Abstract
Background
Risk factors for bloodstream infection in patients with COVID-19 in the intensive care unit (ICU) remain unclear. The purpose of this systematic review was to study the risk factors for BSI in patients admitted to ICUs for COVID-19.
Methods
A systematic search was performed on PubMed, EMBASE, Cochrane Library, and Web of Science up to July 2024. Data were reported as combined odds ratio (OR) for categorical variables and weighted mean difference (WMD) for continuous variables.
Results
6914 studies were retrieved, of which 55 were included in the meta-analysis. Men (OR = 1.28, 95% CI: 1.10–1.50, P = 0.006), high SAPS II score (WMD = 6.43, 95% CI: 0.23–12.63, P = 0.042), diabetes (OR = 1.34, 95% CI: 1.04–1.73, P = 0.022), tracheal intubation (OR = 8.68, 95% CI: 4.68–16.08, P < 0.001), mechanical ventilation (OR = 22.00, 95% CI: 3.77-128.328, P < 0.001), ECMO (OR = 2.70, 95% CI: 1.17–6.26, P = 0.020), central venous cannulation (OR = 9.33, 95% CI: 3.06–28.43, P < 0.001), prolonged ICU stay (WMD = 10.37, 95% CI: 9.29–11.44, P < 0.001), methylprednisolone use (OR = 2.24, 95% CI: 1.24–4.04, P = 0.008), and the combination of methylprednisolone and Tocilizumab (OR = 4.54, 95% CI: 1.09–18.88, P = 0.037) were risk factors for ICU-BSI in COVID-19 patients.
Conclusion
We identified 10 risk factors for ICU-BSI in COVID-19 patients. In future studies, these factors can be combined to establish a more comprehensive and accurate prediction model for ICU-BSI in COVID-19 patients. Targeted measures can be taken earlier to control BSI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10420-1.
Keywords: Bloodstream infection, COVID-19, ICU, Meta-analysis
Background
COVID-19 has had a severe impact on global public health systems and remains one of the most severe epidemics now over the world. As of January 2023, WHO reported about 750 million confirmed cases of COVID-19 globally, covering about 6.8 million deaths [1]. The virus is constantly mutating, and the number of infected people continues to grow rapidly. According to statistics from outpatient and emergency clinics in various hospitals, about 40–50% of the patients are prone to serious illnesses and thus require hospital admission. Of these, 10–20% of patients are prone to critical illnesses and need to be admitted to intensive care units (ICUs) for supportive treatment. Consequently, the number of COVID-19 patients admitted to ICUs has markedly increased, and in some places, temporary ICUs have even been built in large numbers. Previous studies have shown that bloodstream infection (BSI) may occur in approximately 7% of COVID-19 inpatients [2]. The incidence of BSI in COVID-19 patients in ICUs is 10–50%, which is a significant increase and a new record and is four times higher than that in non-COVID-19 patients [3]. BSI can lead to bacterial or viral infections in various organs. In poorly treated or severe cases, it may lead to sepsis, resulting in fever and generalized pain, which gravely impacts the health. In severe cases, it can lead to the risk of death. ICU-BSI increases the risk of 30-day mortality by 40% [4]. In addition, resuscitation therapy such as mechanical ventilation and endotracheal intubation in ICU increases the risk of BSI and poses a great challenge to anti-infective treatment [5–7]. COVID-19 infection may become a small-scale recurrent epidemic pattern in the future. Thereby, it is necessary to stratify patients at risk of BSI and take timely measures to reduce the occurrence of BSI.
Recent studies found that gender, SAPS II score, underlying medical complications [e.g., diabetes mellitus (DM), hypertension], treatment-related factors (e.g., mechanical ventilation, intubation, ECMO), and drug-related factors (e.g., Tocilizumab, Methylprednisolone) might be associated with an increased risk of BSI in critically ill patients with COVID-19 in ICUs. However, the conclusions of various studies about the risk factors for BSI are inconsistent. Regarding gender, most studies found that men had a higher risk of developing BSI, which reached 60–70%, and men accounted for most COVID-19 admissions to ICUs [8–13]. Some studies concluded that gender was not associated with the risk of infection [14–18]. Interestingly, 2 studies found the risk of BSI was equal for males and females [19, 20]. DM, as the most common underlying disease in human beings, is statistically associated with a higher risk of BSI in most studies [8, 10, 13, 15–18, 20, 21]. However, other studies concluded that DM did not lead to a higher risk of BSI [9, 11, 12]. Additionally, the conclusions regarding hypertension were not clear and consistent. In most studies, statistical analyses suggested that hypertension caused a higher risk of BSI [10–13, 16, 18, 19]; whereas some studies indicated no direct correlation between hypertension and the risk of BSI [8, 17, 20]. Therefore, in this study, we aimed to determine the risk factors for BSI in COVID-19 patients in ICUs through a systematic review and meta-analysis.
Methods
This paper was designed and revised following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and was registered in the PROSPERO (CRD42023416813).
Search strategy
PubMed, Embase, Web of Science, and Cochrane Library were comprehensively searched for English papers up to July 2024, while references in related literature were manually checked. The following keywords were utilized to screen published clinical information on risk factors for BSI in COVID-19 patients in ICUs: “intensive care unit”, “covid-19”, “sepsis”, and “bloodstream infection”. All literature retrieved was imported into EndnoteX9 for paper screening. The search process was undertaken by two reviewers and any disagreements were addressed through discussion. (Table S1)
Eligibility criteria
Inclusion criteria covered: (1) study type: observational study; (2) patients: with COVID-19 in ICUs; (3) study content: risk factors for BSI in COVID-19 patients in ICUs. Exclusion criteria encompassed: (1) letters, reviews, conference proceedings, commentaries, and papers with unavailable full text and unsuitable types of publication; (2) with no available primary data; (3) published not in English.
Data extraction
Based on the eligibility criteria, articles imported into EndnoteX9 were initially screened by reviewing the titles and abstracts. Ineligible articles were excluded, and the remaining articles were read through the full text to screen the eligible ones for meta-analysis. Relevant research data were extracted. All procedures were performed by two researchers (Ting Jiang and Jun Wang). The following data were extracted: (1) general information: authors, publication date, study area, study design, and period; (2) study characteristics: sample size, mean age, and gender distribution; (3) risk factors: SAPS II, hypertension, DM, chronic pulmonary disease, liver disease, immunosuppression, chronic kidney disease, heart disease, and tumors; and (4) treatment records: medication administration, treatment modalities, and duration of treatment. In studies where some information was lacking, we attempted to contact the authors by phone or email. In case of disagreement in literature screening and data extraction, a third researcher (Wei Wang) was consulted.
Quality assessment
Two researchers independently assessed the study quality based on the Newcastle-Ottawa Scale (NOS) for cohort studies and case-control studies. The NOS scale covered three dimensions and eight items, with a maximum score of 9 points. A score < 4 was defined as low quality, 4–6 as moderate quality, and ≥ 7 as high quality. The higher score implied a lower risk of bias. The quality of cross-sectional studies was evaluated using a scale recommended by the Agency for Healthcare Research and Quality. The scale consisted of 11 items. Answers included yes, no, and unclear. For the answer of “yes”, the item was assigned a score of 1. The higher the score, the higher the quality: low quality = 0–3; medium quality = 4–7; high quality = 8–11. If two researchers disagreed with quality assessment, a third researcher arbitrated.
Statistical analyses
Statistical analyses were implemented using Stata 15.0 software. Categorical variables were analyzed using odds ratio (OR) while continuous variables using weighted mean difference (WMD). Data from the original studies were transformed before meta-analysis if they were not described as mean and standard deviation. All effect sizes were represented as 95% confidence intervals (CI). Heterogeneity was analyzed using I2. If there was no significant statistical heterogeneity between outcomes (P ≥ 0.1, I2 ≤ 50%), a meta-analysis was performed using a fixed-effects model, otherwise, using a random-effects model. For highly heterogeneous results, sensitivity analyses were performed on the results to validate the stability and reliability of the results. Sensitivity analyses were conducted on all outcomes. By eliminating the articles one by one, the stability of the remaining results was observed. For risk factors that included ≥ 10 articles, the Egger test was adopted to determine whether there was publication bias.
Results
Screening results
The database searches retrieved 9099 relevant English articles. 6914 articles were obtained after duplicates were removed. Subsequently, the titles and abstracts were read to exclude the ineligible studies, leaving 301 studies. Finally, after reading the full text, 55 studies were enrolled in the meta-analysis [3, 5–58]. The screening process is represented in a PRISMA flowchart. (Fig. 1).
Fig. 1.
Flowchart of the search strategy
Characteristics
The 55 included studies comprised 48 cross-sectional studies [5–9, 11, 12, 14, 15, 17, 18, 20–23, 26–58] (Table 1), 6 cohort studies [3, 10, 13, 16, 24, 25], and 1 case-control study [19] (Table 2), including 25,939 patients ranging in age from 18 to 94 years across Italy, CHN, UK, US, Spain, France, Greece, Austria, Singapore, India, Germany, Turkey, Switzerland, Sweden, and Portugal. The meta-analysis results of the research indicators are shown in Table 3. Among them, 48 cross-sectional studies had average AHRQ scale scores > 7, and 6 cohort studies and 1 case-control study had average NOS scores > 7, implying that these articles were all of high quality.
Table 1.
