Abstract
Background
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become the most common chronic liver disease worldwide. The pan-immune-inflammation value (PIV) has been proposed as a biomarker for assessing immune status and inflammation. There is currently no evidence regarding the effect of PIV on the risk of MASLD. This study aimed to investigate the association between PIV and MASLD.
Methods
The cross-sectional study included 6462 adults aged ≥ 20 years from the National Health and Nutrition Examination Survey 2017–2020. PIV was calculated based on blood count data. Weighted multivariable logistic regression was employed to calculate the odds ratio (OR) and 95% confidence interval (CI) to investigate the association of PIV and MASLD. Restricted cubic spline (RCS) analysis was conducted to explore the dose-response relationship between PIV and MASLD. Stratified and sensitivity analyses were performed to confirm the robustness of our findings.
Results
Among 6462 participants, 2458 were diagnosed with MASLD. Positive associations between LnPIV and MASLD were observed in all three models (Model 1: OR = 1.46, 95% CI: 1.28–1.66, P < 0.001; Model 2: OR = 1.41, 95% CI: 1.24–1.60, P < 0.001; Model 3: OR = 1.39, 95% CI: 1.16–1.65, P = 0.004). When PIV was classified into quartiles, both Q3 and Q4 exhibited significantly increased risks of MASLD compared with the reference Q1 in full adjusted Model 3 (Q3: OR = 1.63, 95% CI: 1.20–2.22, P = 0.012; Q4: OR = 1.76, 95% CI: 1.28–2.41, P = 0.008; P for trend = 0.002). RCS analysis did not show a nonlinear relationship between LnPIV and MASLD (P = 0.093 for nonlinearity). Stratified analysis showed a consistent positive association between LnPIV and MASLD in all subgroups, and sensitivity analyses supported the reliability of these results.
Conclusions
Higher PIV levels are significantly associated with an increased prevalence of MASLD, indicating that PIV is a potentially effective inflammatory marker for assessing MASLD in participants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03584-2.
Keywords: Pan-immune-inflammatory value, NHANES, MASLD, Cross-sectional study, Inflammation
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is characterized by excessive fat accumulation in liver cells [1]. It has become the most common chronic liver disease worldwide, with the prevalence in the general population estimated to be 31.3–38.7% in the US, 26% in Japan, and 27.5–47.2% in South Korea [2]. Individuals with MASLD not only face a heightened risk of progressing to severe liver conditions such as metabolic dysfunction-associated steatotic hepatitis, advanced liver fibrosis, cirrhosis, and hepatocellular carcinoma, but also have an increased risk of hypertension, type 2 diabetes, cardiovascular diseases (CVDs), chronic kidney disease, metabolic syndrome, and colorectal cancer [3–8]. These conditions impose a significant economic and healthcare burden. Therefore, it is essential to identify simple, easily accessible, and cost-effective biomarkers for early detection of high-risk MASLD individuals.
Although the pathogenesis of hepatic steatosis is not fully elucidated, substantial evidence suggests that inflammation and immunity play a significant role [9]. Recently, the pan-immune-inflammation value (PIV) has been proposed as a biomarker that reflects local or systemic immune status and inflammatory response [10]. PIV provides a more comprehensive assessment of inflammation than traditional indicators like neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) by incorporating complete blood count (CBC)-derived inflammatory cells, thus offering a more holistic view of the inflammatory state [11]. Initially, PIV was found to be strongly correlated with prognosis in various cancers [12–17]. Subsequent studies have shown that PIV can also be employed to evaluate the onset, monitor progression, and predict outcomes in CVDs, stroke, autoimmune disorders, infections, and other conditions [18–23]. Additionally, one study revealed that PIV is a better inflammatory marker than systemic immunity index for NAFLD assessment [24]. Currently, research on the relationship between PIV and MASLD is limited. Therefore, this study aimed to investigate the association of PIV with MASLD using data from the National Health and Nutrition Examination Survey (NHANES) 2017–2020.
Methods
Study design and population
NHANES, conducted by the National Center for Health Statistics (NCHS), is a research program designed to evaluate the health and nutritional status of US adults and children. It employs a multi-stage cluster probability sampling method and uniquely combines interviews with physical examinations. The interviews cover demographic, dietary, socioeconomic, and health-related topics. The examination section includes medical, dental, and physiological assessments, along with laboratory tests performed by trained healthcare professionals.
