Abstract
Extracellular vesicles (EVs) are membrane-bound vesicles that are shed or secreted from the cell membrane and enveloped by a lipid bilayer. They possess stability, low immunogenicity, and non-cytotoxicity, exhibiting extensive prospects in regenerative medicine (RM). However, natural EVs pose challenges, such as insufficient targeting capabilities, potential biosafety concerns, and limited acquisition pathways. Although engineered EVs demonstrate excellent therapeutic efficacy, challenges such as low production yield and the complexity of engineering modifications constrain their further clinical applications. Bacteria have advantages such as rapid proliferation, diverse gene editing methods, mature cultivation techniques, and relatively easy preparation of bacterial EVs (BEVs), which can be used to effectively address the challenges currently encountered in the field of EVs. This review provides a description of the biogenesis and pathophysiological functions of BEVs, and strategies for optimizing BEVs preparation to attain efficiency and safety are discussed. An analysis of natural characteristics of BEVs is also conducted to explore how to leverage their advantages or mitigate their limitations, thereby overcoming constraints on the application of BEVs in RM. In summary, engineered BEVs possess characteristics such as high production yield, excellent stability, and high drug-delivering capabilities, laying the foundation for their application in RM.
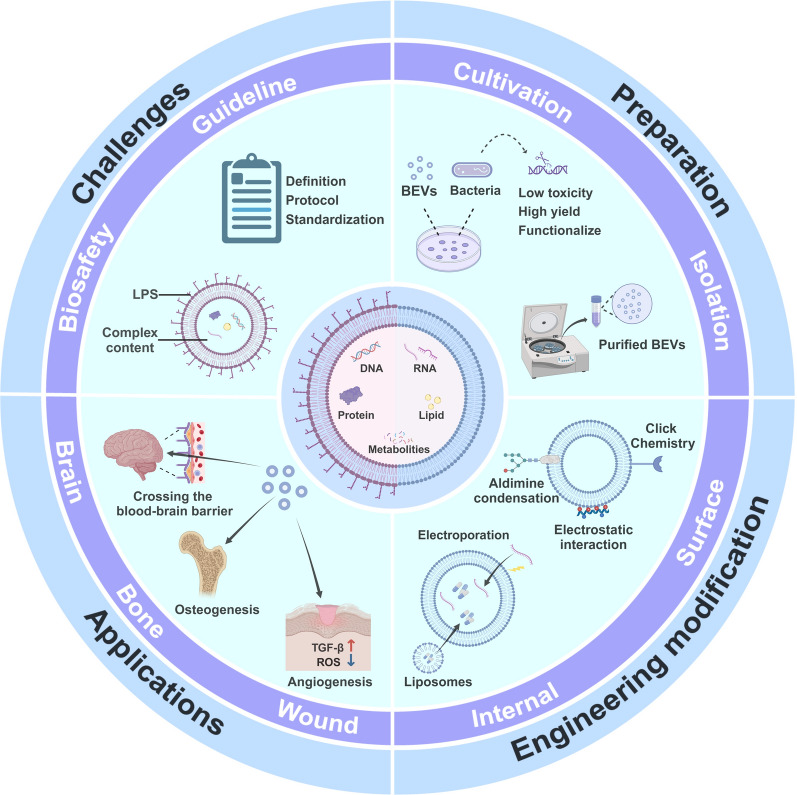
Graphical Abstract
Keywords: Bacterial extracellular vesicles, Outer membrane vesicles, Engineering strategy, Regenerative medicine, Drug delivery
Highlights
Elucidated the advantages and limitations of naturally occurring bacterial extracellular vesicles for regenerative medicine.
Provided an overview of the preparation, characterization, and engineering strategies of bacterial extracellular vesicles, and explored from these perspectives how to alleviate the application limitations of bacterial extracellular vesicles in regenerative medicine.
Discussed the current challenges and potential applications of bacterial extracellular vesicles.
Summarized the immense potential of engineered bacterial extracellular vesicles as innovative therapies in regenerative medicine, particularly from the perspectives of drug delivery, tissue regeneration, and antibacterial effects.
Introduction
Extracellular vesicles (EVs) are membrane-bound vesicles shed or secreted from the cell membrane and encapsulated by a lipid bilayer [1]. Both eukaryotic cells derived from animals and plants, as well as prokaryotic cells including bacteria and archaea, are capable of releasing nanoscale EVs [2]. EVs play a critical messenger role in intercellular communication by mediating the delivery of proteins, lipids, carbohydrates, nucleic acids, and other bioactive molecules, being important in various pathological and physiological processes, encompassing the propagation of infections, cancer progression, tissue repair, and angiogenesis [3]. The first report of EVs mediating cell-to-cell communication through the delivery of functional mRNA was documented in 2007 [4].Subsequently, the potential of EVs to play a role in tissue engineering and regenerative medicine (RM) became clear. For instance, mesenchymal stem cell-derived human EVs (MSC-hEVs) have been investigated for regenerative therapies involving the skin, bone tissue, and cardiovascular system [5]. Compared to conventional regenerative therapies, EVs present a novel strategy for cell-free therapy, offering advantages such as stability, biocompatibility, and absence of cellular toxicity. However, certain challenges remain that need to be addressed, including the insufficient targeting ability of natural EVs, nonspecific accumulation within organs, low production yield, and limited acquisition methods [6]. The engineering Strategy of natural EVs offers potential solutions to address these challenges. For instance, engineered mesenchymal stem cell-derived EVs have demonstrated promising targeting capabilities and therapeutic efficacy. However, limitations such as insufficient production yields and complexities associated with engineering modifications still hinder their clinical translation [7]. There is an urgent need to explore alternative approaches that can compensate for the limitations of EVs in the field of RM.
Bacterial EVs (BEVs) offer a new perspective to address the aforementioned issues, as bacteria possess advantages such as rapid proliferation, diverse gene editing methods, mature cultivation techniques, and relatively straightforward isolation and purification of BEVs [8]. These features are conducive to the engineering transformation of BEVs and their subsequent clinical translation. Bacteria have a significant impact on human health and disease. Furthermore, bacterial therapy has the potential to utilize synthetic biology to improve bacterial characteristics, thereby facilitating their specific applications in RM [9, 10]. An increasing body of evidence suggests that bacteria-host communication involves bacteria delivering bioactive molecules to host cells through the secretion of BEVs, eliciting corresponding functional responses [8, 11]. Moreover, it is of paramount importance that engineered attenuated bacterial strain-derived EVs exhibit low immunogenicity, and appropriate oral or intravenous administration does not induce chronic systemic toxicity or other adverse effects [12]. Furthermore, the application of BEVs, particularly those generated by probiotics, has gained increasing attention in the fields of drug delivery, vaccines, tumors and diagnostics [13]. For example, GSK's Bexsero, an EV-based vaccine formulation, has already been successfully used in clinical applications [14], providing a solid basis for the safe clinical use of other BEV formulations.
In summary, engineered BEVs exhibit advantageous features such as high production yields, excellent stability, and potent drug-loading capabilities, making them highly promising for applications in RM.
Biology and pathology of BEV
Categories and biological composition of BEVs
Classification and secretion mechanisms of BEVs
Just like other cellular organisms, bacteria are capable of spontaneously releasing nanoscale membrane vesicles into the extracellular environment [15]. The gram staining technique categorizes bacteria into two major groups, gram positive bacteria and gram negative bacteria. Based on this classification, BEVs are also divided into two categories: gram positive EVs derived from gram positive bacteria and gram negative EVs derived from gram negative bacteria. The cell membrane of gram negative bacteria consists of a thinner layer of peptidoglycan (PG) surrounded by the inner membrane (IM) and the outer membrane (OM) [16]. In contrast, gram positive bacteria possess a single, thick layer of PG in their cell wall [17]. This implies that the cell wall of gram positive bacteria is inherently less flexible, which hinders the formation of vesicles and suggests that gram positive and gram negative bacteria employ different mechanisms for BEVs secretion. Typically, the major distinction in the composition of BEVs derived from gram positive and gram negative bacteria is the presence of lipopolysaccharide (LPS), which is commonly associated with bacterial infections, inflammation, and immune responses [18].
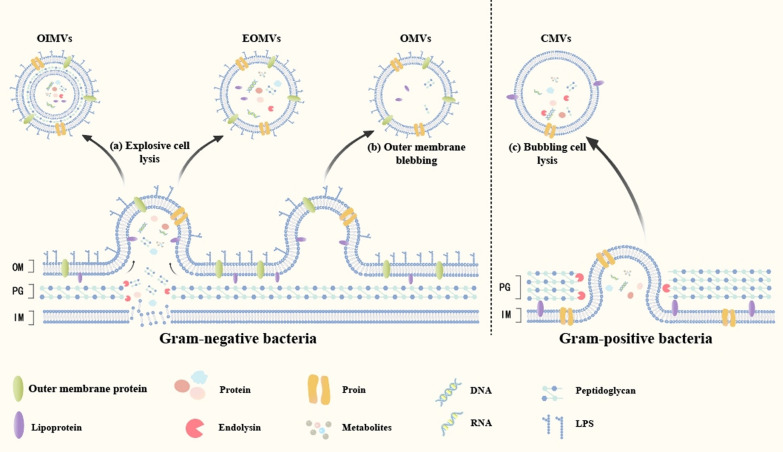
Gram negative bacteria exhibit two distinct pathways for BEVs biogenesis, which lead to the formation of different types of vesicles. As shown on the left side of the dashed line in Fig. 1. The process of explosive cell lysis corresponds to the production of outer-inner membrane vesicles (OIMVs)/explosive outer membrane vesicles (EOMVs), whereas outer membrane budding corresponds to the generation of outer membrane vesicles (OMVs) [19, 20]. The current hypothesis suggests that explosive cell lysis is triggered by the degradation of peptidoglycan cell walls by endolysins or other processes that weaken OM-PG interactions. Upon peptidoglycan degradation, bacteria undergo aggregation and lysis, leading to the accumulation of membrane fragments that give rise to OIMVs and EOMVs, the latter of which may or may not contain cytoplasmic components [16]. OMVs originate from protrusions of the OM. Under the influence of factors such as imbalances in PG biosynthesis or the insertion of hydrophobic molecules into the OM, the balance between the inner and outer layers of the OM is disrupted, resulting in curvature [21]. This leads to the formation of gaps between the OM and the PG, causing the OM to bud and eventually pinch off, releasing OMVs [22]. The integrity of the IM is not compromised during OMV formation; therefore, components within the IM do not enter the OMVs.
Fig. 1.
Biogenesis of Bacterial Extracellular Vesicles (BEVs). Gram negative bacteria exhibit two distinct pathways for BEVs biogenesis: a Explosive cell lysis corresponds to the generation of Outer-Inner Membrane Vesicles (OIMVs) and Explosive Outer Membrane Vesicles (EOMVs); b Outer membrane blebbing corresponds to the generation of Outer Membrane Vesicles (OMVs). Gram positive bacteria exhibit one pathway for BEVs biogenesis: c Bubbling cell lysis corresponds to the generation of Cytoplasmic Membrane Vesicles (CMVs)
Although the exact mechanism by which gram positive bacteria release BEVs has not been confirmed, their secretion has been established. Currently, gram positive bacteria are believed to generate BEVs primarily via "budding cell lysis”. As shown on the right side of the dashed line in Fig. 1. Antibiotics that weaken the cell wall or phage-derived endolysins stimulate the cytoplasmic membrane of gram positive bacteria to protrude through cell wall pores, leading to the production of cytoplasmic membrane vesicles (CMVs) [23]. which contain both cell membrane and cytoplasmic components.
Interestingly, channel proteins in the cell wall can regulate the secretion of BEVs through pore size or thickness [24]. and certain enzymes that increase bacterial cell wall permeability also promote the release of BEVs [25]. Furthermore, changes in the external environment, such as temperature, mechanical stimulation, chemical stimulation, and quorum sensing, may also influence their release [26, 27]. This indicates that the secretion of BEVs is jointly regulated by multiple factors, including environmental influences and the actions of the bacteria themselves. For convenience, in this review, "BEVs" is used to represent all vesicle structures produced by bacteria mentioned above (Fig. 1).
Biological composition of BEVs
BEVs carry various bioactive substances such as proteins, lipids, and nucleic acids [28]. Some markers found in BEVs are shown in Table 1. Through proteomic studies, it has been discovered that BEVs contain a large number of proteins, including various periplasmic proteins and enzymes [29]. Certain proteins have cytotoxic effects on host cells, such as Shiga toxin and hemolytic phospholipase C [30, 31]. Other enzymes that regulate host cell metabolism, including InsP6 phosphatase, have also been found in BEVs [32].
Table 1.
Screened BEVs markers and their primary functions
| Belong to | Marker | Major function | Refs. |
|---|---|---|---|
| Membrane proteins | ObfA | Maintain membrane stability and biofilm formation | [37] |
| OmpA | Maintain membrane stability and immune responses | [38] | |
| OmpC | Regulating Membrane permeability and immune responses | [39] | |
| OmpF | Transport of small molecule substances | [40] | |
| OmpT | Protein hydrolysis and processing | [41] | |
| OmpU | Adhesion, immune responses and biofilm formation | [42] | |
| TolC | Drug resistance and toxin secretion | [43] | |
| MmpL 3 | Lipid transport and virulence transmission | [44] | |
| Tol-Pal complexes | Maintain membrane stability and vesicle formation | [28] | |
| Periplasmic protein | NlpI | Regulating vesicle formation | [29] |
| SurA | Assembly of membrane proteins | [41] | |
| Skp | Assembly of membrane proteins | [41] | |
| Lipid components | Phosphatidylethanolamine | Maintain membrane stability | [45] |
| phosphatidylinositol | Maintain membrane stability and vesicle formation | [45] | |
| Phosphatidylglycerol | Maintain membrane stability | [33] | |
| Cardiolipin | Maintain membrane stability and vesicle formation | [33] | |
| Enzymes | InsP6 phosphatase | Regulate host cell metabolism | [32] |
| β-lactamase | Resistance to antibiotics and transmission of resistance | [46] | |
| Lipopolysaccharide | Lipid A | Immune escape and immune responses | [34] |
| Core oligosaccharide | Connecting lipid A and O antigens | [34] | |
| O-antigen | Immune responses and virulence transmission | [47] | |
| Other toxins | Peptidoglycan | Immune responses | [48] |
| Hemolysin | Disrupting the host cell membrane | [31] | |
| IbeA | Increase the permeability of the blood–brain barrier | [43] | |
| Shiga toxin | Inducing inflammation and cell apoptosis | [30] |
Lipids are important components of BEVs that influence their functions. Zachary et al. showed that BEVs of Salmonella Typhimurium contain phosphatidylglycerol and cardiolipin [33], which can tether lipid A (a component of LPS) to the bacterial surface through hydrophobic interactions. When bacteria are under stress, these lipid A components modulate their structure, thereby increasing their resistance and promoting immune evasion [34].
BEVs deliver bacterial DNA and RNA to host cells and bind to specific small molecules, playing a role in regulating gene expression and delivering genetic information between bacteria [35, 36]. In summary, BEVs deliver various bioactive substances to recipient cells, thereby playing a significant role in bacterial virulence, immune evasion, host transcriptional gene regulation, and host cell immune modulation.
Pathological and physiological functions of BEVs
Safety is a prerequisite for applying BEVs in RM. Therefore, understanding the roles of natural BEVs in pathological and physiological processes is crucial. This will help develop targeted strategies based on specific toxic and beneficial biological characteristics, leading to the production of safe and effective engineered BEVs for RM. In general, BEVs play a significant role in bacterial-host interactions and bacterial infections, influencing the host immune system, aiding in the formation of bacterial biofilms, transferring antibiotic resistance between bacteria, and eliminating competitive microorganisms.
Bacterial–host interaction mediated by BEVs
Bacterial–host interactions mediated by BEVs generate adverse effects on the host by eliciting immune responses, inflammatory reactions, and cytotoxic reactions. These factors affect the application of natural BEVs in RM.
