Abstract
An accumulating body of evidence suggests a bidirectional relationship between sleep and cardiovascular (CV) health. A high level of evidence has linked obstructive sleep apnea (OSA) with cardiovascular disease (CVD). Accordingly, clinical sleep medicine emphasizes the diagnosis and treatment of OSA in the context of promoting CV health. While continuous positive airway pressure (CPAP), the mainstay treatment for OSA, is effective in improving several sleep-related quality-of-life outcomes and leads to modest reductions in blood pressure, there is currently insufficient evidence to justify using CPAP alone for improving CVD outcomes in OSA. Sleep physicians are uniquely positioned to expand their focus beyond the evaluation of OSA and administering CPAP, in efforts to enhance the CV health of sleep patients. Herein, we suggest the role of sleep physicians as CV preventionists. Key focus areas for managing CV risk beyond CPAP therapy in OSA include identifying comorbid disorders that are vital for optimizing CV health. This involves risk-stratifying patients and providing appropriate counseling, referrals, and treatment as appropriate for comorbid sleep conditions such as insomnia and insufficient sleep, comorbid CV risk factors including hypertension, dyslipidemia, metabolic dysfunction-associated steatohepatitis, as well as counseling for weight management programs, smoking, and alcohol cessation. We urge sleep clinicians to play an active and integral role in optimizing the CV health of patients with sleep disorders.
Keywords: OSA, cardiovascular medicine, obesity
Statement of Significance.
The field of sleep medicine has emphasized the impact of impaired sleep, particularly obstructive sleep apnea (OSA), on adverse cardiovascular (CV) health. However, there is insufficient evidence that continuous positive airway pressure alone improves CV outcomes. Herein, we share our perspective that sleep physicians should consider their role as CV preventionists by providing appropriate counseling, referrals, and treatment as appropriate for comorbid sleep conditions and CV risk factors. This includes behavioral counseling for insomnia, weight management and pharmacologic treatment of obesity, management of hypertension and hyperlipidemia, treatment of metabolic dysfunction-associated steatohepatitis, as well as smoking cessation. Reimagination of the sleep physician’s role to be more inclusive of other preventative health strategies will benefit the overall CV health of OSA patients.
Background
The field of sleep medicine has emphasized the impact of impaired sleep, particularly through obstructive sleep apnea (OSA), on adverse cardiovascular (CV) health. Observational studies have suggested that both insufficient sleep duration and OSA are independently associated with increased cardiometabolic and CV diseases (CVD) [1, 2]. Continuous positive airway pressure (CPAP) has been the mainstay therapy in OSA management. Addressing adherence to CPAP, including troubleshooting mask interface and pressure adjustment, has been a major role of sleep clinicians in managing OSA. While clinical medicine emphasizes improving CV health through improving CPAP adherence, there is insufficient evidence to justify CPAP alone for improving CV health outcomes in OSA. Furthermore, insomnia is also an independent CV risk factor [3]. It is our experience that clinical sleep practices primarily focus on the diagnosis and treatment of OSA as a means of CV risk reduction.
While CPAP is effective in improving several sleep-related quality-of-life outcomes, and leads to modest reductions in blood pressure (BP), studies have failed to demonstrate a CV benefit in the prevention of composite CV outcomes [4]. We advocate for an expanded role of sleep providers in addressing CV health beyond the narrow focus of merely ordering sleep testing followed by CPAP prescriptions for patients with OSA.
An encounter with a sleep physician provides a unique opportunity to not only evaluate sleep but also address CV health associated with OSA and sleep disruption. The objective of this perspective is to address these gaps and share practical suggestions to empower sleep clinicians to go beyond focusing on CPAP in efforts to improve the CV health of sleep patients.