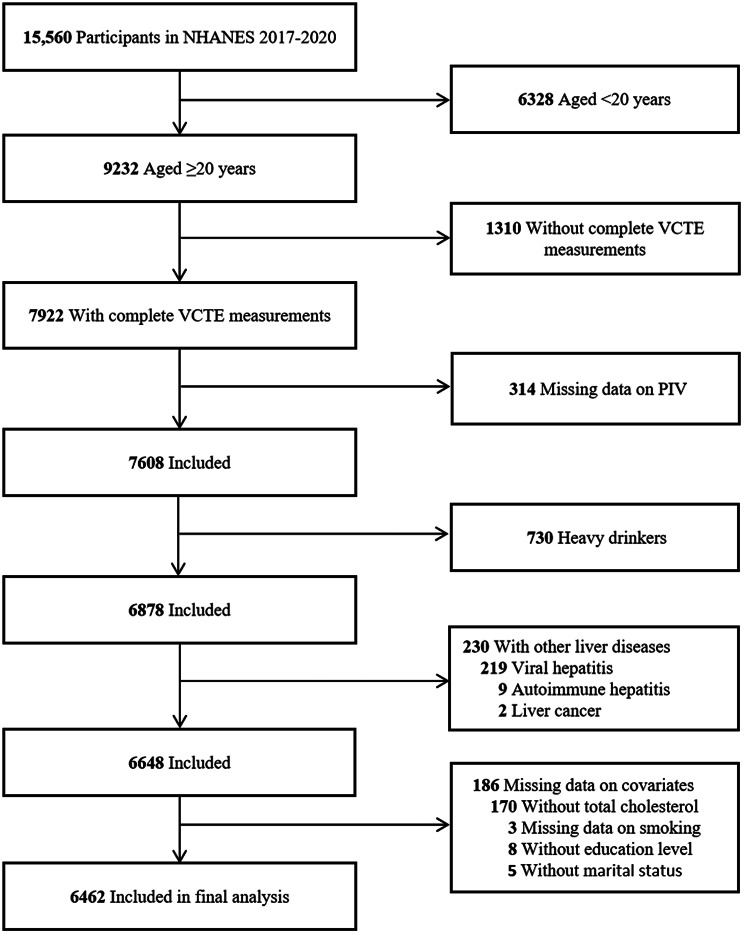
Among the initial 15,560 participants in NHANES 2017–2020, we excluded individuals younger than 20 years (n = 6328), those lacking complete liver ultrasound vibration-controlled transient elastography (VCTE) measurements (n = 1310), those with missing PIV data (n = 314), heavy drinkers (defined as > 30 g/day for men and > 20 g/day for women; n = 730), individuals with other liver diseases (n = 230), and those with missing covariate data (n = 186). Ultimately, 6462 individuals were included in the analyses. The detailed study flowchart is depicted in Fig. 1.
Fig. 1.
Flow chart of participant selection. NHANES, National Health and Nutrition Examination Survey; VCTE, vibration controlled transient elastography; PIV, pan-immune-inflammation value
Definition of MASLD
VCTE was performed with the FibroScan® 502 V2 Touch (Echosens) device to measure controlled attenuation parameter (CAP) and liver stiffness measurement values for assessing liver steatosis and fibrosis. The examination required a fasting period of at least 3 h and a minimum of 10 valid stiffness measurements, with the interquartile range/median of liver stiffness being 30% or less, which were considered the criteria for a complete examination. Hepatic steatosis was defined by a median CAP value of at least 285 dB/m [25]. MASLD was defined as having hepatic steatosis and excluding excessive alcohol consumption (≥ 30 g/day for males and ≥ 20 g/day for females), and meeting at least one of the following cardiometabolic risk factors: (1) body mass index (BMI) ≥ 25 kg/m² (≥ 23 kg/m² for Asians) or waist circumference (WC) ≥ 94/80 cm (male/female); (2) fasting blood glucose ≥ 100 mg/dL or glycosylated hemoglobin ≥ 5.7%, or a history of type 2 diabetes, or currently receiving treatment for type 2 diabetes; (3) blood pressure ≥ 130/85 mmHg or receiving antihypertensive treatment; (4) plasma triglycerides ≥ 150 mg/dL or undergoing lipid-lowering treatment; (5) plasma high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL for men or < 50 mg/dL for women, or receiving lipid-lowering treatment [26].
Calculation of PIV
At the Mobile Examination Center, NHANES professionals utilized the Beckman Coulter DxH 800 device to measure complete blood cell counts, expressed as ×10³ cells/µL. The formula for calculating PIV was: platelet count × neutrophil count × monocyte count / lymphocyte count [27]. Due to the skewed distribution of PIV, a natural logarithmic (Ln) transformation was applied.
Covariates
Confounding factors were chosen based on previous studies [24, 28] and theoretical rationale. This research included several covariates that might be associated with PIV and MASLD, including sex (male/female), age, race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and others), education level (high school or below, some college or associate’s degree, college graduate or above), marital status (married/living with a partner, widowed/divorced/separated, never married), poverty income ratio (PIR), smoking, physical activity, Healthy Eating Index 2015 (HEI-2015), total cholesterol, and total energy intake. Smoking status was categorized as never, former, or current based on whether participants had smoked at least 100 cigarettes in their lifetime and their current smoking habits [29]. Physical activity levels were divided into no (< 1 MET-h/week), low (1–48 MET-h/week), and high (> 48 MET-h/week) [30]. HEI-2015 scores were calculated based on 24-hour dietary recalls, ranging from 0 to 100, with higher scores representing better diet quality [31]. Detailed scoring criteria are available in Supplementary Table S1.
Statistical analysis
We employed NHANES-recommended weighting method in our statistical analyses to guarantee the sample’s national representativeness. R version 4.3.2 software (R Foundation for Statistical Computing, Vienna, Austria) was utilized to analyze and process data. A two-sided P value < 0.05 was considered statistically significant. We reported continuous variables as weighted means and standard errors (SEs), while categorical variables were displayed as weighted percentages and the respective 95% confidence intervals (CIs). Weighted multivariable logistic regression models with different levels of adjustment were used to calculate the odds ratios (ORs) and 95% CIs to examine the association between PIV and the risk of MASLD. Model 1 did not adjust for any covariates. Model 2 adjusted for sex, age, and race/ethnicity. Model 3 was a fully adjusted model that accounted for all covariates, including sex, age, race/ethnicity, education level, marital status, PIR, smoking, physical activity, HEI-2015, total cholesterol, and total energy intake.
We conducted restricted cubic spline (RCS) analysis with four knots (at the 5th, 35th, 65th, and 95th percentiles) to explore the nonlinear relationship between LnPIV and MASLD. Likelihood ratio tests were used to evaluate nonlinearity. Furthermore, stratified analyses were performed based on sex, age (20–39 years, 40–59 years, and ≥ 60 years), race/ethnicity, education level, marital status, PIR (< 1.30, 1.30–3.50, and ≥ 3.50), smoking, and physical activity.
