Take Home Message
This study protocol shows the structured design of the randomised controlled phase 3 “PreAct” trial. It hypothesises the use of a fitness tracker–based prehabilitation programme to increase the physical activity of patients prior to radical cystectomy, as measured by the average number of steps per day.
1. Introduction
With approximately 573 000 newly diagnosed cases and 213 000 deaths worldwide in 2020, bladder cancer is a common malignancy in both men and women [1]. Radical cystectomy (RC) with bilateral pelvic lymphadenectomy is considered the standard procedure in the curative treatment of muscle-invasive bladder cancer (MIBC) [2], providing the best outcomes in terms of cancer control for patients with MIBC [3], [4], [5]. Consequently, it shows cancer-specific 10-yr survival rates of 71.5% (pT2) and 43.7% (pT3) [6]. However, with one in five patients experiencing a serious complication in the postoperative course, RC is still associated with significant morbidity and mortality [7], [8]. Vetterlein et al [8] demonstrated that almost every patient experiences at least one complication and every third even more than five complications in the first 30 postoperative days (PODs). Using the Clavien-Dindo classification (CDC) [9], Hautmann et al [7] reported grade 3–5 complications in 21.8% of patients who underwent RC with placement of an ileum neobladder. In both studies, the three most common types of complications were related to the urogenital and gastrointestinal tracts as well as local or systemic infections [7], [8]. Especially in elderly and multimorbid cystectomy patients, the seriousness of previous diseases and their effect on physical constitution, described as frailty, is predominant [10], [11], [12]. In an effort to improve the postoperative outcome of this patient group, there are various approaches to the perioperative process.
A recent meta-analysis of randomised controlled trials (RCTs) showed comparable outcomes of robot-assisted and open RC in terms of overall and recurrence-free survival [13]. In addition, for many patients, autonomy and quality of life after surgery are more important than simply prolonging life [14]. Especially with regard to autonomy, it will be necessary to actively involve patients in their treatment and recovery process. Multimodal concepts such as the ERAS protocols, which are based on standardised pre- and postoperative patient care, have already found their way into urology [15]. RC patients thus benefit from shorter hospital stays and a faster recovery of bowel function [15]. However, these programmes are often burdensome, requiring planning and supervision by medical staff, and are costly.
In this context, various studies evaluated the benefit of a preoperative increase in physical activity in terms of prehabilitation before surgery. Rahota et al [16] were able to show that a multimodal prehabilitation programme prior to robotic-assisted radical prostatectomy, consisting of moderate physical activity, individual patient counselling, and 1-d patient education, led to significantly shorter hospital stays as well as fewer postoperative complications and hospital readmissions. In addition, such programmes have the potential to effectively reduce patient anxiety in both the preoperative and the postoperative course [17]. Despite these promising results, there are certain limitations in the implementation of the programmes. On the one hand, these extend over a period of several weeks [17], [18] and involve a multidisciplinary approach [16], [18], resulting in higher personnel and cost requirements. On the other hand, more complex urological procedures are condensed to tertiary care referral and specialised centres [18]. Hence, long travel times to the facilities where the prehabilitation programme is being carried out were the main reason for refusing to participate in such a programme [17], [18], [19]. At the same time, the few programmes that were carried out from home show poor adherence control [19] and are again difficult for patients to integrate into their daily lives [20]. Therefore, it is important to develop a prehabilitation programme that can also be carried out at home and integrated into everyday life for an older, oftentimes multimorbid, patient population and that is easy to monitor for caregivers, allowing for easy patient contact.
There is evidence from the field of colorectal surgery suggesting that a preoperative increase in physical activity, based primarily on walking and breathing exercises, has a positive impact on postoperative recovery that is largely independent of other factors [21]. Preoperative improvement in physical activity was associated with maintenance or improvement in initial physical activity postoperatively [21]. The majority of patients who showed this effect participated in an exercise programme based on breathing and walking exercises [21]. In the context of postoperative mobilisation, the number of steps a patient takes differs significantly from the assessment of the medical staff [22]. Daskivich et al [22] suggest that even small differences in the postoperative number of steps can have clinical relevance for the further course. Accordingly, a precise method is needed to measure the individually very different numbers of steps. This supports the clinical use of fitness wristbands to continuously record patients' steps. A significant activity-increasing effect could already be observed postoperatively in orthopaedic patients through the feedback function of the fitness wristband alone [23]. Furthermore, patients show a high level of compliance in the fulfilment of activity goals that are communicated with the help of a fitness wristband [24]. To our knowledge, no data currently exist in the literature of an RCT investigating the preoperative use of fitness wristbands with the exclusive specification of a daily step target to prepare patients for the upcoming stresses of RC.
Therefore, the PreAct trial will investigate whether the preoperative implementation of a fitness tracker–based prehabilitation programme prior to RC leads to a significant increase in the preoperative physical activity, as measured by the average number of steps per day.
2. Methods
2.1. Trial registration
The PreAct trial was registered on clinicaltrials.gov under the official title: “Preoperative Physical Activity Improvement With the Use of Activity Trackers in Patients Undergoing Radical Cystectomy for Bladder Cancer: A Randomized Controlled Trial” (PreAct; unique protocol ID: 2021-677; ClinicialTrials.gov: NCT06416319).