BEVs stimulate the activity of epithelial, endothelial, and various immune cells by stimulating the host immune system through immunogenic molecules such as LPS, flagellar proteins, and peptidoglycans [48]. Notably, immune responses mediated by BEVs are not entirely detrimental. A study focusing on Bacteroides thetaiotaomicron revealed that BEVs induce the production of immunoregulatory interleukin-10 (IL-10) by colonic cells in healthy individuals, playing a crucial role in maintaining immune homeostasis related to the microbial involvement [49]. Similarly, BEVs produced by Bacteroides fragilis are implicated in immune homeostasis. These BEVs mediate their anti-inflammatory effects by promoting the secretion of IL-10 from macrophages and regulatory T cells via a TLR2-dependent pathway [50]. The regulation of BEVs to elicit appropriate immune stimulation while concurrently avoiding detrimental effects on the host immune system constitutes a challenge that needs to be addressed for their effective utilization in RM. The utilization of BEVs can manipulate the host's immune response and potentially confer additional beneficial effects on the host [51].
BEVs disseminate virulence factors into host cells and modulate the expression of inflammatory cytokines such as IL-10, IL-6, and TNF-α, promoting pro-inflammatory responses [52, 53]. BEVs produced by Pseudomonas aeruginosa induce inflammatory responses in macrophages and endothelial cells through the TLR2 and TLR4 pathways, triggering pulmonary inflammation [54]. Bacillus subtilis BEVs enhance macrophages' secretion of pro-inflammatory cytokines IL-6 and TNF-α. Furthermore, the expression levels of these inflammatory factors increase with increasing concentrations of BEVs [55]. Interestingly, BEVs secreted by certain probiotic bacteria suppress the inflammatory responses. For instance, BEVs derived from the probiotic Escherichia coli Nissle 1917 (EcN) in the gastrointestinal tract contribute to modulating host immune responses and facilitating the degradation of intestinal contents, simultaneously treating inflammatory bowel diseases by promoting tight junctions [56]. The aforementioned evidence underscores the intricacy of BEVs in the inflammatory response, presenting their potential to become a distinctive anti-inflammatory therapeutic strategy. However, the utilization of BEVs as therapeutic agents in RM requires a comprehensive assessment. Strict control measures must be implemented to address various potential negative factors and minimize the occurrence of adverse reactions.
BEVs induce toxic responses in host cells by delivering virulence factors, such as leukocidins, hemolysins, and exotoxins. BEVs derived from P. aeruginosa carry various virulence factors, including alkaline phosphatase, proteases, and hemolysins [57]. BEVs derived from Staphylococcus aureus contain hemolysins, leukocidins, and exfoliative toxins [58]. These constituents inflict damage on host cells. These results indicate that the virulence factors present in BEVs significantly compromise the overall health of an organism, greatly limiting their applicability. Addressing this crucial issue is imperative if BEVs are to be applied in RM. Current solutions include the use of low-toxicity strains such as probiotics to obtain their corresponding low-toxicity BEVs, thereby reducing cellular cytotoxic responses [59]. Alternatively, direct engineering modifications of BEVs can be employed to prevent or minimize these cytotoxic reactions, thereby significantly enhancing their safety profile [60].
Bacterial infection mediated by BEVs
Biofilms provide a conducive environment for the prolonged colonization of bacteria, enhancing their virulence and antibiotic resistance [61]. Many chronic diseases, such as otitis media and periodontal diseases, are associated with bacterial biofilms [62, 63]. It is noteworthy that BEVs mediating intercellular signaling among bacteria are capable of either promoting or inhibiting the development of bacterial biofilms [64, 65]. Currently, there is no definitive solution to completely address the issue of biofilm formation. If it is possible to leverage the biological characteristics of BEVs to interfere with the signal transmission that promotes biofilm formation, disrupting the formation of biofilms at the source would be advantageous for the treatment of chronic diseases.
BEVs mediate the death of other bacteria, resulting in a survival advantage for parental strain by killing the competing bacteria. For instance, BEVs of P. aeruginosa deliver cell wall protein hydrolases to degrade peptidoglycans, effectively eliminating competitive bacteria [66]. This provides a novel strategy for antibacterial therapy. On one hand, BEVs serve as a carrier for antibiotic drug delivery, enhancing the uptake of antibiotics and thereby exerting a more potent bactericidal effect [67, 68]. On the other hand, the inherent antibacterial properties of BEVs complement and reinforce the overall bactericidal effectiveness of the treatment [67]. The application of BEVs demonstrates high efficacy in the eradication of pathogenic bacteria within infected wounds, effectively attenuating or even arresting the progression of infections [68]. This presents a novel approach to address the emerging challenges of antibiotic resistance resulting from the widespread misuse of antibiotics in the current healthcare landscape.
In conclusion, the roles of BEVs in bacterial infections have a dual nature. Effectively harnessing their antibacterial characteristics while concurrently mitigating the potential negative consequences associated with BEVs is a pressing challenge [69]. Given the influential factors at play when applying BEVs in RM, rigorous testing of their active components is imperative. Failure to do so may result in the emergence of resistant bacterial strains, leading to further dissemination of infections and hindering tissue regeneration.
Preparation of BEVs
Given the challenges faced by natural BEVs in RM applications, particularly concerning safety and yield, this review discusses how to enhance the preparation processes of BEVs to align with the anticipated clinical demands in the future.
Bacterial culture
Cultivation of appropriate parental bacterial strains is crucial prior to the isolation and purification of BEVs. In 2009, Lee et al. first isolated gram positive EVs from the culture supernatants of S. aureus and B. subtilis [70]. These BEVs exhibit a diameter ranging from 20 to 100 nm and possess spherical lipid bilayer membrane structures. Clostridium perfringens and Streptococcus pneumoniae, among other gram negative bacteria, also produce BEVs [71–73]. In general, all bacterial species produce BEVs. However, considering the toxicity concerns associated with natural BEVs, it is a common practice to employ modified strains with reduced toxicity or less virulent bacteria, such as probiotics, as cultivation organisms. EcN and other mutant strains (msbA and msbB) that generate BEVs lacking intact LPS components exhibit diminished endotoxicity and immunogenicity when interacting with human cells, thereby demonstrating heightened safety [59].
In addition, it is essential to consider environmental factors such as temperature, culture medium composition, mechanical stimuli, biological agents, and non-environmental factors such as genetic influences on the secretion of BEVs [74]. Although adjustments to these factors may enhance the production of BEVs, it is crucial to modify BEVs generation with the utmost stringency. Environmental changes induce changes in the composition of BEVs, introducing uncertainty and the possibility of generating potentially harmful substances [75].
Vesicles released by bacteria at different growth stages may exhibit diverse compositions. For instance, early-formed BEVs by B. thetaiotaomicron are predominantly generated in a non-lytic manner and contain a higher proportion of lipoproteins. Late-stage BEVs are primarily formed through lytic mechanisms and may potentially carry non-selective cargo, which could impact downstream processing (Fig. 2) [76]. Therefore, the collection of BEVs at the appropriate stage of bacterial culture is also crucial (Table 2).
Fig. 2.
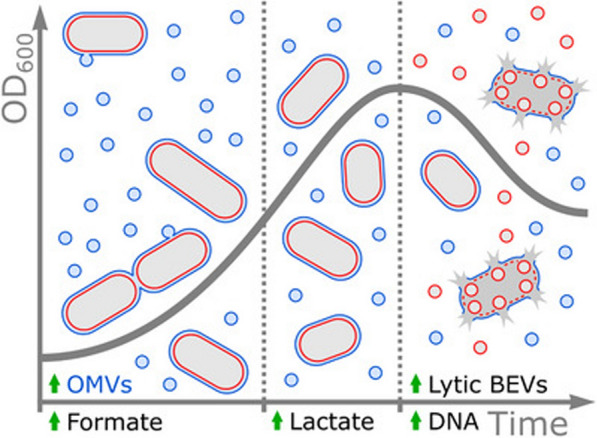
The secretion of Bacterial Extracellular Vesicles (BEVs) from B. thetaiotaomicron is divided into three stages: in the first stage, cell size increases, BEVs are formed non-lytically, and are accompanied by the release of formate. In the second stage, cell size decreases, BEV production decreases and is accompanied by the release of lactate. In the final stage, the number of cells decreases, BEVs are mainly formed lytically, and are accompanied by the release of DNA [76].
Reproduced with permission from Wiley Publications, copyright 2024
Table 2.
Different methods for isolating and charactering BEVs
| Isolation methods | ||||
|---|---|---|---|---|
| Based on | Methods | Advantages | Disadvantages | Refs. |
| Size | Size exclusion chromatography | High purity; preserves bioactive characteristics | limited application in mass production | [77] |
| Density | Density gradient centrifugation | High purity | High cost; time-consuming | [78] |
| Size and density | Ultracentrifugation | Simple and economical process; great homogeneity | Limited efficiency; limited purity | [79] |
| Size and morphology | Ultrafiltration | Faster isolation; high BEVs yields; high biological activity | Limited purity; potential damage to BEVs; potential filter blockage | [80] |
| Tangential Flow Filtration | Higher yield than UF | High cost | [81] | |
| Protein precipitation | ExoPRISM | Simple process; suitable for large-scale separation | Limited purity; sample pretreatment is required | [82] |
| Receptor-ligand binding | Affinity chromatography | High purity | High cost; affects activity; receptor required | [83] |
| Charactered methods | ||||
|---|---|---|---|---|
| Analysis results | Methods | Advantages | Disadvantages | Refs. |
| Size | DLS | Suitable for describing the average size and size distribution of BEVs | Not efficient with polydisperse samples and containing big EVs | [84] |
| Size and density | NTA | Compatible fluorescence detectors; automated sample measurement | Hardware and software can affect the accuracy of the results | [85] |
| TRPS | High sensitivity and reproducibility | Size distributions need parameterized; Incompatible fluorescence detectors | [86] | |
| AF4-MALS | Suited to measure the size of polydisperse samples | Potential sample loss; High purity required | [87] | |
| Size and morphology | TEM | High-resolution | Dehydrating preparations causing shrinkage of EVs | [88] |
| Protein characteristics | WB | Suitable for detecting multiple target proteins | Protein extraction process required and time-consuming | [89] |
| ELISA | Convenient reagent kit | High-cost | [90] | |
| Size, quantity and protein characteristics | SP-IRIS | Particles down to 50 nm can be detected; multi-channel measurement | Specific biomark are required; High disposable costs | [91] |
| Flow cytometry | Compatible fluorescence detectors and fast characterization | Limited potential for sorting nano-sized vesicles | [92] | |
Isolation of BEVs
Following appropriate bacterial cultivation, the initial removal of bacteria from the culture medium at low temperatures can be achieved through low-speed centrifugation (2000–10,000g) and aseptic filtration (0.2–0.45 μm) [93]. Subsequently, one or a combination of the following methods can be employed for further isolation and purification of BEVs.
Ultracentrifugation (UC) is one of the most widely employed techniques for isolating BEVs and is considered the gold standard. It allows for the size- and density-based separation and purification of BEVs, making it applicable for isolating particles of varying sizes and densities from solutions, such as organelles, vesicles, cell debris, and BEVs [94]. To isolate particles of the same size but different densities, density gradient centrifugation (DGC) can be employed for BEVs isolation [95]. This method yields higher BEVs purity compared to conventional UC approaches; however, it comes with the drawbacks of high cost and extended processing time.
Ultrafiltration (UF) represents another low-cost method for BEVs isolation. This technique utilizes nanomembranes with varying molecular weight cutoffs to isolate EVs based on molecular size and morphology [96]. Owing to the occasional significant overlap in size ranges between BEVs and the proteins and cell fragments they are isolated from, in series configuration and sequential ultrafiltration, a majority of particles are retained between the membranes, resulting in a reduction in the purity of BEVs. Tangential flow filtration (TFF) prevents particle entrapment, allowing for the retention of more BEVs and enhancing the purity of the target product [97]. The selection of filtration membranes also impacts the purity of the final product. Generally, regenerated cellulose membranes are the preferred choice; however, in samples with high protein concentrations, polyethersulfone membranes can be used to enhance the purity of the end product [98].
Size-exclusion chromatography (SEC) purifies BEVs without compromising their integrity, purity, or biological activity [99]. It allows for the separation of BEVs from soluble protein impurities. The effectiveness of SEC in purifying BEVs may surpass that of DGC [100]. Importantly, SEC also eliminates unbound molecules from chemical modifications [101], filtering out residual impurities from post-engineering modifications of BEVs, thereby enhancing the safety of the final product.
Immunoaffinity chromatography (IAC) leverages specific antibodies that target various BEVs surfaces for separation and purification, thus producing highly purified BEVs. Immobilized metal affinity chromatography (IMAC) is one of the most commonly employed methods for the purification of recombinant proteins [102]. Alves et al. obtained, through engineered modifications of EcN [103], His-tagged mutant bacteria. BEVs produced by these mutants demonstrated excellent capture efficiencies for IMAC purification. Furthermore, they could separate cargo-loaded BMVs from non-loaded BEVs, effectively enhancing the concentration of active drugs in the final product.
A combination of these separation methods eliminates contaminants from complex culture media and further enhances the purity of BEVs. The isolation of Akkermansia muciniphila-derived BEVs was achieved by combining UF and DGC, resulting in significantly elevated BEVs purity [104]. Following BEVs isolation and purification, it is advisable to either use them immediately or store them at − 80 °C until needed [105]. Storage under 4 °C conditions should not exceed one week [106]. As BEVs exceed the designated storage period, alterations in their components may occur, potentially leading to latent toxic side effects [107]. Simultaneously, the consideration of measures such as freeze-drying or the incorporation of additives can be explored to enhance the storage stability of BEVs during transportation [108]. However, it is imperative to exercise caution, ensuring that these interventions do not perturb the effective components carried by BEVs, nor compromise their characterization.
Characterization of BEVs
Following BEVs isolation, it is imperative to ascertain the characteristics and concentration of the final product. Currently, techniques such as transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and flow cytometry can be employed to characterize BEVs and elucidate their size, shape, and concentration. It is noteworthy that, regardless of the chosen method for separation and purification, utmost attention must be paid to preserving the integrity of the BEVs structure and the activity of the biomolecules. These factors significantly affect the biological characteristics of BEVs.
NTA is a methodology employed to measure particle size and concentration in BEVs formulations. This technique facilitates the measurement of particles as small as 50 nm [109]. NTA exhibits suboptimal accuracy in estimating the particle concentration and size [85]. This phenomenon may result from the inability of the NTA system to discriminate between BEVs particles and other fragments present in the suspension. Additionally, factors such as instrumentation, software versions, and improper operational procedures also contribute to measurement errors [110, 111]. Therefore, when employing NTA for BEVs characterization, rigorous sample separation and dilution are imperative before detection. Strict training of personnel is essential to ensure the accuracy of operations. It is recommended to employ a combination of various separation and purification methods before characterization to enhance the product purity and minimize errors.
Flow cytometry is typically employed to detect particles larger than 500 nm [112], which exceed the general size range of BEVs. However, Puca et al. successfully characterized BEVs using an optimized flow cytometry approach [113], demonstrating rapid and reproducible results. This achievement establishes the feasibility of employing flow cytometry to characterize BEVs.
TEM operates on principles akin to optical microscopy and utilizes the diffraction patterns generated by the scattering of electrons to examine the samples. This technique allows for qualitative measurements of the size and purity of BEVs [80]. Dynamic light scattering (DLS) is employed for the measurement of nanoparticle size and is particularly well-suited for delineating the average size and size distribution of BEVs [84].
Because of the absence of specific biomarkers in natural BEVs, many studies on natural BEVs have not employed protein detection methods for characterization. However, for engineered BEVs, protein detection techniques such as WB and ELISA are essential [114]. These methods detect the expression of specific proteins in engineered BEVs, aiding the identification of the effectiveness of engineered modification strategies.