Two sensitivity analyses were also conducted to ensure the robustness of our findings. First, we conducted a repeated analysis defining hepatic steatosis as a median CAP value of 263 dB/m or higher (90% sensitivity) [32, 33]. Second, we applied multivariate multiple imputation with chained equations to address missing values in PIR, HEI-2015, and total energy intake.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics of the study population. Among the 6462 participants included, 2458 (37.40%) were diagnosed with MASLD. The mean (se) age was 48.43 (0.64) years, with females comprising 51.96% and non-Hispanic whites accounting for 61.80%. The range of PIV across the four quartiles (Q1 to Q4) was as follows: Q1 (< 153.00), Q2 (153.00-239.13), Q3 (239.14-375.22), and Q4 (≥ 375.23). There were significant differences in age, race/ethnicity, education level, PIR, smoke status, and physical activity across the PIV quartiles (all P < 0.05). Older participants, non-Hispanic whites, moderately education level, and never smoker were associated with higher PIV levels. In contrast, low income, low intensity physical activity, and poor dietary habits were associated with higher PIV levels.
Table 1.
Baseline characteristics of participants in NHANES 2017-2020a
| Characteristic | Total | Q1 | Q2 | Q3 | Q4 | P value |
|---|---|---|---|---|---|---|
| Nb | 6462 | 1615 | 1616 | 1615 | 1616 | |
| Sex | ||||||
| Male | 48.04 (46.05–50.03) | 46.36 (42.53–50.24) | 50.91 (47.49–54.32) | 48.30 (44.13–52.50) | 46.49 (42.55–50.48) | 0.289 |
| Female | 51.96 (49.97–53.95) | 53.64 (49.76–57.47) | 49.09 (45.68–52.51) | 51.70 (47.50-55.87) | 53.51 (49.52–57.45) | |
| Age, y | 48.43 (0.64) | 46.98 (0.77) | 47.11 (0.83) | 48.80 (0.85) | 50.34 (0.71) | < 0.001 |
| Age strata, y | ||||||
| 20–39 | 35.92 (33.14–38.80) | 39.65 (35.36–44.11) | 37.84 (33.54–42.34) | 34.56 (30.87–38.45) | 32.66 (28.71–36.87) | 0.009 |
| 40–59 | 34.16 (32.11–36.27) | 33.19 (29.58–36.99) | 35.42 (30.52–40.65) | 35.44 (32.70-38.28) | 32.55 (28.36–37.04) | |
| ≥ 60 | 29.92 (26.67–33.39) | 27.16 (23.12–31.61) | 26.74 (22.46–31.51) | 30.00 (25.34–35.11) | 34.79 (30.89–38.92) | |
| Race and ethnicity | ||||||
| Mexican American | 9.08 (6.73–12.15) | 9.49 (6.23–14.19) | 10.11 (7.28–13.89) | 9.21 (7.22–11.68) | 7.74 (5.49–10.80) | < 0.001 |
| Other Hispanic | 7.92 (6.39–9.78) | 8.31 (6.50-10.57) | 8.28 (6.58–10.37) | 7.28 (5.58–9.46) | 7.92 (5.66–10.98) | |
| Non-Hispanic White | 61.80 (56.13–67.17) | 48.26 (39.50-57.13) | 60.35 (55.99–64.55) | 66.03 (59.86–71.71) | 69.34 (62.52–75.41) | |
| Non-Hispanic Black | 11.07 (8.40-14.46) | 20.91 (15.65–27.36) | 10.86 (8.25–14.17) | 7.72 (5.64–10.47) | 6.98 (5.05–9.57) | |
| Others | 10.12 (7.95–12.81) | 13.03 (9.87–17.02) | 10.40 (7.79–13.74) | 9.75 (7.49–12.60) | 8.03 (5.74–11.11) | |
| Education level | ||||||
| High school or less | 3.95 (3.12–4.99) | 4.17 (3.05–5.67) | 4.12 (2.87–5.89) | 4.16 (3.38–5.12) | 3.42 (2.53–4.60) | 0.003 |
| Some college or associates degree | 34.44 (30.86–38.21) | 28.91 (24.47–33.79) | 33.16 (28.60-38.06) | 36.79 (32.26–41.58) | 37.53 (32.30-43.07) | |
| College graduate or above | 61.61 (57.67–65.41) | 66.93 (61.72–71.74) | 62.72 (57.83–67.36) | 59.04 (54.13–63.78) | 59.05 (53.60-64.29) | |
| Marital status | ||||||
| Married/Living with Partner | 61.77 (58.49–64.94) | 63.01 (59.17–66.69) | 60.63 (54.64–66.32) | 62.26 (58.41–65.96) | 61.37 (56.99–65.57) | 0.865 |
| Widowed/Divorced/Separated | 18.54 (16.86–20.34) | 16.93 (15.22–18.79) | 18.91 (16.09–22.11) | 19.18 (16.13–22.65) | 18.81 (16.38–21.51) | |
| Never married | 19.69 (17.28–22.35) | 20.06 (16.93–23.61) | 20.45 (16.02–25.74) | 18.56 (15.89–21.56) | 19.82 (16.52–23.59) | |
| Poverty income ratio | 3.12 (0.06) | 3.15 (0.1) | 3.12 (0.08) | 3.20 (0.09) | 3.01 (0.06) | 0.041 |
| Poverty income ratio categories | ||||||
| < 1.3 | 18.71 (17.50-19.98) | 20.03 (17.22–23.17) | 18.61 (16.25–21.22) | 18.48 (16.04–21.20) | 18.02 (16.02–20.21) | 0.012 |