Studies characteristics and quality. (cross-sectional study)
| Study | Published time | Country | Study design | Patients | Age | Gender(male) | AHRQ grade |
|---|---|---|---|---|---|---|---|
| Amit, M. et al. [22] | 2020 | Israel | Cross-sectional study | 156 | 72(60–82) | 108/48 | 9 |
| Bonazzetti, C et al. [14] | 2020 | Italy | Cross-sectional study | 89 | 61.5(53.1–68.7) | 69/20 | 8 |
| Fu, Guoping et al. [23] | 2020 | CHN | Cross-sectional study | 51 | 60.94 ± 14.87(25–87) | 27/14 | 8 |
| Giacobbe, D. R et al. [8] | 2020 | Italy | Cross-sectional study | 78 | 66 IQR 57–70 | 60/18 | 9 |
| Anwar, Asad et al. [26] | 2021 | UK | Cross-sectional study | 44 | 17–77 M59.5(IQR 50.5–64.5)/21–80 M59(IQR 49–67.5) | 34/10 | 8 |
| Bardi, Tommaso et al. [27] | 2021 | Spain | Cross-sectional study | 140 | 61(57–67) | 108/32 | 8 |
| d’Humières, C et al. [15] | 2021 | FRANCE | Cross-sectional study | 197 | 59(50–68) | 148/49 | 8 |
| Dupuis, Claire et al. [28] | 2021 | FRANCE | Cross-sectional study | 303 | 61(53–70) | 239/64 | 9 |
| Grasselli, G. et al. [29] | 2021 | Italy | Cross-sectional study | 774 | 62 (54–68) | 597/177 | 9 |
| Karruli, A. et al. [9] | 2021 | Italy | Cross-sectional study | 32 | 68 [55.25–75] | 23/9 | 9 |
| Kokkoris, S. et al. [30] | 2021 | Greece | Cross-sectional study | 50 | Median age 64 | 36/14 | 8 |
| Llitjos, Jean-Francois et al. [5] | 2021 | FRANCE | Cross-sectional study | 176 | 63 (55–73) | 134/42 | 8 |
| Ong, C. C. H. et al. [31] | 2021 | Singapore | Cross-sectional study | 71 | 52(39–66) | 59/12 | 9 |
| Ramos, Rafael et al. [32] | 2021 | Spain | Cross-sectional study | 213 | 61(52–71) | 110/103 | 8 |
| Roedl, Kevin et al. [33] | 2021 | Germany | Cross-sectional study | 223 | 69 (58–77.5) | 163/60 | 8 |
| Rollas, Kazim et al. [17] | 2021 | Turkey | Cross-sectional study | 38 | NR | NR | 9 |
| Søgaard, K. K et al. [34] | 2021 | Switzerland | Cross-sectional study | 41 | 64.8(54.7–72.1) | 31/10 | 9 |
| Suarez-de-la-Rica, A. et al. [35] | 2021 | Spain | Cross-sectional study | 107 | 62.2 ± 10.6 | 76/31 | 8 |
| Yakar, Mehmet Nuri et al. [36] | 2021 | Turkey | Cross-sectional study | 249 | 71(61–80) | 172/77 | 9 |
| Yao, Ren-qi et al. [6] | 2021 | CHN | Cross-sectional study | 35 | 64(59–67) | 25/10 | 8 |
| Zamora-Cintas, M. I. et al. [37] | 2021 | Spain | Cross-sectional study | 54 | NR | NR | 8 |
| Zhang, J. et al. [18] | 2021 | CHN | Cross-sectional study | 32 | 63.34 ± 12.48 | 20/12 | 9 |
| Ahlstrom, Bjorn et al. [38] | 2022 | Swedish | Cross-sectional study | 7382 | 63 (53–72) | 5191/2191 | 9 |
| Brücker, W. et al. [39] | 2022 | Germany | Cross-sectional study | 61 | 66.4 ± 13.3 | 34/27 | 9 |
| Caiazzo, L.et al. [40] | 2022 | Italy | Cross-sectional study | 89 | 68.1 ± 9.3 | 66/23 | 8 |
| Cidade, Jose Pedro et al. [41] | 2022 | Portugal | Cross-sectional study | 118 | 63.3 ± 13.1 | 87/31 | 8 |
| Ćurčić, M. et al. [42] | 2022 | Croatia | Cross-sectional study | 692 | NR | NR | 8 |
| da Costa, R. L. et al. [43] | 2022 | Brazil | Cross-sectional study | 191 | 69.66 ± 16.13 | 116/75 | 8 |
| De Bruyn, A. et al. [44] | 2022 | Belgium. | Cross-sectional study | 94 | 69.65 ± 11.29 | 55/39 | 9 |
| DeVoe, C. et al. [45] | 2022 | US | Cross-sectional study | 126 | 58.1 ± 17.9 | 85/41 | 8 |
| Erbay, Kubra et al. [11] | 2022 | Turkey | Cross-sectional study | 85 | 67.23 ± 13.05 | 54/31 | 8 |
| Kozlowski, Bartosz et al. [46] | 2022 | Poland | Cross-sectional study | 172 | 67.76 ± 11.16 | 112/60 | 8 |
| Kurt, Ahmet Furkan et al. [12] | 2022 | Turkey | Cross-sectional study | 470 | 66 ± 14.87 | 301/169 | 9 |
| Lepape, Alain et al. [47] | 2022 | FRANCE | Cross-sectional study | 4465 | 63.30 ± 11.68 | 3132/1333 | 8 |
| Mantzarlis, K. et al. [20] | 2022 | Greece | Cross-sectional study | 84 | 68.85 ± 12.17 | 56/28 | 9 |
| Mustafa, Z. U. et al. [48] | 2022 | Pakistan | Cross-sectional study | 636 | NR | 398/238 | 7 |
| Pandey, M. et al. [49] | 2022 | UK | Cross-sectional study | 299 | NR | 101/198 | 9 |
| Roda, Silvia et al. [50] | 2022 | Italy | Cross-sectional study | 22 | 61.36 ± 10.30 | 20/2 | 8 |
| Routsi, C. et al. [51] | 2022 | Greece | Cross-sectional study | 600 | NR | NR | 8 |
| Russo, A. et al. [52] | 2022 | Italy | Cross-sectional study | 32 | 62.50 ± 10.99 | 21/11 | 8 |
| Seitz, T. et al. [53] | 2022 | Austria | Cross-sectional study | 117 | 57.2 ± 11.9 | 72/45 | 8 |
| Torrecillas, Miriam et al. [7] | 2022 | Spain | Cross-sectional study | 220 | 63.65 ± 12.69 | 169/51 | 8 |
| Alenazi, T. A. et al. [54] | 2023 | Saudi Arabia | Cross-sectional study | 118 | 60.97 ± 16.32 | 74/43 | 8 |
| Alessandri, F. et al. [55] | 2023 | Italy | Cross-sectional study | 138 | 62.20 ± 15.36 | 97/41 | 9 |
| Bedenić, B. et al. [56] | 2023 | Croatia | Cross-sectional study | 118 | 71 years (range 25–94) | 78/40 | 8 |
| Bonazzetti, C. t al. [21] | 2023 | Italy | Cross-sectional study | 537 | 64.65 ± 11.15 | 402/135 | 9 |
| Guanche Garcell, H. et al. [57] | 2023 | Cuban | Cross-sectional study | 130 | NR | NR | 8 |
| Taysi, M. R. et al. [58] | 2023 | Turkey | Cross-sectional study | 205 | 68.4 ± 13.1 | 119/86 | 8 |
AHRQ Agency for Healthcare Research and Quality, NR Not reported
Table 2.
Studies characteristics and quality. (Cohort study and case-control study)
| Study | Published time | Country | Study design | Patients | Age | Gender (male) | NOS grade |
|---|---|---|---|---|---|---|---|
| Cataldo, M. A et al. [3] | 2020 | Italy | Cohort study | 57 | 62 ± 13 | 41/16 | 7 |
| Garcia, Pedro David Wendel et al. [24] | 2020 | European | Cohort study | 639 | 63 (53–71) | 480/159 | 8 |
| Zhang, H. et al. [25] | 2020 | CHN | Cohort study | 38 | 64.76 ± 13.76 | 32/6 | 7 |
| Massart, N. et al. [10] | 2021 | France, Switzerland, Belgium | Cohort study | 4010 | NR | NR | 8 |
| Palanisamy, N. et al. [16] | 2021 | India | Cohort study | 750 | 60 ± 17.71 | 562/188 | 8 |
| Bartoszewicz, M. et al. [13] | 2023 | Poland | Cohort study | 201 | 66.1 ± 12.1 | 114/87 | 8 |
| Dupper, A. C. et al. [19] | 2022 | US | Case-control study | 96 | 64.91 ± 9.51 | 57/39 | 8 |
NOS Newcastle-Ottawa Scale, NR Not reported
Table 3.
