Abstract
Background
Geraniums (Pelargonium) are among the most popular flowers worldwide. Viral infection is one of the main problems of the genus Pelargonium, and the production of virus-free mother plants is necessary for large-scale geranium propagation and exchange. Meristem culture and thermotherapy are two effective procedures that have been widely adopted to produce healthy virus-free plant stocks. The present study explores the efficiency of a combination of these two methods for virus eradication in two important Pelargonium species, Pelargonium X hortorum (‘Zonal’) and Pelargonium × domesticum (‘Regal’).
Method
For this purpose, RT-PCR have been performed using universal and specific primers of Tombusviridae and Bromoviridae virus families as well as Pelargonium Flower Break Virus (PFBV). Bud explants were taken from ‘Zonal’ and ‘Regal’ and were cultured in MS medium supplemented with different compositions of plant growth regulators (PGRs) as follow: A: (1 mgl− 1 Kin, 1 mgl− 1 BA, and 0.2 mgl− 1 NAA), B: (0.5 mgl− 1 Kin, 0.5 mgl− 1 BA, and 1 mgl− 1 NAA), and C: (1.5 mgl− 1 Kin and 1.5 mgl− 1 BA). After 10 days (16:8 h of light and dark photoperiod) incubation at 38 °C, the meristem (0.3 mm) of the in vitro raised plantlets were cultured on MS medium under sterile conditions. The ribonucleic acid of meristem derived plantlets was subjected to RT-PCR to detect any viral infections using universal primers for the Tombosviridae family and specific primers for PFBV species.
Results
Pelargonium species exhibited varying responses to the PGR treatments. Specifically, the highest bud sprouting, plantlet regeneration, plantlet height, and root number were recorded in ‘Zonal’ and ‘Regal’ pelargoniums when cultured in media A and C, respectively. Although viral infection was confirmed in bud-derived plantlets using RT-PCR, thermotherapy and meristem culture resulted in the generation of 70% and 60% tombusviridae-free plantlets in ‘Regal’ and ‘Zonal’ Pelargoniums, respectively. The virus-free plantlets were propagated using the approved protocol.
Conclusion
These findings underscore the significance of utilizing suitable PGRs for in vitro regeneration of each Pelargonium species. The results of this investigation revealed that RT-PCR using universal and specific primers is a reliable sensitive virus detection procedure that coupled with culturing the heat-treated meristem can result in successful viral eradication in Pelargonium species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-06027-y.
Keywords: Ornamental plant, Meristem culture, Thermotherapy, Pathogen free plants, RT-PCR, Tissue culture medium
Background
Geraniums (Pelargonium spp., Geraniaceae family) are among the most popular and versatile plants used both for indoor and outdoor ornamental (potted plants, garden plants, and hanging baskets) and medicinal purposes [1]. This plant ranks third in the international trade of flowering potted plants [2]. The genus Pelargonium includes approximately 250 species that represent great diversity in terms of size, color of leaves and flowers, growth habit, and type of flowering [3]. These wide varieties make it an attractive choice for landscape designs. Pelargonium X hortorum and Pelargonium X domesticum, respectively known as ‘Zonal’ and ‘Regal’ geraniums, are two popular species of this genus that have been widely cultivated across different regions.
Consumer acceptance of potted and garden ornamental plants can rely heavily on the shape of the plants, as the compact and dense appearance of such plants is preferred by customers [4]. Internal contamination by various microorganisms is a major factor leading to a significant reduction in the quality of clonally propagated plants, which, in turn, negatively impacts customer satisfaction. Geraniums are susceptible to diseases such as bacterial blight (caused by Xanthomonas campestris pv. pelargonii) and botrytis (caused by Botrytis cinerea). Infection by these microorganisms can lead to significant damage during the plant’s growth and development stages [5]. Unlike other microorganisms such as fungi, bacteria, and nematodes, whose activities may be restricted to specific periods in the plant life cycle, viral pathogens can integrate into the plant’s structure and perpetuate their damage through clonal propagation across generations [6]. Additionally, due to the structural simplicity of viruses, there is no effective chemical control strategy against these infectious agents. Viral infections generally do not cause total plant destruction but rather lead to reduced growth, vigor, and quality. This manifests as phenotypic malformations, reproductive organ abortion, stunting, and chlorotic and necrotic spotting on leaves [7]. Therefore, viruses are among the most serious challenges in the plant kingdom, becoming particularly destructive in asexually propagated species.
Geraniums are typically propagated vegetatively from cuttings, making them susceptible to viral diseases, which pose a significant threat to their production [8]. To date, at least 18 different viruses have been isolated from naturally infected geraniums worldwide [9], and widespread viral infections by Pelargonium species have been documented in various countries [7]. Tombusviridae and Bromoviridae are among the main families of plant-infectious viruses, some of which have been isolated from geraniums. The Pelargonium flower break virus (PFBV), a member of the Tombusviridae family, is recognized as one of the most prevalent viruses affecting Pelargonium species. The contamination with this virus has progressively increased over recent decades, primarily due to its efficient transmission mechanisms, including mechanical inoculation, vegetative propagation, and irrigation practices [9, 10]. Furthermore, given that most geraniums infected with this virus are asymptomatic [8], substantial attention needs to be paid to distinguishing phenotypically healthy plants that are used as mother blocks for asexual propagation.
Selecting and developing healthy, virus-free materials is a straightforward and cost-effective ways to prevent viral infections [11]. Tissue culture-based technology provides an unprecedented tool for eliminating viruses from plant materials and producing virus-free materials [12]. Meristem culture is the method of choice that has been widely used for virus eradication in asexually propagated plant species [13, 14]. Because of the lack of vascular tissues and the higher rate of meristem growth compared to virus cell-to-cell transmission, the meristem tip region is usually pathogen-free and it is expected that in vitro culture of growing meristem will give rise to healthy virus-free plantlets [6].
Thermotherapy, another method that has been used to eradicate viruses or reduce virus titers in different plant species [14–16]. This technique involves subjecting infected plant tissues (including shoot tips or entire plants) to elevated temperatures, generally ranging from 34 °C to 38 °C, for a duration of 2 to 4 weeks [15]. Combining meristem culture with other therapies, such as thermotherapy, is more effective for virus elimination from explants and enhances the chance of regeneration true-to-type, virus-free stock plantlets [12, 17].
The present study aimed to optimize an efficient method for sterile culture establishment, proliferation, virus elimination, confirmation, and healthy plantlet regeneration in two popular Pelargonium species by combining meristem culture with thermotherapy.
Materials and methods
Plant materials
Two Pelargonium X hortorum and Pelargonium X domesticum species expressing virus symptoms were collected from the greenhouses of Mahallat (Markazi province, Iran, 33.9115° N, 50.4525° E) and Varamin (Tehran province, Iran, 35.3252° N, 51.6472° E) regions, and were transferred to the experimental greenhouse of the Ornamental Plant Research Center of Iran, in Mahallat city (Supplementary Fig. 1). Moreover, some plants that were apparently healthy and had no signs of viral infection were also collected. In total 72 plants (with or without virus symptoms) were collected from different greenhouses and were transferred to the experimental greenhouse and maintained under optimum condition. After adequate growth of plants, 40 samples were used for further investigation (Supplementary Fig. 2).