2.2. Protocol version
This manuscript contains a detailed description of the study protocol of the PreAct trial in its latest amendment from August 3, 2023.
2.3. Trial design and study setting
The PreAct trial is a bicentric, open-label, parallel-group, randomised controlled phase 3 superiority trial conducted at the Department of Urology and Urosurgery of the University Medical Center Mannheim in Baden-Württemberg, Germany, and at the Urological Clinic München-Planegg in Bayern, Germany (ClinicalTrials.gov: NCT06416319; ethical approval: 2021-677). All staff members involved in the study are members of the Mannheim University Medical Centre, the Mannheim Medical Faculty of the University of Heidelberg, or the Urological Clinic München-Planegg. The trial commenced on March 14, 2023. Based on the results of a pilot study, we plan to recruit 164 patients over a period of 24 mo. The study participants will be assigned to the intervention or control group by block randomisation using a 1:1 allocation rate. Recruitment is scheduled to be completed on January 14, 2025.
2.4. Eligibility criteria
The inclusion criteria are as follows:
-
1.
Scheduled RC with bilateral pelvic lymphadenectomy and one of the different forms of urinary diversion in patients with bladder cancer
-
2.
Patient age ≥18 yr and capacity to consent
-
3.
Mobile patient who is not dependent on a walking aid
-
4.
The patient declares his or her consent by signing and dating the consent form before the surgical intervention
The exclusion criteria are as follows:
-
1.
Karnofsky index ≤70% (at 70%: cares for oneself, normal activity not possible, and not able to work) [25]
-
2.
American Society of Anesthesiologists (ASA) physical status classification [26]: ASA >3
-
3.
ASA 1 and 2 if acute or chronic diseases of the musculoskeletal system or the central nervous system are involved, resulting in symptomatic restriction of motor and/or, in the last case, neurological function (healing ruptures and fractures, Morbus Parkinson's disease, multiple sclerosis, etc.)
-
4.
Emergency RC
2.5. Interventions
2.5.1. Prehabilitation period
The preoperative period begins at the time of consent to participate in the study and signing of the consent form on the day of admission in the urology outpatient clinic, and lasts until the day of RC before transfer to the operating theatre. This period covers 7–10 d at our corresponding clinics. It should be noted that the prehabilitation period for the PreAct trial must by definition be at least 7 d. On average, this period is between 7 and 10 d, but can be extended to up to 14 d depending on the organisational planning of the premedication and surgery appointments in the respective clinics. This supports the idea of integrating the PreAct trial into clinical workflows without disrupting them.
2.5.2. Equipment of the study participants
The study participants, regardless of whether they are in the intervention or control group, each will receive a wearable fitness wristband, with pedometer function, and an accompanying smartphone (intervention: Versa 4, control: Charge 4, fitbit). A messaging app and a corresponding fitness app of the fitness wristband are installed on this smartphone. The smartphone has mobile data and a Bluetooth function, which are switched on throughout the pre- and postoperative periods to ensure permanent synchronisation between the fitness wristband and the smartphone. For each pair of fitness wristband and smartphone, an account is created in the fitness app and the messaging app with a corresponding password and e-mail address. This allows the pairs to be assigned gradually to new study participants without the names of the study participants appearing in the fitness or messaging app, ensuring that the study participants' data are always anonymous from third parties.
2.5.3. Implementation in the intervention group
The fitness wristbands of the patients in the intervention group are fully functional and display the daily step count numerically and graphically with a bar that fills up as the step target is approached. In addition, the fitness wristbands vibrate as an indication that the step target is reached. The patients' daily step target is 8000 steps and is set on the patients' fitness wristbands as the step target to be achieved. As part of the pilot study, a step target of 10 000 steps per day was set in accordance with the general consensus for a health-promoting lifestyle [27]. However, it was found that this step target was too high for some physically weaker patients, potentially leading to a demotivating effect, as these patients would no longer pursue the step goal. Consequently, the step target for the final PreAct trial was adjusted to 8000 steps per day in order to strike a balance between stronger and weaker walkers. Furthermore, the 6-min walk test (6MWT) [28] is performed in the corresponding urology outpatient clinic on the day of premedication after the patient's admission into the study, in order to record the basic endurance and functional levels of the RC patients. This is performed in both groups.
During the defined prehabilitation period of 7–10 d before surgery, the investigator sends the following messages at given times to the smartphone belonging to the corresponding fitness wristband, which immediately forwards the messages to the patient's fitness wristband using the Bluetooth function:
-
1.
8.00 a.m.: indication of the daily step target of 8000 steps
-
2.
11.00 a.m., 2.00 p.m., and 5.00 p.m.: viewing of the daily step count, and corresponding encouragement to be even more active or to continue to be active
-
3.