| 1.3–3.5 | 35.89 (34.06–37.76) | 32.75 (28.65–37.14) | 36.05 (31.38-41.00) | 33.06 (28.40-38.07) | 40.91 (37.10-44.84) | |
| ≥ 3.5 | 45.40 (43.34–47.48) | 47.22 (41.37–53.14) | 45.34 (40.08–50.71) | 48.46 (42.54–54.42) | 41.06 (37.08–45.17) | |
| Smoking | ||||||
| Never | 14.78 (12.30-17.65) | 11.74 (9.41–14.55) | 12.09 (10.15–14.34) | 15.85 (12.44–19.98) | 18.47 (15.01–22.51) | < 0.001 |
| Former | 25.06 (22.83–27.43) | 21.20 (17.45–25.50) | 26.77 (23.49–30.32) | 25.67 (22.13–29.56) | 25.90 (22.97–29.06) | |
| Current | 60.16 (57.45–62.80) | 67.07 (62.58–71.27) | 61.14 (57.14-65.00) | 58.48 (55.35–61.55) | 55.63 (51.64–59.55) | |
| Physical activity | ||||||
| No | 20.60 (19.20-22.08) | 18.08 (15.74–20.68) | 18.86 (16.15–21.91) | 20.97 (17.51–24.92) | 23.73 (21.23–26.43) | 0.004 |
| Low intensity | 42.02 (40.04–44.04) | 42.55 (38.80-46.38) | 39.72 (36.25–43.29) | 41.93 (39.05–44.86) | 43.78 (39.76–47.89) | |
| High intensity | 37.37 (35.46–39.33) | 39.38 (36.06–42.80) | 41.43 (37.61–45.34) | 37.10 (34.07–40.23) | 32.49 (28.44–36.81) | |
| MASLD | ||||||
| No | 62.60 (60.31–64.84) | 71.55 (69.07–73.92) | 66.41 (61.65–70.85) | 58.47 (53.78–63.01) | 56.34 (51.85–60.72) | < 0.001 |
| Yes | 37.40 (35.16–39.69) | 28.45 (26.08–30.93) | 33.59 (29.15–38.35) | 41.53 (36.99–46.22) | 43.66 (39.28–48.15) | |
| HEI-2015 score | 49.86 (0.47) | 52.01 (0.78) | 50.74 (0.57) | 48.46 (0.44) | 48.74 (0.64) | < 0.001 |
| Total cholesterol, mg/dL | 186.64 (1.41) | 187.00 (2.21) | 186.39 (1.9) | 188.05 (2.21) | 185.23 (1.78) | 0.738 |
| Total energy intake, kcal/d | 2102.39 (15.52) | 2093.38 (35.06) | 2128.21 (29.77) | 2154.69 (40.08) | 2035.40 (29.45) | 0.055 |
| PIV | 321.29 (8.05) | 108.30 (1.27) | 195.77 (1.21) | 300.16 (1.5) | 615.35 (12.39) | < 0.001 |
| LnPIV | 5.54 (0.02) | 4.63 (0.01) | 5.27 (0.01) | 5.70 (0) | 6.34 (0.01) | < 0.001 |
NHANES, National Health and Nutrition Examination Survey; PIV, pan-immune-inflammation; MASLD, metabolic dysfunction-associated steatotic liver disease; HEI, healthy eating index
a Continuous variables are reported as weighted mean (standard error), while categorical variables are reported as weighted percentage with 95% confidence interval
b Numbers of each stratum may not add up to the total population due to missing data
c Others include Non-Hispanic Asian, other non-Hispanic, and multi-race individuals
Association between PIV and MASLD
As presented in Table 2, positive associations between LnPIV and MASLD were observed in all three models (Model 1: OR = 1.46, 95% CI: 1.28–1.66, P < 0.001; Model 2: OR = 1.41, 95% CI: 1.24–1.60, P < 0.001; Model 3: OR = 1.39, 95% CI: 1.16–1.65, P = 0.004). When PIV was classified into quartiles, both Q3 and Q4 exhibited significantly increased risks of MASLD compared with the reference Q1 in full adjusted Model 3 (Q3: OR = 1.63, 95% CI: 1.20–2.22, P = 0.012; Q4: OR = 1.76, 95% CI: 1.28–2.41, P = 0.008; P for trend = 0.002). RCS analysis did not show a nonlinear relationship between LnPIV and MASLD after adjusting for multiple covariates (P = 0.093 for nonlinearity, Fig. 2).
Table 2.
Association of PIV with MASLD
| Model | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| LnPIV | 1.46 (1.28–1.66) | < 0.001 | 1.41 (1.24–1.60) | < 0.001 | 1.39 (1.16–1.65) | 0.004 |
| PIV quartile | ||||||
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.27 (1.02–1.59) | 0.036 | 1.22 (0.97–1.54) | 0.081 | 1.20 (0.86–1.67) | 0.204 |
| Q3 | 1.79 (1.43–2.23) | < 0.001 | 1.72 (1.39–2.13) | < 0.001 | 1.63 (1.20–2.22) | 0.012 |
| Q4 | 1.95 (1.57–2.42) | < 0.001 | 1.87 (1.52–2.31) | < 0.001 | 1.76 (1.28–2.41) | 0.008 |
| P for trend | < 0.001 | < 0.001 | 0.002 | |||
PIV, pan-immune-inflammation; MASLD, metabolic dysfunction-associated steatotic liver disease; OR, odds ratio; CI, confidence interval
a Crude model
b Adjusted for sex, age, and race/ethnicity
c Additionally adjusted for education level, marital status, poverty income ratio, smoking, physical activity, HEI-2015, total cholesterol, and total energy intake
Fig. 2.