RNA extraction, cDNA synthesis and PCR reaction
Leaf samples were collected from both infected (20 pots) and apparently healthy (20 pots) plants and used for RNA extraction based on the method described by Masoomi-Aladizgeh et al. [18]. The quality and quantity of the extracted RNA were assessed using a spectrophotometr (Specgene, Jenway, Techne, 6300) and subsequently analyzed via 1% agarose gel electrophoresis. The first complementary DNA strand (cDNA) was synthesized with 1 µg of total RNA and 0.5 µM random hexamer primers or each of the specific reverse primers of viruses (Table 1) using Easy cDNA Synthesis Kit (Pars Toos biotechnology, Iran) according to the manufacturer’s instructions. The reaction was performed in a thermocycler (iCycler, Bio-Rad, USA) at 42 °C for 60 min. The resulting cDNA (1.5 µl) was used as a template for Polymerase Chain Reaction (PCR) in a mixture consisting of 7.5 µl of Red Master Mix (Amplicon Company, Denmark) and 1 mM of each forward and reverse primer (Table 1). The reaction mixture was adjusted to a final volume of 15 µl with sterile distilled water and performed as follows: 94 °C for 5 min, 35 cycles of (95 °C for 30 s, specific primer annealing temperature (50–62 °C) for 30 s, 1 min at 72 °C), and final extension at 72 °C for 10 min. The PCR products were run on a 1% agarose gel and the target bands were purified using a GF-1 Gel DNA Recovery kit (Sina colon company, Iran). All purified bands were sent to Pishgam Biotech Company (Tehran, Iran) for sequencing analysis.
Table 1.
The primers that were used for amplication of viral ribonucleic fragments
| Viruses, virus family | Primer name | Primer sequence 5’  3’ 3’ |
Annealing temperature (°C) | Fragment location | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|---|
| Cucumoviridae | IlarF5/IlarR7 | F: GCNGGWTGYGGDAARWCNAC R: AMDGGWAYYTGYTYNGTRTCACC | 51 | Methyltransferase motif, RNA1 | 300 | [20] |
| Tombusviridae | CarmoIIF/CarmVIR | R: AARGTVGACCCGWVCCNMGNGTNATHCAACC F: GMMCTGCAGNACRCARTCRTCNCCRTTRTT | 50 | RdRp gene | 500 | [21] |
| PFBV | CH1/CH2 | F: ATGGTGGTAATGGGGGGTTCTTGGGTTG R: TTCCCGGGGGGTTGTTTGTTTGTTAG | 62 | 3’ end include Coat Protein gene | 1500 | [22] |
Tissue culture establishment
Following the PCR test, the virus-infected plants were utilized as mother blocks for bud explants collection and subsequent in vitro experiments. Mother plants were treated with pesticides (Imidacloprid 35% SC, repeated monthly) and fungicides (Propiconazole 25% EC, repeated every 2 weeks) to avoid in vitro contamination.
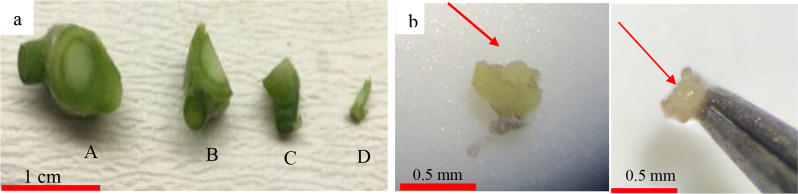
To avoid fungal and bacterial contamination, the effects of explant size and hot-water treatment were examined. Different approaches have been employed to simultaneously reduce and eliminate bacterial and fungi contamination for bud explants (Table 2). The sterilization step started with water prewashing by adding 2 or 4 droplets of Tween-20 for 30 min, followed by 25 min incubation of explants in a water bath at 42 °C. Next, the explants were treated with 70% alcohol (30 s) and sodium hypochlorite (3%) (20 min), respectively. Different sizes of bud explant (0.3 cm, 0.5 cm, 0.8 cm, and 1 cm) were utilized to determine the optimum size of contamination-free, regenerable bud explants (Fig. 1, Left). Finally, the explants were rinsed at least three times with distilled water and were established on the surface of MS [19] culture medium without any plant growth regulators. After four weeks keeping in the establishment stage, the contamination free explants were transferred to the MS medium supplemented with 2 g.l− 1 activated charcoal and different plant growth regulators as follow: medium A (1 mg.l− 1 Kinetin, 1 mg.l− 1 BA, and 0.2 mg.l− 1 NAA); medium B (0.5 mg.l− 1 Kinetin, 0.5 mg.l− 1 BA, and 1 mg.l− 1 NAA), and medium C (1.5 mg.l− 1 Kinetin, and 1.5 mg.l− 1 BA). The explant-containing culture vessels (6 × 10 cm, 220 cc) were incubated in a growth chamber with a 16 h light photoperiod (60 mol.m− 2 s− 2) and 8 h dark period at 25 ± 1 °C. Sub-culturing was performed at two-week intervals to supply fresh medium for the explants.
Table 2.
Different protocols that were used for the surface sterilization of bud explant and their efficiency for sterile culture establishment
| Protocol | Step | Efficiency of sterile culture establishment (%) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| A | Bud explant excision and washing with tap water and detergent for 30 min | Ethanol 70% for 30 s | Sodium hypochlorite 3% for 20 min | Rinsing with autoclaved distilled water | - | - | 0 |
| B | Bud explant excision and washing with tap water and detergent for 30 min | Treatment with Benomyl fungicide 1 g/1000 L) for 10 min | Ethanol 70% for 30 s | Sodium hypochlorite 3% for 20 min | Rinsing with autoclaved distilled water | - | 2 |
| C | Bud explant excision and washing with tap water and detergent for 30 min | Treatment with Benomyl fungicide 1 g/1000 L) for 10 min | Ethanol 70% for 30 s | Sodium hypochlorite 3% for 20 min | Treatment with antibiotic gentamicin 2% for 3 min | Rinsing with autoclaved distilled water | 20 |
| D | Bud explant excision and washing with tap water and detergent for 30 min | Hot water (42 °C) treatment for 25 min | Ethanol 70% for 30 s | Sodium hypochlorite 3% for 20 min | Rinsing with autoclaved distilled water | - | 90 |
Fig. 1.
a: The bud explants that was examined to determine their optimum size for controlling fungal and bacterial contaminations and for inducing plantlet regeneration. The sizes are as follow: A: 1 cm, B: 0.8 cm, C: 0.5 cm and D: 0.3 cm. b: Meristem explant that were excised after thermotherapy (0.3 mm in size)
Thermotherapy and excision of meristems
The in vitro-raised shoots with the appropriate length (at least 6 cm) were tested again for virus infection using RT-PCR. Leaf samples were collected from 10 plantlets of each Pelargonium species under sterile conditions, and their ribonucleic acid was subjected to RT-PCR. Plants that tested positive for viral infection via RT-PCR were subsequently transferred to a phytotron and incubated at 38 °C for 10 days under heat treatment. Shoot tips of the heat-treated plantlets were harvested and considered for meristem excision (Fig. 1b). Meristem isolation was conducted by removing leaf primordial using sterile forceps and needles with the help of a stereomicroscope. The meristematic dome of shoot tip with different leaf primordial, measuring 0.3 mm, were carefully excised and cultured on appropriate medium that demonstrated the best response in the initial experiment for each species (Fig. 1).
The in vitro-raised plantlets from the meristem culture were subsequently used for the final viral detection test using RT-PCR. The virus-free, healthy plants obtained from this process were then utilized as mother blocks for explant preparation, aimed at cloning a group of healthy, pathogen-free plants (Supplementary Fig. 3).