Outcomes of meta-analysis
| Risk factors | No. of studies | Heterogeneity Analysis | Statistical model | statistical method | Effect estimate | P | |
|---|---|---|---|---|---|---|---|
| I² | P | (95%CI) | |||||
| Hypertension | 10 | 70.4% | 0.000 | Random-effects | OR | 1.30(0.92,1.83) | 0.131 |
| Chronic pulmonary disease | 11 | 23.4% | 0.221 | Fixed-effects | OR | 1.07(0.90,1.29) | 0.443 |
| Diabetes | 12 | 50.2% | 0.024 | Random-effects | OR | 1.34(1.04,1.73) | 0.022* |
| Gender | 14 | 0.0% | 0.059 | Fixed-effects | OR | 1.28(1.10,1.50) | 0.006* |
| Liver disease | 6 | 2.3% | 0.402 | Fixed-effects | OR | 0.86(0.47,1.58) | 0.635 |
| Immunosuppressive diseases | 5 | 29.9% | 0.222 | Fixed-effects | OR | 1.11(0.88,1.40) | 0.375 |
| Chronic kidney disease | 6 | 0.0% | 0.751 | Fixed-effects | OR | 1.20(0.78,1.84) | 0.411 |
| Heart disease | 10 | 0.0% | 0.550 | Fixed-effects | OR | 1.00(0.85,1.17) | 0.957 |
| Tocilizumab | 9 | 34.3% | 0.144 | Fixed-effects | OR | 1.04(0.74,1.46) | 0.815 |
| Tumors | 9 | 10.2% | 0.350 | Fixed-effects | OR | 1.04(0.78,1.37) | 0.807 |
| ECMO | 4 | 74.1% | 0.009 | Random-effects | OR | 2.70(1.17,6.26) | 0.020* |
| Tracheal intubation | 4 | 67.8% | 0.025 | Random-effects | OR | 8.68(4.68,16.08) | < 0.001* |
| Mechanical ventilation | 2 | 0.0% | 0.385 | Fixed-effects | OR | 22.00(3.77,128.328) | 0.001* |
| Methylprednisolone | 2 | 13.5% | 0.282 | Fixed-effects | OR | 2.24(1.24,4.04) | 0.008* |
| Methylprednisolone + Tocilizumab | 2 | 71.0% | 0.063 | Random-effects | OR | 4.54(1.09,18.88) | 0.037* |
| Steroids | 3 | 87.6% | 0.000 | Random-effects | OR | 1.17(0.15,9.23) | 0.882 |
| Remdesivir | 2 | 54.4% | 0.139 | Random-effects | OR | 0.80(0.14,4.41) | 0.794 |
| Dexamethasone | 2 | 10.2% | 0.291 | Fixed-effects | OR | 1.64(0.85,3.15) | 0.139 |
| Renal replacement therapy | 2 | 97.9% | 0.000 | Random-effects | OR | 0.86(0.11,6.57) | 0.882 |
| Central venous catheterization | 2 | 0.0% | 0.559 | Fixed-effects | OR | 9.33(3.06,28.43) | < 0.001* |
| Length of stay in ICUs | 8 | 0.0% | 0.712 | Fixed-effects | WMD | 10.37(9.29,11.44) | < 0.001* |
| SAPS II score | 2 | 58.3% | 0.122 | Random-effects | WMD | 6.43(0.23,12.63) | 0.042* |
WMD Weight mean difference, OR Odds ratio, CI Confidence interval
*P < 0.05
Result synthesis
Patient-related factors
Gender
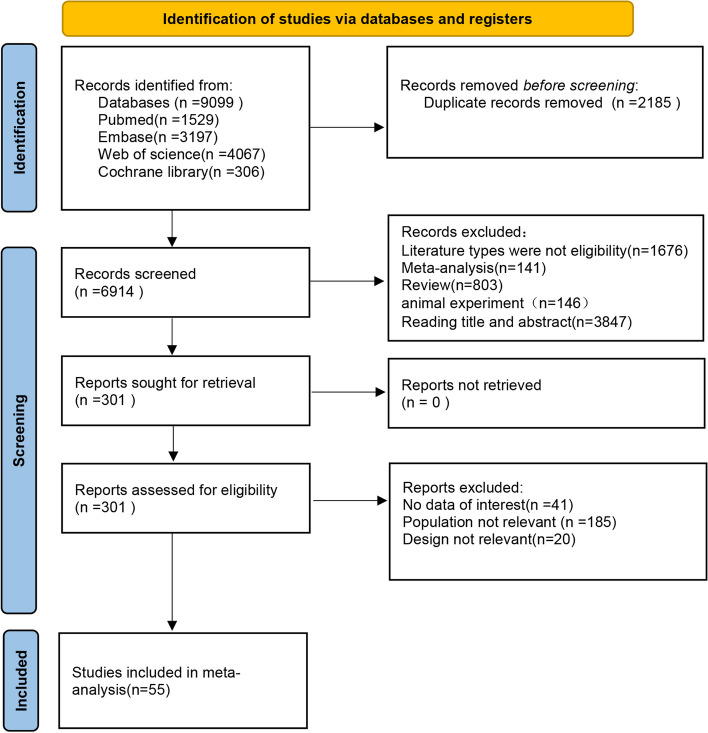
Fourteen studies [8–21] explored the correlation between gender and BSI in COVID-19 patients in ICUs. A pooled analysis showed that male COVID-19 patients in ICUs were 28% more likely to develop BSI (OR = 1.28, 95% CI: 1.10–1.50, P = 0.006, I2 = 0.0%). (Fig. 2)
Fig. 2.
Forest plot of univariate data associating BSI risk with (A) gender; (B) SAPS II score; (C) diabetes; (D) hypertension and (E) chronic pulmonary disease for patients with COVID-19 in ICU
SAPS II score
Two studies [10, 15] analyzed the correlation between SAPS II scores and ICU-BSI in COVID-19 patients. Meta-analysis showed that higher SAPS II scores were positively correlated with an increased incidence of BSI in COVID-19 patients in ICUs (WMD = 6.43, 95% CI: 0.23–12.63, P = 0.042, I2 = 58.3%). (Fig. 2)
DM
Twelve studies [8–13, 15–18, 20, 21] investigated the correlation between DM and BSI in COVID-19 patients in ICUs. Most of these articles did not indicate whether DM was a risk factor for BSI. Our pooled analysis unraveled that DM increased the occurrence of BSI in COVID-19 patients in ICUs by 34% (OR = 1.34, 95% CI: 1.04–1.73, P = 0.022, I2 = 50.2%). (Fig. 2)
Hypertension
There were conflicting results about the association between hypertension and BSI in COVID-19 patients in ICUs. Ten studies [8, 10–13, 16–20] were involved with mixed results. Meta-analysis demonstrated no correlation between hypertension and BSI in COVID-19 patients in ICUs (OR = 1.30, 95% CI:0.92–1.83, P = 0.131, I2 = 70.4%). (Fig. 2)
Chronic pulmonary disease
Because COVID-19 mainly attacked the respiratory system, we extensively investigated the correlation between chronic pulmonary disease and BSI in COVID-19 patients in ICUs through 11 studies [8–12, 15–17, 19–21]. Meta-analysis showed no correlation between chronic pulmonary disease and BSI in COVID-19 patients in ICUs (OR = 1.07, 95% CI: 0.90–1.29, P = 0.443, I2 = 23.4%). (Fig. 2)
Liver disease
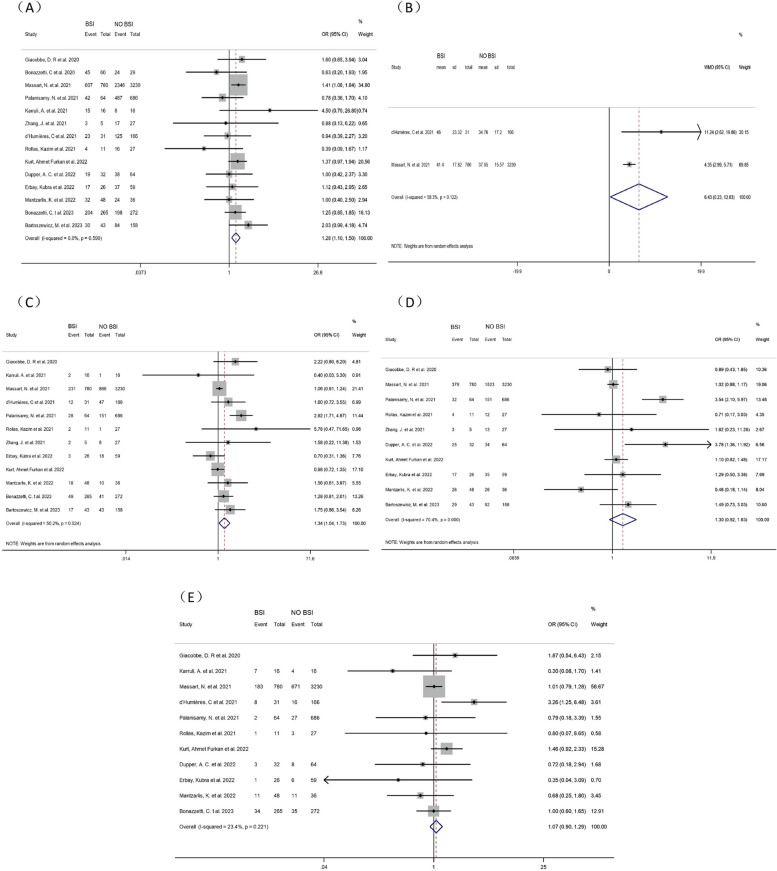
Six studies [8, 9, 12, 15, 16, 21] investigated the correlation between liver disease and ICU-BSI in COVID-19 patients. Meta-analysis showed no correlation between liver disease and BSI in COVID-19 patients in ICUs (OR = 0.86, 95% CI: 0.47–1.58, P = 0.635, I2 = 2.25%). (Fig. 3)
Fig. 3.