Current Evidence Regarding Treatment of OSA With CPAP and CV Outcomes
Observational, non-randomized data have suggested that in patients with severe OSA, the use of CPAP treatment may reduce the risk of nonfatal and fatal CV events [5]. Mechanistically, the use of CPAP has also been shown to improve C-reactive protein, catecholamines, and 24-hour BP in those with severe OSA [6]. Among patients with OSA and comorbid hypertension, CPAP results in approximately 2–4 mm Hg of systolic BP reduction [7]. Although early studies suggested that CPAP may be promising for CV risk reduction in OSA, randomized-control trials in the last decade have not demonstrated a meaningful CV benefit with the use of CPAP [8–10]. One limitation to these findings may be related to challenges with adherence to CPAP therapy, where average CPAP adherence was limited to 3–4 hours per night. Further investigations have suggested that investigating specific endophenotypes in OSA subpopulations may be helpful in identifying those for whom CPAP may lower CV risk. For example, those with OSA and excessive daytime sleepiness may be at a higher risk for CVD events and potentially derive benefit from CPAP, though others have challenged this notion [7, 11, 12]. Additionally, patients with OSA and varying physiological responses may manifest CV risk differently. For example, patients with more pronounced pulse arrival time shortening (the time between the R wave and pulse arrival on photoplethysmography) may have increased CV risk [13]. Patients who exhibit an elevated heart rate response to respiratory events (ΔHR) may be at an increased risk of CV morbidity, and derive a greater benefit from CPAP [14, 15].
Emerging Role of the Sleep Specialist as a CV Preventionist
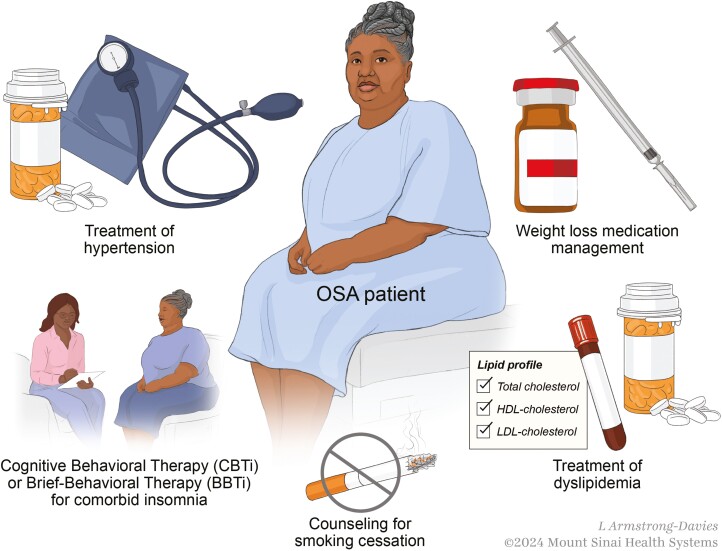
Sleep medicine providers must prioritize preventive and therapeutic interventions alongside CPAP to effectively reduce CV risk. Sleep specialists are well suited to address CV health because patients presenting with sleep symptoms commonly exhibit a high burden of CV risk. Beyond OSA, patients with other sleep symptoms or disorders such as insufficient sleep (short sleep), insomnia, and primary hypersomnia also have increased CV risk [16–18]. A recent systematic review confirms that there is little in the OSA literature focused on the prevention of OSA and other sleep-related and CV-modifiable risk factors. Thus, by concurrently addressing other comorbid risks associated with sleep disorders, sleep specialists can meaningfully contribute to improving CV health (Figure 1). In this context, we emphasize CV risks that sleep specialists can effectively manage as part of their routine patient care.
Figure 1.
Holistic/integrated approach to patient with sleep apnea and cardiometabolic disease (L. Armstrong-Davies, ©2024 Mount Sinai Health Systems).
Weight Management in OSA
Diet, exercise, and bariatric surgery
Weight-loss interventions in OSA are associated with improvements in OSA severity, cardiometabolic comorbidities, and quality of life [19]. The American Thoracic Society recommends tailored weight management strategies in the routine treatment of adults with OSA who are overweight or obese. In particular, comprehensive lifestyle intervention, which includes a program to support a reduced-calorie diet, increased physical activity, and behavioral counseling has been associated with weight loss, reductions in OSA severity, and improvement in daytime sleepiness in patients with OSA who are overweight or obese [20–22]. There is emerging evidence that virtual/web-based behavioral weight-loss programs from physician referral can also provide significant weight loss to over half of the patients studied [23]. Behavioral weight interventions for adults with obesity have also yielded improvements in obesity in a primary care setting and broader integration into specialty care may enhance these benefits [24]. Referral to virtual/web-based weight-loss programs is not time intensive and may be accomplished with the potential for lasting results [24].