Experimental design and statistical analysis
Data were subjected to an analysis of variance (ANOVA) to evaluate significant differences among treatments. The experiment was conducted using a factorial with three variables: plant species, culture medium, and sampling time. A completely randomized design was employed, consisting of four replications (vessels) and five explants (observations) in each replication. Statistical analysis was performed using the SAS software (Ver. 9.4) and the means were compared using Duncan Multiple Range Test (DMRT) at P ≤ 0.05.
Results
RT-PCR test for virus detection
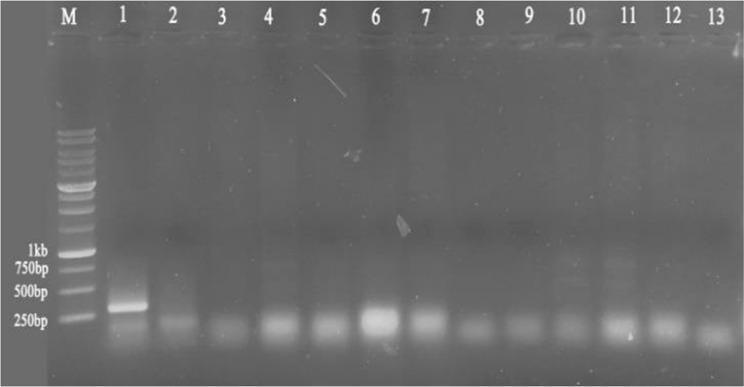
None of the Pelargonium samples were affected by viruses belonging to Bromoviridae, and RT-PCR testing for this family of viruses was negative for all examined Pelargonium samples, while the positive control, Cucumber Mosaic Virus (CMV)-infected Peperomia magnifolia, amplified the respective fragment (Fig. 2).
Fig. 2.
RT-PCR amplification of virus specific fragments using degenerate primer IlarF5/IlarR7 for Bromoviridae. M: 1 kb size marker (GenRuler™ 1 kb DNA ladder, Fermentas). Numbers are as follow: 1: positive control Cucumber Mosaic Virus (CMV)-infected Peperomia magnifolia; 2: negative control (healthy Peperomi magnifolia); 3–13: Pelargonium samples
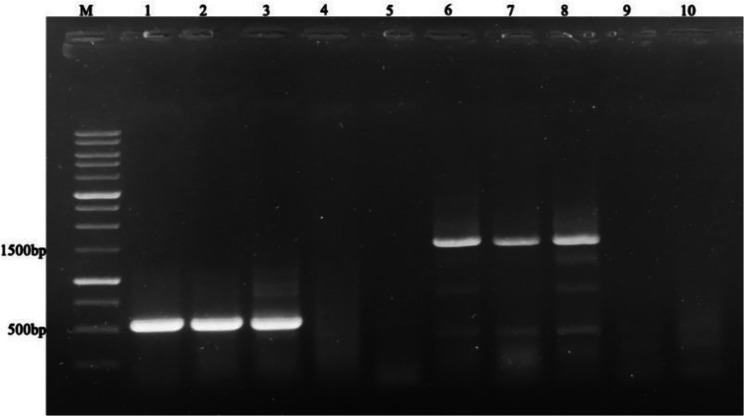
The RT-PCR test of Pelargonium samples yielded positive results for the Tombusviridae family, with a corresponding fragment of 500 bp amplified using a universal degenerate primer (CarmoIIF/CarmVIR) (Fig. 3).
Fig. 3.
RT-PCR amplification of Tombusviridae family using universal primer of Tombusviridae family (CarmoIIF/CarmVIR) (Lanes 1–5) and specific primer for PFBV (CH2/CH1) (Lanes 6–10). M: GenRuler™ 1 kb DNA ladder (Fermentas); Lanes 1–3: Pelargonium samples; Lane 4: Negative control (plantlet derived from meristem tip culture of Pelargonium), Lane 5: water, 6–8: PFBV specific primer on Pelargonium samples; Lane 9: negative control, Lane 10: water
Amplification of the 3’ end of PFBV virus
Following detection of PFBV in the examined samples, a PFBV-specific primer (CH2/CH1) that amplifies the 3’ end of virus genome was used. Gel electrophoresis showed that the expected 1500 bp fragment was amplified using a specific primer (Fig. 3). According to our results, the PFBV virus was detected in some Pelargonium samples, including those that had no visual symptoms of this virus. The 1500 bp fragments of three Pelargonium samples P6, P19, and P105 (without symptoms) that were amplified with CH2/CH1 specific primers were sequenced and their comparison with the sequences available in GenBank showed that these isolates belonged to the PFBV virus. The complete sequence of the CP gene were subjected to BLASTn analysis, and the results showed nucleotide similarities of > 99% the sequences of different isolates of Pelargonium flower break virus (PFBV) that were available in GenBank. These three viral isolates was registered in the GenBank database with the accession numbers MT843891 (P6), MT843892 (P19), and MT843893 (P105) (Supplementary Table 1).
Establishment of in vitro culture and plantlet regeneration
While gentamicin has been employed to eliminate bacterial contamination in geranium bud explants, hot water treatment at 42 °C remains the only effective method for the complete eradication of bacterial contamination. Moreover, our results revealed that the removal of adjacent tissues and the use of smaller explants resulted in reduced fungal and bacterial contamination (Table 3). Explants measuring 0.3 cm exhibited the lowest levels of fungal and bacterial contamination in both geranium species under in vitro culture. In contrast, the contamination rate increased with the size of explants, as all cultures containing explants of 1 cm were affected by either fungal or bacterial contamination. Furthermore, we observed that the in vitro growth of explants measuring 0.3 and 0.5 cm was superior to that of explants measuring 0.8 cm and 1 cm in both geranium species (Table 3).
Table 3.
Effect of bud explant size on the contamination and growth potential in two Pelargonium species
| In vitro culture parameters | Geranium species | Size of bud explant (cm) | |||
|---|---|---|---|---|---|
| 1 | 0.8 | 0.5 | 0.3 | ||
| In vitro fungal and bacterial contamination (%) | Regal | 100 | 87 | 43 | 8 |
| Zonal | 100 | 90 | 51 | 13 | |
| In vitro culture viability (%) | Regal | 14 | 55 | 72 | 80 |
| Zonal | 19 | 62 | 88 | 87 | |
After establishing a practical procedure for removing bacterial and fungal contamination from small bud explants (≥ 0.3 cm) (Table 3), the explants were cultured in MS medium with various PGRs treatments. Growth-related parameters were evaluated at different intervals: 20, 40, and 60 days after culture establishment.
According to the results, bud sprouting of explants was significantly affected (P ≤ 0.01) by species, medium, sampling time, and their interaction (Table 4). The highest percentage of bud sprouting for ‘Zonal’ geranium was recorded 20 days after culture in medium A. Conversely, the lowest percentage of bud sprouting was observed 60 days after culture in medium C (Fig. 4). However, media B and C proved to be more effective for bud sprouting of ‘Regal’ geraniums compared to medium A. Although the bud sprouting rate of both species decreased over time in all culture media, the decline rate was more pronounced in ‘Zonal’ geraniums compared to ‘Regal’ geraniums.
Table 4.