Forest plot of univariate data associating BSI risk with (A) liver disease; (B) chronic kidney disease; (C) heart disease; (D) immunosuppressive disease and (E) tumors for patients with COVID-19 in ICU
Chronic kidney disease
Seven studies [9–11, 15, 19–21] investigating the association between chronic kidney disease and BSI in COVID-19 patients in ICUs were included. One article was excluded by sensitivity analysis [10] and therefore six articles were included in the meta-analysis. It showed no correlation between chronic kidney disease and BSI in COVID-19 patients in ICUs (OR = 1.20, 95% CI: 0.78–1.84, P = 0.411, I2 = 0.0%). (Fig. 3)
Heart disease
Ten studies [9–11, 13, 15, 16, 19–21] investigating the correlation between heart disease and BSI among COVID-19 patients in ICUs were included. Meta-analysis showed no correlation between heart disease and BSI in COVID-19 patients in ICUs (OR = 1.00, 95% CI: 0.85–1.17P = 0.957, I2 = 0.0%). (Fig. 3)
Immunosuppressive diseases
All five studies [9, 15, 16, 19, 21] showed no correlation between immunosuppression and ICU-BSI in COVID-19 patients. Meta-analysis also showed no correlation between immunosuppression and BSI in COVID-19 patients in ICUs (OR = 1.11, 95% CI: 0.88–1.40, P = 0.375, I2 = 29.9%). (Fig. 3)
Tumors
Nine studies [8–11, 17, 19–21] investigating the correlation between tumors and BSI in COVID-19 patients in ICUs were included. Meta-analysis showed no correlation between tumors and BSI in COVID-19 patients in ICUs (OR = 1.04, 95% CI: 0.78–1.37, P = 0.807, I2 = 10.2%). (Fig. 3)
Treatment-related factors
Tracheal intubation
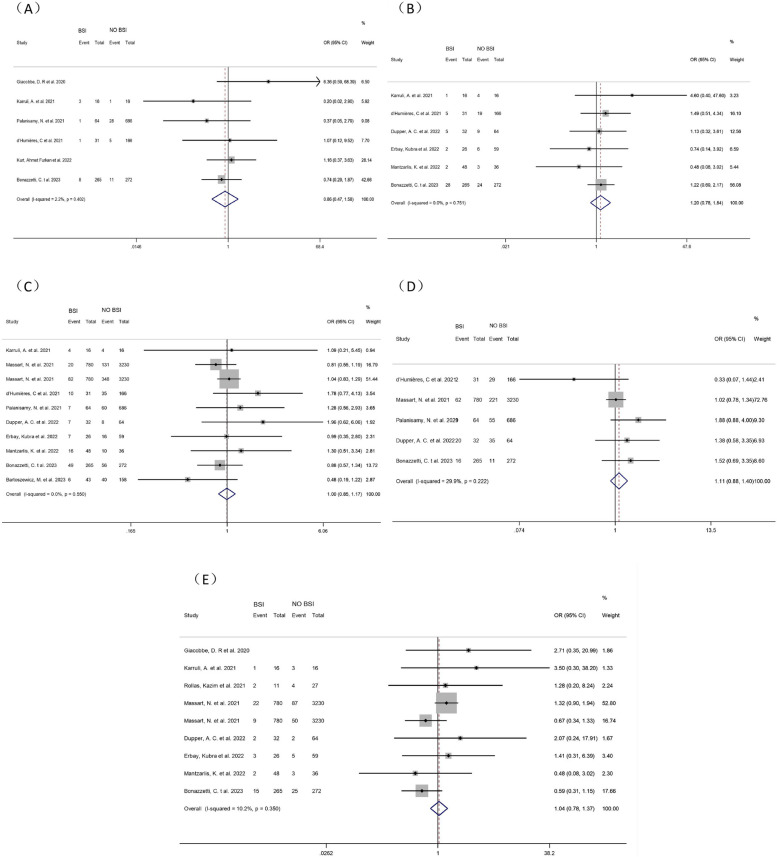
Four studies [10, 15, 16, 21] were included to investigate the association between tracheal intubation and ICU-BSI in COVID-19 patients. Meta-analysis revealed that tracheal intubation increased the risk of BSI in COVID-19 patients in ICUs by nearly 9-fold (OR = 8.68, 95% CI: 4.68–16.08, P < 0.001, I2 = 67.8%). (Fig. 4)
Fig. 4.
Forest plot of univariate data associating BSI risk with (A) tracheal intubation; (B) mechanical ventilation; (C) ECMO; (D) CVC; (E) RRT and (F) Length of stay in ICU for patients with COVID-19 in ICU
Mechanical ventilation
The correlation between mechanical ventilation and BSI in COVID-19 patients in ICUs was investigated by three studies [14, 16, 17]. Since no heterogeneity was found (P = 0.147, I2 = 47.9%), a fixed-effects model was adopted and unraveled marked differences (OR = 4.98, 95% CI: 2.73–9.08, P < 0.001). After sensitivity analysis, the heterogeneity was greatly reduced (P = 0.385, I2 = 0.0%) when the study of Palanisamy, N et al. [16] was excluded. The main source of heterogeneity might be the large sample size of their study, which tended to lead to unstable results compared to other studies with small sample sizes. Thus, this study was excluded because it led to a significant bias. The pooled analysis after exclusion using a fixed-effect model (OR = 22.00, 95% CI: 3.77–128.328, p < 0.001) showed statistically significant differences. The meta-analysis showcased that mechanical ventilation increased the risk of BSI by 22 times in COVID-19 patients in ICUs. The excluded study by Palanisamy, N et al. also showed that mechanical ventilation could increase the risk of BSI by 4-fold, in agreement with our results. (Fig. 4)
ECMO
Many critically ill patients have used ECMO for supportive care. Including four studies [10, 12, 18, 55], we explored the correlation between ECMO and BSI among COVID-19 patients in ICUs. Meta-analysis manifested that ECMO increased the risk of BSI in COVID-19 patients in ICUs by nearly three times (OR = 2.70, 95% CI: 1.17–6.26, P = 0.020, I2 = 74.1%). (Fig. 4)
Central venous catheterization (CVC)
Two studies [11, 18] investigated the correlation between CVC and ICU-BSI in COVID-19 patients. Meta-analysis showed that CVC increased the Catheter-related BSI (OR = 9.33, 95% CI: 3.06–28.43, P < 0.001, I2 = 0.0%). (Fig. 4)
Renal replacement therapy (RRT)
Two studies [10, 12] investigating the correlation between RRT and BSI in COVID-19 patients in the ICU were included. Meta-analysis showed no correlation between BSI and RRT in COVID-19 patients in ICUs (OR = 0.86, 95% CI: 0.11–6.57, P = 0.882, I2 = 97.9%). (Fig. 4)
Length of stay in ICUs
Eight studies were included [12–14, 17, 18, 20, 21, 53], all of which showed a strong correlation between the length of stay in ICUs and the occurrence of BSI in COVID-19 patients in ICUs. A meta-analysis showed that the longer the ICU stay, the higher the risk of BSI in COVID-19 patients in the ICU (WMD = 10.37, 95% CI:9.29–11.44, P < 0.001, I2 = 0.0%). (Fig. 4)
Medication-related factors
Tocilizumab
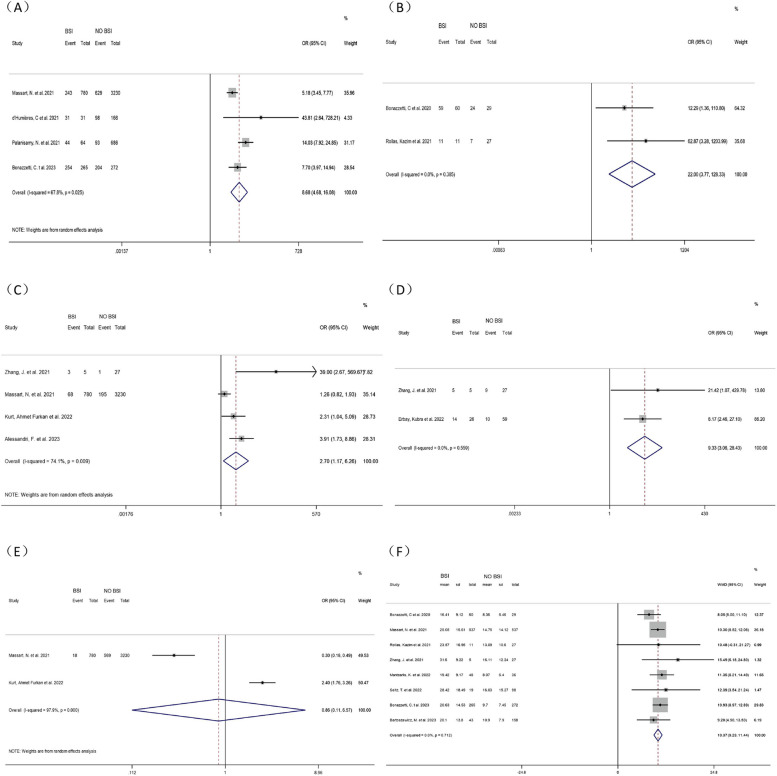
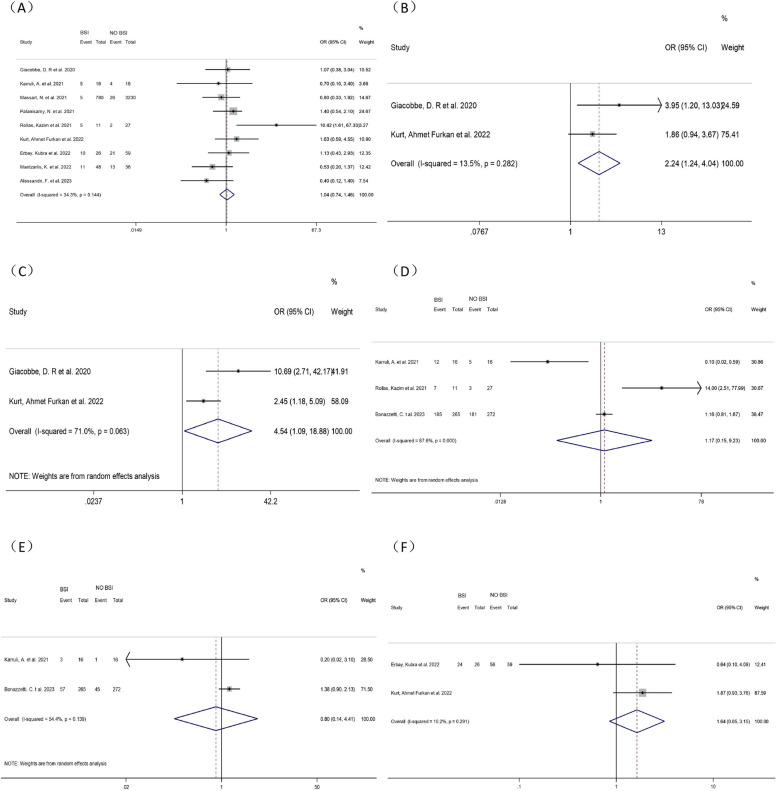
The correlation between Tocilizumab and BSI in COVID-19 patients was investigated in 10 studies [8–12, 16, 17, 20, 21, 55]. One article was excluded by sensitivity analysis [21] and therefore nine articles were enrolled in the meta-analysis. There was no correlation between Tocilizumab and BSI in COVID-19 patients in ICUs (OR = 1.04, 95% CI: 0.74–1.46, P = 0.815, I2 = 34.3%). However, this may explain why Tocilizumab is widely used for severe and critically ill COVID-19 patients in ICUs under the guidance of guidelines. (Fig. 5)
Fig. 5.
