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Wang, J., Wan, J. & Wang, D. Hollow Multishelled Structures for Promising Applications: Understanding the Structure–Performance Correlation. Acc. Chem. Res.52, 2169–2178 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Gu, J. et al. Synergizing metal–support interactions and spatial confinement boosts dynamics of atomic nickel for hydrogenations. Nat. Nanotech.16, 1141–1149 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Zhang, Z. et al. Atomically Dispersed Cobalt Trifunctional Electrocatalysts with Tailored Coordination Environment for Flexible Rechargeable Zn–Air Battery and Self-Driven Water Splitting. Adv. Energy Mater.10, 2002896 (2020). [Google Scholar]
- 43.Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B48, 13115–13118 (1993). [DOI] [PubMed] [Google Scholar]
- 44.Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett.77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- 46.Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys.132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B13, 5188–5192 (1976). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.