Analysis of variance for the effects of the culture medium on the traits of two Pelargonium species at different sampling times
| Source of variance | df | Mean square | |||
|---|---|---|---|---|---|
| Bud sprouting | Plantlet number | Plantlet height | Root number | ||
| Medium | 2 | 89.288** | 91.103** | 36.10** | 19.11** |
| Species | 1 | 85.520** | 91.444** | 75.84** | 74.4ns |
| Time | 2 | 0.215** | 69.309** | 88.181** | 24.561** |
| Medium × Species | 2 | 96.756** | 91.378** | 49.61** | 63.171** |
| Medium × Time | 4 | 22.197** | 24.2ns | 32.4** | 52.3ns |
| Species × Time | 2 | 07.124ns | 46.21** | 97.23** | 57.2ns |
| Medium × Species × Time | 4 | 19.160* | 80.20** | 47.25** | 63.54** |
| Error | 36 | 44.44 | 20.2 | 70.0 | 72.1 |
| CV (%) | 60.9 | 87.15 | 24.16 | 31.23 | |
**: Significance at 1% of probability; *: significance at 5% of probability; ns: no significance differences
Fig. 4.
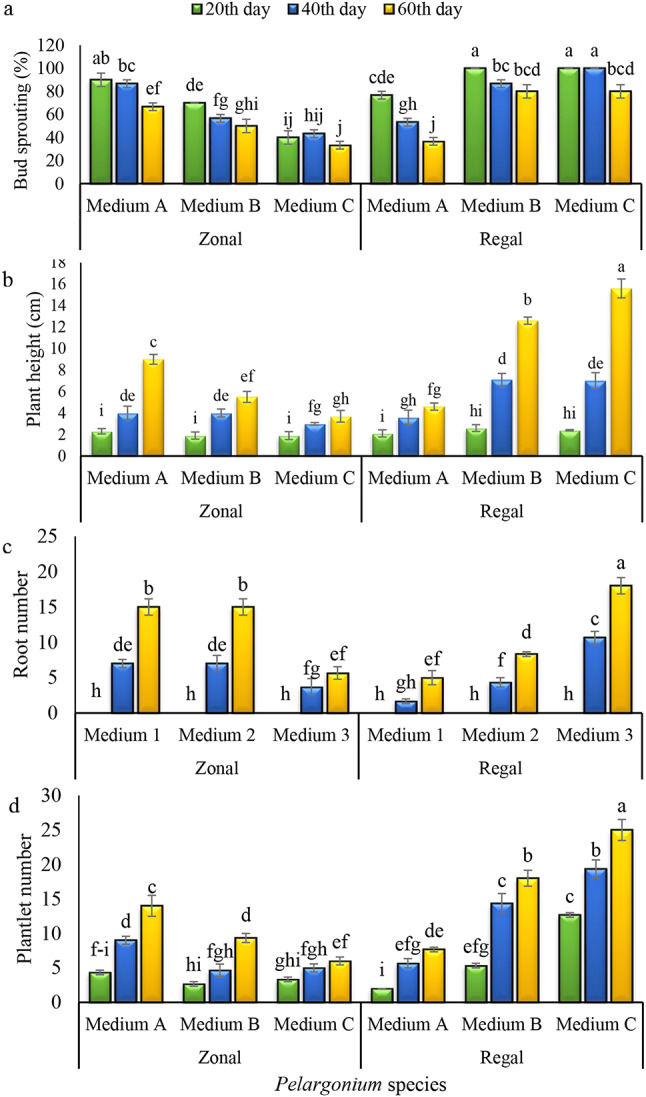
Effects of medium and Pelargonium species on the bud sprouting (a), plant height (b), root number (c), and plantlet regeneration (d) during a period of 60 days. (Columns with at least one common letter do not have a significant difference based on the Duncan Test at the probability level of 0.05. medium A = 1 mg.l− 1 kin + 1 mg.l− 1 BA + 0.2 mg.l− 1 of NAA; medium B = 0.5 mg.l− 1 kin + 0.5 mg.l− 1 BA + 1 mg.l− 1 NAA; medium C = 1.5 mg.l− 1 kin + 1.5 mg.l− 1 of BA)
The number of plantlets was also affected by these three main factors (Table 4). Except for the medium and time, the interactions among the remaining main factors significantly affected the number of regenerated plantlets (P ≤ 0.01) (Table 4). The maximum and minimum number of plantlets for ‘Zonal’ species was attained in the medium A and medium C, respectively (Fig. 4). However, completely different results were observed for the ‘Regal’ samples, with the highest number of plantlets obtained in medium C and the lowest in medium A, respectively (Fig. 4). Based on these results, the plantlet regeneration capacity was significantly higher in ‘Regal’ compared to ‘Zonal’. Both cultivars exhibited an increasing trend in the number of plantlets during the sampling period, with the highest levels recorded at 60 days after culture (Fig. 4).
Plantlet height was also significantly affected by the culture medium, sampling time, plant species, and their interactions (Table 4). This trait exhibited a pattern similar to that of plantlet number, with the highest values for ‘Zonal’ and ‘Regal’ species recorded in medium A and medium C, respectively (Fig. 4). As expected, plantlet height increased during the experiment in both species, and the longest shoots were recorded at 60 days after culture in all treatments (Fig. 4).
The two Pelargonium species exhibited differential responses to the concentration of PGRs. The growth-related parameters of in vitro-derived shoots were statistically influenced by both the composition and concentration of PGRs in the medium. The MS medium supplemented with 1 mg.l− 1 Kin, 1 mg.l− 1 BA, and 0.2 mg.l− 1 NAA resulted in the highest bud sprouting rate, plantlet number, plantlet height, and root induction in ‘Zonal’ Pelargonium. Conversely, an auxin-free medium containing higher levels of cytokinin sources (1.5 mg.l− 1 kin and 1.5 mg.l− 1 BA) led to maximum proliferation and development in ‘Regal’ Pelargonium.
To reduce costs and accelerate plantlet regeneration via a tissue culture approach, the root induction phase for the micro-shoots was conducted in the same medium as the shoot elongation phase. Our data revealed that the root induction capacity was not statistically different between the two Pelargonium species (Table 4). According to our results, all plantlets in the defined medium produced sufficient roots to be transferred to adaptation conditions.
The effects of the medium and time on root production were significant (P ≤ 0.01). The highest root numbers for ‘Zonal’ geranium were attained in media A and B at 60 days after culture (Fig. 4), while medium C resulted in the maximum root production in ‘Regal’ geranium. Root formation at the microscale was detectable from 40 days after culture and reached its highest level at 60 days after culture in both species and all treatments. According to the results, the number of roots in both cultivars increased over time in all media, but this increase was more pronounced in ‘Regal’ compared to ‘Zonal’ plantlets (Fig. 4).
Virus eradication by thermotherapy and meristem culture
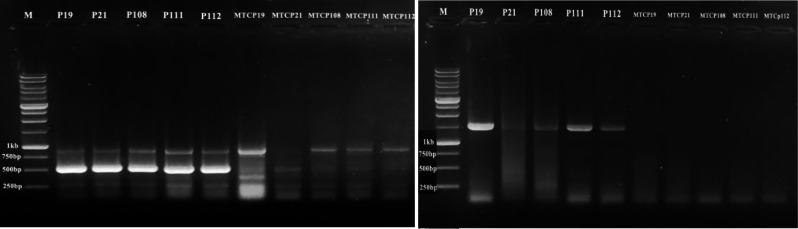
Out of the in vitro plantlets subjected to thermotherapy, 20 samples of each species (virus contamination approved by RT-PCR) were subsequently selected and their meristems were cultured in the selected culture medium. The findings indicate that the eradication rates of the Tombusviridae family were 70% in ‘Regal’ geraniums and 60% in ‘Zonal’ geranium when a combination of thermotherapy and meristem culture techniques was employed (Table 5). The heat-treated meristem culture-derived plantlets were tested for viral contamination using both universal primers for the Tombusviridae family (CarmoIIF/CarmVIR) and specific primers for PFBV (CH1/Ch2), and neither universal primer-derived (500 bp) fragments nor specific primer-derived (1500 bp) fragments of the virus genome were detected in the healthy meristem culture-derived plantlets (Fig. 5).