Forest plot of univariate data associating BSI risk with (A) Tocilizumab; (B) Methylprednisolone; (C) Methylprednisolone and Tocilizumab combination; (D) Steroids; (E) Remdesivir and (F) Dexamethasone for patients with COVID-19 in ICU
Methylprednisolone
Two studies [8, 12] investigated the association between Methylprednisolone and ICU-BSI in COVID-19 patients. Meta-analysis signified that Methylprednisolone was linked with BSI in COVID-19 patients in ICUs (OR = 2.24, 95% CI: 1.24–4.04, P = 0.008, I2 = 13.5%). Meanwhile, we found that the combination of Methylprednisolone and Tocilizumab significantly increased the risk for BSI in COVID-19 patients in ICUs (OR = 4.54, 95% CI: 1.09–18.88, P = 0.037, I2 = 71%). (Fig. 5)
Steroids
All the studies on the risk of steroid use on ICU-BSI in COVID-19 patients were included, and the results were found only in 3 studies [9, 17, 21]. Meta-analysis showed no correlation between steroid use and BSI in COVID-19 patients in ICUs (OR = 1.17, 95% CI: 0.15–9.23, P = 0.882, I2 = 87.6%). This may be related to the fact that steroids are widely used as they are believed to improve the recovery of patients. (Fig. 5)
Dexamethasone
Two studies [11, 12] investigated the association between Dexamethasone and ICU-BSI in COVID-19 patients. Meta-analysis showed no correlation between Dexamethasone use and BSI in COVID-19 patients in ICUs (OR = 1.64, 95% CI: 0.85–3.15, P = 0.139, I2 = 10.2%) (Fig. 5).
Remdesivir
The correlation between Remdesivir and ICU-BSI in COVID-19 patients was investigated in 2 studies [9, 21]. Meta-analysis showed no correlation between Remdesivir and BSI in COVID-19 patients in ICUs (OR = 0.80, 95% CI: 0.14–4.41, P = 0.794, I2 = 54.4%). This may be related to the fact that Remdesivir is considered a potent drug for the treatment of COVID-19, with significant efficacy, and therefore is more widely used for severe and critically ill patients in ICUs. (Fig. 5)
Sensitivity analysis
The stability of the results of the remaining articles was estimated by excluding each article in turn. Sensitivity analyses for gender, SAPS II score, DM, hypertension, chronic pulmonary disease, liver disease, heart disease, immunosuppressive disease, tumor, tracheal intubation, ECMO, CVC, RRT, length of stay in ICUs, and the use of Methylprednisolone, Steroids, and Remdesivir revealed that the results were relatively stable. In the sensitivity analysis of mechanical ventilation, the study by Palanisamy, N et al. [16] greatly impacted the results, so the results were pooled after the exclusion of that article, and the results were more stable. Similarly, in the sensitivity study of chronic kidney disease, it was found that Massart, N et al. [10] greatly influenced the results. After excluding the article and re-combining the results, the results were more stable. In the sensitivity study on the use of Tocilizumab, the study by Bonazzetti, C et al. [21] greatly influenced the results. The results were more stable when the article was excluded, and the results were re-combined. The sensitivity analyses of other factors implied stable and insignificant changes, so these studies were retained. (Figs. S1, S2, S3)
Publication bias
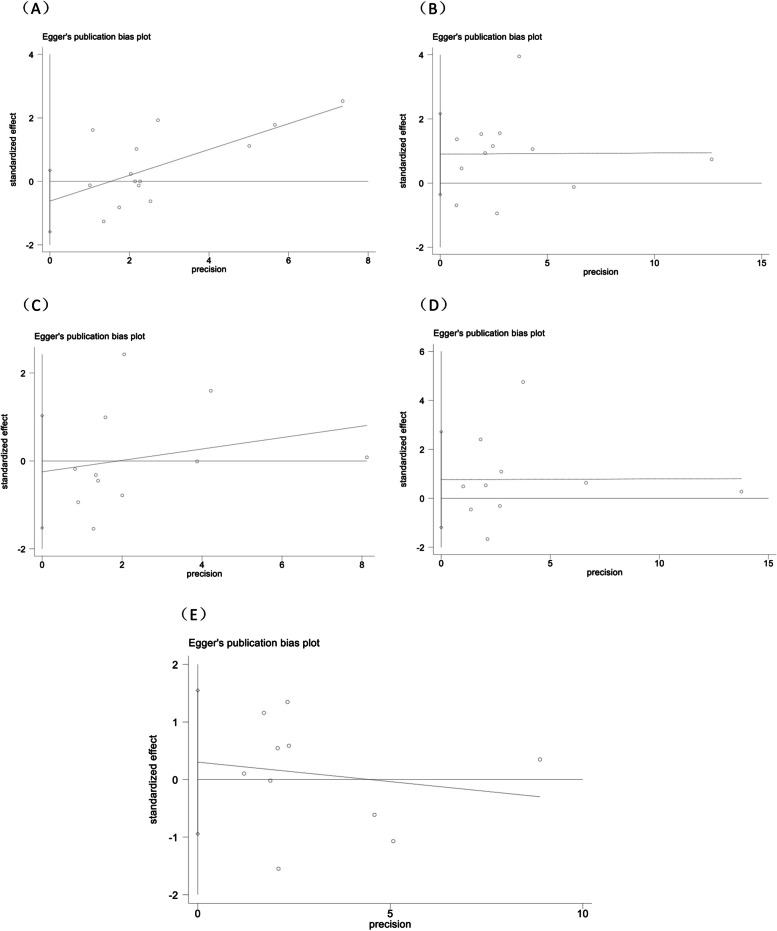
Publication bias was examined using the Egger test for the risk factor containing ≥ 10 articles. There was no publication bias for men (P = 0.187), DM (P = 0.142), hypertension (P = 0.396), heart disease (P = 0.592), and chronic pulmonary disease (P = 0.671). (Fig. 6)
Fig. 6.
Publication bias of univariate data associating BSI risk with (A) gender; (B) diabetes; (C) chronic pulmonary disease; (D) hypertension and (E) heart disease for patients with COVID-19 in ICU
Discussion
In this meta-analysis, we aimed to identify risk factors for BSI in COVID-19 patients in ICUs. Among the studies with available data, 55 published English studies [3, 5–58] investigating risk factors associated with BSI in COVID-19 patients in ICUs were included. Our findings showed that male, DM, tracheal intubation, mechanical ventilation, CVC, ECMO, Methylprednisolone use, higher SAPS II score, and longer ICU stay were risk factors for ICU-BSI in COVID-19 patients. In addition, hypertension, chronic pulmonary disease, liver disease, chronic kidney disease, heart disease, immunosuppression, tumor, RRT, and the use of Tocilizumab, Steroids, and Remdesivir neither increased nor decreased the risk of ICU-BSI in COVID-19 patients.