Pharmacologic management of obesity and the role of incretins
Weight loss and aerobic exercise are essential components of OSA treatment and CV risk reduction. Excess body weight is estimated to be responsible for over half of moderate-to-severe OSA [25, 26]. In the Wisconsin-Sleep cohort, a 10% reduction in weight decreased the AHI (apnea–hypopnea index) by 32% [26]. While CPAP has not demonstrated a reduction in CVD risk, a 10% or more weight loss from baseline can result in a 21% or more reduction in CVD [27].
Glucagon-like peptide 1 (GLP-1) based therapies have emerged as a powerful pharmacologic tool in weight loss. GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) are gastrointestinal peptides also known as incretin hormones. Liraglutide was the first GLP-1 receptor agonist (GLP-1RA) approved for obesity. In a trial of patients without diabetes, weight loss was significantly greater in the 3 mg liraglutide group (−8.0 vs. −2.6 kg in the placebo group) [28]. Semaglutide is also a once-weekly GLP-1RA. Studies have demonstrated weight loss in individuals with and without type 2 diabetes [29]. There is also evidence of CVD benefits with both liraglutide and semaglutide for secondary CVD prevention. In the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial, CV events occurred less frequently in the semaglutide group (6.5% vs. 8% in placebo, HR 0.80, p < 0.001). Tirzepatide, the most recently approved GLP-1, is a dual GIP and GLP-1RA with significant efficacy for weight reduction. A meta-analysis of six tirzepatide trials found that tirzepatide 15 mg resulted in a mean −20.9% change in body weight compared with −3.9% in the placebo group [30, 31].
These promising medications have been recently investigated in patients with OSA and obesity. In a small randomized, double-blind trial of nondiabetic patients with obesity and moderate-to-severe OSA, liraglutide as an adjunct to diet/exercise reduced the mean AHI compared with placebo (−12.2 vs. −6.1 events/hour) [32]. SURMOUNT-OSA is a larger randomized, placebo-controlled 52-week phase 3 clinical trial, evaluating the safety and efficacy of tirzepatide for the treatment of patients with obesity and moderate-to-severe OSA [33]. This study showed that in patients with moderate-to-severe OSA and obesity, tirzepatide provided a robust improvement in the AHI, reduction in hypoxic burden, improvement in BP, as well as sleep-related patient-reported outcomes. These results are promising, not only because of the improvement in OSA severity, but also the adjunct promise of CVD risk reduction. Randomized controlled trials over the last decade have not demonstrated a CVD risk reduction with CPAP in moderate-to-severe OSA [10]. The improvement in OSA is most likely attributed to significant weight loss (−18% vs. −1% change in body weight from the baseline), which was one of the secondary outcomes of the study. While weight loss improves OSA in some cases, it may resolve OSA in others [34]. Thus, it is important to recognize that the beneficial effect on OSA by GLP-1 is likely through weight loss.
Behavioral therapies for insufficient sleep and insomnia
The American Academy of Sleep Medicine (AASM) recommends 7–9 hours of sleep per night. Both insomnia and short sleep duration below 6 hours have been linked with adverse CV outcomes [35]. Sleep clinicians should take a thorough approach to investigating the underlying factors contributing to short sleep duration and insomnia. One effective intervention that can be employed for insomnia is cognitive behavioral therapy for insomnia (CBTi), which is regarded as the first-line treatment for the condition [36]. CBTi is an evidence-based behavioral intervention with a focus on changing the physiological factors, behaviors, and thoughts that contribute to insomnia [37]. One challenge that has emerged with CBTi is that healthcare providers may struggle to connect patients with professionals skilled in this discipline. This difficulty is often due to a shortage of certified CBT-I providers and sleep psychologists, along with limited insurance coverage and the financial burden associated with accessing CBT-I services. Telehealth has emerged as an option for patients after the coronavirus disease 2019 (COVID-19) pandemic to in-part bridge this gap and has been demonstrated to be effective in improving sleep duration and sleep efficiency [38]. When a sleep clinician is not able or skilled in the provision or cannot offer a timely referral for CBTi, there is promising evidence for the benefit of brief behavioral intervention, which can be implemented by any trained sleep clinician, in improving sleep quality and duration [39]. These interventions are also important to consider in patients with OSA, as 30%–40% of patients with OSA are felt to have significant symptoms of insomnia [40]. This new overlap syndrome has been referred to as COMISA, or Comorbid Insomnia and Sleep Apnea [41]. Recognizing this newly identified overlap is crucial, as the combination of these conditions may result in higher morbidity and mortality compared with each disorder alone [42].