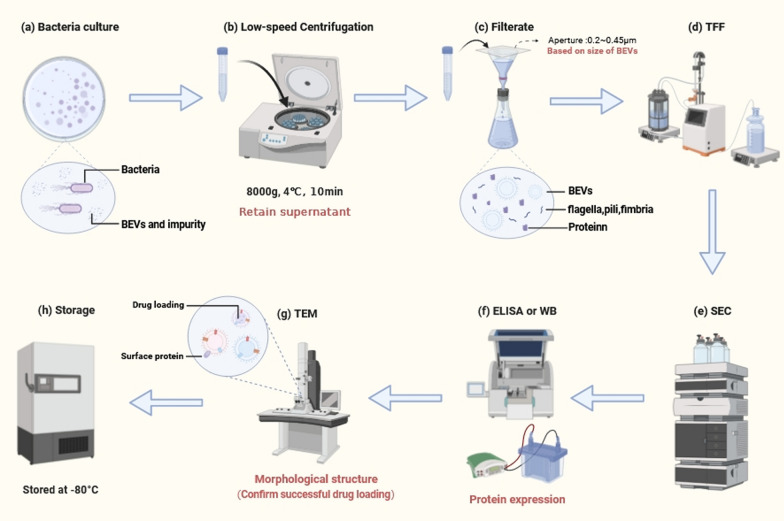
Reagent kits are convenient tools for assessing the pertinent indicators in BEVs formulations. For instance, the BCA reagent kit facilitates the determination of protein concentration in BEVs formulations, whereas the recombinant C-factor endotoxin assay kit enables quantitative analysis of endotoxin levels (Fig. 3) [115].
Fig. 3.
The preparation of Bacterial Extracellular Vesicles (BEVs). a Cultivating bacteria under optimal conditions. b Clarifying bacterial culture medium by low-speed centrifugation and retain supernatant. c Filtration of the supernatant to preliminary remove bacteria and other impurities. d Select an appropriately sized tangential flow filtration (TFF) device to concentrate BEVs. e Size-exclusion chromatography (SEC) is used to increase the purity of EVs and remove non-vesicular protein. f Protein detection methods such as WB and ELISA are used to detect the expression of specific proteins in BEVs. g Transmission electron microscopy (TEM) is utilized for examining the morphological characteristics of BEVs. h Finally, BEVs are stored under appropriate conditions. (Created with biorender.com)
BEVs in RM
Overview of RM
Advancements and challenges in RM
RM is a comprehensive concept that employs various methodologies based on biological and engineering principles to repair and regenerate tissues and organs with compromised functionality. The objective is to restore these structures to normalcy in both form and function, ultimately enhancing the quality of life of patients [116]. Modalities such as organ transplantation, tissue engineering, stem cell therapy, miRNA therapy, and the like fall within the purview of RM [117].
Currently, the RM field is replete in terms of opportunities and challenges. When addressing the treatment of specific diseases, the issues of efficiency and safety have emerged as the most challenging facets within the realm of RM [118]. Stem cell therapy, a crucial component of RM, has been extensively researched for several years [119]. However, allogeneic stem cells face the challenge of immune system elimination, requiring the use of immunosuppressants by patients to overcome this hurdle. This practice results in significant side effects, including severe complications such as infections [120]. Although the use of autologous stem cells circumvent these drawbacks, factors such as high cost and low production efficiency impede their clinical applicability [121]. Furthermore, the clinical application of stem cell therapy has resulted in various side effects, including tumor formation, immunological rejection, and genetic instability [120, 122]. In conclusion, the pivotal challenge in the therapeutic application of stem cell therapy within the field of RM currently lies in the lies in the biosafety, complex operation, and high-cos.
Thus, EVs are promising alternatives to whole-cell therapy. MSC-hEVs deliver miRNAs to target cells, thereby promoting wound healing in diabetic rat models [123]. Although engineered EVs from stem cells have demonstrated high targeting capabilities and therapeutic efficacy, the low extraction efficiency and complexity of engineering modifications limit the clinical application of MSC-hEVs [124]. Therefore, the current field of RM urgently requires a safe and efficient therapeutic approach to address the limitations associated with both stem cells and extracellular vesicles.
How engineered BEVs address the current challenges in RM
Engineered BEVs are regarded as next-generation drug delivery platforms owing to their outstanding characteristics, including stable payload capacity, unique nanoscale structure, and excellent biocompatibility [125]. The current shortcomings in drug delivery materials within the field of RM persist. Synthetic nanomaterials, despite achieving significant success in preclinical trials, face challenges such as low biocompatibility, material-related toxicity, and high production costs, thereby limiting their specific clinical applications [126, 127]. With an in-depth understanding of bacteria-based drug delivery systems, the application of BEVs in RM has emerged as a novel trend. Bacteria exhibit high yields and use mature cultivation methods. Compared with MSC-hEVs, the engineering modification of bioactive BEVs is relatively simpler [128]. Additionally, the direct extraction of BEVs from bacteria effectively circumvents the medical and ethical concerns associated with stem cells or other RM therapies. It also eliminates cellular mutation issues and immune rejection reactions commonly associated with stem cells [129]. We have summarized the current engineering methods of BEVs that may be applied in RM, and the results are shown in Table 3.
Table 3.
The currently available BEV engineering strategies for RM
| Engineering methods | BEVs from | Biological effects | Refs. |
|---|---|---|---|
| Membrane-coated | E. coli K1 | Cross the blood–brain barrier | [130] |
| Membrane-coated | L. animalis | Ultrasound-driven anti-infection | [131] |
| Membrane-coated | E. coli Nissle 1917 | Anti-inflammatory | [132] |
| Incubation | E. coli Nissle 1917 | Anti-inflammatory | [133] |
| Incubation | E. coli ATCC 25922 | High yield and purity | [134] |
| Electroporation | E. coli K12 | Promoted neural repair | [135] |
| Electroporation | P. aeruginosa | Drug loading | [136] |
| Electroporation | E. coli Nissle 1917 | Bone targeting ability | [128] |
| Synthetic biology technology | E. coli Nissle 1917 | Bone targeted osteogenic effect | [137] |
| CRISPR-Cas9 | E. coli Nissle 1917 | Gene therapy | [138] |
| CRISPR/Cas9 | E. coli MG1655 | Non-inflammatory | [59] |
| Recombinant DNA technology | E. coli MG1655 | Targeting and bactericidal properties | [139] |
| Recombinant DNA technology | E. coli K12 | Improved biocompatibility | [140] |
| Ultrasound | E. coli | High yield and low immunogenicity | [38] |
| Single emulsion evaporation | E. coli DH5α | Sonodynamic therapy | [39] |
Surface engineering of BEVs enhances their targeting characteristics and increases the local drug concentration in target tissues [141], thereby reducing side effects and maximizing therapeutic efficacy. Simultaneously, certain BEVs, owing to their unique biological properties, traverse the intestinal and blood–brain barriers [142], facilitating drug delivery to sites that are challenging for conventional carriers. This offers an effective vehicle for regenerative therapies targeting specific locations.
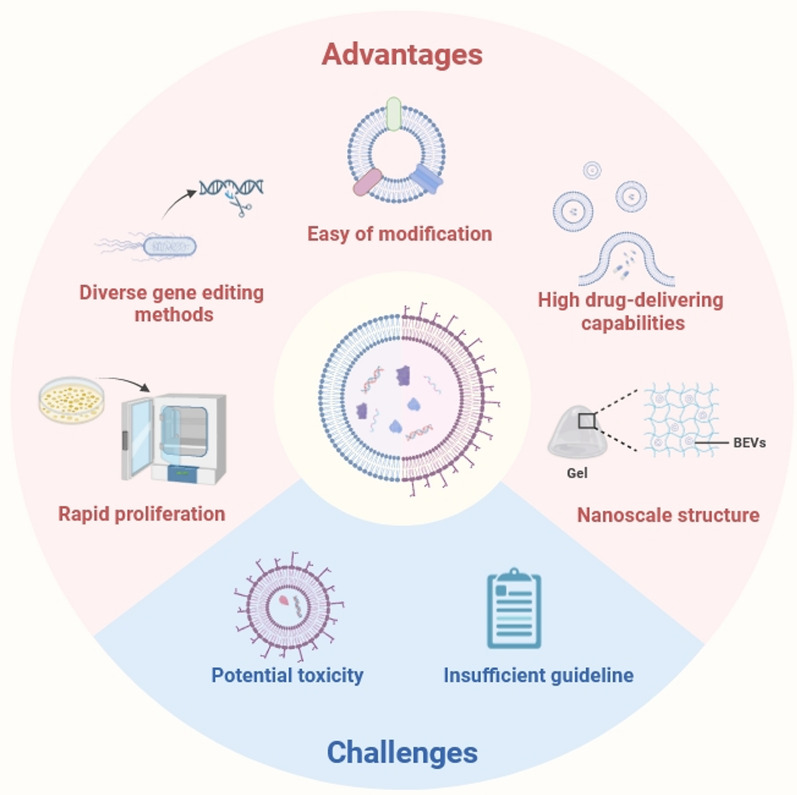
The internal engineering modification of BEVs enables them to carry drugs or active substances that promote regeneration, effectively protecting the biological effects of the payloads [143]. It is worth noting that the potential toxicity of endogenous proteins in BEVs is one of the major challenges hindering their use in RM [144]. The application of the CRISPR-Cas9 system can reduce the quantity of endogenous proteins in BEVs while relatively increasing their internal loading space, thereby reducing the inherent toxicity and enhancing their loading capacity [144]. Other engineering methods, such as protein display technology, sonication, and freeze–thaw techniques, can also be employed to modify natural BEVs [145, 146]. Recently, the concept of synthetic biology has been introduced, which facilitates the rational design of complex biological systems [147]. This can serve as a reference for constructing engineered BEVs, combining the aforementioned engineering methods in a scientific and efficient manner to address the limitations of natural BEVs. As a result, BEVs have the potential to become one of the safe and effective drug carriers in the field of RM (Fig. 4).
Fig. 4.
Advantages and main challenges of bacterial extracellular vesicles (created with biorender.com)
Integration of BEVs with tissue engineering
Tissue engineering is an emerging interdisciplinary field that combines cell biology and engineering to construct tissues or organs and serves as a burgeoning discipline to substitute for natural tissues or organs. As a pivotal branch of RM, it has achieved success in the regenerative treatment of organs [148]. In recent years, rapid progress has been made in the application of bioactive materials in tissue engineering. Hydrogel materials with a structure resembling that of the natural extracellular matrix are commonly acknowledged as promising biomaterial scaffolds in tissue engineering because of their potential for tissue regeneration [149]. They simulate the extracellular matrix space, providing a suitable three-dimensional environment for various cellular functions, including adhesion, migration, proliferation, and differentiation.
Interestingly, because of the nanoparticulate nature of EVs, they can be more effectively loaded onto bioactive materials than into cells [150]. When the hydrogel scaffold loaded with engineered BEVs reaches the target site, the encapsulated engineered BEVs undergo a slow release. This controlled release mechanism governs the therapeutic scope of drugs, prolongs their efficacy, and ultimately leads to more effective tissue repair. Reka et al. observed that employing 3D tissue culture enhances EV production [151], thereby potentially further increasing the BEVs yield. Benefiting from the characteristics of bioactive scaffold materials, the utilization of 3D bioprinting technology to load engineered attenuated strains or low-toxicity strains, such as probiotics, onto scaffold materials provides a sustained supply of beneficial BEVs [152]. The application of this technique in regenerative therapy represents a broad prospect for tissue engineering.
BEVs regulatory processes in RM
BEVs administration pathways
To ascertain the safety of engineered BEVs for application in RM, it is of utmost importance to investigate the specific administration processes and underlying mechanisms. Current administration strategies for BEVs can be categorized as systemic or local. Systemic approaches include intravenous injection, oral administration, and intranasal delivery, whereas local routes involve direct injection of BEVs suspensions or loading BEVs into biomaterials [153–155].
Current treatments for systemic diseases based on EVs primarily rely on intravenous injections. Intravenous administration is the fastest and most widely applied method of drug delivery in this context [156]. Simultaneously, local administration is less practical for certain deep-seated tissues. This challenge can be addressed by engineering the surfaces of BEVs to enhance their targeting characteristics. Alternatively, additional measures, such as photodynamic therapy can be employed to achieve systemic delivery while limiting the impact on specific local tissues [154], thereby significantly reducing the potential side effects of the administered drugs. However, intravenous injection poses a challenge to drug clearance, limiting the ability of BEVs to reach the target site and exert therapeutic effects [157]. Further research is required to address this issue. Engineered BEVs exhibit certain adverse effects. Feng et al. observed that the direct injection of high concentrations of BEVs into the vascular system may lead to fatal outcomes [156]. Therefore, when employing intravenous injection as the route of administration, strict control of the dosage and purity of BEVs is imperative.
Oral administration is a relatively safe therapeutic approach that enhances patient compliance. Jones et al. discovered that BEVs could be internalized by intestinal epithelial cells and sorted into endolysosomal vesicles [153]. Subsequently, they traverse the intestinal epithelium to enter the systemic circulation. During this process, BEVs safeguard the internal cargo, allowing it to reach organs, including the brain, through the bloodstream [135, 158]. This demonstrates the possibility that BEVs enter the systemic circulation through oral administration, exerting their effects on various tissues and organs. Although BEVs traverse the gastrointestinal barrier in healthy individuals, the presence of gastrointestinal enzymes and the low pH environment still pose obstacles to the oral administration of BEVs [159]. Liu et al. developed a dopamine polymerization-mediated decoration method to protect probiotics from the influence of gastric acid and bile salts [132]. Similar designs may potentially apply to BEVs to enhance their oral efficiency and augment their effectiveness in the gastrointestinal context. Moreover, when intestinal epithelial cells are designated as target cells, oral administration is deemed appropriate.
A localized administration route is highly beneficial for targeted therapy of specific tissues. By anchoring BEVs to biomaterials, the scope of BEVs action can be controlled, minimizing potential systemic adverse reactions caused by the drug [160]. This approach aims to achieve sustained release, thereby improving therapeutic efficacy. More importantly, the bioscaffold material not only offers adhesion for surrounding cell regeneration but also serves as a supportive structure by providing a filling effect at the defect site. This, in turn, provides the necessary space for tissue regeneration [161]. The integration of advanced multiphase biomaterial scaffolds with the temporospatial release of BEVs holds significant promise for a wide range of applications [162]. This approach provides sustained and tailored physiological stimuli to achieve long-term regenerative effects.
Internalization of BEVs
The internalization of BEVs is noteworthy once they reach the targeted administration site through various pathways. Specific internalization mechanisms may vary depending on the cell or BEVs type involved. Several primary pathways for the internalization of BEVs have been proposed [163]. These include phagocytosis, macropinocytosis, membrane fusion, caveolae-mediated processes, and clathrin-mediated endocytosis, which are facilitated by grid proteins. These internalization mechanisms are not mutually exclusive; they can coexist simultaneously [164]. In particular, endocytosis is considered the primary mode by which mammalian host cells take up BEVs.
The internalization of BEVs is influenced by various factors, with size as a contributing element. Lorinda et al. observed that BEVs ranging from 20 to 100 nm preferentially undergo caveolae-mediated endocytosis to enter epithelial cells [165]. In contrast, BEVs with sizes between 90 and 450 nm enter host epithelial cells through macropinocytosis and endocytosis. In addition, proteins or LPS carried on the surface or within BEVs may interact with or bind to receptors present on lipid rafts [166]. This interaction can facilitate the adhesion and entry of BEVs. For instance, BEVs possessing O-antigens enter cells through a TLR2-mediated lipid raft-dependent pathway, whereas BEVs lacking O-antigens are internalized via clathrin-mediated endocytosis facilitated by grid proteins [47, 167].
In addition to the intrinsic factors of BEVs, the host cell type also affects BEVs internalization. In immune phagocytic cells such as neutrophils, dendritic cells, and macrophages, the primary pathway for BEVs internalization is phagocytosis [168]. In non-phagocytic cells such as epithelial cells, BEVs can be internalized through macropinocytosis, clathrin-mediated endocytosis facilitated by grid proteins, and lipid raft-mediated processes [169, 170]. After entering host cells, the cargo of BEVs are released through pathways mediated by early endosomes. Different cellular responses are then induced based on the composition and quantity of the cargo. Furthermore, BEVs directly interact with cell surface receptors, inducing diverse cellular responses through various signaling pathways [156].