Patient-related factors
Our analyses showed that male COVID-19 patients were at a higher risk of BSI in ICUs and that males also made up most of the ICU admission population. A previous study by Zamora-Cintas, M. et al. [37] highlighted that male patients had a higher risk of BSI, in line with our results. COVID-19 virus infection mainly affects pulmonary function, resulting in more patients with pulmonary dysfunction being admitted to the hospital, and more severe patients need to be admitted to ICUs, which makes them more susceptible to BSI. However, we did not study whether smoking was a risk factor for BSI in COVID-19 patients in ICUs and the proportion of men who smoke., which might require more data support. Our findings also showed that the SAPS II score directly reflected the risk of BSI in COVID-19 patients in ICUs, in agreement with the findings of Massart, N et al. [10]. SAPS II score, as an important evaluation component in ICUs, to some extent, also reflects infection indicators, which is directly related to our study. In short, a higher SAPS II score indicates a more severe condition and a worse prognosis [59]. DM is a common chronic underlying disease in clinical practice. Our results showed that DM was also an associated risk factor for ICU-BSI in COVID-19 patients. This may be related to diverse complications associated with DM and the poor resistance of diabetic patients, which makes them susceptible to a variety of related infections [60] and directly increases the risk of BSI [61].
Treatment-related factors
Our findings suggested that tracheal intubation substantially increased the risk of ICU-BSI in COVID-19 patients, in support of the findings of Bonazzetti, C et al. [14] and Rollas, Kazim et al. [17]. Tracheal intubation is a common resuscitation technique in ICUs and is essential to save patients in respiratory distress. It ensures that the patient receives an adequate supply of oxygen and provides mechanical ventilation support to maintain normal respiratory function [62]. This makes mechanical ventilation support also a possible risk factor for BSI in COVID-19 patients in ICUs. Invasive treatment is highly likely to cause airway damage, and tracheal intubation may introduce bacteria or other pathogens, increasing the risk of infection in patients. It is also easy for micro-aspiration to occur after tracheal intubation, leading to lung infections [63]. All these directly increase the risk of BSI. COVID-19 has become a specific infection that involves the pathophysiology of the lungs, including endothelial and epithelial changes, pulmonary embolism, and microvascular thrombosis. In addition, secondary infectious injury can cause acute lung injury and prolong mechanical ventilation [5]. Zhang, J et al. showed that multiple invasive treatments were important risk factors for BSI. Early extubation and regular assessment of infection should be done, therefore early anti-infective therapy is important [18]. CVC is widely used in the resuscitation of severe and critically ill patients, which is conducive to the measurement of central venous pressure, long-term medication, and large and rapid rehydration, thus preventing venous damage and repeated puncture. However, a common complication of CVC is deep vein thrombosis, which also leads to the invasion of external bacteria and infection. Patients present with persistent low-grade fever, and the simultaneous presence of bacteria and thrombus can exacerbate the infection [64]. Our study revealed that prolonged CVC substantially increased the risk of ICU-BSI in COVID-19 patients. Therefore, there is a need for timely monitoring of the situation and increased measures for infection control and nursing care for CVC. ECMO serves as an important therapeutic tool to provide continuous extracorporeal respiratory and circulatory function for critically ill patients presenting with cardiopulmonary failure. Some studies showed that infections were highly susceptible to occurring after the use of ECMO, which was related to the fact that patients with low immunity were susceptible to systemic hematogenous infections, thus dramatically increasing the risk of fungal infections [65, 66]. This was in general agreement with the results of our study. Meanwhile, ECMO may result in renal failure in about 50% of patients, which may require RRT [67]. However, our study found that RRT was not a risk factor for ICU-BSI in COVID-19 patients, which may need to be supported by more data. In particular, intuitive data in our study pointed out that the longer the treatment duration in ICUs, the higher the risk of BSI in COVID-19 patients. The possible reasons are as follows: first, the treatment time reflects the severity of the patient’s condition, and a longer ICU stay may mean that the patient’s condition is more critical; second, the longer the treatment time, the higher the chance of nosocomial infections [11], which is closely related to the prolonged use of antibiotics and ward management; third, severe and critically ill patients in ICUs have low resistance and need to be left with various passages during treatment, and most COVID-19 patients have coughing symptoms, which is prone to aerosol dissemination and transmission of infectious disease between the patients, thus greatly increasing the risk of BSI [68].
Medication-related factors
In our study, the use of Tocilizumab, Remdesivir, Steroids, and Dexamethasone did not correlate with the risk of BSI in COVID-19 patients in ICUs, but the use of Methylprednisolone and the combination of Methylprednisolone and Tocilizumab directly increased the risk of ICU-BSI in COVID-19 patients. This is associated with the fact that patients receiving glucocorticoid therapy are more likely to require ventilatory support, vasopressors, and RRT [28, 52]. Glucocorticoids are widely used and effective drugs during hospitalization, especially in ICU, due to their anti-inflammatory and immunosuppressive effects. Glucocorticoids inhibit the inflammatory chemotaxis of cells and the rate of phagocytosis to reach inflammation sites. In addition, glucocorticoids increase the stability of cells so that cell membranes are less likely to rupture, and cells are less likely to release lysosomal enzymes to phagocytose bacteria to destroy inflammatory foci, thus decreasing the body’s immunity and making it susceptible to viral or bacterial infections [69, 70]. Glucocorticoids will also directly inhibit the body’s immune function, thus inhibiting the body’s fever, so that the fever symptoms are not obvious, which in turn masks the severity of the disease and delays the diagnosis and treatment, leading to further deterioration of the condition [28]. Moreover, glucocorticoids also inhibit mucosal exudation and inflammatory exudation. For patients with respiratory tract infections, glucocorticoids inhibit the exudation of inflammatory secretions, so that patients reduce coughing, which is not conducive to discharging bacterial sputum out of the body through coughing. Additionally, it delays the detection and treatment, thus aggravating the infection and increasing the risk of BSI greatly [19]. Therefore, it is crucial to monitor the use of glucocorticoids rationally according to the condition [52].
Incidence of ICU-BSI in COVID-19 patients
Meta-analysis unraveled that the incidence of BSI in COVID-19 patients in ICUs was 19.9%, similar to the currently reported 10–50% incidence rate. Compared with previous studies, in which 7% of COVID-19 hospitalized patients may experience BSI [2], the incidence of BSI in COVID-19 patients in ICUs increased nearly threefold. The incidence of ICU-BSI in COVID-19 patients varied in different studies, mainly because the occurrence of BSI lies in the detection of blood cultures. Also, it is somewhat difficult to exclude sampling contamination and detection contamination, and most patients in ICUs receive various types of medications, which may affect the detection of BSI [15, 71].
Strengths and limitations
This is the first systematic review analyzing risk factors for ICU-BSI in COVID-19 patients, and the data were reviewed by two investigators to ensure accuracy. By incorporating an extensive array of papers with high quality, our findings provide an accurate and reliable framework for promptly identifying the risk of BSI occurrence in COVID-19 patients in ICUs. In addition, our findings provide more comprehensive references of risk factors for ICU-BSI in COVID-19 patients for clinical treatment, which is a guide for early prevention of BSI.
However, some limitations need discussion. First, the studies covered diverse ethnicities, populations, methods, and periods of investigation, which is reflected in heterogeneity. However, this may be due to differences in study design rather than actual differences in outcome measures. Therefore, we used sensitivity analyses and random-effects models to verify the result stability in the presence of high heterogeneity. Second, the diagnosis of BSI in COVID-19 patients treated with Tocilizumab may be difficult because patients often do not have fever and have low serum levels of typical inflammatory markers, requiring further study. Third, Data on diseases such as diabetes and oncology did not have specific types of data, so specific rich data are needed to study their association with BSI. Fourth, few of the included studies analyzed the impact of post-invasive treatment care measures on the occurrence of BSI in COVID-19 patients in ICUs. Therefore, in the future, more assessments of the impact of treatment details on ICU-BSI in COVID-19 patients and randomized controlled trials are needed to enhance the reliability.
Conclusion
Our findings showed that males, higher SAPS II scores, DM, tracheal intubation, mechanical ventilation, ECMO, CVC, longer ICU stays, and Methylprednisolone use increased the risk of ICU-BSI in COVID-19 patients. It is imperative for future research to integrate these factors into a comprehensive predictive assessment framework to identify and intervene promptly in COVID-19 patients at high risk for ICU-BSI to improve treatment outcomes and promote patient health recovery.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Writing - original draft preparation: [Jun Wang, Ting Jiang]; Writing - review and editing: [Jun Wang, Ting Jiang]; Conceptualization: [Jun Wang, Ting Jiang]; Methodology: [Jun Wang, Ting Jiang]; Formal analysis and investigation: [Jun Wang, Ting Jiang]; Resources: [Jun Wang, Ting Jiang]; Supervision: [Jun Wang, Ting Jiang], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that they did not receive any funding from any source.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Wang and Ting Jiang contributed equally to this work and should be considered as co-first authors.