Hypertension, chronic kidney disease, metabolic dysfunction-associated steatohepatitis, hyperlipidemia, smoking cessation, and alcohol cessation
Although often the purview of primary care and other specialists, sleep medicine providers can improve CV outcomes by identifying those with comorbid hypertension, hyperlipidemia, chronic kidney disease, and metabolic dysfunction-associated steatohepatitis through basic laboratory analysis, followed by a prompt referral to the appropriate specialist. There is emerging evidence that statins may reduce CV risk after CPAP initiation in those with hyperlipidemia [43].
Cigarette smoking is a major risk factor for CVD. Patients who quit smoking have a lower 5-year CVD risk compared with those who continue to smoke [44]. Smoking may reduce the amount of slow-wave sleep as well as rapid eye movement (REM) sleep and may exacerbate insomnia. Furthermore, the reduced amount of sleep achieved based on the effects of nicotine may also interfere with the ability of patients to abstain from nicotine [45]. Improving sleep quality may even be a method by which to enhance the efforts of patients to abstain from cigarette smoking [46]. The CV risk in the OSA population continues to be above that experienced by never smokers, although remains below the risk of those that continue to smoke [44]. It is postulated that individuals with untreated, undiagnosed OSA may use the stimulating effects of nicotine from cigarettes to partially counteract their sleep disturbances and excessive daytime sleepiness [47].
In addition to cigarette smoking, alcohol usage also has dramatic effects on sleep. Although alcohol reduces sleep latency, it increases sleep fragmentation and decreases overall REM sleep [48]. Alcohol consumption has also been linked to increased CV risk of which the increase in risk occurs immediately after consumption [49]. Screening for alcohol use and cigarette smoking often takes place during the patient’s assessment, presenting an additional opportunity for the sleep physician to enhance the CV health of patients with sleep disorders.
Current and future landscape of the sleep specialists’ practice as a cardiac preventionist
There is limited data regarding the current landscape of sleep physicians’ engagement beyond OSA diagnosis and CPAP therapy in their efforts to address CV health in patients with OSA or other sleep disorders. Our collective observation at the receiving end of consultations has been that diagnosing OSA and managing CPAP therapy represent the primary tasks in a sleep clinician’s daily practice.
A recent systematic review confirms that there is little in the OSA literature focused on the prevention of OSA and modifiable risk factors [50]. Out of all the publications, 720 examined risk factors and prevention of OSA, while 23 674 articles focused on OSA therapeutics. We can only infer that the disproportionately limited focus in the published literature on lifestyle modifications and behavioral interventions also influences provider practices when treating OSA [50].
Clinical sleep medicine is evolving from simply focusing on OSA diagnosis and CPAP prescription in patients with OSA, to the identification and management of modifiable sleep-related and traditional CV risk factors. As such, sleep clinicians are uniquely positioned to play a more active role in optimizing CV health in their patients. While the extent of their involvement may be open to debate, we believe that a more holistic approach—incorporating care beyond OSA and engaging in behavioral and lifestyle counseling for CV health—can significantly improve the CV outcomes of patients with sleep disorders. We recommend that rather than the sleep specialist taking on the role of the primary care physician, they should work collaboratively with other healthcare providers to address CV risk factors that intersect with sleep disorders, ultimately benefiting the patient. Referral pathways may also need to be evaluated, as these may vary depending on practice setting, healthcare system, and country. This operational aspect of sleep medicine is rarely discussed in medical literature, yet it could be a crucial focus as the field navigates its next steps.