8.00 p.m.: feedback on the number of steps achieved that day and encouragement for the following day
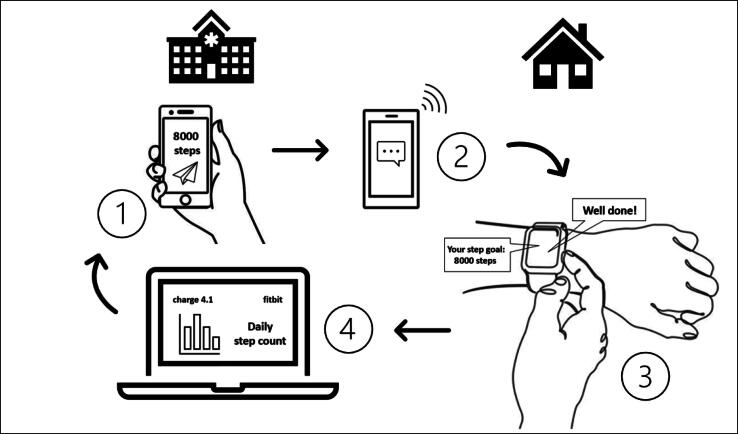
The smartphones of the patients in the intervention group are not locked. In addition, there is unrestricted access to the messaging and fitness app and the corresponding fitness data (see Fig. 1).
Fig. 1.
Graphical representation of the implementation of the intervention: (1) investigator sends daily step target and exercise instructions from the hospital to the patient's smartphone; (2) smartphone receives messages from the investigator on the way/at the patient's home and forwards these to the patient's fitness wristband; (3) vibration of the fitness wristband and direct display of step target, exercise instructions, and feedback on the patient's wrist, and (4) permanent synchronisation of activity data, storage of these data in the fitbit account, and the possibility of permanent hospital-internal access to the patient's activity profile.
2.5.4. Implementation in the control group
The displays of the fitness wristbands of the control group are covered with black tape and do not allow viewing of the fitness data on the wrist. The smartphones of the patients in the control group are not locked, and there is unrestricted access to the messaging app. However, the fitness app is locked using an applocker app, so there is no access and no possibility to view the fitness data. The patients in the control group do not receive a daily step goal, nor an exercise reminder or feedback at the end of the day. Thus, the patients in the control group simply wear the covered fitness wristband throughout the prehabilitation period without any feedback on their own fitness data. In addition, any reminder function such as vibration when the step target is reached is turned off.
2.5.5. Postoperative period
The fitness wristband is put on immediately after the RC at the end of surgical incision closure and removed again on the morning of the 4th POD during morning rounds. Here, the protocol is the same for both groups, in the sense that no step target is specified here. However, the fitness wristbands of the control group are still covered. The same requirements apply to the patients as in the prehabilitation period with regard to the permanent wearing of the fitness wristbands.
2.6. Outcomes
2.6.1. Primary outcome
The primary endpoint of the study is the increase in physical activity in the intervention group, measured by the average number of steps per day.
2.6.2. Secondary outcomes
Based on a previously conducted pilot study, the PreAct trial was powered and statistically designed as an RCT, with the primary endpoint focusing on the potential increase in the average number of steps per day in the period before RC. All secondary endpoints analysed below are exploratory, aimed at determining a potential correlation between the patients' preoperative physical activity and the secondary endpoints. In this context, certain endpoints such as postoperative complications and particularly intraoperative parameters (estimated blood loss, operating time, need for blood transfusions, and feasibility of urinary diversion) are largely influenced by other factors, such as the experience of the surgeon and surgical team, as well as the surgical technique.
2.6.2.1. Total number of steps
On the morning of the operation, the fitness wristband is removed and the steps taken during the preoperative period are evaluated.
2.6.2.2. Patients' postoperative physical activity on the first 3 PODs
After surgical incision closure, the fitness wristband is put on again in the operating theatre. Patients wear it for the first 3 PODs until it is removed again on the morning of the 4th POD during morning rounds. The 1st POD is defined as the 1st day immediately after the RC, starting at 12 a.m. Recording is completed at the end of the 3rd POD at 12 a.m. The overall number of steps and the average number of steps per day on the first 3 PODs are recorded. In particular, step counts in the early PODs after surgery may be an important predictor of the length of hospital stay, as suggested by Daskivich et al [22]. This outcome is recorded as a parameter for the degree of postoperative mobilisation of the patients and is also examined in relation to the average preoperative step counts per day. Accordingly, it can be investigated whether patients with a higher preoperative step count are more active in the early postoperative period during the first days after RC. As the publication by Daskivich et al [22] shows, this can be seen as an important indicator for the further postoperative course.
2.6.2.3. Postoperative complications
Postoperative complications are recorded on the day of discharge, POD 30, and POD 90 using the CDC [9] and the comprehensive complication index (CCI) [29]. The latter, in particular, has proved itself for the documentation of postoperative complications in urological surgery [30]. All complications are documented and classified according to their highest CDC grade. Complications with CDC grade ≥3a, corresponding to a CCI of ≥26.2, are defined as severe complications. Furthermore, the total number of complications that have occurred up to the specified time points is captured.
2.6.2.4. Perioperative parameters
-
1.
Operating time (in minutes, incision—surgical incision closure)
-
2.
Blood loss (in millilitres)
-
3.
Required transfusion of blood products (number of red blood cell concentrates, platelet concentrates, and frozen fresh plasma)
-
4.
Feasibility of the planned urinary diversion (yes/no)
-
5.
Conversion rate if the planned urinary diversion is not feasible
-
6.