References
- 1.Pontali E, Filauro F. Repurposing an “Old” drug for the treatment of COVID-19-related cytokine storm. J Clin Med. 2023;12(10):3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ippolito M, Simone B, Filisina C, Catalanotto FR, Catalisano G, Marino C, Misseri G, Giarratano A, Cortegiani A. Bloodstream infections in hospitalized patients with COVID-19: a systematic review and Meta-analysis. Microorganisms. 2021;9(10):2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cataldo MA, Tetaj N, Selleri M, Marchioni L, Capone A, Caraffa E, Caro AD, Petrosillo N. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming collateral effect. J Global Antimicrob Resist. 2020;23:290–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B, Ruckly S, Dumenil AS, Kallel H, Argaud L, et al. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74(2):131–41. [DOI] [PubMed] [Google Scholar]
- 5.Llitjos J-F, Bredin S, Lascarrou J-B, Soumagne T, Cojocaru M, Leclerc M, Lepetit A, Gouhier A, Charpentier J, Piton G, et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: a multicentre retrospective cohort study. Ann Intensive Care. 2021;11(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao R-q, Ren C, Ren D, Li J-x, Li Y, Liu X-y, Huang L, Liu Y, Peng M, Feng Y-, et al. Development of septic shock and prognostic assessment in critically ill patients with coronavirus disease outside Wuhan, China. World J Emerg Med. 2021;12(4):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torrecillas M, Gumucio VD, Padulles A, Tubau F, Marco D, Shaw E, Fernandez-Huerta M, Maisterra K, Grau I, Petito MM, et al. Antimicrobial use and aetiology of bloodstream infections in critically ill patients during early stages of SARS-CoV-2 pandemic. Infect Prev Pract. 2022;4(4):100241–100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, Crea F, De Maria A, Dentone C, Di Biagio A, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karruli A, Boccia F, Gagliardi M, Patauner F, Ursi MP, Sommese P, De Rosa R, Murino P, Ruocco G, Corcione A, et al. Multidrug-resistant infections and outcome of critically ill patients with Coronavirus Disease 2019: a single Center experience. Microb drug Resist (Larchmont NY). 2021;27(9):1167–75. [DOI] [PubMed] [Google Scholar]
- 10.Massart N, Maxime V, Fillatre P, Razazi K, Ferré A, Moine P, Legay F, Voiriot G, Amara M, Santi F, et al. Characteristics and prognosis of bloodstream infection in patients with COVID-19 admitted in the ICU: an ancillary study of the COVID-ICU study. Ann Intensive Care. 2021;11(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erbay K, Ozger HS, Guzel Tunccan O, Gaygisiz U, Buyukkoruk M, Sultanova F, Yildiz M, Boyaci Dundar N, Aydogdu M, Bozdayi G, et al. Evaluation of prevalance and risk factors for bloodstream infection in severe coronavirus disease 2019 (COVID-19) patients. Antimicrob Stewardship Healthc Epidemiology: ASHE. 2022;2(1):e30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurt AF, Mete B, Urkmez S, Demirkiran O, Dumanli GY, Bozbay S, Dilken O, Karaali R, Balkan II, Saltoglu N, et al. Incidence, risk factors, and prognosis of bloodstream infections in COVID-19 patients in intensive care: a single-center observational study. J Intensive Care Med. 2022;37(10):1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartoszewicz M, Czaban SL, Bartoszewicz K, Kuzmiuk D, Ladny JR. Bacterial bloodstream infection in critically ill patients with COVID-19: a retrospective cohort study. Ther Adv Infect Dis. 2023;10:20499361231207178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonazzetti C, Morena V, Giacomelli A, Oreni L, Casalini G, Galimberti LR, Bolis M, Rimoldi M, Ballone E, Colombo R, et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2020;49(1):e31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d’Humières C, Patrier J, Lortat-Jacob B, Tran-Dinh A, Chemali L, Maataoui N, Rondinaud E, Ruppé E, Burdet C, Ruckly S, et al. Two original observations concerning bacterial infections in COVID-19 patients hospitalized in intensive care units during the first wave of the epidemic in France. PLoS ONE. 2021;16(4):e0250728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palanisamy N, Vihari N, Meena DS, Kumar D, Midha N, Tak V, Sharma A, Bohra GK, Kothari N, Dutt N, et al. Clinical profile of bloodstream infections in COVID-19 patients: a retrospective cohort study. BMC Infect Dis. 2021;21(1):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollas K, Ersan G, Zincircioglu C, Sahar I, Caliskan T, Guldogan IK, Saritas A, Uzun U, Senoglu N. Septic shock in patients admitted to intensive care unit with COVID-19 pneumonia. Eurasian J Pulmonol. 2021;23(2):95–100. [Google Scholar]
- 18.Zhang J, Lan P, Yi J, Yang C, Gong X, Ge H, Xu X, Liu L, Zhou J, Lv F. Secondary bloodstream infection in critically ill patients with COVID-19. J Int Med Res. 2021;49(12):3000605211062783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupper AC, Malik Y, Cusumano JA, Nadkarni D, Banga J, Caban AB, Twyman K, Obla A, Patel D, Mazo D, et al. Longer steroid treatment increases secondary bloodstream infection risk among patients with COVID-19 requiring intensive care. Infect Dis Clin Pract. 2022;30(4):IPC-0000000000001188. [Google Scholar]
- 20.Mantzarlis K, Deskata K, Papaspyrou D, Leontopoulou V, Tsolaki V, Zakynthinos E, Makris D. Incidence and risk factors for blood stream infection in mechanically ventilated COVID-19 patients. Antibiotics (Basel, Switzerland). 2022;11(8):1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonazzetti C, Rinaldi M, Giacomelli A, Colombo R, Ottolina D, Rimoldi SG, Pagani C, Morena V, Ridolfo AL, Vatamanu O, et al. Risk factors associated with bacteremia in COVID-19 patients admitted to intensive care unit: a retrospective multicenter cohort study. Infection. 2023;51(1):129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amit M, Sorkin A, Chen J, Cohen B, Karol D, Tsur AM, Lev S, Rozenblat T, Dvir A, Landau G, et al. Clinical course and outcomes of severe Covid-19: a national scale study. J Clin Med. 2020;9(7):2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu G, Deng J, Xiang J, Liu Y, Xing D. Clinical characteristics and prognosis in 51 severe cases of COVID-2019. Jounral Chongqing Med Univ. 2020;45(7):948–55. [Google Scholar]
- 24.Garcia PDW, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, Roche-Campo F, Schuepbach RA, Hilty MP, Investigators R-I. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, Chen H, Guo M, Chen S, Sun F, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infections. 2020;9(1):1958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anwar A, Ramos-Bascon N, Crerar-Gilbert A, Barnes N, Madden B. A specialised cardiorespiratory team approach in the intensive care management of COVID-19 patients: benefit on mortality, diagnosis and management. Clin Med. 2021;21(2):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardi T, Pintado V, Gomez-Rojo M, Escudero-Sanchez R, Azzam Lopez A, Diez-Remesal Y, Martinez Castro N, Ruiz-Garbajosa P, Pestana D. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40(3):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupuis C, de Montmollin E, Buetti N, Goldgran-Toledano D, Reignier J, Schwebel C, Domitile J, Neuville M, Ursino M, Siami S, et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: a multicenter cohort study of the OUTCOMEREA network. PLoS ONE. 2021;16(8):e0255644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, Bellani G, Biagioni E, Bonfanti P, Bottino N, et al. Hospital-Acquired infections in critically ill patients with COVID-19. Chest. 2021;160(2):454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokkoris S, Papachatzakis I, Gavrielatou E, Ntaidou T, Ischaki E, Malachias S, Vrettou C, Nichlos C, Kanavou A, Zervakis D, et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect. 2021;107:95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong CCH, Farhanah S, Linn KZ, Tang YW, Poon CY, Lim AY, Tan HR, Binte Hamed NH, Huan X, Puah SH, et al. Nosocomial infections among COVID-19 patients: an analysis of intensive care unit surveillance data. Antimicrob Resist Infect Control. 2021;10(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos R, de la Villa S, Garcia-Ramos S, Padilla B, Garcia-Olivares P, Pinero P, Garrido A, Hortal J, Munoz P, Caamano E, et al. COVID-19 associated infections in the ICU setting: a retrospective analysis in a tertiary-care hospital. Enfermedades infecciosas y microbiologia clinica. 2021;41(5):278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roedl K, Jarczak D, Thasler L, Bachmann M, Schulte F, Bein B, Weber CF, Schaefer U, Veit C, Hauber H-P, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Australian Crit Care. 2021;34(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Søgaard KK, Baettig V, Osthoff M, Marsch S, Leuzinger K, Schweitzer M, Meier J, Bassetti S, Bingisser R, Nickel CH, et al. Community-acquired and hospital-acquired respiratory tract infection and bloodstream infection in patients hospitalized with COVID-19 pneumonia. J Intensive care. 2021;9(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez-de-la-Rica A, Serrano P, De-la-Oliva R, Sánchez-Díaz P, Molinero P, Falces-Romero I, Ferrando C, Rello J, Maseda E. Secondary infections in mechanically ventilated patients with COVID-19: an overlooked matter? Revista Esp De Quimioterapia: Publicacion Oficial de la Sociedad Esp De Quimioterapia. 