Table 5.
Screening of virus families in ‘Regal’ and ‘Zonal’ in vitro-raised plantlets after thermotherapy and meristem culture
| Pelargonium species | Heat treatment For 10 days |
Size of meristem (mm) | Efficiency of PFBV free plantlet generation |
|---|---|---|---|
| Regal | 38 °C | 0.3 | 20/14 |
| Zonal | 38 °C | 0.3 | 20/12 |
Fig. 5.
Detection and confirmation of Tombusviridae and PFBV virus elimination using heat treatment and meristem tip culture in five geranium plantlets. Left: Tombusviridae virus detection in geranium samples before (P19, P21, P108, P111, P112) and after (MTCP19, MTCP21, MTCP108, MTCP111, MTCP112) heat treatment and meristem tip culture. Right: PFBV virus detection in geranium samples before (P19, P21, P108, P111, P112) and after (MTCP19, MTCP21, MTCP108, MTCP111, MTCP112) heat treatment and meristem tip culture. M: 1 kb size marker (GenRuler™ 1 kb DNA ladder, Fermentas)
Discussion
Production of virus-free plants is necessary to ensure large-scale high-quality stock plant propagation in nurseries and the global exchange of healthy germplasm [23]. The development of an efficient method for the production of virus-free plants is crucial for the successful propagation of vegetatively propagated species. This advancement is a significant factor contributing to the success of the ornamental plant industry [6, 24]. Consequently, the effectiveness of tissue culture for clonal plant propagation can vary depending on the genetic disposition of plant species, necessitating the assessment of different media compositions to identify the optimal culture conditions that promote robust growth [25]. It is well established that the combination and concentrations of PGRs in culture media significantly influence the proliferation and growth-related parameters of plantlets cultured in vitro [26–28]. Our results revealed that the MS medium successfully supported the proliferation and plantlet regeneration of both Pelargonium species. This result agrees with those reported for other Pelargonium species, as the optimum proliferation and growth of in vitro explants were achieved in MS medium [29–34].
The available literature support our findings that various species as well as different genotypes within the same species and explants from the same plant, necessitate distinct concentrations and types of PGRs to achieve optimal proliferation and plantlet generation [26, 35, 36]. Furthermore, our data indicate that the inclusion of low concentrations of an auxin source in the proliferation medium positively affects ‘Zonal’ species, resulting in higher proliferation rates compared to auxin-free medium. These findings align with previous research highlighting the beneficial effects of trace levels of auxin in combination with optimal concentrations of cytokinins in media designed for proliferation and axillary shoot induction [12, 28, 37].
Similarly, Espino et al. [38] examined the effects of BA and NAA on the regenerative capacities of four distinct Begonia species. Their findings revealed notable variations in regenerative abilities among the species assessed. Furthermore, the authors indicated that for the regeneration of B. semperflorens, the application of a combination of NAA with kinetin, as well as BA with NAA, proved to be more effective than kinetin alone. Ezeibekwe et al. [39] observed that a mixture of 2 mg.l− 1 BA and 0.5 mg.l− 1 NAA significantly enhanced foliar proliferation and extends the vegetative phase of the plant. Accordingly, optimal bud sprouting in Pelargonium graveolens was achieved in a medium supplemented with 5 mg.l− 1 kinetin and 1 mg.l− 1 NAA [40]. Moreover, the authors noted that a lower concentration of NAA was associated with an increase in branch proliferation [40]. These observations suggest that the presence of NAA in the culture medium may play a critical role in promoting branch development and elongation.
Previous studies have highlighted the beneficial effects of kinetin and BA on the proliferation and shoot formation across various plant species [25]. In the case of Pelargonium, the optimal concentration of BA for shoot multiplication varies between 1 and 5 mg.l− 1, depending on the type of explant and the specific genotype being utilized [29, 33, 34]. BA concentrations exceeding this range inhibit shoot formation and elongation, induce callus formation, and consequently reduce the number of plantlets generated.
However, the highest level of proliferation and plantlet regeneration for ‘Regal’ Pelargonium was achieved in an auxin-free medium supplemented with the highest concentrations of kinetin and BAP. Gomes et al. [36] reported that inclusion of NAA in the culture medium did not positively affect the proliferation of Arbutus unedo L., and optimal outcomes were achieved in an NAA-less culture medium, which aligns with the findings of our research on ‘Regal’ Pelargonium.
The Pelargonium shoots, including those grown in the medium devoid of exogenous auxin, successfully produced root under in vitro condition. These observations are consistent with previous reports on other Pelargonium species [41, 42]. Although some Pelargonium species are considered hard-rooting plants [33], it has been reported that hormone-free MS medium induces the maximum functional roots in Pelargonium graveolens compared to auxin-containing media [41–43]. However, there are also reports indicating that the inclusion of auxin in the culture media positively affects root formation in various Pelargonium species under in vitro conditions [29, 33, 40, 44]. These discrepancies were evident in our observations, as the highest root formation for ‘Zonal’ Pelargonium was recorded in NAA-supplemented MS medium, whereas the auxin-free medium led to the highest root induction in ‘Regal’ Pelargonium. These conflicting findings led us to conclude that Pelargonium species have different PGR requirements for achieving optimal root induction. This variation is likely due to differences in their genetic backgrounds and endogenous hormone levels.
Our observations revealed that size of explants had a substantial impact on in vitro contamination and growth of the explants. The size, type, and developmental stages of explants are among the main factors that substantially affect the success of tissue culture [45]. The significant effects of explant size on culture contamination and regrowth capacity have been well documented [46, 47]. For tissue culture establishment, larger-sized explants are generally more responsive, exhibit higher regeneration capacity, and grow more vigorously than smaller-sized explants. Instead, they are more prone to surface and internal contamination, which can lead to the exudation of additional phenolic substances and pose significant constraints during the establishment of tissue culture systems [48]. Our observations indicated that the lowest contamination and the highest survival rate for both geranium species were recorded in bud explants measuring 0.3 cm in size.
Our data revealed that a combination of thermotherapy and meristem culture effectively eradicated various viruses from the two geranium species. These findings are consistent with previous reports on the effectiveness of combining meristem culture and thermotherapy for virus elimination in different plant species [12, 14]. It was found that meristem tips measuring 0.3 mm, when coupled with thermotherapy, led to the generation of 70% and 60% tombusviridae-free plantlets in Regal and Zonal geraniums, respectively. In meristem culture, the size of explants is critically important and plays a major role in achieving the desired outcome. On the other hand, selecting the optimum size of the meristem tip is a significant challenge in producing virus-free plantlets under in vitro conditions. Small-sized meristems may not grow successfully in vitro, while larger meristems may be infected with virus particles [15]. It is well documented that some viruses can invade the meristematic tissues, with microorganisms being detected in meristems as small as 0.2 mm [49, 50]. Consistent with our findings, a combination of meristem culture (0.5–0.6 mm) and thermotherapy resulted in success rates ranging from 50% to100% in eradicating various viruses in apple cultivar ‘Oregon Spur-II’ [12].