In conclusion, different target cells and BEVs may be involved in distinct internalization and mechanisms of action. This aspect should be considered carefully when applying BEVs to RM. Designing BEVs with appropriate sizes and characterization for specific cell types is crucial for achieving optimal therapeutic effects.
Engineering strategies of BEVs
Intercellular communication is a crucial pillar of both tissue engineering and RM. The messenger role of EVs in intercellular communication has sparked significant interest in the potential applications of EVs in tissue engineering and RM [171]. BEVs influence host functions by transmitting information and crucial molecules such as proteins, nucleic acids, and lipids [172]. Therefore, employing various engineering strategies to modify BEVs, altering their contents or surface-associated proteins, enables BEVs to exert diverse functions. Subsequently, these modified BEVs are synthesized with composite multifunctional biomaterials with specific biological functionalities [88, 173]. Importantly, the unique biological mechanisms of BEVs, such as vesicular cell death and explosive cell lysis, enable BEVs to carry molecules from the surrounding environment during their formation [174]. This provides a convenient pathway for drug loading in the context of BEVs.
Currently, the modification strategies for BEVs fall into two main categories. The first involves the modification of the parental bacteria to obtain engineered strains, thereby generating customized BEVs. The second category entails various engineering modifications applied to BEVs to achieve customized functionality. In addition, a combination of these two approaches is feasible, wherein further engineering modifications can be applied to BEVs produced by engineered bacterial strains, resulting in BEVs with specific functionalities.
Utilization of BEVs generated by engineered bacteria in RM
Engineered bacteria generate BEVs with diminished toxicity
Previously, the mitigation of BEVs toxicity induced by LPS often involved treatment with detergents, such as deoxycholate and Brij-96 [175]. However, this method may damage the lipoproteins on the surface of BEVs, causing disruption of their natural characteristics [176]. The CRISPR-Cas9 system or λ-red recombination engineering system is a crucial tool for modifying bacterial genes and altering the characteristics of BEVs [177]. The deletion of certain bacterial genes enhances their natural characteristics for better application in RM. The absence of specific genes does not compromise the integrity and stability of bacterial membranes [178]. This ensures that the removal of BEVs toxicity does not impair its biological properties.
Thomas et al. utilized the λ-red recombination engineering system to develop hypervesiculating E. coli Nissle [140]. This engineered strain produced BEVs with enhanced targeting characteristics and improved biocompatibility, making it an excellent carrier. Similarly, the knockout of genes, such as msbA, msbB, lpxLl, and lpxM, or the overexpression of genes encoding lipid A deacylase in gram negative bacterial strains yields low toxicity BEVs [59, 179–181]. This results in the production of efficient and safe BEVs carriers, subsequently loaded with specific contents to form multifunctional BEVs that play a crucial role in RM.
Engineered bacteria generate BEVs with specific functionalities
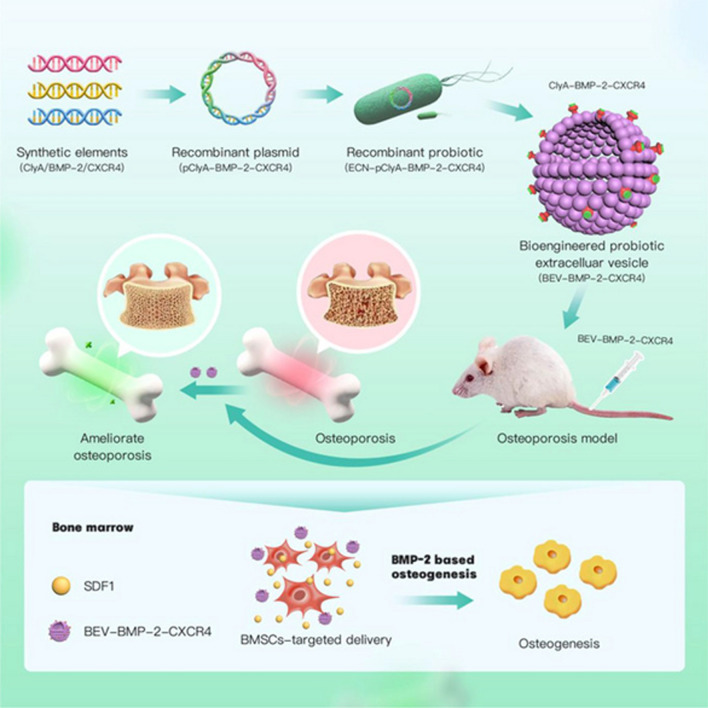
The latest advances in genetic engineering have made it possible to modify the natural cell membrane structure to generate more targeted cell membranes, laying the foundation for the design of BEVs based on synthetic biology [147]. Through engineered modifications of the parental bacterial strain, exogenous proteins or antigenic peptides can be present on the surface of BEVs to achieve specific functionalities. Zhao et al. employed recombinant DNA technology to display various antigens on BEVs to facilitate natural immune responses [182]. Su et al. constructed a recombinant probiotic EcN-pET28a-ClyA-BMP-2-CXCR4, where the surface protein ClyA of their BEVs was fused and overexpressed with BMP-2 and CXCR4 [137]. This formulation exhibited excellent bone-targeting properties and facilitated bone function in vivo, demonstrating the potential of BEVs in bone regeneration therapy (Fig. 5).
Fig. 5.
The engineered Escherichia coli Nissle 1917-pET28a-ClyA-BMP-2-CXCR4, generated through genetic recombination techniques, is capable of displaying BMP-2 and CXCR4 on the surface of its produced Bacterial Extracellular Vesicles (BEVs), exhibiting excellent bone-targeting and osteogenic properties [137].
Reproduced with permission from Wiley Publications, copyright 2024
Overexpression of specific genes generates BEVs with specific functionalities. Lu et al. used lactobacilli capable of overexpressing the vascular endothelial VEGF to create a delivery system [183]. This delivery system confines the bacteria to the wound site and promotes angiogenesis in the surrounding injured tissue [184]. Host–bacterial communication reliant on the secretion of BEVs. This suggests that the regenerative effects observed in this delivery system are likely attributable to the BEVs produced by lactobacilli. This review enumerates bacteria that have been discovered to possess regenerative functions. It is conceivable that BEVs with corresponding functionalities can be generated through engineered modifications of these bacteria. This serves as a reference for the potential application of engineered BEVs in RM and the specific investigation of their mechanisms of action (Table 4).
Table 4.
Selected bacteria with regenerative function
| Functions | Bacteria | Related cells or targets | Vitro or vivo | Refs. |
|---|---|---|---|---|
| Skin regeneration | Staphylococcus aureus | Keratinocytes, IL-1R-MyD88 signaling | Vitro and vivo | [185] |
| Wound healing | Lactobacillus delbrueckii | Fibroblast cells, TGF-β1, FAK | Vitro | [186] |
| Staphylococcus epidermidis | CD8+ T cells, TH17, CXCL 10, IFN | Vitro and vivo | [187] | |
| Lactobacillus plantarum | Phagocyctes, AHLs | Vitro and vivo | [188] | |
| Pseudomonas mendocina | Fibroblasts cells, endothelial cells | Vitro and vivo | [189] | |
| Bone regeneration | Bifidobacterium longum | IL-10, OclnOCLN gene, VEGFa | Vivo | [190] |
| Lactococcus lactis | Mesenchymal stem cells, BMP-2 | Vitro | [191] | |
| Propionibacterium acnes | Mesenchymal stem cells, TLR2 | Vitro and vivo | [192] | |
| Lactobacillus casei | IL-6, IL-10, NF-κB, TNF-α | Vivo | [193] | |
| Cartilage regeneration | Bifidobacterium adolescentis | Callus osteoclasts, IL-16, IL-6 | Vivo | [194] |
| Follicle regeneration | Staphylococcus aureus | Keratinocytes, HIF-1α, IL-1β | Vitro and vivo | [195] |
| Lactobacillus casei | Macrophages, TNF-α, IL-6 | Vitro and vivo | [196] | |
| Nerve regeneration | Mycobacterium leprae | Schwann cells, Twist-1, Sox 2, Msx2 | Vitro and vivo | [197] |
| Intestinal regeneration | Early-life microbiota | Intestinal stem cell, Erdr1, Wnt signaling | Vitro and vivo | [198] |
| Lactobacillus reuteri | Intestinal epithelia, R-spondins | Vitro and vivo | [199] | |
| Stem cell proliferation | Lactobacillus rhamnosus | Intestinal stem cells, IL-17, NF-κ B | Vitro and vivo | [200] |
| Remyelination | Bifidobacterium breve | HO-1, Nrf-2, Olig2 | Vivo | [201] |
| Liver regeneration | Mycobacterium leprae | Wnt/β-catenin signaling, FOXA1/2, HGF | Vivo | [202] |
| Angiogenesis | Synechococcus elongatus | Keratinocytes and fibroblasts, IL-6 | Vitro and vivo | [203] |
| Akkermansia muciniphila | Type H vessels, M-CSF, CSF1 | Vivo | [204] | |
| Cardiac repair | Lactobacillus probiotic | Myeloid cells, SCFA | Vivo | [205] |
| Re-epithelialization | Streptococcus thermophilus | Human immortal keratinocyte line, NOS2 | Vitro | [206] |
| Stem cell mobilization | Bacillus coagulans | Immune cells, G-CSF, IL-1β | Vitro | [207] |
| Stem cell migration | Lactobacillus acidophilus | Mesenchymal stem cells, VCAM-1, VEGF | Vitro | [208] |
Engineered bacteria improve the yield of BEVs
Differential gene expression affects the BEVs yield. For instance, a comparative study between two strains of P. aeruginosa, PA01 and PA14, under identical growth conditions reported a significantly higher production of BEVs by PA14 than by PA01 [74]. The yield of BEVs can be increased through overexpression or silencing of respective genes. Specific mutations associated with the attenuation of OM-PG connections have been identified to improve the yield of BEVs. These mutations include those in the OmpA, rmpM, and LPP genes [209–211].
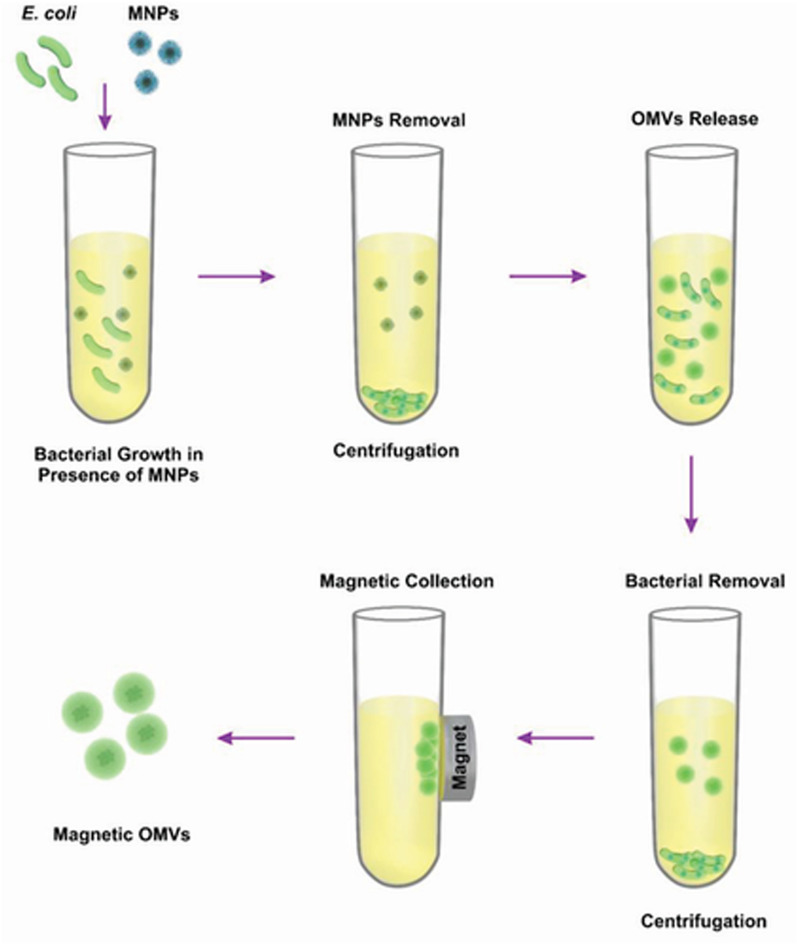
Building upon this foundation and incorporating the optimization of culture conditions further enhances the production yield of BEVs. This advancement has the potential to address the challenges of clinical translation posed by an insufficient EVs yield. Shi et al. cultured E. coli in the presence of magnetic iron oxide nanoparticles (MNPs), resulting in the formation of BEVs containing MNP [134]. The BEVs yield obtained by magnetic harvesting was 60 times higher than that obtained by ultracentrifugation. In addition to the enhanced yield, the BEVs obtained using this method can also leverage magnetic targeting to specific sites (Fig. 6).
Fig. 6.
A method for enhancing the yield and purity of bacterial extracellular vesicles (BEVs) through co-cultivation of E. coli with magnetic iron oxide nanoparticles (MNPs) and utilizing magnetic force for harvesting [134].
Reproduced with permission from Wiley Publications, copyright 2022
Although engineered bacteria yield reusable parental strains, significantly enhancing production efficiency, rigorous testing of various properties of the final product is essential. This ensures that their physicochemical properties and biological safety, among other aspects, remain unchanged during large-scale production processes.
Engineering of isolated BEVs for applications in RM
In addition to engineering the parental bacterial strains, engineering modifications can also be applied to isolated BEVs. This strategy helps circumvent potential issues related to environmental changes during bacterial cultivation [212]. Furthermore, it represents a more direct and stable approach to obtaining functionalized BEVs. Current strategies for modifying BEVs are still in the exploratory phase, whereas modifications of mammalian EVs (MEVs) have reached a relatively mature stage. Considering the similar membrane structures of BEVs and MEVs, this review introduces the engineering methods that have been applied to MEVs and BEVs.
Incubation
Drug loading into BEVs can be achieved through incubation, which represents a relatively convenient engineering strategy. This method is typically employed to load small hydrophobic molecules with a positive charge, as these molecules can traverse the lipophilic membrane [213]. Under special circumstances, the stability of the membrane can be altered by modifying the incubation environment strategy, thereby allowing the entry of other biomolecules into BEVs [214]. In a previous study, the photosensitizer Ce6 and the chemotherapeutic drug doxorubicin were encapsulated within BEVs [215]. Subsequently, a direct incubation approach was employed to load them into macrophages for laser-triggered release, combining phototherapy, chemotherapy, and immunotherapy.
Similarly, Yao et al. introduced plasmid-liposome complexes into a solution containing EVs and incubated them at 37 °C for 12 h, yielding engineered EVs [216]. These engineered EVs were successfully utilized for delivery into MSCs using the CRISPR-Cas9 system, offering a potential avenue for in vivo gene editing procedures. Co-incubating liposomes loaded with target molecules and EVs to generate engineered EVs represents a novel loading strategy for drugs that face challenges in penetrating the EV membrane. By leveraging the biological characteristics of liposomes and lipophilic membranes of EVs, this approach offers an improved means of achieving drug loading.
Saponification
Saponins induce the formation of small pores on the lipid membrane surface [217], in a process referred to as saponification. Saponification can assist in loading cargo into EVs, thereby enhancing the loading efficiency. Importantly, this process does not alter the size and zeta potential of EVs, thereby facilitating the preservation of their inherent biological characteristics [218, 219]. However, it is essential to note that the use of saponins for assisted engineering modification of EVs should be approached with caution because of their inherent toxicity [220]. Following this manipulation, it is advisable to eliminate any residual impurities from the solution to ensure the safety of the final product.
Physical methods
Physical methods for engineering EVs include electroporation, ultrasound, extrusion, freeze–thaw, and other techniques. These methods disrupt the integrity of the membrane and facilitate the entry of cargo into the EVs.