Conclusion
Sleep clinicians have often prioritized managing OSA through sleep studies and CPAP implementation, placing significant emphasis on these approaches in their practice. While this is certainly important, especially in patients with symptomatic OSA, this focus may inadvertently minimize attention to other comorbid disorders, such as insomnia and circadian rhythm disorders, which can also significantly impact CV disease risk. While addressing and treating symptomatic OSA remains critical, implementing CPAP therapy to merely improve CV risks is controversial based on the current body of evidence. We envision the sleep clinician of the future as a preventionist. This holistic approach to sleep care involves not only comprehensive sleep management but also offering appropriate behavioral and lifestyle counseling. It includes established CV risk reduction strategies and appropriate referrals and consultations for weight management, hypertension, and hyperlipidemia, and supporting smoking cessation efforts.
Contributor Information
William J Healy, Division of Pulmonary, Critical Care, and Sleep Medicine, Medical College of Georgia at Augusta University, Augusta, GA, USA.
Vaishnavi Kundel, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Pam R Taub, Division of Cardiology, Department of Medicine, University of California San Diego, San Diego, CA, USA.
Yeilim Cho, VISN20 Northwest Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Puget Sound Health Care System, UW Department of Psychiatry and Behavioral Sciences, Seattle, WA, USA.
Sara J Healy, Division of Endocrinology and Metabolism, Medical College of Georgia at Augusta University, Augusta, GA, USA.
Younghoon Kwon, Division of Cardiology, University of Washington, Seattle, WA, USA.
Disclosure Statement
Financial disclosure: Y.K. has received funding from NIH R21HL167126 and R01HL158765. Nonfinancial disclosure: None declared.
Author Contributions
William Healy (Conceptualization [equal], Project administration [equal], Supervision [equal], Writing—original draft [equal], Writing—review & editing [equal]), Vaishnavi Kundel (Writing—original draft [equal], Writing—review & editing [equal]), Pam Taub (Writing—original draft [equal], Writing—review & editing [equal]), Yeilim Cho (Writing—original draft [equal], Writing—review & editing [equal]), Sara Healy (Writing—original draft [equal], Writing—review & editing [equal]), and Younghoon Kwon (Conceptualization [equal], Writing—original draft [equal], Writing—review & editing [equal])
Data Availability
No new data were generated or analyzed in support of this article.
References
- 1. Yin J, Jin X, Shan Z, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9):e005947. doi: https://doi.org/ 10.1161/JAHA.117.005947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han B, Chen WZ, Li YC, Chen J, Zeng ZQ.. Sleep and hypertension. Sleep Breath. 2020;24(1):351–356. doi: https://doi.org/ 10.1007/s11325-019-01907-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Javaheri S, Redline S.. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. doi: https://doi.org/ 10.1016/j.chest.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ni YN, Lei F, Tang X, Liang Z, Thomas RJ.. The association between the effective apnea-hypopnea index and blood pressure reduction efficacy following CPAP/oxygen treatment. Sleep Med. 2024;117:46–52. doi: https://doi.org/ 10.1016/j.sleep.2024.02.046 [DOI] [PubMed] [Google Scholar]
- 5. Marin JM, Carrizo SJ, Vicente E, Agusti AGN.. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: https://doi.org/ 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 6. Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF.. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: https://doi.org/ 10.1164/rccm.200703-500OC [DOI] [PubMed] [Google Scholar]
- 7. Labarca G, Schmidt A, Dreyse J, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): systematic review and meta-analysis. Sleep Med Rev. 2021;58:101446. doi: https://doi.org/ 10.1016/j.smrv.2021.101446 [DOI] [PubMed] [Google Scholar]
- 8. McEvoy RD, Antic NA, Heeley E, et al. ; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: https://doi.org/ 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 9. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E.. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: https://doi.org/ 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 10. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, et al. ; Spanish Sleep Network. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8(4):359–367. doi: https://doi.org/ 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- 11. Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI.. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506. doi: https://doi.org/ 10.1164/rccm.201808-1509OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eulenburg C, Celik Y, Redline S, et al. Cardiovascular outcomes in adults with coronary artery disease and obstructive sleep apnea with versus without excessive daytime sleepiness in the RICCADSA clinical trial. Ann Am Thorac Soc. 2023;20(7):1048–1056. doi: https://doi.org/ 10.1513/AnnalsATS.202208-676OC [DOI] [PubMed] [Google Scholar]