The findings of this investigation indicated that incubating in vitro-raised shoots at 38 °C for 10 days was effective in restricting viral contamination in geranium species. Preliminary tests revealed that plants treated at higher temperatures exhibited wilting symptoms and could not withstand the heat treatment, possibly due to delicate and subtle nature of in vitro-raised plantlets compared to their in vivo-developed counterparts (data not presented). Our observations are consistent with previous reports on the positive impacts of thermotherapy in reducing the titer of viruses in plant tissues [12, 51–53]. Due to variations in plant physiology and growth conditions, the optimal temperature and duration for thermotherapy can vary significantly among different plant species. According to Knapp et al. [54], virus distribution is less localized in actively growing apples compared to those in the stationary growth phase, which later accumulate higher virus titers. Additionally, host-virus interactions can result in varying distribution patterns of viruses in different plant organs [12]. It is well established that most viruses are sensitive to high temperature. Available records indicate that thermotherapy can eradicate approximately half of the viruses that affect horticultural plants [12]. The mechanisms by which heat treatment reduces virus titer have seldom been scrutinized. However, early studies suggested that high temperatures might accelerate plant growth and inhibit viral replication and movement, thus resulting in virus elimination from the meristem tip [55]. More recent findings indicate that viral RNA silencing could be significantly enhanced during thermotherapy [51, 56]. It has been shown that high temperature significantly enhance RNA silencing and viral RNA degradation in the shoot tips of raspberry [52].
It was observed that some Pelargonium samples were infected with an asymptomatic form of the PFVB virus, with contamination verified only after the RT-PCR testing. Although most common members of the Tombusviridae family cause symptomatic virus infections, there are several reports of different members of this family that exhibite no obvious symptoms on host plants [57]. This type of virus infection is common and easily maintained in perennial plants, posing a threat to adjacent plant communities, as it can be transmitted by vectors, pollen, and equipment, spreading to new hosts and causing disease symptoms upon transition from a latent to an active state [57]. Our findings align with those of previous studies that reported symptomless PFVB infections in different Pelargonium species from other countries [7, 9, 58]. According to Alonso and Borja [7], more than 59% of 800 virus-symptomless geranium plants collected from different regions of Spain were found to be infected with at least one of the geranium viruses, with Pelargonium line pattern virus (PLPV) and PFVB being the most common. Bouwen and Matt [9] also detected PFBV infection in geranium samples with no signs of virus infection. Symptomless virus infections present a serious challenge during the vegetative propagation of plants, as they can lead to excessive spread of the virus within the clonally propagated population. In fact, asymptomatic virus infection is of immense importance in vegetatively propagated plants because such plants may be used as mother blocks for clonal propagation. The quality and performance of the resulting clones may be negatively affected by virus reactivation, causing disease development. These findings highlight that growers and producers should not rely on the absence of viral symptoms to ensure geranium plants are virus-free. As demonstrated in our work and previous reports [7], many asymptomatic plants are actually virus-infected.
In addition to the developing efficient and reliable procedures for identifying and discriminating virus-infected plants, researchers have focused on creating effective methods to eradicate viruses from plants [59]. The availability of reliable, rapid, and sensitive virus detection procedures is crucial, especially for vegetatively propagated plants, to prevent the transmission of viruses from symptomless infected plants to uninfected ones [7]. According to our data, RT-PCR using both universal and specific primers is an effective method for virus indexing in Pelargonium species.
Our results showed that none of the investigated geranium samples were infected with viruses belonging to the Bromoviridae family. The RT-PCR test for detecting Bromoviridae family members was negative for all examined Pelargonium samples, while the positive control, Cucumber Mosaic Virus (CMV)-infected Peperomia magnifolia, successfully amplified the respective fragment (Fig. 2). Cucumber mosaic virus has already been reported in geraniums in Iran [60].
Conclusion
MS medium supplemented with different PGRs was used for the micropropagation of the two Pelargonium species. According to our results, the size of the explants substantially affected the contamination rate and explant growth. The treatment of bud explants with hot water at a temperature of 42 °C for a duration of 25 min effectively controlled bacterial contamination. It was found that Pelargonium species responded differently to the PGR treatments and had different requirements for shoot multiplication and plantlet regeneration. Our investigation revealed that an augmented concentration of BA corresponded to enhanced bud growth.
To ensure virus elimination in Pelargonium, a combination of thermotherapy (incubation of in vitro raised shoots at 38 °C for 10 days) and meristem culture procedures was successfully employed on in vitro-raised plantlets and led to virus eradication. An efficient, cost-effective one-step protocol for meristem culture and plantlet regeneration was developed, which can be utilized commercially for virus-free mother plants and clonal propagation of ‘Zonal’ and ‘Regal’ Pelargonium. Moreover, our data validated the effectiveness of RT-PCR as a powerful technique for virus indexing and facilitated the production of virus-free plant materials. In conclusion, our findings not only highlight the critical role of specific PGR treatments and hot water treatment for sterilization in the micropropagation of Pelargonium species but also establish a reliable protocol for producing virus-free plants, thereby contributing to the advancement of commercial clonal micropropagation in Pelargonium.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- RT-PCR
Reverse transcription polymerase chain reaction
- kinetin
Kin
- BA
Benzyl adenine
- NAA
1-Naphthaleneacetic acid
- MS
Murashige and Skoog
Author contributions
MKA, HB and SK conceived and designed the study; MKA and HB contributed to literature research; DK performed the experiments and collected the data; MKA and AZ analyzed and interpreted the data; Visualization was performed by MKA and AZ; MKA and AZ were major contributors in writing the manuscript; MKA, HB and SK guided all aspects of the research project and revised the manuscript; All authors read and approved the final manuscript.
Funding
Horticultural Sciences Research Institute (HSRI) of Iran.
Data availability
The gene sequences data were deposited into the Gene Expression Omnibus database under accession number GSE85337 and are available NCBI database with the accession numbers of MT843891, MT843892, and MT843893, and the following URL of: https://www.ncbi.nlm.nih.gov/nuccore/MT843891.1/, https://www.ncbi.nlm.nih.gov/nuccore/MT843892.1/, and https://www.ncbi.nlm.nih.gov/nuccore/MT843893.1/, respectively.The experimental data that support the findings of this study are available in Figshare with following doi: 10.6084/m9.figshare.27542190. Other the data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maryam Karimi Alavijeh, Email: mkarimia61@gmail.com.
Abolkarim zarei, Email: zarei@jahromu.ac.ir.
References
- 1.Saraswathi J, Venkatesh K, Baburao N, Hameed Hilal M, Rani AR. Phytopharmacological importance of Pelargonium species. J Medic Plants Res 2011:5(13):2587–98.
- 2.Altobaishi SF, Almana FA, Abd-ElGawad AM, Al-Yafrsi MA, Elhindi KM. Ivy Geranium (Pelargonium peltatum (L.) L’Hér.) Plant growth and flowering as affected by mineral or biofertilizer with or without compost amendment. Agriculture. 2023;13(5):1106. 10.3390/agriculture13051106. [Google Scholar]
- 3.Szutt A, Dołhańczuk-Śródka A, Sporek M. Evaluation of chemical composition of essential oils derived from different Pelargonium species leaves. Ecol Chem Eng S. 2019;26(4):807–16. 10.1515/eces-2019-0057. [Google Scholar]
- 4.Khandan-Mirkohi A, Kazemi F, Babalar M, Naderi R. Effect of different levels of nitrogen in nutrient solution on the qualitative and quantitative traits of geranium (Pelargonium hortorum cv. Bulles eye). J Crops Improv. 2014;16(1):157–68. 10.22059/jci.2014.51949. [Google Scholar]
- 5.Dunbar KB. Geranium tissue culture for the development of bacterial blight resistance. Ph.D. Thesis. Michigan State University. 1990.