Dose-response relationship between PIV and MASLD. Adjusted for sex, age, race/ethnicity, education level, marital status, poverty income ratio, smoking, physical activity, HEI-2015, total cholesterol, and total energy intake. The black solid line and shaded area represent estimates and their corresponding 95% CIs, respectively. Vertical dotted lines indicate the minimal threshold for the beneficial association with estimated OR = 1. OR was calculated for each unit increase in the natural logarithm of PIV (LnPIV). PIV, pan-immune-inflammation value; MASLD, metabolic dysfunction-associated steatotic liver disease; OR, odds ratio; CI, confidence interval
Stratified and sensitivity analyses
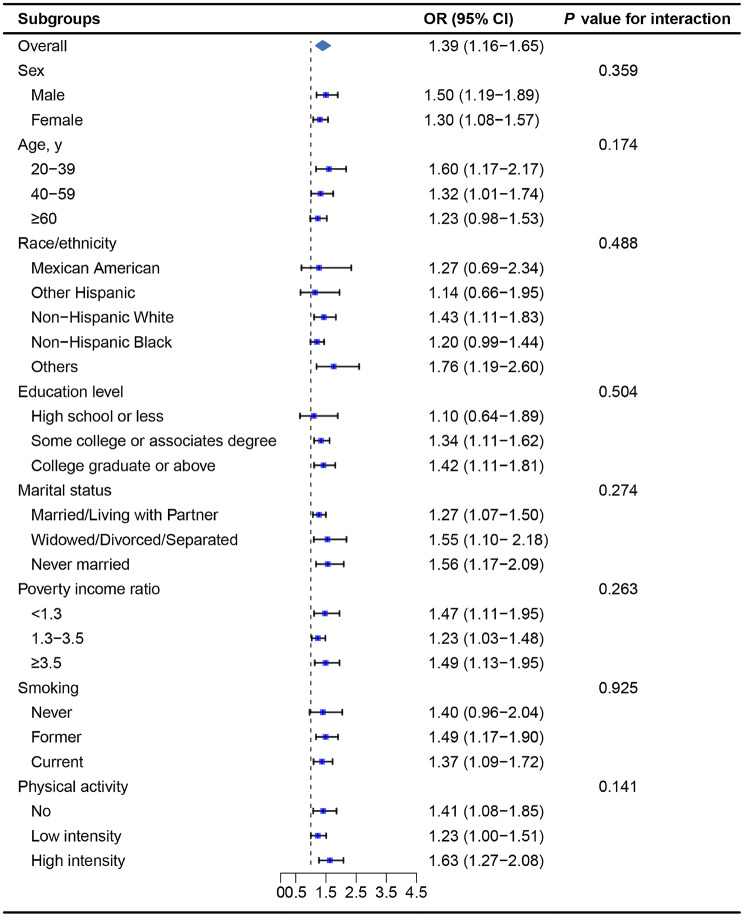
As shown in Fig. 3, a consistent positive association between LnPIV and MASLD was observed in all subgroups stratified by sex, age, race/ethnicity, education level, marital status, PIR, smoking, and physical activity (P for interaction < 0.05).
Fig. 3.
Stratified analysis of the association between PIV and MASLD. ORs were calculated for each unit increase in the natural logarithm of PIV (LnPIV). Each stratification was adjusted for sex, age, race/ethnicity, education level, marital status, poverty income ratio, smoking, physical activity, HEI-2015, total cholesterol, and total energy intake except the stratification factor itself. PIV, pan-immune-inflammation value; MASLD, metabolic dysfunction-associated steatotic liver disease; OR, odds ratio; CI, confidence interval
We also performed two sensitivity analyses in this study (Table 3). First, we used the median CAP value of 263 dB/m as the cutoff for defining hepatic steatosis. A positive association between LnPIV and MASLD were observed in Model 3 (OR = 1.32, 95% CI: 1.08–1.61, P = 0.014). When PIV was divided into quartiles, both Q3 and Q4 showed significantly increased risks of MASLD compared to Q1 in Model 3 (Q3: OR = 1.67, 95% CI: 1.42–2.45, P = 0.020; Q4: OR = 1.55, 95% CI: 1.10–2.16, P = 0.023; P for trend = 0.010). Additionally, the results remained robust after employing multiple imputation to address missing covariates in the repeated analysis.
Table 3.
Sensitivity analysis of the association of PIV with MASLDa
| CAP ≥ 263 dB/m | Multiple imputationb | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| LnPIV | 1.32 (1.08–1.61) | 0.014 | 1.36 (1.15–1.59) | 0.004 |
| PIV quartile | ||||
| Q1 | Reference | Reference | ||
| Q2 | 1.16 (0.86–1.55) | 0.241 | 1.20 (0.90–1.59) | 0.151 |
| Q3 | 1.67 (1.42–2.45) | 0.020 | 1.62 (1.22–2.15) | 0.010 |
| Q4 | 1.55 (1.10–2.16) | 0.023 | 1.78 (1.31–2.42) | 0.006 |
| P for trend | 0.010 | 0.001 | ||
PIV, pan-immune-inflammation; MASLD, metabolic dysfunction-associated steatotic liver disease; CAP, controlled attenuation parameter; OR, odds ratio; CI, confidence interval
a Adjusted for sex, age, race/ethnicity, education level, marital status, poverty income ratio, smoking, physical activity, HEI-2015, total cholesterol, and total energy intake
b Missing data for HEI-2015, poverty income ratio, and total energy intake were imputed
Discussion
In this study, we found a positive association between PIV and the risk of MASLD. Higher PIV quartiles were associated with a higher incidence of MASLD. Specifically, for each one unit increase in LnPIV, the likelihood of MASLD increased by 39% (OR = 1.39, P = 0.004). RCS analysis did not show a nonlinear relationship between LnPIV and MASLD. Stratified analysis suggested that the association between PIV and MASLD prevalence remained consistent across all subgroups (P for interaction > 0.05). Sensitivity analysis also confirmed the robustness of our findings.