Electroporation is primarily employed for loading hydrophilic molecules such as DNA and RNA. The loading efficiency of this method depends on the sizes of both the cargo and EVs [221]. Larger EVs often have the capability to encapsulate a greater amount of linear and plasmid DNA. Electroporation inserts nanoparticles of any size below 10 nm into P. aeruginosa-derived BEVs [136]. This finding serves as a reference for efficient drug loading. Importantly, this method does not compromise the biological properties of BEVs [222], thereby facilitating the preservation of membrane activity.
Extrusion primarily utilizes fluid pressure to disrupt membrane integrity, allowing drugs to enter during vesicle formation. This method yields a higher production rate of engineered BEVs. However, challenges such as size heterogeneity exist, and the process may potentially have negative effects on the membrane structure [223]. Currently, research has shown that there are differences in the composition and subsequent biological effects between BEVs produced by extrusion and those naturally secreted by bacteria [224]. Therefore, further assessment of their safety effects should be conducted after production.
Ultrasound has been employed for the engineering modification of BEVs. Park et al. conducted high-pH solution treatment of BEVs from E. coli, which led to the separation of membrane fragments from cellular components [38]. Subsequent ultrasound treatment of the membrane fragments resulted in the formation of spherical synthetic cell-derived vesicles. BEVs produced through this method exhibit similar morphology and size to natural BEVs, demonstrating lower cytotoxicity and the ability to induce appropriate immune responses.
The combined application of physical methods and other engineering approaches can further enhance the drug loading efficiency. Haney et al. employed ultrasound, extrusion, and freeze–thaw techniques separately to incubate EVs [225]. The results demonstrate that the combined use of these techniques with incubation effectively increased the cargo-loading efficiency of EVs. Notably, engineered EVs treated with ultrasound and extrusion exhibit the highest levels of activity.
Chemical methods
In addition to the methods mentioned above, engineering modifications of the BEVs outer membrane proteins can be achieved through two categories of chemical methods: covalent and non-covalent modifications.
Commonly employed strategies for covalent modification include click chemistry, aldehyde-amine condensation, and amidation. Click chemistry involves conjugating targeting ligands, such as antibodies and peptides, to the membrane surface of EVs to enhance their ability to target specific cells [226]. This method offers advantages such as rapid reaction time, mild reaction conditions, and high specificity. Importantly, during the modification process, the stability of the EVs size is maintained. However, excessive modification of EV surfaces may inhibit the functionality of EV proteins. Therefore, during processing, consideration should be given to whether the activity of the target functional proteins is altered [227]. Aldehyde-amine condensation and amidation enable the conjugation of EVs with aptamers. Aptamers, short oligonucleotides or peptides with high specificity and affinity, can be attached to EVs using these reactions [228]. Upon binding with aptamers, EVs exhibit excellent targeting capabilities, making them suitable for precise diagnostics or personalized therapy. However, natural BEVs lack specific biomarkers, which hinders smooth conjugation reactions. This requires further foundational research to uncover additional potential binding sites on BEVs surfaces. In addition, whether covalent modification affects other sites on BEVs surfaces requires further elucidation, as this has implications for the safety, efficacy, and biocompatibility of the product.
Non-covalent modifications primarily involve altering the receptors or proteins on the surface of EVs through electrostatic interactions, receptor-ligand binding, or other methods. Multivalent electrostatic interactions exploit the characteristic negative zeta potential of EV surfaces, allowing the binding of highly charged cations to the negatively charged EV surface [229]. Zhan et al. developed a multifunctional delivery system by coupling magnetic molecules and the endosome-disruptive peptide L17 E to the membrane of EVs through ligand-receptor coupling and electrostatic interactions [230]. This system exhibits enhanced tumor-targeting characteristics and improved endosomal escape capability, demonstrating significant inhibition of tumor growth.
The removal of residual materials after engineering modification is also a critical issue [231]. This requires the further separation and purification of BEVs. Subsequent characterization of engineered BEVs was performed using TEM and WB. These methods detect the expression of target proteins to ensure that membrane integrity and activity are maintained.
Clinical prospects of engineered BEVs in RM
Although the specific applications of BEVs in RM are currently limited, they have substantial potential. This review aims to analyze how future applications of BEVs may be applied in RM.(Fig. 7).
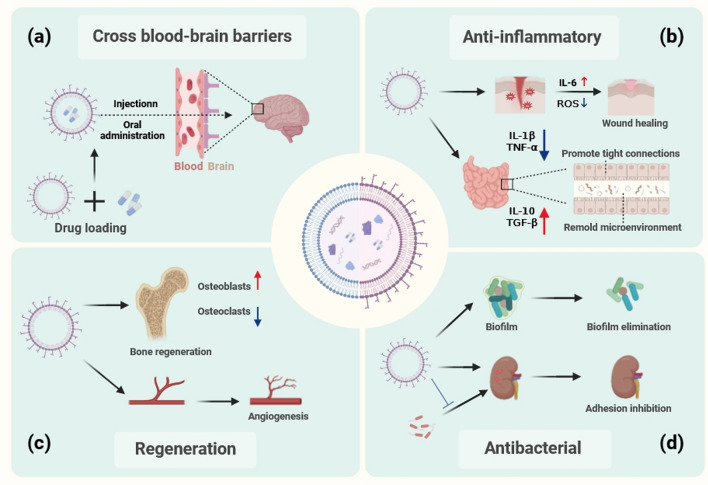
Fig. 7.
Clinical prospects of engineered Bacterial Extracellular Vesicles (BEVs) in regenerative medicine. a BEVs possess the ability to traverse the blood–brain barrier, serving as effective carriers to transport drugs. b BEVs exhibit anti-inflammatory properties and can ameliorate the local inflammatory microenvironment. c BEVs have the capability to directly promote tissue regeneration. d BEVs possess antibacterial properties, capable of inhibiting bacterial adhesion and proliferation during tissue regeneration processes. (created with biorender.com)
Antibacterial properties of BEVs
Bacterial infection adversely affects the regenerative process in the tissue repair environment of implantable biomaterials, and in severe cases, may even pose a threat to life [232]. There is an urgent need for a novel antibacterial strategy to address the widespread issue of antibiotic misuse and resistance. BEVs of certain bacteria can prevent the formation of bacterial biofilms by inhibiting bacterial adhesion [65]. This is crucial for applications in implant materials and organ transplantation. The formation of biofilms exacerbates bacterial infections, potentially leading to bacteremia [233]. This is detrimental to the survival of transplanted organs and ultimately results in the failure of organ transplantation surgeries.
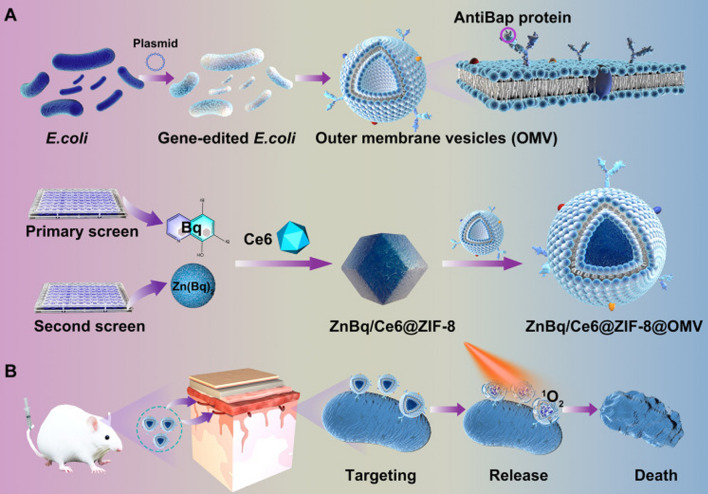
More importantly, BEVs have been demonstrated to be applicable for targeted antibacterial therapy. Yuan et al. obtained an msbB mutant E. coli strain through genetic editing and fused the ClyA encoding region with a targeting antibody fragment to produce BEVs capable of targeting A. baumannii. Furthermore [139], by subsequently coating the engineered BEVs onto the surface of ZnBq/Ce6@ZIF-8, a novel nanomedicine was developed, demonstrating excellent targeting and antibacterial performance in both in vitro and in vivo experiments (Fig. 8). In summary, the strategy of using engineered BEVs for specific targeted antibacterial activity holds great potential for enhancing the efficacy of various antimicrobial agents.
Fig. 8.
By employing genetic editing techniques, bacterial extracellular vesicles were obtained with targeting functionality towards A. baumannii, and further combination with metal–organic frameworks materials exhibited excellent in vivo targeting and bactericidal properties [139].
Copyright © 2024, The American Association for the Advancement of Science
Anti-inflammatory properties of BEVs
Wound healing typically involves four steps: hemostasis, inflammation, proliferation, and remodeling. These processes must occur within the appropriate sequence and timeframe. Prolonged inflammation hinders wound healing [234]. BEVs produced by certain probiotic bacteria in the intestinal tract can alleviate inflammation. The anti-inflammatory properties of BEVs may facilitate wound healing. BEVs produced by Akkermansia muciniphila can improve colitis symptoms by modulating the immune environment in intestinal tissues and regulating the production of the proinflammatory cytokine IL-6 [235]. Similarly, Hao et al. discovered that BEVs produced by Synechococcus elongatus PCC 7942 may induce vascularization and wound healing by promoting the expression of IL-6 [203]. Importantly, BEVs formulations exhibit enhanced activity compared with bacterial cultures, demonstrating a superior capability to facilitate wound healing.
After appropriate engineering modifications, the BEVs of these bacteria may be better utilized in RM. For instance, Li et al. successfully utilized BEVs secreted by EcN to encapsulate manganese dioxide nanozymes. These engineered BEVs adhere to inflamed epithelia [133], eliminate excessive reactive oxygen species in the intestinal tract, reshape the intestinal microenvironment, and demonstrate superior therapeutic effects compared with commercial inflammatory bowel disease drugs. Therefore, it can serve as an effective platform for treating these diseases.
Regenerative properties of BEVs
Direct utilization of the innate properties of BEVs to promote tissue repair and regeneration is a relatively convenient approach. The natural bone-targeting characteristics and osteogenic properties of certain BEVs have garnered attention. The BEVs produced by Proteus mirabilis significantly affect apoptotic signaling pathways and mitochondrial function, exerting a bone-protective role through the inhibition of osteoclastogenesis and bone resorption [236]. The intestinal-bone axis effect mediated by gut microbiota BEVs offers possibilities for the utilization of intestinal strains, especially probiotics, in bone regeneration therapy. Liu et al. used engineered BEVs derived from EcN for siRNA delivery [128]. These BEVs demonstrated robust bone-targeting capabilities and promote the osteogenic differentiation of bone marrow mesenchymal stem cells, ultimately reversing osteoporosis. These therapeutic approach, based on engineered bacteria or BEVs, offers novel strategies for RM.
BEVs properties related to traversing the blood–brain barrier
The presence of the blood–brain barrier hinders the entry of most drugs into the brain, posing significant challenges for the treatment of many neurological disorders [237]. Interestingly, certain BEVs possess the ability to traverse the blood–brain barrier [130], serving as effective carriers to transport drugs across the barrier and achieving breakthrough advancements in regenerative therapy for neurological disorders. Recently, Pan et al. encapsulated pioglitazone (PGZ) in BEVs to form engineered BEVs@PGZ nanocarriers [135]. These particles inherit the biological characteristics of BEVs, demonstrating their ability to traverse the blood–brain barrier, be engulfed by neutrophils, and suppress ferroptosis while inhibiting nucleotide oligomerization domain-like receptor protein 3 inflammasome activation. The effective mitigation of reperfusion injury ultimately plays a neuroprotective role and promotes neural repair.
Challenges and prospects
Issues in the regulatory framework
Owing to the advantages of BEVs in drug loading, production efficiency, and cell-free therapy, there is promising potential for their application in RM. However, before widespread application, numerous challenges must be overcome. Standardization of production and isolation methods is a critical issue that needs to be addressed for the clinical application of BEVs. It is necessary to standardize the manufacturing processes of BEVs to ensure that laboratories obtain relatively consistent test results. This standardization is advantageous for subsequent drug production and development. The standardization of BEVs isolation, characterization, and dosing is imperative. As mentioned earlier, variations in isolation methods, operational standards, and even instrument software can result in inconsistent detection outcomes for the final product, hindering reproducibility.
Despite the existence of various separation methods for BEVs, most of them involve centrifugation and ultrafiltration, which require significant amounts of time. Conversely, commercially available assay kits, although more convenient, present challenges such as high cost and low purity. Further research on the foundational mechanisms of BEVs formation is required to develop more efficient separation and extraction protocols. Standardized parameters and guidelines of BEVs can serve as fundamental references for diverse laboratories. For example, the Minimal Information for Studies of Extracellular Vesicles was published by the International Society for Extracellular Vesicles [238]. This can reduce variations in detection outcomes arising from different methodologies. Furthermore, the establishment of optimal administration routes tailored to specific therapeutic goals to achieve rational biodistribution remains to be addressed. This necessitates the consideration of the metabolic aspects of BEVs and the efficiency of BEVs absorption by target cells.
Toxicity concerns associated with natural BEVs
The inherent toxicity and immunogenicity of natural BEVs constitute the primary challenges in the development of BEVs-based drug delivery systems. Despite the enhanced safety of the delivery system facilitated by BEVs produced from attenuated and low-toxicity bacterial strains, the potential side effects remain a significant concern. Nevertheless, a substantial number of disease treatments based on BEVs have yielded significant efficacy. This also attests to the fact that the intravenous or oral administration of a certain dose of BEVs does not elicit conspicuous toxicity. It is noteworthy that BEVs at appropriate concentrations not only do not induce cytotoxic responses but also exert beneficial immunostimulatory effects. Significant progress has been made in bone-regeneration therapies using BEVs. At present, strategies to mitigate the toxicity of BEVs include the manipulation of parent strains through systems based on CRISPR-Cas9 or λ-red recombination engineering. Alternatively, direct treatment of BEVs with high lysozyme and high pH or encapsulation with pH-sensitive calcium phosphate attenuates the toxicity of BEVs [239].
Another issue lies in the current focus on BEVs derived from gram negative bacteria, with relatively limited exploration of BEVs from gram positive bacteria. This disparity may be partly attributed to the thicker and single-layered cell wall structure of gram positive bacteria, which may impede BEVs production and result in comparatively lower yields. However, gram positive bacteria-derived BEVs lacking lipopolysaccharides significantly reduce the likelihood of safety concerns. Furthermore, the BEVs generated during biofilm growth may represent a potential avenue for acquiring low-toxicity BEVs. BEVs produced by bacteria within biofilms, in comparison to planktonic bacteria, lack cytoplasmic proteins and toxic factors. Moreover, biofilm-derived BEVs are approximately half the size of planktonic BEVs (with diameters of 45 and 86 nm, respectively) [240, 241]. These distinctive characteristics of biofilm-derived BEVs may present additional potential applications in RM. Implementing the aforementioned strategies to further attenuate the toxicity of BEVs will significantly enhance their applicability in RM.
Author contributions
JG and ZH designed the content and wrote the first draft; QW, JG and ZT produced the figures; YH, MH and HL produced the table; JG, MW, WC and YM revised the manuscript. BJ were responsible for supervision of the entire project as the corresponding author.
Funding
This work was supported by the Science and Technology Project of Guangzhou City (201802020018), the Guangdong Science and Technology Program (2019A1515010408), Clinical New Technology Project of Southern Medical University Stomatological Hospital (NTP202304), Key Project of Guangdong Province General Higher Education in Key Areas (2023ZDZX2009) and Science research cultivation program of stomatological hospitalSouthern medical university (PY2022015).
Availability of data and materials
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiming Guo and Zhijie Huang have contributed equally.