- 13. Kwon Y, Wiles C, Parker BE, et al. Pulse arrival time, a novel sleep cardiovascular marker: the multi-ethnic study of atherosclerosis. Thorax. 2021;76(11):1124–1130. doi: https://doi.org/ 10.1136/thoraxjnl-2020-216399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azarbarzin A, Zinchuk A, Wellman A, et al. Cardiovascular benefit of continuous positive airway pressure in adults with coronary artery disease and obstructive sleep apnea without excessive sleepiness. Am J Respir Crit Care Med. 2022;206(6):767–774. doi: https://doi.org/ 10.1164/rccm.202111-2608OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azarbarzin A, Sands SA, Younes M, et al. The sleep apnea–specific pulse-rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med. 1546;203(12):1546–1555. doi: https://doi.org/ 10.1164/rccm.202010-3900oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Black J, Reaven NL, Funk SE, et al. The Burden of Narcolepsy Disease (BOND) study: health-care utilization and cost findings. Sleep Med. 2014;15(5):522–529. doi: https://doi.org/ 10.1016/j.sleep.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 17. Ben-Joseph RH, Saad R, Black J, et al. Cardiovascular Burden of Narcolepsy Disease (CV-BOND): a real-world evidence study. Sleep. 2023;46(10). doi: https://doi.org/ 10.1093/sleep/zsad161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park JA, Yoon JE, Liu X, et al. Cardiovascular implications of sleep disorders beyond sleep apnea. Curr Sleep Med Rep. 2024;10(3):320–328. doi: https://doi.org/ 10.1007/s40675-024-00302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hudgel DW, Patel SR, Ahasic AM, et al. ; American Thoracic Society Assembly on Sleep and Respiratory Neurobiology. The role of weight management in the treatment of adult obstructive sleep apnea. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(6):e70–e87. doi: https://doi.org/ 10.1164/rccm.201807-1326ST [DOI] [PubMed] [Google Scholar]
- 20. Carneiro-Barrera A, Amaro-Gahete FJ, Guillén-Riquelme A, et al. Effect of an interdisciplinary weight loss and lifestyle intervention on obstructive sleep apnea severity: the INTERAPNEA randomized clinical trial. JAMA Netw Open. 2022;5(4):e228212. doi: https://doi.org/ 10.1001/jamanetworkopen.2022.8212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–2275. doi: https://doi.org/ 10.1056/NEJMoa1306187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin CF, Ho NH, Hsu WL, Lin CH, Wang YH, Wang YP.. Effects of aerobic exercise and resistance training on obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med. 2024;20(11):1839–1849. doi: https://doi.org/ 10.5664/jcsm.11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas JG, Leahey TM, Wing RR.. An automated internet behavioral weight-loss program by physician referral: a randomized controlled trial. Diabetes Care. 2014;38(1):9–15. doi: https://doi.org/ 10.2337/dc14-1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madigan CD, Graham HE, Sturgiss E, et al. Effectiveness of weight management interventions for adults delivered in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2022;377:e069719. doi: https://doi.org/ 10.1136/bmj-2021-069719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young T, Peppard PE, Taheri S.. Excess weight and sleep-disordered breathing. J Appl Physiol (1985). 2005;99(4):1592–1599. doi: https://doi.org/ 10.1152/japplphysiol.00587.2005 [DOI] [PubMed] [Google Scholar]
- 26. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J.. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: https://doi.org/ 10.1001/jama.284.23.3015 [DOI] [PubMed] [Google Scholar]
- 27. Gregg E, Jakicic J, Blackburn G, et al. ; Look AHEAD Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. doi: https://doi.org/ 10.1016/S2213-8587(16)30162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pi-Sunyer X, Astrup A, Fujioka K, et al. ; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: https://doi.org/ 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 29. Wilding JPH, Batterham RL, Calanna S, et al. ; STEP 1 Study Group. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: https://doi.org/ 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 30. Jastreboff AM, Aronne LJ, Ahmad NN, et al. ; SURMOUNT-1 Investigators. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: https://doi.org/ 10.1056/NEJMoa2206038 [DOI] [PubMed] [Google Scholar]