Over the past few decades, PIV has been extensively studied in the field of oncology [10, 34–36]. A meta-analysis involving 30 studies and 8799 patients with malignant tumors indicated that pre-treatment PIV can serve as an effective and non-invasive prognostic biomarker for overall survival in cancer patients [37]. Another meta-analysis focused on breast cancer also yielded similar results [38]. In recent years, the prognostic value of PIV has been recognized in non-cancer diseases such as frailty [39], hypertension [40], myocardial infarction [41], heart failure [42], and kidney disease [43]. Currently, there is limited research on PIV in the context of steatotic liver disease. A retrospective study involving only 133 obese children and adolescents aged 6 to 18 confirmed that elevated PIV levels were linked to the presence and severity of hepatic steatosis [44]. Another study indicated that higher PIV levels were associated with an increased risk of NAFLD and liver fibrosis, especially in individuals under 60 years old [24]. However, both studies utilized the definition of NAFLD rather than MASLD, and one study had a notably limited sample size. Our research not only adopted the latest recognized definition of steatotic liver disease but also leveraged the large sample size of the NHANES database, enhancing the credibility of the results.
A recent study has revealed that fat accumulation plays a critical role in the development of NAFLD and metabolic-associated fatty liver disease in young adult males, even among non-obese individuals [45]. This accumulation can trigger inflammation and immune responses within the body. Increasing evidence underscores the significance of the host immune response in the pathogenesis of MASLD [46]. Neutrophils, monocytes, lymphocytes, and platelets all contribute to the development of this disease. Neutrophils contribute to liver cell damage and inflammation by forming neutrophil extracellular traps, releasing pro-inflammatory factors (such as tumor necrosis factor-α, interleukin-1β, and interleukin-6) and reactive oxygen species, and promoting ferroptosis [47]. Monocytes, after differentiating into macrophages, enhance liver inflammation and fibrosis by releasing pro-inflammatory factors, activating the CCR2 signaling pathway, and promoting lipid accumulation [48]. Lymphocytes influence liver inflammation and fibrosis through immune regulation, activation of intestinal innate lymphoid cells and CD8+ T cells, and the function of regulatory T cells [49, 50]. Platelets exacerbate liver inflammation and damage by producing soluble factors (such as platelet factor 4, platelet-derived growth factor, and transforming growth factor-β), interacting with immune cells, promoting fibrosis, and forming microthrombi [51]. The interaction of these cells, along with systemic inflammation and immune dysfunction, collectively contributes to the pathogenesis of MASLD. Higher PIV values indicate greater potential inflammation and poorer immune response capability, which is associated with an increased risk of MASLD, making our study finding unsurprising.
There are several strengths in this study. Our research is the first to investigate the association between PIV and MASLD. Additionally, all data we utilized were collected from NHANES, which has a standardized process for data collection to ensure accuracy. Furthermore, we explored the dose-response relationship between PIV and MASLD. However, this study has several limitations that warrant consideration. First, its cross-sectional design limited our ability to establish a causal relationship between PIV and MASLD. Second, NHANES data, while comprehensive, has inherent limitations, such as reliance on self-reported information and selection biases. Third, our analysis was based on participants from a single country, which may affect the generalizability of the findings to other populations. Fourth, although we effectively controlled for various cardiometabolic factors (e.g. smoking, physical activity, and HEI-2015) that could influence the relationship between PIV and MASLD through multiple mechanisms, such as directly affecting the inflammatory state and fat metabolism, other covariates like genetic factors and environmental exposures were not adequately explored in this study. Fifth, we used VCTE instead of liver biopsy to diagnose steatotic liver. While liver biopsy is considered the gold standard, it is neither feasible nor practical for large-scale population studies. VCTE is regarded as a suitable tool due to its significant sensitivity and specificity [52]. Finally, current guidelines recommend non-invasive serological scoring followed by imaging techniques for MASLD patients [53]. Although our findings have potential clinical applications, we did not construct a risk prediction model that incorporates PIV. Future research should address this issue and conduct prospective studies to assess the role of PIV in the progression and treatment response of MASLD.
Conclusions
Our research identified high PIV levels as an independent risk factor for MASLD, with elevated PIV levels being associated with an increased prevalence of MASLD. Further prospective studies are warranted to investigate the causal relationship underlying this observation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the National Center for Health Statistics of the Centers for Disease Control and Prevention for sharing the NHANES data.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- VCTE
Vibration controlled transient elastography
- PIV
Pan-immune-inflammation value
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- OR
Odds ratio
- CI
Confidence interval
- HEI
Healthy eating index
- CAP
Controlled attenuation parameter
Author contributions
L.H., Y.W., and J.Z. contributed to conception and design of the study. J.Z. provided administrative support. L.H., Z.N., and Q.Y. collected and analyzed the data. W.H. and J.L. prepared the tables and figures. L.H. and Z.N. drafted the manuscript. Q.Y., W.H., J.L., and Y.W. critically revised the manuscript. All authors read and approved the final manuscript.
Funding
The study was funded by the Medical and Health Guiding Project of Xiamen (Grant No. 3502Z20224ZD1009).
Data availability
Publicly available datasets were analyzed in this study. The data underlying this article are available in NHANES (https://www.cdc.gov/nchs/nhanes/index.htm).
Declarations
Ethics approval and consent to participate
The ethics review board of the National Center for Health Statistics (NCHS) approved all NHANES protocols, and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lian-Zhen Huang, Ze-Bin Ni, and Qi-Rong Yao contributed equally to this work.
Contributor Information
Yan-Qing Wang, Email: xiaosirui2010@163.com.