Preoperative placement of an epidural catheter and its POD of removal
-
7.
Perioperative pain management: postoperative need for antipyretic and/or opioid analgesics recorded until POD 5
-
8.
Postoperative need for gastrointestinal prokinetics recorded until POD 5
-
9.
POD of the return to the urology ward
-
10.
POD of the first bowel movement
-
11.
POD of the first mobilisation
-
12.
Duration of the postoperative stay on the intermediate care unit (in days)
-
13.
Duration of the postoperative stay on the intensive care unit (in days)
2.6.2.5. Patient-reported outcome measures
The study participants' health-related quality of life is assessed using the SF-36 questionnaire [31] on the day of premedication in the urological admission, on the day of discharge, and on PODs 30 and 90.
2.6.2.6. Length of hospital stay
The total length of hospital stay in days is recorded on the day of discharge.
2.6.2.7. Readmission rate
The number of days alive and out of hospital up to POD 90 is recorded, as, for example, it was defined in the iROC trial by Catto et al [32].
2.7. Participant timeline
On the day of the premedication appointment in the urology outpatient clinic, approximately 7–10 d before the RC, patients are informed about the possibility of participating in the trial. This takes place after the informative discussion and the clinical examination by a urology specialist, ensuring the feasibility of RC before voluntary participation in the study is offered. The patients are fully informed about the course of the PreAct trial in both randomisation groups. If patients agree to participate in the study, they will be allocated to the intervention or control group according to a computer-generated list. The patients arrive on the ward the day before the RC. The prehabilitation period ends on the morning of the day of the operation, when the fitness wristband is removed at 7 a.m. on the ward before the patient is taken to the operating theatre. The fitness wristband is put back on in the operating theatre immediately after the surgical incision closure. Documentation of the postoperative step counts begins with the start of the 1st POD at 12 a.m. and lasts exactly 72 h. The fitness wristband is finally removed on the morning of POD 4 at 7 a.m. during early rounds. Health-related quality of life is also recorded using the SF-36 questionnaire [31] on the day of discharge, as well as on PODs 30 and 90. For PODs 30 and 90, the questionnaires are sent to patients by e-mail or post (Table 1) [33].
Table 1.
Participant timeline adapted from the original SPIRIT 2013 statement [33]
| Study period |
||||||||
|---|---|---|---|---|---|---|---|---|
| Enrolment | Prehabilitation | Surgery | Postoperative period |
|||||
| Time point | Premedication | 7–10 d before RC | RC | POD 4 | POD 6 | Discharge | POD 30 | POD 90 |
| Enrolment | ||||||||
| Eligibility screen | × | |||||||
| Informed consent | × | |||||||
| Allocation | × | |||||||
| Interventions | ||||||||
| Feedback | ||||||||
| No feedback | ||||||||
| Assessments | ||||||||
| Primary endpoint (steps per day) | × | |||||||
| Steps overall during prehabilitation | × | |||||||
| Intraoperative parameters | × | |||||||
| Postoperative step count per day and overall on PODs 1–3 | × | |||||||
| Postoperative parameters recorded until POD 5 | × | |||||||
| Patient-reported outcome measures (SF-36 questionnaire) | × | × | × | |||||
| Postoperative complications | × | × | × | |||||
| Length of hospital stay | × | |||||||
| Readmission rate | × | |||||||
| Reoperation and reintervention rate | × | |||||||
POD = postoperative day; RC = radical cystectomy.
2.8. Sample size
Before the start of the final PreAct trial, we conducted a pilot study with 14 RC patients to determine the general step count in this population without intervention. On average, a step count of 5467 steps (standard deviation 3088) per day was determined for the patient population in the pilot study. It was assumed that a 25% increase (= 1367 steps) in the average number of steps per day in the intervention group compared with the average in the control group would be clinically relevant. Assuming a power of 80% and a two-sided significance level of 5%, we determined a sample size of 164 patients. The sample size will be increased by 10% to account for potential dropouts. After completion of the follow-up of the first 100 trial participants, we plan to conduct an interim analysis. Subsequently, a sample size recalculation will be performed according to which the sample size will be adjusted or the trial will be cancelled for reasons of clinical meaningfulness.
2.9. Assignment of interventions
2.9.1. Allocation sequence generation
The allocation sequence was created using the programme R and the corresponding R-package for generating allocation sequences for RCTs. The allocation is done in blocks of a certain size, unknown to the investigator, and is divided into intervention or control. After conducting the pilot study, the patient population was stratified into the following groups:
-
1.
Gender (male vs female)
-
2.
Age (≥70 yr defined as old and <69 yr defined as young).
2.9.2. Allocation concealment mechanism
According to the defined stratification groups, randomisation cards were printed out and packed in nonopaque, sealed envelopes by the creator of the allocation sequence who was otherwise not involved in the trial. Thus, any prior inspection of the envelope is impossible. The envelopes remain sealed until the patient agrees to participate in the study and are opened only after the patient has given consent. Only the respective stratum is labelled on the envelope itself for the investigator. Admission and allocation to the randomisation group are performed by the admitting investigator, who was not involved in the generation of the allocation sequence and has no knowledge of the size of the randomisation blocks.