References
- 1.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Wang X, Wang Z, Deng L, Wang Y, Tang Y, Luo L, Leung EL. Extracellular vesicles as a novel mediator of interkingdom communication. Cytokine Growth Factor Rev. 2023;73:173. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L, Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21(5):379–99. [DOI] [PubMed] [Google Scholar]
- 4.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 5.Bjørge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—a new paradigm for tissue repair. Biomater Sci. 2017;6(1):60–78. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–59. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J, Wang X, Jing Y, Chen X, Su J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6(9):2905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater. 2022;14:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H, Niu L, Yu L, Jin K, Zhang J, Liu J, Zhu X, Wu Y, Zhang Y. Cancer phototherapy with nano-bacteria biohybrids. J Control Release. 2023;360:133–48. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinidoust Z, Mostaghaci B, Yasa O, Park B-W, Singh AV, Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Geng Z, Su J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Extracell Vesicles Circ Nucleic Acids. 2022;3(2):63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Jiang W, Zheng C, Shao D, Liu Y, Huang S, Han J, Ding J, Tao Y, Li M. Oral delivery of bacteria: basic principles and biomedical applications. J Control Release. 2020;327:801–33. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Rozovsky S, Chen W. Engineering multi-functional bacterial outer membrane vesicles as modular nanodevices for biosensing and bioimaging. Chem Commun (Camb). 2017;53(54):7569–72. [DOI] [PubMed] [Google Scholar]
- 14.Stejskal L, Thistlethwaite A, Ramirez-Bencomo F, Rashmi S, Harrison O, Feavers IM, Maiden MCJ, Jerse A, Barnes G, Chirro O, Chemweno J, Nduati E, Cehovin A, Tang C, Sanders EJ, Derrick JP. Profiling IgG and IgA antibody responses during vaccination and infection in a high-risk gonorrhoea population. Nat Commun. 2024;15(1):6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini-Giv N, Basas A, Hicks C, El-Omar E, El-Assaad F, Hosseini-Beheshti E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front Cell Infect Microbiol. 2022;12: 962216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shockman GD, Barren JF. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37(1):501–27. [DOI] [PubMed] [Google Scholar]
- 18.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17(1):13–24. [DOI] [PubMed] [Google Scholar]
- 20.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schertzer JW, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. MBio. 2012;3(2): e00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT. Biogenesis of bacterial membrane vesicles. Mol Microbiol. 2009;72(6):1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun. 2017;8(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13(10):620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. 2018;9(1):1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan CA, Hunt JR, Kremer N, Krasity BC, Apicella MA, McFall-Ngai MJ, Ruby EG. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. Elife. 2014;3: e01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devos S, Van Putte W, Vitse J, Van Driessche G, Stremersch S, Van Den Broek W, Raemdonck K, Braeckmans K, Stahlberg H, Kudryashev M, Savvides SN, Devreese B. Membrane vesicle secretion and prophage induction in multidrug-resistant Stenotrophomonas maltophilia in response to ciprofloxacin stress. Environ Microbiol. 2017;19(10):3930–7. [DOI] [PubMed] [Google Scholar]
- 28.Zheng K, Feng Y, Li L, Kong F, Gao J, Kong X. Engineered bacterial outer membrane vesicles: a versatile bacteria-based weapon against gastrointestinal tumors. Theranostics. 2024;14(2):761–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroniger T, Otto A, Becher D. Proteomic analysis of bacterial (outer) membrane vesicles: progress and clinical potential. Expert Rev Proteomics. 2018;15(8):623–6. [DOI] [PubMed] [Google Scholar]
- 30.Dutta S, Iida K, Takade A, Meno Y, Nair GB, Yoshida S. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol Immunol. 2004;48(12):965–9. [DOI] [PubMed] [Google Scholar]
- 31.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5(4): e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stentz R, Osborne S, Horn N, Li AW, Hautefort I, Bongaerts R, Rouyer M, Bailey P, Shears SB, Hemmings AM, Brearley CA, Carding SR. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014;6(4):646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalebroux ZD, Matamouros S, Whittington D, Bishop RE, Miller SI. PhoPQ regulates acidic glycerophospholipid content of the Salmonella Typhimurium outer membrane. Proc Natl Acad Sci USA. 2014;111(5):1963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol. 2013;11(7):467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan R, Zhou Y, Chen X, Zhong X, He F, Peng W, Li L, Wang X, Xu Y. Porphyromonas gingivalis outer membrane vesicles promote apoptosis via msRNA-regulated DNA methylation in periodontitis. Microbiol Spectr. 2023;11(1): e0328822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston EL, Zavan L, Bitto NJ, Petrovski S, Hill AF, Kaparakis-Liaskos M. Planktonic and biofilm-derived pseudomonas aeruginosa outer membrane vesicles facilitate horizontal gene transfer of plasmid DNA. Microbiol Spectr. 2023;11(2): e0517922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebenberger SP, Cakar F, Chen YC, Pressler K, Eberl L, Schild S. The activity of the quorum sensing regulator HapR is modulated by the bacterial extracellular vesicle (BEV)-associated protein ObfA of Vibrio cholerae. J Extracell Vesicles. 2024;13(9): e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KS, Svennerholm K, Crescitelli R, Lässer C, Gribonika I, Lötvall J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J Extracell Vesicles. 2021;10(9): e12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Liang S, Chen S, Ma T, Chen M, Niu C, Leng Y, Wang L. Bacterial outer membrane vesicle-cancer cell hybrid membrane-coated nanoparticles for sonodynamic therapy in the treatment of breast cancer bone metastasis. J Nanobiotechnol. 2024;22(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Terrasse R, Bafna JA, Benier L, Winterhalter M. Electrophysiological characterization of transport across outer-membrane channels from gram-negative bacteria in presence of lipopolysaccharides. Angew Chem Int Ed Engl. 2020;59(22):8517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rath P, Hermann A, Schaefer R, Agustoni E, Vonach JM, Siegrist M, Miscenic C, Tschumi A, Roth D, Bieniossek C, Hiller S. High-throughput screening of BAM inhibitors in native membrane environment. Nat Commun. 2023;14(1):5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potapova A, Garvey W, Dahl P, Guo S, Chang Y, Schwechheimer C, Trebino MA, Floyd KA, Phinney BS, Liu J, Malvankar NS, Yildiz FH. Outer membrane vesicles and the outer membrane protein OmpU govern Vibrio cholerae biofilm matrix assembly. MBio. 2024;15(2): e0330423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitz W, Montero DA, Pardo M, Araya D, De la Fuente M, Hermoso MA, Farfán MJ, Ginard D, Rosselló-Móra R, Rasko DA, Del Canto F, Vidal RM. Characterization of adherent-invasive Escherichia coli (AIEC) outer membrane proteins provides potential molecular markers to screen putative AIEC strains. Int J Mol Sci. 2022;23(16):9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babii S, Li W, Yang L, Grzegorzewicz AE, Jackson M, Gumbart JC, Zgurskaya HI. Allosteric coupling of substrate binding and proton translocation in MmpL3 transporter from Mycobacterium tuberculosis. MBio. 2024;15: e0218324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naradasu D, Miran W, Sharma S, Takenawa S, Soma T, Nomura N, Toyofuku M, Okamoto A. Biogenesis of outer membrane vesicles concentrates the unsaturated fatty acid of phosphatidylinositol in Capnocytophaga ochracea. Front Microbiol. 2021;12: 682685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua Y, Wang J, Huang M, Huang Y, Zhang R, Bu F, Yang B, Chen J, Lin X, Hu X, Zheng L, Wang Q. Outer membrane vesicles-transmitted virulence genes mediate the emergence of new antimicrobial-resistant hypervirulent Klebsiella pneumoniae. Emerg Microbes Infect. 2022;11(1):1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donoghue EJ, Sirisaengtaksin N, Browning DF, Bielska E, Hadis M, Fernandez-Trillo F, Alderwick L, Jabbari S, Krachler AM. Lipopolysaccharide structure impacts the entry kinetics of bacterial outer membrane vesicles into host cells. PLoS Pathog. 2017;13(11): e1006760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giordano NP, Cian MB, Dalebroux ZD. Outer membrane lipid secretion and the innate immune response to gram-negative bacteria. Infect Immun. 2020;88(7):e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durant L, Stentz R, Noble A, Brooks J, Gicheva N, Reddi D, O’Connor MJ, Hoyles L, McCartney AL, Man R, Pring ET, Dilke S, Hendy P, Segal JP, Lim DNF, Misra R, Hart AL, Arebi N, Carding SR, Knight SC. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome. 2020;8(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12(4):509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–87. [DOI] [PubMed] [Google Scholar]
- 52.Lapinet JA, Scapini P, Calzetti F, Pérez O, Cassatella MA. Gene expression and production of tumor necrosis factor alpha, interleukin-1beta (IL-1beta), IL-8, macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect Immun. 2000;68(12):6917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribeiro de Freitas MC, Elaine de Almeida P, Vieira WV, Ferreira-Machado AB, Resende JA, LúciadaSilva V, Diniz CG. Inflammatory modulation and outer membrane vesicles (OMV) production associated to Bacteroides fragilis response to subinhibitory concentrations of metronidazole during experimental infection. Anaerobe. 2022;73: 102504. [DOI] [PubMed] [Google Scholar]
- 54.Park KS, Lee J, Jang SC, Kim SR, Jang MH, Lötvall J, Kim YK, Gho YS. Pulmonary inflammation induced by bacteria-free outer membrane vesicles from Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2013;49(4):637–45. [DOI] [PubMed] [Google Scholar]
- 55.Kim YM, Lee KS, Kim WM, Kim M, Park HO, Choi CW, Han JS, Park SY, Lee KS. Hydrochloric acid-treated Bacillus subtilis ghosts induce IL-1 beta, IL-6, and TNF-alpha in murine macrophage. Mol Cell Toxicol. 2022;18(2):267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behrouzi A, Mazaheri H, Falsafi S, Tavassol ZH, Moshiri A, Siadat SD. Intestinal effect of the probiotic Escherichia coli strain Nissle 1917 and its OMV. J Diabetes Metab Disord. 2020;19(1):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi DS, Kim DK, Choi SJ, Lee J, Choi JP, Rho S, Park SH, Kim YK, Hwang D, Gho YS. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics. 2011;11(16):3424–9. [DOI] [PubMed] [Google Scholar]
- 58.Bielaszewska M, Marejková M, Bauwens A, Kunsmann-Prokscha L, Mellmann A, Karch H. Enterohemorrhagic Escherichia coli O157 outer membrane vesicles induce interleukin 8 production in human intestinal epithelial cells by signaling via Toll-like receptors TLR4 and TLR5 and activation of the nuclear factor NF-κB. Int J Med Microbiol. 2018;308(7):882–9. [DOI] [PubMed] [Google Scholar]
- 59.Gujrati V, Kim S, Kim S-H, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525–37. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y, Li Y, Liu G, Xu J, Xu J. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 2020;14(12):16698–711. [DOI] [PubMed] [Google Scholar]
- 61.Wang W, Chanda W, Zhong M. The relationship between biofilm and outer membrane vesicles: a novel therapy overview. FEMS Microbiol Lett. 2015;362(15):fnv117. [DOI] [PubMed] [Google Scholar]
- 62.Hathroubi S, Servetas SL, Windham I, Merrell DS, Ottemann KM. Helicobacter pylori biofilm formation and its potential role in pathogenesis. Microbiol Mol Biol Rev. 2018;82(2): e0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nibali L, Sousa V, Davrandi M, Spratt D, Alyahya Q, Dopico J, Donos N. Differences in the periodontal microbiome of successfully treated and persistent aggressive periodontitis. J Clin Periodontol. 2020;47(8):980–90. [DOI] [PubMed] [Google Scholar]
- 64.Esoda CN, Kuehn MJ. Pseudomonas aeruginosa leucine aminopeptidase influences early biofilm composition and structure via vesicle-associated antibiofilm activity. MBio. 2019;10(6): e02548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Hoffmann JP, Baker SM, Bentrup KHZ, Wimley WC, Fuselier JA, Bitoun JP, Morici LA. Inhibition of Streptococcus mutans biofilms with bacterial-derived outer membrane vesicles. BMC Microbiol. 2021;21(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadurugamuwa JL, Mayer A, Messner P, Sára M, Sleytr UB, Beveridge TJ. S-layered Aneurinibacillus and Bacillus spp. are susceptible to the lytic action of Pseudomonas aeruginosa membrane vesicles. J Bacteriol. 1998;180(9):2306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu S, Huang Y, Yan J, Li Y, Wang J, Yang YY, Yuan P, Ding X. Bacterial outer membrane-coated mesoporous silica nanoparticles for targeted delivery of antibiotic rifampicin against Gram-negative bacterial infection in vivo. Adv Funct Mater. 2021;31(35):2103442. [Google Scholar]
- 68.MacDonald KL, Beveridge TJ. Bactericidal effect of gentamicin-induced membrane vesicles derived from Pseudomonas aeruginosa PAO1 on gram-positive bacteria. Can J Microbiol. 2002;48(9):810–20. [DOI] [PubMed] [Google Scholar]
- 69.Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, Kim YK, Roh TY, Gho YS. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob Agents Chemother. 2013;57(6):2589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9(24):5425–36. [DOI] [PubMed] [Google Scholar]
- 71.Brown L, Kessler A, Cabezas-Sanchez P, Luque-Garcia JL, Casadevall A. Extracellular vesicles produced by the gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol Microbiol. 2014;93(1):183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Y, Kong Q, Roland KL, Curtiss R. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int J Med Microbiol. 2014;304(3–4):431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martín-Peña R, González-Reyes JA, Jiménez-Munguía I, Gómez-Gascón L, Fernández J, Luque-García JL, García-Lidón C, Estévez H, Pachón J, Obando I, Casadevall A, Pirofski LA, Rodríguez-Ortega MJ. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014;106:46–60. [DOI] [PubMed] [Google Scholar]
- 74.Florez C, Raab JE, Cooke AC, Schertzer JW. Membrane distribution of the pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. MBio. 2017;8(4):1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, Van Balkom BW. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1(1):18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juodeikis R, Martins C, Saalbach G, Richardson J, Koev T, Baker DJ, Defernez M, Warren M, Carding SR. Differential temporal release and lipoprotein loading in B. thetaiotaomicron bacterial extracellular vesicles. J Extracell Vesicles. 2024;13(1): e12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soares M, Pinto MM, Nobre RJ, de Almeida LP, da Graça Rasteiro M, Almeida-Santos T, Ramalho-Santos J, Sousa AP. Isolation of extracellular vesicles from human follicular fluid: size-exclusion chromatography versus ultracentrifugation. Biomolecules. 2023;13(2):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim W, Lee EJ, Bae IH, Myoung K, Kim ST, Park PJ, Lee KH, Pham AVQ, Ko J, Oh SH, Cho EG. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J Extracell Vesicles. 2020;9(1):1793514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byts N, Makieieva O, Zhyvolozhnyi A, Bart G, Korvala J, Hekkala J, Salmi S, Samoylenko A, Reunanen J. Purification of bacterial-enriched extracellular vesicle samples from feces by density gradient ultracentrifugation. Methods Mol Biol. 2023;2668:211–26. [DOI] [PubMed] [Google Scholar]