- 6.Krishna R, Ansari WA, Khandagale K, Benke AP, Soumia PS, Manjunathagowda DC, Gawande SJ, Ade AB, Mokat DN, Singh M. Meristem culture: a potential technique for in vitro virus-free plants production in vegetatively propagated crops. In: Chandra Rai A, Kumar A, Modi A, Singh M, editors. Advances in plant tissue Culture Current developments and Future trends. London: Academic; 2022. pp. 325–43. [Google Scholar]
- 7.Alonso M, Borja M. High incidence of Pelargonium line pattern virus infecting asymptomatic Pelargonium spp. in Spain. Eur J Plant Pathol. 2005;112(2):95–100. [Google Scholar]
- 8.Franck A, Loebenstein G. Virus and virus-like diseases of pelargonium in Israel. In: VIII International Symposium on Virus Diseases of Ornamental Plants, Czech Republic, Prague, 31–40. 1992.
- 9.Bouwen I, Maat DZ. Pelargonium flower-break and Pelargonium line pattern viruses in the Netherlands; purification, antiserum preparation, serological identification, and detection in pelargonium by ELISA. Neth J Plant Pathol. 1992;98(2):141–56. [Google Scholar]
- 10.Gallard A, Mallet R, Chevalier M, Grapin A. Limited elimination of two viruses by cryotherapy of Pelargonium apices related to virus distribution. Cryo Lett. 2011;32(2):111–22. [PubMed] [Google Scholar]
- 11.Kakareka N, Volkov Y, Tolkach V, Sapotskyi M, Shchelkanov M. Possibilities of obtaining and controlling virus-free material in the process of selection and seed production of main crops. IOP Conf Ser Earth Environ Sci. 2021;937:032108. 10.1088/1755-1315/937/3/032108. [DOI]
- 12.Vivek M, Modgil M. Elimination of viruses through thermotherapy and meristem culture in apple cultivar ‘Oregon Spur-II’. Virus Dis. 2018;29(1):75–82. 10.1007/s13337-018-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faccioli VC, Marani F. Virus elimination by meristem tip culture and tip micrografting. In: Hadidi A, Khetarpal RK, Koganezawa H, editors. Plant virus disease control. St. Paul: APS; 1998. pp. 346–80. [Google Scholar]
- 14.Wang MR, Hamborg Z, Blystad DR, Wang QC. Combining thermotherapy with meristem culture for improved eradication of onion yellow dwarf virus and shallot latent virus from infected in vitro-cultured shallot shoots. Ann Appl Biol. 2020;178:142–9. [Google Scholar]
- 15.Wang MR, Cui ZH, Li JW, Hao XY, Zhao L, Wang QC. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods. 2018;18:87. 10.1186/s13007-018-0355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakabonge G, Nangonzi R, Tumwebaze BS, Kazibwe A, Samukoya C, Baguma Y. Production of virus-free cassava through hot water therapy and two rounds of meristem tip culture. Cogent Food Agric. 2020;6(1):1800923. [Google Scholar]
- 17.Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J. Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue Organ Cult. 2015;121(2):435–43. [Google Scholar]
- 18.Masoomi-Aladizgeh F, Jabbari L, Khayam Nekouei R, Aalami A. A simple and Rapid System for DNA and RNA isolation from diverse plants using Handmade Kit. Environ Sci Biol Mat Sci. 2016. 10.21203/RS.2.1347/V2. [Google Scholar]
- 19.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473–97. [Google Scholar]
- 20.Huang KS, Li SL, Sun JH, Wang YC, Jan FJ, Chen TC. Development of a generic method for inspection of tospoviruses. Eur J Plant Pathol. 2018;150:457–69. [Google Scholar]
- 21.Untiveros M, Perez-Egusquiza Z, Clover G. PCR assays for the detection of members of the genus Ilarvirus and family bromoviridae. J Virol Methods. 2010;165(1):97–104. 10.1016/j.jviromet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Morozov SY, Ryabov E, Leiser RM, Zavriev S. Use of highly conserved motifs in plant virus RNA polymerases as the tags for specific detection of carmovirus-related RNA-dependent RNA polymerase genes. Virology. 1995;207:312–5. [DOI] [PubMed] [Google Scholar]
- 23.Bettoni JC, Fazio G, Carvalho Costa L, Hurtado-Gonzales OP, Rwahnih MA, Nedrow A, Volk GM. Thermotherapy followed by shoot tip cryotherapy eradicates latent viruses and apple hammerhead viroid from in vitro apple rootstocks. Plants. 2022;11:582. 10.3390/plants11050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suman R, Singh MK, Rishi N, Chandel V. Century of plant virus management: A way forward. In Plant RNA Viruses. 2023;1 (pp. 591–606). Academic Press.
- 25.Kim SH, Zebro M, Jang DC, Sim JE, Park HK, Kim KY, Bae HM, Tilahun S, Park SM. Optimization of plant growth regulators for in vitro mass propagation of a disease-free ‘Shine Muscat’ grapevine cultivar. Curr Issues Mol Biol. 2023;45:7721–33. 10.3390/cimb45100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahraroo A, Zarei A, Banalar M. In vitro regeneration of the isolated shoot apical meristem of two commercial fig cultivars ‘Sabz’ and ‘Jaami-e-Kan’. Biocatal Agric Biotechnol. 2019;1:743–9. 10.1016/j.bcab.2019.01.024. [Google Scholar]
- 27.Khamushi M, Dehestani-Ardakani M, Zarei A, Kamali Aliabad K. An efficient protocol for micropropagation of old cypress of Abarkuh (Cupressus sempervirens var. Horizontalis [Mill.]) Under in vitro condition. Plant Cell Tissue Organ Cult (PCTOC). 2019;138:597–601. 10.1007/s11240-019-01645-z. [Google Scholar]
- 28.Karimi Alavijeh M, Safi S, Zarei A. An efficient method for economic micropropagation of three aquatic plant species (Lobelia cardinalis, Staurogyne repens and Alternanthera reineckii). Aquacult Int. 2023;31:1623–36. 10.1007/s10499-022-01044-w. [Google Scholar]
- 29.Gupta R, Gupta SK, Banerjee S, Mallavarapu GR, Kumar S. Micropropagation of elite cultivars of rose- scented Geranium (Pelargonium graveolens L.’ Herit.) For industrial production of propagules. Indian J Biotechnol. 2002;1:286–91. [Google Scholar]
- 30.Zhou J, Ma G, Bunn E, Zhang X. In Vitro shoot organogenesis from Pelargonium Citrosum Vanleenii leaf and petiole explants. Floriculture Orna Biotech. 2007;1(2):147–9. [Google Scholar]
- 31.Benazir JF, Suganthi R, Chandrika P, Mathithumilan B. In vitro regeneration and transformation studies on Pelargonium graveolens (Geranium) an important medicinal and aromatic plant. J Med Plant Res. 2013;7(38):2815–22. [Google Scholar]
- 32.Hanus-Fajerska E, Mrzygłód A, Wiszniewska A, Koźmińska A, Stolarczyk P. Establishment of an in vitro culture of Pelargonium × domesticum cultivars characterized by different growth requirements. J Biotech Comp Biol Bionan. 2015;97(2):203–7. [Google Scholar]
- 33.Ebrahimzadeh A, Fathollahzadeh M, Hassanpouraghdam MB, Aazami Mavaloo MA. Micropropagation of Pelargonium odoratissimum (L.) L’Her. Through petioles and leaves. Rev Fac Agron (LUZ). 2021;38:261–78. 10.47280/RevFacAgron(LUZ).v38.n2.03. [Google Scholar]