2.9.3. Blinding
The PreAct trial is designed as an open-label RCT, and there is no blinding on the part of the investigator, surgeon, or patient. This is necessary in order to adequately inform potential patients in the intervention group about the principle of the intervention prior to randomisation, as the randomisation group is not known until the patient has given his/her consent.
2.10. Data collection, management, and analysis
2.10.1. Data collection methods
All study data are recorded by the responsible investigator immediately at the specified study visits in a case report form (CRF). If a patient withdraws from the study before the data collection has been completed, all patient data from the completed study visits will be recorded. By permanently synchronising the respective fitness wristband with the corresponding smartphone via the Fitbit app, step counts are recorded in real time. Furthermore, all step counts can be viewed permanently in the corresponding Fitbit account as soon as these have been synchronised. Step counts are stored for up to 30 d without synchronisation, even if synchronisation is missing.
2.10.2. Statistical methods
All included patients will be incorporated into the final descriptive analysis of the study. For the primary endpoint, confirmatory testing is performed using the t test for independent groups on the intention-to-treat population. All secondary endpoints are reported using descriptive statistics, for the overall cohort as well as stratified by treatment group and subgroups. In the case of continuous data, the mean and median with the standard deviation and interquartile range are reported. Absolute and relative frequencies are given for categorical variables. Group comparisons are carried out for continuous data using the t test or the Mann-Whitney test, or for categorical variables using the chi-square or Fisher test. The analysis is carried out according to the intention-to-treat, per-protocol, and as-treated principles. If possible, statistical graphs will be used to visualise the data. No imputation of missing data will be performed, and data loss will be minimised through consistent documentation. Finally, 95% confidence intervals will be provided for effect estimates.
2.11. Safety and harms
The prehabilitation programme presented in the PreAct trial is based on the idea that patients should increase their physical activity through simple walks and a more active approach to everyday life. Additionally, as mentioned in the eligibility criteria, patients with limited walking ability due to neurological or musculoskeletal disorders, as well as those dependent on a walking aid are excluded from the study. No adverse events related to the prehabilitation programme were observed during the pilot study. During admission before RC, patients will be interviewed whether they have experienced any adverse events, such as falls, during the prehabilitation programme, before being called to the operating theatre. If the patient had already presented at a participating clinic due to such an event or had to reschedule the RC, this information is recorded earlier. In the case of an adverse event, the date, time, and type of event are documented in the CRF. Furthermore, we use the Common Terminology Criteria for Adverse Events (CTCAE) [34] to categorise the event. We are aware, that CTCAE was originally designed for oncological trials; however, as adverse events should be captured, we will implement this to our trial.
2.12. Ethics and dissemination
2.12.1. Research ethics approval
The PreAct trial is being conducted as a bicentric trial at the Department of Urology and Urosurgery at the University Medical Centre Mannheim and the Urology Clinic Munich-Planegg. A positive vote in favour of the ethics application has been received from the Ethics Committee II of the University of Heidelberg under the number 2021-677. The Ethics Committee II of the University of Heidelberg has also voted in favour of the amendment to initiate a second centre in Munich-Planegg. The prerequisite for acceptance of the amendment was the successful acceptance of the ethics application by the Ethics Committee of the Bavarian Medical Association.
2.12.2. Protocol amendments
Any changes to the study protocol will be identified immediately with an update of the protocol version and the date of the current version. In addition, the changes are communicated immediately to both responsible ethics committees in the form of an amendment, which must be approved by them. Following the initiation of a second centre in Munich-Planegg, the study protocol is in version 1.1 dated 03.08.2023.
2.13. Funding
The PreAct trial is funded by the Dr. Rolf M. Schwiete Foundation. This is a nonprofit foundation based in Mannheim, which was established in 2013 and has been recognised as a foundation with legal capacity under civil law since 2014. The Dr. Rolf M. Schwiete Foundation is not involved in the clinical planning, implementation, or evaluation of this trial, or in the drafting of the trial protocol.
3. Summary
RC with bilateral pelvic lymphadenectomy is considered the gold standard in the treatment of MIBC with curative intent [2]. Numerous studies demonstrate the high morbidity and mortality rates, which are due to the invasiveness of the procedure [7], [8], [35], [36]. As the population of cystectomy patients is typically older [37], [38] and will become even older in the future due to an ageing society [39], the preparation of these patients for the upcoming operation is becoming increasingly more important, alongside the improvement of intra- and postoperative standards. For this reason, several studies have taken the approach of preparing this physically impaired patient group for the upcoming operation by applying a preoperative prehabilitation programme. While some trials on the use of a prehabilitation programme prior to RC or other major surgical procedures in urology already exist, data on the use of a fitness tracker–based prehabilitation programme before RC, which can easily be integrated into everyday life and carried out from home, are scarce. The integration of prehabilitation programmes into everyday life and the removal of barriers that prevent urological patients from participating, especially when these programmes are too complex, have already been examined in various studies prior to major urosurgical interventions [40]. However, further well-structured RCTs are needed to investigate and optimise the feasibility, potential benefits on perioperative outcomes, and the safety of these programmes [40].