Jin-Yan Zhang, Email: zjywyq2002@163.com.
References
- 1.Simon TG, Roelstraete B, Khalili H, Hagstrom H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70(7):1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miwa T, Tajirika S, Imamura N, Adachi M, Horita R, Hanai T, et al. Prevalence of Steatotic Liver Disease based on a New nomenclature in the Japanese Population: A Health Checkup-based cross-sectional study. J Clin Med. 2024;13(4):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022;31(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pustjens J, van Kleef LA, Janssen HLA, de Knegt RJ, Brouwer WP. Differential prevalence and prognostic value of metabolic syndrome components among patients with MASLD. JHEP Rep. 2024;22(12):101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73(4):691–702. [DOI] [PubMed] [Google Scholar]
- 6.Bilson J, Mantovani A, Byrne CD, Targher G. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 2024;50(1):101506. [DOI] [PubMed] [Google Scholar]
- 7.Choe EK, Kang HY. The association between platelet-related parameters and nonalcoholic fatty liver disease in a metabolically healthy nonobese population. Sci Rep. 2024;14(1):6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalligeros M, Henry L, Younossi ZM. Metabolic dysfunction-associated steatotic liver disease and its link to cancer. Metabolism. 2024;160:156004. [DOI] [PubMed] [Google Scholar]
- 9.Schwärzler J, Grabherr F, Grander C, Adolph TE, Tilg H. The pathophysiology of MASLD: an immunometabolic perspective. Expert Rev Clin Immunol. 2024;20(4):375–86. [DOI] [PubMed] [Google Scholar]
- 10.Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, et al. The Pan-immune-inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. 2020;123(3):403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murat B, Murat S, Ozgeyik M, Bilgin M. Comparison of pan-immune-inflammation value with other inflammation markers of long-term survival after ST-segment elevation myocardial infarction. Eur J Clin Invest. 2023;53(1):e13872. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Wang L, Yang X, Chen Q, Cheng X. Clinical utility of preoperative pan-immune-inflammation value (PIV) for prognostication in patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2023;123:110805. [DOI] [PubMed] [Google Scholar]
- 13.Lin F, Zhang LP, Xie SY, Huang HY, Chen XY, Jiang TC, et al. Pan-immune-inflammation Value: a New Prognostic Index in operative breast Cancer. Front Oncol. 2022;12:830138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang N, Hou T, Zhang S, Ling J, Jiang S, Xie Y, et al. Prognostic significance of pan-immune-inflammation value (PIV) in nasopharyngeal carcinoma patients. Heliyon. 2024;10(2):e24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambichler T, Said S, Abu Rached N, Scheel CH, Susok L, Stranzenbach R, et al. Pan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. J Cancer Res Clin Oncol. 2022;148(11):3183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topkan E, Selek U, Kucuk A, Pehlivan B. Low Pre-chemoradiotherapy Pan-immune-inflammation Value (PIV) measures Predict Better Survival outcomes in locally advanced pancreatic adenocarcinomas. J Inflamm Res. 2022;15:5413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucuk A, Topkan E, Ozkan EE, Ozturk D, Pehlivan B, Selek U. A high pan-immune-inflammation value before chemoradiotherapy indicates poor outcomes in patients with small-cell lung cancer. Int J Immunopathol Pharmacol. 2023;37:3946320231187759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin C, Li X, Luo Y, Zhang C, Zuo D. Associations between pan-immune-inflammation value and abdominal aortic calcification: a cross-sectional study. Front Immunol. 2024;15:1370516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Şen F, Kurtul A, Bekler Ö. Pan-immune-inflammation Value is independently correlated to impaired coronary Flow after primary percutaneous coronary intervention in patients with ST-Segment Elevation myocardial infarction. Am J Cardiol. 2024;211:153–9. [DOI] [PubMed] [Google Scholar]
- 20.Han W, Yi HJ, Shin DS, Kim BT. Pan-immune-inflammation value predict delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2024;121:47–52. [DOI] [PubMed] [Google Scholar]
- 21.Tutan D, Doğan AG. Pan-immune-inflammation Index as a biomarker for rheumatoid arthritis progression and diagnosis. Cureus. 2023;15(10):e46609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tunca O, Kazan ED. A new parameter predicting steroid response in idiopathic IgA nephropathy: a pilot study of pan-immune inflammation value. Eur Rev Med Pharmacol Sci. 2022;26(21):7899–904. [DOI] [PubMed] [Google Scholar]
- 23.Ayaz ÇM, Turhan Ö, Yılmaz VT, Adanır H, Sezer B, Öğünç D. Can the pan-immune-inflammation value predict gram negative bloodstream infection-related 30-day mortality in solid organ transplant patients? BMC Infect Dis. 2024;24(1):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang R, Hua Y, Hu X, Hong Z. The pan immune inflammatory value in relation to non-alcoholic fatty liver disease and hepatic fibrosis. Clin Res Hepatol Gastroenterol. 2024;48(7):102393. [DOI] [PubMed] [Google Scholar]
- 25.Chai C, Chen L, Deng MG, Liang Y, Liu F, Nie JQ. Dietary choline intake and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2017–2018. Eur J Clin Nutr. 2023;77(12):1160–6. [DOI] [PubMed] [Google Scholar]
- 26.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;1(6):1966–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coste SC, Orășan OH, Cozma A, Negrean V, Sitar-Tăut AV, Filip GA, et al. Metabolic dysfunction-associated steatotic liver disease: The associations between inflammatory markers, TLR4, and Cytokines IL-17A/F, and Their Connections to the Degree of Steatosis and the Risk of Fibrosis. Biomedicines. 2024;21;12(9):2144. [DOI] [PMC free article] [PubMed]