- 34.Masood A, Ashraf Z, Khan SA, Akhtar G, Hassan A, Shah MM, Shah SM, Sajjad Y. In vitro propagation of Geranium Wallichianum: an indigenously threatened medicinal plant in Pakistan. Kuwait J Sci. 2023;50:5–11. 10.1016/j.kjs.2023.02.002. [Google Scholar]
- 35.Karimi Alvijeh M, Ebadi A, Zarei A, Omidi M. Somatic embryogenesis from anther, whole flower, and leaf explants of some grapevine cultivars. Plant Tissue Cult Biotech. 2016;26(2):219–30. 10.3329/ptcb.v26i2.30572. [Google Scholar]
- 36.Gomes F, Simões M, Lopes ML, Canhoto JM. Effect of plant growth regulators and genotype on the micropropagation of adult trees of Arbutus unedo L. (strawberry tree). New Biotechnology. 2010;27(6):882–892. [DOI] [PubMed]
- 37.Mohamad ME, Awad AA, Majrash A, Abd Esadek OA, El-Saadony MT, SaadeAhmed AM, Gendy AS. In vitro study on the effect of cytokines and auxins addition to growth medium on the micropropagation and rooting of Paulownia species (Paulownia hybrid and Paulownia tomentosa). Saudi J Biol Sci. 2022;29(3):1598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espino FJ, Linacero R, Rueda J, Vazquez AM. Shoot regeneration in four Begonia genotypes. Biol Plant. 2004;48(1):101–4. [Google Scholar]
- 39.Ezeibekwe IO, Ezenwaka CL, Mbagwu FN, Unamba CIN. Effects of combination of different levels of Auxin (NAA) and cytokinin (BAP) on in vitro propagation of Dioscorea rotundata L.(White Yam). J Mol Gen. 2009;1(2–4):18–22. [Google Scholar]
- 40.Saxena G, Banerjee S, Rahman L, Mallavarapu GR, Sharma S, Kumar S. An efficient in vitro procedure for micropropagation and generation of somaclones of rose scented Pelargonium. Plant Sci. 2000;155(2):133–40. [DOI] [PubMed] [Google Scholar]
- 41.Ghanem SA, Sobhy A, El-Kazzaz AA, et al. Factors affecting callus production of Pelargonium graveolens in vitro. Egypt J App Sci. 2005;20(10B):595–604. [Google Scholar]
- 42.Rabuma T. In vitro propagation of geranium (Pelargonium graveolens L.) from nodal culture. Res Reviews: J Agr Sci Tech. 2015;4(2):23–34. [Google Scholar]
- 43.Helen D, Yves D, Richard RB. Clonal propagation of Pelargonium x Hotorum through tissue culture: effects salt dilution and growth regulator concentration. Can J Plant Sci. 1993;73:871–8. [Google Scholar]
- 44.Zuraida AR, Shukri MA, Ayu Nazreena O, Zamri Z. Improved micropropagation of biopesticidal plant, Pelargonium radula via direct shoot regeneration. Am J Res Commun. 2013;1(1):1–12. [Google Scholar]
- 45.Hesami M, Adamek K, Pepe M, Jones AMP. Effect of explant source on phenotypic changes of in vitro grown cannabis plantlets over multiple subcultures. Biology (Basel). 2023;13(3):443. 10.3390/biology12030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baek HJ, Kim HH, Cho EG, Chae YA, Engelmann F. Importance of explant size and origin and of preconditioning treatments for cryopreservation of garlic shoot apices by vitrification. Cryo Lett. 2003;24(6):381–8. [PubMed] [Google Scholar]
- 47.Karjadi AK, Karjadi Gunaeni N. The effect of antiviral Ribavirin, explant size, varieties on growth and development in potato meristematic. IOP Conf Series: Earth Environ Sci. 2022;985:012022. 10.1088/1755-1315/985/1/012022. [Google Scholar]
- 48.Permadi N, Akbari SI, Prismantoro D, Indriyani NN, Nurzaman M, Alhasnawi AN, Doni F, Julaeha E. Traditional and next-generation methods for browning control in plant tissue culture: current insights and future directions. Curr Plant Biol. 2024 Mar;27:100339.
- 49.Hu GJ, Dong YF, Zhang ZP, Fan XD, Ren F, Li ZN. Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot tip grafting and in vitro meristem culture. J Phytopathol. 2017;165:701–6. [Google Scholar]
- 50.Bhardwaj SV, Rai SJ, Thakur PD, Handa A. Meristem tip culture and heat therapy for production of apple mosaic virus free plants in India. Acta Hort. 1998;472:135–40. [Google Scholar]
- 51.Chellappan P, Vanitharani R, Ogbe F, Fauquet CM. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005;138:1828–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang QC, Cuellar WJ, Rajam Aki M, Hirata Y, Valkonen JPT. Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol Plant Pathol. 2008;9:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benke AP, Krishna R, Khandagale K, Gawande S, Shelke P, Dukare S, Dhumal S, Singh M, Mahajan V. Efficient elimination of viruses from garlic using a combination of shoot meristem culture, thermotherapy, and chemical treatment. Pathogens. 2023;12:129. 10.3390/pathogens12010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knapp E, Hanzer V, Weiss H, Camara Da, Machado A, Wang Q, Weiss B, Katinger H. Laimer Da Camara Machado M. Distribution of apple chlorotic leafspot virus in apple shoots cultivated in vitro. Acta Hortic. 1995;386:187–94. [Google Scholar]
- 55.Cooper VC, Walkey DGA. Thermal inactivation of cherry leaf-roll virus in tissue cultures of Nicotiana rustica raised from seeds and meristem tips. Ann Appl Biol. 1978;88:273–8. [Google Scholar]
- 56.Qu F, Ye XH, Hou GC, Sato S, Clemente TE, Morris TJ. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana Benthamiana. J Virol. 2005;79:15209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi H, Fukuhara T, Kitazawa H, Kormelink R. Virus latency and the impact on plants. Front Microbiol. 2019;10:2764. 10.3389/fmicb.2019.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei MS, Li GF, Ma J, Kong J. First report of Pelargonium flower break virus infecting Pelargonium plants in China. Plant Dis. 2015;99(5):735. [Google Scholar]
- 59.Milošević S, Cingel A, Jevremović S, Stanković I, Bulajić A, Krstić B, Subotić A. Virus elimination from ornamental plants using in vitro culture techniques. Pestic Phytomed. 2012;27(3):203–11. 10.2298/PIF1203203M. [Google Scholar]
- 60.Saidi A, Safaeizadeh M. First report of Cucumber mosaic virus infecting geraniums (Pelargonium spp.) in Iran. Asian J Plant Pathol. 2011;5:163–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The gene sequences data were deposited into the Gene Expression Omnibus database under accession number GSE85337 and are available NCBI database with the accession numbers of MT843891, MT843892, and MT843893, and the following URL of: https://www.ncbi.nlm.nih.gov/nuccore/MT843891.1/, https://www.ncbi.nlm.nih.gov/nuccore/MT843892.1/, and https://www.ncbi.nlm.nih.gov/nuccore/MT843893.1/, respectively.The experimental data that support the findings of this study are available in Figshare with following doi: 10.6084/m9.figshare.27542190. Other the data that support the findings of this study are available from the corresponding author upon reasonable request.