- 80.Reimer SL, Beniac DR, Hiebert SL, Booth TF, Chong PM, Westmacott GR, Zhanel GG, Bay DC. Comparative analysis of outer membrane vesicle isolation methods with an Escherichia coli tolA mutant reveals a hypervesiculating phenotype with outer-inner membrane vesicle content. Front Microbiol. 2021;12: 628801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellis TN, Leiman SA, Kuehn MJ. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun. 2010;78(9):3822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sunkara V, Park J, Han J, Del Río JS, Cho HJ, Oh IJ, Cho YK. Exosome precipitation by ionic strength modulation: ExoPRISM. ACS Appl Mater Interfaces. 2023;15(49):56807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, Zhou X, Sang Y, Liang X, Liu J, Pan S, Wang L. Identification of a specific surface epitope of OmpC for Escherichia coli O157:H7 with protein topology facilitated affinity mass spectrometry. Appl Microbiol Biotechnol. 2021;105(18):6819–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neyroud AS, Chiechio RM, Moulin G, Ducarre S, Heichette C, Dupont A, Budzynski M, Even-Hernandez P, Faro MJL, Yefimova M, Marchi V, Ravel C. Diversity of extracellular vesicles in human follicular fluid: morphological analysis and quantification. Int J Mol Sci. 2022;23(19):11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bachurski D, Schuldner M, Nguyen PH, Malz A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M, Pogge von Strandmann E. Extracellular vesicle measurements with nanoparticle tracking analysis—an accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles. 2019;8(1):1596016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coumans FA, van der Pol E, Böing AN, Hajji N, Sturk G, van Leeuwen TG, Nieuwland R. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J Extracell Vesicles. 2014;3:25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vogel R, Savage J, Muzard J, Camera GD, Vella G, Law A, Marchioni M, Mehn D, Geiss O, Peacock B, Aubert D, Calzolai L, Caputo F, Prina-Mello A. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: who is up to the challenge? J Extracell Vesicles. 2021;10(3): e12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8(1):626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li CC, Hsu WF, Chiang PC, Kuo MC, Wo AM, Tseng YJ. Characterization of markers, functional properties, and microbiome composition in human gut-derived bacterial extracellular vesicles. Gut Microbes. 2023;15(2):2288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu T, Liu N, Wang Y, Li T, Zhang M. Differential expression of coagulation pathway-related proteins in diabetic urine exosomes. Cardiovasc Diabetol. 2023;22(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arab T, Mallick ER, Huang Y, Dong L, Liao Z, Zhao Z, Gololobova O, Smith B, Haughey NJ, Pienta KJ, Slusher BS, Tarwater PM, Tosar JP, Zivkovic AM, Vreeland WN, Paulaitis ME, Witwer KW. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J Extracell Vesicles. 2021;10(6): e12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van der Pol E, de Rond L, Coumans FAW, Gool EL, Böing AN, Sturk A, Nieuwland R, van Leeuwen TG. Absolute sizing and label-free identification of extracellular vesicles by flow cytometry. Nanomedicine. 2018;14(3):801–10. [DOI] [PubMed] [Google Scholar]
- 93.Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z, Su J. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J. 2022;450: 138309. [Google Scholar]
- 94.Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterization of extracellular vesicles. Am J Reprod Immunol. 2021;85(2): e13367. [DOI] [PubMed] [Google Scholar]
- 95.Kim W, Lee EJ, Bae I-H, Myoung K, Kim ST, Park PJ, Lee K-H, Pham AVQ, Ko J, Oh SH. Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J Extracell Vesicles. 2020;9(1):1793514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tulkens J, De Wever O, Hendrix A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat Protoc. 2020;15(1):40–67. [DOI] [PubMed] [Google Scholar]
- 97.He L, Zhu D, Wang J, Wu X. A highly efficient method for isolating urinary exosomes. Int J Mol Med. 2019;43(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270(2):211–26. [DOI] [PubMed] [Google Scholar]
- 99.Hong C-S, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5(1):29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehanny M, Koch M, Lehr CM, Fuhrmann G. Streptococcal extracellular membrane vesicles are rapidly internalized by immune cells and alter their cytokine release. Front Immunol. 2020;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens M, Heijnen HFG, van Bergen En Henegouwen PMP, Vader P, Schiffelers RM. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85. [DOI] [PubMed] [Google Scholar]
- 102.Cheung RCF, Wong JH, Ng TB. Immobilized metal ion affinity chromatography: a review on its applications. Appl Microbiol Biotechnol. 2012;96:1411–20. [DOI] [PubMed] [Google Scholar]
- 103.Alves NJ, Turner KB, DiVito KA, Daniele MA, Walper SA. Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a His-tag mutant. Res Microbiol. 2017;168(2):139–46. [DOI] [PubMed] [Google Scholar]
- 104.Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, Wan TF, Yue T, Tan YJ, Yin H. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. 2021;8(9):2004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sivanantham A, Jin Y. Impact of storage conditions on EV integrity/surface markers and cargos. Life (Basel). 2022;12(5):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richter M, Fuhrmann K, Fuhrmann G. Evaluation of the storage stability of extracellular vesicles. J Vis Exp. 2019;147: e59584. [DOI] [PubMed] [Google Scholar]
- 107.Schulz E, Goes A, Garcia R, Panter F, Koch M, Müller R, Fuhrmann K, Fuhrmann G. Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J Control Release. 2018;290:46–55. [DOI] [PubMed] [Google Scholar]
- 108.Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, Carr B, Redman CWG, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomed Nanotechnol Biol Med. 2011;7(6):780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vestad B, Llorente A, Neurauter A, Phuyal S, Kierulf B, Kierulf P, Skotland T, Sandvig K, Haug KBF, Øvstebø R. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles. 2017;6(1):1344087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee H, Kang H, Kang M, Han C, Yi J, Kwon Y, Park J. Heterogeneous subcellular origin of exosome-mimetic nanovesicles engineered from cells. ACS Biomater Sci Eng. 2020;6(11):6063–8. [DOI] [PubMed] [Google Scholar]
- 112.Danielson KM, Estanislau J, Tigges J, Toxavidis V, Camacho V, Felton EJ, Khoory J, Kreimer S, Ivanov AR, Mantel PY, Jones J, Akuthota P, Das S, Ghiran I. Diurnal variations of circulating extracellular vesicles measured by nano flow cytometry. PLoS ONE. 2016;11(1): e0144678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puca V, Ercolino E, Celia C, Bologna G, Di Marzio L, Mincione G, Marchisio M, Miscia S, Muraro R, Lanuti P, Grande R. Detection and quantification of eDNA-associated bacterial membrane vesicles by flow cytometry. Int J Mol Sci. 2019;20(21):5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci USA. 2010;107(7):3099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dauros Singorenko P, Chang V, Whitcombe A, Simonov D, Hong J, Phillips A, Swift S, Blenkiron C. Isolation of membrane vesicles from prokaryotes: a technical and biological comparison reveals heterogeneity. J Extracell Vesicles. 2017;6(1):1324731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Edgar L, Pu T, Porter B, Aziz JM, La Pointe C, Asthana A, Orlando G. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020;107(7):793–800. [DOI] [PubMed] [Google Scholar]
- 117.Shang L, Zhao Y, Cui W. Regenerative medicine entering a new era. Small. 2022;18(36): e2204625. [DOI] [PubMed] [Google Scholar]
- 118.Kim D-S, Bae S. Impact and challenges of enactment for advanced regenerative medicine in South Korea. Front Bioeng Biotechnol. 2022;10: 972865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv. 2018;36(4):1111–26. [DOI] [PubMed] [Google Scholar]
- 120.Yamanaka S. Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell. 2020;27(4):523–31. [DOI] [PubMed] [Google Scholar]
- 121.Voga M, Adamic N, Vengust M, Majdic G. Stem cells in veterinary medicine—current state and treatment options. Front Vet Sci. 2020;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kesidou D, da Costa Martins PA, De Windt LJ, Brittan M, Beqqali A, Baker AH. Extracellular vesicle miRNAs in the promotion of cardiac neovascularisation. Front Physiol. 2020;11: 579892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17(5):1723–33. [DOI] [PubMed] [Google Scholar]
- 124.Whitford W, Guterstam P. Exosome manufacturing status. Future Med Chem. 2019;11(10):1225–36. [DOI] [PubMed] [Google Scholar]
- 125.Xie J, Li Q, Haesebrouck F, Van Hoecke L, Vandenbroucke RE. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022;40(10):1173–94. [DOI] [PubMed] [Google Scholar]
- 126.Cao P, Xu ZP, Li L. Tailoring functional nanoparticles for oral vaccine delivery: recent advances and future perspectives. Compos B Eng. 2022;236: 109826. [Google Scholar]
- 127.Furtado M, Chen L, Chen Z, Chen A, Cui W. Development of fish collagen in tissue regeneration and drug delivery. Eng Regen. 2022;3(3):217–31. [Google Scholar]
- 128.Liu H, Zhang H, Wang S, Cui J, Weng W, Liu X, Tang H, Hu Y, Li X, Zhang K, Zhou F, Jing Y, Su J. Bone-targeted bioengineered bacterial extracellular vesicles delivering siRNA to ameliorate osteoporosis. Compos B Eng. 2023;255: 110610. [Google Scholar]
- 129.Yang D, Park SY, Park YS, Eun H, Lee SY. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020;38(7):745–65. [DOI] [PubMed] [Google Scholar]
- 130.Chen H, Zhou M, Zeng Y, Miao T, Luo H, Tong Y, Zhao M, Mu R, Gu J, Yang S, Han L. Biomimetic lipopolysaccharide-free bacterial outer membrane-functionalized nanoparticles for brain-targeted drug delivery. Adv Sci (Weinh). 2022;9(16): e2105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li S, Yue Y, Wang W, Han M, Wan X, Li Q, Chen X, Cao J, Zhang Y, Li J, Li J, Cheng L, Yang J, Wang D, Zhou Z. Ultrasound-activated probiotics vesicles coating for titanium implant infections through bacterial cuproptosis-like death and immunoregulation. Adv Mater. 2024;36: e2405953. [DOI] [PubMed] [Google Scholar]
- 132.Pan C, Li J, Hou W, Lin S, Wang L, Pang Y, Wang Y, Liu J. Polymerization-mediated multifunctionalization of living cells for enhanced cell-based therapy. Adv Mater. 2021;33(13): e2007379. [DOI] [PubMed] [Google Scholar]
- 133.Li J, Sun M, Liu L, Yang W, Sun A, Yu J, Liu D, Zhao W, Cheng M, He Z, Gu Z, Sun J. Nanoprobiotics for remolding the pro-inflammatory microenvironment and microbiome in the treatment of colitis. Nano Lett. 2023;23(18):8593–601. [DOI] [PubMed] [Google Scholar]
- 134.Shi R, Dong Z, Ma C, Wu R, Lv R, Liu S, Ren Y, Liu Z, van der Mei HC, Busscher HJ, Liu J. High-yield, magnetic harvesting of extracellular outer-membrane vesicles from Escherichia coli. Small. 2022;18(48): e2204350. [DOI] [PubMed] [Google Scholar]
- 135.Pan J, Wang Z, Huang X, Xue J, Zhang S, Guo X, Zhou S. Bacteria-derived outer-membrane vesicles hitchhike neutrophils to enhance ischemic stroke therapy. Adv Mater. 2023;35(38): e2301779. [DOI] [PubMed] [Google Scholar]
- 136.Ayed Z, Cuvillier L, Dobhal G, Goreham RV. Electroporation of outer membrane vesicles derived from Pseudomonas aeruginosa with gold nanoparticles. SN Appl Sci. 2019;1:1–9. [Google Scholar]
- 137.Liu H, Song P, Zhang H, Zhou F, Ji N, Wang M, Zhou G, Han R, Liu X, Weng W, Tan H, Wang S, Zheng L, Jing Y, Su J. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. 2024;13(4): e12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin S, Han S, Wang X, Wang X, Shi X, He Z, Sun M, Sun J. Oral microto-nano genome-editing system enabling targeted delivery and conditional activation of CRISPR-Cas9 for gene therapy of inflammatory bowel disease. ACS Nano. 2024;18:25657. [DOI] [PubMed] [Google Scholar]
- 139.Wei X, Xue B, Ruan S, Guo J, Huang Y, Geng X, Wang D, Zhou C, Zheng J, Yuan Z. Supercharged precision killers: Genetically engineered biomimetic drugs of screened metalloantibiotics against Acinetobacter baumanni. Sci Adv. 2024;10(12): eadk331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas SC, Madaan T, Kamble NS, Siddiqui NA, Pauletti GM, Kotagiri N. Engineered bacteria enhance immunotherapy and targeted therapy through stromal remodeling of tumors. Adv Healthc Mater. 2022;11(2): e2101487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gudbergsson JM, Jønsson K, Simonsen JB, Johnsen KB. Systematic review of targeted extracellular vesicles for drug delivery—considerations on methodological and biological heterogeneity. J Control Release. 2019;306:108–20. [DOI] [PubMed] [Google Scholar]
- 142.Han EC, Choi SY, Lee Y, Park JW, Hong SH, Lee HJ. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 2019;33(12):13412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alves NJ, Turner KB, Daniele MA, Oh E, Medintz IL, Walper SA. Bacterial nanobioreactors-directing enzyme packaging into bacterial outer membrane vesicles. ACS Appl Mater Interfaces. 2015;7(44):24963–72. [DOI] [PubMed] [Google Scholar]
- 144.Zanella I, König E, Tomasi M, Gagliardi A, Frattini L, Fantappiè L, Irene C, Zerbini F, Caproni E, Isaac SJ. Proteome-minimized outer membrane vesicles from Escherichia coli as a generalized vaccine platform. J Extracell Vesicles. 2021;10(4): e12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mancini F, Micoli F, Necchi F, Pizza M, Berlanda Scorza F, Rossi O. GMMA-based vaccines: the known and the unknown. Front Immunol. 2021;12: 715393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Q, Li D, Pan X, Liang Y. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J Nanobiotechnol. 2023;21(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Richard JRK, Alexander JW, Paul SF. Opportunities for engineering outer membrane vesicles using synthetic biology approaches. Extracell Vesicles Circul Nucleic Acids. 2023;4(2):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scott L, Jurewicz I, Jeevaratnam K, Lewis R. Carbon nanotube-based scaffolds for cardiac tissue engineering-systematic review and narrative synthesis. Bioengineering (Basel). 2021;8(6):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xi Y, Ge J, Guo Y, Lei B, Ma PX. Biomimetic elastomeric polypeptide-based nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano. 2018;12(11):10772–84. [DOI] [PubMed] [Google Scholar]
- 150.Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. 2021;22(2):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, Wollacott R, Sapp E, Dubuke ML, Li X, Shaffer SA, DiFiglia M, Wang Y, Aronin N, Khvorova A. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y, Xia X, Liu Z, Dong M. The next frontier of 3D bioprinting: bioactive materials functionalized by bacteria. Small. 2023;19(10): e2205949. [DOI] [PubMed] [Google Scholar]
- 153.Jones EJ, Booth C, Fonseca S, Parker A, Cross K, Miquel-Clopés A, Hautefort I, Mayer U, Wileman T, Stentz R, Carding SR. The uptake, trafficking, and biodistribution of bacteroides thetaiotaomicron generated outer membrane vesicles. Front Microbiol. 2020;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Y, Wu J, Qiu X, Dong S, He J, Liu J, Xu W, Huang S, Hu X, Xiang D-X. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioactive Materials. 2023;20:548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thapa HB, Müller AM, Camilli A, Schild S. An intranasal vaccine based on outer membrane vesicles against SARS-CoV-2. Front Microbiol. 2021;12: 752739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Feng Q, Ma X, Cheng K, Liu G, Li Y, Yue Y, Liang J, Zhang L, Zhang T, Wang X, Gao X, Nie G, Zhao X. Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater. 2022;34(40): e2206200. [DOI] [PubMed] [Google Scholar]
- 157.Xing Y, Yerneni SS, Wang W, Taylor RE, Campbell PG, Ren X. Engineering pro-angiogenic biomaterials via chemoselective extracellular vesicle immobilization. Biomaterials. 2022;281: 121357. [DOI] [PubMed] [Google Scholar]
- 158.Jang SC, Kim SR, Yoon YJ, Park KS, Kim JH, Lee J, Kim OY, Choi EJ, Kim DK, Choi DS, Kim YK, Park J, Di Vizio D, Gho YS. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small. 2015;11(4):456–61. [DOI] [PubMed] [Google Scholar]
- 159.Zingl FG, Thapa HB, Scharf M, Kohl P, Müller AM, Schild S. Outer membrane vesicles of vibrio cholerae protect and deliver active cholera toxin to host cells via porin-dependent uptake. MBio. 2021;12(3): e0053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arigita C, van den Berg J, Wensink K, van Steenbergen M, Hennink WE, Crommelin DJ, Kersten GF, Jiskoot W. Immunogenicity of meningococcal PorA formulations encapsulated in biodegradable microspheres. Eur J Pharm Sci. 2004;21(2–3):131–41. [DOI] [PubMed] [Google Scholar]