The ENHANCE trial (NCT0548073) is a multicentre trial with a total of eight participating study centres [41]. With a planned case number of 154 patients, it is one of the largest RCTs to date on prehabilitation prior to cystectomy [42]. It is investigating the superiority of a multimodal prehabilitation programme consisting of physical exercises, a nutritional plan, psychological support, and smoking cessation with regard to the occurrence of perioperative CDC ≥2 complications compared with the standard procedure [41]. The strengths of the study clearly lie in the multicentre design and a multimodal prehabilitation approach of up to 4 wk prior to RC, thoroughly preparing the patients for the upcoming operation. On the contrary, this results in high personnel and resource expenditure [42]. Furthermore, such a programme places high demands on patients and can therefore cause problems in terms of sufficient compliance [42]. Accordingly, there are similar limitations to those in the aforementioned studies [17], [18], [19], [20]. The activity-enhancing effect on the overall population of patients with bladder cancer undergoing RC will be investigated, with a particular focus on the patients' baseline physical condition, as measured by existing comorbidities and their ability to cope actively with everyday life. The validated metrics used are the Charlson Comorbidity Index [43], the ASA physical status classification [26], the Karnofsky Index [25] for recording preoperative performance status in oncological patients, and the SF-36 questionnaire [31] for recording health-related quality of life. Moreover, the 6MWT [28] is carried out at the premedication appointment to assess the patient's basic endurance and functionality status. After the prehabilitation period is completed and at the time of RC, an activity profile emerges for each individual patient. This profile consists of the daily step counts, average number of steps per day, and total step count during the prehabilitation. As suggested by Hunter et al [40], this also provides the opportunity to create a perioperative risk profile for each individual patient based on preoperative physical activity [40].
In addition, the PreAct trial is designed to fit seamlessly into the clinical routines of the participating hospitals and demonstrate the usability of fitness trackers in a clinical setting rather than improving morbidity. Such endpoints will also be analysed, but as secondary outcomes. Should a trend towards improved perioperative parameters emerge, this could serve as the basis for a potential phase 3 trial with the primary endpoint of improving intra- and postoperative parameters. Another factor requiring future improvement is the establishment of effective infrastructures for sending personalised morning step goals, movement reminders, and end-of-day feedback. Currently, this is managed by the study physician and is feasible within the study’s design. For broader implementation, personalised notifications using automated algorithms could be beneficial.
In an effort to improve patient compliance, study approaches such as the PRIMER trial (NCT05790850) already exist, taking advantage of the remote fitness tracker functions to move prehabilitation programmes to home settings [42]. Furthermore, various studies have shown that it is clinically feasible to record postoperative step counts and specify step targets to encourage patients to move, at least in the postoperative period [23], [44]. We will examine the step count during the first 3 PODs to assess whether it may serve as an early predictor of the postoperative course. However, since the sample size calculation was based on the preoperative average daily step count of our patient population, the step count during the first 3 PODs should be considered exploratory. Further RCTs focused on this effect in the postoperative period are needed.
There is also the question of whether such elaborate prehabilitation concepts actually lead to a reduction in postoperative complications [45]. While there is important evidence that these programmes improve postoperative functional capacity [18], [21], the effective reduction of postoperative complications is primarily due to the improvement of surgical procedures [46], [47], [48] and the number of cases in surgical centres [49], [50]. In the EXPELLIARMUS trial (DRKS00016755), for example, no reduction in postoperative complications was achieved, at least for the postoperative use of fitness trackers with specification of a step target [44]. The usefulness of a prehabilitation programme therefore lies in a fundamental cost-benefit analysis with regard to the preoperative effort and the postoperative benefit of the programme [45]. However, it is a fact that patients will have to be more involved in the organisation of their recovery process in the future [14]. For example, in terms of mental health and quality of life, these programmes can have a complementary positive effect where surgical methods showed no improvement [17], [45], [48]. Given its brief duration of 7–10 d, the prehabilitation programme investigated in this study primarily aims to prove the concept of an easy intervention to increase preoperative activity. At this stage, its primary objective is not to validate improvements in intra- and postoperative outcomes.
In the PreAct trial, the step target was adjusted to 8000 steps based on the experience of the previously conducted pilot study. A study by Mayo et al [21] also suggests that preoperative improvement in physical activity, primarily through walking and breathing exercises, can lead to maintenance or improvement in postoperative physical activity. To establish an appropriate and motivating step target for all patients in the intervention group, the target of 8000 steps was set, which is also in line with the recommendations for people older than 65 yr or with chronic diseases [27].
In summary, the prehabilitation programme described in the PreAct trial primarily aims to increase preoperative physical activity through the applied fitness tracker–based intervention. It is a very simple intervention that is easy for patients to integrate into their everyday lives and enables them to potentially contribute to their own recovery in the period immediately prior to surgery. Thus, patients are included in the preoperative preparation without demanding too much of them at the same time. Consequently, prehabilitation should not be seen as a stand-alone element, but as part of a holistic concept comprising preoperative preparation, sophisticated intraoperative surgical standards, and optimal postoperative aftercare.
Author contributions: Karl-Friedrich Kowalewski had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kowalewski, Kriegmair, Kilz, Abate.