- 28.Li Y, Yang P, Ye J, Xu Q, Wu J, Wang Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024;22(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Luo C, Tan D, Li Y. J-shaped associations of pan-immune-inflammation value and systemic inflammation response index with stroke among American adults with hypertension: evidence from NHANES 1999–2020. Front Neurol. 2024;15:1417863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian X, Xue B, Wang B, Lei R, Shan X, Niu J, et al. Physical activity reduces the role of blood cadmium on depression: a cross-sectional analysis with NHANES data. Environ Pollut. 2022;304:119211. [DOI] [PubMed] [Google Scholar]
- 31.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian X, Ding N, Su Y, Qin J. Comparison of obesity-related indicators for nonalcoholic fatty liver Disease diagnosed by transient Elastography. Turk J Gastroenterol. 2023;34(10):1078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atzori S, Pasha Y, Maurice JB, Taylor-Robinson SD, Campbell L, Lim AKP. The Accuracy of Ultrasound Controlled Attenuation parameter in diagnosing hepatic Fat Content. Hepat Med. 2023;15:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamanouchi K, Maeda S. The efficacy of Inflammatory and Immune markers for Predicting the prognosis of patients with stage IV breast Cancer. Acta Med Okayama. 2023;77(1):37–43. [DOI] [PubMed] [Google Scholar]
- 35.Feng J, Wang L, Yang X, Chen Q, Cheng X. Pretreatment pan-immune-inflammation value (PIV) in Predicting Therapeutic Response and Clinical outcomes of Neoadjuvant Immunochemotherapy for esophageal squamous cell carcinoma. Ann Surg Oncol. 2024;31(1):272–83. [DOI] [PubMed] [Google Scholar]
- 36.Liao W, Li J, Feng W, Kong W, Shen Y, Chen Z, et al. Pan-immune-inflammation value: a new prognostic index in epithelial ovarian cancer. BMC Cancer. 2024;24(1):1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hai-Jing Y, Shan R, Jie-Qiong X. Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: an updated meta-analysis of 30 studies. Front Nutr. 2023;10:1259929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng HW, Wang T, Yu GC, Xie LY, Shi B. Prognostic role of the systemic immune-inflammation index and pan-immune inflammation value for outcomes of breast cancer: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2024;28(1):180–90. [DOI] [PubMed] [Google Scholar]
- 39.Okyar Baş A, Güner M, Ceylan S, Hafızoğlu M, Şahiner Z, Doğu BB, et al. Pan-immune inflammation value; a novel biomarker reflecting inflammation associated with frailty. Aging Clin Exp Res. 2023;35(8):1641–9. [DOI] [PubMed]
- 40.Wu B, Zhang C, Lin S, Zhang Y, Ding S, Song W. The relationship between the pan-immune-inflammation value and long-term prognoses in patients with hypertension: National Health and Nutrition Examination Study, 1999–2018. Front Cardiovasc Med. 2023;10:1099427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Liu J, Liu L, Cao S, Jin T, Chen L, et al. Association of systemic inflammatory response index and pan-immune-inflammation-value with long-term adverse Cardiovascular events in ST-Segment Elevation myocardial infarction patients after primary percutaneous coronary intervention. J Inflamm Res. 2023;16:3437–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inan D, Erdogan A, Pay L, Genc D, Demırtola AI, Yıldız U, et al. The prognostic impact of inflammation in patients with decompensated acute heart failure, as assessed using the pan-immune inflammation value (PIV). Scand J Clin Lab Invest. 2023;83(6):371–8. [DOI] [PubMed] [Google Scholar]
- 43.Zhang F, Li L, Wu X, Wen Y, Zhan X, Peng F, et al. Pan-immune-inflammation value is associated with poor prognosis in patients undergoing peritoneal dialysis. Ren Fail. 2023;45(1):2158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demiröz Taşolar S, Çiftçi N. Role of pan immune inflammatory value in the evaluation of hepatosteatosis in children and adolescents with obesity. J Pediatr Endocrinol Metab. 2022;27(12):1481–86. [DOI] [PubMed] [Google Scholar]
- 45.Miwa T, Francisque C, Tajirika S, Hanai T, Imamura N, Adachi M, et al. Impact of body fat accumulation on metabolic dysfunction-associated fatty liver disease and nonalcoholic fatty liver disease in Japanese male young adults. Hepatol Res. 2023;53(8):691–700. [DOI] [PubMed] [Google Scholar]
- 46.Sawada K, Chung H, Softic S, Moreno-Fernandez ME, Divanovic S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023;35(11):1852–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19(2):177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heymann F, Trautwein C, Tacke F. Monocytes and macrophages as cellular targets in liver fibrosis. Inflamm Allergy Drug Targets. 2009;8(4):307–18. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Li J, Wu S, Cheng L, Shen Y, Ma W, et al. Type 3 innate lymphoid cell: a new player in liver fibrosis progression. Clin Sci (Lond). 2018;132(24):2565–82. [DOI] [PubMed] [Google Scholar]
- 50.Zheng C, Wang L, Zou T, Lian S, Luo J, Lu Y, et al. Ileitis promotes MASLD progression via bile acid modulation and enhanced TGR5 signaling in ileal CD8+ T cells. J Hepatol. 2024;80(5):764–77. [DOI] [PubMed] [Google Scholar]
- 51.Min Y, Hao L, Liu X, Tan S, Song H, Ni H, et al. Platelets fine-tune effector responses of naïve CD4 + T cells via platelet factor 4-regulated transforming growth factor β signaling. Cell Mol Life Sci. 2022;18(5):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81(3):492–542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. The data underlying this article are available in NHANES (https://www.cdc.gov/nchs/nhanes/index.htm).