2021;34(4):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakar MN, Ergan B, Ergun B, Kucuk M, Canturk A, Ergon MC, Gezer NS, Yaka E, Comert B, Gokmen AN. Clinical characteristics and risk factors for 28-day mortality in critically ill patients with COVID-19: a retrospective cohort study. Turk J Med Sci. 2021;51(5):2285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zamora-Cintas MI, López DJ, Blanco AC, Rodriguez TM, Segarra JM, Novales JM, Ferriol MFR, Maestre MM, Sacristán MS. Coinfections among hospitalized patients with covid-19 in the first pandemic wave. Diagn Microbiol Infect Dis. 2021;101(3):115416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlstrom B, Frithiof R, Larsson I-M, Strandberg G, Lipcsey M, Hultstrom M. A comparison of impact of comorbidities and demographics on 60-day mortality in ICU patients with COVID-19, sepsis and acute respiratory distress syndrome. Sci Rep 2022;12(1):15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brücker W, Mahabadi AA, Hüschen A, Becker J, Daehnke S, Möhlenkamp S. Clinical characteristics and determinants of mortality in coronavirus disease 2019 (COVID-19) patients on an intensive care unit-a retrospective explorative 1-year all-comers study. J Thorac Disease. 2022;14(5):1319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caiazzo L, Temperoni C, Canovari B, Simonetti O, Montalti R, Barchiesi F. Secondary infections in critically ill patients with COVID-19: a retrospective study. Antibiot (Basel Switzerland). 2022;11(11):1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cidade JP, Coelho LM, Costa V, Morais R, Moniz P, Morais L, Fidalgo P, Tralhao A, Paulino C, Nora D, et al. Septic shock 3.0 criteria application in severe COVID-19 patients: an unattended sepsis population with high mortality risk. World J Crit care Med. 2022;11(4):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ćurčić M, Tarle M, Almahariq H, Hleb S, Havaš J, Pražetina M, Lasić H, Dolenc E, Kukoč A, Mihelčić A, et al. Distribution of pathogens and predictive values of biomarkers of inflammatory response at ICU admission on outcomes of critically ill COVID-19 patients with bacterial superinfections—observations from National COVID-19 Hospital in Croatia. Diagnostics. 2022;12(9):2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Costa RL, Lamas CDC, Simvoulidis LFN, Espanha CA, Moreira LPM, Bonancim RAB, Weber JVLA, Ramos MRF, Silva ECF, de Oliveira LP. Secondary infections in a cohort of patients with COVID-19 admitted to an intensive care unit: impact of gram-negative bacterial resistance. Rev Inst Med Trop Sao Paulo. 2022;64:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Bruyn A, Verellen S, Bruckers L, Geebelen L, Callebaut I, De Pauw I, Stessel B, Dubois J. Secondary infection in COVID-19 critically ill patients: a retrospective single-center evaluation. BMC Infect Dis. 2022;22(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeVoe C, Segal MR, Wang L, Stanley K, Madera S, Fan J, Schouest J, Graham-Ojo R, Nichols A, Prasad PA, et al. Increased rates of secondary bacterial infections, including Enterococcus bacteremia, in patients hospitalized with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2022;43(10):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozlowski B, Kubiak-Pulkowska J, Palka J, Bozilow D, Zajac M, Deptula A. Healthcare-associated infections in COVID-19 ICU patients - two-centre study. Cent Eur J Public Health. 2022;30(3):196–200. [DOI] [PubMed] [Google Scholar]
- 47.Lepape A, Machut A, Bretonniere C, Friggeri A, Vacheron C. Effect of SARS-CoV-2 infection and pandemic period on healthcare-associated infections acquired in intensive care units Clin Microbiol Infection: Official Publication. Eur Soc Clin Microbiol Infect Dis. 2022;29(4):530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustafa ZU, Tariq S, Iftikhar Z, Meyer JC, Salman M, Mallhi TH, Khan YH, Godman B, Seaton RA. Predictors and outcomes of Healthcare-Associated Infections among patients with COVID-19 admitted to Intensive Care Units in Punjab, Pakistan; findings and implications. Antibiotics. 2022;11(12):1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey M, May A, Tan L, Hughes H, Jones JP, Harrison W, Bradburn S, Tyrrel S, Muthuswamy B, Berry N, et al. Comparative incidence of early and late bloodstream and respiratory tract co-infection in patients admitted to ICU with COVID-19 pneumonia versus Influenza A or B pneumonia versus no viral pneumonia: wales multicentre ICU cohort study. Crit Care (London England). 2022;26(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roda S, Seminari E, Pieri TC, Sachs M, Di Matteo AM, Belliato M, Corbella M, Degani A, Piralla A, Bruno R. Sars-Cov 2 versus flu: ECMO-associated bloodstream infections. new Microbiol. 2022;45(1):35–9. [PubMed] [Google Scholar]
- 51.Routsi C, Meletiadis J, Charitidou E, Gkoufa A, Kokkoris S, Karageorgiou S, Giannopoulos C, Koulenti D, Andrikogiannopoulos P, Perivolioti E, et al. Epidemiology of candidemia and fluconazole resistance in an ICU before and during the COVID-19 pandemic era. Antibiot (Basel Switzerland). 2022;11(6):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo A, Gavaruzzi F, Ceccarelli G, Borrazzo C, Oliva A, Alessandri F, Magnanimi E, Pugliese F, Venditti M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection. 2022;50(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seitz T, Holbik J, Grieb A, Karolyi M, Hind J, Gibas G, Neuhold S, Zoufaly A, Wenisch C. The role of bacterial and fungal superinfection in critical COVID-19. Viruses. 2022;11(6):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alenazi TA, Shaman MSB, Suliman DM, Alanazi TA, Altawalbeh SM, Alshareef H, Lahreche DI, Al-Azzam S, Araydah M, Karasneh R, et al. The impact of Multidrug-Resistant Acinetobacter baumannii infection in critically ill patients with or without COVID-19 infection. Healthc (Basel Switzerland). 2023;11(4):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alessandri F, Ceccarelli G, Migliara G, Baccolini V, Russo A, Marzuillo C, Ceparano M, Giordano G, Tozzi P, Galardo G, et al. High incidence of Candidemia in critically ill COVID-19 patients supported by veno-venous extracorporeal membrane oxygenation: a retrospective study. J fungi (Basel Switzerland). 2023;9(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedenić B, Bratić V, Mihaljević S, Lukić A, Vidović K, Reiner K, Schöenthaler S, Barišić I, Zarfel G, Grisold A. Multidrug-resistant Bacteria in a COVID-19 hospital in Zagreb. Pathogens (Basel Switzerland). 2023;12(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guanche Garcell H, Al-Ajmi J, Villanueva Arias A, Abraham JC, Felipe Garmendia AM, Fernandez Hernandez TM. Impact of the COVID-19 pandemic on the incidence, etiology, and antimicrobial resistance of healthcare-associated infections in a critical care unit in Western Qatar. Qatar Med J. 2023;2023(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taysi MR, Yildirim F, Simsek M, Dural HI, Sencan I. Secondary infections in critical patients with COVID-19 Associated ARDS in the ICU: frequency, microbiologic characteristics and risk factors. J Coll Physicians Surgeons–Pakistan: JCPSP. 2023;33(2):181–7. [DOI] [PubMed] [Google Scholar]
- 59.Roggeveen LF, Guo T, Fleuren LM, Driessen R, Thoral P, van Hest RM, Mathot RAA, Swart EL, de Grooth HJ, van den Bogaard B, et al. Right dose, right now: bedside, real-time, data-driven, and personalised antibiotic dosing in critically ill patients with sepsis or septic shock-a two-centre randomised clinical trial. Crit Care (London England). 2022;26(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.John EE, Roy S, Eapen JJ, Karuppusami R, Jose N, Mani SSR, Johny J, Alam R, Yusuf S, Thomas A, et al. Bacterial infection-related glomerulonephritis in patients with diabetes. Nephrol (Carlton). 2023;28(11):597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan L, Vaghari-Tabari M, Malakoti F, Moein S, Qujeq D, Yousefi B, Asemi Z. Quercetin: an effective polyphenol in alleviating diabetes and diabetic complications. Crit Rev Food Sci Nutr. 2023;63(28):9163–86. [DOI] [PubMed] [Google Scholar]
- 62.De Jong A, Myatra SN, Roca O, Jaber S. How to improve intubation in the intensive care unit. Update on knowledge and devices. Intensive Care Med. 2022;48(10):1287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aquino-Martinez R, Hernández-Vigueras S. Severe COVID-19 lung infection in older people and Periodontitis. J Clin Med. 2021;10(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayer S, Bonhag C, Jenkins P, Cornett B, Watts P, Scherbak D. Probiotic-Associated Central venous catheter bloodstream infections lead to increased mortality in the ICU. Crit Care Med. 2023;51(11):1469–78. [DOI] [PubMed] [Google Scholar]
- 65.Selçuk ÜN, Sargın M, Baştopçu M, Mete EMT, Erdoğan SB, Öcalmaz Ş, Orhan G, Aka SA. Microbiological spectrum of nosocomial ECMO infections in a Tertiary Care Center. Braz J Cardiovasc Surg. 2021;36(3):338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carelli S, Dell’Anna AM, Montini L, Bernardi G, Gozza M, Cutuli SL, Natalini D, Bongiovanni F, Tanzarella ES, Pintaudi G, et al. Bloodstream infections in COVID-19 patients undergoing extracorporeal membrane oxygenation in ICU: an observational cohort study. Heart lung: J Crit care. 2023;62:193–9. [DOI] [PubMed] [Google Scholar]
- 67.Selewski DT, Wille KM. Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin Dial. 2021;34(6):537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crawford C, Vanoli E, Decorde B, Lancelot M, Duprat C, Josserand C, Jilesen J, Bouadma L, Timsit JF. Modeling of aerosol transmission of airborne pathogens in ICU rooms of COVID-19 patients with acute respiratory failure. Sci Rep. 2021;11(1):11778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruscoli S, Puzzovio PG, Zaimi M, Tiligada K, Levi-Schaffer F, Riccardi C. Glucocorticoids and COVID-19. Pharmacol Res. 2022;185:106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papic I, Bistrovic P, Cikara T, Busic N, Keres T, Ortner Hadziabdic M, Lucijanic M. Corticosteroid dosing level, incidence and profile of bacterial blood stream infections in hospitalized COVID-19 patients. Viruses. 2024;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munro C, Zilberberg MD, Shorr AF. Bloodstream infection in the intensive care unit: evolving epidemiology and microbiology. Antibiot (Basel Switzerland). 2024;13(2):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.