- 31. de Mesquita YLL, Pera Calvi I, Reis Marques I, et al. Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: a meta-analysis of randomized controlled trials. Int J Obes (Lond). 2023;47(10):883–892. doi: https://doi.org/ 10.1038/s41366-023-01337-x [DOI] [PubMed] [Google Scholar]
- 32. Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40(8):1310–1319. doi: https://doi.org/ 10.1038/ijo.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malhotra A, Grunstein RR, Fietze I, et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med. 2024;391(13):1193–1205. doi: https://doi.org/ 10.1056/NEJMoa2404881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuna ST, Reboussin DM, Strotmeyer ES, et al. ; Sleep AHEAD Research Subgroup of the Look AHEAD Research Group. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the sleep AHEAD study. Am J Respir Crit Care Med. 2021;203(2):221–229. doi: https://doi.org/ 10.1164/rccm.201912-2511OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Itani O, Jike M, Watanabe N, Kaneita Y.. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: https://doi.org/ 10.1016/j.sleep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 36. Morin CM, Jarrin DC.. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2022;17(2):173–191. doi: https://doi.org/ 10.1016/j.jsmc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 37. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255–262. doi: https://doi.org/ 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasan F, Tu YK, Yang CM, et al. Comparative efficacy of digital cognitive behavioral therapy for insomnia: a systematic review and network meta-analysis. Sleep Med Rev. 2022;61:101567. doi: https://doi.org/ 10.1016/j.smrv.2021.101567 [DOI] [PubMed] [Google Scholar]
- 39. Kwon M, Wang J, Wilding G, Dickerson SS, Dean GE.. Brief behavioral treatment for insomnia: a meta-analysis. Behav Sleep Med. 2022;20(6):674–694. doi: https://doi.org/ 10.1080/15402002.2021.1982715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Ren R, Lei F, et al. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2019;45:1–17. doi: https://doi.org/ 10.1016/j.smrv.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 41. Sweetman AM, Lack LC, Catcheside PG, et al. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med Rev. 2017;33:28–38. doi: https://doi.org/ 10.1016/j.smrv.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 42. Lechat B, Loffler KA, Wallace DM, et al. All-cause mortality in people with co-occurring insomnia symptoms and sleep apnea: analysis of the Wisconsin sleep cohort. Nat Sci Sleep. 2022;14:1817–1828. doi: https://doi.org/ 10.2147/NSS.S379252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah R, Patel N, Emin M, et al. Statins restore endothelial protection against complement activity in obstructive sleep apnea: a randomized clinical trial. Ann Am Thorac Soc. 2023;20(7):1029–1037. doi: https://doi.org/ 10.1513/AnnalsATS.202209-761OC [DOI] [PubMed] [Google Scholar]
- 44. Duncan MS, Freiberg MS, Greevy RA, Kundu S, Vasan RS, Tindle HA.. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA. 2019;322(7):642–650. doi: https://doi.org/ 10.1001/jama.2019.10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patterson F, Grandner MA, Malone SK, Rizzo A, Davey A, Edwards DG.. Sleep as a target for optimized response to smoking cessation treatment. Nicotine Tob Res. 2019;21(2):139–148. doi: https://doi.org/ 10.1093/ntr/ntx236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matthews JA, Sallis HM, Dyer ML, McConville R, Isotalus H, Attwood AS.. Associations between self-reported sleep quality, fatigue severity, factors associated with successful cessation, and cessation beliefs among regular smokers. Nicotine Tob Res. 2024;26(7):835–842. doi: https://doi.org/ 10.1093/ntr/ntad231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pataka A, Kotoulas S, Kalamaras G, et al. Does smoking affect OSA? What about Smoking cessation? J Clin Med. 2022;11(17):5164. doi: https://doi.org/ 10.3390/jcm11175164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB.. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539–549. doi: https://doi.org/ 10.1111/acer.12006 [DOI] [PubMed] [Google Scholar]
- 49. Mostofsky E, Chahal HS, Mukamal KJ, Rimm EB, Mittleman MA.. Alcohol and immediate risk of cardiovascular events: a systematic review and dose-response meta-analysis. Circulation. 2016;133(10):979–987. doi: https://doi.org/ 10.1161/CIRCULATIONAHA.115.019743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Correa EJ, Conti DM, Gozal D, O’Connor-Reina C.. Preventive medicine in obstructive sleep apnea—a systematic review and a call to action. Sleep. 2024;47. doi: https://doi.org/ 10.1093/sleep/zsae164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this article.