- 161.Chen Y, Huang J, Chen R, Yang L, Wang J, Liu B, Du L, Yi Y, Jia J, Xu Y, Chen Q, Ngondi DG, Miao Y, Hu Z. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10(3):1454–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nie X, Shi C, Chen X, Yu C, Jiang Z, Xu G, Lin Y, Tang M, Luan Y. A single-shot prophylactic tumor vaccine enabled by an injectable biomembrane hydrogel. Acta Biomater. 2023;169:306–16. [DOI] [PubMed] [Google Scholar]
- 163.O’Donoghue EJ, Krachler AM. Mechanisms of outer membrane vesicle entry into host cells. Cell Microbiol. 2016;18(11):1508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Caruana JC, Walper SA. Bacterial membrane vesicles as mediators of microbe–microbe and microbe–host community interactions. Front Microbiol. 2020;11:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Turner L, Bitto NJ, Steer DL, Lo C, D’Costa K, Ramm G, Shambrook M, Hill AF, Ferrero RL, Kaparakis-Liaskos M. Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front Immunol. 2018;9:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Furuta N, Tsuda K, Omori H, Yoshimori T, Yoshimura F, Amano A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect Immun. 2009;77(10):4187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schaar V, de Vries SP, Perez Vidakovics ML, Bootsma HJ, Larsson L, Hermans PW, Bjartell A, Mörgelin M, Riesbeck K. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell Microbiol. 2011;13(3):432–49. [DOI] [PubMed] [Google Scholar]
- 168.Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10(13): e12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, Wai SN, Oscarsson J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cañas MA, Giménez R, Fábrega MJ, Toloza L, Baldomà L, Badia J. Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS ONE. 2016;11(8): e0160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pan Z, Sun W, Chen Y, Tang H, Lin W, Chen J, Chen C. Extracellular vesicles in tissue engineering: biology and engineered strategy. Adv Healthc Mater. 2022;11(21): e2201384. [DOI] [PubMed] [Google Scholar]
- 172.Chen C-Y, Rao S-S, Yue T, Tan Y-J, Yin H, Chen L-J, Luo M-J, Wang Z, Wang Y-Y, Hong C-G. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci Adv. 2022;8(15):eabg8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kashyap D, Panda M, Baral B, Varshney N, Bhandari V, Parmar HS, Prasad A, Jha HC. Outer membrane vesicles: an emerging vaccine platform. Vaccines (Basel). 2022;10(10):1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10(11):1689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mancini F, Rossi O, Necchi F, Micoli F. OMV vaccines and the role of TLR agonists in immune response. Int J Mol Sci. 2020;21(12):4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Walsh RM, Hochedlinger K. A variant CRISPR-Cas9 system adds versatility to genome engineering. Proc Natl Acad Sci USA. 2013;110(39):15514–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schwechheimer C, Rodriguez DL, Kuehn MJ. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. Microbiologyopen. 2015;4(3):375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gujrati V, Prakash J, Malekzadeh-Najafabadi J, Stiel A, Klemm U, Mettenleiter G, Aichler M, Walch A, Ntziachristos V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun. 2019;10(1):1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman MF. LPS remodeling triggers formation of outer membrane vesicles in salmonella. MBio. 2016;7(4): e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci USA. 2013;110(4):1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, Qin H, Qin Y, Chen L, Li C, Liang J, Li Y, Xu J, Han X, Anderson GJ, Shi J, Ren L, Zhao X, Nie G. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12(1):2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu Y, Li H, Wang J, Yao M, Peng Y, Liu T, Li Z, Luo G, Deng J. Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing. Adv Funct Mater. 2021;31(48):2105749. [Google Scholar]
- 184.Chen C-Y, Du W, Rao S-S, Tan Y-J, Hu X-K, Luo M-J, Ou Q-F, Wu P-F, Qing L-M, Cao Z-M. Extracellular vesicles from human urine-derived stem cells inhibit glucocorticoid-induced osteonecrosis of the femoral head by transporting and releasing pro-angiogenic DMBT1 and anti-apoptotic TIMP1. Acta Biomater. 2020;111:208–20. [DOI] [PubMed] [Google Scholar]
- 185.Wang G, Sweren E, Liu H, Wier E, Alphonse MP, Chen R, Islam N, Li A, Xue Y, Chen J, Park S, Chen Y, Lee S, Wang Y, Wang S, Archer NK, Andrews W, Kane MA, Dare E, Reddy SK, Hu Z, Grice EA, Miller LS, Garza LA. Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe. 2021;29(5):777-791.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Remaggi G, Bottari B, Bancalari E, Catanzano O, Neviani E, Elviri L. Lactobacillus delbrueckii subsp. bulgaricus derivatives for 3D printed alginate/hyaluronic acid self-crosslinking hydrogels: Manufacturing and wound healing potential. Int J Biol Macromol. 2023;242: 124454. [DOI] [PubMed] [Google Scholar]
- 187.Di Domizio J, Belkhodja C, Chenuet P, Fries A, Murray T, Mondéjar PM, Demaria O, Conrad C, Homey B, Werner S, Speiser DE, Ryffel B, Gilliet M. The commensal skin microbiota triggers type I IFN–dependent innate repair responses in injured skin. Nat Immunol. 2020;21(9):1034–45. [DOI] [PubMed] [Google Scholar]
- 188.Valdéz JC, Peral MC, Rachid M, Santana M, Perdigón G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005;11(6):472–9. [DOI] [PubMed] [Google Scholar]
- 189.Owji N, Mandakhbayar N, Gregory DA, Marcello E, Kim HW, Roy I, Knowles JC. Mussel inspired chemistry and bacteria derived polymers for oral mucosal adhesion and drug delivery. Front Bioeng Biotechnol. 2021;9: 663764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roberts JL, Golloshi M, Harding DB, Conduah M, Liu G, Drissi H. Bifidobacterium longum supplementation improves age-related delays in fracture repair. Aging Cell. 2023;22(4): e13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hay JJ, Rodrigo-Navarro A, Petaroudi M, Bryksin AV, García AJ, Barker TH, Dalby MJ, Salmeron-Sanchez M. Bacteria-based materials for stem cell engineering. Adv Mater. 2018;30(43): e1804310. [DOI] [PubMed] [Google Scholar]
- 192.Silveira GDP, Ishimura ME, Teixeira D, Galindo LT, Sardinha AA, Porcionatto M, Longo-Maugéri IM. Improvement of mesenchymal stem cell immunomodulatory properties by heat-killed propionibacterium acnes via TLR2. Front Mol Neurosci. 2018;11:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang C, Xue S, Wang Y, Yu D, Hua L, Guo C, Wang D, Lei M. Oral administration of Lactobacillus casei Shirota improves recovery of hand functions after distal radius fracture among elder patients: a placebo-controlled, double-blind, and randomized trial. J Orthop Surg Res. 2019;14(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roberts JL, Liu G, Darby TM, Fernandes LM, Diaz-Hernandez ME, Jones RM, Drissi H. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed Pharmacother. 2020;132: 110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang G, Sweren E, Andrews W, Li Y, Chen J, Xue Y, Wier E, Alphonse MP, Luo L, Miao Y, Chen R, Zeng D, Lee S, Li A, Dare E, Kim D, Archer NK, Reddy SK, Resar L, Hu Z, Grice EA, Kane MA, Garza LA. Commensal microbiome promotes hair follicle regeneration by inducing keratinocyte HIF-1α signaling and glutamine metabolism. Sci Adv. 2023;9(1): eabo7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dou Z, Li B, Wu L, Qiu T, Wang X, Zhang X, Shen Y, Lu M, Yang Y. Probiotic-functionalized silk fibroin/sodium alginate scaffolds with endoplasmic reticulum stress-relieving properties for promoted scarless wound healing. ACS Appl Mater Interfaces. 2023;15(5):6297–311. [DOI] [PubMed] [Google Scholar]
- 197.Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152(1–2):51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Abo H, Chassaing B, Harusato A, Quiros M, Brazil JC, Ngo VL, Viennois E, Merlin D, Gewirtz AT, Nusrat A, Denning TL. Erythroid differentiation regulator-1 induced by microbiota in early life drives intestinal stem cell proliferation and regeneration. Nat Commun. 2020;11(1):513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu H, Xie S, Miao J, Li Y, Wang Z, Wang M, Yu Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes. 2020;11(4):997–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen L, Li S, Peng C, Gui Q, Li J, Xu Z, Yang Y. Lactobacillus rhamnosus GG promotes recovery of the colon barrier in septic mice through accelerating ISCs regeneration. Nutrients. 2023;15(3):672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hasaniani N, Ghasemi-Kasman M, Halaji M, Rostami-Mansoor S. Bifidobacterium breve probiotic compared to Lactobacillus casei causes a better reduction in demyelination and oxidative stress in cuprizone-induced demyelination model of rat. Mol Neurobiol. 2023;61:498. [DOI] [PubMed] [Google Scholar]
- 202.Hess S, Kendall TJ, Pena M, Yamane K, Soong D, Adams L, Truman R, Rambukkana A. In vivo partial reprogramming by bacteria promotes adult liver organ growth without fibrosis and tumorigenesis. Cell Rep Med. 2022;3(11): 100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yin H, Chen CY, Liu YW, Tan YJ, Deng ZL, Yang F, Huang FY, Wen C, Rao SS, Luo MJ, Hu XK, Liu ZZ, Wang ZX, Cao J, Liu HM, Liu JH, Yue T, Tang SY, Xie H. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics. 2019;9(9):2678–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu JH, Yue T, Luo ZW, Cao J, Yan ZQ, Jin L, Wan TF, Shuai CJ, Wang ZG, Zhou Y, Xu R, Xie H. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis Model Mech. 2020;13(11): dmm043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tang TWH, Chen HC, Chen CY, Yen CYT, Lin CJ, Prajnamitra RP, Chen LL, Ruan SC, Lin JH, Lin PJ, Lu HH, Kuo CW, Chang CM, Hall AD, Vivas EI, Shui JW, Chen P, Hacker TA, Rey FE, Kamp TJ, Hsieh PCH. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139(5):647–59. [DOI] [PubMed] [Google Scholar]
- 206.Lombardi F, Palumbo P, Mattei A, Augello FR, Cifone MG, Giuliani M, Cinque B. Soluble Fraction from Lysates of Selected Probiotic Strains Differently Influences Re-Epithelialization of HaCaT Scratched Monolayer through a Mechanism Involving Nitric Oxide Synthase 2. Biomolecules. 2019;9(12):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jensen GS, Cash HA, Farmer S, Keller D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. J Inflamm Res. 2017;10:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shojaeian A, Mehri-Ghahfarrokhi A, Banitalebi-Dehkordi M. Increased in vitro migration of human umbilical cord mesenchymal stem cells toward acellular foreskin treated with bacterial derivatives of monophosphoryl lipid A or supernatant of Lactobacillus acidophilus. Hum Cell. 2020;33(1):10–22. [DOI] [PubMed] [Google Scholar]
- 209.Gerritzen MJH, Maas RHW, van den Ijssel J, van Keulen L, Martens DE, Wijffels RH, Stork M. High dissolved oxygen tension triggers outer membrane vesicle formation by Neisseria meningitidis. Microb Cell Fact. 2018;17(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Valeru SP, Shanan S, Alossimi H, Saeed A, Sandström G, Abd H. Lack of outer membrane protein A enhances the release of outer membrane vesicles and survival of vibrio cholerae and suppresses viability of Acanthamoeba castellanii. Int J Microbiol. 2014;2014: 610190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kulp AJ, Sun B, Ai T, Manning AJ, Orench-Rivera N, Schmid AK, Kuehn MJ. Genome-wide assessment of outer membrane vesicle production in Escherichia coli. PLoS ONE. 2015;10(9): e0139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim J-Y, Doody AM, Chen DJ, Cremona GH, Shuler ML, Putnam D, DeLisa MP. Engineered bacterial outer membrane vesicles with enhanced functionality. J Mol Biol. 2008;380(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49(1):1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Blokhina SV, Sharapova AV, Ol’khovich MV, Volkova TV, Perlovich GL. Solubility, lipophilicity and membrane permeability of some fluoroquinolone antimicrobials. Eur J Pharm Sci. 2016;93:29–37. [DOI] [PubMed] [Google Scholar]
- 215.Li Y, Wu J, Qiu X, Dong S, He J, Liu J, Xu W, Huang S, Hu X, Xiang DX. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact Mater. 2023;20:548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh). 2018;5(4):1700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ciechomska IA, Tolkovsky AM. Non-autophagic GFP-LC3 puncta induced by saponin and other detergents. Autophagy. 2007;3(6):586–90. [DOI] [PubMed] [Google Scholar]
- 218.Hettich BF, Bader JJ, Leroux JC. Encapsulation of hydrophilic compounds in small extracellular vesicles: loading capacity and impact on vesicle functions. Adv Healthc Mater. 2022;11(5): e2100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. [DOI] [PubMed] [Google Scholar]
- 220.Goh WJ, Lee CK, Zou S, Woon EC, Czarny B, Pastorin G. Doxorubicin-loaded cell-derived nanovesicles: an alternative targeted approach for anti-tumor therapy. Int J Nanomed. 2017;12:2759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm. 2015;12(10):3650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. [DOI] [PubMed] [Google Scholar]
- 223.Zhang YF, Shi JB, Li C. Small extracellular vesicle loading systems in cancer therapy: current status and the way forward. Cytotherapy. 2019;21(11):1122–36. [DOI] [PubMed] [Google Scholar]
- 224.Xiao T, Ma Y, Zhang Z, Zhang Y, Zhao Y, Zhou X, Wang X, Ge K, Guo J, Zhang J, Li Z, Liu H. Tailoring therapeutics via a systematic beneficial elements comparison between photosynthetic bacteria-derived OMVs and extruded nanovesicles. Bioact Mater. 2024;36:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fan Z, Xiao K, Lin J, Liao Y, Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15(47):1903761. [DOI] [PubMed] [Google Scholar]
- 227.Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, Smith-Jones P, Anchordoquy TJ. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25(10):1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yi K, Rong Y, Huang L, Tang X, Zhang Q, Wang W, Wu J, Wang F. Aptamer-exosomes for tumor theranostics. ACS Sens. 2021;6(4):1418–29. [DOI] [PubMed] [Google Scholar]
- 229.Kaddour H, Panzner TD, Welch JL, Shouman N, Mohan M, Stapleton JT, Okeoma CM. Electrostatic surface properties of blood and semen extracellular vesicles: implications of sialylation and HIV-induced changes on EV internalization. Viruses. 2020;12(10):1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q, Wang Y, Liu C, Qiu M, Yuan X, Zhao J, Hou X, Kang C. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. 2020;10(17):7889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Richter M, Vader P, Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev. 2021;173:416–26. [DOI] [PubMed] [Google Scholar]
- 232.Seo JJ, Mandakhbayar N, Kang MS, Yoon JY, Lee NH, Ahn J, Lee HH, Lee JH, Kim HW. Antibacterial, proangiogenic, and osteopromotive nanoglass paste coordinates regenerative process following bacterial infection in hard tissue. Biomaterials. 2021;268: 120593. [DOI] [PubMed] [Google Scholar]
- 233.Sen T, Thummer RP. The impact of human microbiotas in hematopoietic stem cell and organ transplantation. Front Immunol. 2022;13: 932228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE. 2013;8(10): e76520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang T, Mo L, Ou J, Fang Q, Wu H, Wu Y, Nandakumar KS. Proteus mirabilis vesicles induce mitochondrial apoptosis by regulating miR96-5p/Abca1 to inhibit osteoclastogenesis and bone loss. Front Immunol. 2022;13: 833040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Price EH, de Louvois J, Workman MR. Antibiotics for Salmonella meningitis in children. J Antimicrob Chemother. 2000;46(5):653–5. [DOI] [PubMed] [Google Scholar]
- 238.Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku’ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2): e12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Qing S, Lyu C, Zhu L, Pan C, Wang S, Li F, Wang J, Yue H, Gao X, Jia R, Wei W, Ma G. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32(47): e2002085. [DOI] [PubMed] [Google Scholar]
- 240.Toyofuku M, Roschitzki B, Riedel K, Eberl L. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J Proteome Res. 2012;11(10):4906–15. [DOI] [PubMed] [Google Scholar]
- 241.Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188(16):5945–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.
Not applicable.