Acquisition of data: Kowalewski, Kilz, Antoniewicz, Haney, Wieland, Abate, Egen, Studier-Fischer, Kriegmair, Westhoff, Honeck, Worst, Michel.
Analysis and interpretation of data: Kowalewski, Kilz, Haney, Studier-Fischer, Egen.
Drafting of the manuscript: Kowalewski, Kriegmair, Kilz.
Critical revision of the manuscript for important intellectual content: Kowalewski, Kilz, Antoniewicz, Haney, Wieland, Abate, Egen, Studier-Fischer, Kriegmair, Westhoff, Honeck, Worst, Michel.
Statistical analysis: Kowalewski, Kilz, Haney, Studier-Fischer, Egen.
Obtaining funding: Kowalewski, Kriegmair, Kilz, Abate, Egen.
Administrative, technical, or material support: Michel, Honeck, Kriegmair, Kowalewski, Westhoff, Worst.
Supervision: Michel, Honeck, Kriegmair, Kowalewski.
Other: None.
Financial disclosures: Karl-Friedrich Kowalewski certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: The PreAct trial is funded by the Dr. Rolf M. Schwiete Stiftung (nonprofit, financial funding).
Associate Editor: M. Carmen Mir
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe,AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms, Langversion 2.0. https://www.leitlinienprogramm-onkologie.de/leitlinien/harnblasenkarzinom/.
- 3.Hautmann R.E., Volkmer B.G., Gust K. Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3) World J Urol. 2009;27:347–351. doi: 10.1007/s00345-009-0402-4. [DOI] [PubMed] [Google Scholar]
- 4.Stein J.P., Penson D.F. Invasive T1 bladder cancer: indications and rationale for radical cystectomy. BJU Int. 2008;102:270–275. doi: 10.1111/j.1464-410X.2008.07743.x. [DOI] [PubMed] [Google Scholar]
- 5.Denzinger S., Fritsche H.-M., Otto W., Blana A., Wieland W.-F., Burger M. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol. 2008;53:146–152. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Abdollah F., Sun M., Schmitges J., et al. Stage-specific impact of pelvic lymph node dissection on survival in patients with non-metastatic bladder cancer treated with radical cystectomy. BJU Int. 2012;109:1147–1154. doi: 10.1111/j.1464-410X.2011.10482.x. [DOI] [PubMed] [Google Scholar]
- 7.Hautmann R.E., de Petriconi R.C., Volkmer B.G. Lessons learned from 1,000 neobladders: the 90-day complication rate. J Urol. 2010;184:990–994. doi: 10.1016/j.juro.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 8.Vetterlein M.W., Klemm J., Gild P., et al. Improving estimates of perioperative morbidity after radical cystectomy using the European Association of Urology quality criteria for standardized reporting and introducing the comprehensive complication index. Eur Urol. 2020;77:55–65. doi: 10.1016/j.eururo.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Clavien P.A., Barkun J., De Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 10.Chappidi MR, Kates M, Patel HD, et al. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Elsevier; 2016. p. 256. e1–6. [DOI] [PMC free article] [PubMed]
- 11.Sathianathen N.J., Jarosek S., Lawrentschuk N., Bolton D., Konety B.R. A simplified frailty index to predict outcomes after radical cystectomy. Eur Urol Focus. 2019;5:658–663. doi: 10.1016/j.euf.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Palumbo C., Knipper S., Pecoraro A., et al. Patient frailty predicts worse perioperative outcomes and higher cost after radical cystectomy. Surg Oncol. 2020;32:8–13. doi: 10.1016/j.suronc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Kowalewski K.-F., Wieland V.L., Kriegmair M.C., et al. Robotic-assisted versus laparoscopic versus open radical cystectomy—a systematic review and network meta-analysis of randomized controlled trials. Eur Urol Focus. 2023;9:480–490. doi: 10.1016/j.euf.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Glance L.G., Osler T.M., Neuman M.D. Redesigning surgical decision making for high-risk patients. N Engl J Med. 2014;370:1379. doi: 10.1056/NEJMp1315538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyson M.D., Chang S.S. Enhanced recovery pathways versus standard care after cystectomy: a meta-analysis of the effect on perioperative outcomes. Eur Urol. 2016;70:995–1003. doi: 10.1016/j.eururo.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahota R.G., Salin A., Gautier J.R., et al. Prehabilitation program before robotic radical prostatectomy improves perioperative outcomes and continence recovery. BJU Int. 2022;130:357–363. doi: 10.1111/bju.15666. [DOI] [PubMed] [Google Scholar]
- 17.Santa Mina D., Hilton W.J., Matthew A.G., et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol. 2018;27:289–298. doi: 10.1016/j.suronc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Minnella E.M., Awasthi R., Bousquet-Dion G., et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus. 2021;7:132–138. doi: 10.1016/j.euf.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Briggs L.G., Reitblat C., Bain P.A., et al. Prehabilitation exercise before urologic cancer surgery: a systematic and interdisciplinary review. Eur Urol. 2022;81:157–167. doi: 10.1016/j.eururo.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Jensen B.T., Petersen A.K., Jensen J.B., Laustsen S., Borre M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol. 2015;49:133–141. doi: 10.3109/21681805.2014.967810. [DOI] [PubMed] [Google Scholar]
- 21.Mayo N.E., Feldman L., Scott S., et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150:505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 22.Daskivich T.J., Houman J., Lopez M., et al. Association of wearable activity monitors with assessment of daily ambulation and length of stay among patients undergoing major surgery. JAMA Network Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Walt N., Salmon L.J., Gooden B., et al. Feedback from activity trackers improves daily step count after knee and hip arthroplasty: a randomized controlled trial. J Arthroplasty. 2018;33:3422–3428. doi: 10.1016/j.arth.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Symer M.M., Abelson J.S., Milsom J., McClure B., Yeo H.L. A mobile health application to track patients after gastrointestinal surgery: results from a pilot study. J Gastrointest Surg. 2017;21:1500–1505. doi: 10.1007/s11605-017-3482-2. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson T., Boyd N., Feinstein A. Scientific problems in clinical scales, as demonstrated in the Karnofsky index of performance status. J Chronic Dis. 1979;32:661–666. doi: 10.1016/0021-9681(79)90096-1. [DOI] [PubMed] [Google Scholar]
- 26.American Society of Anesthesiologists. ASA physical status classification system. 21.11.2021. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- 27.Tudor-Locke C., Bassett D.R. How many steps/day are enough? Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed]
- 29.Slankamenac K., Graf R., Barkun J., Puhan M.A., Clavien P.-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 30.Kowalewski K.-F., Müller D., Mühlbauer J., et al. The comprehensive complication index (CCI): proposal of a new reporting standard for complications in major urological surgery. World J Urol. 2021;39:1631–1639. doi: 10.1007/s00345-020-03356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware J.E., Jr, Sherbourne C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 32.Catto J.W., Khetrapal P., Ricciardi F., et al. Effect of robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA. 2022;327:2092–2103. doi: 10.1001/jama.2022.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan A.-W., Tetzlaff J.M., Altman D.G., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Cancer Institute Division of Cancer Treatment & Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- 35.Shabsigh A., Korets R., Vora K.C., et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–176. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Stimson C., Chang S.S., Barocas D.A., et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010;184:1296–1300. doi: 10.1016/j.juro.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Ronckers C, Spix C, Trübenbach C, et al. Krebs in Deutschland für 2019/2020. https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_2023.pdf?__blob=publicationFile.
- 38.Galsky M.D. How I treat bladder cancer in elderly patients. J Geriatr Oncol. 2015;6:1–7. doi: 10.1016/j.jgo.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Deutscher Ärzteverlag GmbH. Demographischer Wandel fordert Urologen. https://www.aerzteblatt.de/nachrichten/79455/Demografischer-Wandel-fordert-Urologen.
- 40.Hunter H., Bennington-McKay N., Sher J., Psutka S.P., Lin C. Emerging role of mobile applications and wearable devices for prehabilitation in urologic oncology. Eur Urol Focus. 2024;10:20–22. doi: 10.1016/j.euf.2023.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Akdemir E., Sweegers M.G., Vrieling A., et al. Protocol: EffectiveNess of a multimodal preHAbilitation program in patieNts with bladder canCEr undergoing radical cystectomy: protocol of the ENHANCE multicentre randomised controlled trial. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-071304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rion C., Branger N., Maubon T., Rybikowski S., Pignot G., Walz J. Prehabilitation in urology: trial update. Eur Urol Focus. 2024;10:8–10. doi: 10.1016/j.euf.2023.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 44.Mihaljevic AL, CHIR-Net SIGMA Study Group. Postoperative complications and mobilization following major abdominal surgery with vs. without fitness tracker-based feedback (EXPELLIARMUS): a student-led multicenter randomized controlled clinical trial of the CHIR-Net: SIGMA Study Group. Ann Surg 2024;280:202–11. [DOI] [PMC free article] [PubMed]
- 45.Giese M., Butea-Bocu M., Huber J., Groeben C. Prehabilitation prior to radical cystectomy. Urologie. 2023;62:1034–1040. doi: 10.1007/s00120-023-02172-8. [DOI] [PubMed] [Google Scholar]
- 46.Kowalewski K.-F., Neuberger M., Abate M.A.S., et al. Randomized controlled feasibility trial of robot-assisted versus conventional open partial nephrectomy: the ROBOCOP II study. Eur Urol Oncol. 2024;7:91–97. doi: 10.1016/j.euo.2023.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Parekh D.J., Reis I.M., Castle E.P., et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391:2525–2536. doi: 10.1016/S0140-6736(18)30996-6. [DOI] [PubMed] [Google Scholar]
- 48.Yaxley J.W., Coughlin G.D., Chambers S.K., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 49.Cacciamani G.E., Barzi A., Eppler M.B., et al. The impact of facility surgical caseload volumes on survival outcomes in patients undergoing radical cystectomy. Cancers. 2022;14:5984. doi: 10.3390/cancers14235984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groeben C., Koch R., Baunacke M., Borkowetz A., Wirth M.P., Huber J. In-hospital outcomes after radical cystectomy for bladder cancer: comparing national trends in the United States and Germany from 2006 to 2014. Urol Int. 2019;102:284–292. doi: 10.1159/000496347. [DOI] [PubMed] [Google Scholar]